Abstract
Skin aging results in increased susceptibility to injury and impaired wound healing. Proliferation of fibroblasts is reduced in aged dermis, which contributes to delays in wound closure. Age-associated differences are regulated, in part, by local or systemic factors such as the IGF-1/IGF1R system. The aim of this study was to determine if expression and activation of IGF1R in aged human dermal fibroblasts, when compared to young fibroblasts, is associated with altered proliferative capacity in a 3D collagen matrix that better simulates the dermal extracellular matrix in vivo. The proliferation of young and aged human dermal fibroblasts in 3D collagen and its association with baseline levels of IGF1R expression were measured. The effect of stimulation and inhibition of Erk phosphorylation on the proliferative capacity of fibroblasts in a 3D collagen matrix was defined.
Our results show that proliferation and Erk phosphorylation is reduced in aged dermal fibroblasts relative to young fibroblasts. Activation of Erk phosphorylation in aged fibroblasts is associated with a significant increase in fibroblast proliferation in 3D collagen.
Introduction
Skin aging results in increased susceptibility to injury, reduced wound healing (McCullough and Kelly 2006) and increased risk of wound dehiscence and infection (Farage et al. 2009). Age-associated differences in dermal proliferation, thickness, follicle patterning, and immune cell abundance are regulated, in part, by local or systemic factors such as Insulin like Growth Factor 1 (IGF-1) (Giangreco et al. 2008). IGF-1 is a mitogenic factor for dermal fibroblasts (Martin 1997) and promotes the interaction of dermal fibroblasts with the extracellular matrix during tissue injury and repair (Lewis et al. 2010). The effects of IGF-1 are mediated by Insulin like Growth Factor-1 Receptor (IGF1R), a ubiquitous cell-surface tyrosine kinase receptor (Werner et al. 2008). The role of IGF-1/IGF1R in longevity is well established (Junnila et al. 2013). Moreover, IGF-1 levels (Park and Buetow 1991) and IGF1R expression (Sonntag et al. 1999) decrease in many organs with age. Patients with primary IGF-1 deficiency demonstrate signs of early skin aging, such as dry, thin and wrinkled skin (Zouboulis and Makrantonaki 2011).
Dermal fibroblasts are the primary cell type utilized to study proliferative responses that are relevant to cutaneous healing. Punch biopsies obtained repeatedly over the life span found that in vitro proliferative capacity of dermal fibroblasts mimics wound repair in vivo (Bruce and Deamond 1991). Fibroblasts in the dermis are surrounded by a 3 dimensional (3D) extracellular matrix comprised primarily of collagen I. It is now understood that cells in a 3D matrix are distinct from cells cultured on tissue culture plastic both morphologically (Cukierman et al. 2001) as well as in their molecular composition (Zamir et al. 1999). Consequently, the study of fibroblasts in 3D collagen is thought to better represent cellular behaviors in vivo (Damodarasamy et al. 2010; Egles et al. 2010; Fisher et al. 2009; Reed et al. 2001; Shi et al. 2010).
The aim of this study was to evaluate if expression and activation of IGF1R and its main downstream signaling pathways are associated with the proliferative capacity of aged and young fibroblasts. In addition, this study was novel in that it was conducted in 3D collagen, a better simulation for examining cellular processes relevant to dermal repair in vivo.
Methods
Cell lines
Early passage human dermal fibroblasts from six healthy aged male donors (mean age=83yrs), AG04152 (82yrs), AG04382 (81yrs), AG04383 (80yrs), AG04387 (80yrs), AG04064 (92yrs), AG04057 (81yrs) and five healthy young males (mean age=29yrs) AG13153 (30yrs), AG11747 (22yrs), AG11242 (30yrs), AG05415 (29yrs), AG10046 (31yrs), AG08790 (31yrs) (NIA Aging Cell Repository, Coriell Institute) were grown as previously described (Reed et al. 2001).
Determining young and aged phenotype
Expression of p21 and p16
Total cellular RNA was isolated from fibroblasts plated in 5% DMEM o/n using Trizol (Invitrogen, Grand Island, NY). RNA purity and integrity was assessed by spectrophotometric analysis. A total of 1 μg of RNA was reverse-transcribed using an iScript kit (Bio-Rad Laboratories, Hercules, CA). RT-PCR was performed using an ABI 7900 RT-PCR instrument with SYBR Green Master Mix (Bio-Rad) for human P16, p21.
P16 F: cgccatttgctagcagtgtga P16 R: acattcatgtgggcatttc
P21F:atgaaattcaccccctttcc P21R: aggtgaggggactccaaagt
GAPDH F: ggcctccaaggagtaagacc GAPDH R: aggggtctacatggcaactg
All experiments were performed in triplicate and normalized to GAPDH mRNA (Turabelidze et al. 2010). Fluorescent signals were analyzed during each of 40 cycles consisting of denaturation (95°C, 15 seconds), annealing (54°C, 15 seconds). Relative quantitation was calculated using the comparative threshold cycle method. Expression of p21 protein (Santa Cruz, Dallas, TX) by western blots was performed as described below.
Senescence associated β-gal
Fibroblasts were grown to subconfluence on 35 mm cell culture dishes, washed with PBS, and fixed with 2% Formaldehyde/2%glutaraldehyde in PBS for 10 min. After 2 rinses in PBS, cells were incubated with beta-galactosidase substrate staining solution (150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 40 mM citric acid, and 12 mM sodium phosphate, pH 6.0, containing 1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside)) (Invitrogen) for 24 h at 37°C. Positive cells were identified by blue/green-staining, and % positive cells were calculated from the number of positive cells divided by the total number of cells (Dimri et al. 1995).
Cell area
Approximately 100 fibroblast cells were plated in chamber slides in 5% DMEM o/n. Cells were fixed with 10% neutral buffer formalin for 10 min and stained with 2% crystal violet after 24 h and imaged. Cell area was calculated using imageJ.
Eleven cell lines were able to grow in DMEM with 5% FBS: AG13153 (25, 10); AG11747 (34, 16); AG11242 (38, 9); AG10046 (23, 11); AG5415 (38, 21); AG04383 (38, 14); AG04382 (47, 14); AG04152 (38, 12); AG04387 (24, 11); AG04057 (33, 9); AG04064 (50, 16) (for each cell line the numbers in parenthesis are the replicative potential passage number (until terminal senescence (Reed et al. 2001)) and the passage number used for figure 1, respectively). Cell lines were ranked in order from highest to lowest for each marker of aging and each line was given an average rank score. When cell lines had equivalent average rank scores, the line with the lowest (for young fibroblasts) or highest (for aged fibroblasts) chronological age was chosen.
Figure 1. Screening fibroblast cell lines for young and aged phenotypes.
Expression of p16, p21, senescence associated β-gal and measures of cell area were performed. Results for each cell line are presented followed in parentheses by the relative rank (from highest to lowest) of the cell line in relation to the other cell lines. The last column is the average rank score for all four measurements. Three fibroblast cell lines with the highest average ranking were from aged donors (AG04383, AG04387, AG04064) and the three with the lowest ranking were from young donors (AG11747, AG11242, AG5415).
Preparation of collagen extracts and 3D collagen gels
Young adult (5-6 mo) C57Bl/6 male mice were obtained from the NIA Rodent Colony (aged mouse colony, Charles River Laboratories, Wilmington, MA). For each extraction, an equivalent wet weight of tendon tissue was isolated from each euthanized mouse. The isolated tendons from 5 young mice were pooled, hydrated briefly in phosphate- buffered saline (PBS), rinsed in acetone and 70% isopropanol, macerated, and stirred gently overnight at 4°C in 0.05 N acetic acid. Subsequently, collagen extracts were centrifuged for 15 min at 4,000 × g to remove undissolved material. Total protein content was quantified by a BCA assay (Thermo Fisher Scientific Inc., Waltham, MA) and specific collagen content was quantified by the Sircol assay (Accurate Chemical and Scientific Corp., Westbury, NY).
Gels were prepared from collagen extracts by combining 1 volume of extract, 1/9 volume of 10-strength NaHCO3- saturated Medium 199 (Invitrogen Corp., Grand Island, NY ) and sufficient Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen Corp., Grand Island, NY), and FBS to yield a solution with final collagen and FBS concentrations of 0.6 mg/ml and 1%, respectively. Polymerization of the collagen solution was carried out by a 60-min incubation at 37°C and 100% humidity (Damodarasamy et al. 2010). For purposes of analyzing cell number, cells were placed on the polymerized 3D collagen, which has been shown to elicit changes similar to being in the 3D matrix (Haas et al. 1998).
Cell proliferation assays
2D:
Each fibroblast cell line was plated overnight in 5% DMEM in triplicate and proliferation measured at 24, 48 and 72 h using cell titer proliferation reagent (Promega, Madison, WI).
3D:
Collagen gels (35 μl volume) were polymerized in each well of a 96-well plate. Subsequently, an additional 100 μl of each fibroblast cell line in triplicate were plated on top of each gel at a concentration of 5 × 104 cells/ml. The number of cells growing on the gel were measured after 24, 48 and 72 h of culture with a Cultrex® 3D Culture Cell Proliferation Assay Kit (Trevigen Inc., Gaithersburg, MD).
IGF1R expression
An enzyme-linked immunosorbent assay (ELISA) was used to quantify IGF1R expression in 3D collagen matrix, as western blots were not sufficiently sensitive to detect baseline levels. Briefly, after 48 h fibroblasts in 3D collagen were pelleted by centrifugation and collected. The collagen was removed and the cell pellet lysed in NP40 buffer with appropriate protease and phosphatase inhibitors (Sigma, Saint Louis, MO). Samples were analyzed by the Omnikine Human IGFR-1R ELISA kit using a sandwich ELISA method as per the manufacturer's protocol (Assay Biotech, Sunnyvale, CA).
Western blots
Fibroblasts were plated on 3D collagen gels. After 48 h, collagen gels were centrifuged at 12,000g for 10min at 4°C. The supernatant (which includes the collagen) was removed and the cell pellets were lysed in NP40 buffer with appropriate protease and phosphatase inhibitors (Sigma, Saint Louis, MO). Lysates were prepared for electrophoresis as described (Shi et al. 2010). Equal amounts of protein were subjected to 10% SDS-PAGE, followed by transfer to nitrocellulose. After incubation with 5% milk, blots were probed with antibodies (1-5μg/ml) against Insulin Like Growth Factor 1 Receptor (IGF1R), phospho-IGF1R, Erk1, phospho-Erk1/2 (Thr202/Tyr204), Akt, phospho-Akt (Ser473) and normalized to GAPDH (Cell Signaling, Danvers, MA and Santa Cruz, Dallas, TX).
IGF-1 stimulation and MAPK inhibition
Aged and young fibroblasts were placed on a 3D matrix as described and serum starved overnight (DMEM + FBS 0.5%). To determine the optimal doses, preliminary experiments in which cells were exposed to IGF-1 (1ng/ml-100ng/ml, R&D systems) and UO126 (10uM-100uM, Sigma) in a range of concentrations were used to determine the lowest doses that produced Erk phosphorylation (IGF-1@50ng/ml) and inhibition of Erk phosphorylation (UO126@50um) in 3D collagen. Proliferation assays and western blots were performed as described above. For inhibition of Akt phosphorylation we have used LY294002 (Sigma).
Statistics
Tests for significance between young and aged groups, and with and without stimulation with IGF-1 were performed using a 2 tailed students t-test with unequal variance. Significance was defined by p<0.05.
Results
Screening fibroblast cell lines for young and aged phenotypes
In order to select three cell lines that represent a young adult phenotype and three cell lines that represent an aged adult phenotype, we screened the eleven cell lines for established changes in expression of markers (increased p16 and p21, senescence associated β-gal) and cell morphology (larger cell area) that are associated with aging. Although we are not studying the presence or absence of senescent cells (Lee et al. 2006), accumulation of senescence associated β-gal, as well as increases in levels of the Cyclin-Dependent Kinase Inhibitors p21 and p16, are often observed during cellular aging (Campisi 2005). Cell areas of cultured fibroblasts from aged hosts are larger than from young hosts and are another marker of cellular aging (Schneider and Mitsui 1976). Relative expression of p21 protein levels was higher in the six aged cell lines when compared to the five young cell lines (p=0.03 data not shown). Results of the screening are presented in Figure 1. Not surprisingly, the three fibroblast lines with the lowest ranking for features of aging were from young donors (AG11747, AG11242, AG5415) and the 3 lines with the highest average ranking for aging were from aged donors (AG04383, AG04387, AG04064). As these 6 dermal fibroblast lines well-represented key features of young and aged cells, respectively, these cells were used in the subsequent experiments.
Proliferative capacity on 3D collagen, but not on 2D tissue culture plastic, is highly correlated with IGF1R expression
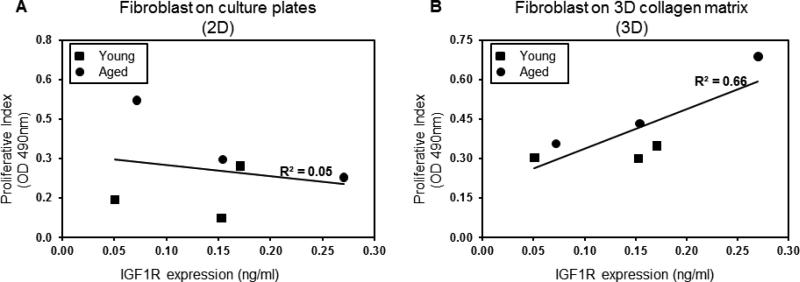
Regardless of the donor age, dermal fibroblasts lose their chronologic phenotype with respect to replicative capacity when proliferation is measured in vitro (Cristofalo et al. 1998). IGF1R expression is an important determinant of fibroblast proliferation in the skin (Okubo et al. 2003) as well as in extra-dermal organs (Reiss et al. 1995). We sought to test the utility of a three dimensional milieu, relative to traditional tissue culture plastic, to simulate the behavior of fibroblasts in the ECM in vivo. For each cell line, proliferation at 72 h reflected proliferation at 24 h and 48 h, in both 2D and 3D conditions. The proliferative capacity (as measured at 72 h relative to time 0) in a flat, two-dimensional tissue culture plate (2D) or in a 3D collagen matrix was compared to IGF1R expression for each of the fibroblast cell lines. The results demonstrated that proliferation on 2D tissue culture plastic is not correlated with IGF1R expression (R2=0.05 Figure 2a.) (R2 or coefficient of determination, is a dimensionless value between 0 and 1 in which 1 is full correlation and 0 is no correlation), but proliferation on a 3D collagen matrix was highly correlated with IGF1R expression (R2=0.66 Figure 2b.)
Figure 2. Proliferative capacity on 3D collagen is highly correlated with IGF1R expression.
Proliferation of human dermal fibroblasts on 2D tissue culture is not correlated with IGF1R expression (R2=0.05 Figure 2a.). In contrast, proliferative activity of the same cells on a 3D collagen matrix was highly correlated with IGF1R expression (R2=0.66 Figure 2b). R2, or coefficient of determination, is a dimensionless value between 0 and 1 in which 1 is full correlation and 0 is no correlation. Cell lines from aged donors are represented by circles, cell lines from young donors are represented by squares.
Baseline Erk phosphorylation is reduced in aged fibroblasts in a 3D collagen matrix
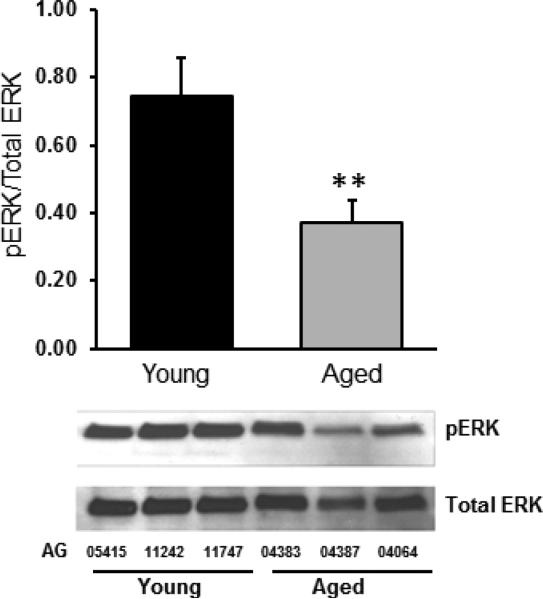
Since baseline levels of IGF1R were low, it was difficult to evaluate phosphorylation of the IGF1R as a measure of receptor activation. We therefore evaluated the phosphorylation of two key intracellular signals that are activated by IGF1R: the Mitogen-Activated Protein Kinase (MAPK) pathway and the phosphoinositide 3-kinase (PI3K) pathway. To evaluate activation of the MAPK pathway we measured total and phosphorylated Ras-dependent extracellular signal-regulated kinase (Erk). PI3K was measured by Akt and phosphorylated Akt as discussed below. Baseline expression of total Erk was not different between aged and young fibroblasts. Phosphorylated (active) fraction of Erk (expressed as the fraction of phosphorylated Erk from total Erk), in the aged fibroblasts was approximately half that of young fibroblasts (0.37 vs 0.75 *=p<0.002, Figure 3).
Figure 3. Erk phosphorylation is reduced in aged fibroblasts in a 3D collagen matrix.
Baseline phosphorylated (active) fraction of Erk (expressed as the fraction of phosphorylated Erk from total Erk) in the aged fibroblasts was approximately half that of young fibroblasts (0.37 vs 0.75, ** p<0.002). Lower panel shows a representative western blot from the three aged donors (AG04383, AG04387, AG04064) and the three young donors (AG11747, AG11242, AG5415).
Proliferation of fibroblasts on a 3D collagen matrix is correlated with Erk phosphorylation
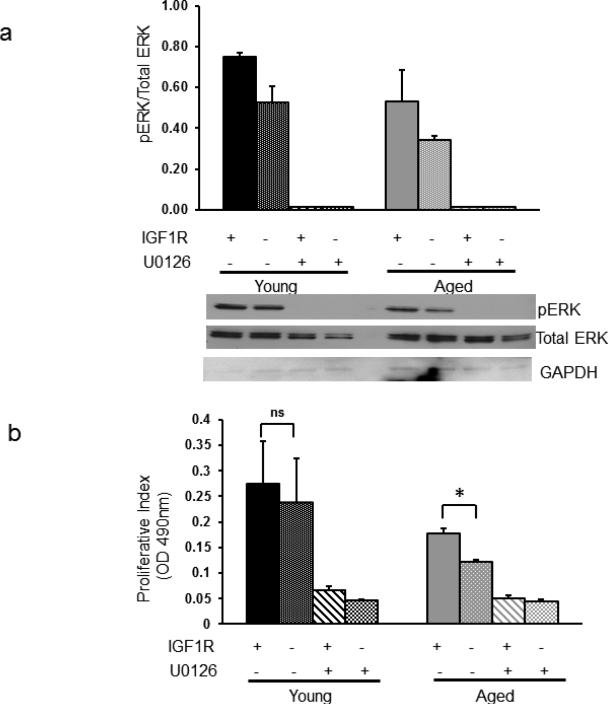
To determine whether Erk phosphorylation levels are associated with changes in proliferation of fibroblasts in 3D collagen matrix, we increased and decreased Erk phosphorylation with exposure to a stimulator (IGF-1) and an inhibitor (UO126) of Erk phosphorylation (Figure 4A). Increasing Erk phosphorylation by stimulation with IGF-1 (50ng/ml) in aged fibroblasts significantly increased proliferation (p=0.01, Figure 4B). Addition of UO126 (50uM) reduced proliferation when cells were exposed to IGF-1 and when treated with UO126 alone. The effect of IGF-1 on young fibroblasts was a non-significant increase in phosphorylation and proliferation (p=0.78). As expected inhibition of Erk phosphorylation reduced proliferation in young fibroblasts similar to that seen in aged fibroblasts. Phosphorylation of Akt after serum starvation and IGF-1 stimulation in young fibroblasts was slightly higher when compared to aged fibroblasts, but the difference was not significant (p=0.53). Furthermore, inhibition of Akt phosphorylation (with LY294002) did not reduce proliferative capacity in the fibroblasts (data not shown).
Figure 4. Proliferation of fibroblasts on a 3D collagen matrix is correlated with Erk phosphorylation.
Figure 4a. Stimulation with IGF-1 (50ng/ml) increased Erk phosphorylation in human dermal fibroblasts. Exposure to UO126 (50uM) almost completely blocked Erk phosphorylation without changing the expression of total Erk. Cells were exposed to four conditions (from left to right in each group: 1). Stimulation with IGF-1; 2). No stimulation or inhibition; 3). Stimulation with IGF-1 and inhibition with UO126; 4). Inhibition with UO126. Upper panel shows average results with standard deviations for the three young donors and the three aged donors. Lower panel shows a representative western blot. Figure 4b. Stimulation with IGF-1 (50ng/ml) in aged fibroblasts significantly increased proliferation (* p=0.01). The effect of IGF-1 on young fibroblasts was a non-significant (ns, p=0.78) increase in proliferation. Addition of UO126 (50uM) reduced proliferation of young and aged fibroblast when exposed to IGF-1 and when treated with UO126 alone. Average results with standard deviations for the three young donors and the three aged donors are shown.
Discussion
The availability and reproducibility of human dermal fibroblasts are invaluable to studies of wound healing and have led to key discoveries in the biology of aging, such as the Hayflick limit (Shay and Wright 2000). We used four key measures of cellular aging (p21, p16, senescence associated β-gal and cell size), to identify those dermal cell lines that best represented the young and aged phenotypes. The four different characteristics were weighted equally as no single marker is sufficiently indicative. The close match of the characteristics with chronological age (cell lines with aged phenotype were from aged donors and those with young phenotypes were from young donors) allowed us to proceed with cells that were best representative of aging in vivo. Interestingly, none of the markers reliably predicted the chronological age of the donors by itself (although a threshold of >60% positive cells for senescence associated β-gal correctly identified all the aged donors). Combining the four characteristics resulted in all young fibroblast cell lines ranking higher than all the aged fibroblast cell lines. In addition, the combination score correctly assigned the youngest donor to the highest rank (22 years old, rank 1), and the oldest donor to the lowest rank (92 years old, rank 11).
The potential for IGF-1 in cutaneous wound healing has long been recognized (Hamon et al. 1993; Tsuboi et al. 1995). The actions of IGF-1 are mediated by IGF1R, which is coupled to several intracellular second messenger pathways including the ras-raf-MAPK and PI3K signaling cascades (Bentov and Werner 2004). We found a strong correlation between expression of IGF1R and the proliferative index in human fibroblasts (regardless of the age of the donor) on 3D collagen matrix, but not on 2D tissue culture plastic. Levels of IGF1R expression were generally less in the aged fibroblasts when compared to young fibroblasts, but differences were not statistically significant. IGF1R levels in the human dermal fibroblasts were fairly low; consequently it was difficult to use western blot to detect a change in phosphorylation of the IGF1R as a measure of activation. We therefore investigated the phosphorylation of the main downstream signaling pathways activated by IGF1R. The Ras-dependent extracellular signal-regulated kinase (Erk) MAPK pathway is responsible for integrating extracellular environmental signals. MAPK is activated by mitogenic factors, differentiation stimuli and cytokines (Meloche and Pouyssegur 2007). Our results showed that, at baseline, there is less phosphorylation of MAPK in aged fibroblasts relative to young fibroblasts. A decline in MAPK activation with age has been observed in other cell types (Liu et al. 1996; Williamson et al. 2003), but has not been previously reported in human dermal fibroblasts.
To test the hypothesis that IGF-1-induced phosphorylation and activation of the MAPK pathway are responsible for the change in proliferative capacity in dermal fibroblasts, we stimulated the fibroblasts with IGF-1 and exposed them to the specific MAPK pathway inhibitor, UO126. Exposure to UO126 (at a dose that inhibited phosphorylation of Erk without toxicity) caused a significant reduction in proliferation, even in cells stimulated with IGF-1. IGF-1 significantly increased proliferation in aged fibroblasts, but only slightly increased proliferation in young fibroblasts, potentially because Erk phosphorylation at baseline was already high in young cells. Other authors have suggested that hyper-activation of the MAPK pathway can lead to proliferative arrest by increasing the expression of p21 (Maier et al. 2004), induction of mitochondrial cytochrome c, or activation of caspase-8 (Cagnol and Chambard 2010). These studies indicate that unregulated Erk activation may negatively impact proliferation. Stimulation with IGF-1 led to phosphorylation of Akt, which indicates activation of the PI3K pathway. However, inhibition of PI3K (with LY294002) did not change the proliferative capacity of fibroblasts, suggesting that the effect of IGF-1 on fibroblast proliferation is mediated through MAPK. It is noteworthy that even with IGF-1 stimulation; aged fibroblasts did not reach the proliferative capacity observed in young fibroblasts. This underscores the fact that other pathways, in addition to the IGF-1/IGF1R axis, contribute to the age-related disruption of dermal healing. Nevertheless, IGF-1 is clinically available and further exploration of its potential during tissue repair in the aged population is warranted.
There are several limitations of this study: the association between age and IGF1R expression needs to be determined in a larger sample size and also take into account other variables that influence the expression of IGF1R, such as nutritional status (Narasimhan et al. 2009), peptide and steroid hormones, cytokines and transcription factors(for example p53) (Werner and Maor 2006). In addition, the activation of IGF1R was measured indirectly by phosphorylation of the IGF1R cognate intracellular pathways, MAPK and PI3K, which can be activated by other stimuli. Lastly, cell types that are not present in our experimental design (keratinocytes, leukocytes and macrophages) also influence fibroblast behavior in the dermis in vivo.
In summary, these data support the use of the more technically challenging 3D matrix when examining cellular functions that are relevant to aging. We found that proliferation and Erk phosphorylation are reduced in aged dermal fibroblasts relative to young dermal fibroblasts in a 3D collagen matrix. Therapeutic manipulation of IGF1R signaling has already shown clinical utility in other disease states (Miller and Yee 2005). Our finding that Erk phosphorylation in aged fibroblasts is associated with a significant increase in proliferation may suggest a potential therapeutic target for age-related impairments of dermal healing.
Acknowledgments
This work was supported by R03 AG042353 (I.B), ITHS and Department of Anesthesiology & Pain Medicine pilot grant (I.B) and R21 AG33391 (M.J.R)
We would like to thank Dr. Kathryn Houmiel for her technical assistance.
References
- Bentov I, Werner H, IGF IGF receptor and overgrowth syndromes. Pediatr Endocrinol Rev. 2004;1:352–360. [PubMed] [Google Scholar]
- Bruce SA, Deamond SF. Longitudinal study of in vivo wound repair and in vitro cellular senescence of dermal fibroblasts. Exp Gerontol. 1991;26:17–27. doi: 10.1016/0531-5565(91)90058-t. [DOI] [PubMed] [Google Scholar]
- Cagnol S, Chambard J-C. ERK and cell death: Mechanisms of ERK-induced cell death – apoptosis, autophagy and senescence. FEBS Journal. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. doi:10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. doi:S0092-8674(05)00111-X [pii]10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. doi:10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Damodarasamy M, Vernon RB, Karres N, Chang CH, Bianchi-Frias D, Nelson PS, Reed MJ. Collagen extracts derived from young and aged mice demonstrate different structural properties and cellular effects in three-dimensional gels J Gerontol. A Biol Sci Med Sci. 2010;65:209–218. doi: 10.1093/gerona/glp202. doi:10.1093/gerona/glp202 glp202 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egles C, Garlick JA, Shamis Y. Three-dimensional human tissue models of wounded skin. Methods Mol Biol. 2010;585:345–359. doi: 10.1007/978-1-60761-380-0_24. doi:10.1007/978-1-60761-380-0_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10:73–86. doi: 10.2165/00128071-200910020-00001. doi:10.2165/00128071-200910020-00001 1 [pii] [DOI] [PubMed] [Google Scholar]
- Fisher GJ, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. doi:10.2353/ajpath.2009.080599 S0002-9440(10)61269-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–259. doi: 10.1111/j.1474-9726.2008.00372.x. doi:10.1111/j.1474-9726.2008.00372.x ACE372 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- Hamon G, Hunt T, Spencer E. In vivo effects of systemic insulin-like growth factor-I alone and complexed with insulin-like growth factor binding protein-3 on corticosteroid suppressed wounds. Growth regulation. 1993;3:53–56. [PubMed] [Google Scholar]
- Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9:366–376. doi: 10.1038/nrendo.2013.67. doi:10.1038/nrendo.2013.67 nrendo.2013.67 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. doi:ACE199 [pii] 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Travers JB, Somani AK, Spandau DF. The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene. 2010;29:1475–1485. doi: 10.1038/onc.2009.440. doi:10.1038/onc.2009.440 onc2009440 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Age-related decline in mitogen-activated protein kinase activity in epidermal growth factor-stimulated rat hepatocytes. J Biol Chem. 1996;271:3604–3607. [PubMed] [Google Scholar]
- Maier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes & development. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann N Y Acad Sci. 2006;1067:323–331. doi: 10.1196/annals.1354.044. doi:1067/1/323 [pii] 10.1196/annals.1354.044. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. doi:1210414 [pii]10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Miller BS, Yee D. Type I insulin-like growth factor receptor as a therapeutic target in cancer. Cancer research. 2005;65:10123–10127. doi: 10.1158/0008-5472.CAN-05-2752. doi:10.1158/0008-5472.CAN-05-2752. [DOI] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657–666. doi: 10.1016/j.cub.2009.06.013. doi:10.1016/j.cub.2009.06.013 S0960-9822(09)01252-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, et al. Cell Proliferation Activities on Skin Fibroblasts from a Short Child with Absence of One Copy of the Type 1 Insulin-Like Growth Factor Receptor (IGF1R) Gene and a Tall Child with Three Copies of the IGF1R Gene. Journal of Clinical Endocrinology & Metabolism. 2003;88:5981–5988. doi: 10.1210/jc.2002-021080. doi:10.1210/jc.2002-021080. [DOI] [PubMed] [Google Scholar]
- Park GH, Buetow DE. Genes for insulin-like growth factors I and II are expressed in senescent rat tissues. Gerontology. 1991;37:310–316. doi: 10.1159/000213278. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Ferara NS, Vernon RB. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech Ageing Dev. 2001;122:1203–1220. doi: 10.1016/s0047-6374(01)00260-3. doi:S0047-6374(01)00260-3 [pii] [DOI] [PubMed] [Google Scholar]
- Reiss K, Cheng W, Kajstura J, Sonnenblick EH, Meggs LG, Anversa P. Fibroblast proliferation during myocardial development in rats is regulated by IGF-1 receptors. American Journal of Physiology-Heart and Circulatory Physiology. 1995;269:H943–H951. doi: 10.1152/ajpheart.1995.269.3.H943. [DOI] [PubMed] [Google Scholar]
- Schneider EL, Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proceedings of the National Academy of Sciences. 1976;73:3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing Nature reviews. Molecular cell biology. 2000;1:72–76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Shi ZD, Ji XY, Berardi DE, Qazi H, Tarbell JM. Interstitial flow induces MMP-1 expression and vascular SMC migration in collagen I gels via an ERK1/2-dependent and c-Jun-mediated mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H127–135. doi: 10.1152/ajpheart.00732.2009. doi:10.1152/ajpheart.00732.2009 00732.2009 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, et al. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. doi:S0306452298001924 [pii] [DOI] [PubMed] [Google Scholar]
- Tsuboi R, Shi C-M, Sato C, Cox GN, Ogawa H. Co-administration of insulin-like growth factor (IGF)-I and IGF-binding protein-1 stimulates wound healing in animal models. Journal of investigative dermatology. 1995;104:199–203. doi: 10.1111/1523-1747.ep12612755. [DOI] [PubMed] [Google Scholar]
- Turabelidze A, Guo S, DiPietro LA. Importance of housekeeping gene selection for accurate reverse transcription-quantitative polymerase chain reaction in a wound healing model Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2010;18:460–466. doi: 10.1111/j.1524-475X.2010.00611.x. doi:10.1111/j.1524-475X.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H, Maor S. The insulin-like growth factor-I receptor gene: a downstream target for oncogene and tumor suppressor action. Trends Endocrinol Metab. 2006;17:236–242. doi: 10.1016/j.tem.2006.06.007. doi:S1043-2760(06)00104-4 [pii] 10.1016/j.tem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Archives of physiology and biochemistry. 2008;114:17–22. doi: 10.1080/13813450801900694. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. doi:10.1113/jphysiol.2002.036673 2002.036673 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112(Pt 11):1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. doi:10.1016/j.clindermatol.2010.07.001 S0738-081X(10)00116-1 [pii] [DOI] [PubMed] [Google Scholar]