Abstract
SLC4 transporters are membrane proteins that in general mediate the coupled transport of bicarbonate (carbonate) and share amino acid sequence homology. These proteins differ as to whether they also transport Na+ and/or Cl−, in addition to their charge transport stoichiometry, membrane targeting, substrate affinities, developmental expression, regulatory motifs, and protein-protein interactions. These differences account in part for the fact that functionally, SLC4 transporters have various physiological roles in mammals including transepithelial bicarbonate transport, intracellular pH regulation, transport of Na+ and/or Cl−, and possibly water. Bicarbonate transport is not unique to the SLC4 family since the structurally unrelated SLC26 family has at least three proteins that mediate Cl−-HCO3− exchange. The present review focuses on the first of the sodium-dependent SLC4 transporters that was identified whose structure has been most extensively studied: the electrogenic Na+-base cotransporter NBCe1. Mutations in NBCe1 cause proximal renal tubular acidosis (pRTA) with neurologic and ophthalmologic extrarenal manifestations. Recent studies have characterized important structure-function properties of the transporter and how they are perturbed as a result of mutations that cause pRTA. It has become increasingly apparent that the structure of NBCe1 differs in several key features from the SLC4 Cl−-HCO3− exchanger AE1 whose structural properties have been well-studied. In this review, the structure-function properties and regulation of NBCe1 will be highlighted and its role in health and disease will be reviewed in detail.
Keywords: SLC4A4, carbonate, bicarbonate, renal tubular acidosis, transport, acid-base, pH
Overview of the SLC4 Family
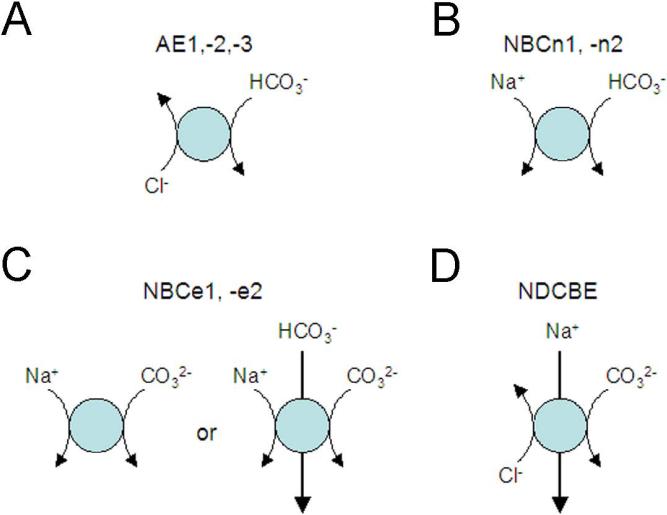
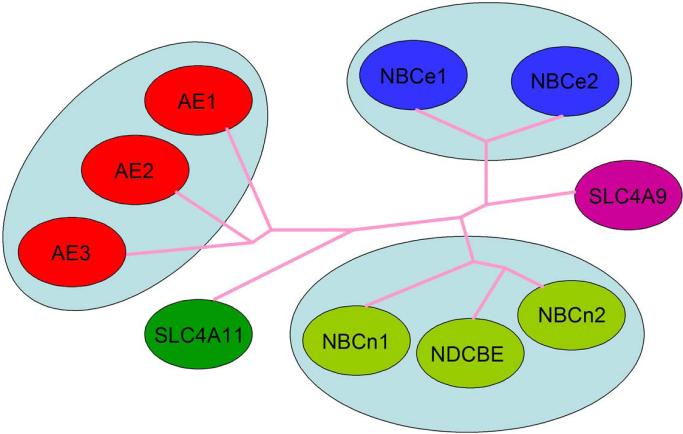
The majority of SLC4 genes encode base transporting proteins that differ in their Na+, −Cl−, and electrogenic properties Fig.1A-D, [61, 91]. 3 homologous transporters, AE1, AE2, and AE3 (SLC4A1, -2,-3 respectively) function as exchangers mediating the electroneutral exchange of Cl− and bicarbonate. A fourth protein encoded by the SLC4A9 gene (originally named AE4) was also initially reported to mediate anion exchange however its amino acid sequence more closely resembles Na+-coupled SLC4 transporters and its function is controversial. NDCBE (encoded by SLC4A8) is an ion exchanger like AE1, AE2, and AE3 yet differs in that it transports Na+ and base in exchange for Cl− electroneutrally. Two transporters, NBCn1 (SLC4A7 gene) and NBCn2 (SLC4A10 gene) are electroneutral Na+-base cotransporters. NBCe1 (SLC4A4 gene) and NBCe2 (SLC4A5 gene) are the two members of the family that mediate electrogenic Na+-base transport. SLC4A11 does not appear to transport HCO3− or CO32− however its function remains controversial having been assigned various transport modes including electrogenic Na+-BO4− cotransport, ion channel activity (Na+, OH−/H+), Na+-coupled OH−/H+ transport, and water channel flux [87, 90, 123]. Of the Na+-coupled SLC4 transporters, NBCe1 is the best understood structurally and is the focus of this review. A dendrogram of the SLC4 transporters is shown in Fig.2.
Figure 1.
SLC4 HCO32-(CO3−) transporters whose function is defined: A) AE1-3: Na+-independent Cl−-HCO3− exchangers; B) NBCn1 and NBCn2: electroneutral Na+-HCO3− transporters. It should be noted that under certain experimental conditions, NBCn2 can also mediate Na+-dependent Cl−-HCO3− exchange; C) NBCe1 and NBCe2: electrogenic Na+-CO32-(HCO3−) transporters with either a 1:2 or 1:3 stoichiometry; D) NDCBE: Na+-driven Cl−-CO3− exchanger. Not depicted is AE4 whose function is not clearly characterized. The SLC4A11 gene product does not transport HCO32−(CO3−) nor is its function clearly defined.
Figure 2.
Dendrogram of SLC4 transporters. Depicted are the protein names of those transporters whose function is well–characterized. In general, proteins with similar function are clustered because function tends to follow structure. Since the function of the proteins encoded by the SLC4A9 (“AE4”) and SLC4A11 (“BTR1” and “NaBC1”) genes remains controversial, the gene name rather than the protein names (that in general refer to a specific function) are depicted.
NBCe1 variants and tissue expression
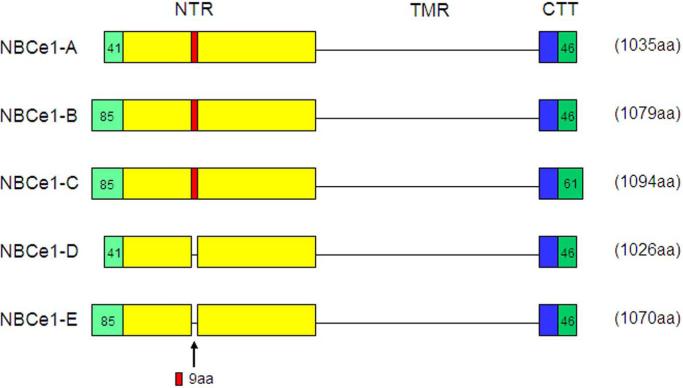
There are 5 variants of NBCe1 (NBCe1-A-E) encoded by the SLC4A4 gene [3] (human chromosome 4q21) that have been reported in mammals, which differ in their extreme N- and C-termini but have an identical transmembrane region (Fig.3;[61, 91]). Three of the variants have been functionally characterized, all of which mediate electrogenic Na+-base cotransport, but differ in their tissue expression, intrinsic activity, and modulation by regulatory factors.
Figure 3.
NBCe1 variants: The N-terminal region (NTR), the common transmembrane region (TMR), and the C terminal tail (CTT) belonging to the 5 known mammalian NBCe1 variants are depicted diagrammatically (not to scale). All variants have an identical TMR but differ in their NTR and CTT. NBCe1-A and –D only differ in their NTR in that the –D variant lacks a stretch of 9 aa (RMFSNPDNG). The NBCe1-B and –E differ in that the latter lacks the same 9 aa cassette. NBCe1-B and –C differ in their CTT where the latter has a unique C-terminus with a type I PDZ motif. NBCe1-D/-E transcripts were detected in mouse reproductive tissues [72] and have not yet been demonstrated at the protein level.
NBCe1-A is highly expressed on the basolateral membrane of kidney in S1 and S2 proximal tubules where it mediates the absorption of bicarbonate [2, 78, 104, 111]. In addition to the proximal tubule, NBCe1-A is also expressed at the protein level in the eye [14, 121] and salivary gland [18]. In turbinate mucosa and nasal polyps, NBCe1-A mRNA is detectable in nasal submucosal glands [64]. A second more widely expressed NBCe1 variant, NBCe1-B, is identical to NBCe1-A except for its extreme N-terminus wherein 85 aa replaces the 41 aa in NBCe1-A (Fig.3). NBCe1-B was originally cloned from pancreas and contributes to transepithelial pancreatic secretion [2, 39, 53, 54, 77]. NBCe1-B has been localized at the transcript and/or protein level (that should also have detected NBCe1-C/-E in some instances) in various tissues including brain, eye, heart, intestine, gall bladder, lung, skeletal muscle, submucosal glands, and nasal mucosa [1, 2, 14, 25, 29, 35, 58, 59, 64, 76, 83, 94, 121, 141]. A third variant originally cloned from rat brain [76, 80], NBCe1-C, has a unique C-terminus with a type I PDZ motif (Fig.3). NBCe1-D and NBCe1-E transcripts more recently isolated from mouse reproductive tissues have the identical sequence as NBCe1-A and NBCe1-B respectively, and differ in that they lack a nine amino-acid cassette in the cytosolic N-terminus (Fig.3; [72]). The latter transcripts have not been demonstrated to be expressed at the protein level nor has their function been reported.
Familial isolated proximal renal tubular acidosis
Hereditary proximal renal tubular acidosis (pRTA) represents a group of diseases that impair proximal tubular bicarbonate absorption [45]. The defect in bicarbonate absorption can be isolated (Table 1) or accompanied by additional proximal tubule transport defects (Table 2). Proximal tubular bicarbonate absorption is an indirect process mediated by the coupling of apical NHE3 (to a lesser extent the apical H+-ATPase) and basolateral NBCe1-A transport (Fig.4A; [16, 44]. The rate of bicarbonate absorption is enhanced by membrane bound CAIV and cytoplasmic CAII that catalyze the hydration/dehydration of CO2 in the lumen and cytoplasm respectively. Currently, mutations in NBCe1 are the only known cause of hereditary pRTA in the absence of other proximal tubule transport defects [52]. Fortuitously, the diagnosis is straightforward because of the unique spectrum of extra-renal manifestations that include short stature, ocular findings (cataracts, band keratopathy, glaucoma), neurological abnormalities (mental retardation, basal ganglia calcifications, migraine headaches), and tooth (enamel) defects that do not occur in other diseases that cause pRTA (Table 1).
Table 1.
Inherited Causes of Isolated pRTA with Asssociated Extrarenal Abnormalities
| Gene | Protein | Inheritance | Renal Phenotype | Extra-Renal Phenotype |
|---|---|---|---|---|
| CA2 | CAII | autosomal recessive | pRTA, dRTA, hypokalemia | osteopetrosis involving skull, axial skeleton, and long bones with widening of metaphyses; growth defect; intracerebral calcification |
| 1SLC4A4 | NBCe1 | autosomal recessive | pRTA, hypokalemia | band keratopathy; glaucoma; cataracts; elevated serum amylase and lipase and enamel defects; intracerebral calcification; decreased IQ; growth defect |
| Unknown gene(s) | unknown | autosomal dominant | pRTA | colomboma; sub-aortic stenosis; decreased radial bone density; thinner iliac cortices; growth defect |
Headaches occur in patients with the R510H, L522P, and R881C missense mutations, 2311delA, and a homozygous C-terminal 65 bp-del. In heterozygous patients with the 65 bp-del and the L522P mutations headaches also occur and have been attributed to a dominant-negative effect.
Table 2.
Disorders Causing pRTA with Additional Proximal Tubule Transport Abnormalities
| Gene | Protein | Inheritance | Disease |
|---|---|---|---|
| ALDOB | aldolase B | autosomal recessive | Hereditary fructose intolerance |
| ARSA | arylsulfatase A | autosomal recessive | Metachromatic leukodystrophy |
| ATP7B | Cu++ transporting ATPase β peptide | autosomal recessive | Wilson's disease |
| 1CLCN5 | 2Cl−/H+ exchanger | X-linked | Dent's disease 1 |
| 2Complex IV | cytochrome C oxidase | N/A | Cytochrome C oxidase deficiency |
| CTNS | cystinosin | autosomal recessive | Cystinosis |
| FAH | fumarylacetoacetase | autosomal recessive | Tyrosinemia type I |
| GALT | galactose-1-phosphate uridylyltransferase | autosomal recessive | Galactosemia |
| MMAB | methylmalonyl CoA mutase | autosomal recessive | Methylmalonic acidemia |
| OCRL1 | PIP2 5-phosphatase | X-linked | Dent's disease 2 |
| OCRL1 | PIP2 5-phosphatase | X-linked | Lowe's syndrome |
| PC | pyruvate carboxylase | autosomal recessive | Pyruvate carboxylase deficiency |
| SLC2A2 | GLUT2 | autosomal recessive | Fanconi-Bickel syndrome |
Dent's disease 1: 60% of patients have mutations in CLCN5 and 15% of patients have mutations in OCRL1. Mutations in OCRL1 also cause Lowe's syndrome.
Potentially involves mutations in several mitochondrial or nuclear encoded genes.
Figure 4.
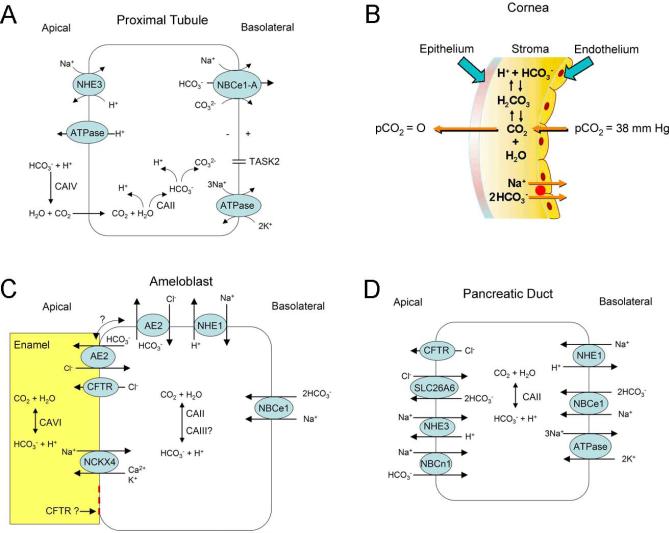
Transport models of various cell types expressing NBCe1-A. A) Proximal tubule cell; B) Corneal epithelial cell; C) Maturation stage ameloblast; D) Pancreatic duct cell. Note that in patients with NBCe1 a mutation, a pancreatic ductal phenotype has not been reported suggesting that pancreatic ductal cells likely have compensatory mechanisms mediating basolateral bicarbonate uptake. Patients typically have an increased blood lipase and amylase suggesting abnormal acinar involvement however unlike the rat, the human pancreatic acinus apparently does not express NBCe1-B [106].
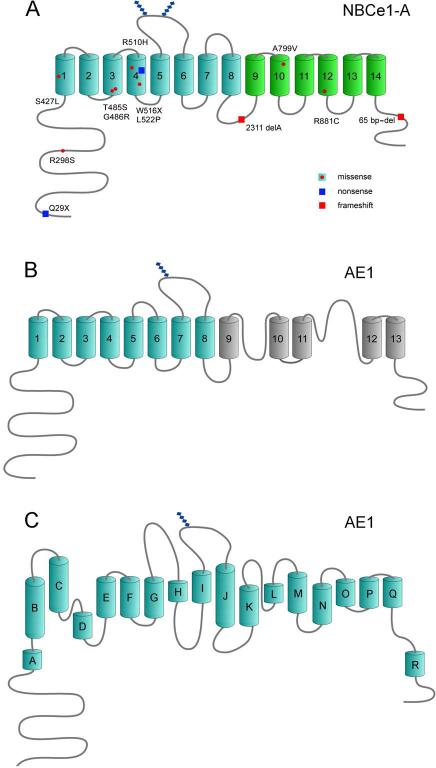
Following the original report by Igarashi et al. [52] a total of eight missense mutations (R298S, S427L, T485S, G486R, R510H, L522P, A799V, and R881C), 2 nonsense mutations (Q29X, W516X), and 2 frameshift deletions (2311 delA, and a C-terminal tail 65bp-del) have been reported (Table 3) [61]. The NBCe1-A-Q29X mutation was originally thought to be variant specific [8, 51] but could theoretically involve NBCe1-D [72] assuming the latter variant is expressed in humans. Interestingly, the patient reported with the NBCe1-A Q29X mutation did not have cataracts or band keratopathy as do patients with mutations common to all variants suggesting that NBCe1-A per se is not involved in these ocular abnormalities. The phenotype in mice with congenital loss/truncated NBCe1 is more severe than in humans and results in decreased survival, volume depletion and colonic obstruction [36, 63]. Ocular and neurologic manifestations have not been reported in mice suggesting that these abnormalities require a longer period of time to become manifest.
Table 3.
1NBCe1-A Mutations Causing Proximal RTA
| Mutation | Location | Classification | Effect of Mutation |
|---|---|---|---|
| Q29X | N-terminal region | nonsense | NBCe1-A protein truncation |
| R298S | N-terminal region | missense | - mistargeting: apical/basolateral membranes -abnormal interaction of the N- terminal region with the cytoplasmic region |
| S427L | TM1 | missense | -mistargeting: predominant apical membrane -abnormal helix packing -decreased GHCO3 -impaired IHCO3 reversal at -Vm |
| T485S | TM3 | missense | - altered ion interaction - electroneutral transport |
| G486R | TM3 | missense | altered ion interaction |
| R510H | TM4 | missense | ER retention |
| W516X | TM4 | nonsense | truncation: all NBCe1 variants |
| L522P | TM4 | missense | ER retension |
| 2311 delA | IL4 | frameshift | truncation: all NBCe1 variants |
| A799V | TM10 | missense | - intracellular retention - decreased GHCO3 - bicarbonate-independent Gcation |
| R881C | TM12 | missense | ER retention (ER) |
| 65 bp-del | C-terminal tail | frameshift | ER retention |
Numbered according to NBCe1-A amino acid sequence
GHCO3: bicarbonate conductance ; Gcation: cation conductance; IHCO3: bicarbonate-dependent current; -Vm: negative plasma membrane voltages
The disease causing mechanism of the known nonsense/frameshift mutations is due to absence of the full-length NBCe1 protein, whereas missense mutations affect the intrinsic function of the transporter and/or impair plasma membrane processing/targeting (Table 3). Unlike the majority of the findings in patients with NBCe1 mutations, migraine headaches have been reported that are mutation specific. Specifically, headaches have been described in patients with R510H, L522P, R881C, 2311 delA, and 65bp-del mutations [117]. Heterozygous family members of a patient with a 65 base-pair C-terminal deletion and the L522P mutation have also been reported to have headaches. It has been hypothesized that headaches in homozygotes may be due to abnormal NMDA-mediated neuronal hyperactivity resulting from ER retained misfolded NBCe1-B in brain astrocytes, whereas in heterozygotes, ER retained mutant-wild type NBCe1-B hetero-oligomer formation may be involved [117, 137].
Unlike AE1 mutations where both autosomal recessive and dominant inheritance has been reported [12], there are no known cases with NBCe1 mutations where only one allele is mutated. Whether individuals heterozygous for NBCe1 mutations have subtle defects in proximal tubule bicarbonate transport and/or mild ocular, brain, growth, and pancreatic enzyme abnormalities is unknown. In addition, there are no reports of naturally occurring gain of function NBCe1 mutations in patients although residues have been identified whose substitution modestly increases the base transport function [4]. Whether various NBCe1 SNPs have functional effects has recently been addressed. Yamazaki et al. examined the NBCe1 SNPs E122G, S356Y, K558R, and N640I and reported that the base transport function of K558R was decreased 41-47% [136]. The impact of these SNPs on more subtle changes in proximal tubule bicarbonate transport and extrarenal cellular function is unknown.
In addition to NBCe1 mutations, familial isolated pRTA with a dominant inheritance has also been described in patients with short stature, and decreased bone density (Table 2) [19, 56, 68]. Mutations in proximal tubule proteins involved in H+/base transport including CA II, CA IV, CA XIV, NBCe1, NHE3, NHE8, NHERF1 and NHERF2, and PAT1(CFEX) were not detected [56]. Intron and promoter region mutations in the genes encoding these proteins have not yet been excluded in these patients.
Extrarenal manifestations in patients with NBCe1 mutations and pRTA
In addressing the pathophysiology of the extrarenal manifestations in patients with NBCe1 mutations, there is currently insufficient data to attribute a specific phenotype to defective NBCe1 function at the cellular level per se versus the effect of chronic systemic acidemia that all patients have prior to treatment. Moreover, we currently lack an understanding of the role of each of the NBCe1 variants in several of the tissues that are affected in patients; specifically the role of the local loss of NBCe1 function in brain (NBCe1-B, NBCe1-C), eye (NBCe1-A, NBCe1-B, and teeth ameloblasts (NBCe1-B). Finally, several of the studies that have examined the tissue protein expression of NBCe1 have used antibodies that could theoretically pick up multiple variants i.e. NBCe1-B/-C-/-E, and therefore the tissue expression of these variants is an area that requires further study. H+/base transport models of some of the cells where NBCe1 proteins are expressed are depicted in Fig.4A-D.
Growth
The decreased growth that is seen in patients with NBCe1 mutations also occurs in other pediatric diseases where metabolic acidosis is present from birth [12, 45]. Although not well recognized, fetal blood is normally more acidic than the maternal circulation and therefore patients with NBCe1 mutations might be exposed to an even more severe metabolic acidosis prior to birth [115]. This consideration has not been previously addressed and could also contribute to growth retardation seen in other causes of metabolic acidosis in early childhood.
Brain
Abnormal brain development leading to a low IQ has not been attributed to acid-base disturbances per se, making it more likely that this phenotype is due to the loss of NBCe1 function in specific cell types in the brain, particularly given the known expression of NBCe1 variants in the cortex and hippocampus [76]. NBCe1 transcripts are also expressed in the striatum that might account for the finding that patients with NBCe1 mutations have calcified basal ganglia [50] as do patients with CAII mutations [17].
Eye
The eye is an additional targeted organ in patients with NBCe1 mutations. Normal lid opening during blinking creates an intermittent loss of CO2 resulting in acute alkalization of the anterior corneal tear coat (Fig.4B). The increased corneal pH would normally be ameliorated by modulation of endothelial cell NBCe1-B transport. Loss of NBCe1-B function is thought to cause an abnormal increase in corneal pH resulting in the precipitation of calcium phosphate (band keratopathy) in the central cornea (region exposed during lid opening). In addition to the cornea, electrogenic Na+-base transport has previously been detected in the toad lens [127] likely mediated by NBCe1-B [14] that is expressed in the lens epithelium and potentially accounts for the poorly understood finding that these patients have cataracts implying that lens transparency is pH-dependent. NBCe1-B is also expressed in the pigmented ciliary body [14] where Woloson et al. has identified an electrogenic Na+-base transport process in rabbit [128]. Exactly how the loss of NBCe1-B activity leads to glaucoma is currently unknown.
Teeth
The abnormal tooth enamel in patients [32] has been attributed to loss of local ameloblast NBCe1-B function leading to abnormal pH regulation of the extracellular matrix during enamel formation (Fig.4C) [63]. Whether the low systemic pH is also playing an important role is not known. In a recent study, mandibular E11.5 explants from NBCe1−/− mice maintained in host kidney capsules of normal for 70 days, resulted in teeth with enamel and dentin with morphological and mineralization properties similar to cultured NBCe1+/+ mandibles suggesting that the cause of the enamel phenotype is primarily to be due to abnormalities in systemic pH [126].
Gastrointestinal Tract
In the pancreas although NBCe1-B is expressed on the ductal cell basolateral cell membrane and mediates base influx (Fig.4D) [39, 53, 54, 77, 106], the loss of NBCe1-B function does not lead to any clinically apparent ductal abnormalities unlike in cystic fibrosis where transepithelial bicarbonate secretion is thought to be impaired [89]. Loss of NBCe1-B somehow leads to an abnormal increase in systemic amylase and lipase in patients suggesting a pH dependency of the secretion and or change in permeability of acinar cells producing these enzymes. Although in rats NBCe1-B is expressed in acinar cells, in humans acinar cell NBCe1-B has not been detected [106]. Although NBCe1-B is also expressed in the intestine, the loss of the transporter does not lead to a severe GI phenotype as it does in mice [36] suggesting that compensatory mechanisms play a more important role in the physiology of the GI tract in humans.
Structure-function properties of NBCe1
Of the 5 variants the structure of NBCe1-A has been most thoroughly studied providing a topologic framework not only for the remaining NBCe1 variants that share an identical transmembrane region, but potentially other Na+-coupled SLC4 bicarbonate transporters. NBCe1-A is a dimer as recently demonstrated in the native kidney in situ using fluorescence image moment analysis and spatial intensity distribution analysis (SpIDA) [109]. Intracellular pH and/or bicarbonate may modulate the dimerization of the N-terminal region [37]. Each monomer is a ~ 140-kDa glycoprotein protein containing 1035 amino acids (Fig.5A) with the ability to independently transport ions [55]. The topologic structure of NBCe1 is conveniently divided into 3 regions: a long N-terminal cytoplasmic region, a large transmembrane region, and a shorter C-terminal cytoplasmic tail. Using cysteine scanning mutagenesis, Zhu et al. analyzed the topologic properties of NBCe1-A showing that it has 14 transmembrane segments (TMs); the N-terminal transmembrane region has 8 TMs homologous to AE1, whereas TMs 10-14 fold significantly differently [148, 150] (Fig.5A,B). The N-terminal region and C-terminal tail of NBCe1 proteins are located in the cytoplasm in all NBCe1 variants and all share a large glycosylated extracellular loop between transmembrane segment (TM) 5 and 6 [26] and a smaller extracellular loop between TMs 7 and 8. The larger loop is not normally accessible to enzymatic digestion suggesting that it is compactly folded within the protein [145]. NBCe1 lacks the two re-entrant loops that were previously reported in AE1 [144]. More recently, the topologic structure of AE1 has been modified based on homology with a prokaryotic ClC protein (Fig.5C) [15, 134]. The extracellular surface of NBCe1-A is compactly folded and the transporter has certain topologic features that resemble the vGLUT and LeuT prokaryotic Na+-coupled transporters [125, 135, 148, 150].
Figure 5.
A) Membrane topology of NBCe1-A [148, 150]. Known mutations throughout the transporter are depicted with the majority localized to the transmembrane region. All NBCe1 variants have a transmembrane region identical to NBCe1-A, which has been most thoroughly studied. B) Membrane topology of AE1 based on Zhu et al. [143, 150]; C) Membrane topology of AE1 based on recent homology with a prokaryotic ClC transporter [15, 134].
Cytoplasmic N-terminal region
A preliminary low resolution crystal of the N-terminal region of NBCe1-A has been reported [38]. Alignment with AE1 showed similar predicted helical regions and hydrophobic residues that can potentially stabilize the N-terminal NBCe1-A dimer interface. The N-terminal cytoplasmic region contains two known NBCe1 mutations: The Q29X nonsense mutation is specific for NBCe1-A (and would be predicted to also truncate NBCe1-D), and the R298S mutation that is common to all NBCe1 variants (Table 3; Fig.5A). The Q29X mutation was the first example of an NBCe1 mutation where potential mutant–specific therapy was demonstrated [8]. In this mutation, a wt-CAG sequence encoding glutamine replaces a UAG stop codon causing premature truncation [51]. Azimov et al. found that in HEK 293-H cells expressing the NBCe1-A-Q29X mutant, the aminoglycoside G418 could induce ribosomal read-though producing full-length functional NBCe1-A protein [8]. These studies also offered the opportunity to treat the eye abnormalities in patients using topical aminoglycosides thereby avoiding their systemic toxicity. Less toxic more potent aminoglycosides with a flexible N-1-AHB group ((S)-2-hydroxy-4-aminobutyl group at the N-1 position) [86] in addition to non-aminoglycoside compounds such Ataluren [93] that induce ribosomal read-through may also prove efficacious.
The N-terminal domain R298S pRTA mutation common to all NBCe1 variants appears to reside in a tightly folded region that may form a “HCO3− tunnel” in the wild-type transporter but whose structure is disrupted in the mutant [22, 49, 52, 117, 148]. Zhu et al. have hypothesized that the N-terminal and transmembrane regions normally interact and that in the mutant, the efficient delivery of base to the ion permeation pathway is perturbed [146] Other groups have shown that the NBCe1-A-R298S and corresponding NBCe1-B-R342S mutant when expressed in MDCK cells have a trafficking defect. Specifically, rather than the typical polarized expression on the basolateral membrane, the arginine to serine mutation mistargets the transporter to both the apical and basolateral membranes [70, 116] suggesting that a targeting signal in the N-terminal domain is perturbed in the mutant.
Transmembrane Region (TM1-14)
TM1 contains 31 amino acids (longer than a standard TM) with an N-terminal cytosolic portion that has a helical conformation connecting the transmembrane portion with the cytoplasmic portion [146]. Structure-function studies of the wild-type transporter have demonstrated that the extracellular C-terminal end of TM1 forms part of the ion permeation pathway where Thr442 (which is located within a 421ITFGGLLG428 motif (NBCe1-A numbering) found in most SLC4 transporters) appears to be part of an extracellular gate involved in ion entry [147]. TM1 contains several key residues including Ala428, AlaP435 in addition to Thr442 that are functionally important and appear to line the ion permeation pathway [147]. Misfolding of the transporter due to substituting Asp416, Gln424, Tyr433, and Asn439 with cysteine causes intracellular retention.
pRTA is caused by a TM1 S427L pRTA mutation which decreases transporter function by 90% and at very negative membrane potentials also blocks the reversal of the direction of the transport current [32]. Ser427 has been recently localized in TM1 to space-confined region wherein the serine side chain hydrophobicity is involved in helix packing ionic /interactions between helices [146]. The S427L functional impairment is caused by conformational change in TM1 associated with a collapse/altered configuration of the ion permeation pathway. An additional mechanism has been reported when the S427L mutant is expressed in MDCK cells, where the mutant transporter is mistargeted to the apical membrane [70].
The T485S and R486R mutations are adjacent substitutions that independently cause pRTA [49, 116, 117, 148, 149]. Unlike all other known NBCe1 mutations, the T485S mutation would not be predicted to cause pRTA because of the structural and chemical similarity between serine and threonine. Zhu et al. hypothesized that Thr485 must therefore reside in a critical region within the transporter either in the aqueous ion permeation pathway or an ion interaction site [149]. Thr485 was subsequently shown to be localized to an aqueous confined region accessible to NEM labeling, and functional sensitivity to MTS reagents was substrate-dependant (due to intra- and extracellular facing conformational changes). These results provided the first evidence that Thr485 is located in an ion interaction site. In addition, the G486R mutation was shown to alter the orientation of Thr485 impairing its labeling with NEM [149].
The T485S mutation decreases the overall base transport function of NBCe1-A by ~ 50%. [49, 116, 117, 148, 149]. The results of whole cell patch clamp experiments indicated that the wild-type transporter functioned as an electrogenic Na+-CO32− cotransporter with one anion interaction site [149]. The T485S mutation converted NBCe1-A from an electrogenic to an electroneutral transporter suggesting that the mutant transporter mediates Na+-HCO3− cotransport [149]. Moreover using NO3− as a surrogate CO32− transport, unlike wt-NBCe1-A, the mutant transporter failed to mediate Na+-driven NO3− [149] suggesting that it favors bicarbonate as a substrate.
The R510H, L522P, and W516X pRTA mutations are located in TM4 that appears to function as a scaffolding helix essential for normal protein folding, and also has potential stop transfer and signal anchor sequences [31, 49, 52, 70, 116, 117, 137, 148]. The W516X nonsense mutation results in a truncated and likely misfolded protein [73]. The R510H and L522P mutations induce misfolding of the mutant proteins and retention in the ER [31, 49, 52, 70, 116, 117, 137, 148]. Why R510H is retained in the ER is currently unclear however it has been hypothesized that the size of the side-chain and/or magnitude of the positive charge is required for ionic interaction between TM4 and neighboring TMs. Given that L522I and L522C are processed to the plasma membrane normally, [137, 148] the L522P substitution likely causes increased TM flexibility resulting in helix disruption, protein misfolding, and ER retention.
TM5 plays an important role in anion interaction and selectivity [139]. Within TM5, Asp555 appears to prevent various anions from being transported by NBCe1-A nonspecifically. In addition to functioning as an electrogenic Na+-base transporter, when glutamate is substituted at Asp555, NBCe1 also mediates the transport of Cl−, NO3−, and SCN−. Residues in TM5 also interact with the well-known stilbene inhibitor 4, 4’-diisothiocyanatostilbene-2,2’-disulfonate (DIDS). DIDS binds from the extracellular surface of the transporter to a KKMIK motif in TM5 [74]. Whether DIDS sterically blocks ionic interaction/permeation through the transporter is currently unknown. DIDS may also prevent substrate induced conformational changes required to mediate ion transport. The apparent affinity between DIDS and NBCe1 is increased at more positive membrane voltages; a phenomenon attributed to voltage dependent changes in transporter conformation [74, 131]. DIDS also inhibits transporter function from the intracellular surface at an undetermined binding site [46]. The mechanism and site of inhibition of other known NBCe1 inhibitors including tenidap [33], benzamil [33], and S0859 [21] has not been studied.
NBCe1 has two extracellular loops that appear to play a functional/structural role. Extracellular loop 3, EL3, located between TM5 and TM6 is the largest (56 amino acids) loop in NBCe1 and other Na+-driven SLC4 transporters, and contains 4 cysteine residues [145] and is glycosylated [26]. The 4 cysteines are intramolecular disufided and form a highly ordered topologically domain [145]. As in the Cys-loop ligand-gated ion channel superfamily, it has been hypothesized that this region in EL3 may bind a ligand(s) that regulates the function of the transporter. The next extracellular loop, EL4, located between TM7 and TM8 can interact with membrane bound CAIV [5] and CAIX [88]. EL4 has also been reported to be involved in the electrogenic properties of NBCe1 [23]. Whether EL4 interacts with residues in the ion permeation pathway thereby modulating the electrogenic properties of NBCe1 remains to be determined.
McAlear et al. proposed that NBCe1-A-TM8 as had previously been shown in AE1 [118] might play an important role in ion permeation [79]. Using cysteine scanning mutagenesis of TM8, pCMBS accessibility was modulated by the presence of substrate ions and the stilbene inhibitor 4.4-dinitro-stilbene-2, 2’-disulfonate (DNDS). Leu750 was reported to play an important role in ion permeation.
Patients with generalized proximal tubule transport impairment (Fanconi's syndrome) and less often with isolated proximal renal tubular acidosis have associated hypokalemia [103]. Several factors including enhanced HCO3− delivery to the collecting duct and an increased aldosterone level due to volume depletion result in enhanced principal cell K+ secretion. In 2001 Deda et al. reported a patient with the phenotype of patients with NBCe1 mutations who had extra-renal K+ loss due to diarrhea and vomiting causing severe acute hypokalemia [30]. It was subsequently shown that the patient had an A799V pRTA mutation that significantly decreased the function of mutant NBCe1-A [49, 117, 148]. Parker et al. showed that the mutant transporter had an associated HCO3−-independent cation leak conductance [92] and hypothesized that given the expression of NBCe1 in skeletal muscles (sarcollema and possibly t-tubules), during severe acute hypokalemia a patient with the A799V mutation would manifest exacerbated muscle weakness.
The TM12 R881C pRTA mutation represents another example where the membrane expression in mammalian cells and Xenopus oocytes differs [49, 117, 120, 148]. When expressed in mammalian cells the R881C substitution induces ER retention suggesting that the transporter is misfolded and that TM12 is involved in helix packing [150]. In Xenopus oocytes the plasma membrane expression of the mutant transporter is decreased [49, 120]. Moreover, the reported functional abnormality of the mutant transporter appears to be entirely due to abnormal plasma membrane expression [120].
Using a TM swapping approach, Chen et al. concluded that TM12 and TM6 form a “functional unit” that plays a role in plasma membrane expression [24]. In these experiments a chimera was formed by replacing TM6 and what was referred to as “TM12” with corresponding TMs from electroneutral NBCn1, and membrane processing was significantly decreased. Given that according to the current topological model rather than TM12 alone, residues from TM12, intracellular loop 6 (IL6), and TM13 were also swapped, and the finding that mixed chimeras per se can potentially fold improperly resulting in ER retention [34] the interpretation of the data is likely premature. Moreover, residues in TM12 are known to play a role in helix packing [150].
Not only does TM12 but the entire C-terminal transmembrane region from TM10-14 (Ala800 and Lys967), plays an important role helix packing and protein folding [150]. The NBCe1 structure is stabilized by 18 residues clustered on the surface of that form intramolecular hydrogen bonds. The loops connecting TMs11-14 are not aqueous exposed and are tightly folded in the protein. Met858 bracketed by Pro857 and Pro858 is the residue where TM11 and 12 are abruptly bent into the lipid bilayer. TM12 and 13 are connected by a cryptic intracellular loop 6 (IL6) which is also tightly folded and likely interacts with the cytoplasmic region. TM12-Lys924 acts as a counter ion and contributes to helix packing. Extracellular loop 7 (EL7; Thr926-Ala929) is likely folded in the transmembrane region since it is minimally exposed to the aqueous media [150].
Cytoplasmic C-terminal tail
Asp960 marks the intracellular lipid/aqueous interface of TM14. A kink is introduced at Pro963 exposing the C-terminal cytoplasmic tail to the cytoplasm [150]. There is a close association between the C-terminal cytoplasmic tail of each monomer in the NBCe1 dimer grouping of two stretches of strongly charged amino acids that may form a regulatory motif [150]. Evidence suggests that the C-terminal tail plays a role in membrane processing and targeting. Specifically, a pRTA 65 bp-del frame shift mutation truncates the C-terminal tail and is retained in the ER in mammalian cells (although not in Xenopus oocytes) [117]. In addition, a C-terminal tail 1010QQPFLSP1015 motif has been identified that functions as a basolateral targeting signal in MDCK cells [71].
Comparison of NBCe1 and AE1
Until the topologic structure of NBCe1 was demonstrated by cysteine scanning mutagenesis studies, it was assumed that NBCe1 and other SLC4 proteins adopted a membrane topology similar to AE1. The differences in the topologic structure of the two proteins likely reflects their functional properties in keeping with the well-characterized differences in the atomic structure of prokaryotic ion exchangers and Na+-coupled substrate transporters. Recent mutagenesis and cryoEM studies have shown that the AE1 fold resembles a prokaryotic ClC protein (Fig.5C) [15, 134] whereas the LeuT and vSGLT prokaryotic Na+-coupled substrate transporters [125, 135] and NBCe1-A share certain features. NBCe1-A and AE1 have structural differences which likely play an important role in their unique functional properties and ion preferences. In NBCe1-A the first transmembrane segment,TM1, plays an important role in forming part of the ion translocation pathway and interacts tightly with the cytoplasmic domain [147]. AE1-TM1 has not been shown to be involved in ion permeation and the AE1 N-terminal cytoplasmic region does not appear to form a substrate access tunnel [110]. The large extracellular loop (EL3) in NBCe1-A and AE1 differ in that in NBCe1-A, EL3 is intra-disulfided and resistant to enzymatic digestion suggesting it is tightly folded tightly and may play an important a role in ligand binding [145]. The C-terminal transmembrane region of NBCe1-A is also tightly folded and lacks reentrant loops that were previously reported in AE1 [144, 150]. In AE1, TM13 and 14 are involved in ion translocation whereas in NBCe1-A, these TMs do not have a similar role [143, 150].
Modulation of NBCe1 Function
Intrinsic structural features
The differential regulation of the function of NBCe1 variants is due in part to intrinsic differences in the structure of their N-terminal domains and their specific interaction with various regulatory factors. McAlear et al. first demonstrated that the unique N-terminus of NBCe1-A differs from other variants in that it has as an autostimulatory domain (ASD) that enhances the function of the transporter through a currently unknown mechanism [80]. Whether the identical N-terminus in the NBCe1-D variant functions similarly has not been studied. Zhu et al. recently reported evidence for interaction between the NBCe1-A N-terminal and transmembrane regions [146]. Whether this interaction is unique to NBCe1-A and/or plays a role in modulating its function via the ASD or a separate mechanism requires further study.
In contrast to the ASD in NBCe1-A, the N-terminus of NBCe1-B and NBCe1-C (and presumably the –E variant) functions as an autoinhibitory domain (AID) [66, 80]. The autoinhibition by AID is stabilized by recruitment of the WNK/SPAK pathway [47]. IRBIT which binds to the NBCe1-B N-terminus at residues 37-65 (a positively charged motif) stimulates the transporter by recruiting protein phosphatase 1 (PP1) that blocks the WNK/SPAK inhibition [47, 66, 138]. PIP2 which can also activate the transporter may bind to the same motif as IRBIT [47]. PIP2 mediated activation of NBCe1-B and –C appears to involve a staurosporine-sensitive kinase [119]. Of interest, PIP2 also activates NBCe1-A (and potentially NBCe1-D) however the mechanism remains undetermined [129].
Magnesium
Mg2+ inhibits the function of NBCe1-B [132, 133] and potentially NBCe1-C/-E. Whether Mg2+ and IRBIT compete for a common binding site or act through independent pathways is currently unknown. Mg2+ has also been shown to cause NBCe1-A rundown in Xenopus oocytes, through a mechanism that has been hypothesized to involve a Mg2+-dependent phosphatase (5′-lipid phosphatase) that dephosphorylates PIP2 to PIP [129]. Wu et al. hypothesized that the inhibition of NBCe1 function by an elevation of intracellular Mg2+ may reduce cellular dysfunction during ischemia [129]. Finally, the Hsp70-like stress 70 protein chaperone STCH interacts with the residues amino acids 96–440 distal to the IRBIT interaction site in NBCe1-B inducing a significant increase in plasma membrane expression [10]. Whether STCH also increases the plasma membrane expression of NBCe1-A/-C/-D/–E is currently unknown. Bae et al. have proposed that STCH stimulation of NBCe1 plasma membrane expression could represent a regulatory pathway that prevents cellular dysfunction during acidemia [10]. The utility of STCH as a therapeutic tool to increase plasma membrane expression remains to be determined.
Phosphorylation state
The phosphorylation state of NBCe1 can affect membrane expression, charge transport stoichiometry, and ion flux through the transporter. In oocytes and proximal tubule cells, ANG II has a biphasic effect on NBCe1 mediated transport [27, 28, 48, 95, 142]. ANG II-induced inhibition of NBCe1-A via the AT1B receptor is mediated by Ca2+--insensitive PKCε leading to decreased NBCe1-A surface expression [94, 95]. In Xenopus oocytes expressing NBCe1-A, Ca2+ also alters the charge transport stoichiometry shifting it from 1:2 to 1:3, possibly via PKC phosphorylation [85]. PKA dependent phosphorylation of NBCe1-A-Ser982 was also reported to shift the charge transport stoichiometry from 1:3 to 1:2 [43]. Thr49 in the unique amino-terminus of NBCe1-B modulates the cAMP-induced increase in cotransporter current without altering its stoichiometry [41]. Accordingly, cAMP has also been found to increase intestinal NBCe1-B mediated transport in part via a change in membrane expression [9, 141]. Both conventional PKCs (PKCαβγ) and a novel PKCδ also participate in constitutive and stimulated (carbachol) endocytosis of NBCe1-A and NBCe1-B [96] providing a potential mechanism for modulating NBCe1-B transport in salivary duct, ileum, and colon [11, 96, 141]. In addition to PKCs, ATP has been hypothesized to phosphorylate NBCe1-A via an unidentified protein kinase thereby increasing the cotransporter current [85].
Carbonic anhydrase
Gross et al. proposed that NBCe1 and carbonic anhydrase II (CAII) form a transport metabolon based on the results of experiments using isothermal titration calorimetry, which showed that CAII binds to NBCe1-A at a C-terminal D986NDD989 motif [40]. Pushkin et al. and Becker et al. provided additional experimental support for interaction between NBCe1 and CAII [13, 99]. CAIV and CAIX were subsequently shown to bind to the extracellular surface of NBCe1-B at EL4 [5, 88]. Other groups however were unable to show a functional interaction with CAII [75, 97, 130]. In comparing these studies, one needs to be cognizant of the techniques/preparations used in addition to the technical differences among the various assays employed. Regardless of whether a metabolon between CA proteins and NBCe1 exists in vivo, there does not appear to be a clinically important interaction between the proteins in that loss of CAII function results in a more mild phenotype than loss of NBCe1 function. Specifically, patients with loss of function mutations in CAII, and mice with targeted disruption of CAII do not have severe pRTA [69, 112]. In addition, patients with CAIV mutations have retinitis pigmentosa (RP17) and pRTA has not been reported [100]. Schueler recently reexamined this question and showed that CAI, CAII and CAIII stimulate NBCe1-A transport in Xenopus oocytes [107] and attributed the CA stimulation of NBCe1-A function to carbonic anhydrase enzymatic activity rather then intramolecular proton shuttling.
Systemic and hormonal factors
Both NaCl and NaHCO3 loading have been shown to reduce the expression of proximal tubule NHE3 and NBCe1-A providing a mechanism for ameliorating both volume overload and metabolic alkalosis during systemic bicarbonate loading [7]. Potassium depletion with concomitant metabolic alkalosis is associated with an increase in proximal tubule renal bicarbonate reabsorption [20, 101, 102] that is potentially due to increased expression of NBCe1-A [6].
Norepinephrine, dopamine, and PTH, and potentially changes in blood pressure have also been reported to modulate the expression of NBCe1. In rats long-term infusion of noradrenaline increased the expression of the transporter [114]. Dopamine decreased the activity of NBCe1-A in rabbit and rat proximal tubules [60]. NBCe1 function is decreased by PTH in the rat possibly via a cAMP whereas in rabbit PTH has no effect [105]. NBCe1 protein expression is increased ~2 fold in the SHR rat in comparison to control WKY rats [113] however the exact mechanism is unknown.
In a rat model of acute renal transplant rejection, NBCe1-A is upregulated [122] whereas the calcineurin inhibitor FK506 decreases NBCe1-A expression [81]. NBCe1-A expression is upregulated in lithium induced distal renal tubular acidosis (dRTA) perhaps as compensatory mechanism [57]. In contrast, ureteral obstruction which induces hyperkalemic dRTA decreases NBCe1-A expression in the proximal tubule [124]. Hypothyroidism is associated with incomplete dRTA in humans, and in a rat hypothyroid model, was associated with decreased NBCe1 abundance [82]. During concomitant NH4Cl loading and hypothyroidism, the expression of NBCe1 increased [82].
NBCe1 charge transport stoichiometry and substrate anion transport: carbonate versus bicarbonate transport
The electrochemical driving force (μ) across NBCe1 proteins dictates the direction of NBCe1 transport. Since in all cells the chemical gradient for Na+ and base (bicarbonate, carbonate) is inward (extracellular to cytoplasm), the charge transport stoichiometry of NBCe1 and the membrane potential are the two major determinants of the overall electrochemical driving force [62]. Based on studies in other species, in the human kidney it is assumed NBC1-A has a charge transport stoichiometry of 1:3 (1 Na+;1 HCO3− :1 CO32−) such that value of μ is positive. In vivo measurements in the rat proximal tubule reported a charge transport stoichiometry of 1:3 [140]. In the isolated perfused rabbit proximal tubule, the value varied from 1:2-1:2.7 depending on the composition of the solutions used [84, 108]. In Necturus proximal tubules in vivo, the charge transport stoichiometry decreases from 1:3 to 1:2 during an acute increase in the PCO2 [98]. Gross et al. reported that the charge transport stoichiometry of NBCe1 is cell-type dependent [42]. Importantly, in heterologous expression systems where the signal/noise ratio is excellent and technical artifacts are minimal, the charge transport stoichiometry of human NBCe1-A in both human HEK293 cells and Xenopus oocytes is 1:2 rather than 1:3 [67, 149]. The question as to whether the charge transport stoichiometry can be modulated in vivo in humans as has been reported in vitro with altered phosphorylation status [43], intracellular Ca2+ [85] and respiratory acid-base parameters [98] is unknown.
In secretory epithelia such as pancreas, salivary glands, and intestine, and non-secretory cells such as astrocytes a charge transport stoichiometry of 1:2 is assumed given that NBCe1-B normally mediates cellular Na+-coupled base influx, and indeed in cultured pancreatic cells a value of 1:2 has been measured [39]. A charge transport stoichiomtery of 1:2 is compatible with either 1 Na+:2 HCO3− transport (2 anion interaction sites) or Na+-CO32− cotransport (1 anion interaction site). Using NO3− as a surrogate for CO3− transport Zhu et al. reported that human NBCe1-A expressed in HEK cells functioning with a 1:2 charge transport stoichiometry functions as Na+-CO32− cotransporter [149]. Preliminary measurements of surface pH in the Xenopus oocyte expression system also suggests that rat NBCe1-A functions as a Na+-CO32− cotransporter [65].
Although measurements will likely never be obtained in native human proximal tubule cells (leaving open the questions as to whether cell-specific factors can modulate the stoichiometry and the exact electrochemical driving force across NBCe1-A in the basolateral membrane in the human proximal tubule), the finding that human NBCe1-A has a charge transport stoichiometry of 1:2 in expression systems with excellent signal/noise, has prompted the re-evaluation of whether a 1:2 charge transport stoichiometry might be sufficient to drive Na+-CO32− efflux in the human proximal tubule in vivo. Using data obtained in the rat proximal tubule since the necessary human data is unavailable, it has recently been shown that of NBCe1-A would have a positive value thereby driving cytoplasmic to peritubular Na+-CO32− efflux; and that the transport process would be very sensitive to changes in small changes in μ [149]. These findings support the notion that that NBCe1 is actually a Na+-CO32− cotransporter whose name should be changed to NCCe1-A (sodium carbonate cotransporter electrogenic 1-A).
Loss of NBCe1-A electrogenicity: Lessons learned in the context of the T485S mutation
It is instructive in this regard, to reconsider the loss of NBCe1 electrogenicity in the context of the T485S mutation in both the proximal tubule and in extra-renal secretory organs [149]. In the proximal tubule, independent of the initial charge transport stoichiometry (which is currently unknown), given the initial inwardly directed substrate ion gradients and the concomitant loss of the effect of the basolateral membrane potential as a driving force, electroneutral Na+-HCO3− influx mediated by the T485S mutant will significantly impair transepithelial bicarbonate absorption. Unlike NBCe1-A, NBCe1-B/-C normally mediate cellular base influx in the cells in which they are expressed. Assuming that the threonine to serine substitution also renders these variants electroneutral with an associated total base transport decrease of ~ 50%, in the steady state, a decrease in cellular base influx would be predicted to result from both the decrease in intrinsic base transport, and in addition, the decreased extracellular bicarbonate concentration (due to renal bicarbonate loss) that is available for transport. Future in vitro and in vivo studies are required to address studies are required Given that many of these abnormalities are predicted to change dynamically, it would be useful to have a conditional transgenic mouse model system to study the effect of the T485S mutation on both the magnitude and direction of renal and extra-renal base transport as a function of time.
Acknowledgements
This work was supported in part by the NIH grants DK077162 and DK058563.
References
- 1.Abdulnour-Nakhoul S, Nakhoul HN, Kalliny MI, Gyftopoulos A, Rabon E, Doetjes R, Brown K, Nakhoul NL. Ion transport mechanisms linked to bicarbonate secretion in the esophageal submucosal glands. Am J Physiol Regul Integr Comp Physiol. 2011;301:R83–R96. doi: 10.1152/ajpregu.00648.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- 3.Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene. 2000;251:109–122. doi: 10.1016/s0378-1119(00)00204-3. [DOI] [PubMed] [Google Scholar]
- 4.Abuladze N, Azimov R, Newman D, Sassani P, Liu W, Tatishchev S, Pushkin A, Kurtz I. Critical amino acid residues involved in the electrogenic sodium-bicarbonate cotransporter kNBC1-mediated transport. J Physiol. 2005;565:717–730. doi: 10.1113/jphysiol.2005.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry. 2003;42:12321–12329. doi: 10.1021/bi0353124. [DOI] [PubMed] [Google Scholar]
- 6.Amlal H, Habo K, Soleimani M. Potassium deprivation upregulates expression of renal basolateral Na+-HCO3− cotransporter (NBC-1). Am J Physiol Renal Physiol. 2000;279:F532–F543. doi: 10.1152/ajprenal.2000.279.3.F532. [DOI] [PubMed] [Google Scholar]
- 7.Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001;60:1824–1836. doi: 10.1046/j.1523-1755.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 8.Azimov R, Abuladze N, Sassani P, Newman D, Kao L, Liu W, Orozco N, Ruchala P, Pushkin A, Kurtz I. G418-mediated ribosomal read-through of a nonsense mutation causing autosomal recessive proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2008;295:F633–F641. doi: 10.1152/ajprenal.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol. 2003;284:G37–G45. doi: 10.1152/ajpgi.00209.2002. [DOI] [PubMed] [Google Scholar]
- 10.Bae JS, Koo NY, Namkoong E, Davies AJ, Choi SK, Shin Y, Jin M, Hwang SM, Mikoshiba K, Park K. Chaperone stress 70 protein (STCH) binds and regulates two acid/base transporters NBCe1-B and NHE1. J Biol Chem. 2013;288:6295–6305. doi: 10.1074/jbc.M112.392001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartolo RC, Harfoot N, Gill M, McLeod BJ, Butt AG. Secretagogues stimulate electrogenic HCO3− secretion in the ileum of the brushtail possum, Trichosurus vulpecula: evidence for the role of a Na+/HCO3− cotransporter. J Exp Biol. 2009;212:2645–2655. doi: 10.1242/jeb.028928. [DOI] [PubMed] [Google Scholar]
- 12.Batlle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant. 2012;27:3691–3704. doi: 10.1093/ndt/gfs442. [DOI] [PubMed] [Google Scholar]
- 13.Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J Biol Chem. 2007;282:13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- 14.Bok D, Schibler MJ, Pushkin A, Sassani P, Abuladze N, Naser Z, Kurtz I. Immunolocalization of electrogenic sodium-bicarbonate cotransporters pNBC1 and kNBC1 in the rat eye. Am J Physiol Renal Physiol. 2001;281:F920–F935. doi: 10.1152/ajprenal.2001.281.5.F920. [DOI] [PubMed] [Google Scholar]
- 15.Bonar P, Schneider HP, Becker HM, Deitmer JW, Casey JR. Three-dimensional model for the human Cl−/HCO3− exchanger, AE1, by homology to the E. coli ClC protein. J Mol Biol. 2013;425:2591–2608. doi: 10.1016/j.jmb.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol. 2006;17:2368–2382. doi: 10.1681/ASN.2006060620. [DOI] [PubMed] [Google Scholar]
- 17.Bosley TM, Salih MA, Alorainy IA, Islam MZ, Oystreck DT, Suliman OS, al Malki S, Suhaibani AH, Khiari H, Beckers S, van Wesenbeeck L, Perdu B, AlDrees A, Elmalik SA, Van Hul W, Abu-Amero KK. The neurology of carbonic anhydrase type II deficiency syndrome. Brain. 2011;134:3502–3515. doi: 10.1093/brain/awr302. [DOI] [PubMed] [Google Scholar]
- 18.Brandes A, Oehlke O, Schumann A, Heidrich S, Thevenod F, Roussa E. Adaptive redistribution of NBCe1-A and NBCe1-B in rat kidney proximal tubule and striated ducts of salivary glands during acid-base disturbances. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2400–R2411. doi: 10.1152/ajpregu.00208.2007. [DOI] [PubMed] [Google Scholar]
- 19.Brenes LG, Brenes JN, Hernandez MM. Familial proximal renal tubular acidosis. A distinct clinical entity. Am J Med. 1977;63:244–252. doi: 10.1016/0002-9343(77)90238-8. [DOI] [PubMed] [Google Scholar]
- 20.Capasso G, Jaeger P, Giebisch G, Guckian V, Malnic G. Renal bicarbonate reabsorption in the rat. II. Distal tubule load dependence and effect of hypokalemia. J Clin Invest. 1987;80:409–414. doi: 10.1172/JCI113087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ch'en FF, Villafuerte FC, Swietach P, Cobden PM, Vaughan-Jones RD. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br J Pharmacol. 2008;153:972–982. doi: 10.1038/sj.bjp.0707667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang MH, DiPiero J, Sonnichsen FD, Romero MF. Entry to “formula tunnel” revealed by SLC4A4 human mutation and structural model. J Biol Chem. 2008;283:18402–18410. doi: 10.1074/jbc.M709819200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LM, Liu Y, Boron WF. Role of an extracellular loop in determining the stoichiometry of Na+-HCO3− cotransporters. J Physiol. 2011;589:877–890. doi: 10.1113/jphysiol.2010.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen LM, Qin X, Moss FJ, Liu Y, Boron WF. Effect of simultaneously replacing putative TM6 and TM12 of human NBCe1-A with those from NBCn1 on surface abundance in Xenopus oocytes. J Membr Biol. 2012;245:131–140. doi: 10.1007/s00232-012-9421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC). Am J Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- 26.Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3− cotransporter (NBCe1). Am J Physiol Renal Physiol. 2003;284:F1199–F1206. doi: 10.1152/ajprenal.00131.2002. [DOI] [PubMed] [Google Scholar]
- 27.Coppola S, Frömter E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. I. Effect of picomolar concentrations. Pflugers Arch. 1994;427:143–150. doi: 10.1007/BF00585953. [DOI] [PubMed] [Google Scholar]
- 28.Coppola S, Frömter E. An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. II. Effect of micromolar concentrations. Pflugers Arch. 1994;427:151–156. doi: 10.1007/BF00585954. [DOI] [PubMed] [Google Scholar]
- 29.De Giusti VC, Orlowski A, Villa-Abrille MC, de Cingolani GE, Casey JR, Alvarez BV, Aiello EA. Antibodies against the cardiac sodium/bicarbonate co-transporter (NBCe1) as pharmacological tools. Br J Pharmacol. 2011;164:1976–1989. doi: 10.1111/j.1476-5381.2011.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deda G, Ekim M, Guven A, Karagol U, Tumer N. Hypopotassemic paralysis: a rare presentation of proximal renal tubular acidosis. J Child Neurol. 2001;16:770–771. doi: 10.1177/088307380101601013. [DOI] [PubMed] [Google Scholar]
- 31.Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis. 2006;12:324–330. [PubMed] [Google Scholar]
- 32.Dinour D, Chang MH, Satoh J, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem. 2004;279:52238–52246. doi: 10.1074/jbc.M406591200. [DOI] [PubMed] [Google Scholar]
- 33.Ducoudret O, Diakov A, Müller-Berger S, Romero MF, Frömter E. The renal Na-HCO3-cotransporter expressed in Xenopus laevis oocytes: inhibition by tenidap and benzamil and effect of temperature on transport rate and stoichiometry. Pflugers Arch. 2001;442:709–717. doi: 10.1007/s004240100594. [DOI] [PubMed] [Google Scholar]
- 34.Fujinaga J, Loiselle FB, Casey JR. Transport activity of chimaeric AE2-AE3 chloride/bicarbonate anion exchange proteins. Biochem J. 2003;371:687–696. doi: 10.1042/BJ20030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garciarena CD, Ma YL, Swietach P, Huc L, Vaughan-Jones RD. Sarcolemmal localisation of Na+/H+ exchange and Na+-HCO3− co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. J Physiol. 2013;591:2287–2306. doi: 10.1113/jphysiol.2012.249664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem. 2007;282:9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 37.Gill HS. pH-sensitive self-associations of the N-terminal domain of NBCe1-A suggest a compact conformation under acidic intracellular conditions. Protein Pept Lett. 2012;19:1054–1063. doi: 10.2174/092986612802762642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill HS, Boron WF. Expression and purification of the cytoplasmic N-terminal domain of the Na/HCO3 cotransporter NBCe1-A: structural insights from a generalized approach. Protein Expr Purif. 2006;49:228–234. doi: 10.1016/j.pep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Gross E, Abuladze N, Pushkin A, Kurtz I, Cotton CU. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO3−:1 Na+. J Physiol. 2001;531:375–382. doi: 10.1111/j.1469-7793.2001.0375i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross E, Pushkin A, Abuladze N, Fedotoff O, Kurtz I. Regulation of the sodium bicarbonate cotransporter kNBC1 function: role of Asp986, Asp988 and kNBC1-carbonic anhydrase II binding. J Physiol. 2002;544:679–685. doi: 10.1113/jphysiol.2002.029777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross E, Fedotoff O, Pushkin A, Abuladze N, Newman D, Kurtz I. Phosphorylation-induced modulation of pNBC1 function: distinct roles for the amino- and carboxy-termini. J Physiol. 2003;549:673–682. doi: 10.1113/jphysiol.2003.042226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol. 2001;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross E, Hawkins K, Pushkin A, Sassani P, Dukkipati R, Abuladze N, Hopfer U, Kurtz I. Phosphorylation of Ser982 in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3− : Na+ stoichiometry from 3 : 1 to 2 : 1 in murine proximal tubule cells. J Physiol. 2001;537:659–665. doi: 10.1111/j.1469-7793.2001.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamm LL, Alperin RJ, P.A. P. Cellular Mechanisms of Unrinary Acidification. In: Alperin RJ, Caplan M, Moe OW, editors. Seldin and Giebisch's The Kidney: Physiology and Pathophysiology. Elsevier/Academic Press; Amsterdam; Boston: 2013. pp. 1917–1978. [Google Scholar]
- 45.Haque SK, Ariceta G, Batlle D. Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant. 2012;27:4273–4287. doi: 10.1093/ndt/gfs493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heyer M, Müller-Berger S, Romero MF, Boron WF, Frömter E. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflugers Arch. 1999;438:322–329. doi: 10.1007/s004240050916. [DOI] [PubMed] [Google Scholar]
- 47.Hong JH, Yang D, Shcheynikov N, Ohana E, Shin DM, Muallem S. Convergence of IRBIT, phosphatidylinositol (4,5) bisphosphate, and WNK/SPAK kinases in regulation of the Na+-HCO3− cotransporters family. Proc Natl Acad Sci U S A. 2013;110:4105–4110. doi: 10.1073/pnas.1221410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horita S, Zheng Y, Hara C, Yamada H, Kunimi M, Taniguchi S, Uwatoko S, Sugaya T, Goto A, Fujita T, Seki G. Biphasic regulation of Na+-HCO3− cotransporter by angiotensin II type 1A receptor. Hypertension. 2002;40:707–712. doi: 10.1161/01.hyp.0000036449.70110.de. [DOI] [PubMed] [Google Scholar]
- 49.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol. 2005;16:2270–2278. doi: 10.1681/ASN.2004080667. [DOI] [PubMed] [Google Scholar]
- 50.Igarashi T, Ishii T, Watanabe K, Hayakawa H, Horio K, Sone Y, Ohga K. Persistent isolated proximal renal tubular acidosis-a systemic disease with a distinct clinical entity. Pediatr Nephrol. 1994;8:70–71. doi: 10.1007/BF00868266. [DOI] [PubMed] [Google Scholar]
- 51.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol. 2001;12:713–718. doi: 10.1681/ASN.V124713. [DOI] [PubMed] [Google Scholar]
- 52.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 53.Ishiguro H, Steward MC, Wilson RW, Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495(Pt 1):179–91. doi: 10.1113/jphysiol.1996.sp021583. DOI Electronic Resource Number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem. 2008;283:26782–26794. doi: 10.1074/jbc.M804006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katzir Z, Dinour D, Reznik-Wolf H, Nissenkorn A, Holtzman E. Familial pure proximal renal tubular acidosis-a clinical and genetic study. Nephrol Dial Transplant. 2008;23:1211–1215. doi: 10.1093/ndt/gfm583. [DOI] [PubMed] [Google Scholar]
- 57.Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frokiaer J, Nielsen S. Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2003;285:F1244–1257. doi: 10.1152/ajprenal.00176.2003. [DOI] [PubMed] [Google Scholar]
- 58.Kreindler JL, Peters KW, Frizzell RA, Bridges RJ. Identification and membrane localization of electrogenic sodium bicarbonate cotransporters in Calu-3 cells. Biochim Biophys Acta. 2006;1762:704–710. doi: 10.1016/j.bbadis.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Kristensen JM, Kristensen M, Juel C. Expression of Na+/HCO3− co-transporter proteins (NBCs) in rat and human skeletal muscle. Acta Physiol Scand. 2004;182:69–76. doi: 10.1111/j.1365-201X.2004.01297.x. [DOI] [PubMed] [Google Scholar]
- 60.Kunimi M, Seki G, Hara C, Taniguchi S, Uwatoko S, Goto A, Kimura S, Fujita T. Dopamine inhibits renal Na+:HCO3− cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int. 2000;57:534–543. doi: 10.1046/j.1523-1755.2000.00873.x. [DOI] [PubMed] [Google Scholar]
- 61.Kurtz I. SLC4 Sodium-driven bicarbonate transporters. In: Alperin RJ, Caplan M, Moe OW, editors. Seldin and Giebisch's The Kidney: Physiology and Pathophysiology. Elsevier/Academic Press; Amsterdam; Boston: 2013. pp. 1837–1860. [Google Scholar]
- 62.Kurtz I, Petrasek D, Tatishchev S. Molecular mechanisms of electrogenic sodium bicarbonate cotransport: structural and equilibrium thermodynamic considerations. J Membr Biol. 2004;197:77–90. doi: 10.1007/s00232-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 63.Lacruz RS, Nanci A, White SN, Wen X, Wang H, Zalzal SF, Luong VQ, Schuetter VL, Conti PS, Kurtz I, Paine ML. The sodium bicarbonate cotransporter (NBCe1) is essential for normal development of mouse dentition. J Biol Chem. 2010;285:24432–24438. doi: 10.1074/jbc.M110.115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SH, Park JH, Jung HH, Lee SH, Oh JW, Lee HM, Jun HS, Cho WJ, Lee JY. Expression and distribution of ion transport mRNAs in human nasal mucosa and nasal polyps. Acta Otolaryngol. 2005;125:745–752. doi: 10.1080/00016480510028519. [DOI] [PubMed] [Google Scholar]
- 65.Lee SK, Grichtchenko II, Boron WF. Distinguishing HCO3− from CO32− transport by NBCe1-A. FASEB J. 2011;25:656.9. [Google Scholar]
- 66.Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na-HCO3 cotransporter NBCe1-B: role of IRBIT vs.amino-terminal truncation. Am J Physiol Cell Physiol. 2012;302:C518–C526. doi: 10.1152/ajpcell.00352.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SK, Boron WF, Parker MD. Substrate specificity of the electrogenic sodium/bicarbonate cotransporter NBCe1-A (SLC4A4, variant A) from humans and rabbits. Am J Physiol Renal Physiol. 2013;304:F883–F899. doi: 10.1152/ajprenal.00612.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemann J, Jr., Adams ND, Wilz DR, Brenes LG. Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int. 2000;58:1267–1277. doi: 10.1046/j.1523-1755.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 69.Lewis SE, Erickson RP, Barnett LB, Venta PJ, Tashian RE. N-ethyl-N-nitrosourea-induced null mutation at the mouse Car-2 locus: an animal model for human carbonic anhydrase II deficiency syndrome. Proc Natl Acad Sci U S A. 1988;85:1962–1966. doi: 10.1073/pnas.85.6.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+:HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis of proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2005;289:F61–F71. doi: 10.1152/ajprenal.00032.2005. [DOI] [PubMed] [Google Scholar]
- 71.Li HC, Worrell RT, Matthews JB, Husseinzadeh H, Neumeier L, Petrovic S, Conforti L, Soleimani M. Identification of a carboxyl-terminal motif essential for the targeting of Na+-HCO3− cotransporter NBC1 to the basolateral membrane. J Biol Chem. 2004;279:43190–43197. doi: 10.1074/jbc.M405780200. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Xu JY, Wang DK, Wang L, Chen LM. Cloning and identification of two novel NBCe1 splice variants from mouse reproductive tract tissues: a comparative study of NCBT genes. Genomics. 2011;98:112–119. doi: 10.1016/j.ygeno.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int. 2011;79:730–741. doi: 10.1038/ki.2010.523. [DOI] [PubMed] [Google Scholar]
- 74.Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3 cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol. 2007;292:C1787–C1798. doi: 10.1152/ajpcell.00267.2006. [DOI] [PubMed] [Google Scholar]
- 75.Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- 76.Majumdar D, Maunsbach AB, Shacka JJ, Williams JB, Berger UV, Schultz KP, Harkins LE, Boron WF, Roth KA, Bevensee MO. Localization of electrogenic Na/bicarbonate cotransporter NBCe1 variants in rat brain. Neuroscience. 2008;155:818–832. doi: 10.1016/j.neuroscience.2008.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marino CR, Jeanes V, Boron WF, Schmitt BM. Expression and distribution of the Na+-HCO3− cotransporter in human pancreas. Am J Physiol. 1999;277:G487–G494. doi: 10.1152/ajpgi.1999.277.2.G487. [DOI] [PubMed] [Google Scholar]
- 78.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na/HCO3 cotransporter in rat and ambystoma kidney. J Am Soc Nephrol. 2000;11:2179–2189. doi: 10.1681/ASN.V11122179. [DOI] [PubMed] [Google Scholar]
- 79.McAlear SD, Bevensee MO. A cysteine-scanning mutagenesis study of transmembrane domain 8 of the electrogenic sodium/bicarbonate cotransporter NBCe1. J Biol Chem. 2006;281:32417–32427. doi: 10.1074/jbc.M607253200. [DOI] [PubMed] [Google Scholar]
- 80.McAlear SD, Liu X, Williams JB, McNicholas-Bevensee CM, Bevensee MO. Electrogenic Na/HCO3 cotransporter (NBCe1) variants expressed in Xenopus oocytes: functional comparison and roles of the amino and carboxy termini. J Gen Physiol. 2006;127:639–658. doi: 10.1085/jgp.200609520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am J Physiol Renal Physiol. 2009;297:F499–509. doi: 10.1152/ajprenal.90489.2008. [DOI] [PubMed] [Google Scholar]
- 82.Mohebbi N, Kovacikova J, Nowik M, Wagner CA. Thyroid hormone deficiency alters expression of acid-base transporters in rat kidney. Am J Physiol Renal Physiol. 2007;293:F416–F427. doi: 10.1152/ajprenal.00391.2006. [DOI] [PubMed] [Google Scholar]
- 83.Moser AJ, Gangopadhyay A, Bradbury NA, Peters KW, Frizzell RA, Bridges RJ. Electrogenic bicarbonate secretion by prairie dog gallbladder. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1683–G1694. doi: 10.1152/ajpgi.00268.2006. [DOI] [PubMed] [Google Scholar]
- 84.Müller-Berger S, Nesterov VV, Frömter E. Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. II. Change of Na-HCO3 cotransport stoichiometry and of response to acetazolamide. Pflugers Arch. 1997;434:383–391. doi: 10.1007/s004240050411. [DOI] [PubMed] [Google Scholar]
- 85.Müller-Berger S, Ducoudret O, Diakov A, Frömter E. The renal Na-HCO3− cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflugers Arch. 2001;442:718–728. doi: 10.1007/s004240100592. [DOI] [PubMed] [Google Scholar]
- 86.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, Belakhov V, Hainrichson M, Chen F, Schacht J, Pilch DS, Ben-Yosef T, Baasov T. Development of novel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem. 2009;52:2836–2845. doi: 10.1021/jm801640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na+ permeable pHi regulator. Am J Physiol Cell Physiol. 2013;305:C716–C727. doi: 10.1152/ajpcell.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orlowski A, De Giusti VC, Morgan PE, Aiello EA, Alvarez BV. Binding of carbonic anhydrase IX to extracellular loop 4 of the NBCe1 Na+/HCO3− cotransporter enhances NBCe1-mediated HCO3- influx in the rat heart. Am J Physiol Cell Physiol. 2012;303:C69–C80. doi: 10.1152/ajpcell.00431.2011. [DOI] [PubMed] [Google Scholar]
- 89.Park HW, Lee MG. Transepithelial bicarbonate secretion: lessons from the pancreas. Cold Spring Harb Perspect Med. 2012;2(10):ii, a009571. doi: 10.1101/cshperspect.a009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+ -coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell. 2004;16:331–341. doi: 10.1016/j.molcel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 91.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev. 2013;93:803–959. doi: 10.1152/physrev.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parker MD, Qin X, Williamson RC, Toye AM, Boron WF. HCO3−-independent conductance with a mutant Na+/HCO3− cotransporter (SLC4A4) in a case of proximal renal tubular acidosis with hypokalaemic paralysis. J Physiol. 2012;590:2009–2034. doi: 10.1113/jphysiol.2011.224733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peltz SW, Morsy M, Welch EM, Jacobson A. Ataluren as an agent for therapeutic nonsense suppression. Annu Rev Med. 2013;64:407–425. doi: 10.1146/annurev-med-120611-144851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perry C, Le H, Grichtchenko II. ANG II and calmodulin/CaMKII regulate surface expression and functional activity of NBCe1 via separate means. Am J Physiol Renal Physiol. 2007;293:F68–F77. doi: 10.1152/ajprenal.00454.2006. [DOI] [PubMed] [Google Scholar]
- 95.Perry C, Blaine J, Le H, Grichtchenko II. PMA- and ANG II-induced PKC regulation of the renal Na+-HCO3− cotransporter (hkNBCe1). Am J Physiol Renal Physiol. 2006;290:F417–F427. doi: 10.1152/ajprenal.00395.2004. [DOI] [PubMed] [Google Scholar]
- 96.Perry C, Baker OJ, Reyland ME, Grichtchenko II. PKCαβγ- and PKCδ-dependent endocytosis of NBCe1-A and NBCe1-B in salivary parotid acinar cells. Am J Physiol Cell Physiol. 2009;297:C1409–C1423. doi: 10.1152/ajpcell.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piermarini PM, Kim EY, Boron WF. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J Biol Chem. 2007;282:1409–1421. doi: 10.1074/jbc.M608261200. [DOI] [PubMed] [Google Scholar]
- 98.Planelles G, Thomas SR, Anagnostopoulos T. Change of apparent stoichiometry of proximal-tubule Na+-HCO3− cotransport upon experimental reversal of its orientation. Proc Natl Acad Sci U S A. 1993;90:7406–7410. doi: 10.1073/pnas.90.15.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pushkin A, Abuladze N, Gross E, Newman D, Tatishchev S, Lee I, Fedotoff O, Bondar G, Azimov R, Ngyuen M, Kurtz I. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J Physiol. 2004;559:55–565. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebello G, Ramesar R, Vorster A, Roberts L, Ehrenreich L, Oppon E, Gama D, Bardien S, Greenberg J, Bonapace G, Waheed A, Shah GN, Sly WS. Apoptosis-inducing signal sequence mutation in carbonic anhydrase IV identified in patients with the RP17 form of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2004;101:6617–6622. doi: 10.1073/pnas.0401529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rector FC, Jr., Bloomer HA, Seldin DW. Effect of Potassium Deficiency on the Reabsorption of Bicarbonate in the Proximal Tubule of the Rat Kidney. J Clin Invest. 1964;43:1976–1982. doi: 10.1172/JCI105071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts KE, Randall HT, Sanders HL, Hood M. Effects of potassium on renal tubular reabsorption of bicarbonate. J Clin Invest. 1955;34:666–672. doi: 10.1172/JCI103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodriguez Soriano J. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol. 2002;13:2160–170. doi: 10.1097/01.asn.0000023430.92674.e5. [DOI] [PubMed] [Google Scholar]
- 104.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3− cotransporter. Nature. 1997;387:409–413. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- 105.Sasaki S, Marumo F. Mechanisms of inhibition of proximal acidification by PTH. Am J Physiol. 1991;260:F833–F838. doi: 10.1152/ajprenal.1991.260.6.F833. [DOI] [PubMed] [Google Scholar]
- 106.Satoh H, Moriyama N, Hara C, Yamada H, Horita S, Kunimi M, Tsukamoto K, Iso ON, Inatomi J, Kawakami H, Kudo A, Endou H, Igarashi T, Goto A, Fujita T, Seki G. Localization of Na+-HCO3− cotransporter (NBC-1) variants in rat and human pancreas. Am J Physiol Cell Physiol. 2003;284:C729–C737. doi: 10.1152/ajpcell.00166.2002. [DOI] [PubMed] [Google Scholar]
- 107.Schueler C, Becker HM, McKenna R, Deitmer JW. Transport activity of the sodium bicarbonate cotransporter NBCe1 is enhanced by different isoforms of carbonic anhydrase. PLoS One. 2011;6:e27167. doi: 10.1371/journal.pone.0027167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seki G, Coppola S, Frömter E. The Na+-HCO3− cotransporter operates with a coupling ratio of 2 HCO3− to 1 Na+ in isolated rabbit renal proximal tubule. Pflugers Arch. 1993;425:409–416. doi: 10.1007/BF00374866. [DOI] [PubMed] [Google Scholar]
- 109.Sergeev M, Godin AG, Kao L, Abuladze N, Wiseman PW, Kurtz I. Determination of membrane protein transporter oligomerization in native tissue using spatial fluorescence intensity fluctuation analysis. PLoS One. 2012;7:e36215. doi: 10.1371/journal.pone.0036215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shnitsar V, Li J, Li X, Calmettes C, Basu A, Casey JR, Moraes TF, Reithmeier RA. A Substrate Access Tunnel in the Cytosolic Domain Is Not an Essential Feature of the Solute Carrier 4 (SLC4) Family of Bicarbonate Transporters. J Biol Chem. 2013;288:33848–33860. doi: 10.1074/jbc.M113.511865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skelton LA, Boron WF, Zhou Y. Acid-base transport by the renal proximal tubule. J Nephrol. 2010;23(Suppl 16):S4–S18. [PMC free article] [PubMed] [Google Scholar]
- 112.Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M, et al. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985;313:139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- 113.Sonalker PA, Tofovic SP, Jackson EK. Increased expression of the sodium transporter BSC-1 in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2004;311:1052–1061. doi: 10.1124/jpet.104.071209. [DOI] [PubMed] [Google Scholar]
- 114.Sonalker PA, Tofovic SP, Bastacky SI, Jackson EK. Chronic noradrenaline increases renal expression of NHE-3, NBC-1, BSC-1 and aquaporin-2. Clin Exp Pharmacol Physiol. 2008;35:594–600. doi: 10.1111/j.1440-1681.2007.04846.x. [DOI] [PubMed] [Google Scholar]
- 115.Spackman T, Fuchs F, Assali NS. Acid-base status of the fetus in human pregnancy. Obstet Gynecol. 1963;22:785–791. [PubMed] [Google Scholar]
- 116.Suzuki M, Vaisbich MH, Yamada H, Horita S, Li Y, Sekine T, Moriyama N, Igarashi T, Endo Y, Cardoso TP, de Sa LC, Koch VH, Seki G, Fujita T. Functional analysis of a novel missense NBC1 mutation and of other mutations causing proximal renal tubular acidosis. Pflugers Arch. 2008;455:583–593. doi: 10.1007/s00424-007-0319-y. [DOI] [PubMed] [Google Scholar]
- 117.Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na+-HCO3− cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci U S A. 2010;107:15963–15968. doi: 10.1073/pnas.1008705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang XB, Kovacs M, Sterling D, Casey JR. Identification of residues lining the translocation pore of human AE1, plasma membrane anion exchange protein. J Biol Chem. 1999;274:3557–3564. doi: 10.1074/jbc.274.6.3557. [DOI] [PubMed] [Google Scholar]
- 119.Thornell IM, Wu J, Liu X, Bevensee MO. PIP2 hydrolysis stimulates the electrogenic Na+-bicarbonate cotransporter NBCe1-B and -C variants expressed in Xenopus laevis oocytes. J Physiol. 2012;590:5993–6011. doi: 10.1113/jphysiol.2012.242479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol. 2006;291:C788–C801. doi: 10.1152/ajpcell.00094.2006. [DOI] [PubMed] [Google Scholar]
- 121.Usui T, Hara M, Satoh H, Moriyama N, Kagaya H, Amano S, Oshika T, Ishii Y, Ibaraki N, Hara C, Kunimi M, Noiri E, Tsukamoto K, Inatomi J, Kawakami H, Endou H, Igarashi T, Goto A, Fujita T, Araie M, Seki G. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. J Clin Invest. 2001;108:107–115. doi: 10.1172/JCI11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Velic A, Hirsch JR, Bartel J, Thomas R, Schroter R, Stegemann H, Edemir B, August C, Schlatter E, Gabriels G. Renal transplantation modulates expression and function of receptors and transporters of rat proximal tubules. J Am Soc Nephrol. 2004;15:967–977. doi: 10.1097/01.asn.0000117287.74203.89. [DOI] [PubMed] [Google Scholar]
- 123.Vilas GL, Loganathan SK, Liu J, Riau AK, Young JD, Mehta JS, Vithana EN, Casey JR. Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum Mol Genet. 2013;22:4579–4590. doi: 10.1093/hmg/ddt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang G, Li C, Kim SW, Ring T, Wen J, Djurhuus JC, Wang W, Nielsen S, Frokiaer J. Ureter obstruction alters expression of renal acid-base transport proteins in rat kidney. Am J Physiol Renal Physiol. 2008;295:F497–F506. doi: 10.1152/ajprenal.00425.2007. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe A, Choe S, Chaptal V, Rosenberg JM, Wright EM, Grabe M, Abramson J. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature. 2010;468:988–991. doi: 10.1038/nature09580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen X, Kurtz I, Paine ML. Rescue of the disrupted enamel phenotype in Slc4a4-null mice using explant organ culture maintained in a living host kidney capsule. FASEB J. 2014 doi: 10.1371/journal.pone.0097318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wolosin JM, Alvarez LJ, Candia OA. HCO3− transport in the toad lens epithelium is mediated by an electronegative Na+-dependent symport. Am J Physiol. 1990;258:C855–C861. doi: 10.1152/ajpcell.1990.258.5.C855. [DOI] [PubMed] [Google Scholar]
- 128.Wolosin JM, Chen M, Gordon RE, Stegman Z, Butler GA. Separation of the rabbit ciliary body epithelial layers in viable form: identification of differences in bicarbonate transport. Exp Eye Res. 1993;56:401–409. doi: 10.1006/exer.1993.1054. [DOI] [PubMed] [Google Scholar]
- 129.Wu J, McNicholas CM, Bevensee MO. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates the electrogenic Na/HCO3 cotransporter NBCe1-A expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 2009;106:14150–14155. doi: 10.1073/pnas.0906303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamada H, Horita S, Suzuki M, Fujita T, Seki G. Functional role of a putative carbonic anhydrase II-binding domain in the electrogenic Na+-HCO3− cotransporter NBCe1 expressed in Xenopus oocytes. Channels (Austin) 2011;5:106–109. doi: 10.4161/chan.5.2.14341. [DOI] [PubMed] [Google Scholar]
- 131.Yamaguchi S, Ishikawa T. Electrophysiological characterization of native Na+-HCO3− cotransporter current in bovine parotid acinar cells. J Physiol. 2005;568:181–197. doi: 10.1113/jphysiol.2005.088633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamaguchi S, Ishikawa T. The electrogenic Na+-HCO3− cotransporter NBCe1-B is regulated by intracellular Mg2+. Biochem Biophys Res Commun. 2008;376:100–104. doi: 10.1016/j.bbrc.2008.08.104. [DOI] [PubMed] [Google Scholar]
- 133.Yamaguchi S, Ishikawa T. IRBIT reduces the apparent affinity for intracellular Mg2+ in inhibition of the electrogenic Na+-HCO3− cotransporter NBCe1-B. Biochem Biophys Res Commun. 2012;424:433–438. doi: 10.1016/j.bbrc.2012.06.127. [DOI] [PubMed] [Google Scholar]
- 134.Yamaguchi T, Ikeda Y, Abe Y, Kuma H, Kang D, Hamasaki N, Hirai T. Structure of the membrane domain of human erythrocyte anion exchanger 1 revealed by electron crystallography. J Mol Biol. 2010;397:179–189. doi: 10.1016/j.jmb.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 135.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 136.Yamazaki O, Yamada H, Suzuki M, Horita S, Shirai A, Nakamura M, Seki G, Fujita T. Functional characterization of nonsynonymous single nucleotide polymorphisms in the electrogenic Na+-HCO3− cotransporter NBCe1A. Pflugers Arch. 2011;461:249–259. doi: 10.1007/s00424-010-0918-x. [DOI] [PubMed] [Google Scholar]
- 137.Yamazaki O, Yamada H, Suzuki M, Horita S, Shirai A, Nakamura M, Satoh N, Fujita T, Seki G. Identification of dominant negative effect of L522P mutation in the electrogenic Na+-HCO3− cotransporter NBCe1. Pflugers Arch. 2013;465:1281–1291. doi: 10.1007/s00424-013-1277-1. [DOI] [PubMed] [Google Scholar]
- 138.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011;121:956–965. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang HS, Kim E, Lee S, Park HJ, Cooper DS, Rajbhandari I, Choi I. Mutation of aspartate 555 of the sodium/bicarbonate transporter SLC4A4/NBCe1 induces chloride transport. J Biol Chem. 2009;284:15970–15979. doi: 10.1074/jbc.M109.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yoshitomi K, Burckhardt BC, Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985;405:360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- 141.Yu H, Riederer B, Stieger N, Boron WF, Shull GE, Manns MP, Seidler UE, Bachmann O. Secretagogue stimulation enhances NBCe1 (electrogenic Na+/HCO3− cotransporter) surface expression in murine colonic crypts. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1223–G1231. doi: 10.1152/ajpgi.00157.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zheng Y, Horita S, Hara C, Kunimi M, Yamada H, Sugaya T, Goto A, Fujita T, Seki G. Biphasic regulation of renal proximal bicarbonate absorption by luminal AT1A receptor. J Am Soc Nephrol. 2003;14:1116–1122. doi: 10.1097/01.asn.0000064700.58048.c1. [DOI] [PubMed] [Google Scholar]
- 143.Zhu Q, Casey JR. The substrate anion selectivity filter in the human erythrocyte Cl−/HCO3− exchange protein, AE1. J Biol Chem. 2004;279:23565–23573. doi: 10.1074/jbc.M401380200. [DOI] [PubMed] [Google Scholar]
- 144.Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem. 2003;278:3112–3120. doi: 10.1074/jbc.M207797200. [DOI] [PubMed] [Google Scholar]
- 145.Zhu Q, Kao L, Liu W, Newman D, Azimov R, Kurtz I. Extracellular loop 3 forms domain-like structure on the surface of NBCe1-A. J Am Soc Nephrol. 2012;23:31A. [Google Scholar]
- 146.Zhu Q, Liu W, Kao L, Azimov R, Newman D, Abuladze N, Kurtz I. Topology of NBCe1 protein transmembrane segment 1 and structural effect of proximal renal tubular acidosis (pRTA) S427L mutation. J Biol Chem. 2013;288:7894–7906. doi: 10.1074/jbc.M112.404533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhu Q, Azimov R, Kao L, Newman D, Liu W, Abuladze N, Pushkin A, Kurtz I. NBCe1-A transmembrane segment 1 lines the ion translocation pathway. J Biol Chem. 2009;284:8918–8929. doi: 10.1074/jbc.M806674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu Q, Kao L, Azimov R, Newman D, Liu W, Pushkin A, Abuladze N, Kurtz I. Topological location and structural importance of the NBCe1-A residues mutated in proximal renal tubular acidosis. J Biol Chem. 2010;285:13416–13426. doi: 10.1074/jbc.M109.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu Q, Shao XM, Kao L, Azimov R, Weinstein AM, Newman D, Liu W, Kurtz I. Missense mutation T485S alters NBCe1-A electrogenicity causing proximal renal tubular acidosis. Am J Physiol Cell Physiol. 2013;305:C392–C405. doi: 10.1152/ajpcell.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu Q, Kao L, Azimov R, Abuladze N, Newman D, Pushkin A, Liu W, Chang C, Kurtz I. Structural and functional characterization of the C-terminal transmembrane region of NBCe1-A. J Biol Chem. 2010;285:37178–37187. doi: 10.1074/jbc.M110.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]