Abstract
Cocaine-dependent individuals show altered brain activation during decision making. It is unclear, however, whether these activation differences are related to relapse vulnerability. This study tested the hypothesis that brain-activation patterns during reinforcement learning are linked to relapse 1 year later in individuals entering treatment for cocaine dependence. Subjects performed a Paper-Scissors-Rock task during functional magnetic resonance imaging (fMRI). A year later, we examined whether subjects had remained abstinent (n=15) or relapsed (n=15). Although the groups did not differ on demographic characteristics, behavioral performance, or lifetime substance use, abstinent patients reported greater motivation to win than relapsed patients. The fMRI results indicated that compared with abstinent individuals, relapsed users exhibited lower activation in (1) bilateral inferior frontal gyrus and striatum during decision making more generally; and (2) bilateral middle frontal gyrus and anterior insula during reward contingency learning in particular. Moreover, whereas abstinent patients exhibited greater left middle frontal and striatal activation to wins than losses, relapsed users did not demonstrate modulation in these regions as a function of outcome valence. Thus, individuals at high risk for relapse relative to those who are able to abstain allocate fewer neural resources to action-outcome contingency formation and decision making, as well as having less motivation to win on a laboratory-based task.
Keywords: Cocaine dependence, Reward, Abstinence, Relapse, Functional magnetic resonance imaging
1. Introduction
Cocaine addiction is a substantial burden for societies worldwide, linked to adverse outcomes such as violence, suicide, and disability, as well as high rates of chronic relapse (Degenhardt and Hall, 2012). A growing literature indicates that brain regions involved in interoception and cognitive control, such as the insular and prefrontal cortices, are dysfunctional in individuals with substance abuse and dependence, and may be involved in the maintenance, escalation, and/or relapse of drug use (Garavan and Hester, 2007; Paulus et al., 2009; Naqvi and Bechara, 2010). Moreover, substance abusers exhibit reduced responsivity to naturally rewarding outcomes in preference for heightened responses to drug rewards, as indexed by altered striatal activity (Volkow et al., 2006). Given prior work on brain dysfunction in substance abusers during decision making (Bolla et al., 2003; Kaufman et al., 2003; Tanabe et al., 2007; Asensio et al., 2010; Hyatt et al., 2012), the study of neural mechanisms involved in the processing of decision-outcome contingencies in cocaine users offers promise in predicting relapse and developing training programs to strengthen patterns of abstinence (Garavan and Hester, 2007; Reske and Paulus, 2011).
Although individuals with cocaine abuse and dependence show higher insula, frontal, and/or striatum activation in response to cocaine-related cues, reflecting heightened attention and interoceptive urges in response to cocaine (Kilts et al., 2001; Wexler et al., 2001; Sinha et al., 2005; Duncan et al., 2007; Prisciandaro et al., 2013; Prisciandaro et al., 2014), cocaine users exhibit attenuation in these regions within the context of non-drug rewards (Tanabe et al., 2007; Asensio et al., 2010; Hyatt et al., 2012) and moral judgments (Verdejo-Garcia et al., 2014), as well as during attention switching and working memory tasks (Bolla et al., 2003; Kaufman et al., 2003; Kubler et al., 2005; Tomasi et al., 2007b) than non-dependent comparison subjects. Moreover, attenuated prefrontal cortex activation is associated with lower inhibitory control in cocaine abusers, which may be involved in their difficulty in disengaging from attention to drug cues (Goldstein et al., 2007). Furthermore, extant research suggests that brain activation in these regions reflects susceptibility to relapse (Garavan and Weierstall, 2012). For example, long-term abstinent cocaine-dependent patients display greater dorsolateral prefrontal cortex (DLPFC) activation than recently abstinent and actively using cocaine-dependent subjects within the context of a response-inhibition task (Connolly et al., 2012). Moreover, greater striatal activation during a cognitive control paradigm is correlated with longer abstinence in cocaine-dependent individuals (Brewer et al., 2008). Similarly, reduced dopamine receptor binding in the striatum predicts poor response to treatment in cocaine-dependent subjects (Martinez et al., 2011) and attenuated insula activation during selective attention predicts relapse in individuals recovering from stimulant dependence (Clark et al., 2014).
Despite prior work examining neural markers of relapse susceptibility in cocaine users, few studies have examined the relationship between prolonged abstinence and brain regions involved in reward-related decision making. Therefore, the present study examined brain and behavioral performance during a reinforcement learning paradigm shown to activate insular, striatal, and frontal regions thought to be impaired in substance dependence (Paulus et al., 2004; Stewart et al., 2013). The striatum is involved in the implementation of contingencies linking choices with rewarding outcomes (Delgado et al., 2005), the insula encodes change in reward and risk variance during reinforcement (Preuschoff et al., 2008), and the prefrontal cortex is involved in encoding and maintaining current task demands (Ridderinkhof et al., 2004). Research demonstrates that healthy individuals exhibit greater insular, striatal and prefrontal activation when learning action-outcome contingencies than when executing contingencies after they have been learned (Paulus et al., 2004; Delgado et al., 2005; Stewart et al., 2013), but it is unclear whether this pattern is disrupted in individuals with cocaine dependence. Given that impairments in these brain regions are evident in cocaine-dependent individuals and extant research suggests that these attenuations may be stronger in stimulant users who relapse (Brewer et al., 2008; Martinez et al., 2011; Connolly et al., 2012; Clark et al., 2014), this study examines whether attenuations in these brain regions during reward learning are associated with cocaine relapse 1 year later.
In the present investigation, early-abstinent cocaine dependent individuals voluntarily enrolled in an inpatient drug treatment facility made choices during two phases of a Paper-Scissors-Rock task (e.g., Paulus et al., 2004; Stewart et al., 2013) involving rewarding and punishing outcomes as follows: (1) in early trials, when decision-outcome contingencies were being acquired; and (2) in late trials, when decision-outcome contingencies were being executed. We hypothesized that compared with cocaine-dependent subjects who maintained sustained abstinence, cocaine-dependent subjects who relapsed would show (1) lower anterior insular, striatal and frontal activation during early trials while learning action-outcome contingencies, reflective of reduced reward encoding; and (2) striatal hypoactivation to winning outcomes, suggestive of diminished reward sensitivity. Furthermore, it was predicted that cocaine users who remained abstinent 1 year later would exhibit modulation in frontal, striatal, and insular regions as a function of learning similar to healthy individuals in prior reward learning studies, whereas relapsed users would not show this flexibility. In addition to testing these specific hypotheses, the present study explored group differences in other brain regions during decision and feedback components of the task.
2. Materials and Methods
2.1. Subjects
The study protocol was approved by the local Human Subjects Review Board and carried out in accordance with the Declaration of Helsinki. Thirty subjects meeting current DSM-IV criteria for cocaine dependence (26 men, 4 women; age: M=43.3 years, SD=10.0 years, range 19-54 years; ethnicity: 54% African American, 43% Caucasian [31% Hispanic], 3% other) were recruited from 28-day inpatient Alcohol and Drug Treatment Programs at the San Diego Veterans Affairs (VA) Medical Center and Scripps Green Hospital (La Jolla, CA). All participants had voluntarily admitted themselves for treatment. Subjects were urine toxicology screened for the presence of drugs (a mandatory procedure of program participation) and were experiencing no substance-withdrawal symptoms. Individuals were informed that this study was examining behavior and brain functioning associated with stimulant dependence and subjects gave written informed consent to participate in a clinical interview session, a functional magnetic resonance imaging (fMRI) session, and a brief follow-up phone interview 1 year later to assess abstinence from cocaine and other substances. All subjects screened negative for drugs at the start of the fMRI session and had been abstinent from cocaine for at least 1 week before the fMRI session. The clinical interview and fMRI sessions were completed while subjects were still actively enrolled in inpatient treatment, and both sessions were also typically completed within the same week (M=5.1±4.4 days apart across all participants). Subjects’ average last cocaine use was approximately 1 month before the clinical interview session (across n=27: M=33.4 days, SD=29.7 days; range=7-129 days).
2.2. Clinical interview session
Lifetime DSM-IV Axis I diagnoses (including substance abuse and dependence) and Axis II antisocial personality disorder (ASPD) were assessed by experienced interviewers using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994), a detailed clinical interview that includes timeline follow-back methods to quantify lifetime drug use based on the number of distinct sessions when drugs were used. Diagnoses were based on consensus meetings with a clinician specialized in substance use disorders (MPP) and trained study personnel. The following were exclusion criteria for all groups: (1) ASPD; (2) current (and past 6 months) Axis I panic disorder, social phobia, post-traumatic stress disorder (PTSD), and major depressive disorder (MDD); (3) lifetime bipolar disorder, schizophrenia, and obsessive-compulsive disorder; (4) current severe medical disorders requiring inpatient treatment or frequent medical visits; (5) use of medications that affect the hemodynamic response within the past 30 days such as antihypertensives, insulin, and thyroid medication; (6) current positive urine toxicology test; and (7) head injuries or loss of consciousness for longer than 5 min. Of the total sample, 28 were right-handed as determined by questions regarding hand use during daily activities.
On the basis of interview data, current patterns of nicotine and alcohol use as well as lifetime uses of amphetamine, cocaine, and marijuana were calculated for each subject. During the interview session, subjects performed the North American Adult Reading Test (Uttl, 2002), a measure of verbal IQ. During this session, subjects also completed questionnaires indexing characteristics previously associated with cocaine abuse and/or dependence. Total scores from the Beck Depression Inventory (BDI) (Beck et al., 1961), Barratt Impulsivity Scale (BIS) (Patton et al., 1995), and Sensation Seeking Scale (SSS-V) (Zuckerman, 1996) were used to index depression, impulsivity and sensation seeking, respectively. To assess current cocaine-craving status, Cocaine Craving Questionnaire (CCQ) (Tiffany et al., 1993) subscales were examined. Independent sample t-tests (two-tailed; SPSS, Chicago, IL) were computed to compare abstinent and relapsed groups on questionnaire, IQ, age, education, clinical symptoms, and drug use variables. In addition, chi-square tests (two-tailed; SPSS) were performed to examine group differences in gender, ethnicity, and percentage of subjects with comorbid nicotine use and/or alcohol and marijuana dependence as well as past PTSD, MDD, and social phobia.
2.3. Follow-up interview at 1 year
Participants were contacted approximately 1 year after the clinical interview session (across n=30: M=370.1 days later; SD=17.7 days). Several approaches that have been successful in large-scale longitudinal studies were used (Twitchell et al., 1992). For example, individuals were contacted by telephone or mail 12 months after the initial evaluation, using addresses of friends or family members, VA records, or the Department of Motor Vehicle records to locate them. For veterans the VA is the principal care center where they receive treatment and help for all medical ailments. Therefore, veterans enter the VA system regularly and repeatedly and thus can more easily be tracked. All 30 subjects were successfully followed up (100% follow-up rate). Sobriety was assessed using a questionnaire based on the SSAGA interview, focusing on the use of illicit drugs during the interval between the date of the neuroimaging session and date of the follow-up. Relapse was defined as any drug use during any time after discharge from the inpatient unit, with the date of relapse defined as the day of first use. A total of 15 subjects (14 men, 1 woman) remained abstinent at 1-year follow-up, whereas the remaining 15 subjects (12 men, 3 women) had relapsed within the past 12 months (number of days abstinent post-treatment discharge: M=80.40, SD=90.91, range=1-300, median=45, mode=30).
2.4. fMRI session: Paper-Scissors-Rock task
The Paper-Scissors-Rock paradigm (Paulus et al., 2004; Stewart et al., 2013) examines how individuals acquire the ability to make decisions associated with advantageous outcomes. This task is based on the well-known Paper-Scissors-Rock game, wherein (1) rock beats scissors, (2) paper beats rock, and (3) scissors beat paper. Subjects were instructed that they were playing against the computer and attempting to maximize points (1 for a win, 0 for a tie, and -1 for a loss). Players were told that they would receive additional payment according to their cumulative point total (each point was worth $1 that subjects were actually paid after completion of the experiment). Unknown to the subject, probability of beating the computer and thus being reinforced (e.g., subject chooses scissors, computer selects paper, scissors beats paper, subject gains 1 point) was predetermined for each response option. A total of 120 trials were presented, consisting of six blocks containing 20 trials each. Within each block, the three possible selections had pre-determined probabilities of having a winning, tying, or losing outcome. The “preferred response” wins on 90% of trials, the “even response” wins 50% of the time, and the “worst response” wins on 10% of trials. Thus, if rock were the preferred response and paper were the worst response in a particular block, then selecting rock would result in a win 90% of the time and selecting paper would result in a win 10% of the time. Unbeknownst to the subject, preferred, even, and worst responses were switched for each of the six blocks presented. Since subjects were instructed to select paper, scissors or rock by pushing the left, middle or right button with the index, middle or ring finger of the right hand, respectively, the statistically optimal corresponding hand position option also changed for each block.
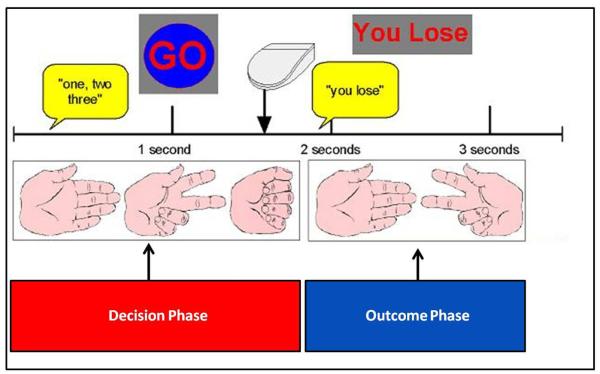
Fig. 1 illustrates that after an initial fixation lasting 2 s, subjects saw pictures of a hand forming paper, scissors, and rock on the computer screen for 1 s and heard the instruction “one, two, three” over MRI compatible, sound-insulated headphones. At 3 s into the trial, subjects were then presented with a “Go” sign, providing the cue to select paper, scissors, or rock. Subjects had 3.5 s to respond, after which the trial timed out. Upon responding, the outcome was presented, wherein the subject saw the computer’s response, and heard “you win,” “you lose,” or “a tie,” and the updated score was displayed at the top of the screen. Nine null trials were interspersed at the beginning, middle, and end of the task as a temporal jitter and trial duration order were optimized to estimate activation during decision making.
Figure 1.
Illustration of Paper-Scissors-Rock paradigm.
2.5. fMRI image acquisition
An fMRI run sensitive to blood oxygenation level-dependent (BOLD) contrast was collected in a randomized fast event-related design using a Signa EXCITE (GE Healthcare, Milwaukee, WI) 3.0 Tesla scanner (T2*-weighted echo planar imaging (EPI) scans, repetition time (TE) =2000 ms, echo time (TE)=32 ms, field of view (FOV) =230mm3, 64×64 matrix, 30 2.6-mm axial slices with 1.4-mm gap, flip angle=90°, 290 whole-brain acquisitions). The fMRI volume acquisitions were time-locked to task onset. A high-resolution T1-weighted image [spoiled gradient recalled (SPGR), TI=450, TR=8 ms, TE=3 ms, flip angle=12°, FOV=250mm3, 192×256 matrix, 172 sagittally acquired slices with 1-mm thickness] was obtained for anatomical reference.
2.6. Behavioral analysis
Responses were obtained using the first three buttons on a four-button response box recorded during each trial to determine response selection (preferred, even, and worst responses). Two linear mixed effects (LME) analyses were performed in R (Pinheiro et al., 2013). The first LME examined group (abstinent, relapsed) and decision time (early trials, late trials) across blocks, with probability of preferred response selection as the dependent variable. The second LME compared group and outcome (wins, ties, losses), with total number of each outcome across blocks as the dependent variable. Subjects were treated as random effects, whereas group, decision time, and outcome were modeled as fixed effects. After the fMRI session, subjects filled out a questionnaire to examine the degree of task-related engagement. The following questions were rated on a 1 (‘None’) to 4 (‘A Lot’) point scale: (1) ‘How interested were you in doing the task?’ (2) ‘How much attention did you pay to the task?’ (3) ‘How much attention did you pay to the computer’s choice?’ and (4) ‘How much attention did you pay to the point total?’ In addition, subjects rated the question ‘How important was it for you to beat the computer?’ on a 1 (‘Not Important at All’) to 5 (‘Very Important’) scale. Answers to these questions were compared between groups using independent sample t-tests.
2.7. fMRI data analysis
2.7.1. Preprocessing
Functional analyses were conducted using AFNI (Cox, 1996). Following reconstruction, differences in slice-acquisition timing were corrected using Fourier interpolation. The time series for each voxel was then motion-corrected (least-squares alignment using three rotational and three translational parameters). Individual EPI datasets were aligned to the T1-weighted anatomical image using local Pearson correlation (Saad et al., 2009). To relate EPI changes to task characteristics, a multivariate regressor approach, described below, was used. Data were inspected to determine successful image alignment and existence of remaining artifacts.
2.7.2. Deconvolution
The first level analysis was carried out using a General Linear Model fitting a set of regressors of interest, as well as a set of nuisance regressors, to the echoplanar time series using the AFNI program 3dDeconvolve. Based on subjects’ learning curves (the frequency of preferred response selection as a function of trial position after each switch) in this sample as well as two additional samples (Stewart et al., 2013; Stewart et al., 2014), groups of trials across blocks during the decision phase of the task depicted in Fig. 1 (from trial onset until response selection, as opposed to the outcome phase) were separated into early trials, defined as trials 1-8, when contingencies (selection of the preferred response, avoidance of the worst response) were being discerned, and late trials, defined as trials 13-20, when contingencies had been established. In addition, based on the behavioral performance of each subject, trials were divided into those resulting in wins, ties, and losses. Deconvolution was then performed, wherein three motion regressors, a baseline and linear drift regressor, two decision time regressors (early trials, late trials), and three outcome regressors (wins, ties, losses) were convolved with a modified hemodynamic response function. The baseline consisted of the inter-trial interval and the null trials interspersed between trial blocks.
2.7.3. Post-deconvolution processing
Voxels were resampled into 4×4×4 mm3 space. Images were spatially filtered using a Gaussian spatial filter (full-width-half-maximum=4 mm) to account for individual anatomical differences. Anatomical images were automatically converted to Talairach space in AFNI, and individual images were then transformed into Talairach space for each subject (resulting in a reduced 40×48×38 voxel matrix). Resultant beta values for regressors of interest were converted to a percent signal change score relative to baseline, which served as the activation measure.
2.7.5. Group analysis: Decision phase
Group effects were subjected to LME analysis, wherein subjects were treated as random effects and group (abstinent, relapsed) and decision (early trials, late trials) were modeled as fixed effects.
2.7.6. Group analysis: Outcome phase
A second LME analysis was computed, wherein subjects were treated as random effects and group (abstinent, relapsed) and outcome (wins, ties, losses) were modeled as fixed effects.
2.7.7. Extraction of significant LME results
A threshold adjustment method based on Monte-Carlo simulations (AFNI’s program AlphaSim) was applied to guard against identifying false positive areas of activation (considering whole-brain voxel size and 4-mm smoothness). For main effects and interactions involving group, AlphaSim identified a minimum cluster volume of 768 μl (12 contiguous voxels) in conjunction with an uncorrected individual voxel significance of P<0.0001 to result in a voxel-wise probability of P<0.05 (P<0.025, two-tailed) corrected for multiple comparisons. The voxelwise threshold for effects of interest were based on the following LME degrees of freedom and F-values: (1) Decision phase group main effect and group by decision interaction: F(1,28)=4.2; (2) Outcome phase group main effect: F(1,28) =4.2 and group by outcome interaction: F(2,56)=3.16. Average percent signal change was then extracted for significant clusters.
2.7.8. Exploratory correlations between brain activation and days of sobriety
Follow-up analyses were conducted to determine whether attenuated activation in relapsers was linearly related to time of relapse. Therefore, within the relapsed group, Pearson correlations were computed between regions of interest (insular, frontal, and striatal regions emerging as significant between groups from the LME analysis) and number of days of posttreatment abstinence. Correction for multiple comparisons was implemented, resulting in a threshold P=0.05/18 regions tested =0.003 significance level.
3. Results
3.1. Subject characteristics
Relapsers did not differ from abstainers on age, education, verbal IQ, cocaine use onset, lifetime drug use, personality measures, current depression symptoms, current patterns of nicotine and alcohol use, or cocaine-craving indices (see Table 1). Moreover, groups endorsed comparable magnitudes of symptoms of DSM-IV antisocial personality disorder, conduct disorder, and attention-deficity/hyperactivity disorder as well as proportionate numbers of subjects with current alcohol and marijuana dependence, past posttraumatic stress disorder, and past social phobia. Relapsers tended to endorse higher rates of past MDD (3/15) than abstainers (0/15). No subjects from either group met criteria for current amphetamine dependence at the time of the clinical interview.
Table 1.
Subject characteristics as a function of group status
| Abstinent (n=15) | Relapsed (n=15) | Group statistics | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristics | M | SD | M | SD | t | df | P |
| Age | 45.5 | 9.4 | 41.1 | 10.4 | 1.2 | 28 | 0.24 |
| VIQ | 101.9 | 10.9 | 105.0 | 9.4 | −0.8 | 28 | 0.41 |
| Education (years) | 13.6 | 1.8 | 13.3 | 2.1 | 0.5 | 28 | 0.64 |
| Age of onset of cocaine use (years) | 25.0 | 8.6 | 21.8 | 6.3 | 1.2 | 28 | 0.25 |
| Recency of cocaine use (days) | 40.9 | 37.8 | 25.4 | 14.8 | 1.4 | 25 | 0.17† |
| Lifetime amphetamine use (sessions) | 201.9 | 536.3 | 1292.4 | 3007.5 | −1.4 | 28 | 0.18† |
| Lifetime cocaine use (sessions) | 15339.3 | 16130.1 | 20246.4 | 24527.1 | −0.6 | 28 | 0.52† |
| Lifetime marijuana use (sessions) | 15718.5 | 21725.1 | 7143.47 | 14874.6 | 1.3 | 28 | 0.22 |
| BDI total | 6.7 | 9.1 | 7.5 | 4.8 | −0.3 | 27 | 0.76 |
| BIS total | 66.6 | 13.1 | 75.2 | 10.2 | −2.0 | 28 | 0.06 |
| SSS total | 19.7 | 7.1 | 22.3 | 7.5 | −0.9 | 25 | 0.38 |
| CCQ desire to use | 49.6 | 10.0 | 45.6 | 10.8 | 1.0 | 25 | 0.33 |
| CCQ plan to use | 51.8 | 4.7 | 46.9 | 10.0 | 1.7 | 25 | 0.12† |
| CCQ anticipate positive outcome | 45.3 | 7.6 | 41.8 | 8.7 | 1.1 | 25 | 0.27 |
| CCQ anticipate relief from withdrawal | 38.3 | 7.4 | 40.9 | 8.1 | −0.9 | 25 | 0.39 |
| CCQ lack of control over use | 35.8 | 4.4 | 30.0 | 9.2 | 2.1 | 25 | 0.06† |
| Lifetime ADHD inattention symptoms | 3.5 | 4.1 | 1.9 | 3.2 | 1.2 | 28 | 0.25 |
| Lifetime ADHD hyperactivity symptoms | 3.9 | 4.0 | 1.9 | 3.3 | 1.4 | 28 | 0.16 |
| Conduct disorder symptoms | 2.6 | 2.8 | 1.8 | 2.0 | 0.9 | 28 | 0.37 |
| Lifetime ASPD symptoms | 1.7 | 1.6 | 1.3 | 1.7 | 0.8 | 28 | 0.44 |
| Current alcohol drinks per week | 25.7 | 52.3 | 20.9 | 24.0 | 0.3 | 26 | 0.75 |
| Current cigarettes per day (smokers) | 14.3 | 8.4 | 15.0 | 5.0 | −0.2 | 15 | 0.82 |
|
| |||||||
| Self-reported post-task ratings | M | SD | M | SD | t | df | P |
|
| |||||||
| Importance in beating computer (1-5) | 4.8 | 0.4 | 3.9 | 1.1 | 2.7 | 26 | 0.02 |
| Interest in playing task (1-4) | 3.8 | 0.4 | 3.9 | 0.4 | −0.6 | 27 | 0.58 |
| Attention paid to task (1-4) | 3.9 | 0.4 | 3.9 | 0.4 | −0.1 | 27 | 0.94 |
| Attention paid to computer’s choice (1-4) | 3.7 | 0.6 | 3.3 | 0.9 | 1.4 | 26 | 0.16 |
| Attention paid to point total (1-4) | 3.9 | 0.4 | 3.3 | 0.8 | 2.1 | 26 | 0.04† |
|
| |||||||
| Demographics/diagnoses | Abstinent (n=15) | Relapsed (n=15) | χ 2 | df | P | ||
|
| |||||||
| % Past MDD | 0% | 20% | 3.8 | 1 | 0.05 | ||
| % Past PTSD | 20% | 7% | 0.9 | 1 | 0.35 | ||
| % Past social phobia | 7% | 7% | 0.1 | 1 | 0.92 | ||
| % Female | 7% | 20% | 1.2 | 1 | 0.28 | ||
| % African American ethnicity | 67% | 40% | 2.1 | 1 | 0.14 | ||
| % Current nicotine smokers | 53% | 60% | 0.1 | 1 | 0.71 | ||
| % Current alcohol dependence | 27% | 33% | 0.2 | 1 | 0.69 | ||
| % Current marijuana dependence | 13% | 7% | 0.4 | 1 | 0.54 | ||
Note: Unequal group variances. VIQ = verbal intelligence quotient. BDI = Beck Depression Inventory. BIS = Barratt Impulsivity Scale. SSS = Sensation Seeking Scale. CCQ = Cocaine Craving Questionnaire. ADHD = attention deficit hyperactivity disorder. ASPD = antisocial personality disorder. MDD = major depressive disorder. PTSD = posttraumatic stress disorder. The abstinent group has missing data for the following number of subjects: n=1 for BDI, CCQ, and n=2 for current alcohol drinks per week and post-task ratings. The relapsed group has missing CCQ for 2 subjects and SSS data for 3 subjects. For the Current Cigarettes Per Day analysis, n=8 abstinent and n=9 relapsed subjects with current tobacco dependence were included.
3.2. Behavioral data
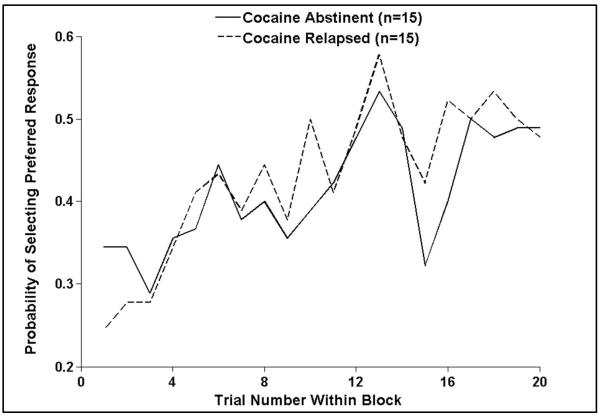
Although no group differences in behavioral data were predicted, given prior research showing little change in behavioral performance during basic decision-making tasks as a function of cocaine abstinence (Verdejo-Garcia et al., 2007; Verdejo-Garcia et al., 2012), the present study explored whether cocaine relapse was associated with poorer reward learning. Results for the decision phase (see Fig. 2) demonstrated that a main effect of decision emerged, wherein subjects selected a greater percentage of preferred responses during late trials (M=48.4%, SD=14.2%) than early trials (M=35.9%, SD=7.2%; F(1,28)=20.6, P<0.001). However, relapsers did not differ from abstainers in percentage of preferred response selection across trials (P=0.68) or between early and late trials (P=0.35). Similarly, results for the outcome phase showed that groups achieved similar numbers of wins, ties, and losses (P=0.33). Both relapsers and abstainers accumulated more wins (M=47.0, SD=9.1) than ties (M=41.0, SD=5.7) or losses (M=31.5, SD=7.1; F(2,54)=31.7, P<0.001; n=1 missing outcome data). Interestingly, abstainers relative to relapsers reported paying more attention to the point total and placing more importance on beating the computer based on post-task questionnaire results (see Table 1). However, both groups reported similar levels of interest and attention in the task. Taken together, there were no behavioral differences during the task, but the perception of task performance differed between relapsers and abstainers.
Figure 2.
Abstinent and relapsed cocaine users demonstrated similar behavioral acquisition (during early trials) and execution (during late trials) of the preferred response averaged across blocks.
3.3. fMRI data
3.3.1. Decision phase
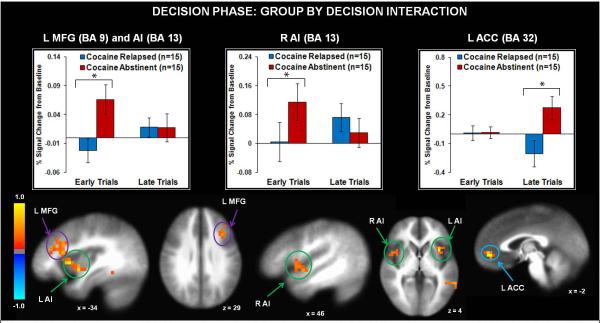
Results for the group main effect (see Fig. 3) indicated that relapsed users displayed lower left anterior insula, left middle frontal gyrus (including DLPFC), bilateral inferior frontal gyrus, and bilateral dorsal/ventral striatum activation than their abstinent counterparts across early and late trials. Relapsed users also exhibited lower bilateral precuneus, left middle occipital gyrus, right inferior parietal lobule, and bilateral temporal cortex activation than abstinent individuals across early and late trials (see Table 2). The group by decision interaction (see Table 3) tested the predictions that (1) relapsed cocaine users would exhibit lower insula, striatum, and prefrontal activation during early trials than abstinent individuals; and (2) abstinent individuals would show decreased activation in these regions as a function of learning, whereas relapsed users would not. Findings (see Fig. 4) indicated that during early trials, relapsed users exhibited lower bilateral middle frontal gyrus (including DLPFC) and anterior insula activation than abstinent users. In contrast, during late trials, relapsed users displayed lower left anterior cingulate cortex (ACC) activation than abstinent users. Finally, within abstinent users, right middle frontal gyrus activation decreased as a function of learning (see Table 3), whereas relapsed users did not differ in right prefrontal activation from early to late trials.
Figure 3.
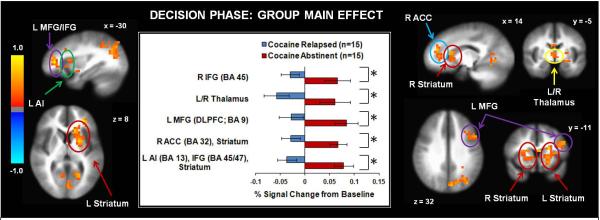
Across early and late trials, replapsed cocaine users exhibited lower activation than abstinent cocaine users in several brain regions including inferior frontal gyrus (IFG), middle frontal gyrus(MFG), anterior cingulate cortex (ACC), anterior insula (Al), thalamus, and dorsal/ventral striatum. L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Error bars denote +/− standard error. Asterisks denote significant group differences. The color bar depicts normalized voxel intensity differences, with positive values indicating greater intensity in the abstinent group than the relapsed group.
Table 2.
fMRI results for decision phase, main effect of group
| Voxels | Vol | x | y | z | L/R | Regions within cluster | BA | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Abstinent > relapsed | ||||||||
| 220 | 14080 | −22 | 19 | 6 | L | Caudate/putamen, lentiform nucleus, inferior frontal gyrus, anterior insula, claustrum |
13/45/47 | 1.58 |
| 14 | 896 | −19 | −19 | 22 | L | Caudate, posterior insula | − | 1.14 |
| 123 | 7872 | 16 | 23 | 9 | R | Caudate/putamen, lentiform nucleus, medial frontal gyrus, anterior cingulate |
32 | 1.41 |
| 42 | 2688 | −43 | 13 | 28 | L | Middle frontal gyrus | 9 | 1.27 |
| 20 | 1280 | −22 | 60 | 9 | L | Middle/superior frontal gyrus | 10 | 1.15 |
| 12 | 768 | −28 | −6 | 56 | L | Middle frontal gyrus | 6 | 1.01 |
| 12 | 768 | −13 | 46 | −10 | L | Medial frontal gyrus | 32 | 1.30 |
| 12 | 768 | 32 | 31 | 8 | R | Inferior frontal gyrus | 45 | 1.16 |
| 18 | 1152 | −44 | −65 | 0 | L | Inferior temporal gyrus, middle occipital gyrus | 37 | 1.11 |
| 12 | 768 | 52 | −15 | 2 | R | Superior temporal gyrus | 22 | 1.21 |
| 12 | 768 | −19 | −41 | −2 | L | Lingual gyrus | 30 | 1.17 |
| 297 | 19008 | −17 | −66 | 23 | L | Precuneus, cuneus, superior occipital gyrus | 7/17/18/ 19/31 |
1.37 |
| 37 | 2368 | 7 | −52 | 51 | L/R | Precuneus | 7 | 1.37 |
| 19 | 1216 | 11 | −75 | 29 | R | Precuneus, cuneus | 18 | 1.15 |
| 69 | 4416 | 40 | −74 | −5 | R | Inferior occipital gyrus, fusiform gyrus | 19 | 1.37 |
| 14 | 896 | −32 | −40 | −20 | L | Culmen | 20 | 1.35 |
| 18 | 1152 | 8 | −8 | 23 | L/R | Cingulate gyrus | 24 | 1.47 |
| 13 | 832 | 0 | −6 | 5 | L/R | Thalamus | − | 1.12 |
| 30 | 1920 | 27 | −54 | 45 | R | Inferior/superior parietal lobule | 7 | 1.13 |
| 12 | 768 | 50 | −40 | 49 | R | Inferior parietal lobule | 40 | 1.09 |
Note: L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Vol = volume in microliters. Talairach coordinates reflect center of mass. All clusters listed were significant at F(1,28) > 4.2 for P<0.05 (0.025 two-tailed) voxelwise corrected for multiple comparisons. Cohen’s d reflects effect size between groups.
Table 3.
fMRI Results for Decision Phase: Group by Decision (Early, Late) Interaction
| Voxels | Vol | x | y | z | L/R | Regions within cluster | BA | Early (E) | Late (L) | Abstinent | Relapsed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early trials: abstinent > relapsed | |||||||||||
| 68 | 4352 | −36 | 16 | 14 | L | Anterior insula Middle frontal gyrus |
9/13 | d=1.02 | NS | NS | L > E |
| 42 | 2688 | 27 | 2 | 44 | R | Middle frontal gyrus Superior frontal gyrus |
6/8 | d=0.68 | NS | E > L | NS |
| 26 | 1664 | −50 | −47 | −4 | L | Middle temporal gyrus | 37 | d=1.16 | NS | E > L | NS |
| 13 | 832 | 36 | −48 | 24 | R | Supramarginal gyrus | 39 | d=1.01 | NS | E > L | NS |
| 24 | 1536 | 46 | 2 | 0 | R | Anterior insula | 13 | d=0.56 | NS | NS | NS |
| 15 | 960 | 39 | 24 | 27 | R | Middle frontal gyrus | 9 | d=0.57 | NS | NS | NS |
| 12 | 768 | −41 | −4 | −11 | L | Superior temporal gyrus | 21 | d=0.61 | NS | NS | NS |
| Late trials: abstinent > relapsed | |||||||||||
| 21 | 1344 | −5 | 46 | −2 | L | Anterior cingulate | 32 | NS | d=1.00 | L > E | E > L |
| Late trials: relapsed > abstinent | |||||||||||
| 13 | 832 | 28 | 4 | 29 | R | Precentral/cingulate gyrus | 6 | d=0.58 | d=0.57 | E > L | NS |
| 14 | 896 | 18 | −20 | 0 | R | Thalamus | NS | d=0.64 | E > L | NS | |
Note: L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Vol = volume in microliters. Talairach coordinates reflect center of mass. All clusters listed were significant at F(1,28) > 4.2 for P<0.05 (0.025 two-tailed) voxelwise corrected for multiple comparisons. d = Cohen’s d effect size between groups.
Figure 4.
Relapsed colcaine users exhibited lower activation than abstinent cocaine users in left middle frontal gyrus (MFG) and bilateral anterior insula (Al) during early trials and anterior cingulate (ACC) during late trials. L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Error bars denote +/− 1 standard error. Asterisks denote significant group differences. The color bar depicts normalized voxel intenstiy differences, with postitive values indicating greater intensity in the abstinent group than the relapsed group.
3.3.2. Outcome phase
Tables 4 and 5 illustrate the group main effect and interaction findings, respectively. The group by outcome interaction tested the hypothesis that relapsed users would demonstrate attenuated striatal activation to winning outcomes. The group main effect demonstrated that relapsed users showed greater activation in right ACC, left superior frontal gyrus (precentral gyrus, frontal pole), and bilateral middle temporal gyrus than abstinent users across all outcomes, whereas abstinent users exhibited greater left posterior insula activation than relapsers (see Fig. 5). Furthermore, the group by outcome interaction (see Table 5) indicated that relapsed users exhibited greater left middle frontal gyrus (including DLPFC) and left dorsal/ventral striatum activation to losses than abstinent users. Furthermore, abstinent users showed greater activation in the latter two regions for wins and ties than losses, but relapsed users did not show similar brain modulation as a function of outcome (see Fig. 6).
Table 4.
fMRI results for outcome phase, main effect of group
| Voxels | Vol | x | y | z | L/R | Regions within cluster | BA | Cohen’s d |
|---|---|---|---|---|---|---|---|---|
| Abstinent > relapsed | ||||||||
| 28 | 1792 | −32 | −51 | 7 | L | Middle occipital gyrus | 30 | 1.43 |
| 15 | 960 | −36 | −41 | 18 | L | Posterior insula/superior temporal gyrus | 13 | 1.27 |
| 14 | 896 | 18 | −51 | −19 | R | Cerebellum (culmen) | − | 1.03 |
| Relapsed > abstinent | ||||||||
| 23 | 1472 | −13 | 27 | 53 | L | Superior frontal gyrus | 6 | 1.21 |
| 17 | 1088 | −24 | 40 | 28 | L | Superior frontal gyrus | 9/10 | 1.07 |
| 16 | 1024 | 9 | 43 | 10 | R | Anterior cingulate | 32 | 1.13 |
| 12 | 768 | −49 | −29 | −1 | L | Middle temporal gyrus | 21 | 1.22 |
| 13 | 832 | 56 | −30 | 0 | R | Middle temporal gyrus | 21 | 1.30 |
| 20 | 1280 | 46 | −9 | −28 | R | Inferior temporal gyrus | 20 | 1.23 |
Note: L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Vol = volume in microliters. Talairach coordinates reflect center of mass. All clusters listed were significant at F(1,28) > 4.2 for P<0.05 (0.025 two-tailed) voxelwise corrected for multiple comparisons. Cohen’s d reflects effect size between groups.
Table 5.
fMRI results for outcome phase: group by outcome (wins, ties, losses) interaction
| Voxels | Vol | x | y | z | L/R | Regions within cluster | BA | Wins (W) |
Ties (T) | Losses (L) | Abstinent | Relapsed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Losses: Abstinent > Relapsed | ||||||||||||
|
| ||||||||||||
| 37 | 2368 | −20 | 37 | 34 | L | Middle frontal gyrus | 9 | NS | NS | d=0.82 | NS | NS |
| 19 | 1216 | −28 | 6 | 2 | L | Lentiform nucleus Putamen | − | NS | NS | d=0.68 | W/T > L | NS |
| 17 | 1088 | 17 | −67 | 7 | R | Cuneus | 30 | NS | NS | d=0.74 | W/T > L | NS |
Note: L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Vol = volume in microliters. Talairach coordinates reflect center of mass. All clusters listed were significant at F(2,56) > 3.16 for P<0.05 (0.025 two-tailed) voxelwise corrected for multiple comparisons. d = Cohen’s d effect size between groups.
Figure 5.
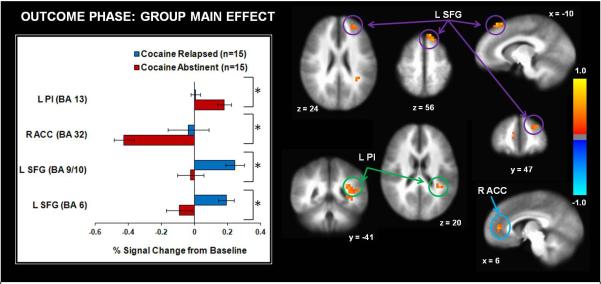
Across wins, ties, and outcomes, compared to abstinent cocaine users, relapsed cocaine users exhibited lower activation in left posterior insula (Pl) but higher activation right anterior cingulate cortes (ACC) and left superior frontal gyrus (SFG). L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Error bars denote +/− 1 standard error. Asterisks denote significant group differences. The color bar depicts normalized voxel intensity differences between groups.
Figure 6.
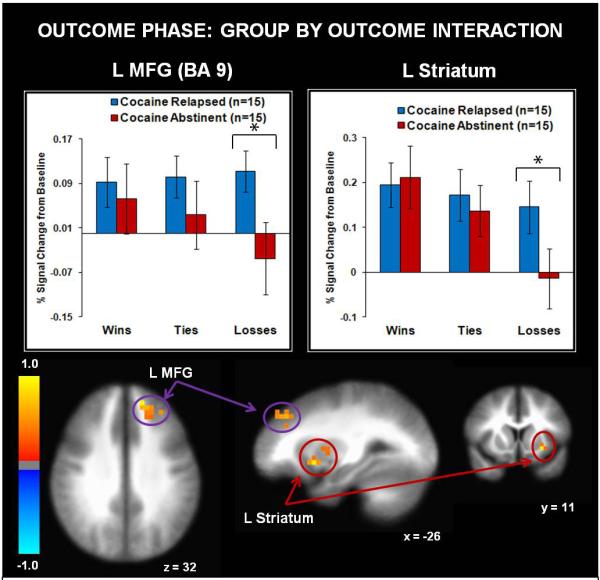
Relapsed cocaine users exhibited greater activation than abstinent cocaine users in left middle frontal gyrus (MFG) and left striatum during losses. L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Error bars denote +/− 1 standard error. Asterisks denote significant group differences. The color bar depicts normalized voxel intensity differences, with postitive values indicating greater intensity in the relapsed group than the abstinent group for losses.
3.3.3. Exploratory correlations between brain activation and days of sobriety
No correlations survived correction for multiple comparisons.
4. Discussion
This study examined three hypotheses involving neural processing differences between abstinent and relapsed cocaine users during reinforcement learning on the basis of literature reporting attenuated neural activation in cocaine addiction during decision making (e.g., Asensio et al., 2010; Bolla et al., 2003; Hyatt et al., 2012; Kaufman et al., 2003; Kubler et al., 2005; Tanabe et al., 2007; Tomasi et al., 2007). Consistent with our first prediction, relapsed cocaine users exhibited lower insular and prefrontal activation during early trials than abstinent users, reflecting reduced recruitment of brain regions involved in the formation of decision-outcome contingency associations. Second, in line with hypotheses, abstinent users exhibited a prefrontal activation decrease from early to late trials as a function of reward learning, whereas relapsed users did not show this modulation. Although it was predicted that relapsed users would show lower striatal activation than abstinent users specifically during learning of reward associations in early trials, cocaine relapse was instead associated with reduced bilateral striatum activation across early and late trials, indicative of altered processing of reward value. Third, although it was predicted that relapsed cocaine users would display lower striatal activation to winning outcomes than abstinent users, results indicated that cocaine relapsers demonstrated greater striatal activation to losses than individuals who were able to maintain abstinence, but groups did not differ in striatal responses to wins. However, whereas abstinent users exhibited greater striatal and frontal activation to wins than losses, relapsed users showed no modulation in these regions as a function of valence. Taken together, attenuated neural processing during the acquisition of action-outcome contingencies as well as reduced reward outcome differentiation via striatal regions may provide biological markers of relapse vulnerability within a sample of chronic cocaine users.
Recent lesion research supports the assertion that the insular cortex is critical for the maintenance of addiction (Naqvi et al., 2007). Moreover, neuroimaging studies indicate that cocaine abusers display insular hyperactivation in response to drug cues and/or craving (Wang et al., 1999; Kilts et al., 2001; Bonson et al., 2002) which is predictive of relapse (Prisciandaro et al., 2013). In contrast, stimulant-dependent individuals show insular attenuation during tracking of non-drug rewards (May et al., 2013; Tanabe et al., 2013) and non-reward related decision-making tasks (Kaufman et al., 2003; Verdejo-Garcia et al., 2014), hypoactivation that is also predictive of relapse (Luo et al., 2013; Clark et al., 2014). The current state of the literature suggests that insular activation reflects context-modulated learning, with both over-recruitment to drug cues and under-recruitment to non-drug cues being problematic with respect to maintaining prolonged sobriety from stimulants. Although the insular cortex is thought to be involved in conditioned visceral responses to drug-related cues via interoceptive integration, the insula subserves many functions and does not exist in isolation. The insular cortex is also involved in cognitive control and task-switching networks that are thought to be dysfunctional in addiction (Paulus and Stewart, 2014). Moreover, recent neuroimaging work indicates that cocaine dependence and relapse are linked to altered connectivity between the insula and regions implicated in reward and executive functioning such as the striatum and prefrontal cortex (Cisler et al., 2013; McHugh et al., 2013). It is simplistic to assume that training the insula to respond less to drug-related stimuli and more to non-drug-related stimuli will automatically increase long-term sobriety in chronic cocaine users, but real-time fMRI could be used in future studies to examine how networks involving the insular cortex can be modulated to influence drug craving, reward, and cognitive control processes.
With regard to brain mechanisms involved in cognitive control, abstinent and relapsed groups also differed in ACC recruitment during decision and outcome phases of reinforcement learning. Relapsed users exhibited lower pregenual ACC activation than abstinent users while making decisions, recruitment which was particularly attenuated during the execution of learned reward contingencies, suggestive of reduced monitoring of reward valuation (Rogers et al., 2004). In contrast, relapsed users exhibited greater pregenual ACC activation than abstinent users in response to all types of feedback in a region that prior work has shown to be linked to processing winning versus losing consequences in healthy adults (Rogers et al., 2004). In addition to ACC hyperactivation to feedback, relapsed cocaine users also exhibited heightened superior frontal/precentral gyrus activation, suggestive of heightened action readiness that is typically seen in healthy adults during reward-related action selection (Ernst et al., 2004). Overall, these findings suggest that reduced monitoring of reward magnitude during action selection paired with increased action readiness and valuation of all outcomes, regardless of reward value, characterizes relapse to cocaine use.
In addition to group differences in insular, frontocingulate, and striatal regions, relapsed cocaine users exhibited lower thalamic activation than abstinent users while making decisions, consistent with prior research showing thalamic reductions in cocaine abusers within the context of working memory tasks (Tomasi et al., 2007b, 2007a; Moeller et al., 2010). Moreover, in the decision phase of the present task, cocaine relapsers demonstrated attenuation in several other regions previously implicated in studies of impaired visual working memory processing in cocaine dependence, such as the precuneus (Kubler et al., 2005; Tomasi et al., 2007a), superior parietal lobule, and middle occipital gyrus (Moeller et al., 2010). Given that the present task required subjects to accumulate visual information regarding the preferred response (e.g., paper, rock, or scissors) over time and maintain this representation in working memory until contingencies were altered, it is not surprising that neural activation in thalamic, parietal, and occipital regions as well as prefrontal cortex differentiated cocaine users as a function of abstinence status. A growing addiction literature indicates that chronic drug use disrupts associative memory circuits involving dopaminergic projections to the striatum and prefrontal cortex, wherein goals involving drug rewards are reinforced at the expense of other goals (Hyman et al., 2006). Our findings suggest that individual differences in brain mechanisms involved in reward learning are involved in the ability to abstain from future cocaine use.
Although this longitudinal study possesses many merits, including examination of self-report, behavioral, and neural indices of relapse vulnerability, it also has several limitations. First, this investigation is confined by small sample sizes, which may have limited the power to detect group differences as a function of relapse, particularly for behavioral measures. It may be the case that fMRI decision-making paradigms do not offer sufficient means to detect behavioral differences given limited response options, consistent with prior work showing comparable or modest differences in behavioral performance in cocaine-dependent individuals as a function of treatment status (Brewer et al., 2008; Connolly et al., 2012; Clark et al., 2014). Moreover, the present task involved manipulation of reward outcome probability, resulting in limited variability of responses (e.g., number of wins allowed for a preferred response); future studies are warranted to examine whether more neural and behavioral indicators of varied responses to reward predict relapse. However, despite no group differences in behavioral learning, abstinent users reported greater attention to point total and greater importance placed on winning than relapsed users, suggestive of greater motivational drive to obtain reward, consistent with greater striatal, insular and DLPFC activation during decision making in abstinent users than in relapsed users. A second limitation is that self-report was the primary measure of relapse, and it is possible that individuals who reported abstinence over the phone may actually have lied to interviewers, despite no incentives to do so. As a result, effect sizes between groups may have been overestimated. However, subjective responses to the follow-up interview were also compared with VA self-report and urine screen records during the follow-up period to support the assertion of sustained sobriety in the 15 participants in the abstinent group. Future investigations of relapse should incorporate urine screens monthly post-treatment and at 1-year follow-up to verify abstinence. Third, the present analyses focused on identifying neural markers of relapse in cocaine-dependent inpatients and therefore did not include a healthy comparison group. Additional research is warranted to determine whether brain activation in prolonged cocaine abstainers resembles that of healthy controls. Fourth, given that all subjects were abstinent from cocaine use for at least 1 week preceding the fMRI session, these results may not generalize to the large population of cocaine users who have not yet achieved short periods of sobriety. Furthermore, although all study inpatients met criteria for current cocaine dependence, pre-scan cocaine abstinence was also highly variable (range: 1-18 weeks); systematic research is needed to determine brain changes as a function of 1 month versus 4 months of sobriety. Fifth, a higher number of relapsers (3/15) endorsed past MDD diagnoses than abstainers (0/15), which may have influenced group differences in brain activation. However, small sample sizes preclude investigation of the role of past MDD within relapsers, and lack of group differences in current depressive symptoms suggests that active sadness and anhedonia were not driving group differences in brain activation. Finally, the majority of patients were was drawn from a VA population and therefore may not generalize to civilians with cocaine dependence, since veterans may present with higher comorbid mental and physical health problems than civilians (Hoerster et al., 2012). However, the present study excluded individuals with severe medical problems including traumatic brain injury as well as lifetime psychotic disorders, current posttraumatic stress disorder, and antisocial personality disorder, which may assist with generalization across populations.
Despite these limitations, this study demonstrates that brain activation during reward learning differentiates cocaine-dependent individuals with sustained abstinence from those who relapsed after a short period of abstinence. It will be important to determine whether intervention programs such as mindfulness meditation (Farb et al., 2013; Paulus et al., 2013; Westbrook et al., 2013), approach-avoidance training (Wiers et al., 2013; Wiers et al., 2014), or instructive cognitive control training (Volkow et al., 2010) can be developed to increase striatal, insular, and/or frontal activation during decision making but diminish responses in these regions to cocaine cues to prevent future cocaine relapse. In summary, our findings derived from longitudinal measures of self-reported drug use indicate that attenuated brain activation in cocaine-dependent individuals reflects relapse vulnerability. Future research is needed to determine whether brain activation changes in abstinent individuals who were formerly dependent on cocaine by collecting fMRI measures at multiple time points along the recovery process.
Acknowledgments
None of the authors report any financial conflict of interest with the content of this manuscript. This work was supported by a grant from the National Institute on Drug Abuse (Grant No. R01 DA018307 to Martin Paulus).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D, Wang GJ, Telang F, Volkow ND, Goldstein RZ. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol and Drugs. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Research: Neuroimaging. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Beatty GK, Anderson RE, Kodituwakku P, Phillips JP, Lane TD, Kiehl KA, Calhoun VD. Reduced fMRI activity predicts relapse in patients recovering from stimulant dependence. Human Brain Mapping. 2014;35(2):414–428. doi: 10.1002/hbm.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug and Alcohol Dependence. 2012;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. American Journal of Addiction. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Social Cognitive and Affective Neuroscience. 2013;8:15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychology Review. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Weierstall K. The neurobiology of reward and cognitive control systems and their role in incentivizing health behavior. Preventative Medicine. 2012;55:S17–23. doi: 10.1016/j.ypmed.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerster KD, Lehavot K, Simpson T, McFall M, Reiber G, Nelson KM. Health and health behavior differences: U. S. Military, veteran, and civilian men. American Journal of Preventative Medicine. 2012;43:483–489. doi: 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Hyatt CJ, Assaf M, Muska CE, Rosen RI, Thomas AD, Johnson MR, Hylton JL, Andrews MM, Reynolds BA, Krystal JH, Potenza MN, Pearlson GD. Reward-related dorsal striatal activity differences between former and current cocaine dependent individuals during an interactive competitive game. PLoS One. 2012;7:e34917. doi: 10.1371/journal.pone.0034917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. European Journal of Neuroscience. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, Hong KI, Sinha R, Mazure CM, Li CS. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. American Journal of Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Dependence. 2013;131:238–246. doi: 10.1016/j.drugalcdep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatalinsula circuits in cocaine addiction: implications for impulsivity and relapse risk. American Journal of Drug and Alcohol Abuse. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Research: Neuroimaging. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Structure and Function. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Tapert SF, Liu TT. Trend detection via temporal difference model predicts inferior prefrontal cortex activation during acquisition of advantageous action selection. Neuroimage. 2004;21:733–743. doi: 10.1016/j.neuroimage.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Frontiers in Psychiatry. 2013;4:137. doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacology, Biochemistry, and Behavior. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: Linear and Nonlinear Mixed Effects Models. 2013.
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addiction Biology. 2014;19(2):240–249. doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug and Alcohol Dependence. 2013;131:44–49. doi: 10.1016/j.drugalcdep.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske M, Paulus MP. The diagnostic and therapeutic potential of neuroimaging in addiction medicine. In: Adinoff B, Stein EA, editors. Neuroimaging in Addiction. John Wiley & Sons, Ltd.; Chichester, UK: 2011. pp. 321–344. [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Connolly CG, May AC, Wittman M, Tapert SF, Paulus MP. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine dependent individuals. Addiction. 2014;109(3):460–471. doi: 10.1111/add.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Flagan TM, May AC, Reske M, Simmons AN, Paulus MP. Young adults at risk for stimulant dependence show reward dysfunction during reinforcement-based decision making. Biological Psychiatry. 2013;73:235–241. doi: 10.1016/j.biopsych.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, Claus E, Thompson LL, Du YP, Banich MT. Reduced neural tracking of prediction error in substance-dependent individuals. American Journal of Psychiatry. 2013;170:1356–1363. doi: 10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Human Brain Mapping. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug and Alcohol Dependence. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Research: Neuroimaging. 2007a;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Research. 2007b;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell GR, Hertzog CA, Klein JL, Schuckit MA. The anatomy of a follow-up. British Journal of Addiction. 1992;87:1327–1333. doi: 10.1111/j.1360-0443.1992.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence. 2007;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, Gonzalez-Saiz F, Fernandez-Calderon F, Bilbao-Acedos I, Perez-Garcia M. Self-regulation and treatment retention in cocaine dependent individuals: a longitudinal study. Drug and Alcohol Dependence. 2012;122:142–148. doi: 10.1016/j.drugalcdep.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Contreras-Rodriguez O, Fonseca F, Cuenca A, Soriano-Mas C, Rodriguez J, Pardo-Lozano R, Blanco-Hinojo L, de Sola Llopis S, Farre M, Torrens M, Pujol J, de la Torre R. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addiction Biology. 2014;19(2):272–281. doi: 10.1111/j.1369-1600.2012.00472.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sciences. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience. 2013;8:73–84. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. American Journal of Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Wiers RW, Walter H, Bermpohl F. Neural correlates of alcohol-approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology. 2014;39(3):688–697. doi: 10.1038/npp.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Gladwin TE, Rinck M. Should we train alcohol-dependent patients to avoid alcohol? Frontiers in Psychiatry. 2013;4:33. doi: 10.3389/fpsyt.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Item revisions in the Sensation Seeking Scale form V (SSS-V) Personality and Individual Differences. 1996;20:515. [Google Scholar]