Abstract
Cerebral white matter (WM) degeneration occurs with increasing age and is associated with declining cognitive function. Research has shown that cardiorespiratory fitness and exercise are effective as protective, even restorative, agents against cognitive and neurobiological impairments in older adults. In this study, we investigated whether the beneficial impact of aerobic fitness would extend to WM integrity in the context of a one‐year exercise intervention. Further, we examined the pattern of diffusivity changes to better understand the underlying biological mechanisms. Finally, we assessed whether training‐induced changes in WM integrity would be associated with improvements in cognitive performance independent of aerobic fitness gains. Results showed that aerobic fitness training did not affect group‐level change in WM integrity, executive function, or short‐term memory, but that greater aerobic fitness derived from the walking program was associated with greater change in WM integrity in the frontal and temporal lobes, and greater improvement in short‐term memory. Increases in WM integrity, however, were not associated with short‐term memory improvement, independent of fitness improvements. Therefore, while not all findings are consistent with previous research, we provide novel evidence for correlated change in training‐induced aerobic fitness, WM integrity, and cognition among healthy older adults. Hum Brain Mapp 34:2972–2985, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, anisotropy, cerebrum, cognition, physical fitness, aging
INTRODUCTION
The growth in the number and proportion of older adults in the United States is unprecedented—it is estimated that adults aged 65 years and older will account for roughly 20% of the U.S. population by 2030. Moreover, the number of Americans diagnosed with dementia is projected to more than double over the same time span [Centers for Disease Control and Prevention and The Merck Company Foundation, 2007]. It is therefore a public health priority to identify the biological mechanisms responsible for cognitive aging as well as preventive measures to counteract its deleterious effects. Epidemiological studies call attention to physical activity as a lifestyle behavior that is associated with decreases in age‐related risks for cognitive impairment and Alzheimer's disease [Barnes et al., 2003; Larson et al., 2006; Lindsay et al., 2002; Richards et al., 2003; Yaffe et al., 2009]. In this study, we assessed white matter (WM) integrity as one potential means by which physical exercise may augment cognition in older adults.
Increasing age is accompanied by several anatomical changes in the brain including a reduction in the integrity of cerebral WM. A number of studies have reported age‐related WM volumetric decline in healthy older adults with a sharp drop in later adulthood, even surpassing that observed in gray matter [Guttmann et al., 1998; Jernigan et al., 2001; Raz, 2000; Raz et al., 2005; Salat et al., 1999]. Whereas research investigating WM volume is a valid first step towards understanding age‐related differences in WM and cognition, it does not shed light on the microstructural properties of the particular brain tissue. Diffusion tensor imaging (DTI) has emerged as an alternate technique for analyzing WM and one that is able to address this gap.
The most commonly used indices of WM integrity derived from DTI are fractional anisotropy (FA) and mean diffusivity (MD). FA is a scalar value that refers to the coherence of the orientation of water diffusion, independent of rate. MD is the average rate of water diffusion across all three eigenvalues, independent of direction. While FA and MD provide summary measures of WM diffusion characteristics, more specific neurobiological inferences may be possible by analyzing the rate of diffusion along the individual eigenvectors. For instance, studies have begun to incorporate additional parameters derived from the individual eigenvalues that may be selectively sensitive to specific neural phenomena. Axial diffusion (AD) refers to the eigenvalue of the primary axis and is hypothesized to reflect axonal differences, that is, axonal damage or loss [Budde et al., 2007; Song et al., 2003]. Radial diffusion (RD) is defined as the average of the two perpendicular eigenvalues and is hypothesized to reflect differences in the degree of myelination [Budde et al., 2007; Nair et al., 2005; Song et al., 2002, 2008, 2010]. Therefore, by investigating distinct patterns of diffusivity among the various measures across the entire human brain, a more nuanced understanding of the neurobiology behind individual differences in WM integrity may be possible [although, see Wheeler‐Kingshott and Cercignani, 2009].
The extensive application of DTI to the study of cerebral WM integrity, cognitive functioning, and aging over the past decade has yielded two general trends: (1) an age‐related anterior to posterior decreasing gradient of decline in FA and (2) an association between decreased integrity of WM and poorer performance on tasks that rely on processing speed and executive functioning [see Madden et al., 2009a, 2001 for a review]. The more pronounced decline in FA in anterior regions of the brain as compared to posterior regions has been demonstrated in a number of cross‐sectional studies [Bennett et al., 2010; Burzynska et al., 2010; Damoiseaux et al., 2009; Head et al., 2004; O'sullivan et al., 2001; Pfefferbaum and Sullivan, 2003; Pfefferbaum et al., 2000; Salat et al., 2005; Sullivan et al., 2001; Yoon et al., 2008]. Additionally, decreased WM integrity is associated with poorer performance on tests that depend on processing speed and executive functioning, independent of age effects [Correia et al., 2008; Davis et al., 2009; O'sullivan et al., 2001; Turken et al., 2008]. Using tractography, Madden et al. 2005 discovered that age differences in the decisional component of a task‐switching paradigm were mediated by FA in regions of the frontoparietal network (the central genu and splenium‐parietal fibers in the right hemisphere).
Given the well‐established phenomena of decreased WM integrity and cognitive functioning accompanying aging as well as the burgeoning population of older adults, it is imperative to consider lifestyle factors that may prevent neural and cognitive decline among older adults. One practice that has consistently been shown to be beneficial to the brain and cognition is physical activity [see Hillman et al., 2008, for a review].
Studies interrogating the associations among cardiorespiratory fitness and/or exercise, aging and WM are few and inconclusive. Colcombe et al. 2006 obtained voxel‐based estimates of WM volume as well as measures of maximal oxygen uptake in a group of older adults, and found that the greatest advantage of fitness was observed in the anterior WM and regions traversing the frontal and posterior parietal lobes. In the context of a six‐month exercise intervention using the same metrics, Colcombe et al. 2009 reported a statistically significant increase in the volume of anterior WM for the aerobic exercisers compared with the nonaerobic exercisers. Due to both studies' volumetric approach to assess WM, however, they were unable to speak to the microstructural properties of the tissue.
Taking the next step, Marks et al. 2007 collected DTI images from 13 younger and 15 older adults. Using an ROI approach to obtain FA values, they found positive correlations between aerobic fitness and FA in two WM areas after controlling for age and sex: the uncinate fasciculus and the cingulum. Based on their preliminary results, the authors concluded that increased cardiorespiratory fitness may be associated with greater WM integrity in specific regions of the brain although a preferential link with prefrontal areas was not observed. The preliminary nature of the Marks et al. study must be emphasized however, due to its significant limitations including the small sample size, the less‐than‐optimal estimation of aerobic fitness, and the cross‐sectional design of the experiment. Finally, a more recent cross‐sectional study with 26 healthy older adults which measured aerobic fitness using a composite measure of VO2 peak derived from a graded maximal exercise test, total time on treadmill, and 1‐min heart rate recovery, reported that greater aerobic fitness was associated with greater WM integrity in portions of the body of the corpus callosum that connect bilateral premotor and prefrontal cortex, and portions of the posterior genu [Johnson et al., in press].
The Colcombe et al. [2006, 2008], Marks et al. 2007, and Johnson et al. (in press) studies are the only empirical investigations to date that have considered the relationships among aging, cardiorespiratory fitness, and cerebral WM. Findings from the first two suggest that aerobic fitness exerts its strongest influence on anterior WM (using VBM) while evidence from the last two used DTI and imply that region‐specific effects may be distributed across the brain. Moreover, none of them incorporated measures of cognition, leaving obscured the nature of the associations among aging, aerobic fitness, WM integrity, and cognitive functioning.
This study was designed to examine the aforementioned associations within the context of a one‐year exercise intervention. The three primary aims were: (1) to investigate the effect of a one‐year aerobic training program on the WM integrity of selected brain regions in older adults, (2) to analyze the pattern of diffusivity changes to better understand the underlying neurobiological substrates, and (3) to explore whether training‐induced improvements in WM integrity would be associated with enhanced cognitive performance independent of fitness gains. To analyze the DTI data, we applied tract‐based spatial statistics [TBSS; Smith et al., 2006] to each individual's images and then extracted the mean value for each diffusion metric (FA, RD, and AD) from the appropriate region of interest (ROI). For our ROI, we focused on prefrontal, parietal, temporal, and occipital WM as defined anatomically by Head et al. 1987.
Based on the limited existing research, we hypothesized that participants assigned to the aerobic exercise condition would exhibit increases in WM integrity compared to their nonaerobic exercise counterparts at the end of the one‐year intervention. Furthermore, we predicted that the beneficial effects would roughly follow an anterior‐posterior gradient, thus counteracting the deleterious impact of aging. We also expected those randomized to the aerobic exercise condition to experience a significantly greater improvement in cognitive performance compared with those in the nonaerobic control group, particularly in the domain of executive functioning [Colcombe and Kramer, 2003]. Lastly, we hypothesized that enhanced WM integrity because of the exercise intervention would be associated with improved cognitive performance. Empirical support for our hypotheses would strengthen the argument that aerobic exercise is an effective antidote for the aging brain and mind, and would shed light on one potential biological mechanism for its influence.
MATERIALS AND METHODS
Participants
The participants were 70 sedentary, community‐dwelling older adults recruited from the greater Champaign‐Urbana area. Eligible participants met the following criteria: (1) were between the ages of 55 and 80 years old, (2) were free from psychiatric and neurological illness, (3) scored > 51 on the modified Mini‐Mental State Exam (mMMSE), (4) scored < 3 on the geriatric depression scale (GDS), (5) scored > 75% right‐handedness on the Edinburgh Handedness Questionnaire, (6) demonstrated normal or corrected‐to‐normal vision of at least 20/40, and (7) were cleared for suitability in the MRI environment, that is, no metallic implants that could interfere with the magnetic field or cause injury, no claustrophobia, and no history of head trauma. The Institutional Review Board of the University of Illinois approved the study and all participants gave written informed consent prior to participating in the study. Additional demographic information is provided in Table 1. Participants in this study represent a subset of participants in previously published studies from the same RCT [e.g., Erickson et al., 2011; Voss et al., 2010].
Table 1.
Participant demographics, mMMSE, fitness, and attendance
| Variable | All | Walk | Stretch | P value |
|---|---|---|---|---|
| N (female) | 70 (45) | 35 (24) | 35 (21) | 0.46 |
| Age (SD) | 64.87 (4.46) | 65.17 (4.40) | 64.57 (4.46) | 0.58 |
| Years of education (SD) | 16.10 (3.00) | 16.07 (3.01) | 16.15 (3.06) | 0.93 |
| mMMSE (SD) | 55.13 (1.78) | 55.06 (1.77) | 55.20 (1.81) | 0.74 |
| Composite VO2 max (mL/kg2) | 21.85 (5.32) | 21.34 (5.11) | 22.37 (5.56) | 0.42 |
| Intervention adherence (% exercise classes attended) | 80.17 (11.62) | 79.43 (11.42) | 80.90 (11.93) | 0.60 |
Measures
Cardiorespiratory fitness assessment
A composite measure of aerobic fitness (VO2 max) was created for each individual by averaging the scores from a graded maximal exercise test and a Rockport one‐mile walk test. Measurements from the graded maximal test and Rockport walk test were highly correlated in our sample, for both preintervention [r (70) = 0.69, P < 0.001] and postintervention [r (70) = 0.71, P < 0.001] measures. Therefore, the composite construct was used to obtain a more precise estimate of the latent variable of interest. Raw group × time means and standard deviations for both aerobic fitness measures, and the composite measure, are available in Table 1 of the Supporting Information.
Graded maximal exercise test.
After obtaining their individual physician's approval to engage in cardiorespiratory fitness testing, participants performed a graded maximal exercise test on a motor‐driven treadmill. The protocol consisted of walking at a speed of 3 mph with the grade of the treadmill increasing 2% every 2 min. Measurements of oxygen uptake, heart rate and blood pressure were continually monitored by a cardiologist and a nurse. Oxygen consumption (VO2) was calculated from expired air sampled at 30‐s intervals until maximal VO2 was reached or the test was terminated due to volitional exhaustion and/or symptom limitation. VO2 max was defined as the highest recorded VO2 value after two of three criteria were met: (1) a plateau in peak VO2 between two or more workloads; (2) a respiratory exchange ratio >1.00, and (3) a heart rate equivalent to their age‐predicted maximum.
Rockport one‐mile walk test.
The Rockport one‐mile walk protocol [Kline et al., 1987] was used as a sub‐maximal estimate of cardiorespiratory fitness. Participants were instructed to complete the one‐mile walk as quickly as possible without running on an enclosed, synthetic track. Trained staff and a nurse supervised the field test. Estimates of VO2 capacity were calculated using sex‐specific formulae that take into account weight, age, heart rate, and time to complete the walk.
Cognitive testing
Five neuropsychological tests were used to assess short‐term memory and executive control processes [Voss et al., 2010].
Short‐term memory.
The forward and backward digit span tasks tested verbal short‐term memory [Bopp and Verhaeghen, 2005].
Forward digit span task.
For this task, a test administrator read a sequence of numbers aloud to the participants (e.g., 4–5). Participants were then instructed to recite the sequence of numbers in the same order (e.g., 4–5). They were given two trials for each list length (e.g., 4–5; 2–4). List lengths began at two and incrementally increased by one number if participants could correctly repeat the sequence for at least one trial. The maximum list length was seven. If participants incorrectly repeated the sequences of a particular list length for both trials, the previous list length was recorded as the dependent variable.
Backward digit span task.
This task was similar to the forward span task, except that the participants were asked to recount the sequence of numbers in reverse order (e.g., 5–4, if the administrator had said 4–5). The number of digits increased by one until the participant consecutively failed in both trials of the same digit span length (minimum length 2, and maximum 7). The last correctly repeated list length was recorded as the dependent variable.
Executive control
Spatial working memory task.
The spatial working memory task provided a measure of the ability to form and retain memories of spatial locations over a delay period. Participants were presented with one, two, or three black dots that appeared at randomly selected locations on the screen for 500 ms. Following the dot display, a fixation cross appeared for 3 s. At the end of the delay, a single red test dot appeared on the screen, either at the same location as one of the previous black dots (match), or at a novel location (nonmatch). Participants had 2 s to decide whether the red test dot matched or did not match the spatial location of one of the initially presented dots, and were asked to indicate their response by pressing the designated key on a computer keyboard (“x” = nonmatch; “m” = match). The entire task consisted of 120 trials (40 trials for each set size divided into 20 match and 20 nonmatch conditions) and was preceded by several practice trials to acquaint the participants with the protocol. The measure for the spatial working memory task was the sum of the normalized average reaction time and error rate, representing overall performance on the task.
Task‐switching
The task‐switching test assessed the ability to switch flexibly focus of attention between multiple task sets. Participants had to switch between judging whether a number was odd or even, and judging whether it was larger or <5 (i.e., high or low). The eligible numbers were 1–4 and 6–9. Numbers were presented individually for 1,500 ms against a pink or blue background in the center of the computer screen with the constraint that the same number did not appear twice in succession. If the background was blue, participants used their left hand to report as quickly as possible whether the number was high (“x” key) or low (“z” key). If the background was pink, they used their right hand to report whether the number was odd (“n” key) or even (“m” key). Participants completed two single task blocks of 24 trials each (one block of odd/even and one block of high/low) and one switching block of 120 trials during which the task for each trial was chosen randomly. Each block was preceded by a series of practice trials to familiarize the participants with the rules.
For this study, the primary executive function measure was local switch cost: the difference in performance for trials when the preceding trial involved the same task (nonswitch trial) and those when the preceding trial was of the other task (switch trial). Mean reaction times were used in the calculations of local switch cost.
Wisconsin card sorting task.
Participants completed a computerized version of the Wisconsin card sorting task (WCST), which assesses multiple components of executive function including working memory, inhibition, and switching capacity. The task requires participants to sort cards by shape, color, or number of objects on the card without explicitly stating which criterion to apply. Participants were asked to match each card that appeared at the bottom of the computer screen with one of the four cards displayed at the top of the screen. They were told that the computer would provide feedback about the accuracy of their decision, but that the examiner could not give them any additional instructions about the task. The primary dependent variable for this task was computed as the average of the standardized number of perseverative responses and perseverative errors residualized on the total number of errors, thereby controlling for overall level of performance (Raz et al., 2003).
MRI acquisition
Diffusion‐weighted images were acquired on a 3 T Siemens Allegra head‐only scanner (Siemens, Erlangen, Germany) with TR = 4,200 ms, TE = 94 ms, and 1.9 mm2 in‐plane resolution. Twenty‐eight 4 mm slices were obtained parallel to the anterior‐posterior commissure plane with no interslice gap. The protocol consisted of a T2‐weighted acquisition (b‐value = 0 s/mm2) followed by a 12‐direction diffusion‐weighted echo planar imaging scan (b‐value = 1,000 s/mm2), repeated six times.
Exercise Intervention
Older adults were randomized to either an aerobic walking group (walk) or flexibility, toning, and balance control group (stretch). There were no significant differences between the two groups in initial level of aerobic fitness, years of education, sex distribution, or program attendance (see Table 1). Both the walking and control programs were 1 year in duration and consisted of three structured 40‐min exercise sessions per week led by a trained exercise leader. The non‐aerobic control program was designed to match its aerobic counterpart in terms of social stimulation without incorporating the element of cardiorespiratory exercise. Participants in both groups met in an indoor gym facility and had the same opportunity to socialize with each other and with the exercise instructor. All preintervention and postintervention measures were administered within 2 weeks of the start and end of the intervention, respectively.
Walking condition
All walking sessions started and ended with ∼5 min of stretching for warming up and cooling down. Participants began by walking continuously for 10 min and proceeded to increase their walking duration in 5‐min increments until a period of 40 min was achieved at week seven. For the remainder of the program, participants walked for 40 min per session. Participants wore heart rate monitors while exercising and were encouraged to stay within their target heart rate zone, which was calculated using the Karvonen formula [Strath et al., 2000] based on the resting and maximum heart rates achieved during the baseline maximal graded exercise test. The target heart rate zone was 50–60% of the maximum heart rate reserve for weeks 1–7 and 60–75% for the remainder of the program. Participants also completed an exercise log at each session and received written feedback forms that summarized the data from their logs every 4 weeks. Participants with low attendance and/or exercise heart rates were encouraged to improve their performance for the following month.
Flexibility, toning, and balance condition
All flexibility, toning, and balance sessions started and ended with warm‐up and cool‐down stretches. During each class, participants engaged in four muscle‐toning exercises, two exercises designed to improve balance, one yoga sequence, and one exercise of their choice. The muscle‐toning exercises incorporated dumbbells and/or resistance bands. To maintain the older adults' interest, a new group of exercises was introduced every three weeks. During the first week, participants focused on familiarizing themselves with the new exercises; during the second and third weeks, they were encouraged to increase the intensity by using more weight and/or adding more repetitions. Participants in the stretching group also completed exercise logs at each session and received monthly feedback forms. They were encouraged to exercise at an appropriate intensity (13–15 on the Borg RPE scale [Borg, 1985]) and to attend as many classes as possible.
Diffusion Data Analysis
DTI data processing
Diffusion data were processed using TBSS v1.2 [Tract‐Based Spatial Statistics, Smith et al., 2006]. First, each participant's data were passed through an automated pipeline consisting of, (1) motion and eddy current correction, (2) removal of nonbrain tissue using the Brain Extraction Tool [Smith, 2002], and (3) local fitting of the diffusion tensor model at each voxel using FMRIB's Diffusion Toolbox v2.0 (FDT: http://www.fmrib.ox.ac.uk/fsl/fdt). The products of the multistep procedure included FA and AD images; RD maps were calculated as the mean of the second and third eigenvalues [Song et al., 2002]. Each participant's FA data were then aligned into a common space using FMRIB's nonlinear registration tool FNIRT [Andersson et al., 2007a, 2007b] and a mean diffusion image was created. Next, the mean FA image was thinned to create an average skeleton representing the centers of the tracts shared by all participants, and the skeleton was thresholded at 0.2. Finally, each participant's aligned FA data was projected onto the skeleton, taking on the FA value from the local center of the nearest relevant tract. AD and RD skeletons for each participant were formed in a similar manner by projecting the analogous data onto the mean skeleton.
ROI analysis
Mean diffusion values were calculated for each subject within four a priori ROIs. The regions that were chosen correspond roughly to the four primary lobes of the telencephalon: the prefrontal lobe, the parietal lobe, the occipital lobe, and the temporal lobe. All ROIs were outlined directly on the mean FA image following the anatomical guidelines detailed in Head et al. 1987. The manually defined ROIs were then used to mask the mean skeleton and an average diffusion value was computed for each ROI for each diffusion measure for each participant (see Fig. 1 for an illustration of the ROIs). We confined our analyses to the four lobar regions to assess general trends across the brain and because the limited number of diffusion directions precluded the isolation of individual tracts with any degree of certainty.
Figure 1.
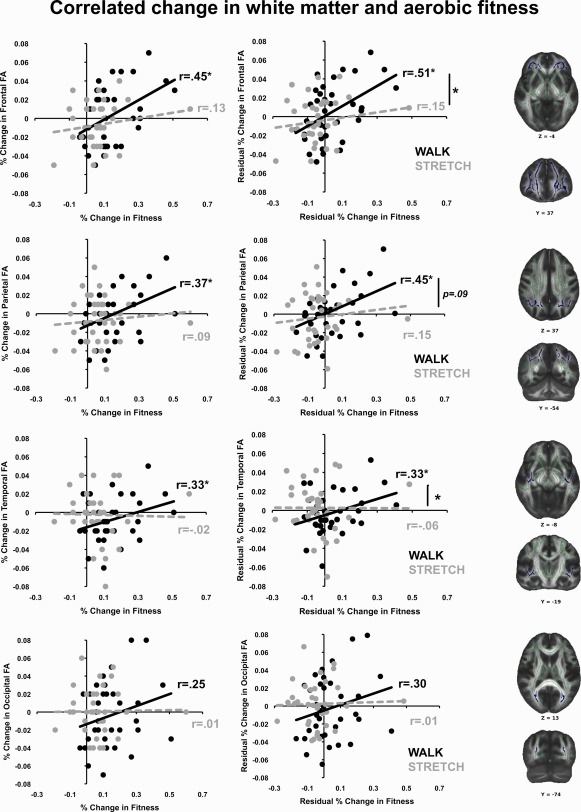
Correlation between change in FA and aerobic fitness. Left and middle columns illustrate the patterns of increased fitness associated with increased regional FA for the walk (black) and the stretch (gray) groups. The left column plots raw data points; the middle column plots residuals, after correction for variance associated with age, sex, and program adherence. The right column illustrates representative views of the four a priori ROIs; ROIs (shown in blue) were manually defined on the mean skeleton (shown in green), according to the anatomical guidelines outlined in Head et al. 1987.
Exploratory whole‐brain analysis
In an exploratory analysis, we assessed whether there were localized group differences in whole‐brain percentage change skeleton images [i.e., (T post – T pre)/T pre] of FA, AD, or RD using a nonparametric independent samples t‐test in FSL. In addition, we examined whether an increase in fitness within each group was differentially associated with percentage change in FA, AD, or RD. The significance threshold for these analyses was set at P < 0.05, corrected for voxel‐wise multiple comparisons with threshold‐free cluster enhancement in FSL.
Statistical Analyses
Effect of intervention group on aerobic fitness and cerebral WM diffusivity
To examine the effect of the exercise intervention group on changes in cardiorespiratory fitness while accounting for baseline fitness levels, we conducted an independent samples t‐test on percentage change in fitness. To evaluate the effect of the controlled trial on regional WM diffusivity while accounting for baseline measures, we conducted repeated‐measures ANOVA on percentage change diffusion measures, with Region as the within‐subjects factor and Group as the between‐subjects factor. A separate ANOVA was conducted for each diffusion metric, that is, FA, RD, and AD. Percentage change scores were calculated for fitness and diffusion measures by subtracting the preintervention value from the postintervention value and dividing the result by the preintervention value [i.e., (T post – T pre)/T pre].
Effect of intervention group on cognitive performance
Due to experimenter error in saving the behavioral files, the WCST neuropsychological test had greater than 10% missing data. Little's chi‐square test for whether values were missing completely at random (MCAR) indicated that test scores for all neuropsychological tests were MCAR (all P > 0.05 for baseline and postintervention). Therefore, instead of excluding these participants from further analyses, all missing data points in neuropsychological scores were imputed using the EM (expectation‐maximization) method, which iteratively computes maximum likelihood estimates of missing data given the available data [Dempster et al., 1977; Schafer and Graham, 2002]. Imputation was conducted for baseline and end‐point data separately such that imputed scores for any missing cognitive measures were generated based on all other original cognitive measures at that time point. For all other tasks, <10% of data were missing at pretest and post‐test (see Supporting Information). However, to limit missing data in change scores in multivariate analyses, all other cognitive measures were also imputed in the same way.
Change in cognitive performance following the intervention was expressed as the postintervention score after controlling (via linear regression) for preintervention performance, this was done to assess the change in cognitive performance relative to each person's baseline performance. Percentage change scores were not calculated on cognitive data since some cognitive measures could be negative at baseline (e.g., corrected WCST perseverative errors). To examine the effect of the exercise intervention group on cognitive performance, we conducted a multivariate analysis of variance of residualized change scores.
Changes in aerobic fitness, WM diffusivity, and cognitive performance
To assess the differential relationship among cardiorespiratory fitness, WM integrity, and cognition for the exercise groups, we performed within‐group partial correlations among percentage change in fitness, percentage change in the regional diffusivity metrics and change scores for each cognitive measure, with age, sex, and adherence as covariates. Given the previous evidence supporting a positive association between aerobic fitness and WM integrity in healthy older adults, hypothesis tests were unidirectional.
RESULTS
Effect of Intervention Group on Aerobic Fitness and Cerebral WM Diffusivity
The intervention significantly improved aerobic fitness levels with older adults in the aerobic exercise group displaying a greater percentage improvement in cardiorespiratory fitness (mean = 14.63%; SD = 13.15%) compared to those in the stretching group (mean = 6.07%; SD = 13.10%), t(68) = 2.73 and P = 0.01.
Prior to examining the effect of the intervention on DTI measures, we performed a confirmatory analysis to test the association between age and preintervention WM integrity while controlling for the variance associated with sex. Age was negatively correlated with FA in the prefrontal [pr(67) = −0.38; P = 0.001], parietal [pr(67) = −0.35; P = 0.001], and temporal [pr(67) = −0.29; P = 0.008] ROIs, and marginally associated with FA in the occipital ROI [pr(67) = −0.18; P = 0.07]. For AD measures, age was positively correlated with AD in the prefrontal [pr(67) = 0.37; P = 0.001] and temporal [pr(67) = 0.28; P = 0.009] ROIs, but not in the parietal [pr(67) = −0.13; P = 0.15] or occipital [pr(67) = 0.02; P = 0.44] ROIs. Finally, for RD measures, age was positively correlated with RD in the prefrontal [pr(67) = 0.44; P < 0.001], parietal [pr(67) = 0.31; P = 0.005], temporal [pr(67) = 0.40; P < 0.001], and occipital [pr(67) = 0.22; P = 0.04] ROIs.
Regarding the effect of the intervention on FA in the various ROI, there was no main effect of exercise group [F(1, 68) = 0.07; P = 0.79]; however, some regions exhibited altered percentage change scores compared with others as evidenced by a significant group × region interaction, [F(1, 68) = 2.91; P = 0.03]. Although, independent samples t‐tests (one‐tailed) conducted to examine this interaction further, with the expectation that the walking group would show increased FA compared with the stretching group, and showed that none of the ROIs exhibited a significant group difference. Only percentage change in prefrontal FA showed a marginal group difference [t(68) = 1.44; P = 0.08]. These results suggest that the pattern of training‐induced changes in FA differed by ROI, and while prefrontal FA showed the greatest trend for differences in WM integrity, no single ROI showed a statistically significant difference between exercise groups. See Table 2 for preintervention and postintervention measures for all ROIs and effect sizes for within‐ and between‐groups change.
Table 2.
DTI measures for preintervention and postintervention
| Walk | Stretch | % Diff difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | d | % diff | Pre | Post | d | % diff | d | |
| FA | |||||||||
| Frontal | 0.425 (.03) | 0.426 (0.03) | 0.03 | 0.24 (3)a | 0.434 (0.03) | 0.431 (0.04) | −0.08 | −0.75 (3)a | 0.33 |
| Parietal | 0.433 (0.03) | 0.433 (0.03) | −0.02 | −0.13 (3) | 0.439 (0.02) | 0.436 (0.03) | −0.13 | −0.78 (3) | 0.22 |
| Temporal | 0.429 (0.02) | 0.426 (0.02) | −0.15 | −0.84 (2) | 0.435 (0.03) | 0.434 (0.03) | −0.03 | −0.26 (3) | −0.23 |
| Occipital | 0.655 (0.05) | 0.652 (0.05) | −0.05 | −0.36 (2) | 0.655 (0.06) | 0.656 (0.06) | 0.02 | 0.11 (3) | −0.18 |
| AD (×10 2) | |||||||||
| Frontal | 0.112 (0.003) | 0.112 (0.003) | −0.04 | 0.14 (2) | 0.112 (0.003) | 0.112 (0.003) | −0.03 | 0.12 (2) | −0.01 |
| Parietal | 0.120 (0.003) | 0.121 (0.004) | −0.18 | 0.55 (2) | 0.120 (0.004) | 0.121 (0.004) | −0.06 | 0.23 (2) | −0.16 |
| Temporal | 0.121 (0.004) | 0.122 (0.004) | −0.15 | 0.53 (2) | 0.122 (0.003) | 0.122 (0.003) | 0.05 | −0.10 (2) | −0.32 |
| Occipital | 0.167 (0.009) | 0.168 (0.010) | −0.08 | 0.43 (3) | 0.167 (0.010) | 0.168 (0.008) | −0.05 | 0.35 (4) | −0.02 |
| RD (×10 2) | |||||||||
| Frontal | 0.058 (0.004) | 0.058 (0.004) | −0.03 | 0.26 (3) | 0.057 (0.005) | 0.057 (0.005) | −0.08 | 0.74 (3) | 0.16 |
| Parietal | 0.061 (0.004) | 0.061 (0.005) | −0.08 | 0.59 (4) | 0.060 (0.003) | 0.061 (0.004) | −0.14 | 0.89 (3) | 0.09 |
| Temporal | 0.062 (0.004) | 0.063 (0.004) | −0.17 | 1.16 (3)b | 0.061 (0.004) | 0.062 (0.004) | −0.02 | 0.13 (2)b | −0.40 |
| Occipital | 0.049 (0.007) | 0.049 (0.008) | −0.10 | 1.67 (7) | 0.049 (0.008) | 0.049 (0.008) | 0.02 | −0.06 (7) | −0.25 |
Note: Standard deviations are in parentheses; %diff = [(T post – T pre)/T pre] × 100. d = Cohen's d effect size calculated from paired t‐test, positive sign for greater white matter integrity at post following pretest; %diff difference d = Cohen's d effect size comparing the means and standard deviations of %difference estimates for each group, positive sign when difference is in favor of the walking group.
P < 0.10 (one‐tailed).
P < 0.05 (one‐tailed).
For AD indices, there was also no main effect of group [F(1, 68) = 0.52; P = 0.48], and this time also no differential effect by region [F(1, 68) = 0.27; P = 0.85]. This was also the case for RD measures; there was no main effect of group [F(1, 68) = 0.46; P = 0.50] or differential effect by region [F(1, 68) = 1.55; P = 0.20]. See Table 2 for a full listing of preintervention and postintervention AD and RD for all ROIs and effect sizes for within‐ and between‐groups change.
Exploratory whole‐brain analyses did not reveal any localized regions of improved WM integrity (FA, AD, or RD) in favor of either the walking or stretching group.
Changes in Aerobic Fitness and Cerebral WM Diffusivity
To evaluate further whether the intervention produced a differential effect on changes in WM diffusivity, we conducted separate Pearson partial correlations between percentage changes in FA, AD, and RD and percentage change in aerobic fitness within each group, controlling for the variance associated with age, sex, and attendance. This analysis probes whether there is a quantitative relationship between change in WM integrity and change in fitness that varies as a function of intervention group despite nonsignificant group‐level differences. Results showed that increased aerobic fitness was associated with significant increases in prefrontal [pr(30) = 0.51; P = 0.001], parietal [pr(30) = 0.45; P = 0.005], and temporal [pr(30) = 0.33; P = 0.03] FA in the walking group, whereas these associations were not significant in the stretching group [prefrontal, pr(30) = 0.15; P = 0.21; parietal, pr(30) = 0.15; P = 0.21; temporal, pr(30) = −0.06; P = 0.36; see Fig. 1]. A one‐tailed Fisher's z‐test for the difference of correlation strengths indicated that the correlations were significantly different for the prefrontal (P = 0.05) and temporal (P = 0.05) ROIs, but were only marginally different for the parietal ROI (P = 0.09). In contrast, there were no significant associations between changes in AD or RD and increased fitness for either the walking or stretching group.
Exploratory whole‐brain analyses did not reveal any localized regions of increased WM integrity (FA, AD, or RD) associated with improved fitness within either the walking or stretching group.
Effect of Intervention Group on Cognitive Performance
Contrary to our predictions, the intervention did not yield a differential change in cognitive performance between the two exercise groups. This was true of all the cognitive measures as a whole (Pillai's trace V = 0.08, F(5, 64) = 1.08, and P = 0.38), and for each of the five cognitive measures independently (forward span, P = 0.99; backward span, P = 0.22; local switch cost, P = 0.65; SPWM, P = 0.91; WCST perseverative errors, P = 0.12).
Changes in Aerobic Fitness, WM Diffusivity, and Cognitive Performance
Since only the prefrontal and temporal FA measures showed significantly greater positive correlations between increased FA and increased aerobic fitness for the walking group compared with the stretching group, we restricted our examination of links between brain and behavior to the prefrontal and temporal ROIs. Similar to the analysis above, we conducted partial correlations to investigate whether there was a quantitative relationship between change in cognitive performance and change in fitness that varied as a function of intervention group despite nonsignificant group‐level differences in cognitive change. Results revealed that increased aerobic fitness was associated with increased backward digit span in the walking group [pr(31) = 0.43; P = 0.01], but not in the stretching group [pr(31) = −0.01; P = 0.48], independent of age, sex, and attendance (see Fig. 2). A one‐tailed Fisher's z‐test for the difference of correlation strengths indicated that the correlations were significantly different (P = 0.03).
Figure 2.
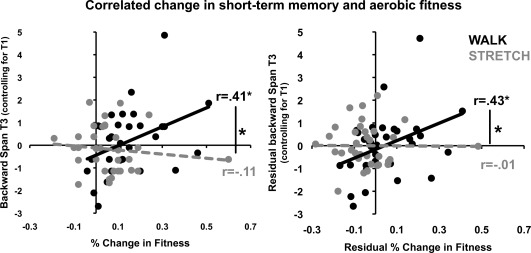
Correlation between change in short‐term memory and aerobic fitness. Figure illustrates the correlation between increased fitness and increased short‐term memory for the walk (black) and the stretch (gray) groups. The left column plots raw data points; the middle column plots residuals, after correction for variance associated with age, sex, and program adherence.
To examine whether changes in prefrontal and temporal FA were associated with improvements in backward digit span in the walking group independent of their common associations with fitness, we performed within‐group partial correlations between percentage change in prefrontal and temporal FA, and change in backward digit span while controlling for the variance associated with age, sex, attendance, and percentage change in fitness. Neither of these correlations was statistically significant (pr = 0.20 and pr = 0.10, respectively), indicating that the training‐induced changes in prefrontal and temporal FA in the walking group could not statistically account for their training‐induced change in cognitive performance. Therefore, mediation analyses to test the hypothesized causal influence of changes in WM integrity on changes in cognition would not be appropriate and were not conducted.
DISCUSSION
This study had three principal objectives. First, we aimed to determine the region‐specific effects of a one‐year aerobic training program on cerebral WM integrity in older adults. Second, we sought to analyze the pattern of diffusivity changes to draw inferences about the biological basis of the observed differences. Lastly, we proposed to examine whether training‐induced increases in WM integrity would be associated with enhanced cognitive performance independent of improvements in fitness.
Improved Aerobic Fitness is Associated with Change in WM Integrity Particularly in Prefrontal and Temporal Brain Regions
The observed pattern of group‐level changes in WM integrity demonstrates that the exercise intervention differentially affected mean FA across brain regions, with the greatest group difference occurring in prefrontal FA. It is interesting to note that the only trend for a statistically significant group difference in FA over the one‐year intervention occurred in the prefrontal cortex (see Table 2). These DTI findings are the first to corroborate results from a study that assessed aerobic training‐induced changes in WM volume in humans using voxel‐based morphometry (VBM) [Colcombe et al., 2006], and a study that showed increased functional connectivity in the prefrontal cortex following one year of aerobic fitness training [Voss et al., 2010].
Additionally, greater aerobic training‐induced increases in fitness were associated with larger changes in mean FA in the prefrontal and temporal cortices compared to nonaerobic training‐induced fitness changes. Therefore, not only was exercise modality critical, but also the magnitude of fitness improvement. While nonhuman animal studies have shown that greater amounts of exercise are associated with increased axonal outgrowth and regeneration [Ghiani et al., 2007; Molteni et al., 2004], this is the first study in humans to show a quantitative relationship between the magnitude of fitness gains and changes in WM integrity.
With regard to the anterior‐posterior diffusivity gradient often documented in WM and aging studies, it appears there may exist an anterior‐to‐posterior slope of differential improvements in FA with enhanced cardiorespiratory fitness. Particularly since age‐related declines in WM integrity are consistently strongest in the prefrontal cortex [e.g., Davis et al., 2009; Head et al., 2004; Sullivan et al., 2010], our results suggest that greater gains in aerobic fitness from moderate walking may be an effective intervention for delaying the effects of normal aging on WM atrophy. This beneficial effect may be due in part to the positive effects of exercise‐induced neurotrophins in promoting axonal regeneration after injury [Ghiani et al., 2007; Molteni et al., 2004]. Thus, to the extent that normal age‐related atrophy is a form of progressive axonal degeneration, exercise may be able to counteract the accelerated injury in prefrontal and temporal cortices by enhancing the production of neurotrophins such as brain‐derived neurotrophic factor (BDNF) that continually facilitate axonal growth and regeneration.
Along these lines, it is interesting that we saw no statistically significant group‐level differences or associations in change between AD and RD measures and aerobic fitness. We examined these diffusion measures as a way to understand more about the microstructural properties of WM alterations that occur as a function of aerobic fitness training. While a recent study found a relationship between greater aerobic fitness and less RD, but not AD, in the body of the corpus callosum in healthy older adults [Johnson et al., in press], these measures have also been criticized for their unreliability in voxels with a high probability of crossing fibers [Wheeler‐Kingshott and Cercignani, 2009]. In this study, our ROIs extended into areas of the skeleton that would be characterized by complex fiber systems. Although TBSS provides a method for minimizing alignment problems of individuals' tracts, it is still possible that voxel estimates of peak AD and RD were somewhat noisy given our extensive ROIs. Although FA is an estimate of fiber orientation that is derived mathematically from the diffusivity measures that generate AD and RD, it is possible that there is some aspect of variability in the diffusivity measures that is reduced in the calculation of FA.
Regarding the precise localization of the effects of aerobic exercise on WM integrity, we found the effects to be largely “nonlocal”; exploratory skeleton‐wise analyses did not reveal specific areas of enhanced WM integrity because of aerobic exercise training. Given that VBM is a method used to detect localized effects and a previous VBM study found positive effects of aerobic training on prefrontal WM volume [Colcombe et al., 2006], we expected our exploratory analyses to uncover specific areas of increased prefrontal FA. However, in contrast to the established sensitivity of FA to WM fiber orientation and coherence, the neurobiological substrate of WM differences in VBM is relatively unknown. Therefore, these results are not necessarily conflicting, but rather, underscore the need for future studies to assess fitness training‐induced changes in WM using higher resolution fiber estimation with greater anatomical resolution, which will enhance sensitivity of the skeleton‐wise analysis. An alternate explanation may be that the effects of aerobic training on WM integrity are characterized by a distributed, global pattern of enhanced structural connectivity. Future research is needed to assess this hypothesis, perhaps through comparison of the effects of fitness training on regional and global measures of anatomical network organization [Hagmann et al., 2008].
Altered Prefrontal and Temporal WM Integrity do not Account for the Relationship Between Improvements in Aerobic Fitness and Short‐Term Memory Performance
The one‐year aerobic exercise intervention yielded improvements in backward digit span performance, particularly in older adults who experienced the greatest fitness gains. While the backward digit span task is sometimes described as a measure of working memory, it has also been shown to be less sensitive to age‐related cognitive decline than working memory tasks that require continual online processing and memory updating [Bopp and Verhaeghen, 2005]. Thus, it may be seen as a measure of short‐term memory that taps more complex memory ability than forward span.
Given results that have linked aerobic fitness to enhanced memory [Chaddock et al., 2010; Kramer et al., 2001], and an extensive literature linking aerobic exercise to increased hippocampal size and function [see Erickson et al., 2011 for review], it is not surprising that increased aerobic fitness was associated with improved backward span performance. However, the nonsignificant correlation between aerobic fitness gains and performance on tasks of higher‐level executive functioning such as task‐switching, WCST, and spatial working memory was surprising given the substantial literature supporting the preferential advantage of aerobic exercise on executive task performance [Colcombe and Kramer, 2003; Smith et al., 2010]. It is possible that the small sample of older adults in this study contributed to the null result. Another possibility is that the tasks used in this study differed in theoretically important ways from tasks that have previously shown positive effects following aerobic exercise training. For example, the task switching in Kramer et al. [2009a, 2009b] was predictable (every third trial) and the cue for task type was visuospatial (upper or lower quadrant). In contrast, in this study, switch trials were unpredictable and were cued by color. Therefore, it may be too general to say that aerobic exercise enhances executive function globally, but rather it may be specific aspects of executive function that are enhanced, such as planning and preparatory processes, inhibition of prepotent responses, and/or interactions between set shifting and visuospatial planning. Future studies are required to further understand how aerobic exercise impacts executive function at more refined levels. Equally important, more research aimed at understanding the mechanisms of exercise effects on brain structure and function are needed and promise to be insightful for understanding the complex relationship between exercise and cognition.
Lastly, although aerobic exercise improved backward digit span performance, exercise‐induced increases in FA were not associated with improvements in backward digit span performance. Potential explanations for this null effect may include lack of statistical power for the cognitive measures employed or that the aerobic training‐induced changes in FA were not sufficient to map onto cognitive improvement. It is also possible that WM changes associated with short‐term memory were more regionally specific than our ROIs were able to detect. Thus, future research is needed to explore further the relationship between changes in WM integrity due to increased aerobic fitness, and cognitive improvement.
LIMITATIONS AND CONCLUSIONS
The results and interpretations from our study should be evaluated within the context of its limitations. Regarding our ROI‐based approach of the diffusion data, although our analysis provided a general examination of trends in WM diffusivity across the brain, we were unable to draw conclusions about the specific fiber tracts and brain networks impacted by improved cardiorespiratory fitness. The application of DTI tractography would offer a more focused and localized treatment of WM integrity as it relates to aging, aerobic fitness and cognitive performance. In addition, in light of the existing aging research that highlights processing speed and executive functioning as cognitive domains particularly linked to WM integrity, future studies should incorporate measures of processing speed in their design.
Furthermore, the effect sizes in this study were generally small and therefore some effects were statistically significant only with one‐tailed hypothesis tests. Although previous research justified the use of one‐tailed hypothesis testing, it is also important to note that more research is needed to understand the clinical significance of such changes in brain structure as a function of exercise training. Even though the effect sizes were generally small, this may still translate to meaningful health benefits. For instance, one study estimated that the annual percent change in FA in the genu is −0.75% (−0.008 absolute change over 2 years) [Barrick et al., 2010]. In this study, the aerobic group showed a 0.24% increase in frontal FA whereas the stretching group showed a −0.75% reduction in frontal FA. This would suggest that the aerobic group had some sparing of typical age‐related decline in frontal FA. What this means for benefits in real‐world function is still unclear. Thus, more research is needed to understand the clinical and real‐world relevance of aerobic training‐induced changes in brain structure.
Despite the aforementioned limitations, this study provides an important and original contribution to this understanding of the plasticity of the aging brain. Aerobic fitness has been implicated as a combatant against the cognitive and neurobiological declines experienced with normal aging [Hillman et al., 2008]. We explored one potential mechanism by which fitness may exert its beneficial influence: through the protection of WM microstructure. Using DTI, we demonstrated that an increase in cardiorespiratory fitness as a result of an aerobic exercise intervention alters WM integrity in regions of the brain that are particularly susceptible to the damaging influence of aging (i.e., prefrontal and temporal lobes). Given the debate about whether cardiovascular fitness is the critical variable associated with the positive benefits of physical activity on cognition [Angevaren et al., 2008; Etnier et al., 2006], this study provides valuable support from a randomized controlled trial that the magnitude of aerobic fitness gains is associated with the magnitude of training‐induced neural plasticity and cognitive improvement. Therefore, our cumulative findings elucidate ways for future studies to understand further the interactions among aerobic fitness, WM integrity, cognition, and aging.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Nancy Dodge, Holly Tracy, the Lifelong Brain & Cognition Laboratory, and the Exercise Psychology Laboratory for their assistance with data collection. They also thank the anonymous reviewers for their helpful comments.
REFERENCES
- Andersson JR, Jenkinson M, Smith S (2007a): TR07JA1: Non‐linear optimisation. Retrieved from http://www.fmrib.ox.ac.uk/analysis/techrep.
- Andersson JR, Jenkinson M, Smith S (2007b): TR07JA2: Non‐linear registration, aka spatial normalisation. Retrieved from http://www.fmrib.ox.ac.uk/analysis/techrep.
- Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L (2008): Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 3:1–70. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB (2003): A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 51:459–465. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS (2010): White matter structural decline in normal ageing: A prospective longitudinal study using tract‐based spatial statistics. Neuroimage 51:565–577. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Howard DV (2010): Age‐related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp 31:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp KL, Verhaeghen P (2005): Aging and verbal memory span: a meta‐analysis. J Gerontol B Psychol Sci Soc Sci 60:P 223–233. [DOI] [PubMed] [Google Scholar]
- Borg G (1985): An introduction to Borg's RPE‐Scale. Ithaca, NY:Movement; p23–25. [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK (2007): Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57:688–695. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR (2010): Age‐related differences in white matter microstructure: Region‐specific patterns of diffusivity. Neuroimage 49:2104–2112. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, The Merck Company Foundation (2007): The State of Aging and Health in America 2007. Whitehouse Station, NJ:The Merck Company Foundation; p38. [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, Pontifex MB, Raine LB, Konkel A, Hillman CH, Cohen NJ, Kramer AF (2010): A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF (2003): Fitness effects on the cognitive function of older adults: A meta‐analytic study. Psychol Sci 14:125–130. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF (2003): Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci 58:176–180. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF (2006): Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61:1166–1170. [DOI] [PubMed] [Google Scholar]
- Correia S, Lee SY, Voorn T, Tate DF, Paul RH, Zhang S, Salloway SP, Malloy PF, Laidlaw DH (2008): Quantitative tractography metrics of white matter integrity in diffusion‐tensor MRI. Neuroimage 42:568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA (2009): White matter tract integrity in aging and Alzheimer's disease. Hum Brain Mapp 30:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R (2009): Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster A, Laird N, Rubin D (1977): Maximum likelihood from incomplete data via the EM algorithm. J Royal Statistical Soc Ser B (Methodological) 39:1–38. [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo AN, Chaddock L, White SM, Wojcicki TR, Mailey EL, McAuley EM, Kramer AF (2011): Reply to Coen et al.: Exercise, hippocampal volume, and memory. Proc Natl Acad Sci USA 108:E90. [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo AN, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey EL, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley EM, Kramer AF (2011): Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier J, Nowell P, Landers D, Sibley B (2006): A meta‐regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev 52:119–130. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Ying Z, de Vellis J, Gomez‐Pinilla F (2007): Exercise decreases myelin‐associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia 55:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS (1998): White matter changes with normal aging. Neurology 50:972–978. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey C, Wedeen V, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ (2004): Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex 14:410–423. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF (2008): Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci 9:58–65. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594. [DOI] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT: Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline GM, Porcari JP, Hintermeister R, Freedson PS, Ward A, McCarron RF, Ross J, Rippe JM (1987): Estimation of VO2max from a one‐mile track walk, gender, age, and body weight. Med Sci Sports Exerc 19:253–259. [PubMed] [Google Scholar]
- Kramer AK, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison C, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A (1999): Ageing, fitness and neurocognitive function. Nature 400:418–419. [DOI] [PubMed] [Google Scholar]
- Kramer AK, Hahn S, McAuley E, Cohen NJ, Banich MT, Harrison C, Chason J, Boileau RA, Bardell L, Colcombe A, Vakil E (2001): Exercise, Aging and Cognition: Healthy Body, Healthy Mind? In: Rogers W, Fisk AD, editors.Human Factors Interventions for the Health Care of Older Adults. Hillsdale, NJ:Erlbaum; p92–120. [Google Scholar]
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W (2006): Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 144:73–81. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, McDowell I (2002): Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol 156:445–453. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW (2009a)Cerebral white matter integrity and cognitive aging: Contributions from diffusion tensor imaging. Neuropsychol Rev 19:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA (2009b)Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks BL, Madden DJ, Bucur B, Provenzale JM, White LE, Cabeza R, Huettel SA (2007): Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci 1097:171–174. [DOI] [PubMed] [Google Scholar]
- Molteni R, Zheng J‐Q, Ying Z, Gómez‐Pinilla F, Twiss JL (2004): Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA 101:8473–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G, Tanahashi Y, Low HP, Billings‐Gagliardi S, Schwartz WJ, Duong TQ (2005): Myelination and long diffusion times alter diffusion‐tensor‐imaging contrast in myelin‐deficient shiverer mice. Neuroimage 28:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57:632–638. [DOI] [PubMed] [Google Scholar]
- Peters A (2002): The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol 31:581–593. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV (2003): Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Mag Reson Med 49:953–961. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M (2000): Age‐related decline in brain white matter anisotropy measured with spatially corrected echo‐planar diffusion tensor imaging. Mag Reson Med 44:259–268. [DOI] [PubMed] [Google Scholar]
- Raz N (2000): Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings In: Craik F, Salthouse T, editors.Handbook of aging and cognition. Mahwah, NJ:Erlbaum; p1–90. [Google Scholar]
- Raz N, Rodrigue KM, Acker JD (2003): Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci 117:1169–1180. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Richards M, Hardy R, Wadsworth ME (2003): Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med 56:785–792. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS (1999): Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol 56:338–344. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM (2005): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26:1215–1227. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW (2002): Missing data: Our view of the state of the art. Psychol Methods 7:147–177. [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh‐Bohmer K, Browndyke JN, Sherwood A (2010): Aerobic exercise and neurocognitive performance: A meta‐analytic review of randomized controlled trials. Psychosom Med 72:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005): Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140. [DOI] [PubMed] [Google Scholar]
- Strath SJ, Swartz AM, Bassett DR, O'Brien WL, King GA, Ainsworth BE (2000): Evaluation of heart rate as a method for assessing moderate intensity physical activity. Med Sci Sports Exerc 32:S465–470. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A (2001): Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport 12:99–104. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A (2010): Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging 31:464–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A, Whitfield‐Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD (2008): Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage 42:1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo A, White SM, Wojcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF (2010): Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler‐Kingshott CAM, Cercignani M (2009): About “axial” and “radial” diffusivities. Magn Reson Med 61:1255–1260. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, Harris TB (2009): Predictors of maintaining cognitive function in older adults: The Health ABC study. Neurology 72:2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW (2008): Region‐specific changes of cerebral white matter during normal aging: a diffusion‐tensor analysis. Arch Gerontol Geriatr 47:129–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


