Abstract
Continuing transmission of human intestinal schistosomiasis depends on the parasite’s access to susceptible snail intermediate hosts (often Biomphalaria glabrata). Transmission fails when parasite larvae enter resistant individuals in wild snail populations. The genetic basis for differences in snail susceptibility/resistance is being intensively investigated as a means to devise novel control strategies based on resistance genes. Reactive oxygen species produced by the snail’s defence cells (haemocytes) are effectors of resistance. We hypothesised that genes relevant to production and consumption of reactive oxygen species would be expressed differentially in the haemocytes of snail hosts with different susceptibility/resistance phenotypes. By restricting the genetic diversity of snails, we sought to facilitate identification of resistance genes. By inbreeding, we procured from a 13–16-R1 snail population with both susceptible and resistant individuals 52 lines of B. glabrata (expected homozygosity ~87.5%), and determined the phenotype of each in regard to susceptibility/resistance to Schistosoma mansoni. The inbred lines were found to have line-specific differences in numbers of spreading haemocytes; these were enumerated in both juvenile and adult snails. Lines with high cell numbers were invariably resistant to S. mansoni, whereas lines with lower cell numbers could be resistant or susceptible. Transcript levels in haemocytes were quantified for 18 potentially defence-related genes. Among snails with low cell numbers, the different susceptibility/resistance phenotypes correlated with differences in transcript levels for two redox-relevant genes: an inferred phagocyte oxidase component and a peroxiredoxin. Allograft inflammatory factor (potentially a regulator of leucocyte activation) was expressed at higher levels in resistant snails regardless of spread cell number. Having abundant spreading haemocytes is inferred to enable a snail to kill parasite sporocysts. In contrast, snails with fewer spreading haemocytes seem to achieve resistance only if specific genes are expressed constitutively at levels that are high for the species.
Keywords: Biomphalaria, Schistosoma, Haemocyte, Haematocrit, Host–parasite, Resistance, Expression, Oxidative
1. Introduction
Schistosomiasis is a group of chronic parasitic diseases afflicting at least 240 million people in over 70 countries (United States of America Centers for Disease Control and Prevention, http://www.cdc.gov/parasiteSchistosomiasis/index.html). Its causative agents, trematodes of the genus Schistosoma, cycle between humans and fresh water snails of which Biomphalaria spp. are important in Africa and South America. Infected humans discharge eggs which hatch upon contact with fresh water, releasing miracidia. These penetrate the snail headfoot/mantle and transform into primary sporocysts near their point of entry. Within susceptible snails, two generations of asexual reproduction result in the production of thousands of cercariae that, when shed from a snail, can infect humans. Susceptible intermediate snail hosts provide protection and nourishment for larval schistosomes as they multiply. In resistant Biomphalaria glabrata snails, which do occur in nature (Newton, 1953; Michelson and DuBois, 1978), the Schistosoma mansoni parasite fails to develop, presumably due to recognition and aggressive activities of the immune system.
Since humans must be exposed to snail-derived larvae in order to be infected with blood flukes, it is crucial to understand the mechanisms which permit or prevent the parasite’s establishment in the snail. Considerable advances have been made toward this goal (Bayne, 2009; Loker, 2010; Moné et al., 2010; Martins-Souza et al., 2011; Hanington et al., 2012; Mitta et al., 2012; Negrão-Corrêa et al., 2012; Blouin et al., 2013; Ittiprasert et al., 2013), yet for both the recognition and the effector phases of the snails’ defence responses, much remains to be learned. Among known facts are that snail size can influence infectivity rates: some strains of B. glabrata are susceptible as juveniles but resistant as adults (Richards et al., 1992), and larger snails exposed to S. mansoni have lower infection levels than smaller snails of the same age (Niemann and Lewis, 1990). Circulating snail haemocytes play a key role in immune surveillance (Oliveira et al., 2010) and will migrate from the haemolymph into the tissues after parasitic infection (Noda and Loker, 1989; Martins-Souza et al., 2009; Barçante et al., 2012). This change is most intense in resistant snails in which larger haemocytes nearly disappear from the haemolymph, while small cells gradually increase (Martins-Souza et al., 2009). Haemocytes are involved in parasite recognition (Negrão-Corrêa et al., 2012), a capability that involves carbohydrate-binding receptors on spreading haemocytes (Fryer et al., 1989; van der Knaap and Loker, 1990; Renwrantz and Richards, 1992; Johnston and Yoshino, 2001; Castillo et al., 2007; Martins-Souza et al., 2011; Mitta et al., 2012). These cells are phagocytic granulocytes and, in resistant snails, they encapsulate schistosomes and the parasites are killed.
In the process of encapsulation, carbohydrate ligand binding by haemocyte receptors initiates production of toxic reactive oxygen species (ROS) (Hahn et al., 2000; Humphries and Yoshino, 2008) and nitric oxide (NO) (Hahn et al., 2001). Additional evidence for the role of ROS in parasite killing is the association of the B allele of the cytoplasmic Cu/Zn superoxide dismutase (sod1) gene in B. glabrata with resistance to S. mansoni, and the fact that snails with the B allele have significantly higher sod1 expression (Bender et al., 2007). Schistosomes produce quantities of proteins that can scavenge ROS, possibly a strategy to avoid oxidative damage (Mourão et al., 2009). Considered together, these facts strongly implicate oxidative stress and nitration in haemocyte-effected snail defences against schistosomes.
As a path toward examining the genes involved in determination of snail resistance (R) or susceptibility (S), we used the hermaphroditic nature of B. glabrata and its ability to self-fertilise to derive more than 50 inbred snail lines from our 13–16-R1 population. The lines, exhibiting approximately 87.5% homozygosity, were phenotyped for resistance to S. mansoni (PR1 strain). The most highly resistant (R) and most highly susceptible (S) lines were further examined for haemocyte numbers and for expression of a panel of genes known to be involved in oxidative stress or otherwise implicated in the R/S phenotype. We hypothesised that higher numbers of haemocytes, or haemocytes which constitutively express relevant defence genes at higher levels would successfully thwart trematode infection. Innate resistance of Aedes aegypti mosquitos to Dengue virus is similarly thought to rely on ‘basal-level immune activation’ of immune-related genes (Sim et al., 2013).
Because the R/S phenotype is influenced by snail size (Richards and Merritt, 1972; Richards et al., 1992), we counted spread haemocytes in small juvenile snails and in larger adults, and determined R/S phenotypes in those two size classes. When snail size, spread haemocyte number, and constitutive haemocyte mRNA levels of selected genes were considered alongside the R/S phenotypes, complex relationships emerged. Increased snail size alone did not guarantee resistance. In adult snails, high numbers of spread cells ensured resistance, but similarly high counts in juvenile snails did not. When mRNA levels were compared in R and S lines of equally low spread cell number, peroxiredoxin1 (prx1) and a gene for an inferred phagocyte oxidase (phox) subunit were twofold higher in R relative to S snails. The gene for allograft inflammatory factor (aif) was also expressed at higher levels in R snail lines, regardless of the numbers of spreading haemocytes characterizing the lines. The first two genes add further evidence of critical roles for ROS-dependent killing mechanisms in this host–parasite system, while aif suggests that constitutive activation of haemocytes may also contribute to the R phenotype.
2. Materials and methods
2.1. Animal care
Animals were handled following protocols approved by the Oregon State University, USA, Animal Care and Use Committee according to requirements outlined in the United States National Research Council Guide for the Care and Use of Laboratory Animals, 8th edition. Animal numbers were held to the minimum required for the research.
2.2. Inbreeding followed by resistance phenotyping
Inbred snail lines were developed from juveniles of our 13–16-R1 (Oregon State University) laboratory population of B. glabrata. The provenance of this strain was described earlier (Bonner et al., 2012). Snails were isolated prior to sexual maturity and allowed to mature and self-fertilise. Two more generations of offspring were subsequently isolated as juveniles for two more full rounds of inbreeding. Fourth generation siblings were used to establish tank colonies (inbred lines).
Inbred lines were maintained in 26 °C de-chlorinated water supplemented with shell hardener (480 μM CaCO3, 82 μM NaCl, 58 μM MgCO3, 13 μM KCl). Filters contained activated charcoal; crushed coral was added to enhance buffering capacity. To minimise the chances of stress-related effects, snails were sampled between 1 and 4 weeks after water change. The snails were fed washed green leaf lettuce ad libitum and kept in an environment with a 12 h light–dark cycle.
To score susceptibility, 12 snails were placed individually in wells with 1.5 mL of artificial spring water (Ulmer, 1970) and exposed for 2 h to five freshly hatched S. mansoni miracidia. The parasite eggs were isolated from hamster livers and miracidia were prepared as previously described (Stibbs et al., 1979). After exposure each snail group was maintained in a tank under dim light (~3 lx) (Steinauer and Bonner, 2012). Infection was confirmed by cercarial shed from individual snails exposed to bright light at 5, 7 and 9 weeks post-exposure. The entire procedure was repeated once and yielded consistent results.
2.3. Spread cell counts
Haemolymph was collected from snails of 5 and 12 mm diameter, and haemocytes that extended pseudopods were counted. To minimise stress, each snail was placed in a small dish containing tank water at 26 °C. Sterile balanced salt solution (CBSS; 48 mM NaCl, 2 mM KCl, 0.5 mM Na2HPO4, 0.6 mM NAHCO3, 5.5 mM glucose, 2.9 mM trehalose; Chernin, 1963) was pre-warmed to 26 °C. Directly prior to bleeding, snail shells were cleaned with de-chlorinated water and cotton swabs, and wiped dry. Cardiac punctures were performed (Bayne et al., 1980) and only haemolymph which pooled on the shell was sampled. Haemolymph from each individual snail was placed on Parafilm™ and shell debris was allowed to settle for 30 s. The clean, upper portion of haemolymph was mixed 1:1 with CBSS and loaded on a Neubauer Improved haemacytometer. After incubation in a 26 °C humid chamber for 30 min, the cells with pseudopodia were counted in five 1 mm squares at 200× magnification. Cell numbers were calculated as cells/μL of haemolymph. Over a period of 6 months, 10 snails of 12 mm diameter and three snails of 5 mm diameter were sampled from each inbred line.
2.4. RNA extraction and cDNA synthesis
Snails were prepared for haemocyte RNA collection as described for cell counts. Each 250 μL sample contained the pooled haemolymph of eight to 12 individual snails. Multiple samples were combined for RNA preparations and the number of samples was dependent on the inbred line’s circulating haemocyte number; a larger total volume was combined for lines with fewer haemocytes. From two to seven sample pools were required from each line to ensure quality RNA in satisfactory amounts. Each 250 μL haemolymph sample was pipetted onto a poly-L-lysine coated well of a 96 well plate (Costar #3997, USA) pre-loaded with 50 μL of CMC medium at 26 °C (22% Schneider’s Drosophila Medium, 7.2 mM glucose, 23.9 mM NaCl, 0.1% Gentamycin™, pH 7.4, 121 mOsm). The plates were centrifuged at 25g for 5 min at room temperature. Samples were inspected with an inverted microscope and were rejected if contamination of any sort was present. Plasma (200 μL) was replaced with 200 μL of CMC medium and centrifugation was repeated. All but 10% of the medium was removed and the haemocytes were lysed in 400 μL of RLT™ buffer (Qiagen, USA) containing 1% β-mercaptoethanol. All steps were completed in 40 min. Total RNA was isolated according to the manufacturer’s protocol for the RNeasy kit™ (Qiagen). The protocol included an on-column DNase treatment (Qiagen). RNA concentrations were measured with a Quant-It RiboGreen RNA Assay Kit™ (Invitrogen, USA) using a standard curve based on rRNA, and RNA integrity was evaluated using an Agilent 2100 Bioanalyzer with an Agilent RNA 6000 Pico Kit® (Agilent Technologies, USA). Two independent RNA samples from pooled individuals were collected over a 6–20 month period for each inbred line. At least 20 ng of snail RNA were added to each 100 μL reverse transcription reaction. Pilot runs revealed that reactions containing less than 20 ng of RNA sometimes failed to give linear responses in message abundance for low-expressing genes as measured by quantitative PCR (qPCR). Reverse transcription (RT) was performed using Superscript VILO RT™ (Invitrogen) with random hexamer primers. The RT programme was 10 min at 25 °C, 60 min at 42 °C, 85 °C for 5 min. Samples were stored at −80 °C.
To confirm the removal of genomic DNA (gDNA), qPCR was performed on RNA samples using primers specific for a sequence within a flanking region of the myoglobin gene (myo). The forward and reverse primers, 5′-TGGATGTTCGCCAATGTTC-3′ and 5′-TGACGTTGACCTGCTTGATG-3′, respectively, were combined at 4 pmol with 0.5 μL of sample RNA in 20 μL of qPCR mixture. The programme was the same as that used to measure cDNA levels. Under these conditions, 35 pg of gDNA could be detected with an amplification threshold of 35. RNA preparations with cycle threshold (Ct) values of ≤35 were considered gDNA positive and were excluded from the study.
2.5. qPCR to determine transcript abundance
qPCR was performed in 96-well optical plates with an ABI Prism 7500 Sequence Detection System™ (Applied Biosystems, USA) using iTaq™ SYBR® Green Supermix with ROX (BioRad, USA). Cycling conditions were: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 56 °C for 1 min, followed by melting curve analysis (from 60 to 95 °C) for single product verification. From each of two independently collected haemocyte pools representing at least 16 snails from each snail line, a minimum of 200 pg of cDNA was loaded with 4 pMol of each primer in 20 μL reaction volumes. The Ct value was determined for each reaction using manual baseline and threshold settings. All qPCRs were carried out in duplicate with a no template control (NTC) included for each primer pair. Raw fluorescence readings were analysed by LinReg (Ramakers et al., 2003) and only reactions with efficiencies above 1.8 were included in the study. The amplification of two reference genes (see Section 2.7) was averaged and used for normalisation in each sample. Relative constitutive expression of each gene was calculated in the form of ΔCt: the difference between gene of interest (g.o.i.) and the mean of two reference genes, adjusted for efficiency.
2.6. Design of primers for genes of interest
Target genes not yet named in GenBank (duox303, gpx65, nox2, phox22, infPhox) were located in the B. glabrata genome as follows: orthologues from other species (including – when available – gastropods Lottia gigantea, Lymnaea stagnalis and Aplysia californica) were retrieved and aligned. Highly conserved regions were located and used to interrogate the B. glabrata genome to discover the homologous sequences. Amplicons obtained with paired primers were sequenced and verified as orthologous to the genes of interest. The inferred phox subunit found with this strategy was initially thought to be a phox47 orthologue, but later analysis revealed that it has limited similarity to that protein. Even though it remains partially characterised, it is included in this report due to its significant correlations with resistance in a subset of snail lines. For other genes, publicly available B. glabrata sequences were retrieved and primers optimised for qPCR (Table 1) were designed using Primer3 on-line (Rozen and Skaletsky, 2000). Supplementary Table S1 explains the reasons for inclusion of each g.o.i. in the study.
Table 1.
The genes in this study are listed. For arginase, catalase, two dual oxidases, three glutathione peroxidases and a peroxiredoxin, the scaffold on which each is encoded in the present assembly (BglaB1) of the Biomphalaria glabrata genome (https://www.vectorbase.org/organisms/biomphalaria-glabrata) is included as part of the gene abbreviation. Supplementary Table S1 further explains the reasons for inclusion of each gene in the study.
| Protein name | Gene abbreviation | Accession # | Primer Sequences: 5′–3′ |
|---|---|---|---|
| Arginase | arg1386 | CX727777 | F-CAGTGCTGGCTCTAGGAGGA R-AGACGGACGTCAGAGGAGTG |
| Catalase | cat42 | JZ482251 | F-TGGTGAACAAAGATGGCAAG R-AGCTTGGCAGCAGCATCAG |
| Dual oxidase 1 | duox584 | CK149203 | F-GCATCGATTTGTTCCATTTGA R-GATGACGGCTGTGTGGCTAT |
| Dual oxidase 2a | duox303 | JZ482249 | F-CCTTGACATCAGCAGCTTCC R-CGTCACTCCGTTCGCTTCTA |
| Glutathione peroxidase 1 | gpx97 | EW999290 | F-TGAAGTCAATGGCAAGAATGC R-GGGAGCATAGCGATTCACTG |
| Glutathione peroxidase 2 | gpx2404 | DW474737 | F-ACTTGAATGTGCTGGCCTTC R-GGCGCACATATCTGAGACCA |
| Glutathione peroxidase 3a | gpx65 | JZ482250 | F-TCTCTGGAAAAATTTAGAGGCAAAG R-GCAAGGAAAGGCCAAAACAT |
| Migration inhibitory factor | mif | GQ118971.1 | F-TGCCAGCCCTGTTCTGTCA R-TCCCTTGAGGTCTTAATCAC |
| NADPH oxidasea | nox2 | JZ482247 | F-TCACCAGTCGAGAGTGTTTGAA R-TGCCACTCTAGGCGTGAGAT |
| p22phoxa | phox22 | JZ482252 | F-TGCCAACTATTCTGGGTGCT R-GGTCTCCTTGGTGGTGGTCT |
| infPhoxa,b | infPhox | JZ482248 | F-TGACACCTTCCCCATTGAAG R-GACATCTTCACACCGAGAAATTTTA |
| Peroxiredoxin 1 | prx1 | CK988723 | F-ATCCCACTTCTTGCTGACAAAA R-AAGCCTGTACAAGGCGAAGA |
| Peroxiredoxin 4 | prx4 | FJ176942 | F-AAACATGGCATCCTCTCTGC R-GCGTTTGGTTTCTTGTGGAT |
| Peroxiredoxin 6 | prx6 | EW997425.1 | F-GTGGGCATGCCTGTTTTC R-TCTGGCTCAAGGCTTACTGC |
| Peroxiredoxin621 | prx621 | DW474756 | F-AAACTGTCTGATTTCAAGGGAAAG R-GGCTGTAATCTCTGTGGGACA |
| T-cell acute lymphocytic leukaemia protein 1c | tal1–100 | AC238540 | F-GCAGCAGAACGTCAATGGAG R-AATGGCCAGCCTCAGAATTT |
| Fibrinogen-related protein 3 | frep3 | AY028461 | F-GCAGACTTGAGCACTAAACAACAA R-CGAGGCAGTTGAAAGATTGG |
| Allograft inflammatory factor | aif | ES744748.1 | F-GTCAAGCTAAGACCCACCTGGAG R-TTGCCGAGTCCTTCAAACAGA |
The snail gene was found using a bioinformatics approach. Sequences from other species were multiply aligned and conserved stretches of the protein were sought in publicly available B. glabrata expressed sequence tag (EST) and genome databases. In silico analyses indicated that functionally essential motifs are encoded in the snail gene.
The sequence infPhox encodes less than a functional protein. See Section 2.6 for the basis of the inferred name.
This putative tal1 orthologue less closely resembles NFkB. Primers were designed based on the B. glabrata sequence most probably encoding the tal1 orthologue.
2.7. Determination of optimum reference genes
Genes whose transcripts are present at consistent levels in a given tissue or species (‘reference’ or ‘housekeeping’ genes) may not be consistent in other tissues or species. In spite of this, genes such as β-actin have often been assumed wrongly to be suitable reference genes across species. Validation of prospective steady-state genes in B. glabrata was deemed helpful for us and others. Extensive primer design and testing of seven prospective ‘steady state’ genes determined the most stable two in our snail haemocytes. Snails from the 13–16-R1 parental stock, from which the lines were derived, were prepared for haemocyte collection as described in Section 2.3. For each sample, the haemolymph of 10–12 individual snails was pooled. RNA samples were prepared as described for the inbred lines. Superscript II RT™ (Invitrogen) with Oligo dT 12–18 primers and RNase Out™ (Invitrogen) was used for each 55 μL RT reaction containing 20 ng of RNA. Following synthesis of cDNA, RNA was removed using RNase H™ (Invitrogen). Samples were stored at −80 °C. From the GenBank (U. S. National Center for Bio-technology Information, http://www.ncbi.nlm.nih.gov/) B. glabrata database we obtained expressed sequence tags (ESTs) for which the submitting scientists had assigned the following names: actin (act), elongation factor-1 alpha (ef1), glyceraldehyde-3-phosphate dehydrogenase 3 (gapdh), guanine nucleotide binding protein beta polypeptide (gnb), 60S ribosomal protein L32 (L32), myoglobin (myo), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon polypeptide (ywhae). Each EST was used to interrogate the same database for additional sequences with overlapping regions of identity. Such sequences were used to assemble contigs. The most highly conserved regions were used with Primer 3 software to design PCR primers. Sequencing primers were designed for the regions flanking the 5′ and 3′ ends of each amplicon. Amplification with the sequencing primers enabled us to verify primer region consensus in our 13–16-R1 snail population. Accession numbers and primer sequences for these (reference) genes are provided in Supplementary Table S2. Real time qPCR was performed in 96-well optical plates with an ABI Prism 7500 Sequence Detection System™ (Applied Biosystems). iTaq™ SYBR® Green Supermix with ROX (BioRad) was combined with cDNA and 4 pmol of each primer in a 20 μL volume. Cycling conditions were: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 59 °C for 35 s. By melting curve analysis, we verified the presence of single products. The Ct value was determined for each reaction using manual baseline and threshold settings. All qPCRs were carried out in triplicate and an NTC was included for each primer pair. Raw fluorescence readings were analysed by LinReg (Ramakers et al., 2003) and only reactions whose efficiencies were above 1.8 were included in the study. Cts were adjusted for plate-to-plate variation and amplification efficiency (effadjusted Ct), then averaged for each gene and analysed using geNorm Visual Basic application for Microsoft Excel (Vandesompele et al., 2002). Two reference genes were selected for measurement in each sample in our qPCR study. P values <0.05 were considered significant.
3. Results
3.1. Procurement of inbred lines
One hundred and forty B. glabrata individuals from our 13–16-R1 population were isolated at the juvenile stage (3–4 mm shell diameter), grown to maturity and allowed to self-fertilise. Isolated juveniles from each of the next three generations were similarly handled. Fourth generation siblings were used to start inbred snail lines. Fifty-two lines were developed from this inbreeding process. Using snails with a shell diameter of 4 mm, the inbred lines were phenotyped for susceptibility to S. mansoni. Nineteen inbred lines with either high or low susceptibility (above 80% or below 20%) were phenotyped for susceptibility again at 12 mm. The exposure dose was constant at five miracidia and infection was confirmed by parasite shedding 5, 7 and 9 weeks later. The inbred lines of B. glabrata described herein (Table 2 and Supplementary Table S4) are available on request, so long as the lines persist.
Table 2.
Fifty-two inbred snail lines were developed from 13–16-R1 laboratory stock of Biomphalaria glabrata. The lines have approximately 88% homozygosity at the allelic level. All inbred Biomphalaria glabrata lines were phenotyped for susceptibility to Schistosoma mansoni at 4 mm shell diameter and, based on those phenotypes, the 10 most susceptible (S) and nine most resistant (R) were re-tested for susceptibility at 12 mm diameter. Lines designated S when juveniles are next to the white bar; the lines that were R at this size are next to the black bar. Spread haemocytes (sprd hem) were also counted at similar sizes of 5 and 12 mm for the 19 lines, and values represent Mean ± S.E. (n = 3, n = 10, respectively). Susceptibility phenotypes at 4 mm diameter were determined for the remaining 33 inbred lines and are reported in Supplementary Table S4.
| Line # | Juvenile
|
Adult
|
|||
|---|---|---|---|---|---|
| % S | sprd hem | % S | sprd hem | ||
|
|
i4 | 0 | 71 ± 28 | 0 | 163 ± 41 |
| i68 | 0 | 129 ± 27 | 0 | 162 ± 36 | |
| i84 | 0 | 31 ± 4 | 0 | 318 ± 54 | |
| i98 | 0 | 107 ± 27 | 0 | 238 ± 51 | |
| i31 | 2 | 61 ± 10 | 0 | 135 ± 13 | |
| i113 | 2 | 85 ± 10 | 0 | 135 ± 19 | |
| i147 | 5 | 99 ± 27 | 0 | 404 ± 42 | |
| i170 | 5 | 39 ± 13 | 0 | 417 ± 43 | |
| i156 | 9 | 11 ± 1 | 0 | 73 ± 14 | |
| i7 | 13 | 5 ±1 | 0 | 122 ± 15 | |
|
|
i172 | 100 | 35 ± 8 | 11 | 148 ± 41 |
| i121 | 89 | 220 ± 51 | 19 | 206 ± 50 | |
| i26 | 100 | 15 ± 9 | 41 | 123 ± 17 | |
| i171 | 89 | 11 ± 3 | 61 | 15 ± 3 | |
| i154 | 98 | 13 ± 9 | 61 | 165 ± 16 | |
| i36 | 100 | 69 ± 4 | 73 | 94 ± 13 | |
| i93 | 100 | 15 ± 3 | 75 | 94 ± 15 | |
| i159 | 87 | 48 ± 5 | 78 | 120 ± 7 | |
| i163 | 95 | 17 ± 10 | 84 | 69 ± 9 | |
3.2. Susceptibility/resistance of juvenile and adult snails to S. mansoni
Snail lines with high resistance (0–13% S) at the juvenile size were also resistant at the larger adult size (Table 2). Previous studies have shown a relationship between increased snail size and resistance (Richards et al., 1992). Two inbred lines that were highly susceptible (89% S, 100% S) at the small size were found to be highly resistant at the large size (81% R, 89%R respectively). Overall, 55% of the snail lines that were highly susceptible at the small size increased in resistance as they grew larger, but we also isolated four lines with high susceptibility at 4 mm (>87% S) which remained predominantly susceptible at 12 mm (>73% S). Although a few more of the adult individuals in these lines remained parasite-free compared with juveniles, the increased size did not guarantee resistance for the majority of snails in those lines.
3.3. Abundance of spreading cells in haemolymph from juvenile and adult snails
The 19 lines were phenotyped for numbers of spread haemocytes at both juvenile (5 mm) and adult (12 mm) sizes (Table 2). Resistant lines at 12 mm were more likely to have spread cell counts over 100 cells/μL (Fisher Exact Test, P = 0.0172, two-tailed) and no susceptible lines were found to have spread cell counts over 200 cells/μL. High numbers of spread cells are associated with a R phenotype (Fig. 1). Both R and S snails were found in lines with low spread haemocyte numbers. The median value for all R lines was 162.7 cells/μL (interquartile range (IQR) = 149.2) which differed significantly (P = 0.01, Mann–Whitney) from the S median of 93.8 cells/μL (IQR = 51.0). Total haemocyte numbers were also counted. In the nine lines with high numbers of haemocytes (>140/μL), spread cells comprised the majority of the population (71% ± 4), whereas in the 10 lines with lower total cell numbers (65–135 cells/μL), the populations of spread and non-spread haemocytes were essentially equal and did not differ between S and R snails (52% ± 7 and 57% ± 2, respectively). The numbers of spread cells in 5 mm snails were not correlated with resistance (P = 0.628, unpaired T-test).
Fig. 1.
Susceptibility (S) phenotypes of 19 inbred Biomphalaria glabrata snail lines and counts of their spread haemocytes/μL (mean + S.E.M.). Data points between the dotted lines all represent %S = 0 and are staggered to show S.E.M. There is a significant and moderately strong correlation (P = 0.0235, r2 = 0.2670) between susceptibility and cell count (Linear Regression Analysis). Resistant snails are more likely to have spread haemocyte counts over 100 cells/μL (Fisher Exact Test, P = 0.0172, two-tailed).
3.4. mRNA abundance for genes of interest
Constitutive haemocyte mRNA expression was analysed by qPCR for seven putatively steady state genes and 18 genes implicated in immune function. Haemolymph from 16 to 24 12 mm snails representing the same inbred line was pooled; the haemocytes were washed and lysed within 40 min of bleeding. RNA from the haemocytes was reverse-transcribed, and the resulting cDNA was amplified in replicate qPCR wells using primers listed in Table 1. The three most stably expressed reference genes under our experimental conditions (Fig. 2) were gnb, L32, and myo. Pair-wise variations between each combination of sequential normalisation factors were calculated using geNorm. This showed that use of two reference genes, L32 and myo, was sufficient for normalisation (Vandesompele et al., 2002).
Fig. 2.
Expression stability measures (M values) for seven candidate reference genes (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, epsilon polypeptide (ywhae); actin (act); elongation factor-1 alpha (ef1); glyceralde-hyde-3-phosphate dehydrogenase 3 (gapdh); guanine nucleotide binding protein beta polypeptide (gnb); 60S ribosomal protein L32 (L32); and myoglobin (myo)) in Biomphalaria glabrata. Using geNorm (Vandesompele et al., 2002), M values were calculated from the average pair-wise variation of each candidate gene in comparison with all other genes. A higher M value represents higher variability in expression. Iterative removal of the least stable gene and recalculation of M values for the remaining genes allows a ranking of genes from least to most stable. Since the calculations are based on ratios, the protocol does not differentiate between the two most stable genes. In our system the most steadily expressed genes are L32 and myo.
The expression levels for the g.o.i. were normalised with the mean of L32 and myo amplified in the same samples. Both plate to plate variation and replicate variation were minimal at <1 amplification cycle. The average relative expression for each g.o.i. is shown in Supplementary Table S3. The gene which differed most in constitutive expression between R and S inbred lines was aif: it was expressed at 1.8-fold higher levels in R lines (P = 0.045, unpaired T-test) (Fig. 3).
Fig. 3.
Constitutive expression of three genes implicated in resistance to Schistosoma mansoni, in haemocytes of Biomphalaria glabrata. (A) Allograft inflammatory factor (aif) is expressed at a significantly higher level in resistant inbred snail lines (P = 0.0456 two-tailed T-test). (B) The gene for the inferred phox subunit of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (infPhox) is expressed at 2.3-fold higher levels in haemocytes from resistant lines compared with susceptible lines of equally low spread cell numbers (P = 0.0261, two-tailed T-test). (C) Peroxiredoxin 1 (prx1) expression is 2.4-fold higher in resistant snails from low spread cell lines (P = 0.0004, two-tailed T-test).
Both R and S phenotypes were seen in snail lines with low spread cell counts. We reasoned that, in contrast to cells from lines with higher cell numbers (in which resistance could be a consequence of cell quantity rather than cell quality), haemocytes from snail lines with fewer cells would be more likely to reveal transcriptional patterns reflective of the R/S phenotypic difference. Therefore, mRNA levels were analysed further in eight inbred lines, four R and four S, whose median cell counts were low and did not differ significantly (R = 108, S = 96; Mann Whitney, P = 0.06). Two genes differed significantly in expression between R and S snails with low cell counts. prx1 was expressed at 2.4-fold higher levels in R than S lines (P = 0.0004, T-Test, two tailed). The gene for an inferred nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunit (infPhox) was 2.3-fold higher in R than S lines (P = 0.0261, T-test, two-tailed) (Fig. 3).
A graphical representation of all expression levels is shown in Fig. 4. Expression levels were ranked as low, medium or high based on an equal division of the gene’s range among all inbred lines. Each gene was ranked independently. Ranked expression levels for all three measured components of the NADPH oxidase system (nox2, phox22 and infPhox component) are shown separately for inbred lines with low spread cell counts (Fig. 4B) and this illustrates that, although nox2 and phox22 expression levels were not significantly different between R and S lines with low cell counts, there was a trend toward higher expression in R lines (Fisher’s Exact Test, P = 0.08).
Fig. 4.
The log transformed gene expression levels among all inbred Biomphalaria glabrata lines, calculated for each gene independently, were divided into three ranges. For each gene, low, medium and high groups are illustrated. Inbred lines are listed from most resistant (i170) to most susceptible (i163), and the spread cell counts (Cell #) and % susceptibility (%S) are shown for each. (A) Heat map for all genes and all inbred lines. (B) Heat map of NADPH oxidase components and peroxiredoxin 1 (prx1) from resistant (R) and susceptible (S) lines with similar spread cell counts. Expression of the gene for the inferred phox subunit (infPhox) is significantly higher in R than S lines. Two other components of the NADPH oxidase complex which were measured, nox2 and phox22, were not significantly higher in R lines but show trends towards higher expression in the R lines. Genes are abbreviated as follows, where bold numbers refer to Contig numbers in a preliminary draft of the snail genome (https://www.vectorbase.org/organisms/biomphalaria-glabrata): aif, allograft inflammatory factor; arg, arginase; c42, catalase 42; d584, dual oxidase 584; d303, dual oxidase 303; frep3, fibrinogen-related protein 3; g2402, glutathione peroxidase 2402; g97, glutathione peroxidase 97; g65, glutathione peroxidase 65; mif, macrophage migration inhibitory factor; nox2, NADPH oxidase 2; phox22, phagocyte oxidase 22; infPhox, inferred phox subunit; prx6, peroxiredoxin 6; prx4, peroxiredoxin 4; prx621, peroxiredoxin 621; prx1, peroxiredoxin 1; tal-1, T-cell acute lymphocytic leukaemia-1.
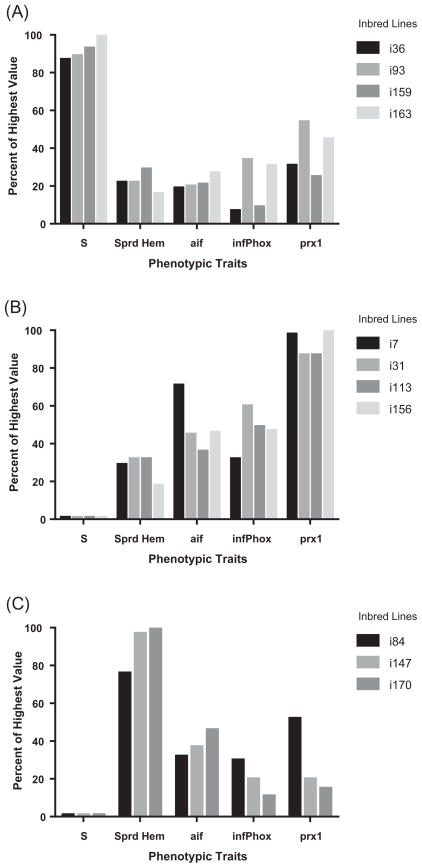
The phenotypic traits that correlated with S or R (Fig. 5) were spread cell count and the expressions of aif, infPhox and prx1. Trait values were calculated as percentages of the highest value in all lines. As shown, the lines with highest susceptibility had lower than average cell counts and uniformly low aif levels which did not differ by more than 10% from one another. Resistant inbred lines with the lowest cell counts had high prx 1 with mid-range levels of aif and infPhox. Highly resistant lines with high cell counts had aif expressions of mid-level which were also consistent among lines. The infPhox and prx1 were expressed more variably in this group. The absence of significant differences in constitutive expression of catalase, the three glutathione peroxidases, peroxiredoxins 6 and 621 or tal1 implies their lack of influence on R/S phenotype (data not shown).
Fig. 5.
Phenotypic traits of inbred Biomphalaria glabrata lines that correlate with susceptibility to Schistosoma mansoni. Traits were measured in 12 mm snails. Trait values were calculated as a percentage of the highest mean of all the lines. (A) The four most susceptible inbred lines (i36, i93, i159, i163) have less than 40% of the highest average spread cell numbers (Sprd Hem) and low allograft inflammatory factor (aif) expression which is consistent among the lines. (B) Four resistant lines (i7, i31, i113, i156) with the lowest numbers of spread cells consistently expressed high peroxiredoxin 1 (prx 1) and similar mid-levels of aif and the inferred phox subunit (infPhox). (C) Three resistant lines (i84, i147, i170) with high numbers of spread cells showed mid to lower levels of expression in nearly all depicted genes.
Correlation analysis was performed between susceptibility and four separate variables (numbers of spread haemocytes and relative expression of infPhox, prx1 and aif). Independently, both cell number (P = 0.020) and aif (P = 0.011) were significantly correlated with susceptibility while prx1 trended toward significance (P = 0.078, Pearson Product Moment Correlation). The gene for inf-Phox did not show significant correlation with susceptibility when analysed with all of the inbred snail lines (P = 0.486). A multiple regression analysis utilizing spread cell number and prx1 expression explained nearly 70% of the variation in S among the 19 snail lines (adjusted r2 = 0.669). However, the addition of aif to the analysis did not significantly improve the ability to predict susceptibility. These results substantiate our idea that prx1 expression is important when cell number is accounted for. In contrast, cell number and aif expression are critical when analysing a broader range of phenotypes.
4. Discussion
The genus Biomphalaria is naturally highly variable (see Bayne, 2009), and natural variation within and among populations within a species can complicate the search for the genetic basis of particular traits. There is ample evidence that, although resistance in snails is controlled by multiple loci, it is strongly influenced by a handful of major-effect loci (Richards and Merritt, 1972; Woolhouse and Webster, 2000). The inbred lines were developed to facilitate further investigations into the genetic causes of resistance or susceptibility to infection. As these lines (Table 2 and Supplementary Table S4) were inbred for three generations, they are likely fixed for genes that mediate resistance/susceptibility. In general, susceptibility has been found to be recessive to resistance (Webster et al., 2001a, b). Because the founding population for this work was predominantly resistant, susceptibility alleles (or combinations of alleles that lead to susceptibility) were expected to exist at low or intermediate frequencies. Thus, inbreeding was also useful in that it exposed recessive alleles in the homozygous state and fixed them in lines where their effects could be studied. The relative homogeneity of each inbred line allowed us to pool haemocyte extracts from several individuals while minimizing the noise generally observed when pooling multiple individuals.
Fifty-two inbred lines of B. glabrata are available for further investigations, although it is important to acknowledge some constraints. First, the R/S phenotypes of these lines have been established with the PR1 strain of S. mansoni that has been cycled in Oregon for more than 30 years. It would be expected that the snails may have different phenotypes when challenged with genetically distinct strains of the parasite (Basch, 1975; Richards et al., 1992; Moné et al., 2010; Mitta et al., 2012). Second, R/S phenotypes can change rapidly, so prior to use of an inbred line, phenotypes should be verified (Richards et al., 1992). Our collaborators (M.S. Blouin, personal communication) are currently using these lines and single nucleotide polymorphism genotyping to map regions of the genome that control the R/S phenotype.
We scored resistance at two different sizes of snail (4 and 12 mm). In approximately half of the S snail lines, resistance increased with size. This is consistent with work done by Richards and Merritt (1972) and confirmed by Lewis et al. (2003). In some lines, increase in resistance with size was marginal however, and we obtained inbred lines which were predominantly susceptible at the larger size, as would also be expected. The isolation of lines with this phenotype allowed us to further examine traits which contribute to susceptibility.
It was not anticipated that the inbred lines would differ so markedly in spread haemocyte numbers. This was recognised early in the process of collecting cells for RNA extraction and resulted in systematic counting of cells for the lines. Haemocyte counts can be highly variable but 10 replicate counts from each line proved that haemocyte number is a heritable trait. With two crucial traits (R/S phenotype and spread haemocyte numbers) scored in both juvenile and adult snails from inbred populations, we then looked for associations between them.
Adults of inbred lines that averaged more than 206 spread cells/μL were resistant to S. mansoni. In those families, spread cells made up more than 70% of the total circulating haemocyte number, which further implicates the spread cell’s role in defence against S. mansoni. We infer that, in adult snails, high numbers of haemocytes (>230/μL) are sufficient to stop a schistosome infection. This is consistent with recent work on B. glabrata which showed increased haemocyte numbers in snails exposed to Plagiorchis elegans (Daoust et al., 2012) and increased resistance to S. mansoni following P. elegans challenge (Zakikhani et al., 2003; Daoust et al., 2012).
Spread cell numbers differed significantly between S and R adult snails but not juvenile snails, although those snail lines which were highly susceptible at both small and large sizes had 220 or fewer spread cells/μL at both sizes. One of the two lines in which highly susceptible juveniles grew into highly resistant adults had the largest spread cell count at both sizes. This illustrates the complexity of mechanisms involved in the R phenotype: neither high cell number nor large size on its own is invariably sufficient to manifest resistance. It may be that snails that are S in spite of high numbers of haemocytes may fail to recognise the parasite (Roger et al., 2008).
Highly resistant snails quickly mount an immune response (van der Knaap and Loker, 1990; Loker 2010). Our previous work revealed evidence of a role for the haemocyte oxidative burst in resistance (Hahn et al., 2000; Goodall et al., 2006; Bender et al., 2007). On that basis, we focused our current work on haemocyte expression of several genes involved in regulating reactive oxygen and nitrogen levels. In addition, after recognizing the significant influence of haemocyte numbers, we also measured mRNA levels for genes which influence haemocyte differentiation and function, together with genes for two secreted humoral factors that have been implicated in R/S, MIF (Baeza Garcia et al., 2010) and FREP3 (Hanington et al., 2012). Other than aif, none emerged as being differently expressed. Typically expressed in phagocytic and granular leucocytes, aif is involved in cell proliferation and migration, and is a marker of activation of both vertebrate macrophages (Zhao et al., 2013) and molluscan haemocytes (Zhang et al., 2013). It is more highly expressed in R snails (Lockyer et al., 2012). In a study of the malarial-mosquito interaction (Molina-Cruz et al., 2013), differences in expression levels of candidate genes involved in infection success were in the range of two to fourfold. Even such small differences in constitutive expression of crucial genes may enable haemocytes to be more capable of destroying parasites. This is consistent with the idea that resistant B. glabrata rely on constitutive, innate defences that are manifested as an immediate attack on penetrating schistosomes (Bayne et al., 2001; Bender et al., 2007). Other studies have shown that additional genes are activated in the defence response that follows exposure to schistosomes (Adema et al., 2010; Lockyer et al., 2012); therefore, while humoral components may contribute to the R/S phenotype, constitutive haemocyte gene expression profiles most probably do.
Snail lines with the highest numbers of spread cells appeared to have lower overall mRNA levels of the target genes (Fig. 4). This was not due to measurement errors since all expression values were adjusted with two reference genes, including one ribosomal, measured in the same sample. These low expression profiles may reflect the cost of gene expression: if cell numbers are sufficient to mount an effective defence response, then each cell can commit fewer resources to transcription of defence related genes. We suggest that high numbers of cells (at the level of the organism) can compensate for unremarkable defence capacities at the level of the individual haemocyte as reflected by the specific genes included in this study.
Regardless of haemocyte number, aif was expressed at significantly higher levels in R lines. aif was identified in an EST study of B. glabrata haemocytes (Mitta et al., 2005). This evolutionarily conserved gene encodes a calcium binding protein that is associated with inflammatory responses in mammals (Pawlik et al., 2011). It is up-regulated in wound repair (Yamamoto et al., 2011) and modulates macrophage activation (Deininger et al., 2002). In three molluscs, oyster, disc abalone and clam, while ubiquitously expressed in tissues, high (often the highest) expression levels were found in haemocytes (Zhang et al., 2011; Zhang et al., 2013; De Zoysa et al., 2010). In our samples, aif was expressed at 1.8 × higher levels in R snail lines (P = 0.045). Its higher expression in more R lines suggests a constitutively higher ‘activation’ of haemocytes in these snails. While it remains to quantitate these observations, it was apparent in our work (personal observations) that haemocytes in these snail lines differed not only in numbers but also in behaviours, with a higher propensity to aggregate in some lines.
High numbers of spread cells (>230/μL) are excellent predictors of resistance. Among snail lines with low numbers of haemocytes, some are highly resistant to the schistosome and other lines are highly susceptible. This dichotomy begs for an explanation. It is probable that ‘low haematrocrit-high resistance’ snails such as lines i7, i31, i113 and i156 either (i) rely on defences that are not haemocyte-dependent (i.e. they may be humoral), or (ii) have haemocytes with high (constitutive) expression of defence-related genes. Snails with low resistance and low haematocrit, it is inferred, both lack the cell numbers to kill efficiently and lack putative humoral defences that might otherwise compensate for the impotence of haemocyte-mediated attacks.
We examined the potential mechanisms for resistance in these lines with low spread cell numbers. Gene expression was evaluated in eight families evenly split between R and S snails which did not differ significantly in their spread cell counts (Mann–Whitney, P = 0.06). Genes for both a prx and infPhox were found to be more highly expressed in R snail families (P = 0.0004 and P = 0.0261, respectively, unpaired T-test).
The findings for prx1 and infPhox echoe a similar change reported for peroxiredoxin 4 (prx4) by Knight et al. (2009). Peroxiredoxins require conserved cysteines to scavenge (detoxify) hydrogen peroxide (H2O2). Their importance is evidenced by knock-out mice in which life spans are decreased and responses to oxidative stress are compromised (Neumann et al., 2003). Peroxiredoxins also can contribute to cell signalling by concentrating H2O2 molecules near membrane protein functional groups (Wood et al., 2003), by chaperoning or acting as cell cycle regulators (Phalen et al., 2006; Trotter et al., 2008), or by disulphide exchange (Veal et al., 2004; Vivancos et al., 2005). The snail prx gene, found to differ in our low cell number lines that also differed in R/S phenotype, codes for a protein which shares amino acid sequence homology with Human Prx1 and 2, and abalone TPX2 (scored 67.5%, 67%, 78.5%, respectively; data not shown). Due to its shared sequence, we suggest that it belongs to the AhpC/Prx1 subfamily (Hall et al., 2010; PREX database (http://csb.wfu.edu/PREX/)). In our view, it is likely that this snail prx1 coded protein is insensitive to high peroxide levels due to the absence of the YF motif in the C terminal helix. Peroxide insensitivity would lower the likelihood that this peroxiredoxin functions in signalling, as does the lack of a cysteine near position 83 which in other species is associated with chaperone activity (Lee et al., 2007). Signalling is still possible through disulphide exchanges which do not require peroxide sensitivity.
The infPhox subunit gene contains features that imply a real, if distant, evolutionary relationship with an NADPH oxidase (NOX) component, perhaps phox47. The components of this macromolecular NOX complex have not previously been reported in B. glabrata. In mammalian white blood cells, the complex assembles in plasma membranes and in phagosomal membranes where it transfers electrons from NADPH and couples them with oxygen to produce superoxide anions. This is the first known report to identify genes for this system in B. glabrata. The putative NOX gene we measured shares sequence homology with vertebrate nox2. Nox2 is regulated by interactions with Phox22 and Phox47. In our study, infPhox was expressed at a significantly higher level in R lines with low spread cell numbers and a functional role for the NADPH oxidase system is further evidenced in the trend towards higher expression of both nox2 and phox22 in the same R lines.
Of the 14 genes relevant to reactive oxygen and nitrogen levels in this study, two (prx1 and infPhox) were expressed at significantly higher levels in low cell number resistant lines, two others (nox2 and phox22) trended toward higher expression in R lines, and one (duox303) trended towards higher expression in S lines. We suggest that the ability to generate reactive intermediates, notably the superoxide anion, may be an essential element in snail resistance, and that generation may fall below or exceed a threshold that is crucial for mounting a successful attack against schistosomes. As the duox enzymes both produce superoxide and participate in cysteine-dependent stabilisation of proteins (Meitzler et al., 2013), the higher expression of duox303 in S lines is enigmatic. Our observations on sod1 mRNAs will be reported elsewhere (unpublished data).
Arginase (the most variably expressed of the genes we measured) did not emerge as differently expressed in the S and R phenotypes. This is not interpreted as sufficient to exclude NO as being influential in snail-schistosome interactions, since levels of intra-cellular NO are subject to additional controls. Also, a second locus encoding arginase is probable, although we saw none of its mRNA in haemocyte extracts.
The strengths of this study lie in its multi-component approach. Given the functional interdependencies among molecular components in a cell, an infection is unlikely to be the result of an aberration in a single gene product. An R/S phenotype reflects several biological processes that interact in a complex network. This study introduced the use of inbred lines of snails to examine resistance in the context of snail size, spread haemocyte number and constitutive haemocyte mRNA levels of several genes linked to immune function. The power of the approach can be seen in the consistent trait patterns among lines with similar R/S phenotypes.
Supplementary Material
Acknowledgments
This work was supported by the United States National Institutes of Health (award AI016137). The Central Services Laboratory of Oregon State University’s Center for Genome Research and Biocomputing assisted in the quality and quantity assessment of nucleic acids, and did the sequencing. We would like to thank Piera Callahan and Douglas Batson for technical assistance. Kaitlin Bonner and Michael Blouin provided constructive feedback on an earlier version of this paper.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijpara.2013.11.004.
Footnotes
Note: Nucleotide sequence data first reported in this paper are available in the GenBank™, EMBL and DDBJ databases under the accession numbers JZ482247 to JZ482252.
References
- Adema CM, Hanington PC, Lun CM, Rosenberg GH, Aragon AD, Stout BA, Lennard Richard ML, Gross PS, Loker ES. Differential transcriptomic responses of Biomphalaria glabrata (Gastropoda, Mollusca) to bacteria and metazoan parasites, Schistosoma mansoni and Echinostoma paraensei (Digenea, Platyhelminthes) Mol Immunol. 2010;47:849–860. doi: 10.1016/j.molimm.2009.10.019. http://dx.doi.org/10.1016/j.molimm.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeza Garcia A, Pierce RJ, Gourbal B, Werkmeister E, Colinet D, Reichhart JM, Dissous C, Coustau C. Involvement of the cytokine MIF in the snail host immune response to the parasite Schistosoma mansoni. PLoS Pathog. 2010;6 (9):e1001115. doi: 10.1371/journal.ppat.1001115. http://dx.doi.org/10.1371/journal.ppat.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barçante TA, Barçante JM, Fujiwara RT, Lima WS. Analysis of circulating haemocytes from Biomphalaria glabrata following Angiostrongylus vasorum infection using flow cytometry. J Parasitol Res. 2012;2012:314723. doi: 10.1155/2012/314723. http://dx.doi.org/10.1155/2012/314723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch PF. An interpretation of snail-trematode infection rates: specificity based on concordance of compatible phenotypes. Int J Parasitol. 1975;5:449–452. doi: 10.1016/0020-7519(75)90012-0. [DOI] [PubMed] [Google Scholar]
- Bayne CJ. Successful parasitism of vector snail Biomphalaria glabrata by the human blood fluke (trematode) Schistosoma mansoni: a 2009 assessment. Mol Biochem Parasitol. 2009;165:8–18. doi: 10.1016/j.molbiopara.2009.01.005. http://dx.doi.org/10.1016/j.molbiopara.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne CJ, Buckley PM, DeWan PC. Macrophage-like hemocytes of resistant Biomphalaria glabrata are cytotoxic for sporocysts of Schistosoma mansoni in vitro. J Parasitol. 1980;66:413–419. [PubMed] [Google Scholar]
- Bayne CJ, Hahn UK, Bender RC. Mechanisms of molluscan host resistance and of parasite strategies for survival. Parasitology. 2001;123 (Suppl):S159–S167. doi: 10.1017/s0031182001008137. [DOI] [PubMed] [Google Scholar]
- Bender RC, Goodall CP, Blouin MS, Bayne CJ. Variation in expression of Biomphalaria glabrata SOD1: a potential controlling factor in susceptibility/ resistance to Schistosoma mansoni. Dev Comp Immunol. 2007;31:874–878. doi: 10.1016/j.dci.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Blouin MS, Bonner KM, Cooper B, Amarasinghe V, O’Donnell RP, Bayne CJ. Three genes involved in the oxidative burst are closely linked in the genome of the snail, Biomphalaria glabrata. Int J Parasitol. 2013;43:51–55. doi: 10.1016/j.ijpara.2012.10.020. http://dx.doi.org/10.1016/j.ijpara.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner KM, Bayne CJ, Larson MK, Blouin MS. Effects of Cu/Zn superoxide dismutase (sod1) genotype and genetic background on growth, reproduction and defense in Biomphalaria glabrata. PLoS Negl Trop Dis. 2012;6 (6):e1701. doi: 10.1371/journal.pntd.0001701. http://dx.doi.org/10.1371/journal.pntd.0001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo MG, Wu XJ, Dinguirard N, Nyame AK, Cummings RD, Yoshino TP. Surface membrane proteins of Biomphalaria glabrata embryonic cells bind fucosyl determinants on the tegumental surface of Schistosoma mansoni primary sporocysts. J Parasitol. 2007;93:832–840. doi: 10.1645/GE-954R.1. [DOI] [PubMed] [Google Scholar]
- Chernin E. Observations on hearts explanted in vitro from the snail Australorbis glabratus. J Parasitol. 1963;49:353–364. [PubMed] [Google Scholar]
- Daoust SP, Rau ME, McCloughlin JD. Plagiorchis elegans (Trematoda) induces immune response in an incompatible snail host Biomphalaria glabrata (Pulmonata: Planorbidae) J Parasitol. 2012;98:1021–1022. doi: 10.1645/GE-3106.1. [DOI] [PubMed] [Google Scholar]
- De Zoysa M, Nikapitiya C, Kim Y, Oh C, Kang DH, Whang I, Kim SJ, Lee JS, Choi CY, Lee J. Allograft inflammatory factor-1 in disk abalone (Haliotis discus discus): molecular cloning, transcriptional regulation against immune challenge and tissue injury. Fish Shellfish Immunol. 2010;29:319–326. doi: 10.1016/j.fsi.2010.04.006. http://dx.doi.org/10.1016/j.fsi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Deininger MH, Meyermann R, Schluesener HJ. The allograft inflammatory factor-1 family of proteins. FEBS Lett. 2002;514:115–121. doi: 10.1016/s0014-5793(02)02430-4. http://dx.doi.org/10.1016/S0014-5793(02)02430-4. [DOI] [PubMed] [Google Scholar]
- Fryer SE, Hull CJ, Bayne CJ. Phagocytosis of yeast by Biomphalaria glabrata: carbohydrate specificity of hemocyte receptors and a plasma opsonin. Dev Comp Immunol. 1989;13:9–16. doi: 10.1016/0145-305x(89)90011-6. [DOI] [PubMed] [Google Scholar]
- Goodall CP, Bender RC, Brooks JK, Bayne CJ. Biomphalaria glabrata cytosolic copper/zinc superoxide dismutase (SOD1) gene: association of SOD1 alleles with resistance/susceptibility to Schistosoma mansoni. Mol Biochem Parasitol. 2006;147:207–210. doi: 10.1016/j.molbiopara.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Hahn UK, Bender RC, Bayne CJ. Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: carbohydrate-specific stimulation. Dev Comp Immunol. 2000;24:531–541. doi: 10.1016/s0145-305x(00)00017-3. [DOI] [PubMed] [Google Scholar]
- Hahn UK, Bender RC, Bayne CJ. Involvement of nitric oxide in killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata. J Parasitol. 2001;87:778–785. doi: 10.1645/0022-3395(2001)087[0778:IONOIK]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hall A, Nelson K, Poole L, Karplus PA. Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid Redox Signal. 2010;15 (3):795–815. doi: 10.1089/ars.2010.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanington PC, Forys MA, Loker ES. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl Trop Dis. 2012;6 (3):e1591. doi: 10.1371/journal.pntd.0001591. http://dx.doi.org/10.1371/journal.pntd.0001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JE, Yoshino TP. Regulation of hydrogen peroxide release in circulating hemocytes of the planorbid snail Biomphalaria glabrata. Dev Comp Immunol. 2008;32:554–562. doi: 10.1016/j.dci.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittiprasert W, Miller A, Su XZ, Mu J, Bhusudsawang G, Ukoskit K, Knight M. Identification and characterisation of functional expressed sequence tagsderived simple sequence repeat (eSSR) markers for genetic linkage mapping of Schistosoma mansoni juvenile resistance and susceptibility loci in Biomphalaria glabrata. Int J Parasitol. 2013;43:669–677. doi: 10.1016/j.ijpara.2013.03.007. http://dx.doi.org/10.1016/j.ijpara.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Yoshino TP. Larval Schistosoma mansoni excretory-secretory glycoproteins (ESPs) bind to hemocytes of Biomphalaria glabrata (Gastropoda) via surface carbohydrate binding receptors. J Parasitol. 2001;87:786–793. doi: 10.1645/0022-3395(2001)087[0786:LSMESG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Knight M, Raghavan N, Goodall C, Cousin C, Ittiprasert W, Sayed A, Miller A, Williams DL, Bayne CJ. Biomphalaria glabrata peroxiredoxin: effect of Schistosoma mansoni infection on differential gene regulation. Mol Biochem Parasitol. 2009;167:20–31. doi: 10.1016/j.molbiopara.2009.04.002. http://dx.doi.org/10.1016/j.molbiopara.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Choi KS, Riddell J, Ip C, Ghosh D, Park JH, Park YM. Human peroxiredoxin 1 and 2 are not duplicate proteins: the unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J Biol Chem. 2007;282:22011–22022. doi: 10.1074/jbc.M610330200. [DOI] [PubMed] [Google Scholar]
- Lewis FA, Patterson CN, Grzywacz C. Parasite-susceptibility phenotypes of F1 Biomphalaria glabrata progeny derived from interbreeding Schistosoma mansoni-resistant and -susceptible snails. Parasitol Res. 2003;89:98–101. doi: 10.1007/s00436-002-0730-4. [DOI] [PubMed] [Google Scholar]
- Lockyer AE, Emery AM, Kane RA, Walker AJ, Mayer CD, Mitta G, Coustau C, Adema CM, Hanelt B, Rollinson D, Noble LR, Jones CS. Early differential gene expression in haemocytes from resistant and susceptible Biomphalaria glabrata strains in response to Schistosoma mansoni. PLoS ONE. 2012;7 (12):e51102. doi: 10.1371/journal.pone.0051102. http://dx.doi.org/10.1371/journal.pone.0051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loker ES. Gastropod immunobiology. Adv Exp Med Biol. 2010;708:17–43. doi: 10.1007/978-1-4419-8059-5_2. http://dx.doi.org/10.1007/978-1-4419-8059-5_2. [DOI] [PubMed] [Google Scholar]
- Martins-Souza RL, Pereira CA, Coelho PM, Martins-Filho OA, Negrão-Corrêa D. Flow cytometry analysis of the circulating haemocytes from Biomphalaria glabrata and Biomphalaria tenagophila following Schistosoma mansoni infection. Parasitology. 2009;136:67–76. doi: 10.1017/S0031182008005155. http://dx.doi.org/10.1017/S0031182008005155. [DOI] [PubMed] [Google Scholar]
- Martins-Souza RL, Pereira CA, Rodrigues L, Araújo ES, Coelho PM, Corrêa A, Jr, Negrão-Corrêa D. Participation of N-acetyl-D-glucosamine carbohydrate moieties in the recognition of Schistosoma mansoni sporocysts by haemocytes of Biomphalaria tenagophila. Mem Inst Oswaldo Cruz. 2011;106:884–891. doi: 10.1590/s0074-02762011000700015. [DOI] [PubMed] [Google Scholar]
- Meitzler JL, Hinde S, Bánfi B, Nauseef WM, Ortiz de Montellano PR. Conserved cysteine residues provide a protein-protein interaction surface in dual oxidase (duox) proteins. J Biol Chem. 2013;288:7147–7157. doi: 10.1074/jbc.M112.414797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson EH, DuBois L. Susceptibility of Bahian populations of Biomphalaria glabrata to an allopatric strain of Schistosoma mansoni. Am J Trop Med Hyg. 1978;27:782–786. doi: 10.4269/ajtmh.1978.27.782. [DOI] [PubMed] [Google Scholar]
- Mitta G, Galinier R, Tisseyre P, Allienne JF, Girerd-Chambaz Y, Guillou F, Bouchut A, Coustau C. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Dev Comp Immunol. 2005;29:393–407. doi: 10.1016/j.dci.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mitta G, Adema CM, Gourbal B, Loker ES, Théron A. Compatibility polymorphism in snail/schistosome interactions: from field to theory to molecular mechanisms. Dev Comp Immunol. 2012;37:1–8. doi: 10.1016/j.dci.2011.09.002. http://dx.doi.org/10.1016/j.dci.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, Ortega C, van Schaijk BC, Sauerwein RW, Taylor-Salmon E, Barillas-Mury C. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340 (6135):984–987. doi: 10.1126/science.1235264. http://dx.doi.org/10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moné Y, Gourba LB, Duva LD, Du Pasquier L, Kieffer-Jaquinod S, Mitta G. A large repertoire of parasite epitopes matched by a large repertoire of host immune receptors in an invertebrate host/parasite model. PLoS Negl Trop Dis. 2010;4(9):e813. doi: 10.1371/journal.pntd.0000813. pii. http://dx.doi.org/10.1371/journal.pntd.0000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mourão MM, Dinguirard N, Franco GR, Yoshino TP. Role of the endogenous antioxidant system in the protection of Schistosoma mansoni primary sporocysts against exogenous oxidative stress. PLoS Negl Trop Dis. 2009;3 (11):e550. doi: 10.1371/journal.pntd.0000550. http://dx.doi.org/10.1371/journal.pntd.0000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrão-Corrêa D, Mattos AC, Pereira CA, Martins-Souza RL, Coelho PM. Interaction of Schistosoma mansoni sporocysts and hemocytes of Biomphalaria. J Parasitol Res. 2012;2012:743920. doi: 10.1155/2012/743920. http://dx.doi.org/10.1155/2012/743920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424 (6948):561–565. doi: 10.1038/nature01819. http://dx.doi.org/10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- Newton WL. The inheritance of susceptibility to infection with Schistosoma mansoni in Australorbis glabratus. Exp Parasitol. 1953;2:242–257. [Google Scholar]
- Niemann GM, Lewis FA. Schistosoma mansoni: influence of Biomphalaria glabrata size on susceptibility to infection and resultant cercarial production. Exp Parasitol. 1990;70:286–292. doi: 10.1016/0014-4894(90)90110-x. [DOI] [PubMed] [Google Scholar]
- Noda S, Loker ES. Effects of infection with Echinostoma paraensei on the circulating haemocyte population of the host snail Biomphalaria glabrata. Parasitology. 1989;98:35–41. doi: 10.1017/s0031182000059667. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Levada PM, Zanotti-Magalhaes EM, Magalhães LA, Ribeiro-Paes JT. Differences in the number of hemocytes in the snail host Biomphalaria tenagophila, resistant and susceptible to Schistosoma mansoni infection. Genet Mol Res. 2010;9:2436–2445. doi: 10.4238/vol9-4gmr1143. [DOI] [PubMed] [Google Scholar]
- Pawlik A, Kurzawski M, Dziedziejko V, Safranow K, Paczkowska E, Maslinski W, Drozdzik M, Gawronska-Szklarz B. Allograft inflammatory factor-1 gene polymorphisms in patients with rheumatoid arthritis. Genet Test Mol Biomarkers. 2011;16:341–534. doi: 10.1089/gtmb.2011.0201. http://dx.doi.org/10.1089/gtmb.2011.0201. [DOI] [PubMed] [Google Scholar]
- Phalen TJ, Weirather K, Deming PB, Anathy V, Howe AK, van der Vliet A, Jönsson TJ, Poole LB, Heintz NH. Oxidation state governs structural transitions in peroxiredoxin II that correlate with cell cycle arrest and recovery. J Cell Biol. 2006;175:779–789. doi: 10.1083/jcb.200606005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339 (1):62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Renwrantz LR, Richards EH. Recognition of beta-glucuronidase by the calcium-independent phosphomannosyl surface receptor of haemocytes from the gastropod mollusc, Helix pomatia. Dev Comp Immunol. 1992;16:251–256. doi: 10.1016/0145-305x(92)90024-7. [DOI] [PubMed] [Google Scholar]
- Richards CS, Merritt JW. Genetic factors in susceptibility of juvenile Biomphalaria glabrata to Schistosoma mansoni infection. Am J Trop Med Hyg. 1972;21:425–434. doi: 10.4269/ajtmh.1972.21.425. [DOI] [PubMed] [Google Scholar]
- Richards CS, Knight M, Lewis FA. Genetics of Biomphalaria glabrata and its effect on the outcome of Schistosoma mansoni infection. Parasitol Today. 1992;8:171–174. doi: 10.1016/0169-4758(92)90015-t. [DOI] [PubMed] [Google Scholar]
- Roger E, Grunau C, Pierce RJ, Hirai H, Gourbal B, Galinier R, Emans R, Cesari IM, Cosseau C, Mitta G. Controlled chaos of polymorphic mucins in a metazoan parasite (Schistosoma mansoni) interacting with its invertebrate host (Biomphalaria glabrata) PLoS Negl Trop Dis. 2008;2 (11):e330. doi: 10.1371/journal.pntd.0000330. http://dx.doi.org/10.1371/journal.pntd.0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sim S, Jupatanakul N, Ramirez JL, Kang S, Romero-Vivas CM, Mohammed H, Dimopoulos G. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in Dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl Trop Dis. 2013;7 (7):e2295. doi: 10.1371/journal.pntd.0002295. http://dx.doi.org/10.1371/journal.pntd.0002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinauer ML, Bonner KM. Host susceptibility is altered by light intensity after exposure to parasites. J Parasitol. 2012;98:1052–1054. doi: 10.1645/GE-3109.1. http://dx.doi.org/10.1645/GE-3109.1. [DOI] [PubMed] [Google Scholar]
- Stibbs HH, Owczarzak A, Bayne CJ, DeWan P. Schistosome sporocystkilling amoebae isolated from Biomphalaria glabrata. J Invert Pathol. 1979;33:159–170. doi: 10.1016/0022-2011(79)90149-6. [DOI] [PubMed] [Google Scholar]
- Trotter EW, Rand JD, Vickerstaff J, Grant CM. The yeast Tsa1 peroxiredoxin is a ribosome-associated antioxidant. Biochem J. 2008;412:73–80. doi: 10.1042/BJ20071634. [DOI] [PubMed] [Google Scholar]
- Ulmer MJ. Laboratory maintenance of parasites. In: MacInnis AJ, Voge M, editors. Experiments and Techniques in Parasitology. W.H. Freeman Co; San Francisco, California: 1970. pp. 143–144. [Google Scholar]
- van der Knaap WP, Loker ES. Immune mechanisms in trematode–snail interactions. Parasitol Today. 1990;6:175–182. doi: 10.1016/0169-4758(90)90349-9. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal EA, Findlay VJ, Day AM, Bozonet SM, Evans JM, Quinn J, Morgan BA. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Vivancos AP, Castillo EA, Biteau B, Nicot C, Ayte J, Toledano MB, Hidalgo E. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci USA. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Davies CM, Ndamba J, Noble LR, Jones CS, Woolhouse ME. Spatio-temporal genetic variability in the schistosome intermediate host Biomphalaria pfeifferi. Ann Trop Med Parasitol. 2001a;95:5155–27. doi: 10.1080/00034980120072239. [DOI] [PubMed] [Google Scholar]
- Webster JP, Davies CM, Hoffman JI, Ndamba J, Noble LR, Woolhouse ME. Population genetics of the schistosome intermediate host Biomphalaria pfeifferi in the Zimbabwean highveld: implications for co-evolutionary theory. Ann Trop Med Parasitol. 2001b;95:203–214. doi: 10.1080/00034980120041062. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Woolhouse ME, Webster JP. In search of the red queen. Parasitol Today. 2000;16:506–508. doi: 10.1016/s0169-4758(00)01820-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Ashihara E, Nakagawa Y, Obayashi H, Ohta M, Hara H, Adachi T, Seno T, Kadoya M, Hamaguchi M, Ishino H, Kohno M, Maekawa T, Kawahito Y. Allograft inflammatory factor-1 is overexpressed and induces fibroblast chemotaxis in the skin of sclerodermatous GVHD in a murine model. Immunol Lett. 2011;30 (135):144–150. doi: 10.1016/j.imlet.2010.10.015. http://dx.doi.org/10.1016/j.imlet.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zakikhani M, Smith JM, Rau ME. Effect of Plagiorchis elegans (Digenea: Plagiorchiidae) infections of Biomphalaria glabrata (Pulmonata: Planorbidae) on a challenge infection with Schistosoma mansoni (Digenea: Schistosomatidae) J Parasitol. 2003;89:70–75. doi: 10.1645/0022-3395(2003)089[0070:EOPEDP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li J, Yu F, He X, Yu Z. Allograft inflammatory factor-1 stimulates hemocyte immune activation by enhancing phagocytosis and expression of inflammatory cytokines in Crassostrea gigas. Fish Shellfish Immunol. 2013;34:1071–1077. doi: 10.1016/j.fsi.2013.01.014. http://dx.doi.org/10.1016/j.fsi.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao J, Li C, Su X, Chen A, Li T, Qin S. Cloning and characterization of allograft inflammatory factor-1 (AIF-1) from Manila clam Venerupis philippinarum. Fish Shellfish Immunol. 2011;30:148–153. doi: 10.1016/j.fsi.2010.09.021. http://dx.doi.org/10.1016/j.fsi.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Yan DJ, Chen ZW. Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell Immunol. 2013;284:75–83. doi: 10.1016/j.cellimm.2013.07.008. http://dx.doi.org/10.1016/j.cellimm.2013.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.