Abstract
There is extensive evidence that activation of the immune system is both necessary and required for the development of Ang II-induced hypertension in males. The purpose of this study was to determine if sex differences exist in the ability of the adaptive immune system to induce Ang II-dependent hypertension and whether central and renal T cell infiltration during Ang II-induced hypertension is sex-dependent. Rag-1−/− mice, lacking both T and B cells, were used. Male and female Rag-1−/− mice received adoptive transfer of male CD3+ T cells 3 weeks prior to 14 day Ang II infusion (490ng/kg/min). Blood pressure was monitored via tail cuff. In the absence of T cells, systolic blood pressure (SBP) responses to Ang II were similar between sexes (Δ22.1mmHg males vs. Δ18mmHg females). After adoptive transfer of male T cells, Ang II significantly increased SBP in males (Δ37.7mmHg, p<0.05) compared to females (Δ13.7mmHg). Flow cytometric analysis of total T cells and CD4+, CD8+, and regulatory Foxp3+-CD4+ T cell subsets identified that renal lymphocyte infiltration was significantly increased in males vs females in both control and Ang II infused animals (p<0.05). Immunohistochemical staining for CD3+ positive T cells in the SFO region of the brain was increased in males compared to females. These results suggest that female Rag-1−/− mice are protected from male T cell-mediated increases in Ang II-induced hypertension as compared to their male counterparts, and this protection may involve sex differences in the magnitude of T cell infiltration of the kidney and brain.
Keywords: Angiotensin II, Hypertension, T-lymphocytes, Sex differences, Subfornical Organ, Kidney
Emerging clinical and experimental data suggest that inflammation, and adaptive immunity in particular, is an important contributor to the development of hypertension1,2. Angiotensin II (Ang II)-induced hypertension has been shown to involve inflammatory mechanisms in the peripheral vasculature, the kidney and the CNS3,4,5,6. Experimental studies have provided extensive evidence that activation of the immune system is both necessary and required for the development of Ang II-induced hypertension in males3. In male mice deficient for the recombinant activating gene-1 (Rag-1−/−), which lack both B and T cells, increases in blood pressure (BP) following Ang II infusion are significantly attenuated compared to wild type mice3,7. When T cells were transferred back into the male Rag-1−/− deficient mice (adoptive transfer), the hypertensive effects of Ang II were restored.
Target organ lymphocyte infiltration is thought to contribute to the development of hypertension in males. Renal infiltration of lymphocytes is known to be associated with increases in BP in Ang II-dependent and salt-sensitive hypertension8,9,10. Recent studies support an essential role for the central nervous system (CNS) and subfornical organ (SFO) in the induction and maintenance of Ang II-dependent hypertension, which is associated with peripheral activation of lymphocytes and tissue infiltration7,11.
To date, however, there is limited information regarding the role of the immune system in the development of hypertension in females. Sex-specific differences in the development of hypertension are well documented12,13,14. It has been proposed that 17β-estradiol delays and/or prevents the onset of cardiovascular disease and hypertension and may function to keep women “cardiovascularly younger” than men of the same age. Similar observations have been made in experimental models of cardiovascular regulation and hypertension15,16,17,18,19. The underlying mechanisms involved in the relative protection of females from hypertension involve multiple end organs and systems including the peripheral vasculature, renal function, central regulation of sympathetic outflow and likely include the adaptive immune system19,20,21.
Both the kidney and the brain are known to be important in the development of Ang II-dependent hypertension. The SFO has dense angiotensin type 1 receptor expression and innervates the parvocellular neurons of the periventricular nucleus (PVN), which are known to be involved in the regulation of sympathetic outflow and BP. Important to the present study, the SFO is richly endowed with sex steroid receptors including estrogen receptors alpha and beta (ER-α and ER-β)22. Previously, we have demonstrated that ER-α receptors are expressed in SFO neurons and it has been shown that 17β-estradiol alters the physiological responses of SFO neurons to Ang II23.
The purpose of the present study was to determine if the sex of the Rag-1−/− host impacts the ability of male T cells to restore the magnitude of the Ang II-induced hypertension. Furthermore, we investigated if T cell infiltration into the kidney and brain was affected by the sex of the Rag-1−/− host following adoptive transfer of male T cells and Ang II infusion.
Methods
Detailed descriptions of the animals, methods and statistics can be found in the online-only Data Supplement. All methods were approved by the University of Arizona Animal Care and Use Committee.
Results
No sex differences were observed in basal SBP and HR in Rag-1−/− mice
Baseline SBP values prior to male T cell adoptive transfer and Ang II infusion were not significantly different between male and female Rag-1−/− mice (Table 1). Mean HR between groups was also similar at baseline.
Table 1.
Baseline hemodynamic measurements in male and female Rag-1−/− mice.
| Group | SBP | HR | |
|---|---|---|---|
| Female | CD3M→Rag1−/− | 106.6 ± 1.5 | 635.4 ± 10.6 |
| Rag1−/−+Ang II | 111.4 ± 1.2 | 607.5 ± 14.6 | |
| CD3M→Rag1−/−+Ang II | 108.8 ± 4.2 | 639.1 ± 11.4 | |
|
| |||
| Male | CD3M→Rag1−/− | 105.7 ± 2.1 | 601.4 ± 19.8 |
| Rag1−/−+Ang II | 101.3 ± 2.4 | 629.6 ± 9.60 | |
| CD3M→Rag1−/−+Ang II | 103.4 ± 2.8 | 641.4 ± 14.9 | |
No sex differences were observed in the magnitude of Ang II-induced hypertension in T cell-deficient Rag-1−/− mice
Ang II infusion significantly increased SBP in both male and female Rag-1−/− mice; however, there were no sex differences in the magnitude of the hypertension (Rag1−/−-M+ Ang II, Δ 22.1±5 vs. Rag1−/−-F+ Ang II, Δ18±4 mmHg, p<0.05 vs. baseline) (Figure 2A). Ang II infusion did not significantly change HR in either sex (Figure 2B).
Figure 2. Effect of Ang II infusion on hemodynamic responses in male and female Rag-1−/− mice with and without male T cell adoptive transfer.
A. SBP response to Ang II was enhanced by the adoptive transfer of male T cells in males, but not in females. B. Mean HR following 14d Ang II infusion (490ng/kg/min) was similar across all groups. *p<0.05 vs. CD3M→Rag1−/−-F+Ang II.
Sex differences were observed in Ang II-induced hypertension in Rag-1−/− mice following adoptive transfer of male T lymphocytes
As reported previously3,7, adoptive transfer of male T cells into male Rag-1−/− mice significantly augmented the Ang II-induced increase in SBP (Rag1−/−-M+ Ang II, Δ22.1±5 vs. CD3M→Rag1−/−-M+ Ang II, Δ37.7±7mmHg, p<0.05) (Figure 2A). In contrast, there were no significant differences in the Ang II-induced SBP response following adoptive transfer of male T cells into female Rag-1−/− mice (Rag1−/−-F+ Ang II, Δ18±4 vs. CD3M→Rag1−/−-F+ Ang II, Δ13.7±7 mmHg) (Figure 2A). There were no sex differences in HR in the Rag-1−/− mice following adoptive transfer of male T cells and Ang II infusion (Figure 2B).
Sex differences in renal T cell infiltration were independent of Ang II infusion in Rag-1−/− mice after adoptive transfer of male T lymphocytes
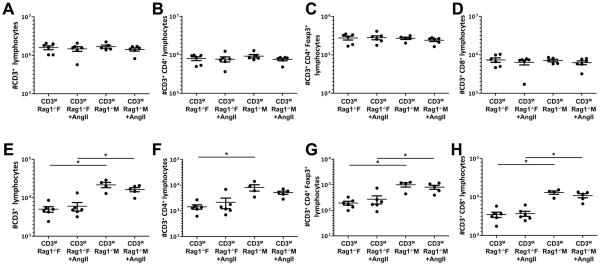
After adoptive transfer of male T cells into male and female Rag-1−/− mice, kidney and brain tissues were analyzed via flow cytometry to determine if there were sex differences in the degree of T cell infiltration in the presence and absence of Ang II infusion. The number of CD3+, CD3+CD4+, CD3+CD8+ or CD3+CD4+Foxp3+ T cells found in the spleen (Figure 3A–D) was similar among all groups, demonstrating that T cell engraftment after adoptive transfer was similar between the sexes.
Figure 3. Effect of Ang II infusion on splenic and renal T-lymphocyte infiltration into male and female Rag-1−/− mice.
Similar splenic infiltration of adoptively transferred CD3+ (A), CD3+-CD4+ (B), CD3+-CD4+-Foxp3+ (C) and CD3+-CD8+ (D) T lymphocytes at the time of sacrifice indicates equal engraftment occurred in both sexes. Male mice demonstrated greater renal infiltration of CD3+ (E), CD3+-CD4+ (F), CD3+-CD4+-Foxp3+ (G) and CD3+-CD8+ (H) T-lymphocytes compared to females at the time of sacrifice, and this sex difference was not affected by Ang II infusion. *p<0.05.
Basal renal T cell infiltration for all T cell subtypes measured was greater in the male compared to the female Rag-1−/− host after adoptive transfer of male T cells (Figure 3E–H); however, Ang II infusion had no effect on renal T cell infiltration in either sex.
Flow cytometric analysis from whole brain homogenates demonstrated a trend for greater T cell infiltration in male compared to female Rag-1−/− mice; however this trend did not reach significance (Figure 4A–D). Furthermore, similarly to the kidney, there was no effect of Ang II on the number of brain infiltrating T cells.
Figure 4. Effect of Ang II infusion on whole brain and SFO T-lymphocyte infiltration into male and female Rag-1−/− mice.
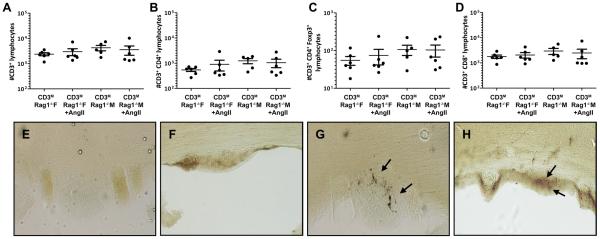
Whole brain homogenate CD3+ (A), CD3+-CD4+ (B), CD3+-CD4+-Foxp3+ (C) and CD3+-CD8+ (D) T-lymphocyte infiltration did not differ between sex or among treatment groups. Representative images of CD3+-stained SFO sections demonstrate attenuated infiltration in female (E, F) compared to male (G, H) mice. Slices shown for each sex are from two different animals.
Sex differences were observed in the Ang II-induced inflammatory response in Rag-1−/− mice after adoptive transfer of male T lymphocytes
Ang II-induced renal inflammation contributes to the hypertensive response in male mice, and is markedly attenuated in females8,9,18. To determine whether altered T cell function contributes to the reduction in Ang II-induced renal inflammation in females, we measured renal expression of the inflammatory cytokines IL-2, TNF-α and MCP-1 using quantitative real time PCR in male and female Rag-1−/− mice ± T cell transfer (Table 2). Renal expression of IL-2, TNF-α and MCP-1 were significantly upregulated by Ang II infusion in CD3M→Rag1−/−-M compared to Rag1−/−-M. Conversely, there were no differences in the expression of these cytokines between CD3M→Rag1−/−-F and Rag1−/−-F after 14d Ang II infusion, suggesting that during Ang II infusion, T cell-dependent renal inflammation is attenuated in females, and may contribute to the female resistance against Ang II-induced hypertension.
Table 2.
Ang II-induced renal cytokine mRNA expression in male and female Rag-1−/− mice
| Male | Female | |||
|---|---|---|---|---|
|
| ||||
| Gene | Rag1−/−+Ang II | CD3M→Rag1−/−+Ang II | Rag1−/−+Ang II | CD3M→Rag1−/−+Ang II |
| TNF-α | 1.00 ± 0.15 | 2.87 ± 0.5* | 1.00 ± 0.19 | 0.68 ± 0.27 |
| MCP-1 | 1.00 ± 0.33 | 2.08 ± 0.33* | 1.00 ± 0.16 | 0.99 ± 0.25 |
| IL-2 | 1.00 ± 0.21 | 2.44 ± 0.36* | 1.00 ± 0.34 | 0.72 ± 0.25 |
Sex differences were observed in T cell infiltration into the SFO of Rag-1−/− mice after adoptive transfer of male T cells and Ang II infusion
Activation of inflammatory processes within the SFO contribute to maintaining Ang II-induced hypertension24,25,26. These inflammatory processes such as Ang II-induced production of reactive oxygen species are inhibited by activation of estrogen receptors27,28. Immunohistochemical techniques were employed to determine if sex differences exist in T cell infiltration into the SFO of Rag-1−/− mice following adoptive transfer of male T cells and Ang II infusion. Photomicrographic visualization demonstrated the degree of CD3+ positive staining in the SFO was greater in male compared to female Rag-1−/− mice following adoptive transfer of male T cells and Ang II infusion (Figure 4E–F).
Sex differences in Ang II-induced glomerular hypertrophy were independent of T cells in Rag-1−/− mice
Glomerular hypertrophy is an early physiologic adaptation of the kidney during hypertension29. Thus, we compared glomerular areas in PAS-stained renal sections to determine if sex differences exist in the susceptibility of the Rag-1−/− host to glomerular hypertrophy after adoptive transfer of male T cells and Ang II infusion (Figures 5A–F). Quantification via ImageJ software revealed that the glomerular area was significantly greater in male compared to female Rag-1−/− mice at baseline (Figure 5G). Ang II infusion caused significant glomerular hypertrophy in both males and females. The glomerular area was greater in the male compared to the female Rag-1−/− host after adoptive transfer of male T cells and Ang II infusion; however, adoptive transfer of male T cells had no impact on the magnitude of the glomerular hypertrophy induced by Ang II in either sex.
Figure 5. Effect of Ang II infusion on renal morphological changes in male and female Rag-1−/− mice with and without male T cell adoptive transfer.
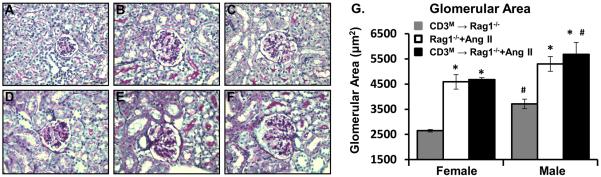
Representative images of PAS-stained renal sections from A. CD3M→Rag1−/−-F, B. Rag1−/−-F+Ang II C. CD3M→Rag1−/−-F+Ang II and D. CD3M→Rag1−/−-M, E. Rag1−/−-M+Ang II F. CD3M→Rag1−/−-M+Ang II. G. Quantification shows that Ang II infusion induced significant glomerular hypertrophy in both males and females; however, this effect was independent of the presence of T cells. *p<0.05 vs. CD3M→Rag1−/−, same sex; #p<0.05 vs. female, same group.
Discussion
The results of this study identify a sex difference in the ability of the adaptive immune system to facilitate Ang II-induced hypertension. The major findings of the present study are: 1) male CD3+ T cell adoptive transfer facilitates a significantly greater Ang II-induced increase in SBP in male compared to female Rag-1−/− mice; 2) Renal T cell infiltration after T cell adoptive transfer is significantly greater in male versus female Rag-1−/− mice; however, this effect was independent of Ang II infusion; and, 3) After adoptive transfer, there was a tendency for increased T cell infiltration in whole brain homogenates of male versus female Rag-1−/− mice and examination of the SFO region identified higher numbers of T cells in males versus females. These results suggest that the pro-hypertensive effects of male T cells on Ang II-induced hypertension are inhibited in the female host and that reduced T cell infiltration of the kidneys and brain could protect females against the hypertensive actions of Ang II.
Emerging evidence concerning the importance of the adaptive immune system in the development of hypertension has been supported from a number of different laboratories and models of hypertension. Guzik et al.3 were among the first to report that male mice lacking T-lymphocytes have a reduced hypertensive response to both Ang II and DOCA-salt. Further, these studies demonstrated that adoptive transfer of T cells, but not B cells, could restore the hypertensive effects of Ang II and DOCA-salt. Similar results on the role of lymphocytes in Ang II-induced hypertension have also been reported by others; however all of these studies were performed in male animals8,9. The present study confirmed and expanded these results by comparing the role of T cells in Ang II-induced hypertension in both sexes. We found that in male Rag-1−/− mice, adoptive transfer of male CD3+ T cells markedly enhanced the hypertensive response to a 14-day infusion of Ang II (490 ng/kg/min) as compared to their response in the absence of T cells. Similar to what has been previously reported, this suggests that the presence of T cells is essential for the full development of hypertension in male mice. However, in females the effect of T cells was absent. The mechanism responsible for the protection of females from the pro-hypertensive effect of T cells is currently unknown.
Previously, we reported that there are sex differences in the ability of Ang II to induce an increase in sympathetic outflow28,30. Following 14 days of Ang II infusion, the drop in MAP during ganglionic blockade with hexamethonium was greater in males than in females, suggesting that the higher levels of hypertension in males were a result of larger increases in sympathetic outflow in response to Ang II infusion. Harrison and colleagues have recently suggested that an increase in sympathetic outflow may be required for T cells to facilitate Ang II-induced hypertension31. Indeed, the removal of the central sympathoexcitatory sites of action for circulating Ang II, via lesioning of the anteroventral third cerebral ventricle (AV3V), prevented not only the hypertension but also Ang II-induced activation of circulating T cells, and vascular inflammation. This study thus suggested that Ang II-induced increases in sympathetic outflow are necessary for the full expression of its proinflammatory effects and T cell activation. In the present study, the inability of male T cells to facilitate Ang II-induced hypertension in female Rag-1−/− mice may be due to reduced levels of sympathoexcitation following Ang II infusion in females compared to their male counterparts30.
Alternatively, the relatively high levels of 17β-estradiol found in cycling females may inhibit the formation of neoantigens, which are purportedly necessary for the hypertensive properties of T cells. This notion is supported by the biphasic model of hypertension proposed by Marvar et al.31, which suggests that increased sympathetic outflow during Ang II infusion may only be directly responsible for an initial modest increase in blood pressure, and that the formation of neoantigens induced by this rise in blood pressure causes the activation and infiltration of T cells to the kidney and vasculature, subsequently increasing cytokine release and promoting the genesis of severe hypertension. In the current study, the T cell-dependent BP response exhibited by males supports this two-phase hypothesis. The hypertensive response to Ang II in both female groups were similar to that in T cell-deficient males, suggesting that the observed protection in females from the T cell-dependent severe hypertension may be the result of a lack of neoantigen formation or T cell activation. Additional studies are necessary to further examine this hypothesis.
Lymphocyte infiltration of the kidney has also been linked with the development of hypertension8,9,10,32,33. It has been suggested that the infiltration of T cells into the kidney in salt-sensitive hypertension, Ang II-induced hypertension and in the spontaneous hypertensive rat (SHR)34 results in increased renal inflammation, including increased renal cytokine and reactive oxygen species production. Subsequent inhibition of the inflammatory response with the immunosuppressive drug, mycophenolate mofetil, reduced hypertension and renal T cell infiltration. Importantly, most of these studies investigating the role of renal T cell infiltration in the development of hypertension were conducted in male animals. However, a recent study by Tipton et al.35 investigated sex differences in renal T cell infiltration in the SHR rat. They reported that lymphocytes are necessary for the development of hypertension in the SHR and that there is a significant difference in the T cell profile in the kidneys between males and females. In these studies, females had greater numbers of CD8+ and T regulatory cells than males, while males had greater numbers of CD4+ and Th17 T cell infiltration. After adoptive transfer of male T cells, the male Rag-1−/− host exhibited significantly greater basal infiltration of renal CD4+, CD8+, and Foxp3+ T cells compared to the female host, despite similar blood pressure between the sexes. Although we did not detect greater T cell infiltration after Ang II infusion in either male or female Rag-1−/− mice, it is possible that Ang II increased the number of activated T cells and that sex difference in the number of activated T cells contributed to the augmented hypertensive response in the male compared to the female host.
The observed sex differences in renal T cell infiltration is likely to be influenced by sex differences in the host hormonal milieu. Upon transfer of male T cells into the female host, the cells become exposed to an ovarian hormone milieu. Thus, the potential for an immunogenic response exists and the female host receiving male T cells could reject the transferred cells due to the presence of the male H-Y antigen. However, FACS analysis of splenic T lymphocyte infiltration confirmed that the transfer of male T cells into female Rag-1−/− recipients was well tolerated and did not generate an immunogenic response, at least over the course of the 5 week experiment. Additionally, the pre-transfer environment of the donor mouse can significantly affect the recipient's hypertensive response7. Because of the known pro-hypertensive properties of male T cells in the genesis of Ang II hypertension, the inability of male T cells to generate a full hypertensive response in female recipients strongly suggests that female resistance to Ang II-induced hypertension involves inhibition of T cell-mediated processes. The mechanisms responsible for preventing T cell-mediated Ang II hypertension in females are unknown, but are likely to involve hormonal differences between the sexes12,13,14.
In a number of different experimental models, Reckellhoff and colleagues36,37,38,39 have shown that testosterone is an important mediator of hypertension and renal injury in males, and that castration slows the onset of hypertension and related renal damage. Recently, a series of studies have offered convincing support for the hypothesis that androgens act in the kidney to increase 20-hydroxyeicosatetraenoic acid (20-HETE), thereby activating an inflammatory cascade that alters renal vascular reactivity and results in the development of hypertension40,41,42. In our studies, adoptively transferred T cells in male Rag-1−/− mice infiltrated the kidney in the absence of Ang II or hypertension. Based on the above mentioned studies into the effects of androgen on 20-HETE production, we hypothesize that our observed sex differences in basal renal T cell infiltration could be due to a higher baseline inflammation, induced by higher testosterone levels in males, which thus serve as a signal for T cell trafficking and predisposing them to renal tissue T cell infiltration. Future studies are needed to clarify the role of circulating androgens in renal T cell infiltration.
We also observed sex differences in T cell trafficking into the brain, specifically into the SFO. The mechanisms responsible for the sex differences in T cell infiltration of the SFO are unknown. In previous studies of Ang II-induced hypertension in mice, we have shown that central infusion of 17β-estradiol attenuates Ang II-induced hypertension in both males and ovariectomized females21,27, suggesting that 17β-estradiol acting on the brain can inhibit Ang II-induced increases in sympathetic outflow and hypertension. We have also shown that 17β-estradiol inhibits Ang II-induced increases in SFO reactive oxygen species43. Thus, differences in circulating 17β-estradiol levels may be one potential mechanism underlying the protection of females from T cell infiltration into the SFO. Future studies are needed to characterize the effects of 17β-estradiol on T cell infiltration into the kidney and SFO, and its ability to facilitate Ang II hypertension.
Supplementary Material
Perspectives.
The present studies suggest that understanding the role of the adaptive immune system in the development of hypertension requires studies be conducted in both male and female animal models. Of the multiple factors that are known to contribute to the development of hypertension, the sex of the subject being studied must be taken into consideration. The clear sex differences that were observed in these studies highlights that translation of our understanding of the underlying physiology of hypertension to the development of new antihypertensive therapies for both men and women will require ongoing physiological studies to include data from both sexes.
Novelty and Significance.
What is New?
There is growing evidence for a critical role of the adaptive immune system in the genesis of hypertension in males. We demonstrate for the first time that the ability of the adaptive immune system to induce hypertension is blunted in females, and coincides with a reduction in T lymphocyte infiltration in the kidney.
What is Relevant?
Females are protected against the genesis of hypertension prior to menopause, however the mechanisms responsible for this protection are unclear.
Summary
Females are protected from the hypertensive properties of T lymphocytes compared to males. Targeting these cells may prove to be an effective treatment against hypertension.
Figure 1. Experimental protocol.
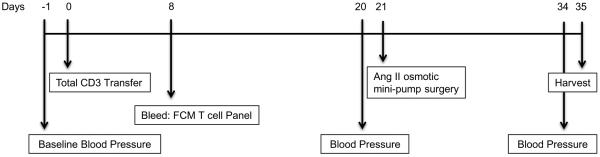
Time line of BP & HR recordings, male T cell adoptive transfer, Ang II infusion and tissue harvesting in male and female Rag-1−/− mice.
Acknowledgments
Sources of Funding This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK073611 (to HLB) and National Institute of Health grant T32 HL007249 (DPP), University of Arizona Sarver Heart Center Heart Disease in Women Research Grant (DPP), and Achievement Rewards for College Scientists (ARCS®) award (DPP).
Footnotes
Disclosures None.
References
- 1.Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33:89–94. doi: 10.1016/j.neubiorev.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benigni A, Cassis P, Remuzzi G. Angiotensin ii revisited: New roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating t lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300:F734–742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin ii concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 11.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of t-lymphocyte activation and vascular inflammation produced by angiotensin ii-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 13.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14:254–260. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman RK, Azar AS, Mulvaney JM, Hinojosa-Laborde C, Haywood JR, Brooks VL. Baroreflex sensitivity varies during the rat estrous cycle: Role of gonadal steroids. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1419–1426. doi: 10.1152/ajpregu.91030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue B, Gole H, Pamidimukkala J, Hay M. Role of the area postrema in angiotensin ii modulation of baroreflex control of heart rate in conscious mice. Am J Physiol Heart Circ Physiol. 2003;284:H1003–1007. doi: 10.1152/ajpheart.00793.2002. [DOI] [PubMed] [Google Scholar]
- 17.Ji H, Zheng W, Menini S, Pesce C, Kim J, Wu X, Mulroney SE, Sandberg K. Female protection in progressive renal disease is associated with estradiol attenuation of superoxide production. Gend Med. 2007;4:56–71. doi: 10.1016/s1550-8579(07)80009-x. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor gpr30 reduces oxidative stress and proteinuria in the salt-sensitive female mren2.Lewis rat. Hypertension. 2011;58:665–671. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reckelhoff JF, Maric C. Sex and gender differences in cardiovascular-renal physiology and pathophysiology. Steroids. 2010;75:745–746. doi: 10.1016/j.steroids.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Maric C, Xu Q, Sandberg K, Hinojosa-Laborde C. Age-related renal disease in female dahl salt-sensitive rats is attenuated with 17 beta-estradiol supplementation by modulating nitric oxide synthase expression. Gend Med. 2008;5:147–159. doi: 10.1016/j.genm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin ii-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–1776. doi: 10.1152/ajpheart.01011.2005. [DOI] [PubMed] [Google Scholar]
- 22.Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–262. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- 23.Xue B, Beltz TG, Johnson RF, Guo F, Hay M, Johnson AK. Pvn adenovirus-sirna injections silencing either nox2 or nox4 attenuate aldosterone/nacl-induced hypertension in mice. Am J Physiol Heart Circ Physiol. 2012;302:H733–741. doi: 10.1152/ajpheart.00873.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the nadph oxidases in the subfornical organ in angiotensin ii-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G, Sarkar P, Peterson JR, Anrather J, Pierce JP, Moore JM, Feng J, Zhou P, Milner TA, Pickel VM, Iadecola C, Davisson RL. Cox-1-derived pge2 and pge2 type 1 receptors are vital for angiotensin-ii-induced formation of reactive oxygen species and ca2+ influx in the subfornical organ. Am J Physiol Heart Circ Physiol. 2013;305:H1451–H1461. doi: 10.1152/ajpheart.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin ii-dependent hypertension requires cyclooxygenase 1-derived prostaglandin e2 and ep1 receptor signaling in the subfornical organ of the brain. Hypertension. 2012;59:869–876. doi: 10.1161/HYPERTENSIONAHA.111.182071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin ii-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;295:H1025–H1032. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue B, Singh M, Guo F, Hay M, Johnson AK. Protective actions of estrogen on angiotensin ii-induced hypertension: Role of central nitric oxide. Am J Physiol Heart Circ Physiol. 2009;297:H1638–1646. doi: 10.1152/ajpheart.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens. 2008;17:266–270. doi: 10.1097/MNH.0b013e3282f88a1f. [DOI] [PubMed] [Google Scholar]
- 30.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin ii-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 31.Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol. 2011;11:156–161. doi: 10.1016/j.coph.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quiroz Y, Johnson RJ, Rodríguez-Iturbe B. The role of t cells in the pathogenesis of primary hypertension. Nephrol Dial Transplant. 2012;27(Suppl 4):iv2–5. doi: 10.1093/ndt/gfs421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2012;39:96–103. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 35.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory t lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol. 2012;303:R359–R367. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reckelhoff JF, Roman RJ. Androgens and hypertension: Role in both males and females? Hypertension. 2011;57:681–682. doi: 10.1161/HYPERTENSIONAHA.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–131. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- 38.Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen-induced oxidative stress on hypertension in male shr. Am J Physiol Regul Integr Comp Physiol. 2007;292:R731–735. doi: 10.1152/ajpregu.00353.2006. [DOI] [PubMed] [Google Scholar]
- 39.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:F771–779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1055–1062. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 41.Ding Y, Wu CC, Garcia V, Dimitrova I, Weidenhammer A, Joseph G, Zhang F, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. 20-hete induces remodeling of renal resistance arteries independent of blood pressure elevation in hypertension. Am J Physiol Renal Physiol. 2013;305:F753–763. doi: 10.1152/ajprenal.00292.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu CC, Schwartzman ML. The role of 20-hete in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin ii-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;295:H1025–H1032. doi: 10.1152/ajpheart.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.