Abstract
The pyrrole-imidazole alkaloids are a group of structurally unique and biologically interesting marine sponge metabolites. Among them, the cyclic dimers have caught synthetic chemists’ attention particularly. Numerous synthetic strategies have been developed and various biosynthetic hypotheses have been proposed for these fascinating natural products. We discuss herein the synthetic approaches and the biosynthetic insights obtained from these studies.
Natural products have been a rich resource for discovering new drugs over the past decades.1 Studying their total synthesis and biosynthesis often leads to the discovery of new chemical reactivities and recognition of structural issues that may be difficult to identify by other means.2,3 The unique chemical properties of dimeric pyrrole-imidazole alkaloids have attracted numerous research groups to study their total synthesis. Several excellent review articles discussing the chemistry of pyrroleimidazole alkaloids have been published.4 This review focuses on the chemistry of cyclic pyrrole-imidazole dimers. In particular, we present an overview our synthetic work5–10 and the biosynthetic insights obtained from these studies.
Introduction
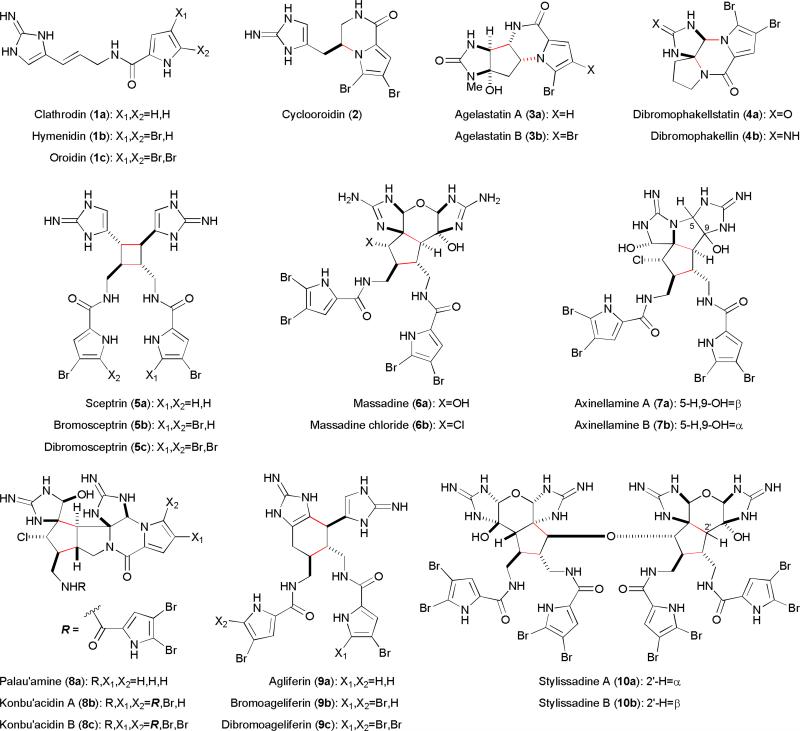
The pyrrole-imidazole alkaloids are a group of densely functionalized, highly nitrogenated natural products widely distributed within a few genera of marine sponges (Phylum Porifera) (Fig. 1). While their biological activities have not been fully investigated, many family members possess anticancer, antimicrobial, antiviral, or immunosuppressive activities. To date, about 100 pyrrole-imidazole alkaloids have been discovered. Although their biosynthesis11,12 is poorly understood, it is generally agreed that these sponge metabolites are derived from clathrodin (1a), hymenidin (1b), and oroidin (1c) through oxidation, cyclization, and dimerization reactions. This family of natural products possesses a diverse set of molecular skeletons, and is often referred to as the oroidin family of alkaloids because 1c is highly abundant in the producing sponges (up to 4.2% dry weight).13
Fig. 1.
Structures of the representative pyrrole-imidazole alkaloids.
Many pyrrole-imidazole alkaloids contain a polycyclic skeleton, making them attractive platforms for developing new synthetic methods and strategies. For example, there are several reports of the synthesis of cyclooroidin (2),14 agelastatins (3),15 and dibromophakellin/dibromophakellstatin (4).16 The most synthetically challenging family members are the cyclic dimers. Structurally, the sceptrins (5) are [2+2] dimers of 1; the massadines (6), axinellamines (7), and palau'amine (8) are [3+2] dimers, and the ageliferins (9) are [4+2] dimers. Numerous synthetic approaches have been reported17–28 and the syntheses of 5–9 have been achieved.8–10,29–35 In particular, the seminal syntheses of 5–9 by the Baran group involve many revolutionary developments at both the strategic and methodological levels.29–33 Currently, there is no report of the synthesis of the stylissadines (10), which are the only known tetrameric family members.
Biosynthetic hypotheses
Many biosynthetic hypotheses have been proposed for the pyrrole-imidazole alkaloids.11 However, much remains to be learned about how sponges assemble these natural products. For example, while it is generally agreed that the biogenic cyclization, oxidation, and dimerization of 1 are catalyzed by enzymes, the corresponding cyclases and oxidases have not been identified although it has been demonstrated that proline and lysine are the amino acid precursors for 1.11h,i
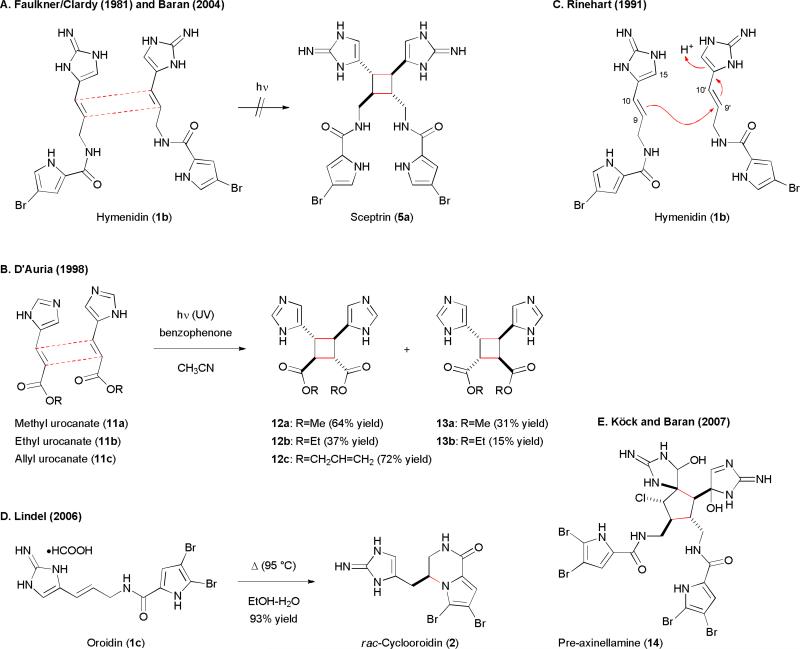
The isolation of the first dimeric pyrrole-imidazole alkaloid sceptrin (5a) was reported by the Faulkner and Clardy groups in 1981.36 They suggested the biosynthesis of 5a would not involve a [2+2] photocycloaddition reaction with 1b because the producing sponges live 20–30 m below the ocean's surface. Sunlight is insufficient at these depths for photocycloaddition reactions. In addition, a direct photoreaction would give rise to racemic 5a while the natural product was found optically active. Furthermore, all attempts to synthetically induce the photodimerization of 1b in either solid-state or solution phase failed to deliver 5a (Fig. 2A).29a,36 The inaccessibility of 5a from 1b via photolysis has been confirmed by the Baran group recently.29a Interestingly, D’Auria and Racioppi have showed that photolysis of urocanic esters (11) in the presence of benzophenone (photosensitizer) gave the desired cyclobutane skeleton of 5a (Fig. 2B).37 Only the head-to-head dimers 12 and 13 were obtained. This regioselectivity was explained by the frontier molecular orbital theory with either a HOMO/LSOMO (primary) or a HSOMO/LUMO (secondary) interaction. The observed stereoselectivities could also be explained by steric effects.
Fig. 2.
Probing the biosynthetic hypotheses of the dimerization of the pyrrole-imidazole alkaloid by biomimetic synthesis.
The Rinehart group and the Kobayashi group reported the isolation of the second type of dimeric pyrrole-imidazole alkaloids ageliferins (9) in 1989 and 1990, respectively.38,39 While Kobayashi considered that ageliferins (9) could be derived from 1b/c through a Diels–Alder reaction followed by olefin isomerization, Rinehart disagreed because natural 9 are optically active. Instead, Rinehart proposed that the dimerization of 1b would be an acid-promoted, stepwise process with the C9-C9’ linkage formed first (Fig. 2C).38b Subsequent C10-C10’ cyclization would then yield sceptrin (5a), while C15-C10’ cyclization would provide ageliferin (9a). However, Lindel found that thermolysis of 1c•HCOOH did not give any dimerization products (Fig. 2D), but instead observed rac-2.14c
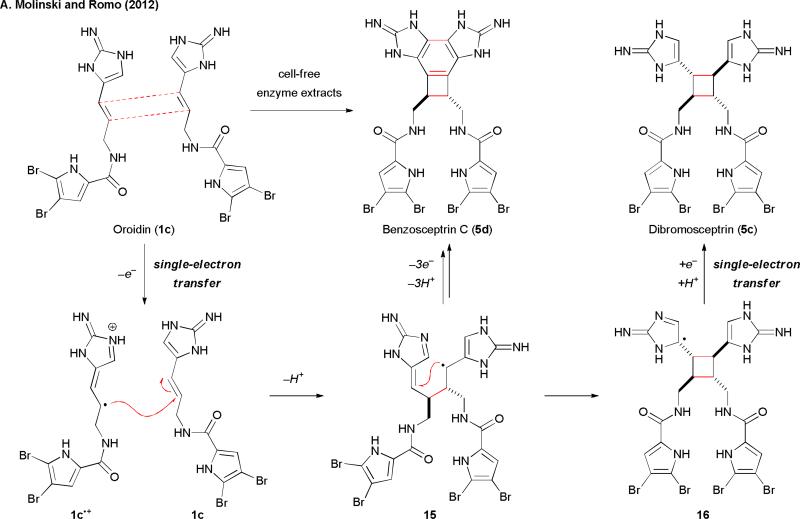
To explain the mismatched electronic properties toward homodimerization, an alternative mechanism involving single-electron transfer (SET) has recently been reported. The Molinski and Romo groups found that cell-free enzyme extracts collected from pyrrole-imidazole alkaloid-producing sponges could promote the dimerization of 1c to give benzosceptrin C (5d) (Fig. 3).12 They suggested that the biosynthesis of 5c and 5d would involve oxidoreductase-catalyzed SET reactions. Specifically, a single-electron oxidation of 1c would give a highly reactive radical cation 1c•+. A radical addition of 1c•+ to another molecule of 1c would then give 15 after loss of a proton. Subsequent cyclization of 15 would yield 16. A single-electron reduction of 16 followed by protonation would then provide 5c. This reversible SET mechanism nicely fits the redox-neutral dimerization of 1c for the biosynthesis of 5c. Similarly, 5d would be formed from 15 through a net three-electron oxidation. Molinski and Romo also suggested that this SET biosynthetic hypothesis could be extended to other pyrrole-imidazole alkaloids, including both the monomeric and dimeric members. They further argued that the high-energy radical species renders the corresponding cyclization reactions kinetically favorable over the two-electron mechanisms.
Fig. 3.
The SET-mediated dimerization biosynthetic hypothesis.
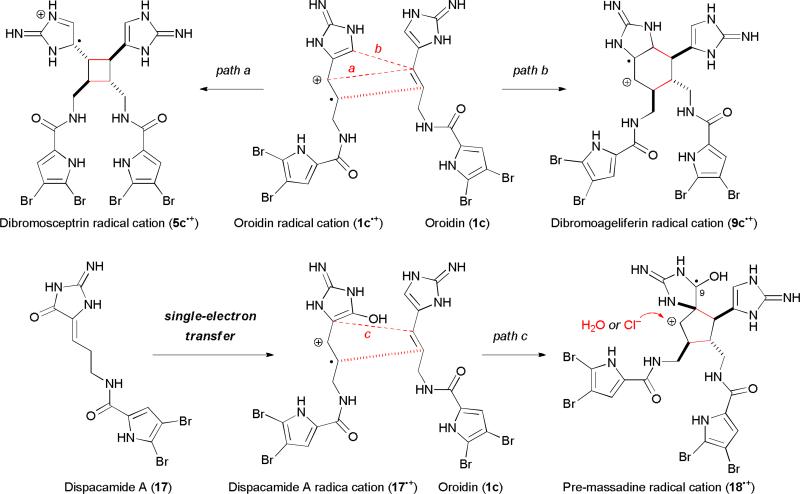
We agree to the biosynthetic hypothesis put forth by Molinski and Romo, and believe that sponges use reversible SET reactions to generate 5–9 through divergent biosyntheses (Fig. 4). We envision that the biosynthesis of dibromosceptrin (5c) would involve a SET of 1c to give 1c•+, which would undergo a [2+2] cycloaddition (stepwise or concerted) with another molecule of 1c to provide 5c•+ (path a), or a [4+2] cycloaddition to provide 9c•+ (path b). A subsequent back-SET and aromatization would then yield 5c and 9c.
Fig. 4.
The divergent SET-mediated dimerization biosynthetic hypothesis.
We further propose that a redox-neutral, reversible SET process was also involved in the formation of the core skeleton of the [3+2] dimers. Unlike the biosyntheses of sceptrins (5) and ageliferins (9) that involve a homodimerization of 1, we believe that the [3+2] dimers involve a heterodimerization of dispacamide A (17) and oroidin (1) (Fig. 4). A SET-oxidation of 17 would give 17•+, which would then undergo a [3+2] cycloaddition with 1c to give “pre-massadine” radical cation (18•+) (path c). Subsequent SET-reduction of 18•+, protonation, and trapping with water or chloride would provide “premassadine” (18-OH) or “pre-massadine chloride” (18-Cl). Oxidation of “pre-massadine chloride” (18-Cl) would give rise to “pre-axinellamine” (14), a common biosynthetic intermediate proposed by Köck and Baran for all [3+2] pyrroleimidazole dimers (Fig. 2E).31,40 Cyclization of 14 would afford massadine chloride (6b), axenellamines (7), and konbu’acidins (8b,c). While massadine (6a) and massadine chloride (6b) could be produced independently, Köck and Baran showed that 6a could be converted to 6b completely by incubating 6a in water at 60 °C for 4 h.40 The retention of stereochemistry could be explained by an aziridinium intermediate that was previously proposed by Romo.19d
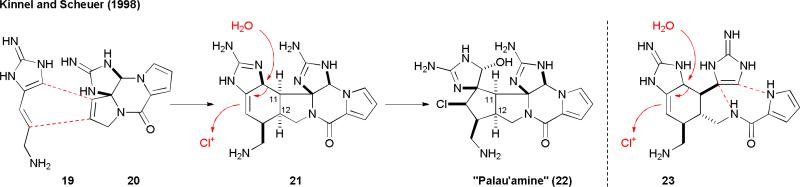
Because enzymes that promote [3+2] cycloaddition reactions remain elusive, it is generally believed that the [3+2] pyrrole-imidazole dimers are produced from skeletal rearrangement of the [4+2] dimers. This biosynthetic hypothesis was first put forth by Kinnel and Scheuer in 1998.41 They proposed that the biosynthesis of “palau’amine” (22) would involve a [4+2] cycloaddition of 3-amino-1-(2-aminoimidazolyl)prop-1-ene (AAPE, 19) and 11,12-dehydrophakellin (20). A chloroperoxidase-mediated oxidative ring contraction of the Diels–Alder adduct 21 would then yield “palau’amine” (22). Interestingly, they noted that this biosynthetic pathway was a “better alternative” suggested by a reviewer.
Recently, the structure of palau'amine has been revised from 22 to 8a. The new trans C-11/C-12 configuration could not be easily explained by the original “Kinnel–Scheuer hypothesis”. We thus proposed a modified scheme that involves the intermediate 23 to reconcile the C-11/C-12 stereochemical issue.7 Specifically, a [4+2] cycloaddition reaction of 19 and clathrodin (1a) would give rise to 23 and set the trans C-11/C-12 stereochemistry. An oxidative bicyclization and a chlorinative ring contraction would then afford 8a.
Total Syntheses
Synthesis of sceptrin
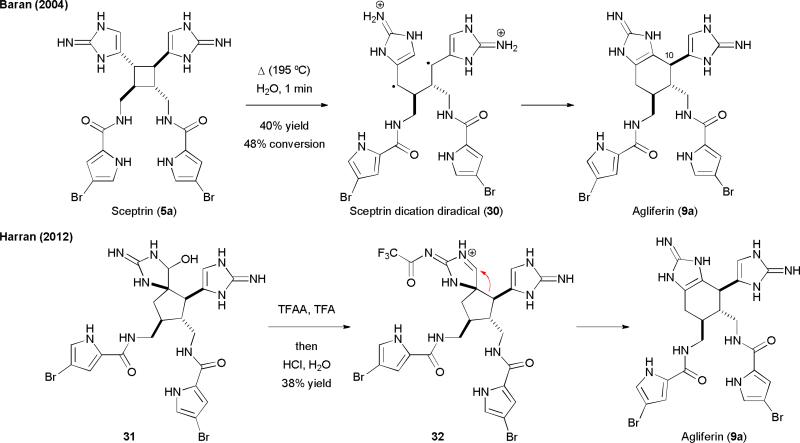
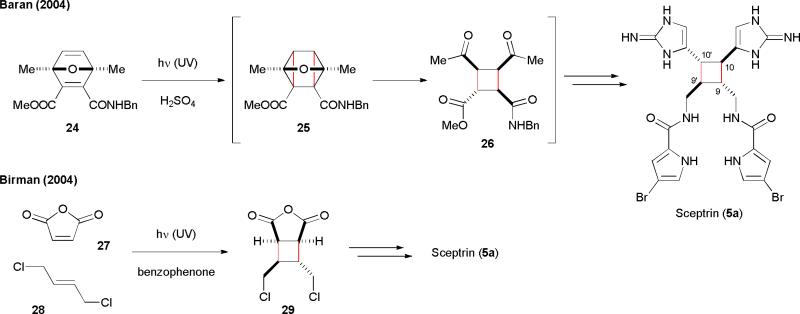
The isolation of sceptrin (5a), a [2+2] pyrrole-imidazole dimer, was first reported by the Faulkner and Clardy groups in 1981.36 Its total synthesis has been accomplished by the Baran and Birman groups in 2004.29,34 As noted by Baran, “unexpected reactivity, intermediate fragility, and forbidding elements of complexity” render 5a a significant synthetic problem. Baran used a 3-oxaquadricyclane rearrangement as the key step to generate the core skeleton of 5a.29 The Baran group also achieved the asymmetric synthesis of 5a with an enzymatic desymmetrization approach to generate 24.30b Photolysis of 24 promoted a [2+2] cycloaddition to form the cyclobutane framework of 5a. Treating the resulting cycloadduct 25 directly with acid initiated the oxaquadricyclane rearrangement reaction to give 26. This practical approach allowed for a gram-scale synthesis of 5a with an impressive 24% overall yield. The Birman group used an intermolecular [2+2] photocycloaddition of 27 and 28 to construct the sceptrin precursor 29. Both the Baran and Birman's approaches involved a cycloaddition reaction to construct the C9-C10 and C9’-C10’ bonds in a “non-biogenetic” fashion.
Synthesis of ageliferin
The isolation and characterization of ageliferins (9a) were first reported by the Rinehart group and the Kobayashi group in 1989 and 1990, respectively.38,39,42 While attempts to synthesize ageliferins by inducing the dimerization of 1 via a thermal Diels–Alder reaction have not been successful,14c the Romo group and the Lovely group successfully used the Diels–Alder reaction to construct the core skeleton of 9a.19,20 The Ohta group also used this Diels–Alder approach to accomplish the synthesis of 12,12’-dimethylageliferin in 2002.21
The first synthesis of 9a was achieved by the Baran group in 2004 using a completely orthogonal synthetic approach.30 Because ageliferin (9a) was always co-isolated with sceptrin (5a), they proposed that 9a was a formal [1,3]-sigmatropic rearrangement product of 5a. Supportive to this hypothesis, they found that microwave-thermolysis of the acid salts of 5a gave 9a stereospecifically (Fig. 7).30 Incubating nat-5a in water at 200 °C for 2 min yielded nat-9a, while thermolysis of ent-5a gave ent-9a.30b A small amount of nagelamide E, the C-10 isomeric natural product of ageliferin (9a) was also obtained. Computational studies by the Houk group indicated that this rearrangement reaction proceeded through dicationic diradical 30.30c The Harran group also reported synthesis of 9a using an acid-promoted ring-expansion reaction.35
Fig. 7.
The total syntheses of ageliferins (9) reported by the Baran and Harran's groups.
Synthesis of axinellamine, massadine and palau'amine
The [3+2] class of pyrrole-imidazole alkaloid dimers are among the most complicated marine alkaloids. Palau'amine (8a), the first known member was isolated by the Kinnel and Scheuer groups in 1993.41 The pyrrole-isomeric congener styloguanidine and the bis-pyrrole congener konbu’acidins (8b,c) were isolated by the Kato group and the Kobayashi group in 1995 and 1997, respectively.43 Unlike other [3+2]-type pyrrole imidazole alkaloid dimers (6–9), they were originally proposed to bear an all-cis stereochemistry on the central all-carbon 5-memebered ring (22) because a trans-fused 5,5-bicyclic system would be significantly strained. However, the Köck, Quinn, and Matsunaga groups independently reported structural revisions of Palau'amine to 8a in 2007.44 The stereochemistry of the central 5-membered ring in 8a is now consistent with that of 6–9.
The second type of [3+2] dimers identified are the axinellamines (7), first reported by the Quinn group in 1999.45 Instead of bearing a phakellin substructure, it possesses a C–N linkage between the two cyclic guanidine units. The isolation of massadine (6a), the third type of the [3+2] dimers, was first reported by the Fusetani group in 2003.46 It contains an ether linkage between the two cyclic guanidine units, and is currently the only [3+2] dimer that does not bear a chlorine atom. The Köck group further discovered that dimers of massadine also exist in nature. They reported the isolation of the first tetrameric pyrrole-imidazole alkaloid stylissadine in 2006,47 and proposed that 6a was derived from massadine chloride (6b) via hydrolysis. Together with the Baran group, they have successfully discovered 6b and demonstrated that slow hydrolysis of 6b gave 6a.40 Finally, donnazoles, discovered by Muñoz and the Al-Mourabit group in 2012, are the fourth type of [3+2] dimers.48 They closely resemble “pre-axinellamine” (14) and bear a hydantoin group without further cyclization.
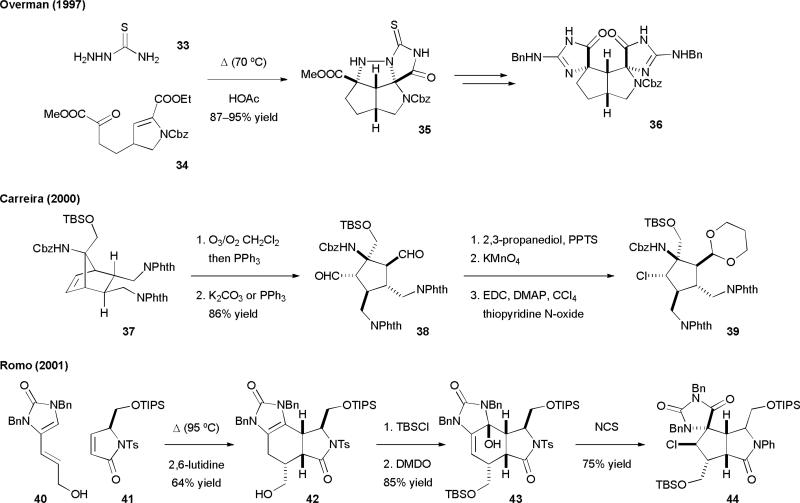
The fascinating structures of 6–9 have inspired many research groups to develop new strategies and methodologies for total synthesis. The first approach was reported by the Overman group.17 They developed a novel azomethine imine cycloaddition reaction to construct the core structure of [3+2] dimers (Fig. 8). Tandem condensation-cycloaddition of 33 and 34 afforded the tetracyclic thiohydantoin 35. Cleavage of the N–N bond followed by installation of the second iminohydantoin group furnished the original proposed Palau'amine skeleton 36. The Carreira group subsequently reported a Diels–Alder cycloaddition/oxidative cleavage approach to construct the core structure of axinellamine (6).18 Ozonolysis of 37, in which the norbonene skeleton was constructed from an intermolecular Diels–Alder reaction, gave 38. The chlorine atom was then introduced through a Barton decarboxylation reaction followed by in situ trapping of a chlorine atom to afford the highly functionalized cyclopentane skeleton 39. The Romo group first demonstrated the use of the Diels–Alder/Kinnel–Scheuer oxidative ring-contraction approach to synthesize the core structure of Palau'amine (8a).19 The intermolecular Diels–Alder reaction of vinylimidazolinone 40 and the reactive dienophile 41 proceeded smoothly to give cyclohexene 42. Oxidation of the imidazolinone group of 42 installed the requisite olefin for the Kinnel–Scheuer rearrangement. Subsequent oxidative chlorination of 43 induced the pinacol-type rearrangement to afford 44. The Lovely group also independently developed a similar biomimetic approach to construct the core skeleton of [3+2] pyrrole-imidazole dimers.20 The Harran group has reported an oxidative enolate coupling/skeletal rearrangement approach to synthesize “axinellamines deficient in halogen”.23 Additional synthetic approaches have been reported by the Austin,22 Gin,24 Gleason,25 Williams,26 Feldman,27 and Nagasawa28 groups.
Fig. 8.
The synthetic approaches of massadines (6)/axinellamines (7)/Palau'amine (8a) reported by the Overman, Carreira, and Romo groups.
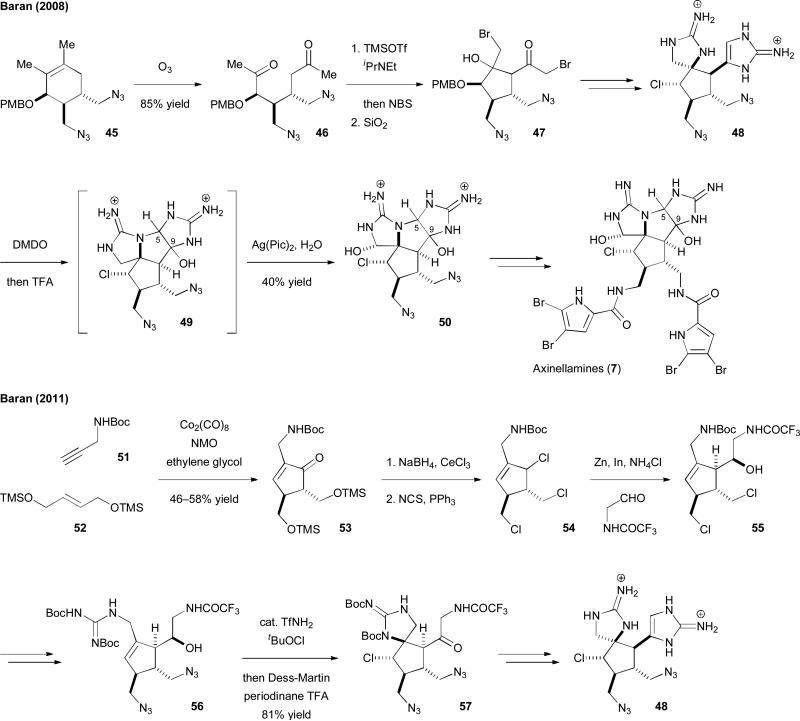
The syntheses of [3+2] pyrrole-imidazole dimers have been accomplished by the Baran group with two different approaches (Fig. 9).31–33 In their first approach, an intramolecular aldol reaction was used to construct the core skeleton and a novel silver(II)-mediated oxidation to adjust the oxidation state of the spirocyclic guanidine. They first established the core skeleton of 45 by a Diels–Alder reaction. Ozonolysis of 45 then gave 46, which was brominated and treated with dry silica gel to induce the intramolecular aldol reaction to yield 47. Installation of the two cyclic guanidines and the chlorine atom provided 48, setting the stage for the key oxidation reactions. Treating 48 with dimethyldioxirane (DMDO) afforded 49 with the complete axinellamine skeleton. Subsequent oxidation with silver(II) picolinate provided 50 with the desired axinellamine oxidation state. Using this approach, they successfully completed the first synthesis of axinellamines (7)31 and massadine (6)32 in 2008. They then developed a macrocyclization approach to construct the highly strained “phakellin” subunit of Palau'amine (8a). The synthesis of the legendary natural product Palau'amine (8a) was therefore achieved for the first time in 2010.33 The Baran group further developed an asymmetric approach in 2011 and accomplished the enantioselective synthesis of 6–8a.33b In the same year, they also reported a new, practical approach that allowed for the gram-scale synthesis of axinellamines (7).31c Some notable discoveries include the intriguing role of ethylene glycol in the Pauson–Khand cycloaddition reaction of 51 and 52 to give 53, the unprecedented combination of In(0) and Zn(0) for promoting a facile Barbier-type reaction of 54 to give 55, and the beneficial effects of trifluoromethanesulfonimidic acid (TfNH2) in the chloroamidation of 56 to give 57. These reports demonstrated once again that total synthesis is a fertile ground for studying chemical reactivities and developing new methodologies.
Fig. 9.
The total syntheses of massadines (6)/axinellamines (7)/Palau'amine (8a) reported by the Baran group.
Our synthetic strategy
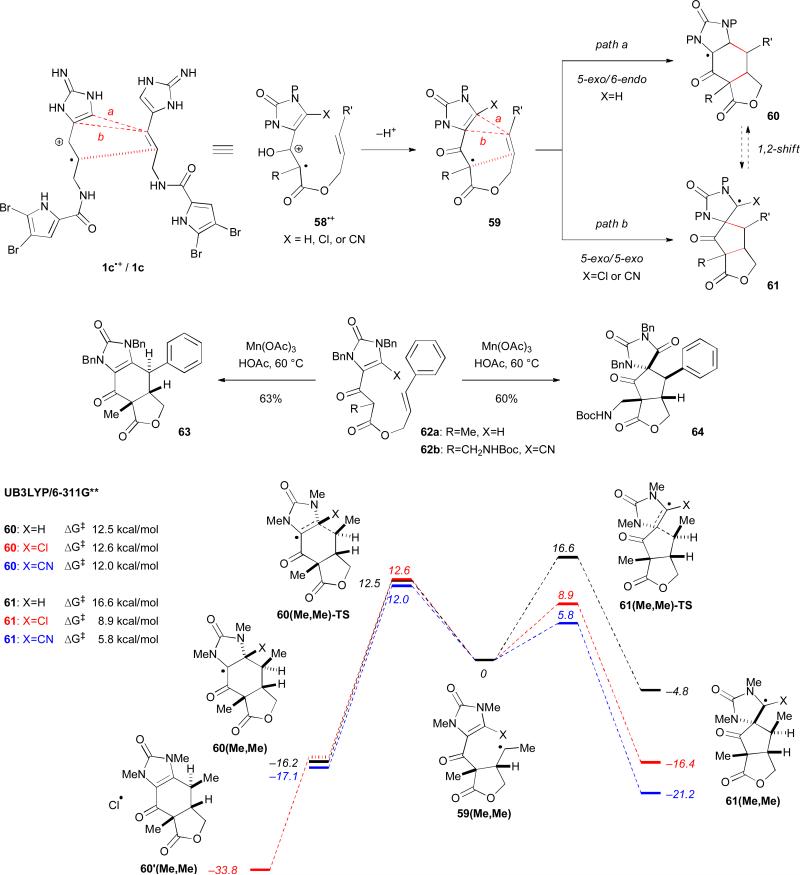
We are interested in developing a biomimetic approach for the synthesis of ageliferins (9a–c) (Fig. 10). Our work started with the design of an oxidative radical cyclization reaction that mimics the dimerization of 1.5 We envisioned that the reaction efficiency could be improved significantly by tethering the two dimerization units and replacing the olefin group with an enol. We used 58•+ that could be readily generated from a single electron oxidation of the corresponding β-ketoester to mimic 1c•+/1c. After losing a proton, radical 59 would undergo a 5-exo cyclization followed by a 6-endo cyclization to afford the ageliferin core skeleton 60, or a 5-exo cyclization followed by another 5-exo cyclization to afford the massadine core skeleton 61. The pathway selectivity could be controlled by the electronic properties of the substituent group X. When X=H, the 5-exo/6-endo ageliferin-cyclization was preferred, giving 60 selectively. When X=Cl or CN, the 5-exo/5-exo massadinecyclization was preferred, giving 61 selectively. For example, oxidation of 62a gave 63 as the only product, while oxidation of 62b gave 64 exclusively. Our computational studies suggested that both 63 and 64 were kinetic products.9
Fig. 10.
Our divergent biomimetic synthetic strategy and the energy diagram of the reaction pathways.
Based on our calculations, the 6-endo cyclization product of 59 is 11.4 kcal/mol more stable than the 5-exo cyclization product when X=H and R,R’=Me, consistent with the reported ESR studies on the captodative stabilization of radicals. The radical stabilization enthalpy of an amino group was estimated to be 3.9 kcal/mol and that of a carbonyl group to be 6.0 kcal/mol.49 Together, these two groups were estimated to provide 20.7 kcal/mol stabilization. The 10.8 kcal/mol captodative stabilization provided by the amino and carbonyl groups was the thermodynamic driving force for the 6-endo cyclization pathway when X=H. The captodative stabilization also greatly contributed to stabilization of the transition states that led to 60 and 61 as judged by their atomic spin densities. Consequently, we predicted that a captodative stabilization could to introduce to the 5-exo cyclization pathway when X=Cl or CN, resulting in a switch of the pathway selectivity. Computational studies indicated that the introduction of a chloro or a cyano group would lower the activation barrier of the 5-exo cyclization pathway without affecting the 6-endo cyclization pathway (Fig. 10). For 60, the 6-endo cyclization pathway was favored by 4.1 kcal/mol when X=H. However, for 61, the 5-exo cyclization pathways was favored by 3.7 and 6.2 kcal/mol when X=Cl and CN, respectively. The 6-endo cyclization was also predicted to undergo a barrierless elimination of the chlorine atom.
Based on these results, we hypothesized that sponges utilize a divergent biosynthetic approach to produce 6–9. The biosyntheses of sceprins (6) and ageliferins (9) would involve a homodimerization of 1, while that of pre-massadine (18) would include a hetereodimerization of 1 and dispacamide A (17) (Fig. 4). A SET-oxidation of 17 would give 17•+. The hydroxyl group of 17•+ would provide additional stabilization for 18•+ and the corresponding transition state to direct the reaction toward [3+2] cyclization.
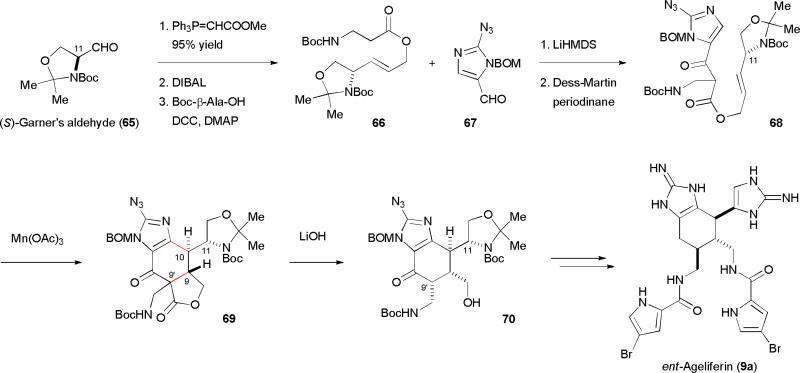
Using this radical cyclization approach, we have successfully completed the synthesis of ageliferins (9a–c) (Fig. 11).8,9 We started our synthesis with (S)-Garner's aldehyde (65) that could be easily prepared from l-serine. Horner–Wadsworth–Emmons homologation of 65 followed by ester reduction and coupling with Boc-β-alanine gave 66. Incorporation of the azidoimidazole group through an aldol reaction with 67 provided allylic β-ketoester 68 after alcohol oxidation. Treating 68 with Mn(OAc)3 induced the key oxidative radical cyclization to deliver the ageliferin core skeleton 69 as a mixture of C-9’ diastereomers. The allylic stereocenter (C-11) of 68 controlled the selective installation of the C-9 and C-10 stereocenters by minimizing the A1,3 strain.50 The two diastereomers of 69 converged upon decarboxylation to give 70. Subsequent installation of the bromopyrrole and aminoimidazole groups yielded ageliferin. The CD spectra of our synthetic sample indicated that we obtained ent-ageliferin while expecting to get nat-ageliferin. Consistently, repeating the synthesis using (R)-Garner's aldehyde as the source of chirality gave nat-ageliferins. Our approach also allows for the differential installation of the pyrrole groups bearing different bromine patterns. In addition to ageliferin (9a), bromoageliferin (9b) and dibromoageliferin (9c) have also been synthesized using this flexible approach.
Fig. 11.
Our synthesis of ageliferin (9a).
Conclusions
Natural products have proved to be useful in providing new chemical entities to target both known and unknown biological domains. Their “privileged skeletons” have also provided precious platforms to study chemical principles. Because natural products often modulate signal transduction pathways and affect cell cycles, studying their chemistry and biology often leads to the development of new therapeutic agents. However, the primary function of many natural products remains unknown, rendering them uninteresting targets from biological perspectives. We believe that natural product evolution is driven by selection for functions and the biosynthetic machineries are purpose-built. In particular, complex natural products produced by lower marine organisms that heavily rely on chemicals for defence and symbiosis would be a rich resource for drug development. The successful identification of the primary functions of natural products and exploration of their therapeutic potentials heavily rely on chemists’ ability to synthesize and modify these natural products. We anticipate that natural product synthesis will continue to play a central role in chemical research and stimulate the development of new chemical tools, offer insights into biosynthesis, and facilitate drug discovery.
Fig. 5.
The Kinnel–Scheuer biosynthetic hypothesis.
Fig. 6.
The total syntheses of sceptrins (5) reported by the Baran and Birman's groups.
Acknowledgements
Financial Support was provided by NIH (NIGMS R01-GM079554), the Welch Foundation (I-1596), and UT Southwestern. C.C. is a Southwestern Medical Foundation Scholar in Biomedical Research.
Notes and references
- 1.a Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Newman DJ, Cragg GM, Snader KM. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]; c Gerwick WH, Moore BS. Chem. Biol. 2012;19:85–98. doi: 10.1016/j.chembiol.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Cordell GA, Colvard MD. J. Nat. Prod. 2012;75:514–525. doi: 10.1021/np200803m. [DOI] [PubMed] [Google Scholar]; e Li JW-H, Vederas JC. Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]; f Koehn FE, Carter GT. Nat. Rev. Drug Discovery. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]; g MacCoss M, Baillie TA. Science. 2004;303:1810–1813. doi: 10.1126/science.1096800. [DOI] [PubMed] [Google Scholar]; h Butler MS. J. Nat. Prod. 2004;67:2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]; i Breinbauer R, Vetter IR, Waldmann H. Angew. Chem. Int. Ed. 2002;41:2878–2890. doi: 10.1002/1521-3773(20020816)41:16<2878::AID-ANIE2878>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 2.a Cannon JS, Overman LE. Angew. Chem. Int. Ed. 2012;51:2–26. doi: 10.1002/anie.201107385. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc. Chem. Res. 2012;45:826–839. doi: 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]; d Ishihara Y, Baran PS. Synlett. 2010:1733–1745. [Google Scholar]; e Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; f Giri R, Shi B-F, Engle KM, Maugelc N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]; g Que L, Jr., Tolman WB. Nature. 2008;455:333–340. doi: 10.1038/nature07371. [DOI] [PubMed] [Google Scholar]; h Clardy J, Walsh C. Nature. 2004;432:829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 3.a Suyama TL, Gerwick WH, McPhail KL. Bioorg. Med. Chem. 2011;19:6675–6701. doi: 10.1016/j.bmc.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Maier ME. Nat. Prod. Rep. 2009;26:1105–1124. doi: 10.1039/b809658a. [DOI] [PubMed] [Google Scholar]; c Usami Y. Mar. Drugs. 2009;7:314–330. doi: 10.3390/md7030314. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Nicolaou KC, Snyder SA. Angew. Chem. Int. Ed. 2005;44:1012–1044. doi: 10.1002/anie.200460864. [DOI] [PubMed] [Google Scholar]
- 4.a Hoffmann H, Lindel T. Synthesis. 2003;35:1753–1783. [Google Scholar]; b Jacquot DEN, Lindel T. Curr. Org. Chem. 2005;9:1551–1565. [Google Scholar]; c Du H, He Y, Sivappa R, Lovely CJ. Synlett. 2006:965–992. [Google Scholar]; d Köck M, Grube A, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2007;46:6586–6594. doi: 10.1002/anie.200701798. [DOI] [PubMed] [Google Scholar]; e Weinreb SM. Nat. Prod. Rep. 2007;24:931–948. doi: 10.1039/b700206h. [DOI] [PubMed] [Google Scholar]; f Arndt H-D, Riedrich M. Angew. Chem. Int. Ed. 2008;47:4785–4788. doi: 10.1002/anie.200801793. [DOI] [PubMed] [Google Scholar]; g Wei Y, Zipse H. Eur. J. Org. Chem. 2008:3811–3816. [Google Scholar]; h Forte B, Malgesini B, Piutti C, Quartieri F, Scolaro A, Papeo G. Mar. Drugs. 2009;7:705–753. doi: 10.3390/md7040705. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Heasley B. Eur. J. Org. Chem. 2009:1477–1489. [Google Scholar]; j Shenvi RA, O'Malley DP, Baran PS. Acc. Chem. Res. 2009;42:530–541. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Al-Mourabit A, Zancanella MA, Tilvi S, Romo D. Nat. Prod. Rep. 2011;28:1229–1260. doi: 10.1039/c0np00013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan X, Chen C. Angew. Chem. Int. Ed. 2006;45:4345–4348. doi: 10.1002/anie.200601208. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Tan X, Chen C. J. Am. Chem. Soc. 2007;129:7768–7769.. doi: 10.1021/ja072844p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Z, Lu J, Wang X, Chen C. Chem. Commun. 2011;47:427–429. doi: 10.1039/c0cc02214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Lu ZMJ, Tan X, Chen C. J. Am. Chem. Soc. 2011;133:15350–15353. doi: 10.1021/ja207386q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Wang X, Tan X, Lu J, Cormier KW, Ma Z, Chen C. J. Am. Chem. Soc. 2012;134:18834–18842. doi: 10.1021/ja309172t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X-L, Ma Z, Wang X, Rodriguez RA, Moore CE, Rheingold AL, Baran PS, Chen C. manuscript in preparation. [Google Scholar]
- 11.a Kitagawa I, Kobayashi M, Kitanaka K, Kido M, Kyogoku Y. Chem. Pharm. Bull. 1982;31:2321–2328. [Google Scholar]; b Braekman J-C, Daloze D, Stoller C, Soest RWMV. Biochem. Syst. Ecol. 1992;20:417–431. [Google Scholar]; c Andrade P, Willoughby R, Pomponi SA, Kerr RG. Tetrahedron Lett. 1999;40:4775–4778. [Google Scholar]; d Lindel T, Hochgürtel M, Assmann M, Köck M. J. Nat. Prod. 2000;63:1566–1569. doi: 10.1021/np000160o. [DOI] [PubMed] [Google Scholar]; e Al Mourabit A, Potier P. Eur. J. Org. Chem. 2001:237–243. [Google Scholar]; f Linington RG, Williams DE, Tahir A, Soest R. v., Andersen RJ. Org. Lett. 2003;5:2735–2738. doi: 10.1021/ol034950b. [DOI] [PubMed] [Google Scholar]; g Travert N, Al-Moura A. J. Am. Chem. Soc. 2004;126:10252–10253. doi: 10.1021/ja047574e. [DOI] [PubMed] [Google Scholar]; h Vergne C, Boury-Esnault N, Perez T, Martin M-T, Adeline M-T, Dau ETH, Al A. Mourabit, Org. Lett. 2006;8:2421–2424. doi: 10.1021/ol0608092. [DOI] [PubMed] [Google Scholar]; i Genta-Jouve G, Cachet N, Holderith S, Oberhänsli F, Teyssié J-L, Jeffree R, Al Mourabit A, Thomas OP. ChemBioChem. 2011;12:2298–2301. doi: 10.1002/cbic.201100449. [DOI] [PubMed] [Google Scholar]
- 12.a Wang Y-G, Morinaka BI, Reyes JCP, Wolff JJ, Romo D, Molinski TF. J. Nat. Prod. 2010;73:428–434. doi: 10.1021/np900638e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Stout EP, Wang Y-G, Romo D, Molinski TF. Angew. Chem. Int. Ed. 2012;51:4877–4881. doi: 10.1002/anie.201108119. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Stout EP, Morinaka BI, Wang Y-G, Romo D, Molinski TF. J. Nat. Prod. 2012;75:527–530. doi: 10.1021/np300051k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a Cafieri F, Carnuccio R, Fattorusso E, Taglialatela-Scafati O, Vallefuoco T. Bioorg. Med. Chem. Lett. 1997;7:2283–2288. [Google Scholar]; b Assmann M, Lichte E, Pawlik JR, Köck M. Mar. Ecol. Prog. Ser. 2000;207:255–262. [Google Scholar]; c Lindel T, Hoffmann H, Hochgürtel M, Pawlik JR. J. Chem. Ecol. 2000;26:1477–1496. [Google Scholar]; d Richelle-Maurer E, De Kluijver MJ, Feio S, Gaudêncio S, Gaspar H, Gomez R, Tavares R, Van de Vyver G, Van Soest RWM. Biochem. Sys. Ecol. 2003;2003:1073–1091. [Google Scholar]
- 14.a Papeo G, Frau MAG-Z, Borghi D, Varasi M. Tetrahedron Lett. 2005;46:8635–8638. [Google Scholar]; b Patel J, Pelloux-Léon N, Minassian F, Vallée Y. Tetrahedron Lett. 2006;47:5561–5563. [Google Scholar]; c Pöverlein C, Breckle G, Lindel T. Org. Lett. 2006;8:819–821. doi: 10.1021/ol0526219. [DOI] [PubMed] [Google Scholar]; d Mukherjee S, Sivappa R, Yousufuddin M, Lovely CJ. Org. Lett. 2010;12:4940–4943. doi: 10.1021/ol1020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a Stien D, Anderson GT, Chase CE, Koh Y.-h., Weinreb SM. J. Am. Chem. Soc. 1999;121:9574–9579. [Google Scholar]; b Feldman KS, Saunders JC. J. Am. Chem. Soc. 2002;124:9060–9061. doi: 10.1021/ja027121e. [DOI] [PubMed] [Google Scholar]; c Hale KJ, Domostoj MM, Tocher DA, Irving E, Scheinmann F. Org. Lett. 2003;5:2927–2930. doi: 10.1021/ol035036l. [DOI] [PubMed] [Google Scholar]; d Domostoj MM, Irving E, Scheinmann F, Hale KJ. Org. Lett. 2004;6:2615–2618. doi: 10.1021/ol0490476. [DOI] [PubMed] [Google Scholar]; e Davis FA, Deng J. Org. Lett. 2005;7:621–623. doi: 10.1021/ol047634l. [DOI] [PubMed] [Google Scholar]; f Trost BM, Dong G. J. Am. Chem. Soc. 2006;128:6054–6055. doi: 10.1021/ja061105q. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Endeshaw MM, Bayer A, Hansen LK, Gautun OR. Eur. J. Org. Chem. 2006:5249–5259. [Google Scholar]; h Ichikawa Y, Yamaoka T, Nakano K, Kotsuki H. Org. Lett. 2007;9:2989–2992. doi: 10.1021/ol0709735. [DOI] [PubMed] [Google Scholar]; i Yoshimitsu T, Ino T, Tanaka T. Org. Lett. 2008;10:5457–5460. doi: 10.1021/ol802225g. [DOI] [PubMed] [Google Scholar]; j Yoshimitsu T, Ino T, Futamura N, Kamon T, Tanaka T. Org. Lett. 2009;11:3402–3405. doi: 10.1021/ol9012684. [DOI] [PubMed] [Google Scholar]; k Wehn PM, Du Bois J. Angew. Chem. Int. Ed. 2009;48:3802–3805. doi: 10.1002/anie.200806292. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Dickson DP, Wardrop DJ. Org. Lett. 2009;11:1341–1344. doi: 10.1021/ol900133v. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Hama N, Matsuda T, Sato T, Chida N. Org. Lett. 2009;11:2687–2690. doi: 10.1021/ol900799e. [DOI] [PubMed] [Google Scholar]; n Movassaghi M, Siegel DS, Han S. Chem. Sci. 2010;1:561–566. doi: 10.1039/c0sc00351d. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Han S, Siegel DS, Morrison KC, Hergenrother PJ, Movassaghi M. J. Org. Chem. 2013;78:11970–11984. doi: 10.1021/jo4020112. [DOI] [PMC free article] [PubMed] [Google Scholar]; p Menjo Y, Hamajima A, Sasaki N, Hamada Y. Org. Lett. 2011;13:5744–5747. doi: 10.1021/ol2023054. [DOI] [PubMed] [Google Scholar]; q Reyes JCP, Romo D. Angew. Chem. Int. Ed. 2012;51:6870–6873. doi: 10.1002/anie.201200959. [DOI] [PMC free article] [PubMed] [Google Scholar]; r Kano T, Sakamoto R, Akakura M, Maruoka K. J. Am. Chem. Soc. 2012;134:7516–7520. doi: 10.1021/ja301120z. [DOI] [PubMed] [Google Scholar]; s Duspara PA, Batey RA. Angew. Chem. Int. Ed. 2013;52:10862–10866. doi: 10.1002/anie.201304759. [DOI] [PubMed] [Google Scholar]
- 16.a Foley LH, Büchi G. J. Am. Chem. Soc. 1982;104:1776–1777. [Google Scholar]; b Wiese KJ, Yakushijin K, Horne DA. Tetrahedron Lett. 2002;43:5135–5136. [Google Scholar]; c Poullennec KG, Romo D. J. Am. Chem. Soc. 2003;125:6344–6345. doi: 10.1021/ja034575i. [DOI] [PubMed] [Google Scholar]; d Wang S, Romo D. Angew. Chem. Int. Ed. 2008;47:1284–1286. doi: 10.1002/anie.200703998. [DOI] [PubMed] [Google Scholar]; e Chung R, Yu E, Incarvito CD, Austin DJ. Org. Lett. 2004;6:3881–3884. doi: 10.1021/ol0490532. [DOI] [PubMed] [Google Scholar]; f Feldman KS, Skoumbourdis AP. Org. Lett. 2005;7:929–931. doi: 10.1021/ol0500113. [DOI] [PubMed] [Google Scholar]; g Jacquot DEN, Zöllinger M, Lindel T. Angew. Chem. Int. Ed. 2005;44:2295–2298. doi: 10.1002/anie.200462252. [DOI] [PubMed] [Google Scholar]; h Zöllinger M, Mayer P, Lindel T. Synlett. 2007:2756–2758. [Google Scholar]; i Imaoka T, Iwamoto O, Noguchi K.-i., Nagasawa K. Angew. Chem. Int. Ed. 2009;48:3799–3801. doi: 10.1002/anie.200806233. [DOI] [PubMed] [Google Scholar]; j Hewlett NM, Tepe JJ. Org. Lett. 2011;13:4550–4553. doi: 10.1021/ol201741r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a Overman LE, Rogers BN, Tellew JE, Trenkle WC. J. Am. Chem. Soc. 1997;119:7159–7160. [Google Scholar]; b Bélanger G, Hong F-T, Overman LE, Rogers BN, Tellew JE, Trenkle WC. J. Org. Chem. 2002;67:7880–7883. doi: 10.1021/jo026282y. [DOI] [PubMed] [Google Scholar]; c Katz JD, Overman LE. Tetrahedron. 2004;60:9559–9568. [Google Scholar]; d Lanman BA, Overman LE, Paulini R, White NS. J. Am. Chem. Soc. 2007;129:12896–12900. doi: 10.1021/ja074939x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a Starr JT, Koch G, Carreira EM. J. Am. Chem. Soc. 2000;122:8793–8794. [Google Scholar]; b Breder A, Chinigo GM, Waltman AW, Carreira EM. Angew. Chem. Int. Ed. 2008;47:8514–8517. doi: 10.1002/anie.200803284. [DOI] [PubMed] [Google Scholar]; c Chinigo GM, Carreira ABEM. Org. Lett. 2011;13:78–81. doi: 10.1021/ol102577q. [DOI] [PubMed] [Google Scholar]; d Breder A, Chinigo GM, Waltman AW, Carreira EM. Chem. Eur. J. 2011;17:12405–12416. doi: 10.1002/chem.201101862. [DOI] [PubMed] [Google Scholar]
- 19.a Dilley AS, Romo D. Org. Lett. 2001;3:1535–1538. doi: 10.1021/ol015864j. [DOI] [PubMed] [Google Scholar]; b Dransfield PJ, Wang S, Dilley A, Romo D. Org. Lett. 2005;7:1679–1682. doi: 10.1021/ol0473602. [DOI] [PubMed] [Google Scholar]; c Dransfield PJ, Dilley AS, Wang S, Romo D. Tetrahedron. 2006;62:5223–5247. [Google Scholar]; d Wang S, Dilley AS, Poullennec KG, Romo D. Tetrahedron. 2006;62:7155–7161. [Google Scholar]; e Zancanella MA, Romo D. Org. Lett. 2008;10:3685–3688. doi: 10.1021/ol801289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a Lovely CJ, Du H, Dias HVR. Org. Lett. 2001;3:1319–1322. doi: 10.1021/ol015687m. [DOI] [PubMed] [Google Scholar]; b Lovely CJ, Du H, He Y, Dias HVR. Org. Lett. 2004;6:735–738. doi: 10.1021/ol036403w. [DOI] [PubMed] [Google Scholar]; c Sivappa R, Hernandez NM, He Y, Lovely CJ. Org. Lett. 2007;9:3861–3864. doi: 10.1021/ol0711568. [DOI] [PubMed] [Google Scholar]
- 21.a Kawasaki I, Sakaguchi N, Fukushima N, Fujioka N, Nikaido F, Yamashita M, Ohta S. Tetrahedron Lett. 2002;43:4377–4380. [Google Scholar]; b Kawasaki I, Sakaguchi N, Khadeer A, Yamashita M, Ohta S. Tetrahedron. 2006;62:10182–10192. [Google Scholar]
- 22.Koenig SG, Miller SM, Leonard KA, Löwe RS, Chen BC, Austin DJ. Org. Lett. 2003;5:2203–2206. doi: 10.1021/ol0344063. [DOI] [PubMed] [Google Scholar]
- 23.a Garrido-Hernandez H, Nakadai M, Vimolratana M, Li Q, Doundoulakis T, Harran PG. Angew. Chem. Int. Ed. 2005;44:765–769. doi: 10.1002/anie.200462069. [DOI] [PubMed] [Google Scholar]; b Li Q, Hurley P, Ding H, Roberts AG, Akella R, Harran PG. J. Org. Chem. 2009;74:5909–5919. doi: 10.1021/jo900755r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ding H, Roberts AG, Harran PG. Angew. Chem. Int. Ed. 2012;51:4340–4343. doi: 10.1002/anie.201200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bultman MS, Ma J, Gin DY. Angew. Chem. Int. Ed. 2008;47:6821–6824. doi: 10.1002/anie.200801969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.a Cernak TA, Gleason JL. J. Org. Chem. 2008;73:102–110. doi: 10.1021/jo701866g. [DOI] [PubMed] [Google Scholar]; b Hudon J, Cernak TA, Ashenhurst JA, Gleason JL. Angew. Chem. Int. Ed. 2008;47:8885–8888. doi: 10.1002/anie.200803344. [DOI] [PubMed] [Google Scholar]
- 26.a Namba K, Kaihara Y, Yamamoto H, Imagawa H, Tanino K, Williams RM, Nishizawa M. Chem. Eur. J. 2009;15:6560–6563. doi: 10.1002/chem.200900622. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Namba K, Inai M, Sundermeier U, Greshock TJ, Williams RM. Tetrahedron Lett. 2010;51:6557–6559. doi: 10.1016/j.tetlet.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a Feldman KS, uriye AYN. Org. Lett. 2010;12:4532–4535. doi: 10.1021/ol1018322. [DOI] [PubMed] [Google Scholar]; b Feldman KS, Nuriye AY, Li J. J. Org. Chem. 2011;76:5042–5060. doi: 10.1021/jo200740b. [DOI] [PubMed] [Google Scholar]
- 28.Fukahori Y, Takayama Y, Imaoka T, Iwamoto O, Nagasawa K. Chem. Asian J. 2012;8:244–250. doi: 10.1002/asia.201200820. [DOI] [PubMed] [Google Scholar]
- 29.a Baran PS, Zografos AL, O'Malley DP. J. Am. Chem. Soc. 2004;126:3726–3727. doi: 10.1021/ja049648s. [DOI] [PubMed] [Google Scholar]; b O'Malley DP, Li K, Maue M, Zografos AL, Baran PS. J. Am. Chem. Soc. 2007;129:4762–4775. doi: 10.1021/ja069035a. [DOI] [PubMed] [Google Scholar]
- 30.a Baran PS, O'Malley DP, Zografos AL. Angew. Chem. Int. Ed. 2004;43:2674–2677. doi: 10.1002/anie.200453937. [DOI] [PubMed] [Google Scholar]; b Baran PS, Li K, O'Malley DP, Mitsos C. Angew. Chem. Int. Ed. 2006;45:249–252. doi: 10.1002/anie.200503374. [DOI] [PubMed] [Google Scholar]; c Northrop BH, O'Malley DP, Zografos AL, Baran PS, Houk KN. Angew. Chem. Int. Ed. 2006;45:4126–4130. doi: 10.1002/anie.200600514. [DOI] [PubMed] [Google Scholar]
- 31.a Yamaguchi J, Seiple IB, Young IS, O'Malley DP, Maue M, Baran PS. Angew. Chem. Int. Ed. 2008;47:3578–3580. doi: 10.1002/anie.200705913. [DOI] [PubMed] [Google Scholar]; b O'Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew. Chem. Int. Ed. 2008;47:3581–3583. doi: 10.1002/anie.200801138. [DOI] [PubMed] [Google Scholar]; c Su S, Rodriguez RA, Baran PS. J. Am. Chem. Soc. 2011;133:13922–13925. doi: 10.1021/ja206191g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su S, Seiple IB, Young IS, Baran PS. J. Am. Chem. Soc. 2008;130:16490–16491. doi: 10.1021/ja8074852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.a Seiple IB, Su S, Young IS, Lewis CA, Yamaguchi J, Baran PS. Angew. Chem. Int. Ed. 2010;49:1095–1098. doi: 10.1002/anie.200907112. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Seiple IB, Su S, Young IS, Nakamura A, Yamaguchi J, Jørgensen L, Rodriguez RA, O'Malley DP, Gaich T, Köck M, Baran PS. J. Am. Chem. Soc. 2011;133:14710–14726. doi: 10.1021/ja2047232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birman VB, Jiang X-T. Org. Lett. 2004;6:2369–2371. doi: 10.1021/ol049283g. [DOI] [PubMed] [Google Scholar]
- 35.Ding H, Roberts AG, Harran PG. Chem. Sci. 2012;4:303–306. doi: 10.1039/C2SC21651E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker RP, Faulkner DJ, Engen DV, Clardy J. J. Am. Chem. Soc. 1981;103:6772–6773. [Google Scholar]
- 37.D'Auria M, Racioppi R. J. Photochem. Photobiol., A. 1998;112:145–148. [Google Scholar]
- 38.a Rinehart KL. Pure Appl. Chem. 1989;61:525–528. [Google Scholar]; b Keifer PA, Schwartz RE, Koker MES, Robert J, Hughes G, Rittschof D, Rinehart KL. J. Org. Chem. 1991;56:2965–2975. 6728. [Google Scholar]
- 39.Kobayashi J, Tsuda H, Murayama T, Nakamura H, Ohizumi Y, Ishibashi M, Iwamura M. Tetrahedron. 1990;46:5579–5586. [Google Scholar]
- 40.Grube A, Immel S, Baran PS, Köck M. Angew. Chem. Int. Ed. 2007;46:6721–6724. doi: 10.1002/anie.200701935. [DOI] [PubMed] [Google Scholar]
- 41.a Kinnel RB, Gehrken H-P, Scheuer PJ. J. Am. Chem. Soc. 1993;115:3376–3377. [Google Scholar]; b Kinnel RB, Gehrken H-P, Swali R, Skoropowski G, Scheuer PJ. J. Org. Chem. 1998;63:3281–3286. [Google Scholar]
- 42.a Williams DH, Faulkner DJ. Tetrahedron. 1996;52:5381–5390. [Google Scholar]; b Eder C, Proksch P, Wray V, Soest R. W. M. v., Ferdinandus E, Pattisina LA, Sudarsono J. Nat. Prod. 1999;62:1295–1297. doi: 10.1021/np990071f. [DOI] [PubMed] [Google Scholar]; c Assmann M, van Soest RWM, Köck M. J. Nat. Prod. 2001:1345–1347. doi: 10.1021/np000482s. [DOI] [PubMed] [Google Scholar]; d Endo T, Tsuda M, Okada T, Mitsuhashi S, Shima H, Kikuchi K, Mikami Y, Fromont J, Kobayashi J. J. Nat. Prod. 2004;67:1262–1267. doi: 10.1021/np034077n. [DOI] [PubMed] [Google Scholar]
- 43.a Kato T, Shizuri Y, Izumida H, Yokoyama A, Endo M. Tetrahedron Lett. 1995;36:2133–2136. [Google Scholar]; b Kobayashi J, Suzuki M, Tsuda M. Tetrahedron. 1997;53:15681–15684. [Google Scholar]
- 44.a Grube A, Köck M. Angew. Chem. Int. Ed. 2007;46:2320–2324. doi: 10.1002/anie.200604076. [DOI] [PubMed] [Google Scholar]; b Buchanan MS, Carroll AR, Addepalli R, Avery VM, Hooper JNA, Quinn RJ. J. Org. Chem. 2007;72:2309–2317. doi: 10.1021/jo062007q. [DOI] [PubMed] [Google Scholar]; c Buchanan MS, arroll ARC, Quinn RJ. Tetrahedron Lett. 2007;48:4573–4574. [Google Scholar]; d Kobayashi H, Kitamura K, Nagai K, Nakao Y, Fusetani N, van Soest RWM, Matsunaga S. Tetrahedron Lett. 2007;48:2127–2129. [Google Scholar]
- 45.a Urban S, Leone P. d. A., Carroll AR, Fechner GA, Smith J, Hooper JNA, Quinn RJ. J. Org. Chem. 1999;64:731–735. doi: 10.1021/jo981034g. [DOI] [PubMed] [Google Scholar]; b Yasuda T, Araki A, Kubota T, Ito J, Mikami Y, Fromont J, Kobayashi J. i. J. Nat. Prod. 2009;72:488–491. doi: 10.1021/np800645q. [DOI] [PubMed] [Google Scholar]; c Köck M, Schmidt G, Seiple IB, Baran PS. J. Nat. Prod. 2012;75:127–130. doi: 10.1021/np200514g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura S, Matsunaga S, Shibazaki M, Suzuki K, Furihata K, van Soest RWM, Fusetani N. Org. Lett. 2003;5:2255–2257. doi: 10.1021/ol034564u. [DOI] [PubMed] [Google Scholar]
- 47.Grube A, Köck M. Org. Lett. 2006;8:4675–4678. doi: 10.1021/ol061317s. [DOI] [PubMed] [Google Scholar]
- 48.Muñoz J, Moriou C, Gallard J-F, Marie PD, Al-Mourabit A. Tetrahedron Lett. 2012;53:5828–5832. [Google Scholar]
- 49.Welle FM, Beckhaus H-D, Rüchardt C. J. Org. Chem. 1997;62:552–558. doi: 10.1021/jo961703v. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann RW. Chem. Rev. 1989;89:1841–1860. [Google Scholar]