Abstract
Type 2 diabetes, often associated with obesity, results from a deficiency of insulin production and action manifested in increased blood levels of glucose and lipids that further promote insulin resistance and impair insulin secretion. Glucolipotoxicity caused by elevated plasma glucose and lipid levels is a major cause of impaired glucose-stimulated insulin secretion from pancreatic β-cells, due to increased oxidative stress, and insulin resistance. Glucagon-like peptide-1 (GLP1), an insulinotropic glucoincretin hormone, is known to promote β-cell survival via its actions on its G-protein-coupled receptor on β-cells. Here, we report that a nonapeptide, GLP1(28–36)amide, derived from the C-terminal domain of the insulinotropic GLP1, exerts cytoprotective actions on INS-1 β-cells and on dispersed human islet cells in vitro in conditions of glucolipotoxicity and increased oxidative stress independently of the GLP1 receptor. The nonapeptide appears to enter preferably stressed, glucolipotoxic cells compared with normal unstressed cells. It targets mitochondria and improves impaired mitochondrial membrane potential, increases cellular ATP levels, inhibits cytochrome c release, caspase activation, and apoptosis, and enhances the viability and survival of INS-1 β-cells. We propose that GLP1(28–36)amide might be useful in alleviating β-cell stress and might improve β-cell functions and survival.
Introduction
Diabetes results from both a deficiency of insulin production and the development of resistance to the actions of insulin (Leahy 2005, Szoke & Gerich 2005). Deficient insulin production is believed to be a consequence of an inadequate mass of functional β-cells in the pancreas; a near absolute deficiency of β-cells in type 1 diabetes, or a relative reduction in type 2 diabetes (T2D; Butler et al. 2007, Nyalakonda et al. 2010). A major contributor to reduced β-cell mass in obesity-related diabetes is increased oxidative stress of multiple organs of the body including pancreatic β-cells. In modern societies, excessive caloric intake exceeding caloric expenditure results in obesity, increased oxidative stress, and insulin resistance. This circumstance manifests in hyperglycemia, hyperlipidemia, hypertension, atherosclerosis, and steatosis of major organs such as the liver (nonalcoholic fatty liver disease), heart (lipotoxic cardiomyopathy), brain (Alzheimer’s disease), and pancreatic islets (diabetes) (Grattagliano et al. 2008, Haas & Biddinger 2009). Chronic exposure to supraphysiological levels of plasma glucose and lipids (glucolipotoxicity) induces oxidative stress that leads to a further decline in the mass and function of β-cells (Maiese et al. 2007, Poitout & Robertson 2008, Pitocco et al. 2010). This constellation of disorders is collectively known as the metabolic syndrome. Increased oxidative stress both impairs functions of β-cells to produce and secrete insulin in response to nutrients (glucose) and shortens their life span (Grattagliano et al. 2008, Haas & Biddinger 2009).
In this study, we report that a nonapeptide, GLP1(28–36)amide, consisting of the C-terminal domain of glucagon-like peptide-1 (GLP1; Tomas & Habener 2010) enters glucolipotoxic-stressed INS-1 β-cells and inhibits stress-induced apoptosis. We showed earlier that the C-terminal nonapeptide, GLP1(28–36)amide, derived from GLP1 enters insulin-resistant mouse hepatocytes and appears to target mitochondria, alleviates oxidative stress, and suppresses excessive gluconeogenesis (Tomas et al. 2011a). In studies in vivo, the nonapeptide curtails weight gain and the development of diabetes in diet-induced obese mice (Tomas et al. 2011b). GLP1 is a peptide hormone derived by its cleavage from proglucagon in the intestinal enteroendocrine L-cells and is secreted in the form of a 30 amino acid peptide, GLP1(7–36)amide, which has potent insulinotropic actions in the augmentation of glucose-dependent insulin secretion. This insulinotropic peptide is rapidly modified (T1/2=1–2 min) to GLP1(9–36)amide by the removal of the N-terminal two amino acids by the diaminopeptidyl peptidase-4 (DPP4; Lovshin & Drucker 2009). The GLP1 C-terminal nonapeptide GLP1(28–36)amide is proposed to be one of the products derived from GLP1 by the actions of the neutral endopeptidase (NEP) 24.11 (neprilysin) or related endopeptidase (Hupe-Sodmann et al. 1995). As it has been recently shown that injury of β-cells in islets, for example, by the β-cell toxin streptozotocin (STZ), induces the formation of GLP1 in α-cells of the islets (Liu et al. 2011), we surmised that the nonapeptide derived from GLP1 might exert cytoprotective actions on pancreatic β-cells similar to its actions on hepatocytes. This study presents evidence that oxidative stress induced by tert-butyl hydro-peroxide (t-BHP), hydrogen peroxide (H2O2), STZ, and glucolipotoxicity mediates the apoptosis of INS-1 β-cells and that GLP1(28–36)amide attenuates stress and prevents their apoptosis. We found that the functional activity of GLP1 (28–36)amide in preventing t-BHP or glucolipotoxicity-induced apoptosis is associated with the preservation of mitochondrial functions. GLP1(28–36)amide inhibits mitochondrial depolarization, cytochrome c release, caspase activation, and apoptosis. These findings raise the possibility that GLP1(28–36)amide might be therapeutically useful in reducing glucolipotoxicity-induced stress in β-cells and thereby improve β-cell functions in T2D.
Materials and Methods
Reagents
GLP1(28–36)amide (FIAWLVKGRamide) was prepared by solid-phase synthesis and purified by sequential HPLC to >98% single-component homogeneity. The nonapeptide was prepared in 0.9% (0.154 M) NaCl solution containing 0.1% (w/v) human serum albumin and stored at 4 °C.
Fluorescent-labeled GLP1(28–36)amide was prepared with the green fluorescence compound, 5-carboxyfluorescein (5-FAM, fluorescein amidite). Verification of the peptides was done by both amino acid composition analysis and mass spectroscopy. MitoTracker fluorophores used were from Molecular Probes (AnaSpec, Fremont, CA, USA). The reduced red MitoTracker fluorophore stains only actively respiring cells (Red CM-H2XRos #7513). The MitoTracker compound used requires oxidation to develop fluorescence emission and fluoresces only in viable cells. All cell culture supplies and fluorescent probes were obtained from Invitrogen. Unless specified, all other reagents were supplied by Sigma–Aldrich.
Human donor islets
Human donor islets were obtained from the National Islet Distribution Center, Des Moines, IA, USA. The discarded human tissue was used after the approval by the Human Studies Committee at Massachusetts General Hospital. Islets were hand-picked from the mixture of islet tissues received and aliquots consisting of 150 islets were dissociated by trypsinization (Liu et al. 2011), plated in 12-well dishes, cultured overnight in DMEM media with 10% fetal bovine serum (FBS), and then treated with 18.25 μM t-BHP alone or with the addition of 0.1–10 μM GLP1(28–36)amide for 24–48 h. In other experiments, dispersed human islet cells were cultured on 96-well dishes for 48 h in the presence of peptide, 30 mM glucose, and 0.3 mM oleate (glucolipotoxicity). Cell viability was then determined by the ATPlite assay.
Cell culture
INS-1 cells were kindly provided by C.B. Wollheim, Geneva. INS-1 cells were grown in RPMI medium containing 10% FBS, 11 mM glucose, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Cells were cultured and maintained at 37 °C and 5% CO2. Cells were trypsinized and subcultured every 3 days. Cells were treated with glucotoxicity media (30–40 mM glucose), glucolipotoxicity media consisting of RPMI media made to 25–30 mM glucose and 0.3 mM oleate, or treated with t-BHP (18.25 μM) or H2O2 (50 μM to 5 mM), inducers of oxidative stress. Oleate was purchased from Sigma–Aldrich as oleic acid-albumin from bovine serum (#O3008). In some experiments, STZ, a β-cell-specific DNA-damaging agent, was added to INS-1 cells at a concentration of 0.5 mM. Cell viability was measured after 18–48 h incubation using the ATPlite assay (Perkin Elmer, Waltham, MA, USA).
Labeling of INS-1 cells with FAM-GLP1(28–36)amide
INS-1 cells were plated on slides and treated for 72 h with 25 mM glucose, 0.3 mM oleate, or regular RPMI medium and then treated for 18 h with 10 μM FAM-labeled GLP1(28–36) peptide (FAM-FIAWLVKGRamide) and 400 nM MitoTracker Red CM-H2Ros in the same medium. In other experiments, cells were incubated for 18 h in media containing 50 μM H2O2 and 10 μM FAM-labeled peptide and 20 nM Mitotracker. Cells were washed, fixated with 70% methanol/30% acetone for 15 min, and mounted with Vectashield mounting medium (Vector Labs, Burlingame, CA, USA). Fluorescent images were captured with a Nikon Optiphot 2 microscope using a Photometric Cool SnapHQ camera (Photometrics, Huntington Beach, CA, USA) and IP Lab 3.6.5 software (Scanalytics, Falls Church, VA, USA).
Measurement of cell viability
INS-1 cells were plated in 96-cell plates at a density of 1×104 cells/well for 24 h. The cells were then incubated with t-BHP (18.25 μM), H2O2 (32 μM), STZ (0.5 mM) alone, or in the presence of GLP1(28–36)amide or the GLP1 receptor agonists GLP1(7–36)amide and exendin-4 for 24–48 h. Cell viability was evaluated by measuring cellular ATP levels using the ATPlite assay (Perkin Elmer).
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was evaluated using the JC-1 Mitochondrial Membrane Potential Assay (Cayman Chemicals, Ann Arbor, MI, USA). In healthy cells with high mitochondrial potential, JC-1 spontaneously forms complexes known as J-aggregates. In apoptotic cells with low membrane potential, JC-1 remains in the monomeric form, which displays the green fluorescence measured by a fluorescent plate reader using excitation/emission wavelengths of 485/535 nm. INS-1 cells (10 000 cells) were plated in chamber slides and treated with t-BHP (18.25 μM) alone or with t-BHP and GLP1(28–36)amide for 48 or 72 h. Cells were loaded with JC-1 for the last 20 min of incubation. Fluorescence intensity was measured with excitation and emission at 485 and 535 nm respectively.
GLP1 receptor activation assay
The Cre-luc reporter plasmid, a cAMP-responsive reporter gene construct, was transfected into INS-1 cells (Clontech). The construct includes a multimerized cAMP response element ligated upstream of the coding sequence for firefly luciferase. Twenty-four hours following transfection, the cells were incubated for an additional 18 h at 37 °C with or without increasing concentrations of GLP1(28–36)amide and other GLP1R agonists and antagonists. The cells were then lysed and light emission was assessed using the LucLite R luciferase kit (Perkin Elmer Life and Analytical Sciences).
Mitochondrial cytochrome c release
Cytochrome c release provides an effective means for detecting apoptosis. INS-1 cells (100 000/well) were either mock untreated (control) or treated with t-BHP (18.25 μM) alone or in combination with 10 μM GLP1(28–36)amide for 72 h. Cells were processed and subsequently prepared for fluorescence microscopy as outlined in the manufacturer’s protocol (Calbiochem, San Diego, CA, USA). Nuclei stained with DAPI (blue) and cytochrome c stained with FITC (green) were visualized using a fluorescence microscope. The loss of green fluorescence correlates with cytochrome c release.
Measurement of caspase activity
Pan caspase activity was assayed using a commercial kit based on fluorochrome-labeled caspase inhibitors (Roche Applied Science). INS-1 cells (5000 cells/well) were treated with t-BHP (18.75 μM) or glucotoxicity media alone or with different concentrations of GLP1(28–36)amide. Cells were labeled with pan-caspase inhibitor. Cells were then rinsed to remove the unbound reagent and fixed. Cell fluorescence was measured by a microplate reader (ex/em=488/520 nm; Molecular Devices, Sunnyvale, CA, USA).
PARP cleavage
Antibody to asparagine-214 of cleaved poly (ADP-ribose) protein (PARP) was obtained from Sigma–Aldrich. The antibody detects endogenous levels of the large fragment (89 kDa) of PARP1 resulting from caspase cleavage. The antibody does not recognize full-length PARP1 or other PARP isoforms. INS-1 cells (100 000) were incubated for 72 h in medium with high glucose and lipid (GLT media) only or in the presence of GLP1(28–36)amide. After the incubation, total cell extracts were analyzed by western blotting with a PARP1 antibody.
DAPI (nuclei fluorescence) and TUNEL staining
For the TUNEL assay, cells seeded in four-well chamber slides (Lab-Tak’ Sigma-Aldrich, St Louis, MO<USA) were treated with t-BHP or glucolipotoxicity media, or with 10 μM GLP1(28–36)amide, at 37 °C for 24 or 72 h. TUNEL assay was performed using DeadEnd Fluorimetric TUNEL System (Promega).
Insulin secretion assay
INS-1cells grown in RPMI 1640 culture medium containing 10% FBS, 1% penicillin/streptomycin, and 5 μl/l β-mercaptoethanol (Invitrogen) were split into 24-well plates for insulin secretion assays. Cells were treated or not with 40 mM glucose for 48 h with or without GLP1(28–36)amide. Insulin secretion was performed in standard extracellular solution (SES) containing 138 mM NaCl, 5.6 mM KCl, 2.6 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES (pH adjusted to 7.4), and 0.05% human serum albumin. Cells were washed with SES containing 2 mM glucose and then treated with 2 or 16 mM glucose in SES with or without 10 nM exendin-4. Insulin was measured in collected SES media with a rat insulin RIA kit (Millipore, Bedford, MA, USA).
Statistical analysis
All data are presented as mean±S.E.M. Differences among groups were compared by two-tailed Student’s t-test.
Results
GLP1(28–36)amide protects INS-1 cells against cytotoxicity induced by H2O2, t-BHP, STZ, or glucolipotoxicity and protects human islets against t-BHP-induced stress
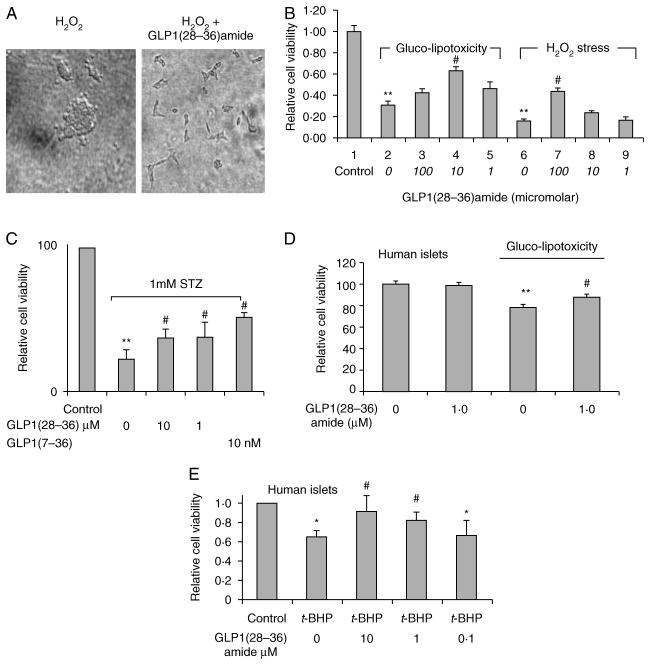
INS-1 cells treated with H2O2 for 3 days demonstrate ~100% cytotoxicity, showing rounded cells with multiple cytoplasmic blebs (Fig. 1A, left). The co-addition of GLP1(28–36)amide visually rescues most cells from apoptosis and show a typical elongated appearance adherent to the culture dish (Fig. 1A, right). A cell viability ATPlite-based study in INS-1 β-cells demonstrates that GLP1(28–36)amide raises cellular ATP levels and enhances cell viability in conditions of increased stress provoked experimentally by glucolipotoxicity and by t-BHP, an elevator of the oxidative stress in cells (Fig. 1B), and by STZ, an inducer of DNA damage-mediated stress (Fig. 1C). Because INS-1 cells are an immortalized β-cell line, we tested the effects of GLP1(28–36)amide on primary dispersed cells prepared from donor human islets. Dispersed cells prepared from human donor islets treated with either high glucose and oleate (Fig. 1D) or with t-BHP for 48 h (Fig. 1E) show a diminution of intracellular ATP levels that is partially reversed in the presence of GLP1(28–36)amide.
Figure 1.
GLP1(28–36)amide attenuates glucolipotoxic and oxidative stress and enhances viability and survival of INS-1 cells. (A) Phase-contrast images of INS-1 cells cultured for 72 h in 5 mM H2O2 without (left) and with (right) 10 μM GLP1(28–36)amide nonapeptide present in the media. (B) GLP1(28–36)amide preserves viability and survival of INS-1 cells against glucolipotoxic and oxidative (H2O2) stress. INS-1 cells were incubated for 6 days in the presence of 25 mM glucose plus 0.3 mM oleate (glucolipotoxicity media, GLT media) or 5 mM H2O2 with or without the concentrations of nonapeptide indicated. Media containing the reagents was replenished at days 2 and 4. Relative cell viability was determined by measurement of cellular ATP levels using the ATPlite assay. (C) GLP1(28–36)amide preserves cell viability in response to STZ. STZ (1.0 mM) was added and INS-1 cells were cultured for 48 h in the presence or absence of the nonapeptide at the concentrations indicated. GLP1(7–36)amide also confers enhanced cell viability. Cell viability was measured by ATPlite assay at 48 h. (D) GLP1(28–36)amide (1.0 μM) increases dispersed human islet cell viability impaired by a 48 h incubation in glucolipotoxic media (30 mM glucose and 0.3 mM oleate). GLP1(28–36)amide has no effect on control cells treated for 48 h with 0.1% BSA (left two bars). (E) Effects of t-BHP and GLP1(28–36)amide on human islet cell viability. Cells dissociated from 150 human islets were treated with 18.25 μM t-BHP alone or with t-BHP and different doses of GLP1(28–36)amide for 48 h. Cell viability was then determined by the ATPlite assay. Cell viability decreased with 18.25 μM t-BHP. Cells co-incubated with GLP1(28–36)amide had higher viability. *P<0.05, **P<0.01 cell stressor (glucolipotoxicity, t-BHP, H2O2, and STZ) vs control. #P<0.05, stressor control vs stressor plus GLP1(28–36)amide (n=6).
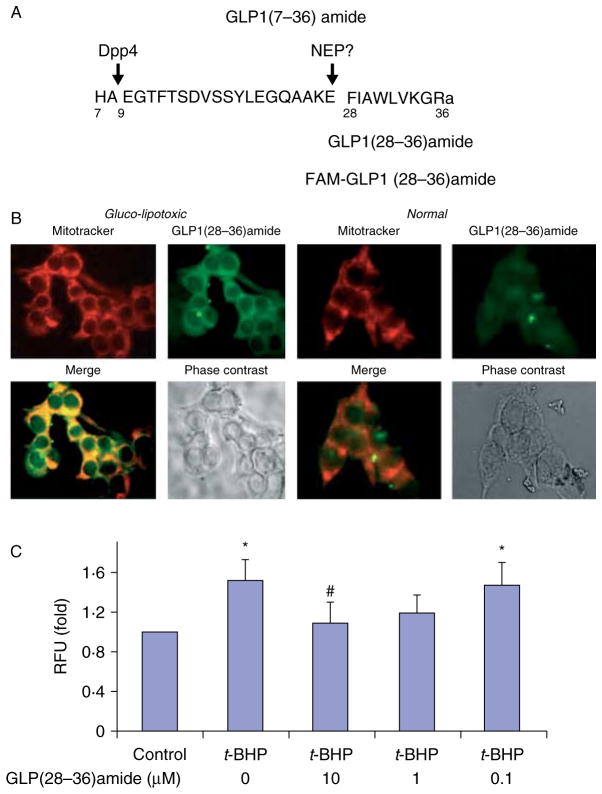
GLP1(28–36)amide nonapeptide enters glucolipotoxic INS-1 β-cells and not normal INS-1 cells
Because oxidative stress originates from mitochondria of cells and earlier we noted that GLP1(28–36)amide appears to readily enter into insulin-resistant hepatocytes (Tomas et al. 2011a), we examined INS-1 cells to determine whether the nonapeptide would gain access into the cells. A fluorescent-labeled FAM-GLP1(28–36)amide (Fig. 2B) and MitoTracker, a fluorescent compound that specifically targets actively respiring mitochondria of viable cells, was used to track the cellular distribution of the peptide after addition to INS-1 cells that were rendered glucolipotoxic by incubation in 25 mM glucose −0.3 mM oleate for 72 h (Fig. 2B) or oxidatively stressed by incubation with 50 μM H2O2 for 18 h (data not shown). The fluorescent-labeled FAM-GLP1(28–36)amide enters cells, and distributes in an intracellular pattern that appears to overlap with that of MitoTracker (Fig. 2B). Because of the small amount of cytoplasm (large nuclei) characteristic of INS-1 cells compared with hepatocytes, it was not possible to determine with certainty whether the FAM-labeled nonapeptide colocalizes with the MitoTracker even at a higher magnification (data not shown). It is worth noting that the FAM-labeled nonapeptide appears to produce some nuclear staining in addition to the cytoplasmic staining (Fig. 2B). The significance of this tentative finding is unknown. The nonapeptide seems not to enter normal, unstressed INS-1 cells (Fig. 2B), a finding consistent with the earlier observations that the cytoprotective actions of the peptide appear to manifest predominantly in insulin-resistant, stressed hepatocytes and not in normal, unperturbed cells (Tomas et al. 2011a).
Figure 2.
GLP1-derived nonapeptide selectively enters glucolipotoxic INS-1 β-cells, targets mitochondria, and protects against oxidative stress-induced loss of mitochondrial membrane potential. (A) Sequence of the insulinotropic form of GLP1, GLP1(7–36)amide, and the C-terminal nonapeptide, GLP1(28–36)amide, derived from it. The peptide was labeled at the amino-terminus with the fluorescent compound 5- FAM. (B) Intracellular transport of fluorescence FAM-labeled GLP1(28–36)amide into glucolipotoxic (left panel), but not normal (right panel), INS-1 cells. Cells were cultured in the presence of 25 mM glucose and 0.3 mM oleate for 72 h. FAM-labeled nonapeptide and MitoTracker were added to the cells for 18 h. (C) GLP1(28–36)amide inhibits t-BHP-induced loss of mitochondrial membrane potential in INS-1 cells. Mitochondrial membrane potential was evaluated using the JC-1 Mitochondrial Membrane Potential Assay. Treatment of INS-1 cells with 18.25 μM t-BHP for 48 h resulted in a loss of mitochondrial potential, as indicated by an increase in green fluorescence intensity of monomeric JC-1, and this was inhibited by concurrent treatment with 10 or 1.0 μM GLP1(28–36)amide. For mitochondrial membrane potential studies, cells were treated with 18.25 μM t-BHP for 48 h. *P<0.05, t-BHP vs control; #P<0.05. t-BHP control vs t-BHP plus GLP1(28–36)amide.
GLP1(28–36)amide prevents t-BHP-induced mitochondrial depolarization
The results described earlier using the mitochondria-targeting marker MitoTracker suggest that GLP1(28–36)amide readily enters cells where it targets mitochondria and might inhibit oxidative stress by modulating oxidative phosphorylation and inhibiting apoptosis. Therefore, we first investigated the effects of GLP1(28–36)amide directly on the modulation of mitochondrial membrane potential. The opening of the mitochondrial permeability transition (MPT) pore and loss of mitochondrial membrane potential have been linked to oxidative cell death caused by t-BHP (Piret et al. 2004). Treatment of INS-1 cells with 50 μM t-BHP for 6 h resulted in a loss of mitochondrial potential. Fluorescence intensity of monomeric JC-1 (green), which accumulates with loss of membrance potential, was significantly higher in cells treated with 18.25 mM t-BHP compared with untreated control cells (Fig. 2C). These observations support the findings using MitoTracker shown in panel B, suggesting that GLP1(28–36)amide enters the INS-1 cells and targets mitochondria.
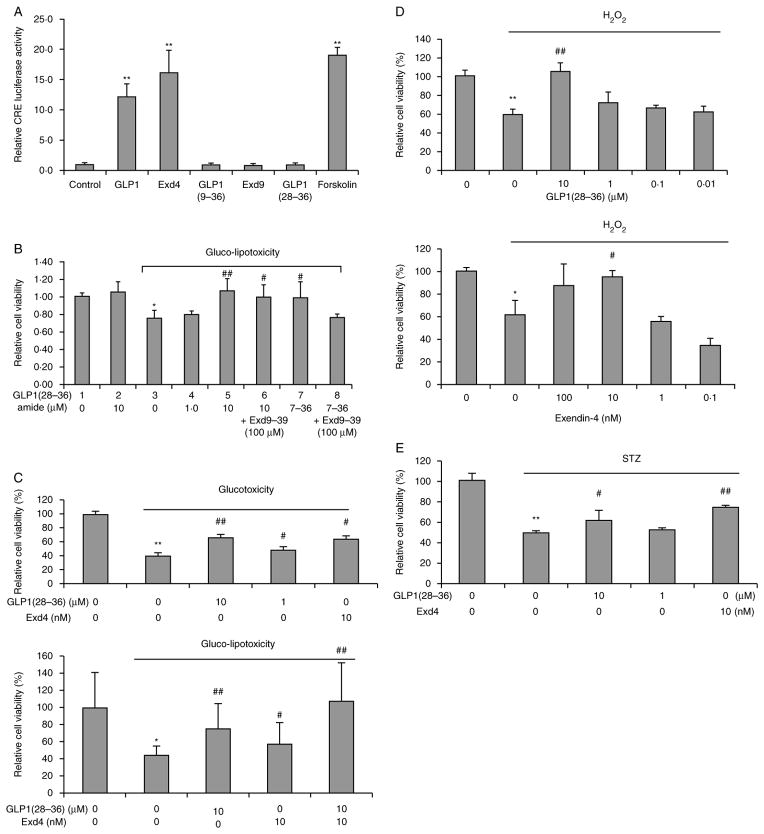
GLP1(28–36)amide nonapeptide does not activate the GLP1 receptor in β-cells
Because it is generally believed that GLP1 peptides must act on the GLP1 receptor, and we observed rapid entry of GLP1(28–36)amide into the INS-1 cells that are known to express GLP1 receptors (Liu & Habener 2008), we examined the effects of GLP1(28–36)amide using the CRE reporter activation assay and the specific GLP1 receptor antagonist, exendin(9–39). Because the GLP1 receptor is coupled to the stimulatory G-protein that activates the formation of cAMP and transcription factor CREB resulting in the activation of reporter gene expression driven by a promoter containing cAMP-responsive enhancer elements (CREs), this assay is used as a GLP1 receptor activation assay (Beinborn et al. 2005). The actions of GLP1(28–36)amide appear to occur independently of the GLP1 receptor because the nonapeptide does not activate the CRE-luciferase receptor reporter assay (Fig. 3A) and its prosurvival actions are not inhibited by the blockade of the GLP1 receptor with the antagonist, exendin(9–39) (Fig. 3B). Notably, in the absence of glucolipotoxicity, the nonapeptide has no effect on cell viability (compare bar 1 vs bar 2, Fig. 3B).
Figure 3.
GLP1(28–36) protects INS-1 cells against glucotoxicity, glucolipotoxicity, H2O2-induced oxidative stress, and STZ-induced cell damage independent of the GLP1R. (A) GLP1 receptor activation assay. A multimerized cAMP response element promoter driving a luciferase transcriptional reporter was used to test GLP1 receptor activation by GLP1 peptides. Forskolin is a control activator of adenylate cyclase. (B) ATPlite cell viability assay: INS-1 cells were cultured in glucolipotoxicity media (30 mM glucose +200 μM oleic acid). Different doses of GLP1(28–26)amide with and without addition of the GLP1 receptor antagonist, exendin(9–39) (Exd9–39), or control vehicle were concomitantly added and replenished at day 2. Cell viability was measured by ATPlite assay at day 4. The known GLP1 receptor agonist, GLP1(7–36)amide (7–36), serves as a control and is antagonized by the co-addition of Exd9–39. (C) Effects of GLP1(28–36)amide and exendin-4 on glucotoxic and glucolipotoxic cells. (D) Cytoprotective effects of GLP1(28–36)amide and exendin-4 on H2O2-induced oxidative stress. (E) GLP1(28–36)amide and the enzyme-resistant GLP1 receptor agonist exendin-4 both protect against STZ-induced (0.5 mM) cell stress. *P<0.05, **P<0.01 cell stressor vs control. #P<0.05, ##P<0.01 stressor control vs stressor plus GLP1(28–36)amide (n=6–12).
Comparison of effects of GLP1(28–36)amide with exendin-4, an enzyme-resistant GLP1 receptor agonist, on cell stress induced by glucotoxicity, glucolipotoxicity, and H2O2
To compare the relative effectiveness of GLP1(28–36)amide with an enzyme-resistant GLP1 receptor agonist, we examined the effects of the nonapeptide to exendin-4, a known potent receptor-dependent agonist, resistant to cleavage by dipeptidyl peptidase-4, with cytoprotective actions on β-cells, on the viability of INS-1 cells rendered either glucotoxic or glucolipotoxic (Fig. 3C). Exendin-4 at a concentration of 10 nM gives a survival response similar to that of 10 μM GLP1(28–36)amide. The co-addition of exendin-4 and the nonapeptide appears to be additive, consistent with different mechanisms of action of the two peptides on cell viability. We propose that in contrast to the receptor agonist exendin-4, GLP1(28–36)amide acts via GLP1 receptor-independent mechanisms.
GLP1(28–36)amide and exendin-4 protection against STZ-induced cell stress
The enzyme-resistant GLP1 receptor agonist, exendin-4, was compared with GLP1(28–36)amide in the enhancement of cell viability in response to the treatment of INS-1 cells with STZ. Both exendin-4 and GLP1(28–36)amide increased cell survival (Fig. 3E).
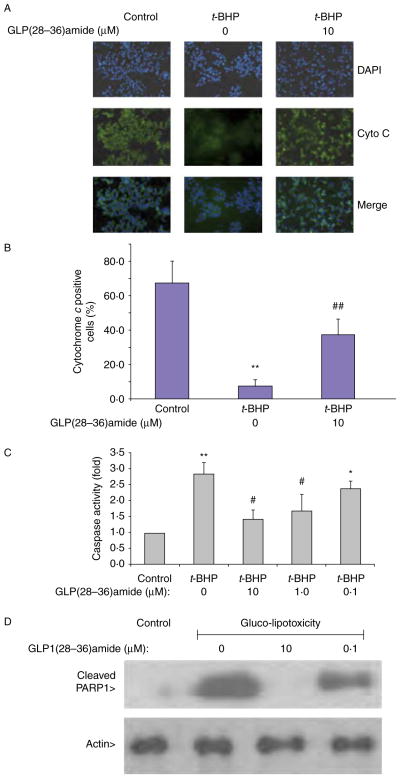
GLP1(28–36)amide protects against apoptosis induced by t-BHP and glucolipotoxicity media
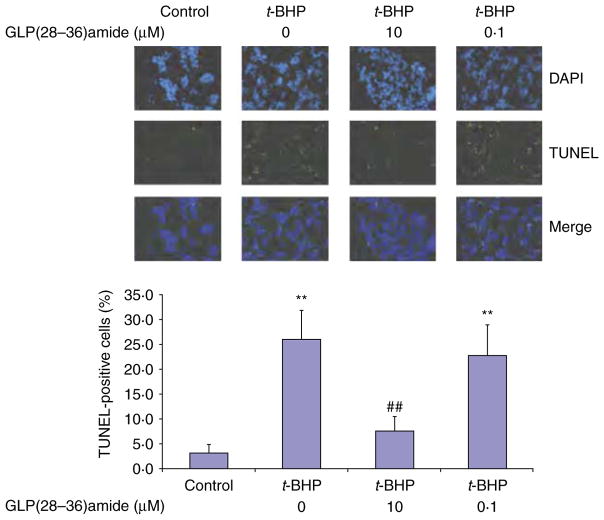
The findings that GLP1(28–36)amide enters stressed INS-1 cells and elevates intracellular ATP levels in response to glucolipotoxic and oxidative stresses suggest that the peptide exerts cytoprotective antiapoptotic actions on the cells. Because a major mitochondrial pathway responsible for cell apoptosis is the cytochrome c-mediated activation of the caspase pathway, we examined the effects of GLP1(28–36)amide in the attenuation of cytochrome c release, caspase activation, and the cleavage of PARP, a major substrate of active caspase. The addition of t-BHP to INS-1 cells stimulated cytochrome c release (loss of green fluorescent cytochrome c), which was inhibited by the addition of the GLP1-derived nonapeptide (Fig. 4A and B). The effects of t-BHP and GLP1(28–36)amide were examined on pan-caspase activity. Cells treated with 18.25 μM t-BHP alone displayed a significant increase in caspase-3 activity compared with the control group. GLP1(28–36)amide decreased caspase-3 activity dose dependently (Fig. 4C) and inhibited the formation of the cleaved PARP protein fragment PARP1 (Fig. 4D). A sensitive assay for assessing apoptosis in INS-1 cells is the TUNEL assay that measures ends of fragmented DNA at mid to late stages of apoptosis (Liu & Habener 2009). The addition of GLP1(28–36)amide to INS-1 cells in the presence of an apoptosis-producing concentration of t-BHP (18.25 μM) inhibited the appearance of TUNEL-positive apoptotic cells (Fig. 5).
Figure 4.
Effects of t-BHP and GLP1(28–36)amide on cytochrome c release, pan-caspase activity, and caspase-mediated cleavage of PARP in INS-1 cells. (A) Cytochrome c release. GLP1(28–36)amide prevents t-BHP-induced cytochrome c release from mitochondria (see Materials and methods section). Treatment of INS-1 cells with t-BHP resulted in rapid loss of cytochrome c by FITC fluorescence (middle), over 72 h at 37 °C. Concurrent treatment with GLP1(28–36)amide reduces the loss of cytochrome c (right). (B) Quantitative analysis of fluorescence image data in (A) plotted as percentage of control cells in which 300 or more cells were analyzed. (C) Caspase activation inhibited by GLP1(28–36)amide (n=6). (D) Formation of cleaved PARP (Asp 214) inhibited by GLP1(28–36)amide. The antibody detects endogenous levels of the large fragment (89 kDa) of PARP1 resulting from caspase cleavage and does not recognize full-length PARP1 or other PARP isoforms. *P<0.05, **P<0.01 t-BHP vs control. #P<0.05, ##P<0.01 t-BHP control vs t-BHP plus GLP1(28–36)amide. Full colour version of this figure available via http://dx.doi.org/10.1530/JOE-11-0328
Figure 5.
TUNEL assay shows inhibition of t-BHP-induced apoptosis by GLP(28–36)amide in INS-1 cells. INS-1 cells were plated in four-well chamber slides and treated with t-BHP alone or with GLP1(28–36)amide for 48 h. Top: representative images of TUNEL staining of INS-1 cells; Bottom: quantification of apoptotic cells (TUNEL positive) expressed as percent apoptotic cells relative to total cells counted. At least, 500 total cells were counted in each experimental condition. **P<0.01 t-BHP vs control. ##P<0.01 t-BHP control vs t-BHP plus GLP1(28–36)amide. Full colour version of this figure available via http://dx.doi.org/10.1530/JOE-11-0328
Lack of effect of GLP1(28–36)amide on insulin secretion
To determine whether GLP1(28–36)amide might improve insulin secretin by INS-1 cells rendered glucotoxic, INS-1 cells were cultured in glucotoxic conditions with and without the addition of GLP1(28–36)amide for 48 h. Insulin secretion was determined in response to a hyperglycemic challenge (2 vs 16 mM glucose) and to 16 mM glucose plus the GLP1 receptor agonist exendin-4 (10 nM) (data not shown). Although there was a tendency for GLP1(28–36)amide to improve insulin secretion, the responses were not significant. These findings suggest that the nonapeptide might not affect the secretory functions of INS-1 cells and that the major actions of the nonapeptide are cytoprotection and survival.
Discussion
In these studies, we report on a cell-penetrating peptide, GLP1(28–36)amide, which selectively enters stressed INS-1 β-cells, appears to target mitochondria and to prevent stress-induced apoptosis in response to glucolipotoxicity media, STZ, and the oxidizing agents H2O2 and t-BHP. We have show that GLP1(28–36)amide promotes the viability and survival of INS-1 cells in the oxidative environment created by the addition of glucolipotoxicity media, and t-BHP. GLP1(28–36)amide also protects dispersed human islet cells by inhibiting a decrease in intracellular ATP levels induced by t-BHP. We provide evidence that GLP1(28–36)amide protects mitochondrial membrane potential and attenuates the release of cytochrome c, caspase activation, and programmed cell death. These studies on β-cells extend observations of the antioxidant and prosurvival actions of GLP1(28–36)amide from insulin-resistant hepatocytes (Tomas et al. 2011a), insulin-sensitive target cells for the actions of insulin, to insulin-producing β-cells of the pancreas.
Oxidative stress plays a key role in promoting mitochondrial cytochrome c release and the induction of apoptosis. The cell-permeable nonapeptide, GLP1(28–36), appears to act as an antioxidant and inhibits MPT, preserves membrane potential, and prevents cytochrome c release and apoptosis caused by t-BHP in INS-1 cells. Oxidative stress induced by t-BHP enhances the gating of Ca2+ in mitochondria, leading to the onset of MPT defined as an opening of the pores in the inner membrane of the mitochondria (Szeto 2006). The opening of pores results in a dissipation of the mitochondrial potential, uncoupling of oxidative phosphorylation, swelling of the mitochondrial matrix, and rupture of the outer mitochondrial membrane (Szeto 2006). These membrane-associated events lead to the release of cytochrome c into the cytosol, where it induces activation of the caspase cascade and apoptotic program. In our studies reported here, we have found that GLP1(28–36)amide appears to prevent MPT-induced oxidative damage to mitochondria and subsequent activation of caspase cascades leading to apoptosis (Szeto 2006). The findings of the effects of the nonapeptide on the stabilization of mitochondrial membrane potential and the inhibition of cytochrome c release, both functions carried out by mitochondria, support the preliminary findings that a fluorescence-labeled non-apeptide appears to enter INS-1 cells and overlaps with the fluorophore MitoTracker, although co-localization of the nonapeptide and MitoTracker was not ascertainable. Our studies reinforce the concept that antioxidants protect β-cells from oxidative stresses generated via different sources. Prolonged exposure of β-cells to high glucose concentrations leads to glucotoxicity and β-cell exhaustion (Moran et al. 1997). In this regard, a recent study demonstrated that GLP1(28–36)amide suppresses elevated levels of reactive oxygen species in glucotoxic hepatocytes in culture (Tomas et al. 2011a).
Whether or not GLP1(28–36)amide is a naturally occurring active peptide in vivo remains unknown at present. Several lines of evidence, however, suggest that the nonapeptide is produced in the body from larger precursor forms of GLP1 by selective enzymatic cleavage. Of relevance to this conjecture, and to our findings from the INS-1 β-cell, are the recently reported actions of GLP1(9–36)amide (Tomas et al. 2010) and GLP1(28–36)amide (Tomas et al. 2011a) on isolated mouse hepatocytes. These GLP1-derived peptides suppress gluconeogenesis and appear to modulate oxidative phosphorylation. Notably, the action of GLP1(9–36)amide in isolated mouse hepatocytes (Tomas et al. 2010) suggests that GLP1(9–36)amide might be a precursor of the active GLP1(28–36)amide nonapeptide, GLP1(28–36)amide may arise from the cleavage of GLP1(9–36)amide by an endopeptidase in the circulation. In support of this notion are the findings that the clearance rate of C-terminal peptide immunoreactivity during infusions of GLP1(7–36)amide in pigs is markedly prolonged by the co-administration of candoxatril, a specific inhibitor of NEPs (Plamboeck et al. 2005). This observation suggests that enzymatic modifications of GLP1(9–36)amide might occur in the circulation resulting in the formation of GLP1(28–36)amide and/or other GLP1-derived peptides. The existing GLP1 immunoassays appear not to cross-react with the GLP1 nonapeptide (Plamboeck et al. 2005). Moreover, the NEP, NEP 24.11, known as neprilysin, cleaves GLP1(7–36)amide into several smaller peptides including the GLP1(28–36)amide nonapeptide, which comprises the C-terminal nine amino acids of GLP1 (Hupe-Sodmann et al. 1995). Our findings that GLP1(28–36)amide appears to have biological actions suggest that the enzyme-resistant forms of GLP1 in use might not clinically possess the full repertoire of GLP1-mediated effects.
Our studies on INS-1 β-cells further support the notion that the actions of GLP1(28–36)amide occur independently of the GLP1 receptor. We found that the pro-survival actions of GLP1(28–36)amide on INS-1 cells are not affected by the GLP1 receptor antagonist, exendin(9–39), and the nonapeptide does not activate the CRE-luciferase assay expressed in INS-1 cells. The CRE-luciferase reporter is accepted as a valid assay for GLP1 receptor activation (Beinborn et al. 2005) and is activated by the known receptor agonists, GLP1(7–36)amide and exendin-4. Furthermore, GLP1(28–36)amide was shown earlier to enter isolated primary hepatocytes and target mitochondria (Tomas et al. 2011a) under conditions in which there is no detectable expression of the GLP1 receptor on hepatocytes (Tomas et al. 2010). The identity of the putative receptor or transporter for GLP1(28–36)amide in β-cells, and hepatocytes, is unknown at present. Possible candidates for such a transporter include one of the solute carriers (Brandsch et al. 2008), an ATP-binding cassette/multidrug resistant-type transporter (Oude Elferink & Zadina 2001), an unidentified opiate peptide transporter (Ganapathy & Miyauchi 2005), and the multifunctional pattern-reading receptor/transporter CD36/fatty acid translocator (Demers et al. 2008). Because the entry of GLP1(28–36)amide appears to be selective to stressed β-cells (Fig. 3B) and stressed insulin-resistant hepatocytes, it is tempting to speculate that the unknown receptor/transporter mechanism might be induced by the conditions of oxidative stress and/or insulin resistance.
Recent evidence suggests that injuries of β-cells in islets, e.g. STZ, induce the expression of GLP1 in the α-cells of the islets (Liu et al. 2011, Whalley et al. 2011) and that GLP1 exerts cytoprotective, pro-survival actions on adjacent β-cells (Liu et al. 2011). As the endopeptidase responsible for the generation of GLP1(28–36)amide from GLP1 is expressed in islets (Zraika et al. 2007), it is tempting to speculate that the GLP1(28–36)amide nonapeptide might be formed in the islets (Hupe-Sodmann et al. 1997) and that some or all the cytoprotective effects of GLP1 on β-cells in islets are mediated by the GLP1-derived nonapeptide.
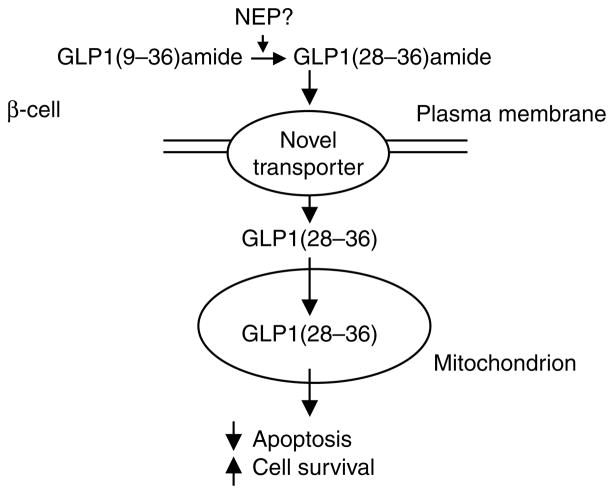
In summary, our current working hypothesis proposes that the GLP1-derived nonapeptide, GLP1(28–36)amide, enters INS-1 β-cells, independently of the GLP1 receptor, where it targets mitochondria and modulates oxidative phosphorylation, inhibits oxidative stress, and suppresses apoptosis (Fig. 6). As glucolipotoxicity and resultant oxidative stress are associated with increased insulin resistance in T2D, GLP1(28–36)amide might prove to be a therapeutic factor that could improve β-cell viability and function as well as to alleviate insulin resistance in insulin-sensitive peripheral tissues.
Figure 6.
Model of hypothesized actions of GLP1(28–36)amide on pancreatic β-cells. GLP1(28–36)amide, formed in the circulation by the cleavage of a precursor GLP1(9–36)amide by an endopeptidase (NEP), enters cells by an unknown novel receptor/transporter pathway. After entry into cells, the nonapeptide gains access to mitochondria where it is proposed to modulate oxidative phosphorylation, reduce oxidative stress, inhibit apoptosis, and promote cell survival.
Acknowledgments
Funding
The studies were supported in part by a grant from the Charles A. Hood Foundation to Z L and a Basic Science Grant from Novo Nordisk to J F H.
The authors thank Karen McManus for expert experimental assistance.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Note added in proof
Since submission of the manuscript we became aware that the nonapeptide, GLP-1(28–36)amide used in the experiments was not completely solubilized. More efficient solubilization of the nonapeptide was accomplished by using vehicles consisting of 20% acetic acid, 0.154 M saline, 0.1% human serum albumin or 0.1 M sodium acetate, 0.1% human serum albumin (pH 4.0). When completely solubilized at low pH, cytoprotective actions of the peptide were observed at concentrations of 0.1–100 nM, rather than at 0.1–10 μM.
References
- Beinborn M, Worrall CI, McBride EW, Kopin AS. A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regulatory Peptides. 2005;130:1–6. doi: 10.1016/j.regpep.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brandsch M, Knütter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. Journal of Pharmacy and Pharmacology. 2008;60:543–585. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]
- Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of β cells in normal physiology, in disease and for therapy. Nature Clinical Practice. Endocrinology & Metabolism. 2007;3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- Demers A, Rodrigue-Way A, Tremblay A. Hexarelin signaling to PPARgamma in metabolic diseases. PPAR Research. 2008;2008:364784. doi: 10.1155/2008/364784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Miyauchi S. Transport systems for opioid peptides in mammalian tissues. AAPS Journal. 2005;7:E852–E856. doi: 10.1208/aapsj070482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattagliano I, Palmieri VO, Portincasa P, Moschetta A, Palasciano G. Oxidative stress-induced risk factors associated with the metabolic syndrome: a unifying hypothesis. Journal of Nutrition and Biochemistry. 2008;19:491–504. doi: 10.1016/j.jnutbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Haas JT, Biddinger SB. Dissecting the role of insulin resistance in the metabolic syndrome. Current Opinion in Lipidology. 2009;20:206–210. doi: 10.1097/MOL.0b013e32832b2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Göke R, Göke B, Thole H, Zimmermann B, Voigt K. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regulatory Peptides. 1995;58:149–156. doi: 10.1016/0167-0115(95)00063-H. [DOI] [PubMed] [Google Scholar]
- Hupe-Sodmann K, Göke R, Göke B, Thole HH, Zimmermann B, Voigt K, McGregor GP. Endoproteolysis of glucagon-like peptide (GLP)-1 (7–36) amide by ectopeptidases in RINm5F cells. Peptides. 1997;18:625–632. doi: 10.1016/S0196-9781(97)00123-X. [DOI] [PubMed] [Google Scholar]
- Leahy JL. Pathogenesis of type 2 diabetes mellitus. Archives of Medical Research. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic β cell proliferation. Journal of Biological Chemistry. 2008;283:8723–8735. doi: 10.1074/jbc.M706105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Habener JF. Stromal cell-derived factor-1 promotes survival of pancreatic β cells by the stabilisation of β-catenin and activation of transcription factor 7-like 2 (TCF7L2) Diabetologia. 2009;52:1589–1598. doi: 10.1007/s00125-009-1384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Stanojevic V, Avadhani S, Yano T, Habener JF. Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances β cell survival. Diabetologia. 2011;54:2067–2076. doi: 10.1007/s00125-011-2181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nature Reviews. Endocrinology. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Current Neurovascular Research. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Zhang HJ, Olson LK, Harmon JS, Poitout V, Robertson RP. Differentiation of glucose toxicity from β cell exhaustion during the evolution of defective insulin gene expression in the pancreatic islet cell line, HIT-T15. Journal of Clinical Investigation. 1997;99:534–539. doi: 10.1172/JCI119190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalakonda K, Sharma T, Ismail-Beigi F. Preservation of β cell function in type 2 diabetes. Endocrine Practice. 2010;21:1–33. doi: 10.4158/EP10112.RA. [DOI] [PubMed] [Google Scholar]
- Oude Elferink RP, Zadina J. MDR1 P-glycoprotein transports endogenous opioid peptides. Peptides. 2001;22:2015–2020. doi: 10.1016/S0196-9781(01)00564-2. [DOI] [PubMed] [Google Scholar]
- Piret JP, Arnould T, Fuks B, Chatelain P, Remacle J, Michiels C. Mitochondria permeability transition-dependent tert-butyl hydroperoxide-induced apoptosis in hepatoma HepG2 cells. Biochemical Pharmacology. 2004;67:611–620. doi: 10.1016/j.bcp.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Review of Diabetic Studies. 2010;7:15–22. doi: 10.1900/RDS.2010.7.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–1890. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocrine Reviews. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS Journal. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke E, Gerich JE. Role of impaired insulin secretion and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Comprehensive Therapy. 2005;31:106–112. doi: 10.1007/s12019-005-0005-y. [DOI] [PubMed] [Google Scholar]
- Tomas E, Habener JF. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends in Endocrinology and Metabolism. 2010;21:59–67. doi: 10.1016/j.tem.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Stanojevic V, Habener JF. GLP-1(9–36)amide metabolite suppression of glucose production in isolated mouse hepatocytes. Hormone and Metabolic Research. 2010;42:657–662. doi: 10.1055/s-0030-1253421. [DOI] [PubMed] [Google Scholar]
- Tomas E, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28–36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regulatory Peptides. 2011a;167:177–184. doi: 10.1016/j.regpep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Tomas E, Wood JA, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28–36)amide inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Regulatory Peptides. 2011b;169:43–48. doi: 10.1016/j.regpep.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic α cells: is this a paracrine mechanism enabling GLP-1 to act on β cells? Journal of Endocrinology. 2011;211:99–106. doi: 10.1530/JOE-11-0094. [DOI] [PubMed] [Google Scholar]
- Zraika S, Hull RL, Udayasankar J, Clark A, Utzschneider KM, Tong J, Gerchman F, Kahn SE. Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes. 2007;56:304–310. doi: 10.2337/db06-0430. [DOI] [PubMed] [Google Scholar]