Abstract
Summary
Ovules are the female reproductive structures that develop into seeds. Angiosperm ovules include one, or more commonly two, integuments that cover the nucellus and female gametophyte. Mutations in Arabidopsis KANADI (KAN) and YABBY polarity genes result in amorphous or arrested integument growth, suggesting that polarity determinants play key roles in ovule development. We show that the Class III Homeodomain Leucine-Zipper (HD-ZIPIII) genes CORONA (CNA), PHABULOSA (PHB), and PHAVOLUTA (PHV) are expressed adaxially in the inner integument during ovule development, independent of ABERRANT TESTA SHAPE (ATS, also known as KANADI4) activity. Loss of function of these genes leads to aberrant integument growth. Additionally, over-expression of PHB or PHV in ovules is not sufficient to repress ATS expression, and can produce phenotypes similar to those of the HD-ZIPIII loss of function lines. The absence of evidence of mutual negative regulation by KAN and HD-ZIPIII transcription factors is in contrast to known mechanisms in leaves. Loss of HD-ZIPIII activity can partially compensate for loss of ATS activity in the ats cna phb phv quadruple mutant, demonstrating that CNA/PHB/PHV act in concert with ATS to control integument morphogenesis. In a parallel pathway, ATS acts with REVOLUTA (REV) to restrict INNER NO OUTER (INO) expression and outer integument growth. Based on these expression and genetic studies we propose a model in which a balance between the relative levels of adaxial/abaxial activities, rather than the maintenance of boundaries of expression domains, is necessary to support laminar growth of the two integuments.
Keywords: integument, adaxial-abaxial polarity, HD-ZIPIII, KANADI, YABBY, Arabidopsis
Introduction
In flowering plants, ovules are critical female reproductive structures that develop into seeds. Angiosperm ovules include one, or more commonly two, integuments that cover the nucellus and female gametophyte. After fertilization the integuments become the seed coat. Ovule ontogeny has been well characterized in Arabidopsis (Robinson-Beers et al., 1992; Schneitz et al., 1995) and many genes involved in ovule development have been identified (Skinner et al., 2004). Recent genetic studies have shown that mutations in putative polarity determinants, such as members of the YABBY and KANADI gene families, result in amorphous or arrested integument growth (Eshed et al., 2001; McAbee et al., 2006; Villanueva et al., 1999), suggesting that such determinants play key roles in ovule development.
ABERRANT TESTA SHAPE (ATS, also referred to as KANADI4 (KAN4)) is a member of the KANADI gene family that is necessary for laminar extension of the inner integument and for maintaining integument separation (McAbee et al., 2006). In ats mutant ovules, the two integuments fail to originate as separate structures, resulting in a single ‘fused’ integument, and leading to aberrant heart-shaped seeds (Léon-Kloosterziel et al., 1994; McAbee et al., 2006). Loss of function of two other KANADI family members, KAN1 and KAN2, results in an amorphous outer integument and a normal inner integument, implying that these polarity determinants are necessary for laminar extension of the outer integument (Eshed et al., 2004; McAbee et al., 2006). A dominant allele of one Class III Homeodomain Leucine-Zipper (HD-ZIPIII) gene, PHABULOSA (PHB), displays incomplete integument formation, implying that these transcription factors may also play important roles in ovule patterning and growth (McConnell and Barton, 1998).
Based on current knowledge regarding the expression and roles of polarity genes during leaf morphogenesis, a model has been proposed to explain initiation and extension of integuments and ovule mutant phenotypes such as ats (McAbee et al., 2006). In leaves, the combined expression of HD-ZIPIII transcription factors provides adaxial identity, while YABBY and KANADI family members are abaxial determinants (Bowman et al., 2002; Emery et al., 2003; Kerstetter et al., 2001; McConnell and Barton, 1998; McConnell et al., 2001; Siegfried et al., 1999). Loss of a polarity function can result in arrested and/or radialized lateral organs, leading to the hypothesis that the juxtaposition of adaxial and abaxial characters is required in order to properly define locations of laminar growth (Bowman et al., 2002; Eshed et al., 2001; Eshed et al., 2004; Waites and Hudson, 1995; Waites et al., 1998). McAbee et al. (2006) proposed that ATS acts as an abaxial determinant in the inner integument, while KAN1 and KAN2 act in conjunction with a YABBY gene (INNER NO OUTER (INO)) to specify abaxial cell fate in the outer integument. Furthermore, McAbee et al. (2006) hypothesized that HD-ZIPIII transcription factors act in both the outer and inner integuments as adaxial determinants.
While there is evidence to support the idea that abaxial determinants are required for initiation and maintenance of integument growth (Eshed et al., 2001; McAbee et al., 2006; Villanueva et al., 1999), a role for adaxial determinants in ovules has not yet been demonstrated. We sought to further define the role of polarity establishment in integument growth through expression analyses, genetic and transgenic studies of HD-ZIPIII and ATS transcription factors.
In addition to their roles in leaves, we have found that CORONA (CNA), PHB, PHAVOLUTA (PHV) and REVOLUTA (REV) also regulate ovule development. We show these HD-ZIPIIIs act in concert with ATS to control patterning and laminar growth of both the inner and outer integument. These results provide evidence that a polarity establishment pathway is required for integument formation, and also reveal differences between the pathways utilized in ovule and leaf development. In light of these new findings, we posit a model in which a balance between levels of polarity determinants acts to mediate integument development.
Results
The HD-ZIPIII genes PHB, PHV, and CNA are expressed in a polar fashion during ovule development
Based on previous genetic studies (Eshed et al., 2001; McAbee et al., 2006), we hypothesized that HD-ZIPIII genes may function as adaxial determinants during integument morphogenesis. There are five HD-ZIPIII genes in Arabidopsis: CNA/ATHB15, PHB, PHV, REV, and ATHB8. The patterns of PHB and REV have been previously examined during ovule development (Sieber et al., 2004a). Herein we focus on expression of PHV, and CNA, and reexamine PHB as a control because of their demonstrated roles in leaf development, and an indication of a role in ovule development (McConnell and Barton, 1998; Sieber et al., 2004a). ATHB8 expression has been previously shown to be primarily associated with vascular development (Prigge et al., 2005) and this gene was therefore not examined in this study. Expression patterns of PHB, PHV, and CNA were examined in wild-type ovules using in situ hybridization with gene-specific probes. In stage 2 ovules (stages according to (Schneitz et al., 1995)) PHB mRNA was detected only in the inner integument, specifically in the cell layer adjacent to the nucellus (Figure 1a and Figure S1a,b). In later stages, hybridization was also seen in the vasculature of wild-type ovules (Figure 1a). This expression pattern is consistent with an earlier report (Sieber et al., 2004a). PHV and CNA were expressed in similar patterns in ovules (Figure 1c and 1e, respectively; Figure S1e, f, i), with inner integument specific expression being present as early as stage 1 ovules (Figure 1e). Altogether the observed PHB, PHV, and CNA in situ hybridizations present an adaxial pattern in the inner integument (as defined in (McAbee et al., 2006)).
Figure 1.
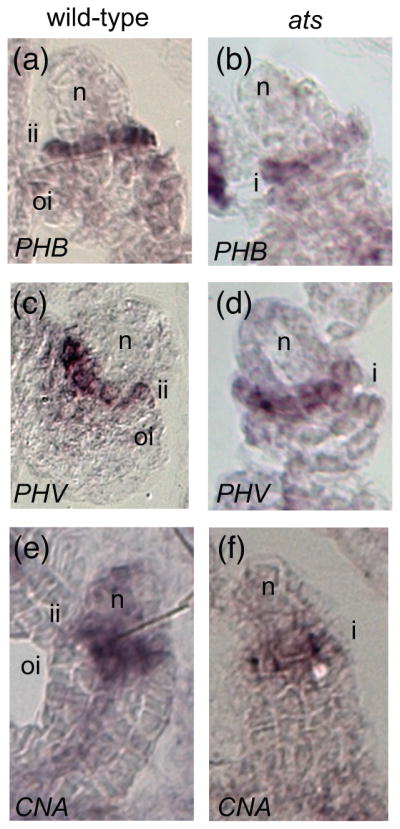
The polar expression patterns of HD-ZIPIII genes are independent of ATS activity. (a), (c), (e) wild-type Ler ovules; (b), (d), (f) ats-1 mutant ovules; (a), (b) in situ hybridization with antisense PHB probe. (c), (d) in situ hybridization with antisense PHV probe. (e) in situ hybridization with antisense CNA probe. Abbreviations: nucellus (n), inner integument (ii), outer integument (oi).
During polarity establishment of leaves, HD-ZIPIII expression patterns are refined through a combination of microRNA regulation and repression by KANADI genes (Bowman et al., 2002; Kidner and Timmermans, 2007). Given that ATS is the only KANADI gene known to be active in the inner integument, we wanted to test whether ATS is required to restrict the expression of PHB, PHV, and/or CNA during ovule development. In loss of function ats ovules we observed expression patterns for PHB, PHV, and CNA that did not differ from those observed in wild type (for PHB compare Figure 1b to 1a; for PHV compare Figure 1d to 1c; for CNA compare Figure 1f to 1e; see Figure S1 for additional comparisons). This suggests ATS activity is not required to delineate PHB, PHV, nor CNA expression patterns during ovule development.
PHB, PHV, and CNA are required for integument morphogenesis
In order to examine the collective role(s) of PHB, PHV, and CNA during ovule development we took a genetic approach. These genes overlap in expression patterns as well as function and therefore single and double mutants appear phenotypically wild type (Prigge et al., 2005). A recent study of these genes described the cna-2 phb-13 phv-11 triple mutant as having “short integuments” in addition to other developmental defects such as extra carpels (Prigge et al., 2005).
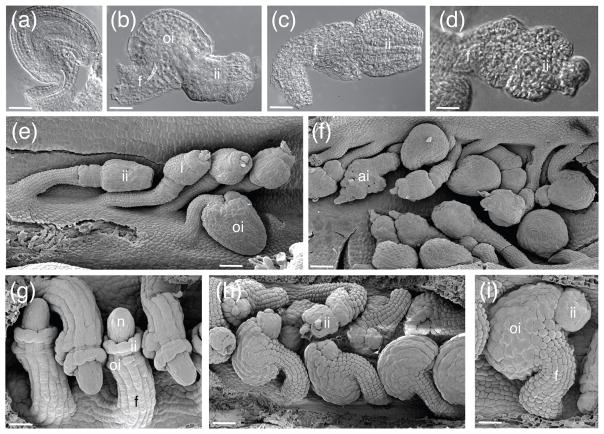
In our examination of cna-2 phb-13 phv-11 we found the ovule phenotype to be highly variable (Figure 2). In some cases, cna-2 phb-13 phv-11 ovules initiated both integuments normally, resulting in nearly wild-type ovules (Figure 2a, e bottom right corner). More frequently, ovules exhibited a protruding inner integument and a superficially shorter outer integument at maturity (Figure 2b, f top right corner). This indicated a disruption in the coordination of timing of initiation and/or growth of the two integuments. Despite this morphogenetic disconnect between the two integuments, the outer integument still retained its anatropous form. We also observed ovules that phenocopied the ovules of ino mutants (Baker et al., 1997; Villanueva et al., 1999) (Figure 2c, e) having an inner integument and an absent or rudimentary outer integument. Ovules with an amorphous, globular structure in place of the integuments and an exposed nucellus were also commonly observed (Figure 2d–f). In general, it appears that the combined loss of CNA, PHB and PHV function leads to abnormal integument development. Surprisingly, this effect is not restricted to the inner integument, as would be predicted from their ovule expression patterns depicted in Figure 1.
Figure 2.
HD-ZIPIII genes are required for integument morphogenesis. (a)–(f) cna phb phv triple mutant ovules; (g)–(i) phv-1d gain-of-function ovules. (a)–(d) whole-mount ovule clearings. (e)–(i) SEMs. Scale bar = 20 μm in (a)–(d), (i). Scale bar = 50 μm in (e), (f), (h). Scale bar = 5 μm in G. Abbreviations: nucellus (n), outer integument (oi), inner integument (ii), funiculus (f), amorphous integument (ai), integument (i).
Gain of function phb-1d mutants display ectopic PHB expression as a result of microRNA insensitivity (Reinhart et al., 2002; Rhoades et al., 2002). In phb-1d ovules the timing of integument initiation and growth is disrupted, producing ovules with elongated inner integuments (McConnell and Barton, 1998; McConnell et al., 2001; Sieber et al., 2004a). We examined phv-1d ovules to compare them to those of phb-1d. In phv-1d plants we observed three classes of ovules: phb-1d-like, ino-like and wild type (Figure 2h, i). Because these dominant mutations represent microRNA-resistant alleles of PHB and PHV (McConnell et al., 2001), these data imply that proper regulation of PHB and PHV expression patterns via miR165/166 action is required for normal integument development. Additionally, these phenotypes were similar to those observed in the cna phb phv triple loss-of-function mutant suggesting that a relative level of adaxial activity produced by PHB, PHV and CNA may directly influence integument morphogenesis.
ATS and HD-ZIPIII functions are required to maintain integument development
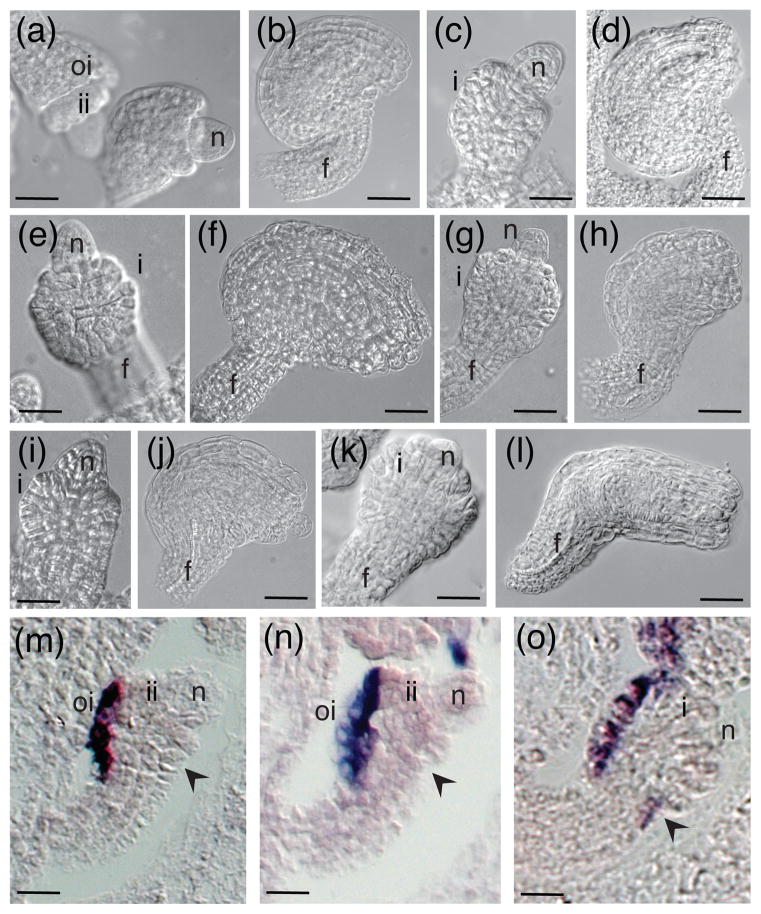
We crossed cna-2 phb-13 phv-11 with ats-3 phb-6 phv-5 rev-9/+ in order to further examine the roles of HD-ZIPIII genes in the context of ATS function. We were able to identify ats cna phb phv and ats cna phb phv rev/+ mutants in the F2 population by PCR-based genotyping. While less than 10% of ats cna phb phv ovules had a wild type appearance (Figure 3d) no wild-type ovules were observed in ats cna phb phv rev/+. More frequently, ats cna phb phv ovules exhibited arrested outer integument growth (Figure 3e, f) and partial inner integument growth (Figure 3e, f; note the naked nucellus in 3f and the amorphous inner integument in 3e). Loss of one copy of REV in this mutant background enhances these defects, producing ovules with arrested inner and outer integument growth (Figure 3g, h). While both mutant combinations displayed a range of phenotypes, the ats cna phb phv rev/+ mutants were more severely affected than the ats cna phb phv mutants (compare Figure 3g–h to d–f) suggesting that REV activity does contribute to integument growth.
Figure 3.
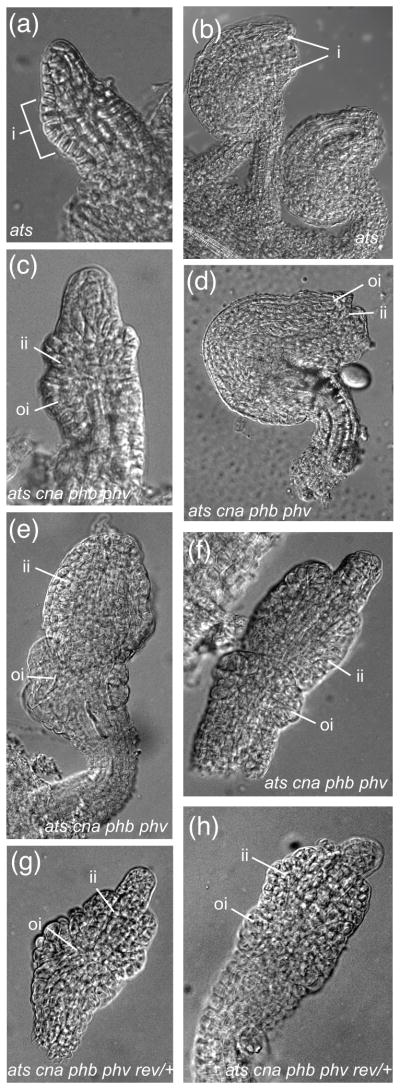
Loss of function of CNA, PHB, and PHV can suppress the Ats− phenotype. (a)–(b) whole-mount clearings of ats; (c)–(f) whole-mount clearings of ats cna phb phv ovules; (g)–(h) whole-mount clearings of ats cna phb phv rev/+ ovules. Scale bar = 5 μm in (a), (c). Scale bar = 10 μm in (b), (d)–(h). Abbreviations: nucellus (n), integument (i), inner integument (ii), outer integument (oi), funiculus (f).
Notably, the combined loss of CNA, PHB, and PHV function can suppress the integument fusion Ats− phenotype. In ats ovules the integuments are congenitally fused, resulting in a unitegmic ovule (McAbee et al., 2006)(Figure 3a). However ats cna phb phv and ats cna phb phv rev/+ ovules initiated separate inner and outer integuments (Figure 3c and e–h, respectively; compare Figure 3a to Figure 3c). It is worthwhile to note that this suppression is not observed in other mutant combinations tested, i.e. ats phb phv rev/+ plants (see Figure 4), suggesting that loss of CNA activity (in the absence of PHB and PHV) may be a predominant component of the suppression phenotype. Together these mutant analyses provide genetic evidence that CNA, PHB, PHV, and REV together with ATS are required to sustain normal integument growth.
Figure 4.
Genetic interactions between HD-ZIPIII genes and ATS. (a), (b) phb phv rev/+; (c), (d) ats single mutant; (e), (f) ats phv double mutant; (g), (h) ats phb double mutant; (i), (j) ats phb phv triple mutant; (k), (l) ats phb phv rev/+. (m) wild-type Ler. (n) phb phv rev/+. (o) ats phb phv rev/+. (a)–(l) whole mount clearings of ovules. (m)–(o) in situ hybridizations with antisense INO probe. Scale bar = 5 μm in (a), (c), (e), (g), (i), (k), (m)–(o). Scale bar = 10 μm in (b), (d), (f), (h), (j), (l). Black arrow in (m)–(o) indicates the gynoapical side of the ovule. Abbreviations: nucellus (n), inner integument (ii), outer integument (oi), funiculus (f), integument (i).
ATS and HD-ZIPIII genes interact to negatively regulate INO expression
Of all five HD-ZIPIII genes, only REV has been shown to be expressed in both integuments (Sieber et al., 2004). Given this difference in expression domains, we wanted to examine roles of REV in the absence of ATS. We crossed phb-6 phv-5 rev-9/+ plants, which have wild-type ovules (Figure 4a, b), with ats-3 (Figure 4c, d) in order to examine whether or not these genes act in the same genetic pathway during integument development. In the segregating F2 progeny from an ats-3/+ phb-6/+ phv-5/+ rev-9/+ parent we were able to evaluate the phenotypes of desired mutant combinations after PCR-based genotyping. These mutant studies suggest that PHB, PHV and REV may have overlapping activities with ATS, as ats phv (Figure 4e, f), ats phb (Figure 4g, h) and ats phb phv (Figure 4i, j) mutants all show an intermediate phenotype toward symmetrical integument growth (compare Figure 4f, h, j to 4l). Additionally, ats phb phv rev/+ ovules are more severely affected than ats phb phv ovules (compare Figure 4k to 4i, and 4l to 4j), and exhibit completely symmetrical outer integument development (Figure 4h, l). These mutant combinations also have significantly reduced seed set compared to wild type (compare Figure S2d to S2e; S2f). Furthermore, suppression of ats does not occur in the ats phb phv rev/+ mutant, which is in contrast to the ats phb phv cna quadruple mutant (Figure 3). This phenotypic difference suggests that both CNA and REV may act in concert with PHB and PHV in different manners during ovule development. While this hypothesis is consistent with previously demonstrated differences in function between CNA and REV (Prigge et al., 2005) it is important to note that it is based on our limited ability to examine only partial loss of REV function in the absence of PHB and PHV because true phb phv rev triple mutants are seedling lethal (Emery et al., 2003).
The ats phb phv rev/+ ovule morphology is reminiscent of superman (sup) ovules, wherein symmetrical growth results from ectopic INO expression on the gynoapical side of the developing ovule (Meister et al., 2002). We examined expression of INO in wild type, phb phv rev/+ and ats phb phv rev/+ ovules to see if similar expression could account for the observed phenotype. As in wild type, INO was expressed only on the gynobasal side of phb phv rev/+ ovules (Figure 4n compared to m). In contrast, ats phb phv rev/+ ovules showed ectopic INO expression on the gynoapical side of the ovule (Figure 4o, arrow) in addition to the normal gynobasal location. The expression pattern of INO in ats ovules was not found to differ from that in wild type ovules (McAbee et al., 2006), so the observed difference in expression patterns represent a synergistic effect between ATS and REV, PHB, and PHV.
ATS expression is unaltered in phb-1d ovules
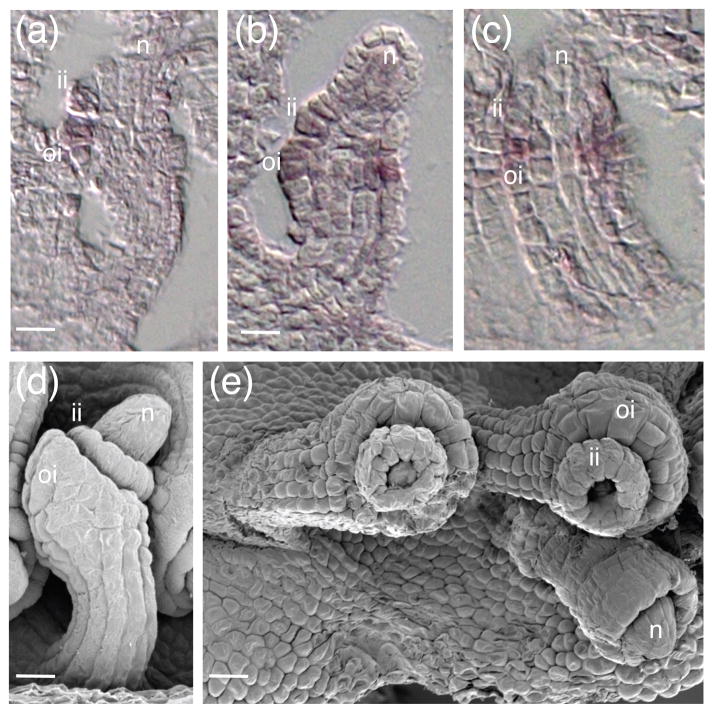
Antagonism between KAN and HD-ZIPIII genes occurs during leaf development to delineate abaxial-adaxial boundaries (Bowman et al., 2002; Kidner and Timmermans, 2007). In gain of function alleles of HD-ZIPIII such as phb-1d and phv-1d, leaf tissue becomes adaxialized via repression of abaxial factors, and KAN expression is reduced (Emery et al., 2003; Eshed et al., 2001; Eshed et al., 2004; Kerstetter et al., 2001). We examined ATS expression in phb-1d ovules using in situ hybridization to see if a similar mechanism may be acting in ovules. In wild type ovules, ATS expression is first seen in the boundary cells between the inner and outer integument (Figure 5a) and later becomes restricted to the inner integument (McAbee et al., 2006). ATS is expressed normally in phb-1d and phv-1d ovules (Figure 5b, c and data not shown), implying that at least these HD-ZIPIIIs are not sufficient to negatively regulate ATS when ectopically expressed during integument development. This suggests that the canonical repressive interactions between KAN and HD-ZIPIII genes in lateral organs may not be reiterated in ovules, at least with respect to ATS and PHB or PHV.
Figure 5.
ATS is expressed normally in phb-1d mutant ovules. (a), (d) wild-type Ler ovules; (b), (c), (e) phb1d ovules. (a)–(c) in situ hybridizations with antisense ATS probe. (d)–(e) SEMs. Scale bars = 5 μm. Abbreviations: nucellus (n), inner integument (ii), outer integument (oi).
Ectopic expression of ATS can arrest growth of the outer integument
Misexpression of KAN genes can lead to arrest of organ growth (Emery et al., 2003; Kerstetter et al., 2001). We used the LHG4≫OP system (Liu and Meinke, 1998; Moore et al., 1998) to ectopically express ATS across the chalaza during ovule development under the control of the ANT promoter, which is active in the region from which both integuments will form (Elliott et al., 1996). A line harboring a PANT:LHG4 transgene (Gross-Hardt et al., 2002) was crossed into POP:ATS and the resulting ANT≫ATS F1 plants were evaluated. Two phenotypic classes of ovules were observed in these F1 plants: wild type (Figure 6b) and ino-like (Figure 6c, d). The ino-like ovules had an inner integument and a reduced/absent outer integument (Figure 6c, d). The lack of complete penetrance of the ino-like phenotype may be due to weak expression from either the ANT:LHG4 and/or OP:ATS transgenes. These data suggest that expression of ATS in the chalaza can lead to outer integument arrest, possibly through negative regulation of INO, which would be consistent with our mutant studies (Figure 4).
Figure 6.
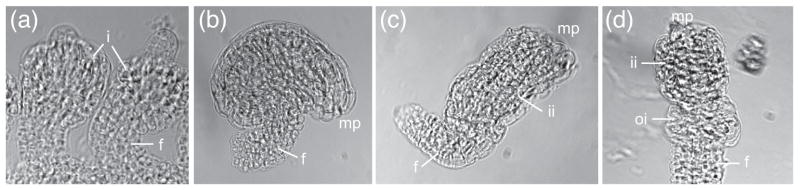
Ectopic expression of ATS can arrest growth of the outer integument. (a)–(d) whole-mount clearings of ANT≫ATS ovules. (a) integument initiation. (b)–(d) mature ovule phenotypes. (b) wild-type ovule. (c)–(d) ino-like ovules. Abbreviations: integuments (i), inner integument (ii), outer integument (oi), micropyle (mp), funiculus (f).
Discussion
Adaxial expression of HD-ZIPIII genes in the inner integument
While roles for KAN (ATS, KAN1 and KAN2) and YABBY (INO) genes in ovule development have been described, the functions of adaxial determinants in this process are less well understood. In leaves, HD-ZIPIII expression domains are adjacent to KAN expression domains and this arrangement promotes laminar growth (Eshed et al., 2001; Kidner and Timmermans, 2007). Using in situ hybridization we show that the HD-ZIPIII genes PHB, PHV and CNA were expressed in a polar fashion in the inner integument, with their mRNA accumulating in the cell layer adjacent to the nucellus (which later differentiates into the endothelium). Thus PHB, PHV, and CNA expression was juxtaposed with ATS expression in ovules (Figure 1), consistent with the polarity model proposed by McAbee et al. (2006). In contrast to the other three examined HD-ZIPIII genes, REV is expressed broadly in both integuments (Sieber et al., 2004b) and thus does not conform to the proposed model.
HD-ZIPIII expression expands abaxially in leaves of kan mutants, demonstrating that KAN genes negatively regulate HD-ZIPIII expression and contribute to confinement of HD-ZIPIII expression to adaxial domains (Bowman et al., 2002; Eshed et al., 2001; Eshed et al., 2004; Kerstetter et al., 2001). However, in ovules it appears that PHB, PHV, and CNA expression patterns are not governed by ATS activity because they are unaltered in ats ovules (Figure 1).
McAbee et al. (2006) hypothesized that an absence of abaxial function in ats mutants created a single adaxial/abaxial boundary in the ovule, rather than two separate boundaries, which led to the formation of a single integument. They further hypothesized that loss of ATS activity could lead to an expansion of the “adaxial” factor(s) expression domain. Our observation that PHB, PHV, and CNA expression patterns are unchanged in ats ovules (Figure 1), conflicts with this later hypothesis. Rather, it appears that loss of the ATS abaxial boundary function appears to be sufficient to produce the observed integument fusion.
Since ATS is not responsible for patterning PHB, PHV, or CNA expression in ovules, what factor(s) could contribute to the difference in their expression patterns from that of REV in this structure? In leaves, the patterning of HD-ZIPIII mRNA accumulation occurs in part through negative regulation by miR165/166 (Kidner and Timmermans, 2007). In ovules, the PHB promoter appears to be active in both integuments based on GUS activity in the phb-6 enhancer trap line (Figure S4). Because this promoter activity does not match the mRNA distribution pattern (Figure 1 and Sieber et al. (2004b)), miR165/166 could contribute to restricting PHB expression in ovules. In addition, the miRNA-resistant phb-1d mutant is seen to expand in expression domain relative to the wild-type (Sieber et al., 2004b) further implicating miRNA in regulation of this gene. Although all five HD-ZIPIII genes share the miR165/166 recognition sequence, miR166g has been shown to have differential affects on HD-ZIPIII transcripts (Williams et al., 2005). Overexpression of miR166g in the jabba-1d mutant led to down-regulation of PHB, PHV, and CNA mRNAs while REV expression was increased (Williams et al., 2005). Based on these data, one hypothesis that could account for the differences in PHB, PHV, and CNA mRNA distributions compared to the REV expression domain could be differential sensitivity to miR165/166 action.
The LITTLE ZIPPER (ZPR) proteins, a novel family of leucine zipper-containing proteins, have recently been proposed to negatively influence HD-ZIPIII activity and expression (Kim et al., 2008; Wenkel et al., 2007). The roles of ZPR genes in ovules have not yet been evaluated, but differential activity of ZPR proteins on the HD-ZIPIII genes provides another hypothesis for the different expression domains of REV and PHB/PHV/CNA.
HD-ZIPIII genes are required for patterning and growth during ovule development
Loss of PHB, PHV, and CNA led to abnormal ovule development characterized by arrested or amorphous inner and outer integuments. It is curious that both integuments were affected when expression of these genes was only detected in the inner integument (Figure 1 and Sieber et al. (2004b)). There are several possible explanations for this combination of observations. It is possible that in situ hybridization (Figure 1 and Sieber et al. (2004b)) is insufficiently sensitive to detect low levels of PHB, PHV, and/or CNA mRNA that may be present in the outer integument. Another possibility derives from the order and timing of integument formation. Inner integument initiation precedes initiation of the outer integument, and development of both structures is coordinated (Schneitz, 1999). If inner integument patterning is unbalanced, it could impact the quality of outer integument growth by a domino effect. This type of non-cell autonomous action has been described for other transcription factors active in ovules, such as WUSCHEL (Gross-Hardt et al., 2002). An additional influencing factor could be production/perception of hormones, such as auxin. Recent studies on ARF6 and ARF8 indicate that auxin perception and responsiveness contribute to integument formation (Wu et al., 2006). Given that auxin cues during embryogenesis appear to be mediated by KAN and HD-ZIPIII activity via PIN1 localization (Izhaki and Bowman, 2007), the same could be true in ovules, and alterations in inner integument development could alter the hormone environment in ways that would affect initiation and growth of the outer integument.
Reduction in outer integument growth was also observed in both phb-1d (McConnell and Barton, 1998) and phv-1d (Figure 2g–i), and inner integument defects were observed in phv-1d (Figure 2g–i). The over (ectopic) production of the products of these two genes could therefore produce effects that were similar to those resulting from a decrease in HD-ZIPIII function (e. g. Figure 2b). This seemingly paradoxical observation can be explained if an appropriate balance between adaxial and abaxial promoting activities is necessary for proper integument growth, and that an imbalance in either direction results in disruption of this process.
Loss of CNA/PHB/PHV can suppress both aspects of the Ats− phenotype in an ats mutant background (Figure 3). This observation that the simultaneous loss of abaxial and adaxial functions restores inner integument growth and integument separation (Figure 3) indicates that there may be additional ad/abaxial factors that are active in ovules, or that other functions can substitute for the juxtaposition when both classes of factors are absent. Indeed, the loss of REV activity in this background reduces inner integument growth, suggesting that REV may be one of the factors that can compensate for loss of CNA, PHB, and PHV. That loss of adaxial activity can mitigate effects of loss of abaxial activity is consistent with our hypothesis that an appropriate balance between the levels of these two activities is critical for normal laminar extension of the integuments.
We also observed that ectopic expression of ATS in the chalaza during ovule development can lead to arrest of outer integument growth (Figure 6). These data are also consistent with the concept that an appropriate balance between levels of KAN and HD-ZIPIII functions must be maintained in order to promote integument growth, but other mechanisms are also possible. For example, ectopic KAN and ATS expression can lead to meristem arrest (Emery et al., 2003; Kerstetter et al., 2001) and unpublished data), and growth arrest may be a general activity of KAN proteins.
A balance model for the ad/abaxial determinants underlying integument morphogenesis
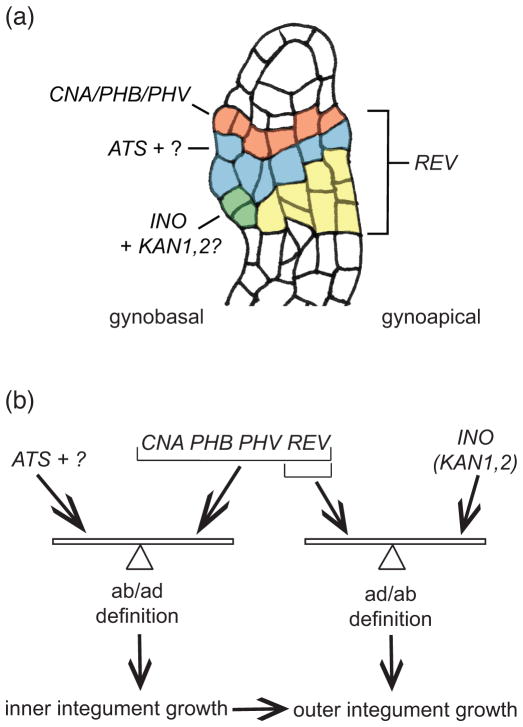
Based on these novel expression and genetic data we have refined our model for integument growth (Figure 7) from what was previously published (McAbee et al., 2006). We had previously proposed that ATS acted in juxtaposition to a hypothetical adaxial function to promote inner integument growth. We can now say with confidence that this adaxial function includes the activities of the HD-ZIPIII family members PHB, PHV, and CNA, which are expressed adaxially in the inner integument (Figure 7a). These expression patterns may be established through a combination of promoter activities, miR165/166 expression and/or ZPR action but are shown to be independent of ATS activity. The adaxial activity also includes REV function, even though this gene is such a role for REV comes from comparison of ats cna phb phv rev/+ ovules to ats cna phb phv ovules (Figure 3). The loss of one copy of REV enhances the ats cna phb phv phenotype by negatively impacting inner integument growth. ATS is shown to be a critical component of the abaxial function, but we hypothesize the existence of an additional abaxial function because inner integument growth and integument separation are restored by certain mutant combinations with ats. These results can be explained by an ab/adaxial juxtaposition model if the model is modified to hypothesize that integument growth depends on a proper balance between abaxial and adaxial activities, rather than the absolute levels of the activities (Figure 7b). The observation that ATS and HD-ZIPIII expression in the inner integument was not altered when either factor was absent show that, in contrast to the situation in leaves, the ab and adaxial function are not mutually suppressive.
Figure 7.
The collective roles of polarity determinants in ovule development comprise the balance model. (a) Expression summary of polarity determinants required for integument growth. During early ovule development CNA, PHB, and PHV (red), REV (all colored regions), ATS (blue), “?” unknown abaxial factor(s) which may share expression domain with ATS, and INO (green) mRNAs are specifically patterned along the chalaza. KAN1 and KAN2 (green) are predicted to be expressed in a similar fashion to INO. Once these patterns are established, persistent expression/action of all these transcription factors is required for proper integument growth. (b) The proposed polarity balance model. In the inner integument, ATS and the proposed additional abaxial factor (“?”) act in balanced opposition to CNA/PHB/PHV and REV to promote inner integument growth. Disruption of this balance by decrease or increase in either function alters the growth plane, leading to aberrant integument growth. Initiation of the inner integument precedes outer integument initiation in Arabidopsis, and thus growth of the outer integument is directly influenced by inner integument morphogenesis. In the outer integument, abaxial activities provided by INO, KAN1, and KAN2 act in balance with REV to control morphogenesis.
The earlier model (McAbee et al., 2006) and the model for leaf development (Bowman et al., 2002; Kidner and Timmermans, 2007) would predict a progressive loss of integument growth with progressive loss of ab/adaxial factors. However, our observation that loss of HD-ZIPIII activity can partially compensate for loss of ATS activity is inconsistent with these models. The revised balance model can explain these results and provide an explanation of how similar phenotypes can result from loss of function and ectopic expression of HD-ZIPIII genes. Whether such a balance mechanism is also acting in leaves, or if it represents a further difference between leaves and integuments has yet to be determined. Another aspect of the balance model relates to the coordinated growth of the inner and outer integuments. Loss of apparently inner integument specific gene functions leads to a disruption in outer integument growth (Figure 2, Figure 3 and (McAbee et al., 2006)), suggesting that inner integument growth positively contributes to outer integument growth. A final aspect of the model is that the pattern and growth are self-reinforcing. Once an initial state of appropriately balanced ad/ab definition is achieved it will be subsequently maintained. This could explain the range of phenotypes observed among ovules of individual polarity mutants, where small stochastic variations in the initial levels of determinants could result in different final outcomes.
Patterning roles of REV and ATS during integument morphogenesis
While a more complete understanding of REV function in ovules is lacking due to the inability to examine phb phv rev ovules, we have found that in the absence of ATS phb phv rev/+ ovules become sup-like (Figure 4). We attribute this phenotype to ectopic INO expression (Figure 4). Because we do not observe this same phenotype in ats phb phv cna ovules, and REV is expressed differently from its paralogs in ovules, we propose that REV may have a unique function in conjunction with ATS in patterning INO expression during integument initiation. This mechanism may be connected with SPOROCYTELESS/NOZZLE action, which was previously shown to act with ATS to regulate INO (Balasubramanian and Schneitz, 2002). While KAN1 and KAN2 (the only other KANADI genes that appear to play a role in ovule development) also participate in control outer integument formation, expression patterns of these genes in ovules have not been observable to date (Kelley and Gasser, unpublished data).
How leaf-like are integuments?
The fossil record indicates that the origin of ovules was contemporaneous with the origin of leaves (Andrews, 1963; Gasser et al., 1998; Herr, 1995). According to the telome theory, the inner integument is homologous to lateral sterile or sterilized structures (born on a reproductive telome truss, with the nucellus being homologous to the apical fertile telome). The outer integument was gained later in plant history, on the stem lineage leading to angiosperms, possibly by transformation from a leaf-like structure known as a cupule (Doyle, 2006; Gasser et al., 1998).
Separate origins for both integuments are also supported at the molecular level as the control of inner and outer integument development occurs through different genes ((McAbee et al., 2006; Skinner et al., 2004) and this study). For instance, ATS and CNA/PHB/PHV drive inner integument growth while INO and KAN1/2 regulate outer integument growth, in both cases REV is an apparent additional participant (Figure 7). Thus the inner integument shares process homology with leaves, in that it is not directly derived from a leaf but it does utilize the same set of gene classes (KANADI and HD-ZIPIII) to control development. On the other hand, the outer integument is believed to share structural homology with leaves (being most likely derived from a cupule (Doyle, 2006)). The outer integument developmental genetic program would, therefore, be expected to also include a YABBY, in this case the diverged family member INO. If REV acts as the corresponding adaxial factor in the outer integument, it may do so independent of a delineated ad/abaxial expression boundary (Sieber et al., 2004b). Current phylogenetic analyses of these three gene families (KAN, HD-ZIPIII and YABBY) are consistent with these hypotheses, with the origin of KAN and HD-ZIPIII lineages pre-dating seeds and leaves and YABBY genes originating with seed plants (Floyd and Bowman, 2007).
Together, our studies, and those of previous researchers (Balasubramanian and Schneitz, 2002; McAbee et al., 2006; Sieber et al., 2004b), demonstrate that while there are similarities between integuments and leaves, there are also marked differences. Although these organs have distinct evolutionary origins, a common set of polarity determinants appears to have been serially utilized in both sets of structures. The differences in the precise roles and interactions of the determinants in each structure may represent differences from the time of their origin or their derived states resulting from subsequent structural diversifications.
Experimental Procedures
Plant material and cultivation
Arabidopsis plants were grown under long-day conditions as previously described (McAbee et al., 2006). Unless otherwise stated, the alleles used in this study were ats-3 (McAbee et al., 2006); phb-6, phv-5, rev-9 (Emery et al., 2003); and cna-2 (Prigge et al., 2005). Triple mutant cna-2 phb-13 phv-11 seed was a kind gift from Steven Clark (Prigge et al., 2005). phb-6 phv-5 rev-9/+, ANT:LHG4, and OP:ATS seeds were a kind gift from John Bowman.
To create ats-3 phb-6 phv-5 and ats-3 phb-6 phv-5 rev-9/+ plants phb-6 phv-5 rev-9/+ pistils were pollinated with ats-3 pollen on four independent plants. Ten (10) F1 plants from each cross were genotyped for ATS/ats-3, PHB/phb-6, PHV/phv-5, and REV/rev-9 alleles using the polymerase chain reaction (PCR) (see Table S1 for a list of primers used in this study). Three of the F1 ats-3/+ phb-6/+ phv-5/+ rev-9/+ plants were allowed to self-pollinate to create segregating F2 populations. Approximately 360 Basta-resistant (rev-9 carries a Basta resistance marker) F2 plants were genotyped for ATS/ats-3, PHB/phb-6, PHV/phv-5, and REV/rev-9 alleles using PCR. Seeds from these plants were evaluated for ats-3 or wild type morphology. From this population we identified three ats-3 phb-6/+ phv-5 rev-9/+ individuals. F3 (and subsequent generations) ats-3 phb-6 phv-5 and ats-3 phb-6 phv-5 rev-9/+ plants were identified by PCR-based genotyping.
To create ats cna phb phv and ats cna phb phv rev/+ plants cna-2 phb-13 phv-11 pistils were pollinated with ats-3 phb-6 phv-5 rev-9/+ pollen on two plants. Five F1 plants were genotyped for REV/rev-9; three of the five were rev-9/+. These three individuals were further genotyped for ATS/ats-3, CNA/cna-2, phb-13/phb-6, and phv-11/phv-5; all three were confirmed to be ats-3/+ cna-2/+ phb-13/phb-6 phv-11/phv-5. The resulting segregating F2 population (193 individuals) from a self-pollinated ats-3/+ cna-2/+ phb-13/phb-6 phv-11/phv-5 rev-9/+ plant contained 14 sterile plants and 18 plants with an abnormal seedling phenotype (Figure S3): 1–2 radialized cotyledons; hyperaccumulation of anthocyanins in cotyledons; no shoot apical meristem and swollen hypocotyls. These phenotypes are similar to the previously described phv rev and the phb phv rev phenotypes (Prigge et al., 2005) and occurred at approximately a 1 in 16 frequency. We completed genotyping on 96 of the remaining 193 individuals for ATS/ats-3, CNA/cna-2, phb-6, phv-11/phv-5, and REV/rev-9. Among these 96 F2 plants we identified one ats cna phb-6 phv individual and four ats cna phb-6 phv rev/+ individuals; these individuals were used for phenotypic analyses.
To create an ectopic ATS expression line we used the pOpL two-component system (Liu and Meinke, 1998; Moore et al., 1998). The ANT:LHG4 line was previously described (Schoof et al., 2000). The OP:ATS line was created by cloning the ATS cDNA into the 10-OP BJ36 vector (Moore et al., 1998) using BamHI as the 5′ site and HindIII as the 3′ site; the OP:ATS cassette was then cloned into pMLBART as a NotI fragment and then transformed by the floral dip method (Clough and Bent, 1998) into Ler (Eshed and Bowman, unpublished). We crossed ANT:LHG4 into OP:ATS to generate ANT≫ATS plants. Ovules in the resulting F1 progeny from 3 different individuals were phenotyped.
DNA Extraction and Genotyping
Genomic DNA was extracted from Arabidopsis leaf tissue using 2X CTAB buffer (2% cetyl-trimethyl-ammonium bromide (CTAB), 1.4 M NaCl, 100 mM Tris-HCl pH 8.0, 20 mM EDTA) followed by chloroform extraction and DNA precipitation with isopropanol at room temperature. DNA pellets were washed with 70% ethanol, re-suspended in 100 μl of sterile water and stored at −20°C. Genotyping was performed by PCR with 2.0 μl of genomic DNA in a 25 μl reaction with either GoTaq Master Mix (Promega, http://www.promega.com) or ExTaq DNA Polymerase (Takara, http://www.takara-bio.com).
Oligonucleotides
Primers used in this study for genotyping are listed in Table S1.
Microscopy
Whole mount clearings were prepared by dissecting ovules from carpels using needles and clearing for 2–3 days in a couple drops of Hoyer’s solution (7.5% gum arabic (w/v), 6 M chloral hydrate, 5% (v/v) glycerol) under a coverslip according to Liu & Meinke (1998). Ovules were photographed on a Zeiss (http://www.zeiss.com) Axioplan microscope with Normarski optics using a Zeiss Axiophot camera.
For scanning electron micrography (SEM), tissue was fixed and critical point dried as previously described (McAbee et al., 2006) and imaged on a Philips XL 30 SEM (FEI Company, http://www.fei.com).
Light micrographs were taken on a Kodak DC290 (Kodak, http://www.kodak.com) camera mounted on a Zeiss SV8 stereomicroscope.
All images were acquired digitally and edited in Adobe Photoshop CS2 (http://www.adobe.com).
In situ hybridization
Digoxigenin (DIG)-labeled antisense probes for in situ hybridization were synthesized using plasmids purified with a Qiagen Miniprep Kit (Qiagen, http://www.qiagen.com) as previously described (McAbee et al., 2006). For antisense PHB probe pGEM-PHB (Williams et al., 2005) was linearized with SphI and transcribed with SP6 RNA Polymerase (Promega). For antisense PHV probe pGEM-PHV (Williams et al., 2005) was linearized with NotI and transcribed with T7 RNA Polymerase (Promega,). For antisense CNA probe pGEM-CNA (Williams et al., 2005) was linearized with SphI and transcribed with SP6 RNA Polymerase (Promega). For antisense INO probe pJMV86 (Villanueva et al., 1999) was linearized with XhoI and transcribed with T7 RNA Polymerase (Promega). For antisense ATS probe pBS-KAN4 (a gift of John Bowman) was linearized with HindIII and transcribed with T7 RNA Polymerase (Promega). Tissue fixation and in situ hybridization were performed as previously described (McAbee et al., 2006), with the following modifications: inflorescences were fixed in FAA for 2 hours at room temperature prior to dehydration in ethanol and embedding in Paraplast-Xtra (Fisher Scientific, http://www.fishersci.com). Slides were hybridized with approximately 10 pg of DIG-labeled probe overnight at 53°C. Immunological detection of the DIG-labeled probes was performed with a DIG Nucleic Acid Detection Kit (Roche, http://www.roche.com) according to manufacturer’s instructions. Following detection, slides were rinsed in sterile water and mounted with Crystal Mount (Fisher Scientific) and a coverslip prior to being photographed under DIC using a Zeiss Axiophot camera attached to a Zeiss Axioplan microscope. Digital images were edited using Adobe Photoshop CS2.
Seed set measurements
Measurements of seed number per silique represent the average values from three individual plants (biological replicates). Each biological replicate is comprised of the average value obtained from five siliques (technical replicates). For statistical analysis of genetic effects, seed number per silique was compared by Kruskal-Wallis one way ANOVA on ranks, with pairwise comparisons (Student-Newman-Keuls Method), using Sigma Stat v3.5 (Systat, http://www.systat.com).
Supplementary Material
Figure S1. The polar expression patterns of HD-ZIPIII genes are independent of ATS activity.
Figure S2. Siliques of ats phb phv rev/+ plants have reduced seed set.
Figure S3. Mutant seedling phenotypes observed in segregating F2 progeny from a selfed ats/+ cna/+ rev/+ phb phv plant.
Figure S4. PHABULOSA promoter activity measured by GUS staining in phb-6 ovules.
Table S1. Oligonucleotides used in this study for genotyping.
Acknowledgments
The authors would like to thank John Bowman (Biological Sciences, Monash University), Jennifer Fletcher (PGEC, University of California, Berkeley) and Steven Clark (MCDB, University of Michigan) for generously sharing seeds and plasmids; Justin Walley, Alexandra Arreola and Quynh Do for technical assistance. We would also like to acknowledge John Bowman, Simon Chan (Plant Biology, University of California, Davis), Jim Doyle (Evolution and Ecology, University of California, Davis) and members of the Gasser lab for valuable discussion. This work was supported by National Science Foundation grant IOS0419531 (to CSG) and National Institutes of Health traning grant T32M07337 (to DRK).
References
- Andrews HNJ. Early seed plants. Science. 1963;142:925–931. doi: 10.1126/science.142.3594.925. [DOI] [PubMed] [Google Scholar]
- Baker SC, Robinson-Beers K, Villanueva JM, Gaiser JC, Gasser CS. Interactions among genes regulating ovule development in Arabidopsis thaliana. Genetics. 1997;145:1109–1124. doi: 10.1093/genetics/145.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Schneitz K. NOZZLE links proximal-distal and adaxial-abaxial pattern formation during ovule development in Arabidopsis thaliana. Development. 2002;129:4291–4300. doi: 10.1242/dev.129.18.4291. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Eshed Y, Baum SF. Establishment of polarity in angiosperm lateral organs. Trends Genet. 2002;18:134–141. doi: 10.1016/s0168-9525(01)02601-4. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Doyle JA. Seed ferns and the origin of angiosperms. Journal of the Torrey Botanical Society. 2006;133:169–209. [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Baum SF, Perea JV, Bowman JL. Establishment of polarity in lateral organs of plants. Curr Biol. 2001;11:1251–1260. doi: 10.1016/s0960-9822(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004;131:2997–3006. doi: 10.1242/dev.01186. [DOI] [PubMed] [Google Scholar]
- Floyd SK, Bowman JL. The ancestral developmental tool kit of land plants. International Journal of Plant Sciences. 2007;168:1–35. [Google Scholar]
- Gasser CS, Broadhvest J, Hauser BA. Genetic analysis of ovule development. Ann Rev Plant Physiol Plant Mol Biol. 1998;49:1–24. doi: 10.1146/annurev.arplant.49.1.1. [DOI] [PubMed] [Google Scholar]
- Gross-Hardt R, Lenhard M, Laux T. WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev. 2002;16:1129–1138. doi: 10.1101/gad.225202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr JM. The origin of the ovule. Am J Bot. 1995;82:547–564. [Google Scholar]
- Izhaki A, Bowman JL. KANADI and class IIIHD-zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell. 2007;19:495–508. doi: 10.1105/tpc.106.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. KANADI regulates organ polarity in Arabidopsis. Nature. 2001;411:706–709. doi: 10.1038/35079629. [DOI] [PubMed] [Google Scholar]
- Kidner CA, Timmermans MCP. Mixing and matching pathways in leaf polarity. Current Opinion in Plant Biology. 2007;10:13–20. doi: 10.1016/j.pbi.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim SG, Lee M, Lee I, Park HY, Seo PJ, Jung JH, Kwon EJ, Suh SW, Paek KH, Park CM. HD-ZIP III activity is modulated by competitive inhibitors via a feedback loop in Arabidopsis shoot apical meristem development. Plant Cell. 2008;20:920–933. doi: 10.1105/tpc.107.057448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Keijzer CJ, Koornneef M. A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell. 1994;6:385–392. doi: 10.1105/tpc.6.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Meinke DW. The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant Journal. 1998;16:21–31. doi: 10.1046/j.1365-313x.1998.00268.x. [DOI] [PubMed] [Google Scholar]
- McAbee JM, Hill TA, Skinner DJ, Izhaki A, Hauser BA, Meister RJ, Venugopala Reddy G, Meyerowitz EM, Bowman JL, Gasser CS. ABERRANT TESTA SHAPE encodes a KANADI family member, linking polarity determination to separation and growth of Arabidopsis ovule integuments. Plant J. 2006;46:522–531. doi: 10.1111/j.1365-313X.2006.02717.x. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Barton K. Leaf polarity and meristem formation in Arabidopsis. Development. 1998;125:2935–2942. doi: 10.1242/dev.125.15.2935. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- Meister RJ, Kotow LM, Gasser CS. SUPERMAN attenuates positive INNER NO OUTER autoregulation to maintain polar development of Arabidopsis ovule outer integuments. Development. 2002;129:4281–4289. doi: 10.1242/dev.129.18.4281. [DOI] [PubMed] [Google Scholar]
- Moore I, Galweiler L, Grosskopf D, Schell J, Palme K. A transcription activation system for regulated gene expression in transgenic plants. Proc Natl Acad Sci USA. 1998;95:376–381. doi: 10.1073/pnas.95.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 2005;17:61–76. doi: 10.1105/tpc.104.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes Dev. 2002;16:1616–1626. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/s0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- Robinson-Beers K, Pruitt RE, Gasser CS. Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell. 1992;4:1237–1249. doi: 10.1105/tpc.4.10.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz K. The molecular and genetic control of ovule development. Curr Opin Plant Biol. 1999;2:13–17. doi: 10.1016/s1369-5266(99)80003-x. [DOI] [PubMed] [Google Scholar]
- Schneitz K, Hulskamp M, Pruitt RE. Wild-type ovule development in Arabidopsis thaliana: a light microscope study of cleared whole-mount tissue. Plant J. 1995;7:731–749. [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- Sieber P, Gheyselinck J, Gross-Hardt R, Laux T, Grossniklaus U, Schneitz K. Pattern formation during early ovule development in Arabidopsis thaliana. Dev Biol. 2004a;273:321–334. doi: 10.1016/j.ydbio.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Sieber P, Petrascheck M, Barberis A, Schneitz K. Organ polarity in Arabidopsis. NOZZLE physically interacts with members of the YABBY family. Plant Physiol. 2004b;135:2172–2185. doi: 10.1104/pp.104.040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews DN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;128:4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- Skinner DJ, Hill TA, Gasser CS. Regulation of ovule development. Plant Cell. 2004;16:S32–45. doi: 10.1105/tpc.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS. INNER NO OUTER regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes Dev. 1999;13:3160–3169. doi: 10.1101/gad.13.23.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites R, Hudson A. phantastica: a gene required for dorsoventrality of leaves in Antirrhinum majus. Development. 1995;121:2143–2154. [Google Scholar]
- Waites R, Selvadurai HRN, Oliver IR, Hudson A. The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell. 1998;93:779–789. doi: 10.1016/s0092-8674(00)81439-7. [DOI] [PubMed] [Google Scholar]
- Wenkel S, Emery J, Hou BH, Evans MM, Barton MK. A feedback regulatory module formed by LITTLE ZIPPER and HD-ZIPIII genes. Plant Cell. 2007;19:3379–3390. doi: 10.1105/tpc.107.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Grigg SP, Xie MT, Christensen S, Fletcher JC. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development. 2005;132:3657–3668. doi: 10.1242/dev.01942. [DOI] [PubMed] [Google Scholar]
- Wu MF, Tian Q, Reed JW. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development. 2006;133:4211–4218. doi: 10.1242/dev.02602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The polar expression patterns of HD-ZIPIII genes are independent of ATS activity.
Figure S2. Siliques of ats phb phv rev/+ plants have reduced seed set.
Figure S3. Mutant seedling phenotypes observed in segregating F2 progeny from a selfed ats/+ cna/+ rev/+ phb phv plant.
Figure S4. PHABULOSA promoter activity measured by GUS staining in phb-6 ovules.
Table S1. Oligonucleotides used in this study for genotyping.