SUMMARY
CD14 is important in the clearance of bacterial pathogens from lungs. However, the mechanisms that regulate the expression of membrane CD14 (mCD14) on alveolar macrophages (AM) have not been studied in detail. This study examines the regulation of mCD14 on AM exposed to Escherichia coli in vivo and in vitro and explores the consequences of changes in mCD14 expression. The expression of mCD14 was decreased on AM exposed to E. coli in vivo and AM incubated with lipopolysaccharide (LPS) or E. coli in vitro. Polymyxin B abolished LPS effects but only partially blocked the effects of E. coli. Blockade of extracellular signal-regulated kinase pathways attenuated LPS and E. coli-induced decrease in mCD14 expression. Inhibition of proteases abrogated the LPS-induced decrease in mCD14 expression on AM and the release of sCD14 into the supernatants, but did not affect the response to E. coli. The production of TNF-α in response to a second challenge with Staphylococcus aureus or zymosan was decreased in AM following incubation with E. coli but not LPS. These studies show that distinct mechanisms regulate the expression of mCD14 and the induction of endotoxin-tolerance in AM and suggest that AM function is impaired at sites of bacterial infection.
Keywords: alveolar macrophages, CD14, lungs, lipopolysaccharide, rabbit
INTRODUCTION
Gram-negative pneumonia is an important cause of morbidity and mortality in medical patients (1, 2). The innate immune system is responsible for the clearance of bacteria from the lungs, and an effective response results in the clearance of bacterial pathogens with minimal tissue injury. In contrast, an ineffective innate immune response results in bacterial proliferation, increased lung injury, and the development of sepsis and septic shock (3, 4).
Lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria, provides a potent signal to the innate immune system and is often used to model gram-negative infections in vitro and in vivo. However, studies show that whole E. coli causes a more potent and prolonged activation of the innate immune system when compared to equivalent amounts of E. coli LPS (5). Therefore, the activation and subsequent responses of the innate immune system to LPS and whole E. coli may differ.
CD14, the receptor for LPS, is implicated in various immune responses including activation of the innate immune system (6), bacterial phagocytosis (7), and clearance of apoptotic cells (8). Macrophages recognize LPS via membrane CD14 (mCD14) and toll-like receptor-4 (TLR4), a member of the toll-like receptor family which mediate signaling in response to a number of microbial components (9-11). Recognition of LPS by the CD14/TLR4/MD-2 complex activates intracellular signaling pathways involving mitogen-activated protein (MAP) kinases, resulting in the production of proinflammatory cytokines and chemokines (12-14). In rabbits with Escherichia coli pneumonia, blockade of CD14 protected against the deleterious systemic response that occurs in sepsis; however, CD14 blockade resulted in an increased bacterial burden in the lungs (15). This suggests that the CD14 pathway is important in local intrapulmonary host defenses and the clearance of gram-negative bacteria from lungs. However, little is known about the mechanisms that regulate the expression of mCD14 on AM at sites of bacterial infections.
Pre-administration of LPS in vitro or in vivo is known to down-regulate responses to a second LPS challenge [Reviewed in (16) and (17)]. This phenomenon, known as endotoxin-tolerance, results in animals, humans, and cells becoming refractory to a second challenge of LPS after the initial exposure. Initially, it was thought that tolerance was beneficial due to its ability to diminish the inflammatory response to LPS. However, the development of tolerance in patients with sepsis may worsen clinical outcomes due to the development of immune dysregulation (17-19). A potential mechanism whereby endotoxin-tolerance develops is the downregulation of LPS receptors such as mCD14 on macrophages (16, 20).
The objectives of this study were two-fold, first the expression of mCD14 on alveolar macrophages was measured in vivo at sites of bacterial infection and in vitro to define the mechanisms responsible for changes in mCD14 expression. The second objective was to determine whether decreased expression of mCD14 following incubation with LPS or whole E. coli affects the response of AM to a subsequent challenge with E. coli, S. aureus or zymosan. The data show that LPS and whole E. coli regulate the cell surface expression of CD14 and the development of tolerance by distinct mechanisms, and suggest that AM function may be down-regulated at sites of bacterial infection in the lungs.
MATERIALS AND METHODS
Reagents and antibodies
LPS from Escherichia coli serotype 0111:B4 was purchased from List Biological Laboratories (Campell CA). Escherichia coli serotype K-1 was a clinical isolate obtained from a patient with bacteremia due to biliary sepsis (15). The inhibitor of phosphatidylinositol-specific phospholipase ET-18-OCH3, and kinase inhibitors SB203580 (p38 inhibitor), PD98059 (ERK inhibitor), and SP600125 (JNK inhibitor) were purchased from Calbiochem Co. (La Jolla, CA). The protease inhibitor cocktail (P1860) and polymyxin B were obtained from Sigma-Alderich Co. (St. Louis MO). The Vector “Elite” ABC-HP kit was from Vector Laboratories (Burlingame, CA). S. aureus-BODIPY and Zymosan-BODOPY bioparticles were purchased from Molecular Probes (Eugene, OR). The antibodies to ERK and phosphorylated ERK were from Cell Signaling (Beverly, MA). The polyclonal goat anti-rabbit CD14, soluble CD14, polyclonal goat anti-rabbit TNF-α and recombinant rabbit TNF-α were generous gifts of John Mathison (Scripps Research Institute). The limulus amebocyte lysate kit was from Biowhittaker Co. (Walkersville, MD). Complete-RPMI was made up of RPMI-1640 (Biowhittaker, Walkersville, MD) supplemented with 10% fetal calf serum (Hyclone, Logan, UT), 10 mM HEPES (Gibco, Grand Island, NY), 2 mM L-glutamine (Gibco, Grand Island, NY), 50 U/ml penicillin and 50 μg/ml streptomycin (Penn/Strep, Gibco, Grand Island, NY).
Rabbit Model of Bacterial Pneumonia
The Animal Research Committee of the Veterans Affairs Puget Sound Health Care System approved all of the experiments. Female New Zealand White rabbits, specific pathogen free, weighing 3.0-3.5 kg were purchased from Western Oregon Rabbit Company, Philomath, OR and housed in the animal facility until the day of the experiment.
Induction of gram-negative bacterial pneumonia
Rabbits were anesthetized with a combination of intravenous ketamine (10 mg/kg) and xylazine (3 mg/kg) and allowed to breathe spontaneously. To induce pneumonia, anesthetized rabbits were placed prone on a 20-degree incline with the head elevated. Then 1.0 ml of the E. coli suspension (1 × 107 or 1 × 109 CFU/ml) was instilled in the right and left lower lobe bronchus using PE90 tubing (Intramedic, Sparks, MD) advanced through the biopsy channel of a pediatric bronchoscope (Pentax, Tokyo, Japan). Bacteria were mixed with 1% colloidal carbon (Pelikan, Hanover, Germany) to aid in identifying the instilled areas at necropsy [Doerschuk, 1994 #46]. The rabbits were placed on a heated water blanket (Gaymar Industries, Orchard Park, NY) after the instillation to maintain body temperature following anesthesia.
Immunohistochemistry
To prepare tissue for immunohistochemistry, paraffin-embedded lung tissue was sectioned into 4-6 μm slices and water mounted onto charged Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA). Slides were deparaffinized by washing twice in xylene for 5 min and rehydrated by washing twice in 100% ethanol for 3 min, twice in 95% ethanol for 3 min and once in dH2O for 5 min. Endogenous peroxidases were blocked by incubating slides with 0.3% H2O2 in methanol for 60 min, and then rinsed twice with PBS. The slides were rinsed twice with phosphate buffered saline (PBS) for 5 min and the samples digested for 10 min in 0.05% Pronase. Following digestion, the slides were rinsed twice with PBS for 5 min and blocked in PBS containing 5% normal rabbit serum (Vector, Burlingame, CA) and 5% nonfat milk protein (Carnation) for 60 min at room temperature.
Next, CD14 and rabbit alveolar macrophages were visualized in lung tissue sections by immunohistochemistry using 5 μg/ml of polyclonal goat anti-rabbit CD14 antibody and 5 μg/ml of mouse anti-rabbit alveolar macrophage antibody (DAKO, Carpentier, CA). Primary antibodies were incubated with the tissue sections overnight in a moist chamber at 4° C. The slides were rinsed twice with PBS and labeled with an appropriate biotinylated secondary antibody for 2 hours at 4°C. To detect the mouse anti-rabbit macrophage antibody, slides were incubated with biotinylated anti-mouse IgG, rinsed three times in PBS. The samples were labeled with streptavidin-horseradish peroxidase and incubated with tyramide-FITC. After rinsing twice in PBS, any avidin or biotin remaining in the tissues was blocked with the Avidin-Biotin Blocking Kit (Vector Laboratories, Burlingame, CA). To detect rabbit CD14 in tissue, slides were incubated with biotinylated anti-goat IgG for 2 hours and then incubated with streptavidin horseradish peroxidase for 30 min. The sections were rinsed, incubated with biotinyl-tyramide, rinsed again in PBS and stained with streptavidin-Alexa 568 (Molecular Probes, Eugene, OR) at 1:200 and To-Pro-3 (Molecular Probes, Eugene, OR) at 1 μM. The slides were rinsed with PBS and then mounted with Vectashield hard mounting media (Vector Laboratories, Burlingame, CA).
Imaging procedure for confocal microscopy
Visualization of positive immunostaining for CD14 and AM was performed with a Leica TCS-SP confocal microscope with an upright Leica DMR Microscope and Leica Confocal Software (Leica Microsystems, Heidelberg, Germany). Confocal microscopy was used to eliminate the fluorescent light from above and below the plane of focus. Differential interference contrast (DIC) was used to provide structural detail of the lungs. The lasers used were argon (488 nm) for detection of FITC, krypton (568 nm) for the detection of Alexa 568, and helium/neon (633 nm) for the detection of ToPro-3. In order to calculate the optimum PMT setting for each laser line, the glow-over option of the Leica Confocal Software was used in order to maximize signal without over-saturating the image; and to minimize autofluorescence in the tissue. The pinhole for all images was set at 1 Airy Unit. For the negative controls, identical PMT and pinhole settings were used when capturing images.
Bronchoalveolar lavage
The rabbit alveolar macrophages (AM) were obtained by bronchoalveolar lavage (BAL) from healthy rabbits as described (15). The rabbits were anesthetized with a combination of ketamine (10 mg/kg) and xylazine (3 mg/kg) intravenously, then euthanized with pentobarbital and exsanguinated by cardiac puncture. The heart and lungs were removed en bloc and the heart and surrounding soft tissue were carefully removed from lungs. The lungs were lavaged with 5 separate 30-ml aliquots of isotonic saline containing 0.6 mM EDTA at 37° C. The cells were spun at 200 × g to pellet cells and the cells were washed twice with complete-RPMI. The cell viability was determined by trypan blue exclusion, and in all cases the recovered cells were > 95% viable.
Culture of AM
AM were resuspended at 5×105 cells/ml in complete RPMI and placed in 6-well culture dishes in media, allowed to adhere for 60 min, and then washed three times with warm complete-RPMI to remove nonadherent cells. The adherent cells were incubated for 24 hours in complete-RPMI, with PBS, 100 ng/ml LPS, or 1×106 CFU/ml of either live or heat-killed E. coli. To examine the roles of MAP kinases, rabbit AM were preincubated for 30 min with the vehicle control, dimethyl sulfoxide (DMSO), SB203580 (10 μM), PD98059 (30 μM), or SP600125 (25 μM), which are specific inhibitors of P38, ERK and JNK kinases respectively. Then the AM were incubated in the presence of LPS or E. coli for an additional 24 hours. In another set of experiments, the cells were incubated with polymyxin B (10 μg/ml), protease inhibitor cocktail (1:200 dilution) and ET-18-OCH3 (100μM), which is an inhibitor of phosphatidylinositol-specific phospholipase (PI-PLC) for 30 min prior to adding LPS or E. coli. Preliminary studies were performed for each of the inhibitors to identify the maximal inhibitory concentration of each reagent on reversing the decreased mCD14 expression of E. coli-stimulated AM. The maximal inhibitory concentration for each inhibitor was identified as the concentration where the dose response curve reached a plateau and increasing amounts had no further effects on mCD14 expression in AM exposed to E. coli. The maximal inhibitory concentration was then used in all subsequent studies when AM were exposed to LPS or E. coli. Preliminary studies also showed that the concentration of each inhibitor used did not affect mCD14 expression on AM exposed to PBS for 24 h. After 24 hours incubation, the supernatants were collected and saved at −70°C, and the adherent cells were resuspended and analyzed by flow cytometry.
SDS-PAGE and Western Blotting
SDS-PAGE and Western Blotting were performed using a 4 - 15% polyacrylamide gradient gel (BIO-RAD, Hercules, CA) with Tris-glycine PAGE running buffer. Cell lysates (2×106 cells/100 μl) were loaded to the gel at 10 μl/well. The gel was then blotted onto nitrocellulose membrane (Hybond-ECL, Amersham Biosciences, Piscataway, NJ) using a Bjerrum and Schafer-Nielsen buffer (48 mM Tris, 39 mM glycine, 0.037% SDS and 20% methanol, pH9.2). The transblotted membranes were incubated overnight with blocking buffer (I-Block, Tropix, Bedford, MA), then incubated with a rabbit anti-ERK, rabbit anti-phosphorylated ERK, or a goat anti-rabbit CD14 polyclonal antibody or a control goat antibody at 1 μg/ml at room temperature for 1 hour. Finally the membranes were incubated with HRPO labeled mouse anti-rabbit or rabbit anti-goat secondary antibody. Visualization of antibody-antigen complexes was visualized with chemiluminescence detecting reagents as described by the manufacturer (Super-Signal West Femto substrate, Pierce biotechnology, Rockford, IL). Multiple films of the Western-blot were obtained to insure that the bands were not saturated.
Flow cytometry
Rabbit alveolar macrophages were washed twice with PBS containing 0.1% sodium azide and 0.1% BSA then incubated with normal rabbit serum for 20 min on ice to block Fc receptors, and then incubated for 40 min with polyclonal goat anti-rabbit CD14 IgG, or control goat serum at 1:500 dilutions. After washing twice, cells were incubated for 30 min in buffer with 1:100 dilutions of PE-labeled donkey anti-goat antibodies specific to the primary antibodies. Cells were washed twice and examined by flow cytometry (FACScan, Becton Dickinson). At least 10,000 cell signals were obtained in each sample and the data were analyzed by WinMDI 2.8 software (Joseph Trotter, San Diego).
Second Challenge of Rabbit AM with E. coli, S. aureus, or Zymogen
AM were incubated for 24 hours in complete-RPMI with PBS, 100 ng/ml LPS, or 1×106 CFU/ml of live E. coli. The 24-hour cell viability determined by trypan blue exclusion showed that there was no difference in viability between the treatment groups. The cells were washed twice with culture media and then incubated with PBS, BODIPY-labeled E. coli, S. aureus or zymosan for 2 or 4 hours. The bacteria and zymosan were opsonized with heat-inactivated pooled rabbit serum and added to AM at a ratio of 10:1. After 2 hours of incubation, the cells were spun at 200 × g and incubated with 0.1% trypan blue for 1 minute to quench external fluorescence (7). The cells were washed with PBS containing 0.1% sodium azide and 0.1% BSA and phagocytosis was measured by flow cytometry. Phagocytosis was measured as the percentage of cells containing fluorescent particles using the WinMDI 2.8 software. The supernatants of the 4-hour incubation studies were saved at −70°C.
Concentrations of LPS, soluble CD14 and TNF-α
The LPS concentrations in the supernatants of culture fluid were assessed using the quantitative chromogenic limulus amebocyte lysate assay (QCL 1000 Whittaker M.A. Bioproducts, Walkersville, MD) according to the manufacturer’s instructions. The lower limit of LPS detection was 0.1 EU/ml. The concentrations of soluble CD14 (sCD14) and TNF-α in the culture supernatants were measured with rabbit-specific immunoassays (15, 21, 22). The assay sensitivities were sCD14= 5 pg/ml and TNF-α= 10 pg/ml.
Statistics
Differences among groups were determined using one-way ANOVA and paired comparisons between groups were performed using Student’s t test. Analysis of correlation between mCD14 and TNFα was performed using Pearson’s coefficient of correlation. A p value of 0.05 was considered significant. Values are presented as means ± SEM.
RESULTS
Co-localization of CD14 and alveolar macrophages in rabbit lung tissue
To determine whether the cell surface expression of CD14 changes in lungs of rabbits with E. coli pneumonia, immunohistochemistry was performed in paraffin-embedded lung sections from normal rabbits and rabbits E. coli pneumonia for 24 hours. Immunohistochemistry performed with an enzymatic-based detection system and bright field microscopy showed that in normal lungs CD14 was localized primarily to AM and approximately 100% of the AM stained positive. In contrast, there was considerable variability in the immunoreactivity of CD14 on AM from tissue sections obtained from infected rabbits with a consistent finding of decreased or absent immunoreactivity for CD14 on AM (data not shown).
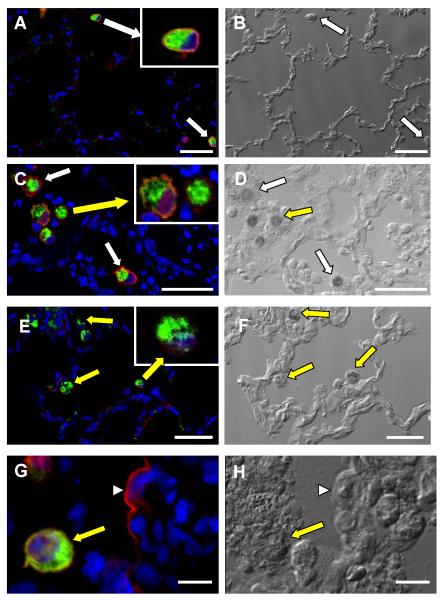
To determine the cellular distribution of CD14 and to increase the sensitivity of antigen detection, immunohistochemistry was performed with Tyramide signal amplification (TSA) and confocal microscopy (23, 24). TSA amplification and confocal microscopy provided similar results to the previous studies and showed that in normal lungs, approximately 100% of normal macrophages had positive staining for CD14. An advantage of confocal microscopy was the ability to localize CD14 expression to the cell surface of AM in normal rabbits (Fig. 1A). In contrast, tissue sections obtained from rabbits with E. coli pneumonia had variable amounts CD14 immunoreactivity, with many AM having little to no mCD14 (Fig. 1C, 1E, 1G). The presence of mCD14 on an AM next to an AM with no immunoreactivity for CD14 is important as it shows that the lack of positive staining is not due to failure of antigen preservation or the detection system (Figure 1C). On occasion, staining of the alveolar septa was observed in rabbits with E. coli pneumonia (Figure 1G). These findings suggest that mCD14 is down-regulated on rabbit AM 24 hours post-exposure to E. coli in vivo.
Figure 1.
Immunofluorescence for CD14 (red) and AM (green) in representative tissue sections obtained from rabbits treated with PBS (A) or live E. coli (C, E, G). Differential interference contrast (DIC) was performed on the same regions to provide structural details (B, D, F, and H). White arrows designate AM where mCD14 is present and yellow arrows identify macrophages where mCD14 is significantly decreased or absent. The insets are enlarged images of the AM designated by the larger arrows. (G) Positive staining for CD14 (red) on an alveolar septal wall (arrowhead). (H) DIC image of G showing location of alveolar macrophage (arrow) and the alveolar septa (arrowhead). Scale bar is 40 μm in A-F and 8 μm in G and H. CD14 was detected using goat anti-rabbit CD14 antibody and Alexa-568 (red), rabbit AM were detected with mouse anti-rabbit macrophage antibody and FITC (green), and nuclei were counterstained with To-Pro (blue).
Effects of LPS and E. coli on expression of membrane CD14 on AM
To confirm observations made with immunohistochemistry and to study the effects of LPS and E. coli on the expression of mCD14 on rabbit AM, experiments were performed in vitro. SDS-PAGE and Western blots as well as flow cytometry were used to measure CD14 expression in AM obtained from normal rabbits and stimulated in the presence of PBS, LPS (100 ng/ml), or E. coli (1×106 CFU/ml) for 24 hours in vitro (Fig. 2). After 24 hours of incubation, more than 85% of the cells were alive by trypan blue exclusion method and no differences in AM viability were observed between the treatment groups. CD14 expression was decreased in Western-Blot analysis of AM incubated with either LPS or E. coli (Figure 2A). There were detectable amounts of CD14 in cell lysates obtained from AM treated with E. coli when the Western blot was incubated with the film for longer periods of time. However, this resulted in saturation of the bands in lanes containing AM lysates from PBS and LPS treated macrophages (data not shown). Studies performed with flow cytometry show that incubation of normal AM with either LPS or E. coli significantly reduced the expression of CD14 on the cell surface (Figure 2B). Incubation with LPS reduced mCD14 by 30% (n=9, p< 0.01), whereas incubation with live E. coli reduced mCD14 expression by 60% without affecting cell viability (n=9, p< 0.01).
Figure 2.
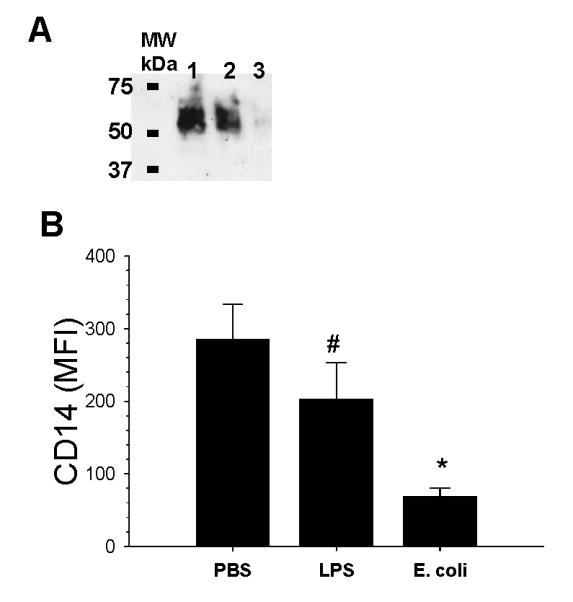
SDS-PAGE and Western blot of CD14 in AM lysates and flow cytometry of mCD14 expression on rabbit AM following incubation with PBS, LPS, or E. coli for 24 hour. (A) Expression of CD14 was measured by SDS-PAGE and Western blotting using a goat anti-rabbit CD14 polyclonal antibody. Lane 1: AM treated with PBS; Lane 2: AM treated with LPS; Lane 3: AM treated with E. coli. (B) Flow cytometry showing the amount of mCD14 present on AM following incubation with PBS, LPS, or E. coli. #: p= 0.001 as compared with 24 hour PBS group. *: p= 0.0007 compared with PBS group. MFI= mean fluorescence intensity. Values are mean ± SEM.
Components of E. coli responsible for downregulation of mCD14 on AM
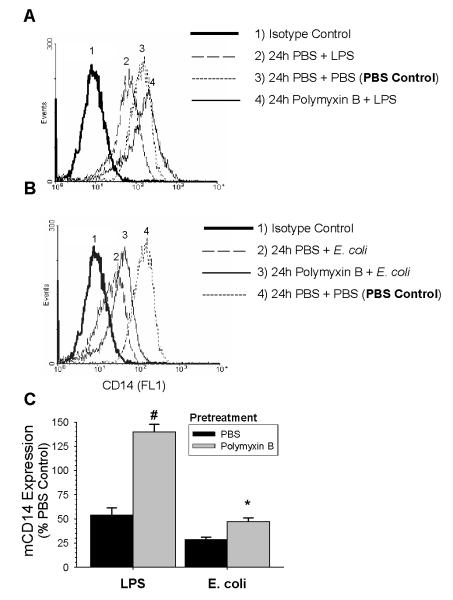
To investigate the role of LPS in the downregulation of mCD14 induced by E. coli, we preincubated cells with polymyxin B (10 μg/ml) for 30 min, and then added either LPS or live E. coli and continued incubating the AM for 24 hours. Preliminary studies showed that this concentration of polymyxin B had maximal inhibitory effects in AM incubated with E. coli for 24 h, but had no effect on the expression of mCD14 on AM incubated with PBS for 24 h. The MFI for mCD14 on AM pre-treated with polymyxin B and then incubated with PBS for 24 hours was 237.7 ±37.7 versus the MFI for mCD14 on AM pre-treated with PBS and then incubated with PBS for 24 h, which was 248.9 ±43.1. Polymyxin B pretreatment completely blocked the ability of LPS to decrease the expression of mCD14 on AM (Fig 3). In contrast, polymyxin B pretreatment returned the cell surface expression of CD14 to 47% of the baseline when AM were incubated with E. coli. Taken together, polymyxin B totally abolished the LPS-induced downregulation of mCD14, but only partially reduced the effects of E. coli. To exclude the possibility that there were extremely high amounts of LPS in the supernatant of AM exposed to E. coli, which were not blocked by 10 μg/ml polymyxin B, we measured the concentration of LPS in the supernatants using the limulus amebocyte lysate assay method. The results showed that the concentrations of LPS in the supernatant of rabbit AM incubated with 4-hour and 24-hour E. coli were 14.18 ± 6.16 ng/ml and 15.02 ± 7.67 ng/ml respectively. Thus, components of E. coli other than LPS appear to contribute to the effects of E. coli on rabbit AM.
Figure 3.
Effect of polymyxin B on LPS or E. coli-induced decrease in mCD14 expression. Cells were pretreated with PBS or polymyxin B [10 ng/ml] for 30 min prior to the addition of PBS, LPS [100 ng/ml], or E. coli (1×106 CFU/ml) for 24 hours. The expression of mCD14 was measured by flow cytometry. (A) Representative histogram of mCD14 expression on rabbit AM pretreated with PBS or polymyxin B and then exposed to PBS or LPS for 24 hours. (B) Representative histogram of mCD14 expression on rabbit AM pretreated with PBS or polymyxin B and then exposed to PBS or E. coli for 24 hours. (C) Data from six separate experiments was normalized as a percentage of the mean fluorescent intensity (MFI) of the PBS control. The black bars are the data from AM pretreated with PBS and the gray bars are the data from AM pretreated with polymyxin B. Values are mean ± SEM. #: p= 0.003 for polymyxin B + 24 hours LPS group vs PBS + 24 hours (n=4). *: p= 0.02 for polymyxin B + 24 hour E. coli vs PBS + 24 hours E. coli (n=6).
Role of E. coli Products on the Expression of mCD14
Porphyromonas gingivalis produces cysteine proteinases that proteolytically cleave CD14 from cell surface of monocytes (20). To determine whether products actively secreted by live E. coli, such as phospholipases or proteases, play a role in the down-regulation of mCD14 on AM, we compared the ability of live and heat-killed E. coli to decrease the cell surface expression of CD14. Surprisingly, there was no difference in the ability of live and heat-killed E. coli to decrease mCD14 on AM (54.01 ± 8.49 vs 59.74 ± 6.16 % for live vs. heat-killed bacterial respectively, as compared with the PBS controls at 24 hours). These data suggest that E. coli decreased expression of mCD14 on AM by a mechanism other than actively secreted bacterial products.
Role of MAP Kinase in LPS and E. coli-induced reduction of mCD14
LPS activation of macrophages increases tyrosine-phosphorylation of several MAP kinases including p38, ERK (Figure 4), and JNK (25-28). The role of MAP kinases in LPS and E coli-induced decrease in mCD14 expression was studied using specific pharmacological inhibitors that blocked the p38, ERK, and JNK kinases. Blockade of the p38 (SB203580) or JNK (SP600125) pathways did not alter the affects of either LPS or live E. coli on mCD14 expression (Figure 4). In contrast, mCD14 expression was returned to 82 ± 6.9% of the baseline value when the ERK pathway was blocked in AM incubated with LPS. Blockade of the ERK pathway returned the expression of mCD14 back to 59 ± 6.6% of baseline in AM incubated with E. coli (Fig 4). Thus, blockade of ERK, but not p38 or JNK attenuated the effects of LPS and E. coli on mCD14 expression and blockade of the ERK pathway was more effective in LPS than in E. coli-treated AM.
Figure 4.
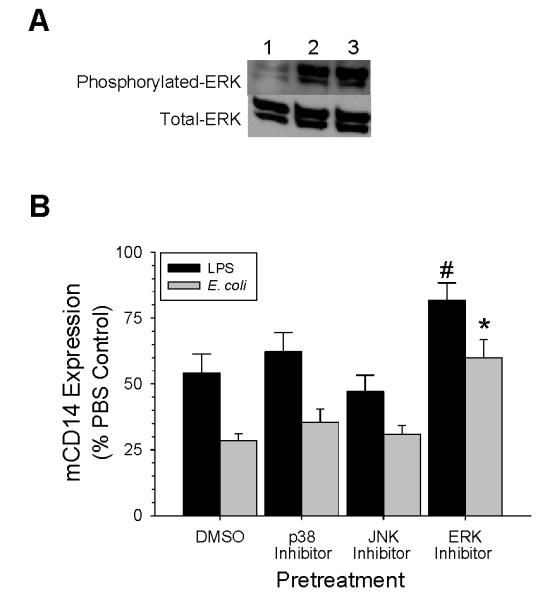
Role of MAP-kinase pathways in the regulation of mCD14 expression on AM exposed to LPS or E. coli for 24 hours in vitro. (A) Western blot analysis of phosphorylated- and total-ERK in cell lysates from AM treated with PBS (Lane 1), LPS (Lane 2), or E. coli (Lane 3) (B) AM were preincubated with either DMSO, 10 μM SB203580 for blockade of p38 pathways, 25 μM SP600125 for blockade of JNK pathways, or 30 μM PD98059 for blockade of ERK pathways for 30 min prior to adding either LPS (100 ng/ml) or E. coli 1×106 CFU/ml for 24 hours. The expression of mCD14 was measured by flow cytometry and the data are expressed as a percentage of the 24 hours PBS control group. Values are mean ± SEM, # p= 0.03 for ERK pathway blockade in LPS-treated AM as compared with DMSO group (n= 4), and * p= 0.006 for ERK pathway blockade in E. coli-treated AM as compared with DMSO group (n= 6).
The role of proteases and phosphatidylinositol-specific phospholipase
CD14 is anchored in the cell membrane by a glycosylphosphatidylinositol (GPI) tail, and PIPLC cleaves CD14 from the cell surface (11). However, Bazil et al (29) found that protease-dependent shedding was the mechanism for PMA–induced downregulation of mCD14 on blood monocytes. To determine whether proteases or PI-PLC decrease the expression of mCD14, AM were treated with either 100 μM of ET-18-OCH3, a specific inhibitor of PI-PLC (30) or a protease inhibitor cocktail that blocks serine, cysteine, aspartic and aminopeptidases for 30 min prior to incubation with LPS or E. coli. Inhibition of proteases blocked the LPS-induced decrease of mCD14 on AM, but did not alter the effects of E. coli on mCD14 expression (Fig. 5). In contrast, inhibition of phospholipase did not prevent the downregulation of mCD14 by either LPS or E. coli (Fig. 5). These data demonstrate that phospholipase activation is not responsible for either LPS or E. coli-induced downregulation of mCD14, whereas a protease is responsible for LPS-induced but not E. coli-induced downregulation of mCD14. To further investigate the involvement of proteases in the shedding of mCD14, we measured the concentrations of sCD14 in the supernatants of the culture media from AM stimulated with LPS or E. coli with or without protease inhibitor pretreatment. LPS treatment significantly increased the amount of sCD14 in the supernatants at 24 hours (Fig. 6), and pretreatment with the protease inhibitor mixture abrogated the LPS effects. In preliminary studies, sCD14 was not directly affected by the protease inhibitor mixture. Thus, the decreased expression of mCD14 on AM stimulated by LPS occurs by protease-dependent cleavage of mCD14. In contrast, inhibition of proteases did not affect the amount of sCD14 in the supernatants of AM incubated with either E. coli or PBS. This suggests that a different mechanism is responsible for the decreased expression of mCD14 when AM are incubated with E. coli.
Figure 5.
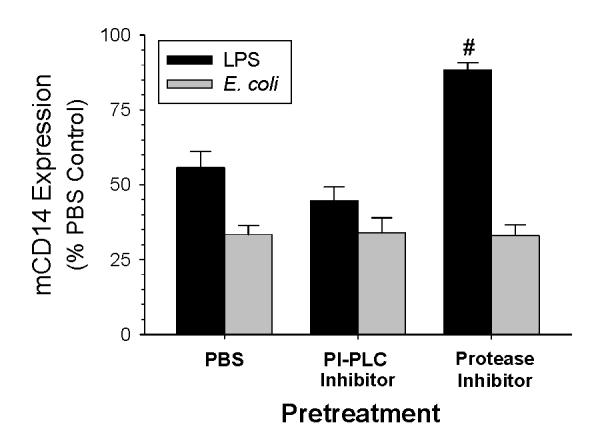
Effects of phospholipase and protease inhibition on mCD14 expression on AM. Rabbit AM were preincubated for 30 minutes with ET-18-OCH3 (100 μM) or a protease inhibitor mixture and then incubated with LPS 100 ng/ml (black bars) or E. coli 1×106 CFU/ml (gray bars) for 24 hours. The expression of mCD14 was measured by flow cytometry and the data are expressed as a percentage of the values of the 24 hour PBS control group. Values are mean ± SEM. #: p= 0.002 as compared to 24 hour LPS +PBS group, n= 4.
Figure 6.
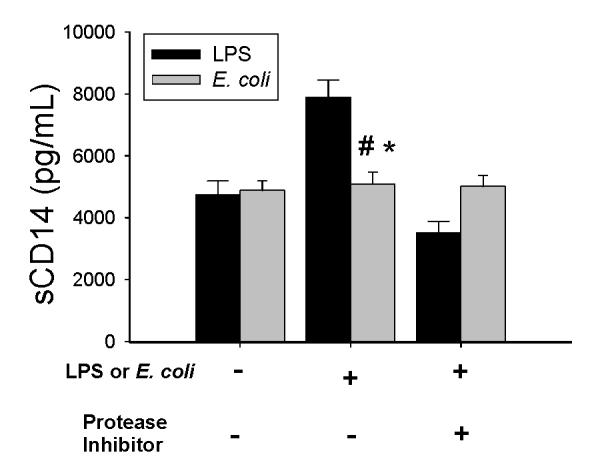
Effects of LPS and E. coli on sCD14 in the AM supernatants. AM were incubated with 100 ng/ml LPS (black bars) or 1×106 CFU/ml E. coli (gray bars) for 24 hours with or without prior treatment with the protease inhibitor mixture for 30 min. The concentration of sCD14 was determined by ELISA. Values are mean ± SEM. #: p = 0.05 as compared to incubation without LPS or protease inhibitors; *: p= 0.01 as compared to incubation with a combination of LPS and protease inhibitors, n= 4.
Effects of LPS and E. coli on the response of alveolar macrophages to a second challenge with S. aureus or zymosan
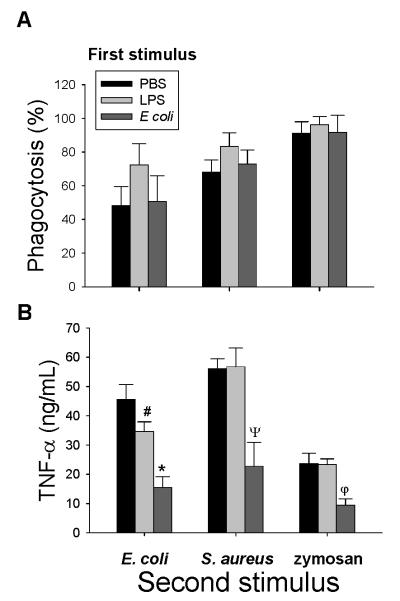
Human monocytes and macrophages exposed to LPS for 3 to 24 hours are rendered tolerant to a second challenge with LPS (17). A potential mechanism whereby endotoxin-tolerance develops is the down-regulation of mCD14 on macrophages (16, 20). In order to compare the effects of LPS and E. coli on the subsequent responses of AM, we incubated AM with E. coli, S. aureus or zymosan after pre-exposure to either LPS or live E. coli. Phagocytosis of E. coli, S. aureus or zymosan was not altered following incubation with LPS or E. coli for 24 hours (Fig 7A). In contrast, the production of TNF-α in response to E. coli was attenuated in AM following 24 hours incubation with either LPS (34.6 ± 3.34 ng/ml) or E coli (15.48 ± 3.71 ng/ml) as compared with PBS (45.63 ± 5.06 ng/ml) (Figure 7B). In addition, there was a positive correlation between the amount of mCD14 on the cell surface of AM and TNF-α production (Figure 8). The production of TNF-α following incubation with S. aureus was significantly reduced in AM pretreated with E. coli (22.78 ± 8.18 ng/ml) but not LPS (56.78 ± 6.39 ng/ml) as compared with PBS (56.15± 3.3 ng/ml (Fig. 7B). Following incubation with E. coli for 24 hours, AM were hyporesponsive to zymosan-induced production of TNF-α (9.44 ± 2.2 ng/ml vs. 23.65± 3.49 ng/ml for PBS, p= 0.03, n= 4). Incubation of AM with LPS (23.35 ±1.9 ng/ml) for 24 hours did not affect the TNF-α response to zymosan. The production of TNF-α in response to a second challenge with S. aureus or zymosan did not show any relationship with the amount of CD14 on the cell surface. When the amount IL-8 was measured in the same biological samples, prior exposure of AM to either LPS or E .coli did not affect the production of IL-8 in response to a second challenge (data not shown).
Figure 7.
Rabbit AM were incubated with PBS, LPS [100ng/ml] or E. coli [1×106 CFU/ml] for 24 hours, and then the second stimulus of BODIPY-labeled E. coli, S. aureus or zymosan was added to the AM at a ratio of 10:1. (A) Phagocytosis of E. coli, S. aureus and zymosan was measured by flow cytometry after incubation of AM with BODIPY-labeled bioparticles for 2 hours. Data are expressed as the percentage of AM which phagocytized fluorescent bioparticles. (B) The concentration of TNF-α in the supernatants of AM cultured with E. coli, S. aureus or zymosan for 4 hours was determined by ELISA. #: p= 0.03, *: p= 0.02, ψ: p=0.009, φ: p=0.03 as compared to pretreatment with PBS, n=4.
Figure 8.
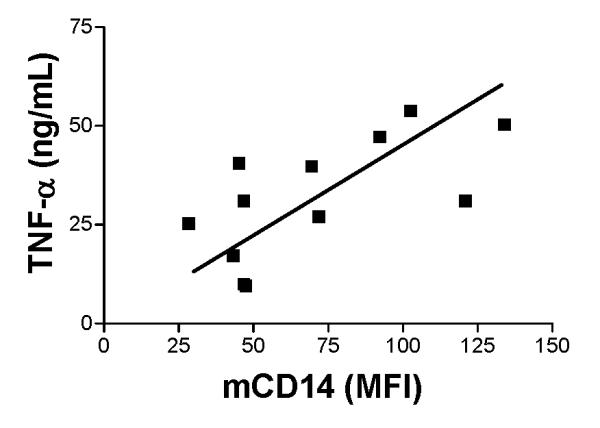
Positive correlation between the production of TNFα and expression of mCD14 on AM exposed to E. coli as the second stimulus. Rabbit AM were pretreated with PBS, LPS, or E. coli for 24 hours and then exposed to E coli and the amount of TNFα produced in response to the second challenge with E. coli was determined. TNFα was measured in AM supernatant using an ELISA for rabbit TNFα and the amount of mCD14 on AM was quantitated with flow cytometry using a goat anti-rabbit CD14 polyclonal antibody. Correlation between mCD14 and TNFα was analyzed using Pearson’s coefficient of correlation (r = 0.065; p = 0.02).
Taken together, phagocytosis and production of IL-8 were preserved in AM following 24 hours incubation with either LPS or E. coli, whereas the production of TNF-α in response to a second challenge with E. coli was impaired after prior incubation with LPS and E. coli. In contrast, hyporesponsiveness of TNF-α production in response to S. aureus or zymosan was observed in AM pretreated with E. coli but not LPS.
DISCUSSION
The purpose of this study was to examine the regulation of mCD14 in bacterial pneumonia and to explore the mechanisms and consequences of changes in mCD14 expression. Studies performed with immunohistochemistry for rabbit CD14 in normal lungs and lungs from rabbits with E. coli pneumonia showed that prior exposure to E. coli decreased the positive staining for mCD14 on alveolar macrophages in vivo. Studies performed in vitro to determine the mechanisms responsible for modifying the expression of mCD14 on AM show that LPS decreased the expression of mCD14 by a protease-dependent mechanism. When compared to LPS, E. coli caused a more profound decrease in mCD14 expression on AM and the decreased expression of mCD14 caused by E. coli did not require proteases. In studies to evaluate the development of tolerance to a second stimulus, the prior incubation of AM with LPS and E. coli resulted in the development of tolerance to E. coli. In contrast, E. coli but not LPS resulted in the development of cross-tolerance to S. aureus and zymosan. The development of cross-tolerance to S. aureus and zymosan did not correlate with changes in the expression of mCD14 on AM suggesting that other mechanisms are responsible for the development of tolerance in macrophages. This suggests that prior exposure to whole E. coli may result in dysregulation of the innate immune response to diverse microbial pathogens in the lungs.
Incubation of AM with LPS and E. coli caused a significant decrease in mCD14 expression on AM (Fig. 1 and 2). Previous studies show that LPS and E. coli alter the amounts of mCD14 on monocytes and macrophages, but the results are contradictory because of differences in cell types, endotoxin concentrations and incubation times (31, 32). In the work of Fahmi and Chaby (33), there was a decrease of LPS-binding sites on macrophages treated with LPS, which is in agreement with our results. In contrast, Landmann and colleagues reported an increase in mCD14 on monocytes after incubation with LPS or E. coli (31). In their study LPS increased the expression of mCD14 on monocytes, whereas in our studies LPS reduced mCD14 expression on rabbit AM in vitro. Monocytes and macrophages, although derived from the same cellular lineage, are distinct in differentiation and maturation. The expression of cell surface markers and cytokine production in response to LPS (34), and the expression of CD14 on the surface of monocytes and macrophages differ (32).
To identify which components of E. coli were responsible for the decreased expression of mCD14, polymyxin B was used to block the effects of LPS and E. coli (Fig. 3). Polymyxin B is a cationic, cyclic peptide antibiotic that inhibits the biological activities of LPS, including binding of LPS to leukocytes (5) and LPS-induced production of cytokines by macrophages (35). The results showed that LPS-induced downregulation of mCD14 expression was completely abolished by preincubation with polymyxin B, whereas the E. coli-induced reduction in expression of mCD14 was only partially attenuated by incubation with polymyxin B. To insure that an excess of LPS in the supernatants of the whole bacteria did not exceed the neutralizing capacity of polymyxin B, the LPS in these supernatants was measured. The LPS in supernatants of whole E. coli was relatively low (15.02 ± 3.43 ng/ml), which is in agreement with other reports (5, 31). Because a large excess of polymyxin B was used relative to the measured concentration of LPS, it is likely that other bacterial components aside from LPS are responsible for the E. coli-induced downregulation of mCD14 expression on rabbit AM.
To determine whether secreted bacterial products were responsible for the decreased expression of mCD14 on rabbit AM, we compared the potency of live and heat-killed E. coli. There was no difference in the ability of live and heat-killed bacteria to decrease the expression of mCD14 on rabbit AM. Therefore, it is unlikely that the effect of live E. coli is due to secreted bacterial products as has been reported for Porphyromonas gingivalis which produces cysteine proteinases that proteolytically cleave CD14 from cell surface of monocytes (20).
Numerous studies have demonstrated that mCD14, which belongs to the family of GPI-anchored glycoproteins, can be released from cells by treatment with PI-PLC (11, 36). However, it also has been reported that protease-dependent shedding causes downregulation of mCD14 on PMA-stimulated monocytes (29). To determine whether PI-PLC or proteases play a role in decreasing the expression of mCD14 we used specific inhibitors to block the effect of these enzymes. Inhibition of PI-PLC did not prevent the decreased expression of mCD14 caused by LPS or E. coli (Fig. 5). In contrast, inhibition of proteases completely blocked the LPS-induced reduction of mCD14, whereas protease inhibitors had no effect on mCD14 expression of AM incubated with E. coli. To confirm this finding, we measured the concentrations of sCD14 in cell culture supernatants. The concentrations of sCD14 were increased by incubation of AM with LPS, but not with live E. coli. Furthermore, the LPS-induced shedding of sCD14 into the AM supernatants was abolished by pretreatment with the mixture of protease inhibitors (Fig. 6). Therefore, the LPS-induced downregulation of mCD14 on rabbit AM appears to be mediated by protease-dependent cleavage of mCD14 from the cell surface and the proteases must derive from the AM following activation with LPS. In contrast, the E. coli-induced decrease of mCD14 on rabbit AM is mediated by mechanisms other than protease-dependent shedding.
A possible mechanism that could account for the decreased expression of mCD14 on AM exposed to E. coli without a reciprocal increase in sCD14, is the internalization of mCD14 during the process of phagocytosis (37, 38). Monocytes have been reported to internalize E. coli using a CD14-dependent pathway (7). In contrast, studies suggest that mCD14 and LPS molecules are internalized independently after association on the cell surface (39). To determine whether phagocytosis was the mechanism responsible for the decreased expression of mCD14, AM were incubated with Bodipy-labeled E. coli at 0, 5, 15, 30, 60, 120, and 240 minutes and bacterial phagocytosis was measured with flow cytometry. This study showed that phagocytosis of E. coli by AM was maximal at 2 hours. When mCD14 was measured at 4 hours, there was no difference in the expression of mCD14 on AM that had been treated with PBS (215.2, +/37.6, MFI), LPS (201.2 ± 47.3, and MFI), or that had maximal uptake of Bodipy-labeled E. coli (223.7 ± 39.2 MFI). This suggests that phagocytosis is not the mechanism responsible for the decreased expression of mCD14 on AM exposed to E. coli. The finding that CD14 expression in cell lysates is significantly decreased in Western blots of AM exposed to E. coli for 24 hours is additional evidence that phagocytosis and internalization of cell surface CD14 is not the mechanism responsible for the decrease in mCD14 (Figure 2A). Future experiments are required to define the precise mechanism responsible for the E. coli-induced downregulation of mCD14 expression
In monocytes and macrophages, MAP kinases are an important family of protein-serine/threonine kinases that mediate responses to LPS (13 77). MAP kinases regulate proliferation and differentiation (40) and in this report we show that the ERK pathway regulates the expression of mCD14 on the macrophage surface. Blockade of the ERK pathway attenuated 60 % of LPS and 44 % of E. coli-induced downregulation of mCD14. In contrast, blockade of the p38 and JNK pathways did not attenuate the effect of LPS or E. coli on mCD14 expression on rabbit AM. This suggests that the ERK pathway plays an important role in regulating the decreased expression of mCD14 when AM are exposed to either LPS or E. coli.
Human monocytes and macrophages exposed to endotoxin for 3 to 24 hours are rendered tolerant and show a suppressed TNFα response when rechallenged with LPS (17). A potential mechanism for the development of endotoxin-tolerance is the decreased cell surface expression of CD14 (16, 20). The production of TNF-α in response to a second challenge with E. coli was attenuated following primary incubation with either LPS or E. coli. In contrast, the production of TNF-α in response to S. aureus or zymosan was impaired only in AM pretreated with E. coli but not LPS. Prior studies have shown that monocytes pretreated with LPS respond normally to a second challenge with S. aureus (41, 42) or zymosan (43), in agreement with our results. However, whole E. coli results in cross-desensitization and development of hyporesponsiveness to a second challenge with S. aureus and zymosan. The results show that the production of TNF-α in response to a secondary challenge with E. coli correlates well with the amount of CD14 on the cell surface of AM, but altered expression of mCD14 could not explain the different responses to secondary challenge with S. aureus or zymosan in LPS and E. coli-pretreated AM. The observation that the production of IL-8 was not decreased in the same supernatants in which TNFα production was significantly decreased suggests differential desensitization of cytokine- and chemokine-signaling pathways, as reported for human monocytes and macrophages and is further evidence that decreased mCD14 is not the mechanism responsible for endotoxin-tolerance (44).
Toll-Like receptor-2 (TLR2) is required for signaling by gram-positive bacteria (45) and zymosan (46), suggesting that decreased cell surface expression of TLR2 could be the mechanism responsible for the cross-tolerance induced by E. coli. The expression of TLR2 on the cell surface of AM is unaffected by 24 hours incubation with either LPS or E. coli (data not shown). Thus, decreased TLR2 expression on rabbit AM does not seem to be involved in the cross-tolerance response. Therefore, it seems likely that the tolerance develops downstream of mCD14 and TLR2 with alterations in signal transduction pathways being a possible explanation (17). The precise signaling pathways responsible for the different responses to LPS and E. coli will need to be clarified.
In conclusion, the expression of mCD14 is decreased on AM from lung sections of rabbits with E. coli pneumonia. Studies performed in vitro show that LPS and E. coli decrease the expression of mCD14 on AM by different mechanisms. LPS induces a protease-dependent shedding of mCD14 into the cell supernatant, whereas E. coli elicits mechanisms other than cleavage of the mCD14 from the surface. In addition, LPS and E. coli induce different responses to a second challenge, with cross-tolerance to gram-positive bacteria or zymosan being developed following exposure of AM to E. coli but not LPS. These studies show that distinct mechanisms regulate the expression of mCD14 and the induction of tolerance in rabbit AM exposed to LPS and E. coli and suggest that AM function is impaired at sites of bacterial infection in the lungs.
ACKNOWLEDGMENTS
Supported in part by NIH grants GM37696, HL30542.
ABBREVIATIONS
- AM
alveolar macrophages
- DIC
Differential interference contrast
- ERK
extracellular signal-regulated kinase
- LPS
lipopolysaccharide
- mCD14
membrane CD14
- MAP
mitogen-activated protein
- PI-PLC
phosphatidylinositol-specific phospholipase
- sCD14
soluble CD14
LITERATURE CITED
- 1.El-Solh AA, Aquilina AT, Dhillon RS, Ramadan F, Nowak P, Davies J. Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med. 2002;166(8):1038–43. doi: 10.1164/rccm.200202-123OC. [DOI] [PubMed] [Google Scholar]
- 2.Park DR, Sherbin VL, Goodman MS, Pacifico AD, Rubenfeld GD, Polissar NL, Root RK. The Etiology of Community-Acquired Pneumonia at an Urban Public Hospital: Influence of Human Immunodeficiency Virus Infection and Initial Severity of Illness. J Infect Dis. 2001;184(3):268–77. doi: 10.1086/322040. [DOI] [PubMed] [Google Scholar]
- 3.Fox-Dewhurst R, Alberts MK, Kajikawa O, Caldwell E, Johnson MC, Skerrett SJ, Goodman RB, Ruzinski JT, Wong VA, Chi EY, Martin TR. Pulmonary and systemic inflammatory responses in rabbits with gram-negative pneumonia. American Journal Of Respiratory And Critical Care Medicine. 1997;155(6):2030–2040. doi: 10.1164/ajrccm.155.6.9196112. [DOI] [PubMed] [Google Scholar]
- 4.Matute-Bello G, Frevert CW, Kajikawa O, Skerrett SJ, Goodman RB, Park DR, Martin TR. Septic shock and acute lung injury in rabbits with peritonitis: failure of the neutrophil response to localized infection. Am J Respir Crit Care Med. 2001;163(1):234–43. doi: 10.1164/ajrccm.163.1.9909034. [DOI] [PubMed] [Google Scholar]
- 5.Katz SS, Chen K, Chen S, Doerfler ME, Elsbach P, Weiss J. Potent CD14-mediated signalling of human leukocytes by Escherichia coli can be mediated by interaction of whole bacteria and host cells without extensive prior release of endotoxin. Infect Immun. 1996;64(9):3592–600. doi: 10.1128/iai.64.9.3592-3600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–57. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 7.Schiff DE, Kline L, Soldau K, Lee JD, Pugin J, Tobias PS, Ulevitch RJ. Phagocytosis of gram-negative bacteria by a unique CD14-dependent mechanism. J Leukoc Biol. 1997;62(6):786–94. doi: 10.1002/jlb.62.6.786. [DOI] [PubMed] [Google Scholar]
- 8.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392(6675):505–9. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 9.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J Leukoc Biol. 2003;74(4):479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 10.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 11.Martin TR, Mongovin SM, Tobias PS, Mathison JC, Moriarty AM, Leturcq DJ, Ulevitch RJ. The CD14 differentiation antigen mediates the development of endotoxin responsiveness during differentiation of mononuclear phagocytes. J Leukoc Biol. 1994;56(1):1–9. doi: 10.1002/jlb.56.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189(11):1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 14.da silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in Close Proximity to Each of the Proteins in Its Membrane Receptor Complex: Transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 2001:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 15.Frevert CW, Matute-Bello G, Skerrett SJ, Goodman RB, Kajikawa O, Sittipunt C, Martin TR. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J Immunol. 2000;164(10):5439–45. doi: 10.4049/jimmunol.164.10.5439. [DOI] [PubMed] [Google Scholar]
- 16.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4(9):903–14. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 17.West MA, Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30(1 Supp):S64–S73. [PubMed] [Google Scholar]
- 18.Wolk K, Docke WD, von Baehr V, Volk HD, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96(1):218–23. [PubMed] [Google Scholar]
- 19.Heagy W, Hansen C, Nieman K, Cohen M, Richardson C, Rodriguez JL, West MA. Impaired ex vivo lipopolysaccharide-stimulated whole blood tumor necrosis factor production may identify "septic" intensive care unit patients. Shock. 2000;14(3):271–6. doi: 10.1097/00024382-200014030-00005. discussion 276-7. [DOI] [PubMed] [Google Scholar]
- 20.Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, Takada H. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol. 2000;165(1):411–8. doi: 10.4049/jimmunol.165.1.411. [DOI] [PubMed] [Google Scholar]
- 21.Kajikawa O, Goodman RB, Johnson MC, Konishi K, Martin TR. Sensitive and specific immunoassays to detect rabbit IL-8 and MCP-1 cytokines that mediate leukocyte recruitment to the lungs. J. Immunol. Methd. 1996;197:19–29. doi: 10.1016/0022-1759(96)00101-9. [DOI] [PubMed] [Google Scholar]
- 22.Kajikawa O, Johnson MC, Goodman RB, Frevert CW, Martin TR. A sensitive immunoassay to detect the alpha-chemokine GRO in rabbit blood and lung fluids. J Immunol Methods. 1997;205(2):135–43. doi: 10.1016/s0022-1759(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 23.Loup F, Weinmann O, Yonekawa Y, Aguzzi A, Wieser HG, Fritschy JM. A highly sensitive immunofluorescence procedure for analyzing the subcellular distribution of GABAA receptor subunits in the human brain. J Histochem Cytochem. 1998;46(10):1129–39. doi: 10.1177/002215549804601005. [DOI] [PubMed] [Google Scholar]
- 24.van Gijlswijk RP, Zijlmans HJ, Wiegant J, Bobrow MN, Erickson TJ, Adler KE, Tanke HJ, Raap AK. Fluorochrome-labeled tyramides: use in immunocytochemistry and fluorescence in situ hybridization. J Histochem Cytochem. 1997;45(3):375–82. doi: 10.1177/002215549704500305. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268(33):25009–14. [PubMed] [Google Scholar]
- 26.Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci U S A. 1996;93(7):2774–8. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouadrhiri Y, Pilette C, Monteiro RC, Vaerman JP, Sibille Y. Effect of IgA on respiratory burst and cytokine release by human alveolar macrophages: role of ERK1/2 mitogen-activated protein kinases and NF-kappaB. Am J Respir Cell Mol Biol. 2002;26(3):315–32. doi: 10.1165/ajrcmb.26.3.4590. [DOI] [PubMed] [Google Scholar]
- 28.Koch A, Giembycz M, Ito K, Lim S, Jazrawi E, Barnes PJ, Adcock I, Erdmann E, Chung KF. MAP-kinase modulation of NF-{kappa}B-induced GM-CSF release from human alveolar macrophages. Am J Respir Cell Mol Biol. 2003 doi: 10.1165/rcmb.2003-0122OC. [DOI] [PubMed] [Google Scholar]
- 29.Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147(5):1567–74. [PubMed] [Google Scholar]
- 30.Powis G, Seewald MJ, Gratas C, Melder D, Riebow J, Modest EJ. Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues. Cancer Res. 1992;52(10):2835–40. [PubMed] [Google Scholar]
- 31.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64(5):1762–9. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antal-Szalmas P. Evaluation of CD14 in host defence. Eur J Clin Invest. 2000;30(2):167–79. doi: 10.1046/j.1365-2362.2000.00610.x. [DOI] [PubMed] [Google Scholar]
- 33.Fahmi H, Chaby R. Desensitization of macrophages to endotoxin effects is not correlated with a down-regulation of lipopolysaccharide-binding sites. Cell Immunol. 1993;150(1):219–29. doi: 10.1006/cimm.1993.1191. [DOI] [PubMed] [Google Scholar]
- 34.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am J Physiol Lung Cell Mol Physiol. 2001;280(1):L58–68. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- 35.Coyne CP, Fenwick BW. Inhibition of lipopolysaccharide-induced macrophage tumor necrosis factor-alpha synthesis by polymyxin B sulfate. Am J Vet Res. 1993;54(2):305–14. [PubMed] [Google Scholar]
- 36.Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141(2):547–52. [PubMed] [Google Scholar]
- 37.Poussin C, Foti M, Carpentier JL, Pugin J. CD14-dependent endotoxin internalization via a macropinocytic pathway. J Biol Chem. 1998;273(32):20285–91. doi: 10.1074/jbc.273.32.20285. [DOI] [PubMed] [Google Scholar]
- 38.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277(49):47834–43. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 39.Antal-Szalmas P, Poppelier MJ, Broekhuizen R, Verhoef J, van Strijp JA, van Kessel KP. Diverging pathways for lipopolysaccharide and CD14 in human monocytes. Cytometry. 2000;41(4):279–88. [PubMed] [Google Scholar]
- 40.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92(17):7686–9. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathison JC, Virca GD, Wolfson E, Tobias PS, Glaser K, Ulevitch RJ. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990;85(4):1108–18. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth J, Aslan T, Storr B, Zeisberger E. Lack of cross tolerance between LPS and muramyl dipeptide in induction of circulating TNF-alpha and IL-6 in guinea pigs. Am J Physiol. 1997;273(4 Pt 2):R1529–33. doi: 10.1152/ajpregu.1997.273.4.R1529. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993;177(2):511–6. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufmann A, Gemsa D, Sprenger H. Differential desensitization of lipopolysaccharide-inducible chemokine gene expression in human monocytes and macrophages. Eur J Immunol. 2000;30(6):1562–7. doi: 10.1002/1521-4141(200006)30:6<1562::AID-IMMU1562>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274(25):17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 46.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401(6755):811–5. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]