Abstract
The enantioseparation of ten mandelic acid derivatives was performed by reverse phase high performance liquid chromatography with hydroxypropyl-β-cyclodextrin (HP-β-CD) or sulfobutyl ether-β-cyclodextrin (SBE-β-CD) as chiral mobile phase additives, in which inclusion complex formations between cyclodextrins and enantiomers were evaluated. The effects of various factors such as the composition of mobile phase, concentration of cyclodextrins and column temperature on retention and enantioselectivity were studied. The peak resolutions and retention time of the enantiomers were strongly affected by the pH, the organic modifier and the type of β-cyclodextrin in the mobile phase, while the concentration of buffer solution and temperature had a relatively low effect on resolutions. Enantioseparations were successfully achieved on a Shimpack CLC-ODS column (150×4.6 mm i.d., 5 μm). The mobile phase was a mixture of acetonitrile and 0.10 mol L-1 of phosphate buffer at pH 2.68 containing 20 mmol L-1 of HP-β-CD or SBE-β-CD. Semi-preparative enantioseparation of about 10 mg of α-cyclohexylmandelic acid and α-cyclopentylmandelic acid were established individually. Cyclodextrin-enantiomer complex stoichiometries as well as binding constants were investigated. Results showed that stoichiomertries for all the inclusion complex of cyclodextrin-enantiomers were 1:1.
Keywords: Mandelic acid derivatives, Chiral separation, Hydroxypropyl-β-cyclodextrin, Sulfobutyl ether-β-cyclodextrin, High performance liquid chromatography
1. Introduction
During the past decades much attention has been paid for the enantioseparation of chiral compounds since most of the bioorganic molecules are stereospecific. It has become a major concern in the modern pharmaceutical technology after the US Food and Drug Administration issued a guideline for research on chiral drugs in 1992 [1]. Development of an efficient enantiomeric separation method is a critical step for both chiral screening protocol and production process. Generally, an optical enantiomer could be obtained by either resolution from racemates or asymmetrical synthesis. Among the various kinds of chiral separation techniques, high performance liquid chromatography (HPLC) has been proven to be the most widely applicable platform for both preparative and analytical purposes [2]. Currently chiral separations by HPLC are usually performed with the following two methods: the direct method, which uses chiral stationary phase or chiral mobile phase additive; and the indirect method, which utilizes derivatization of the racemates. Since the indirect method can only be applicable for some specific samples that could be synthetically modified for enantioseparation, the use of the direct method is generally preferred due to its distinctive advantages for both preparative and analytical separations. However, the method of chiral stationary phase requires an expensive chiral column, especially for preparative separations. Furthermore, a chiral HPLC column can not be universally used due to its strict stereospecificity. As with all chromatographic methods, various chiral stationary phases are particularly suited to specific types of analytes. In comparison, the method of using the chiral mobile phase additive has been widely used in the preliminary research since it can be performed on a conventional achiral column and it is more versatile with relatively lower costs, because different chiral mobile phase additive could be applied for different samples. Therefore, HPLC with chiral mobile phase additive provides a flexible alternative for enantioseparations.
β-Cyclodextrin is a natural cyclic oligosaccharides comprised of seven glucose units joined through α-1, 4 linkages, and it has been heavily exploited in the chiral separations by HPLC, capillary electrochromatography and other techniques. Hydroxypropyl-β-cyclodextrin (HP-β-CD) and sulfobutyl ether-β-cyclodextrin (SBE-β-CD) are synthetically modified derivatives of β-cyclodextrin. They generally show higher solubility in the aqueous solution and better enantiorecognition than native β-cyclodextrin for any class of enantiomers. In recent years many papers have been reported about their applications as chiral selector in various kinds of chiral separation methods, such as HPLC with chiral mobile phase additive [3-9], enantioselective liquid-liquid extraction [10,11], membrane separation [12], capillary electrophoresis [13,14], etc. In our previous studies, several aromatic carboxylic acids were enantioseparated by high speed countercurrent chromatography using HP-β-CD as a chiral selector in the two-phase solvent system, where strong chiral recognition was observed [15-18]. In the present studies, HP-β-CD and SBE-β-CD were used as chiral mobile phase additives in HPLC to enantioseparate ten racemic mandelic acid derivatives, including mandelic acid, 4-bromomandelic acid, 4-methoxymandelic acid, 4-hydroxymandelic acid, 3-chloromandelic acid, 4-hydroxy-3-methoxymandelic acid, 2-chloromandelic acid, α-cyclohexylmandelic acid, α-cyclopentylmandelic acid and α-methylmandelic acid (Fig.1). Mandelic acid derivatives are of great importance since they are used as either starting materials or critical intermediate for synthesis of chiral drugs. Although several literatures could be found about enantioseparation of mandelic acid [19-22], α-cyclohexylmandelic acid [23] and other mandelic acid derivatives [24] by HPLC with native unsubstituted β-cyclodextrin as a chiral selector, β-cyclodextrin was needed to be synthetically combined with stationary phase [19-20] and peak resolution for these racemates were very low [21, 22, 24]. To the best of our knowledge, only one literature is available about enantioseparation of α-cyclohexylmandelic acid by HPLC with HP-β-CD as a chiral mobile phase additive [4]. But, the method introduced in literature [4] required a long retention time of 90 min for chiral separation for the enantiomers, which was not suitable for HPLC analysis. And in aforementioned literature [23], the mobile phase was composed of ternary solvent systems, including aqueous buffer solution, ethanol and acetonitrile, and so its composition was appeared to be slightly complex. At any rate no literatures about enantioseparation of other nine mandelic acid derivatives by HPLC were available when either of HP-β-CD and SBE-β-CD was used as chiral mobile phase additive. In our research, the enantioseparation of ten mandelic acid derivatives by reverse phase HPLC with HP-β-CD or SBE-β-CD as chiral mobile phase additive were performed and eight of them were successfully enantioseparated with high resolution in short retention time.
Fig. 1. Chemical structures of mandelic acid derivatives.
It is well known that some of the mandelic acid derivatives could be enantioseparated by HPLC with a chiral ligand exchange method. For instance, racemic mandelic acid could be well resolved by conventional HPLC column with a chiral ligand L-phenylalanine and copper sulfate as mobile phase additive [25]. Chiral ligand exchange is an efficient method for enantioseparation of mandelic acid derivatives due to its high stereospecificity. However, sometimes it is difficult to get an ideal chromatogram due to the serious interference caused by the UV absorbance of chiral ligand, L-phenylalanine. Furthermore, not all the mandelic acid derivatives could be enantioseparated by this method. In this paper, HP-β-CD and SBE-β-CD were used as the mobile phase additives for the enantioseparation of ten mandelic acid derivatives by HPLC. Eight racemates were successfully enantioseparated with high resolution by HPLC with HP-β-CD or SBE-β-CD as a mobile phase additive. This method is found to be highly preferred due to its stability, efficiency and simplicity. In addition, the inclusion complex formation between enantiomers and cyclodextrin, which plays a critical role in enantioseparation, was evaluated.
2. Experimental Section
2.1. Chemicals and Reagents
Racemic mandelic acid, 4-bromomandelic acid, 4-methoxymandelic acid, α-cyclohexylmandelic acid, α-cyclopentylmandelic acid and 4-hydroxymandelic acid were purchased from J&K Scientific Ltd., Shanghai, China. 3-chloromandelic acid, 4-hydroxy-3-methoxymandelic acid, 2-chloromandelic acid and α-methylmandelic acid were purchased from Tokyo Chemical Industry Co, Ltd., Tokyo, Japan. HP-β-CD and SBE-β-CD (degree of substitution 7.5 and 6.9, respectively) were purchased from Qianhui Fine Chemical & Co. Inc., Shandong, China. Sodium dihydrogen phosphate, phosphoric acid, acetic acid and sodium acetate with analytical grade were purchased from local commercial chemical store. Acetonitrile and methanol used for HPLC analysis were of chromatographic grade. Water used for HPLC study was redistilled.
2.2. Apparatus
The analytical chromatographic studies were performed on a Shimadzu HPLC CLASS-VP Ver.6.1 system (Shimadzu Corporation, Kyoto, Japan) comprised of a Shimadzu SPD10Avp UV detector, a Shimadzu LC-10ATvp multisolvent delivery system, a Shimadzu SCL-10Avp controller, a Shimadzu LC pump, and a CLASS-VP Ver.6.1 workstation. The pH value for buffer solutions was determined with a portable Delta 320-s pH meter (Mettler-Toledo, Greifensee, Switzerland).
The semi-preparative HPLC used was a KNAUER chromatographic system (KNAUER, Germany) comprised of a KNAUER K-2501 UV detector, two KNAUER K-501 HPLC pumps and an EastChrom Plus workstation. The sample loop for preparative HPLC was 2 mL.
2.3. Chromatographic Condition
The chiral separations were performed on a Shimpak CLC-ODS (150×4.6 mm i.d., 5 μm) column. The mobile phase was a mixture of acetonitrile and 0.1 mol L-1 phosphate buffer (pH 2.68 adjusted with phosphoric acid) containing 20 mM of HP-β-CD or SBE-β-CD. The flow rate was 0.6 mL min-1. The mobile phase was filtered through a 0.45 μm filter and sonicated for 20 min prior to use. The wave length of the UV detector was set at 220 nm for all the analytes. The column was operated at the temperature of 25°C unless otherwise specified. The sample injection volume was 20 μL.
2.4. Preparation of Stock Solution
Racemates of ten mandelic acid derivatives were each accurately weighed, transferred to volumertric flasks and dissolved in the aqueous phase of the mobile phase to make individual stock solutions of 2-3 mmol L-1. The solutions were stored at 5°C and brought to room temperature before use.
2.5. Optimization of Chromatographic Condition
The effects of mobile phase composition, including the buffer type, pH and the organic modifier; concentrations of cyclodextrin, and column temperature on the enantioseparation of the ten mandelic acid derivatives were studied. The ability of chiral separation was evaluated by peak resolution along with retention time. Acetate buffer and phosphate buffer solutions were used and the effect of pH and concentration of the buffer on the resolution were investigated. In addition, the effect of organic modifiers, the concentration of cyclodextrin and the column temperature on peak resolution were also evaluated with a wide range.
2.6. Semi-preparative Enantioseparations
The column was Venusil XBP C18, with 10 μm particle size of the packing material, 250 mm × 10 mm I.D. (Bonna-Agela Technologies Co., Ltd., Wilmington, USA). Composition of the mobile phase was identical to that of analytical HPLC but with a flow rate of 1.0 mL min-1. The UV detector was set at 220 nm. The column temperature was ambient.
Recovery of enantiomer from separated fractions: Each collected fraction was acidified with a small volume of concentrated hydrochloric acid and extracted three times with ethyl acetate. The combined organic layers were washed with small amount of water and then dried with anhydrous sodium sulfate and filtered, and the solvent was evaporated under reduced pressure. The residue of the organic layers was spotted on silica gel TLC plates and developed with dichloromethane: methanol: glacial acetic acid (25:1:0.05, v/v/v). The visual detections were done by iodine powder. Rf value of α-cyclopentylmandelic acid and α-cyclohexylmandelic acid were 0.35 and 0.40 respectively and Rf value of HP-β-CD was less than 0.05. The residue was further subjected to a silica gel column chromatography with isocratic elution to remove small amount of cyclodextrins.
3. Results and Discussion
3.1. Chromatographic conditions for the method development
Initially, the effect of pH value of the mobile phase was determined. Mandelic acid derivatives belong to strong organic acids and they are easily dissociated in the aqueous solution to form ionic molecules when pH increases. Our previous studies showed that HP-β-CD could highly recognize the molecular enantiomer of α-cyclohexylmandelic acid in the aqueous buffer solution at low pH, because HP-β-CD shows chiral recognition only for the isomer of free madelic acid derivatives, but not for ionic molecules [15-18]. Therefore, pH value of the mobile phase plays a key role for peak resolution when HP-β-CD is used as chiral mobile phase additive. Results showed that the lower the pH of the buffer was, the higher peak resolution was obtained for all the racemates. Slight increase of pH of the mobile phase led to a great decrease in peak resolutions for all racemates when pH was greater than 3.0, as shown in the Table 1. Thus, pH value of the mobile phase should be strictly controlled below 3.0, and pH 2.68 was selected for the mobile phase in the present study.
Table 1. Effect of aqueous buffer pH value on peak resolution.
| pH | 2.68 | 3.00 | 3.50 | 4.50 |
|---|---|---|---|---|
| α-cyclopentylmandelic acida | 2.40 | 2.25 | 1.44 | 0.00 |
| α-cyclohexylmandelic acida | 2.86 | 2.87 | 2.40 | 0.90 |
| mandelic acidb | 1.29 | 1.21 | 0.98 | 0.35 |
| 4-methoxymandelic acidb | 1.20 | 1.17 | 1.02 | 0.51 |
| 3-chloromandelic acidb | 1.23 | 1.18 | 1.02 | 0.59 |
| 4-bromomandelic acidb | 1.20 | 1.17 | 1.02 | 0.55 |
Note: 0.1 mol L-1 of phosphate buffer (pH 2.68-3.50) or acetate buffer (pH 4.50) containing 20 mmol L-1 of HP-β-CD and acetonitrile
(60:40) and
(95:5),
flow rate:0.6 mL min-1, wavelength: 220 nm, column temperature: 25 °C.
Then, the influence of mobile phase composition on resolution and retention time was investigated using HP-β-CD as a chiral mobile phase additive. Buffer type and organic modifier were first considered. Preliminary experiments showed that the type of buffer, including phosphate buffer and acetate buffer, didn't significantly affect peak resolution and retention time of the analytes. Then, phosphate buffer was used in our study. Table 2 shows the effects of the concentration of buffer on the peak resolutions of six mandelic acid derivatives. No significant change in the resolutions of racemates was found when the buffer concentration was increased while only a slight change was observed for the column pressure, for instance, 6.1-6.7 MPa was observed under 0.01 mol L-1 of phosphate buffer compared with 6.4-7.2 MPa when the concentration was increased to 0.10 mol L-1. Since the resolution was very sensitive with pH change, a relatively high phosphate buffer concentration of 0.10 mol L-1 was selected.
Table 2. Effects of buffer concentration on peak resolution.
| Concentration of buffer (mol L-1) | 0.01 | 0.02 | 0.05 | 0.10 |
|---|---|---|---|---|
| α-cyclopentylmandelic acida | 2.42 | 2.29 | 2.16 | 2.46 |
| α-cyclohexylmandelic acida | 2.73 | 2.70 | 2.60 | 2.96 |
| mandelic acidb | 1.21 | 1.17 | 1.17 | 1.27 |
| 4-methoxymandelic acidb | 1.13 | 1.12 | 1.09 | 1.20 |
| 3-chloromandelic acidb | 1.18 | 1.17 | 1.18 | 1.21 |
| 4-bromomandelic acidb | 1.17 | 1.15 | 1.16 | 1.20 |
Note: Different concentrations of phosphate buffer at pH 2.68 containing 20 mmol L-1 of HP-β-CD and acetonitrile
(60:40) and
(95:5),
flow rate: 0.6 mL min-1, wavelength: 220 nm, column temperature: 25°C.
On the other hand, the organic modifier in the mobile phase greatly affected both resolution and retention time. The two organic solvents frequently used for the HPLC mobile phase composition, methanol and acetonitrile, were compared under otherwise the same chromatographic conditions. Results showed that much longer retention time was needed for methanol than acetonitrile for base line separation of the enantiomers. For instance, with the mobile phase consisting of phosphate buffer at pH 2.68 containing 10 mmol L-1 of HP-β-CD and organic modifier (60:40) for enantioseparation of α-cyclopentylmandelic acid, the retention time was only 16.0-17.0 min when acetonitrile was used as organic modifier whereas 59.3-68.0 min was needed to elute the enantiomers with a slightly higher resolution with methanol used as the organic modifier. Similar results were also been found in the literature [4], indicating that the retention time of the enantiomer of α-cyclohexylmandelic acid was over 90 min when methanol was used as the organic modifier. Therefore, in our studies acetonitrile was preferred as organic modifier for the mobile phase.
For a given flow rate the concentration of acetonitrile in the mobile phase composition significantly affected peak resolution of the enantiomers (Fig.2). The optimum concentration of acetonitrile in the mobile phase was about 40% for α-cyclohexylmandelic acid and α-cyclopentylmandelic acid, which provided an ideal resolution with short retention time. Lower concentration of acetonitrile resulted in better peak resolution, but the retention times were drastically increased. For example, the retention time for enantiomers of α-cyclohexylmandelic acid was 16.8-18.9 min under 40% of acetonitrile compared with 26.9-33.1 min under 30% of acetonitrile in mobile phase composition. For peak resolution of mandelic acid, 4-bromomandelic acid, 4-methoxymandelic acid and 3-chloromandelic acid, the optimum concentration of acetonitrile was only 5% of the mobile phase composition. Higher concentration of over 5% led to no successful enantioseparation of the four racemates. High resolution could be obtained when concentration of acetonitrile was 2% but long retention time was needed. These experimental results could be explained by different chiral recognition ability of HP-β-CD for each of these racemates. It seems that HP-β-CD exhibits higher chiral recognition on the isomers of α-substitution mandelic acids than those on aromatic substitution mandelic acids. Thus relatively lower effect on resolution was observed for α-substitution mandelic acids when the high concentration of organic modifier was used in the mobile phase. As shown in Fig. 2, the resolution decreased from ca. 5.0 to 3.0 for the enantiomers of α-substitution mandelic acids when the concentration of organic modifier was increased from 30% to 40%; whereas for aromatic substitution mandelic acids, resolution decreased from 1.3-1.5 to 0-0.4 when the concentration of organic solvent was increased from 2% to 15%. As a result, 40% and 5% of acetonitrile were selected for α-substitution and aromatic substitution mandelic acids respectively.
Fig.2.
Effects of concentration of acetonitrile on the resolution of the enantiomers. Mobile phase: 0.1 mol L-1 phosphate buffer at pH2.68 with 20 mmol L-1 of HP-β-CD containing different percentage of acetonitrile in the mobile phase.
Effects of separation temperature on the peak resolution were also investigated. Chiral recognition between enantiomer and cyclodextrin is generally resulted from the formation of inclusion complex and this process is greatly influenced by temperature. The chemical interaction between enantiomer and cyclodextrin is most likely to be an exothermic process and low temperature generally favors its enantioseparation by improving its enantioselectivities. Experimental results showed that when column temperature was increased, retention time for all the enantiomers slightly decreased accompanied with great reduction of resolution, especially for α-substitution mandelic acids. Table 3 summarizes the effect of column temperature on resolution of the racemates.
Table 3. Effects of column temperature on peak resolution.
| Column temperature (°C) | 25 | 35 | 45 |
|---|---|---|---|
| α-cyclopentylmandelic acida | 2.46 | 1.92 | 1.57 |
| α-cyclohexylmandelic acida | 2.96 | 2.46 | 2.03 |
| mandelic acidb | 1.27 | 1.17 | 1.08 |
| 4-methoxymandelic acidb | 1.20 | 1.14 | 1.04 |
| 3-chloromandelic acidb | 1.21 | 1.16 | 1.07 |
| 4-bromomandelic acidb | 1.20 | 1.20 | 1.16 |
Note: mobile phase: 0.10 mol L-1 of phosphate buffer at pH 2.68 containing 20 mmol L-1 of HP-β-CD and acetonitrile
(60:40) and
(95:5),
flow rate: 0.6 mL min-1, wavelength: 220 nm.
Finally, the effect of flow rate on peak resolution was examined for a mobile phase consisting of 0.10 mol L-1 phosphate buffer at pH 2.68 containing 20 mmol L-1 of HP-β-CD and acetonitrile. A flow rate of 0.6 mL min-1 was selected as a compromise between resolution and time of analysis.
3.2. Effect of the type and concentration of chiral mobile phase additive
Two chiral mobile phase additives, HP-β-CD and SBE-β-CD, were tested for enantioseparation of ten mandelic acid derivatives. Results showed that both β-cyclodextrin derivatives could be used for complete enantioseparation of α-cyclohexylmandelic acid and α-cyclopentylmandelic acid with the mobile phase composed of 0.10 mol L-1 phosphate buffer at pH 2.68 and acetonitrile (60:40 for HP-β-CD and 90:10 for SBE-β-CD), but HP-β-CD gave much higher resolution with a shorter retention time than that SBE-β-CD for these two racemates. Furthermore, HP-β-CD could also be used for enantioseparation of the following four racemates: mandelic acid, 4-bromomandelic acid, 4-methoxymandelic acid and 3-chloromandelic acid with the mobile phase phosphate buffer at pH 2.68 and acetonitrile (95:5), but no successful enantioseparation of α-methylmandelic acid, 4-hydroxy-3-methoxymandelic acid, 2-chloromandelic acid and 4-hydroxymandelic acid were obtained. However, further investigation demonstrated that α-methylmandelic acid and 4-hydroxy-3-methoxymandelic acid could be successfully enantioseparated by the mobile phase composed of phosphate buffer at pH 2.68 containing 20 mmol L-1 of SBE-β-CD and acetonitrile (90:10 and 98:2, respectively). Therefore, SBE-β-CD was used as chiral mobile phase additive for the enantioseparation of α-methylmandelic acid and 4-hydroxy-3-methoxymandelic acid.
4-Hydroxymandelic acid appears to be most hydrophilic due to its high polarity since it was eluted out with no resolution within several minutes (5.52 min) by the mobile phase composed of phosphate buffer at pH 2.68 and acetonitrile (95:5) with either HP-β-CD or SBE-β-CD as the chiral selector. 2-Chloromandelic acid couldn't be enantioseparated by HPLC with the above mobile phase (95:5 or 100:0) with either of the two chiral mobile phase additives. However, 2-chloromandelic acid was reported to be enantioseparated by HPLC with native β-cyclodextrin as a chiral mobile phase additive when an aqueous buffer at pH 2.1 containing ca. 14 mmol L-1 of β-cyclodextrin with no organic modifier was used as the mobile phase [24]. The mobile phase containing native β-cyclodextrin reported in the literature was also tested in our HPLC column for enantioseparation of 2-chloromandelic acid, but no successful enantioseparation was obtained. Different stationary phase might be needed for successful enantioseparation of 2-chloromandelic acid.
The concentration of chiral mobile phase additive has a significant effect on peak resolution of the optical isomers. Fig. 3 shows the effects of concentration of HP-β-CD on peak resolution of enantiomers of the six racemic mandelic acid derivatives. With the increasing concentration of HP-β-CD, peak resolution reached the maximum value for α-cyclohexylmandelic acid and α-cyclopentylmandelic acid when 60-80 mmol L-1 of HP-β-CD was used, whereas for mandelic acid, 4-bromomandelic acid, 4-methoxymandelic acid and 3-chloromandelic acid the resolution reached the highest value when 10-20 mmol L-1 of HP-β-CD was used as the mobile phase additive. As expected, the retention time was decreased with the increase of concentration of HP-β-CD. Since good enantioseparation could also be achieved for α-cyclohexylmandelic acid and α-cyclopentylmandelic acid when low concentration of HP-β-CD was used, 20 mmol L-1 of chiral mobile phase additive was finally selected for the mobile phase. Here it needs to be pointed out that the optimum concentration of the chiral mobile phase additive was critically dependent on the concentration of organic modifiers in the mobile phase [26].
Fig.3.
Effects of the concentration of HP-β-CD on the resolution of the enantiomers. Experimental conditions: Mobile phase: 0.10 mol L-1 of phosphate buffer at pH2.68 containing different concentration of HP-β-CD and acetonitrile (95:5) for mandelic acid, 4-methoxymandelic acid, 3-chloromandelic acid and 4-bromomandelic acid and (60:40) for α-cyclopentylmandelic acid and α-cyclohexylmandelic acid, flow rate:0.6mL min-1, wavelength: 220 nm, column temperature: 25 °C.
Fig. 4 and Table 4 show the optimum HPLC conditions for enantioseparation of eight mandelic acid derivatives. Except for the two racemates of 2-chloromandelic acid and 4-hydroxymandelic acid, all other racemates could be well enantioseparated using HP-β-CD or SBE-β-CD as the chiral mobile phase additive.
Fig.4.
Chromatograms of the eight mandelic acid derivative enantiomers with the optimized chromatographic conditions. Mobile phase compositions were listed in Table 4. Other chromatographic conditions are described in Experimental sections 2.3.
Table 4. The optimized chromatographic conditions for enantioseparation of racemic mandelic acid derivatives.
| Racemates | Ratio of mobile phase | Chiral mobile phase additive | Resolution (Rs) | Capacity factor k′ | Enantioselectivity α |
|---|---|---|---|---|---|
| α-cyclopentylmandelic acid | 60:40 | HP-β-CD | 2.46 | 3.47; 3.94 | 1.14 |
| α-cyclohexylmandelic acid | 60:40 | HP-β-CD | 2.96 | 4.59; 5.27 | 1.15 |
| mandelic acid | 95:5 | HP-β-CD | 1.27 | 2.66; 3.01 | 1.13 |
| 4-methoxymandelic acid | 95:5 | HP-β-CD | 1.20 | 3.19; 3.62 | 1.13 |
| 3-chloromandelic acid | 95:5 | HP-β-CD | 1.21 | 7.46; 8.34 | 1.12 |
| 4-bromomandelic acid | 95:5 | HP-β-CD | 1.20 | 10.32; 11.67 | 1.13 |
| α-methylmandelic acid | 90:10 | SBE-β-CD | 1.78 | 3.59; 3.97 | 1.11 |
| 4-hydroxy-3-methoxymandelic acid | 98:2 | SBE-β-CD | 1.38 | 1.01; 1.14 | 1.13 |
Note: mobile phase: 0.1 mol L-1 phosphate buffer at pH 2.68: acetonitrile containing 20 mmol L-1 of chiral mobile phase additive; flow rate: 0.6mL min-1, wavelength: 220 nm, column temperature: 25°C.
Enantioseparation of the eight mandelic acid derivatives by semi-preparative HPLC column were also investigated. Only two racemates with high enantioselectivity, α-cyclohexylmandelic acid and α-cyclopentylmandelic acid, could be successfully enantioseparated by semi-preparative HPLC column. No successful preparative enantioseparation for the rest of racemates was obtained due to low enantioselectivity. As shown in Fig.5, 10 mg of racemic α-cyclohexylmandelic acid and α-cyclopentylmandelic acid could be enantioseparated individually by a semi-preparative reverse phase column with the same mobile phase as indicated in Table 4. The fractions from preparative HPLC were analyzed by analytical HPLC. Results showed that purity of enantiomers was over 95%. Recovery of enantiomer was in the range of 66-82% and 3.5-4.4 mg of enantiomer was obtained from the preparative enantioseparation.
Fig. 5.
Semi-preparative HPLC chromatogram for enantioseparation of α-cyclohexylmandelic acid and α-cyclopentylmandelic acid. Column: Venusil XBP C18 (250 mm × 10 mm I.D.); mobile phase: phosphate buffer at pH 2.68 containing 20 mmol L-1 of HP-β-CD and acetonitrile (60:40, v/v); flow rate: 1.0 mL min-1; UV detector: 220 nm; column temperature: ambient.
3.3. Evaluation of inclusion complex formation
Any organic molecules smaller than the cavity of HP-β-CD molecule could undergo non-covalent interactions, including electrostatic interactions, van der Waals forces, and hydrophobic effects, with the atoms lining and trimming inside of the cavity, leading to the inclusion complex formation [27]. A 1:1 stoichiometry for the inclusion complex is assumed in most of the previous studies. Evaluation of inclusion complex formation is important for discussion on chiral separation mechanism because the interpretation of kinetic, synthetic, and chiral interaction data could be significantly altered if multiple cyclodextrin molecules per one analyte complexes were formed. Alternatively, there are also cases where two guest molecules bind to a single cyclodextrin host. The chiral HPLC method with chiral mobile phase additive is an efficient way to determine the binding constant or partition coefficient of a solute to a cyclodextrin molecule. For a 1:1 or 2:1 (cyclodextrin: substrate) complex the following equations could be used for determination of formation constant and the stoichiometry for the inclusion complex [28]:
| (1) |
| (2) |
Where k′ is chromatographic definition of capacity factor, Φ is the phase ratio, A is a stationary phase adsorption site, CD stands for cyclodextrin, and k, k1 and k2 are the respective equilibrium constants. Equations (1) and (2) describe the HPLC retention behavior of solutes bound with one and two cyclodextrin molecules, respectively. Plots of 1/k′ vs. cyclodextrin concentration give a straight line for 1:1 stoichiometry inclusion complex (Eq. 1). In cases where two or more cyclodextrin molecules bind to one solute molecule, traditional equations give curves of increasing slope (Eq. 2). Since the intercept 1/Φk[A] is frequently near zero and inaccurate, the following alternative equation is proposed for equation (2) [28]:
| (3) |
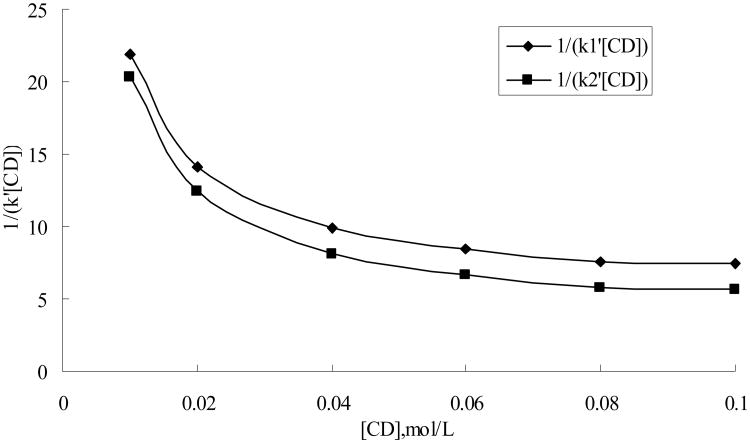
Equation (3) indicates that a plot of 1/k′[CD] vs. cyclodextrin concentration should be linear and have a slope of k1k2/Φk[A] and intercept of k1/Φk[A] for cyclodextrin complexes of 2:1 stoichiometry. But for 1:1 inclusion complexes, plots of 1/k′[CD] vs. cyclodextrin concentration with equation (3) would produce downward curving lines of negative slope, which could be further used to testify the binding ratio.
Table 5 gives the retention data for the six mandelic acid derivatives as a function of concentration of HP-β-CD in the mobile phase. Plots of 1/k′ vs. cyclodextrin concentration for all the enantiomers gave the straight lines as determined by the method of least squares, and the correlation coefficients of all plots were greater than 0.997 indicating that the stoiciometry was 1:1 for all the enantiomer-cyclodextrin inclusion complex as shown in Table 6. Furthermore, Plots of 1/k′[CD] vs. cyclodextrin concentration of equation (3) resulted in downward curving lines of negative slope, which excluded the cases of multiple cyclodextrin molecules combined with one analyte complexes. The representative plots are given in Fig. 6. Therefore, no cyclodextrin multiple complex formations, e.g. 2:1 stoichiometry inclusion complex, were found in the present studies.
Table 5. Variation in the capacity factor (k′) of six mandelic acid derivatives with increasing concentration of HP-β-CD in the mobile phase.
| Racemates | Concentration of HP-β-CD | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0.01 | 0.02 | 0.04 | 0.06 | 0.08 | 0.10 | |
| α-cyclopentylmandelic acid | 4.570 | 3.540 | 2.541 | 1.985 | 1.661 | 1.337 |
| 4.930 | 4.004 | 3.068 | 2.500 | 2.150 | 1.779 | |
| α-cyclohexylmandelic acid | 6.402 | 4.689 | 3.224 | 2.448 | 1.884 | 1.562 |
| 6.976 | 5.354 | 3.901 | 3.085 | 2.415 | 2.049 | |
| mandelic acid | 3.280 | 2.644 | 1.917 | 1.497 | 1.259 | 1.060 |
| 3.599 | 2.982 | 2.203 | 1.743 | 1.477 | 1.251 | |
| 4-methoxymandelic acid | 4.270 | 3.154 | 2.059 | 1.522 | 1.237 | 1.017 |
| 4.757 | 3.561 | 2.348 | 1.744 | 1.422 | 1.165 | |
| 3-chloromandelic acid | 10.580 | 7.387 | 4.516 | 3.238 | 2.575 | 2.091 |
| 11.646 | 8.237 | 5.081 | 3.671 | 2.929 | 2.381 | |
| 4-bromomandelic acid | 15.651 | 10.149 | 5.828 | 4.122 | 3.232 | 2.619 |
| 17.547 | 11.448 | 6.604 | 4.686 | 3.674 | 2.970 | |
Note: For the chromatographic conditions see Fig. 3.
Table 6. Regression equation of inclusion complex and binding constants.
| Racemates | Regression equation | R2 | Binding constants L/mol |
|---|---|---|---|
| α-cyclopentylmandelic acid | 1/k1′=5.7116[CD]+0.1630 | 0.9973 | 35.040 |
| 1/k2′=3.8649[CD]+0.1679 | 0.9973 | 23.019 | |
| α-cyclohexylmandelic acid | 1/k1′=5.3413[CD]+0.1005 | 0.9986 | 53.147 |
| 1/k2′=3.8054[CD]+0.1055 | 0.9984 | 36.070 | |
| mandelic acid | 1/k1′=7.0158[CD]+0.2374 | 0.9996 | 29.553 |
| 1/k2′=5.7685[CD]+0.2214 | 0.9996 | 26.055 | |
| 4-methoxymandelic acid | 1/k1′=8.2885[CD]+0.1526 | 0.9997 | 54.315 |
| 1/k2′=7.1604[CD]+0.1386 | 0.9996 | 51.662 | |
| 3-chloromandelic acid | 1/k1′=4.2546[CD]+0.0513 | 0.9998 | 82.936 |
| 1/k2′=3.7019[CD]+0.0484 | 0.9998 | 76.486 | |
| 4-bromomandelic acid | 1/k1′=3.5276[CD]+0.0291 | 0.9999 | 121.223 |
| 1/k2′=3.0996[CD]+0.0262 | 0.9999 | 118.305 |
Note: For the chromatographic conditions see Fig. 3.
4. Conclusions
The enantioseparation of ten mandelic acid derivatives by reverse phase HPLC with chiral mobile phase additive was investigated where eight racemates including α-cyclohexylmandelic acid, α-cyclopentylmandelic acid, mandelic acid, 4-bromomandelic acid, 4-methoxymandelic acid, 3-chloromandelic acid, α-methylmandelic acid and 4-hydroxy-3-methoxymandelic acid were successfully enantioseparated. The separations were performed with the mobile phase consisted of 0.10 mol L-1 phosphate buffer at pH 2.68 containing 20 mmol L-1 of HP-β-CD or SBE-β-CD with a suitable concentration of acetonitrile. Evaluation of inclusion complex formation showed that the stoichiometry for all inclusion complexes between enantiomer and cyclodextrin was 1:1 and their binding constants were determined.
Acknowledgments
The authors are greatly indebted to National Natural Science Foundation of China (No. 21105090) and Department of Education of Zhejiang Province of China (pd2013031). S.Q. Tong also thanks Personnel Department of Zhejiang University of Technology for providing the visiting scholar program (2011).
References
- 1.FDA'S policy statement for the development of new stereoisomeric drugs. Chirality. 1992;4:338. doi: 10.1002/chir.530040513. [DOI] [PubMed] [Google Scholar]
- 2.Beesley TE, Lee JT. Method development and optimization of enantioseparations using macrocyclic glycopeptide chiral stationary phases. In: Subramanian G, editor. Chiral Separation Techniques. 3rd. Vol. 11. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2007. p. 1. [Google Scholar]
- 3.Xu J, Zhao WX, Ning YW, Bashari M, Chen YH, Jin ZY, Yang N, Xu XM. J Incl Phenom Macro. 2013;76:461. [Google Scholar]
- 4.Shi JH, Su YH, Jiang W. J Chromatogr Sci. 2013;51:8. doi: 10.1093/chromsci/bms097. [DOI] [PubMed] [Google Scholar]
- 5.Ye JC, Yu WY, Chen GS, Shen ZG, Zeng S. Biomed Chromatogr. 2010;24:799. doi: 10.1002/bmc.1365. [DOI] [PubMed] [Google Scholar]
- 6.Su LQ, Li C, Zhang XH, Li YJ. Yaowu Fenxi Zazhi. 2008;28:921. [Google Scholar]
- 7.Leon AG, Olives AI, Martin MA, Castillo B. J Incl Phenom Macro. 2007;57:577. [Google Scholar]
- 8.Jiao FP, Huang KL, Ning FR, Hu WG, Yu JG. Separ Sci Technol. 2006;41:1893. [Google Scholar]
- 9.Ameyibor E, Stewart JT. J Liq Chromatogr R T. 1997;20:855. [Google Scholar]
- 10.Tang KW, Zhang H, Zhang PL. Ind Eng Chem Res. 2013;52:3893. [Google Scholar]
- 11.Tang KW, Yi JM, Huang KL, Zhang GL. Chirality. 2009;21:390. doi: 10.1002/chir.20601. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Cai CQ, Lin YT, Bian YR, Guo HQ, Chen XM. Sep Purif Technol. 2011;79:63. [Google Scholar]
- 13.Qiu B, Chen HJ, Chen GN. Zhongguo Yaoxue Zazhi. 2005;40:1276. [Google Scholar]
- 14.Huang YY, Xiao MT, Shi XN, Meng C, Guo YH. Yaowu Fenxi Zazhi. 2004;24:460. [Google Scholar]
- 15.Tong SQ, Zheng Y, Yan YZ. J Chromatogr A. 2013;1281:79. doi: 10.1016/j.chroma.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Tong SQ, Guan YX, Yan JZ, Zheng B, Zhao LY. J Chromatogr A. 2011;1218:5434. doi: 10.1016/j.chroma.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Tong SQ, Yan JZ, Guan YX, Lu YM. J Chromatogr A. 2011;1218:5602. doi: 10.1016/j.chroma.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Tong SQ, Yan JZ, Guan YX, Fu YE, Ito Y. J Chromatogr A. 2010;1217:3044. doi: 10.1016/j.chroma.2010.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSoi D, Kier LB, Cheng CK, Karnes HT. J Chromatogr A. 2013;1291:73. doi: 10.1016/j.chroma.2013.03.060. [DOI] [PubMed] [Google Scholar]
- 20.Ruan YP, Zhang XM, Chen AQ, Qiu HY, Huang PQ. Sepu. 2004;22:420. [PubMed] [Google Scholar]
- 21.Debowski J, Sybilska D, Jurczak J, Chromatogr J. 1982;237:303. [Google Scholar]
- 22.Debowski J, Sybilska D, Jurczak J. Chromatographia. 1982;16:198. [Google Scholar]
- 23.Hu SS, Wu YZ, Shi MR. Jingxi Huagong. 2004;21:731. [Google Scholar]
- 24.Debowski J, Jurczak J, Sybilska D. J Chromatogr. 1983;282:83. [Google Scholar]
- 25.Wernicke R. J Chromatogr Sci. 1985;23:39. [Google Scholar]
- 26.Herráez-Hernández R, Campíns-Falcó P. J Chromatogr B. 2000;740:169. doi: 10.1016/s0378-4347(00)00103-1. [DOI] [PubMed] [Google Scholar]
- 27.Wong AB, Lin SF, Connors KA. J Pharm Sci. 1983;72:388. doi: 10.1002/jps.2600720417. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong DW, Nome F, spino LA, Golden TD. J Am Chem Soc. 1986;108:1418. [Google Scholar]