Abstract
In this study, we investigated the mechanism of apoptosis induction of obatoclax (GX15-070), a novel BH3 mimetic, in acute myeloid leukemia (AML) cell lines and primary AML samples. Obatoclax inhibited cell growth of HL-60, U937, OCI-AML3 and KG-1 cell lines. Apoptosis induction contributed to the observed antiproliferative effects at concentrations of this agent that mirror its affinity for antiapoptotic Bcl-2 proteins. We demonstrate that obatoclax can promote the release of cytochrome C from isolated leukemia cell mitochondria, and that apoptosis induced by this agent is preceded by the release of Bak from Mcl-1, liberation of Bim from both Bcl-2 and Mcl-1, and the formation of an active Bak/Bax complex. Notably, apoptosis was diminished, but not fully prevented, in the absence of Bak/Bax or Bim suggesting that obatoclax has additional targets that contribute to its cytotoxicity. At growth-inhibitory doses that did not induce apoptosis or decreased viability, obatoclax induced an S/G2 cell cycle block. Obatoclax induced apoptosis in AML CD34+ progenitor cells with an average IC50 of 3.59 +/− 1.23μM although clonogenicity was inhibited at concentrations of 75-100 nM. Obatoclax synergized with the novel BH3 mimetic ABT-737 to induce apoptosis in OCI-AML3 cells, and synergistically induced apoptosis in combination with AraC in leukemic cell lines and in primary AML samples. In conclusion, we show that obatoclax potently induces apoptosis and decreases leukemia cell proliferation and may be used in a novel therapeutic strategy for AML alone and in combination with other targeted agents and chemotherapeutics.
Keywords: obatoclax, apoptosis, AML, Mcl-1, BH3
Introduction
Induction of apoptosis through the intrinsic apoptotic pathway is triggered by activation and oligomerization of the proapoptotic Bcl-2-family proteins Bax and Bak which permeabilize the outer mitochondrial membrane to release apoptogenic factors like cytochrome C, AIF, endoG, and omi/htra2 (1). In order for Bax and Bak to oligomerize, they must first be liberated from antiapoptotic Bcl-2-family proteins, and endogenous proteins that contain a conserved dimerization motif termed BH3, bind to antiapoptotic Bcl-2-family members, and promote the release of Bax and Bak (2). Notably, a recent report demonstrates that antagonizing the antiapoptotic Bcl-2 proteins that sequester Bax and Bak is necessary and sufficient to induce apoptosis (3), explaining the spectacular single agent activity reported for the novel BH3 mimetic ABT-737 in lung cancer xenografts (4). Albeit ABT-737 potently antagonizes most antiapoptotic Bcl-2 family proteins, it does not antagonize Mcl-1, and we have shown accordingly that in acute myeloid leukemia (AML) cells Mcl-1 confers complete resistance to ABT-737 induced apoptosis (4;5). Therefore, it is of great interest to identify agents that can antagonize the antiapoptotic action of Mcl-1.
In this report, we investigate the activity of the novel BH3 mimetic obatoclax (GX15-070) in AML cell lines and primary samples. Obatoclax has been reported to similarly antagonize all antiapoptotic Bcl-2-family proteins (average IC50 3 μM), including Mcl-1 (IC50 2.9 μM) and Bfl-1 (IC50 of 5 μM) (6), and the clinical formulation of this agent is currently being evaluated in several Phase I and Phase II trials. We first sought to determine if apoptosis contributed to the antiproliferative effects of obatoclax, and found that concentrations that antagonize Mcl-1 and Bcl-2, as evidenced by the release of Bak and Bim, induced apoptosis by activation of the intrinsic pathway. However, unlike observed for ABT-737, apoptosis induced by this agent was only partially dependent of Bak/Bax or Bim, suggesting that in cells treated with obatoclax targets other than Bcl-2 proteins contribute to its cytotoxicity. Secondly, we identified that obatoclax can induce an S/G2 cell cycle arrest that mediates its potent growth-inhibitory effects, suggesting that in addition to antagonizing antiapoptotic Bcl-2 proteins, the cycloprodigiosin structure of this agent may have other targets. Lastly, we observed that obatoclax effectively induced apoptosis of primary AML samples, and found that this was associated with release of Bim from Bcl-2. Our observations support the therapeutic use of obatoclax alone and in combination with AraC and ABT-737, and we propose that the liberation of Bim from Bcl-2 may serve as a biomarker of activity of this agent.
Materials and Methods
Reagents and antibodies
Dimethyl sulfoxide (DMSO) and trypan blue were purchased from Sigma Chemical Co. (St. Louis, MO). Annexin V APC was purchased from BD Biosciences (San Jose, CA). The cytochrome c, Bax, Mcl-1, and Bcl-2 antibody used for immunoprecipitation were purchased from BD Biosciences, the Bcl-2 antibody for immunoblotting from Dako (Carpinteria, CA), and the Bak antibody from Upstate (Lake Placid, NY). Bim and activated Bak antibodies were purchased from Calbiochem (San Diego, CA). The Mcl-1 antibody used for immunoprecipitation was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), GAPDH antibody from Chemicon International (Temecula, CA), and goat-anti-mouse and goat anti-rabbit-horse radish peroxidase conjugate secondary antibodies from Bio-Rad (Hercules, CA).
Cell lines and primary AML samples
U937, HL-60, and KG1 cells were purchased from The American Type Culture Collection (ATCC, Rockville, MD). OCI-AML3 cells were kindly provided by Mark Minden (Ontario Cancer Institute, Toronto, ON, Canada); Bak K.O. mouse embryo fibroblasts (MEFs) by Anthony Letai (Dana-Farber Cancer Institute, Boston, MA); and Bim K.O. MEFs by Philippe Bouillet (Walter and Eliza Hall Institute of Medical Research, Melbourne, Victoria, Australia).
Bone marrow or peripheral blood samples were obtained for in vitro studies from patients diagnosed with AML during routine diagnostic workup under informed consent in accordance with regulations and protocols approved by the IRB Committee of The University of Texas M.D. Anderson Cancer Center. Mononuclear cells were separated by Ficoll-Hypaque (Sigma Chemical Co.) density-gradient centrifugation. Cells were either used for colony assays, as described below, or cultured in AIM-V medium (Gibco Laboratories, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, Woodland, CA), 1mM L-glutamine (Gibco Laboratories), and 50ug/ml penicillin/streptomycin (Gibco Laboratories).
Cell culture
U937, HL-60, KG1, and OCI-AML3 cells were cultured in RPMI-1640 (Mediatech Inc., Herndon, VA). MEF cells were cultured in DMEM (Mediatech Inc.). All media was supplemented with 10% FBS, 1mM L-glutamine, and 50ug/ml penicillin/streptomycin. Leukemic cell lines and mononuclear cells from AML patients were cultured at a density of 3.0 × 105 cells/mL in medium supplemented with 10% FBS and treated with either obatoclax or vehicle (DMSO final concentration, 0.1%). Obatoclax was dissolved in DMSO to yield a stock of 10 mM, which was diluted into the culture medium to the indicated concentrations. In all experiments, cells were treated in log-phase growth.
Viability assay
The number of viable cells was assessed using a Vi-CELL XR cell viability analyzer from Beckman Coulter (Fullerton, CA) at 72 h post treatment.
Flow cytometric analysis of apoptosis
Apoptosis was determined by the flow cytometric detection of phosphatidylserine externalization using annexin V APC (BD Biosciences). Briefly, cells were washed twice with binding buffer [10 mmol/L HEPES, 140 mmol/L NaCl, and 5 mmol/L CaCl2 (pH 7.4), all from Sigma Chemical Co.] and stained with APC-conjugated annexin V for 15 minutes at room temperature. Annexin V fluorescence was determined with a Becton Dickinson FACS Calibur or LSRII flow cytometer. Annexin V binds to those cells that express phosphatidylserine on the outer layer of their membrane (7). Patient derived cells from patient samples, were stained with PE labeled anti-CD34 and annexin V APC. The extent of apoptosis was quantified as percentage of Annexin V-positive cells, and the extent of drug-specific apoptosis was assessed by the formula: %specific apoptosis = (test-control) × 100/(100-control) (8).
Western blot analysis
Cells were lysed at a density of 1 × 106/50 μL in protein lysis buffer (0.25 M Tris-HCl, 2% sodium dodecylsulfate, 4% β-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue) and heated at 95°C for 10 minutes. The lysis buffer was supplemented with a protease inhibitor cocktail (Roche Diagnostic Co.). Cell lysates were then loaded onto a 10-12% SDS-PAGE gel (Bio-Rad). After electrophoresis, proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Buckinghamshire, England), followed by immunoblotting. Signals were detected using a PhosphorImager (Storm 860, version 4.0; Molecular Dynamics, Sunnyvale, CA).
Co-Immunoprecipitation
Cells (10 × 106) were washed with 1× PBS and resuspended in ice-cold 1% CHAPS lysis buffer [150 mM NaCl, 10 mM HEPES (pH 7.4), 1% CHAPS and protease inhibitors (Roche)] on ice for 30 minutes. Insoluble debris was removed by centrifugation at 4°C for 10 min at 13,000 rpm. Protein A-coated 96-well strips (Pierce) were washed 3 times with CHAPS lysis buffer. For each 106 cells, 2.5 μg of antibody [(Bcl-2/Bim co-IP: hamster anti-Bcl-2 antibody (6C8, BD); activated bak/bax co-IP: mouse anti-activated bak (Ab1, Calbiochem); Mcl-1/Bak/Bim co-IP: rabbit anti-Mcl-1 (sc-819, Santa Cruz); control: mouse, hamster, or rabbit IgG (Santa Cruz)] was incubated in each well in 100 μL CHAPS lysis buffer with shaking for 1 h at room temperature. The strips were then washed 3 times with CHAPS lysis buffer. The cell extracts (10 × 106 cell equivalent) were added to the antibody-bound wells and shaken overnight at 4°C. The wells were washed 5 times with CHAPS lysis buffer. Immunoprecipitated proteins were solubilized from the protein A- antibody wells with 2× SDS-PAGE sample buffer (0.25 M Tris-HCl pH 6.8, 2% SDS, 10% Glycerol, 4% β-meracptoethanol, 0.02% Bromophenol Blue). The samples were heated for 3 minutes by placing the well-strip directly on a 95°C heating block. Proteins were separated by 12% SDS-PAGE gels, which were then transferred to Hybond-P membranes (Amersham Pharmacia Biotech) and detected by immunoblotting using rabbit anti-Bim (Calbiochem), mouse-anti-Bcl-2 (Dako), rabbit-anti-bak (Upstate), rabbit-anti-bax (BD), mouse-anti-bak (Calbiochem), or mouse-anti-Mcl-1 (BD) antibodies. Signals were detected using a PhosphorImager (Storm 860, version 4.0; Molecular Dynamics, Sunnyvale, CA, USA).
Mitochondrial cytochrome c release
HL60 cells were grown T-175 flasks in RPMI-1640 medium supplemented with 10% FBS to a cell density of 5 × 105 cells/ml. 1 × 108 cells were collected by centrifugation and washed in 10 volumes of ice cold PBS. Cells were resuspended in 10 volumes of ice-cold CEI buffer (20 mM HEPES pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA) and incubated on ice for 10 minutes. The swollen cell suspension was homogenized by forcefully passing through a 24 G needle 6-8 times. One volume of cold CEII buffer (CEI buffer supplemented with 170 mM sucrose, 440 mM mannitol) was added to the cell suspension and gently mixed by inversion followed by centrifugation at 800 rpm for 5 minutes to collect nuclei and unbroken cells. The supernatant was then centrifuged at 3,500 rpm for 10 minutes, and the pellet was washed twice in cold CEII buffer. The mitochondrial pellet was resuspended in 500 μl of M buffer (125 mM KCl, 20 mM HEPES, 10 mM Tris-Cl, 2 mM KPO4, pH 7.2) and maintained on ice. Protein was quantitated from 5 μl of a 1:5 dilution using the BCA method. The purity of the mitochondrial preparations was assessed by Western blot. Fractions were immunoblotted with COXIV and GAPDH to determine the presence of mitochondrial and cytosolic components, respectively (9). Using the above methodology, cross-contamination of cytosolic and mitochondrial fractions was not observed. Mitochondria were then resuspended in M buffer at 0.8 mg/ml protein and equilibrated at room temperature for 2 minutes prior to the addition of obatoclax. The concentration of DMSO in the solution did not exceed 0.2%. Mitochondrial suspensions were incubated for 15 minutes at room temperature, and mitochondria were collected by centrifugation at 14,000 rpm for 5 minutes. The presence of cytochrome c was evaluated by Western blotting of the mitochondrial pellet and the supernatant.
BrdU incorporation
Cells were incubated with BrdU (final concentration 10 μg/ml) for 1 h at 37°C with 5% CO2. Cells were then washed and fixed in cold ethanol. After treatment with RNAse, DNA was partially denatured with heat at 85°C for 10 minutes. Anti-BrdU antibody (BD Biosciences, San Jose CA) and appropriate secondary FITC conjugated antibody were added. Counterstaining for total DNA content was performed using propidium iodide.
AML blast colony and CFU-granulocyte-macrophage assays
AML bone marrow cells were isolated by gradient centrifugation and plated in duplicate at a density of 1-2 × 105 cells/mL in 1% methylcellulose in Iscove’s MDM (Methocult; Stem Cell Technologies, Vancouver, BC, Canada) containing 10% fetal bovine serum and the following human recombinant growth factors: erythropoietin (3 U/mL), IL-6 (10 ng/mL), IL-3 (10 ng/mL), granulocyte-macrophage colony stimulating factor (10 ng/mL), and stem cell factor (50 ng/mL). Obatoclax was added at the start of cultures at concentrations of 50 to 100 nM. In four experiments, mononuclear cells isolated from normal bone marrow (1 × 104 cells/mL) were plated, as described above. The colony-forming capacity of AML and normal samples was evaluated under a stereo or inverted microscope after 8-10 days of culture at 37°C in a 5% CO2 humidified environment. A colony was defined as a cluster of 40 or more cells [blasts (CFU) or erythrocyte (BFU-E), granulocyte, monocyte (CFU-GM) or the mixed population (CFU-GEMM)].
Small interfering RNA (siRNA) transfection
Silencing of Bim and Bak gene expression in leukemic cells was achieved by the siRNA technique. Short interfering RNAs were obtained as duplexes in purified and desalted form (Option C) from Dharmacon. The sense strand of the siRNA silencing bim gene (Bim-siRNA) was GACCGAGAAGGUAGACAAUUGdTdT. Bak ON-TARGETplus SMARTpool (L-003305-00) was purchased from Dharmacon. Non-specific control pool containing 4 pooled non-specific siRNA duplexes was also used as a negative control (referred to as NS-siRNA, Dharmacon-Upstate). Transfection of leukemic cells was carried out by electroporation using the Nucleofection® system (Amaxa, Köln, Germany), following the manufacturer’s instructions. Briefly, 2 × 106 cells were resuspended in 100 μl of T cell nucleofector solution containing 4000 nM of double-stranded siRNAs. After electroporation, 500 μl of cultured medium were added to the cuvette, and the cells were transferred into culture plates containing 1.5 ml pre-warmed culture medium (siRNA final concentration 67 nM).
Statistics
Results are expressed as means ± SEM of two to three replicates unless otherwise indicated. Synergism, additive effects, and antagonism were assessed with the Chou-Talay method (10) and Calcusyn software (Biosoft, Ferguson, MO); the combination index (CI) for each experimental combination was calculated. When CI=1, the equation represents the conservation isobologram and indicates additive effects. CI values < 1.0 indicate an additive effect characteristic of synergism.
Results
Obatoclax induces apoptosis in acute myeloid leukemia cell lines
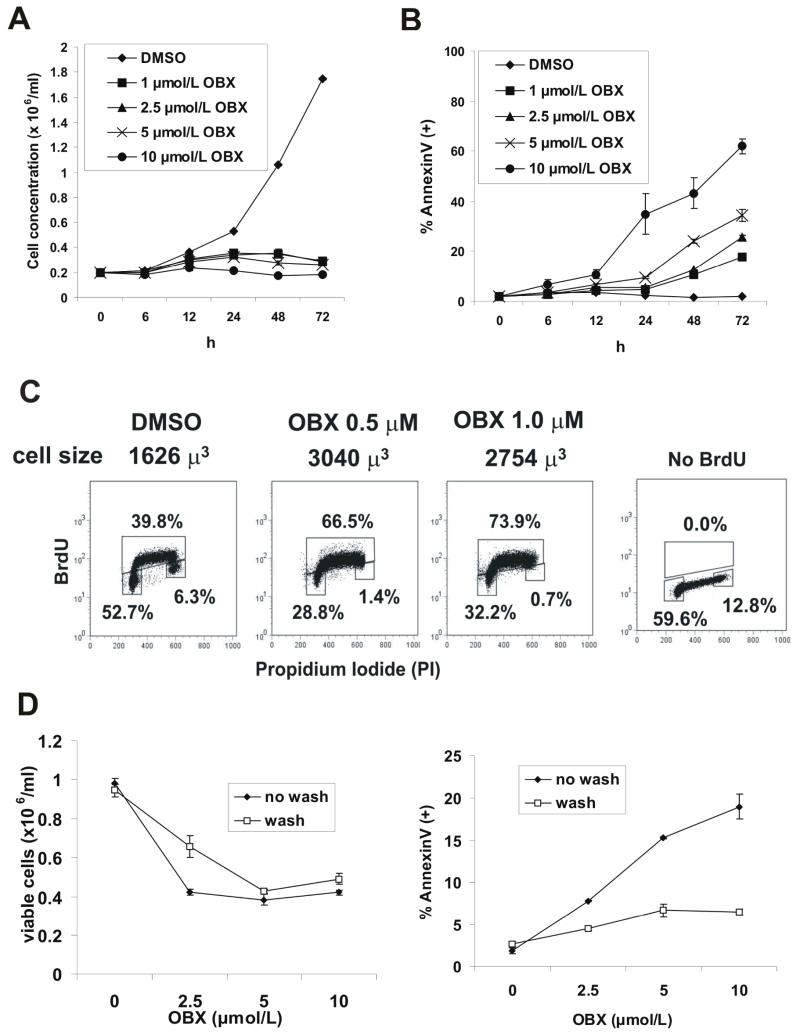
Obatoclax is a novel cycloprodigiosin-derived small molecule BH3 inhibitor that binds with moderate affinity to all antiapoptotic Bcl-2 family members, including Mcl-1, and is currently undergoing Phase I clinical trials in leukemias (11). To evaluate the antileukemia effects of obatoclax, we incubated OCI-AML3 acute myeloid leukemic (AML) cells with various concentrations of obatoclax (0, 0.5, 1, 2.5, 5, and 10 μM) and measured the number of viable cells using a Vi-CELL XR cell viability analyzer (Figure 1A). Our results suggested that obatoclax effectively abrogated the growth of OCI-AML3 cells, and similar results were seen in HL60, KG1, and U937 cells (not shown). To then determine if apoptosis contributes to the antiproliferative effect of GX15-070, OCI-AML3 cells were treated with increasing concentrations of this agent for various times and phosphatydil serine (PS) externalization was monitored by flow cytometry by staining with Annexin V-FITC. As shown in Figure 1B, GX15-070 induced a time- and dose-dependent externalization of phosphatydil serine, that was detected as early as 24 hrs after exposure and at doses that paralleled the reported IC50 values (1.1 – 5.0 μM) for antagonism of Bcl-2 family proteins (6). Interestingly, obatoclax also displayed low-dose antiproliferative properties that were accompanied by a S/G2 cell cycle block as demonstrated by an increase in BrdU labeled S-phase cells concomitant with the disappearance of cells in G2M cell cycle phase, as well as an increase in cell size as determined by the Coulter ViCell XR analyzer (Figure 1 C). To investigate if the apoptotic and antiproliferative effects of obatoclax could be differentiated pharmacokinetically, we performed a washout experiment (Figure 1 D). The results demonstrate that wash out of obatoclax after 1 h exposure prevents the induction of apoptosis (right panel), but does not prevent the observed growth inhibitory effects (left panel), suggesting that the target(s) that mediate apoptosis are different from those mediating mitotic arrest. Nonetheless, our observations suggest that at concentrations of obatoclax that display affinity for antiapoptotic Bcl-2 proteins, apoptosis contributes to the observed antiproliferative effects. Similar results were also found in HL-60 cells (data not shown).
Figure 1.
Obatoclax induces apoptosis in AML. A - OCI-AML3 cells were incubated with various concentrations of obatoclax (0, 1, 2.5, 5, and 10 μM) and the number of viable cells was determined as described in the Materials and Methods. B - OCI-AML3 cells were treated with increasing concentrations of obatoclax for various times and phosphatydil serine (PS) externalization was monitored by flow cytometry by staining with Annexin V-APC. C – OCI-AML3 cells were treated with 0.5 and 1.0 μM of obatoclax for 48 h and BrdU incorporation was quantitated by flow cytometry as described in the Materials and Methods. Cell volume was determined from the average diameter measured by the ViCell XR analyzer. D – Cells were treated with obatoclax for 1 h and washed twice in serum containing media. Cells were then cultured under standard conditions for 48 h, and cell viability and apoptosis were quantitated as described in the Materials and Methods.
Obatoclax induced apoptosis proceeds through the intrinsic apoptotic pathway after neutralization of Mcl-1
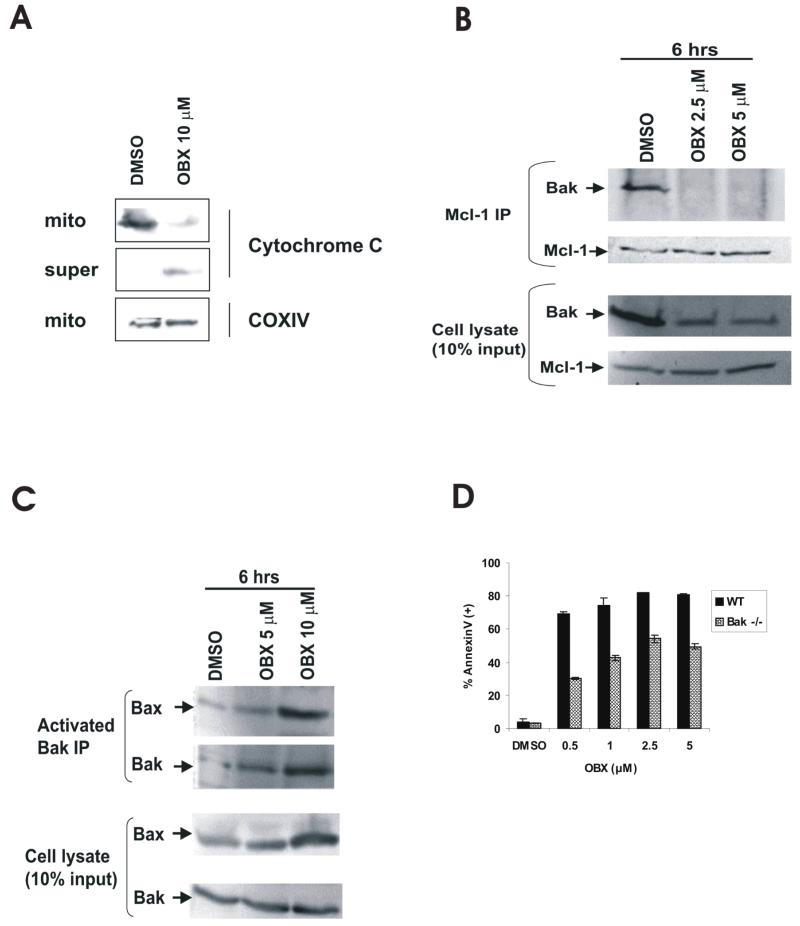
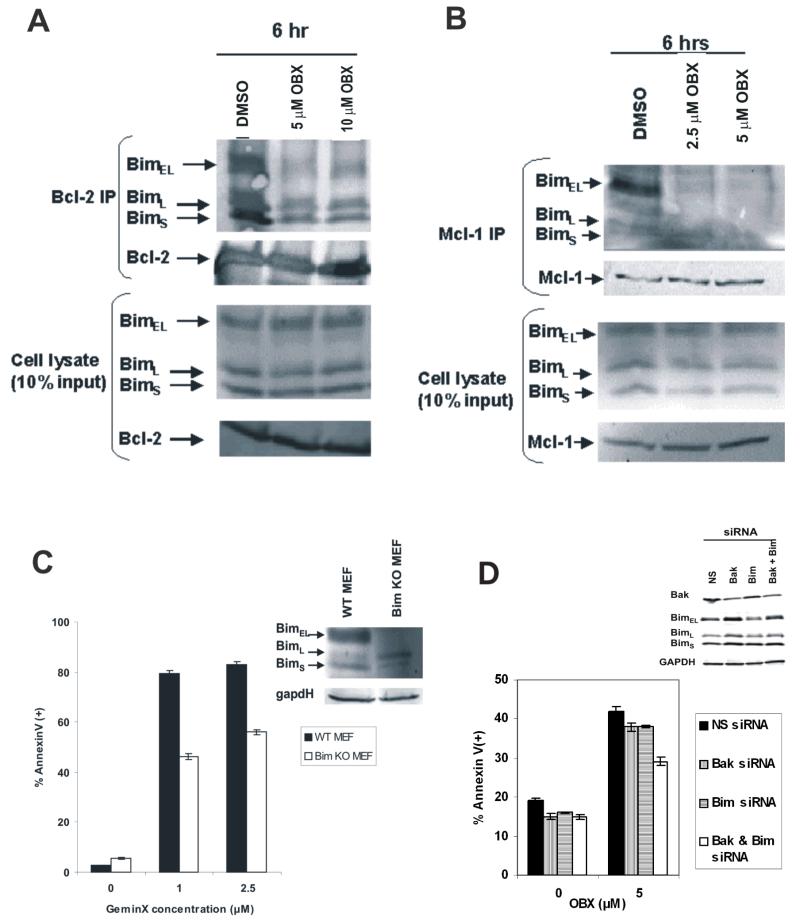
We have previously reported that the BH3 mimetic ABT-737 promotes the release of cytochrome C from isolated HL60 mitochondria (5). To investigate if obatoclax could exert similar effects, we exposed succinate/rotenone-energized HL60 mitochondria to obatoclax (10 μM) for 15 minutes followed by a cold-centrifugation step and assessed the levels of cytochrome C in the pellet and corresponding supernatant. As shown in Figure 2A, obatoclax promotes the release of cytochrome C from isolated mitochondria, suggesting that like ABT-737 this agent induces apoptosis through activation of the intrinsic apoptotic pathway. Similar results were obtained with U937 cell mitochondria (not shown). We then investigated if obatoclax induced activation of the intrinsic pathway involved the release of Bak from the potent antiapoptotic protein Mcl-1, a protein that we have previously reported mediates resistance to ABT-737 (5). Treatment of OCI-AML3 cells with obatoclax resulted in a rapid and complete release of Bak from Mcl-1 (Figure 2B), and this was accompanied by increased expression of activated Bak in a complex with Bax (Figure 2C). Additionally, it was observed that obatoclax-induced apoptosis was decreased, but not completely abolished, in Bak −/− cells (Figure 2D) suggesting that Bak contributes to some extent to cytotoxicity induced by this agent. No further protection from cell death was seen in Bax/Bak −/− MEFs (not shown). Finally, we sought to determine if similar to ABT-737-induced apoptosis, obatoclax-induced apoptosis proceeded in a Bim-independent manner in leukemia cells. We observed that Bim was efficiently released from Bcl-2 and Mcl-1 in OCI-AML3 cells treated with obatoclax (Figures 3A and B), and most interestingly, cells devoid of Bim expression were less susceptible to apoptosis induction by this BH3 mimetic (Figure 3C). These results suggest that in cells treated with obatoclax-free Bim may cooperate with Bak to promote the activation of the intrinsic apoptotic pathway. Indeed, partial knock-down of both, Bim and Bak by siRNA in HL-60 cells partially protected cells from apoptosis (Figure 3D), while cells electroporated with Bak or Bim siRNA alone were minimally protected. Although we were unable to achieve complete knock-down in notoriously difficult to transfect leukemic cells, this data suggest other targets contributing to pro-apoptotic effects of this agent.
Figure 2.
Obatoclax activates the intrinsic apoptotic pathway. A - Succinate/rotenone-energized HL60 mitochondria were exposed to obatoclax (10 μM) for 15 minutes and the levels of cytochrome C in the pellet and corresponding supernatant were determined as described in the Materials and Methods. B - Mcl-1 was immunoprecipitated from OCI-AML3 cells treated with obatoclax for 6 h, and the presence of Bak was determined by Western blot. C – Active Bak was immunoprecipitated from OCI-AML3 cells treated with obatoclax for 6 h, and the presence of Bax was determined by Western blot. D – Wild-type (WT)- or Bak-deficient MEFs were treated with obatoclax for 48 h and Annexin V-positivity was monitored by flow cytometry as described in the Materials and Methods.
Figure 3.
Obatoclax induces release of Bim from anti-apoptotic Bcl-2 and Mcl-1 proteins. A – Bcl-2 was immunoprecipitated from obatoclax treated OCI-AML3 cells and the presence of Bim was investigated by Western blot. B – Mcl-1 was immunoprecipitated from obatoclax treated OCI-AML3 cells and the presence of Bim was investigated as above. C - Bim-deficient MEFs were treated with obatoclax for 48 h and Annexin V-positivity was monitored by flow cytometry as described in the Materials and Methods. D – Bak, Bim, Bak and Bim siRNA or control (NS) siRNA was transfected into HL-60 cells using Amaxa nucleofection, and the levels of Bim/Bak expression were analyzed by Western blotting. Cells were treated with 5μM obatoclax for 48 h, and induction of apoptosis was assessed by annexin V flow cytometry.
Obatoclax synergizes with AraC and ABT-737 in inducing apoptosis in AML cell lines
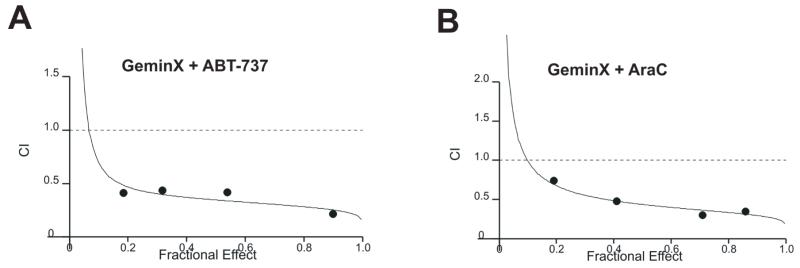
Since the binding affinities of obatoclax to antiapoptotic Bcl-2 proteins are different than those of ABT-737, and each agent binds to a different but overlapping region of the hydrophobic pocket of Bcl-2, we sought to investigate if the combination of both BH3 mimetics could synergistically activate apoptosis. Briefly, ABT-737-resistant OCI-AML3 cells were treated simultaneously with ABT-737 and obatoclax using a fixed ratio (1:1), and Annexin V-positivity was monitored by flow cytometry after 48 h. Isobologram analysis (Figure 4A) revealed that obatoclax and ABT-737 act synergistically in inducing apoptosis (averaged CI values 0.3 +/− 0.03) suggesting that indeed the combination of two BH3 mimetics with different binding characteristics promotes a greater degree of apoptosis than each agent alone. Furthermore, as shown in Figure 4B, obatoclax also synergized with AraC, a frontline chemotherapeutic agent for the treatment of AML, to induce apoptosis in OCI-AML3 cells (fixed 1:1 ratio; averaged CI 0.36 +/− 0.04). Finally, pretreatment with AraC for 24 h, or pretreatment of obatoclax for 24 h did not significantly alter the average CI values for 48 h combination treatment with these agents, suggesting the schedule-independence of their interaction (not shown). Similar results were observed in HL60 cells as well as in a primary AML sample, with averaged CI values for apoptosis induction of 0.062 and 0.43, respectively. These results suggest that like other BH3 mimetics (5;12;13), obatoclax can potentiate the effects of traditional chemotherapy and may offer a therapeutic advantage in combination with other targeted therapy agents.
Figure 4.
Obatoclax synergizes with ABT-737 and AraC to induce cell death in OCI-AML3 cells. A - OCI-AML3 cells were treated simultaneously with ABT-737 and obatoclax using a fixed ratio (1:1), and Annexin V-positivity was monitored by flow cytometry after 48 h and CI values were determined by isobologram analysis. B - ABT-737-resistant OCI-AML3 cells were treated simultaneously with AraC and obatoclax and CI values were determined as above.
Obatoclax induces apoptosis and selectively inhibits colony formation of primary AML cells
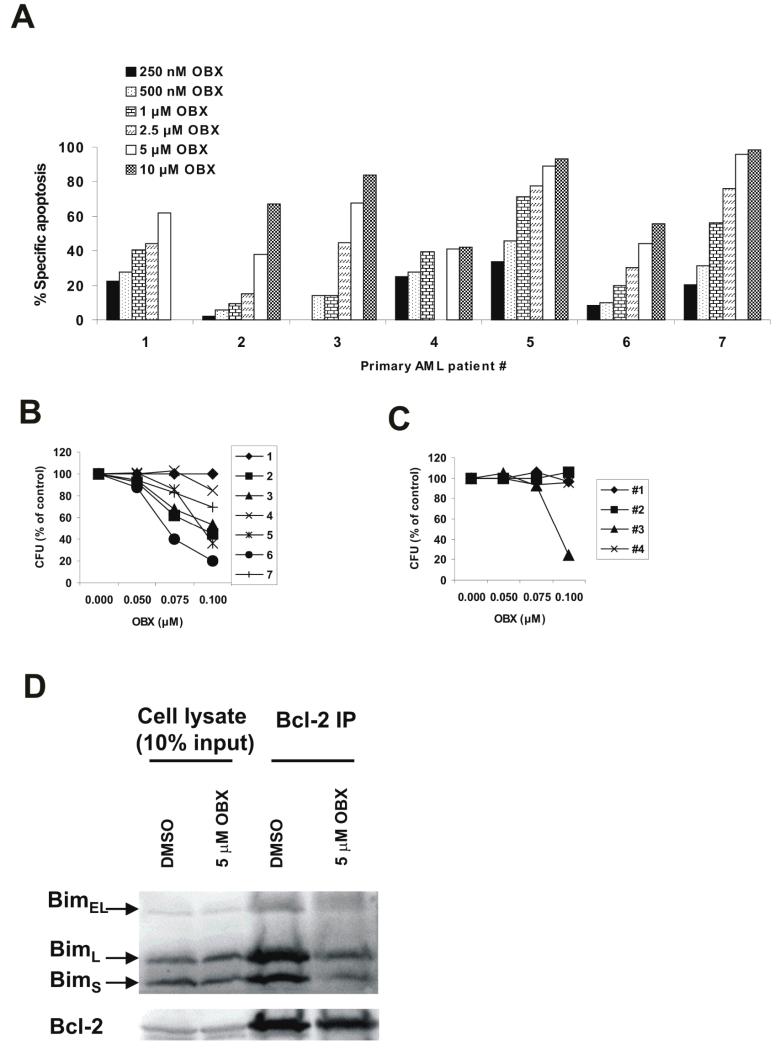
To determine the effects of obatoclax on AML progenitor cells, primary AML samples were treated with increasing concentrations of obatoclax, and annexin V was measured in the CD34-positive compartment by flow cytometry after 24 h (Figure 5A). All samples were obtained from untreated new or relapsed AML patients (Table 1A). Specific apoptosis was induced at 250 nM obatoclax (16.04±4.79%), and increased in a dose-dependent manner up to the highest dose tested (10 μM: 73.48±8.39%). The average IC50 for the seven AML patient samples tested was 3.59±1.23 μM. Additionally, albeit Mcl-1 expression was very low in the primary samples examined, obatoclax was able to efficiently dissociate Bim from Bcl-2 in all 3 primary samples tested (Figure 5D), suggesting that cell death induced by this agent in AML is associated with antagonism of Bcl-2. Furthermore, we investigated the effects of obatoclax on the clonogenicity of untreated or relapsed primary AML samples (Table 1B) in the CFU-blast assay. The formation of surviving AML progenitor colonies was reduced to 77.33±8.41% at 75 nM and 58.45±10.63% at 100 nM (Figure 5B). Colony inhibition in normal bone marrow was only reduced to 98.00±3.22% at 75 nM and 80.63±18.88% at 100 nM (Figure 5C). The average IC50 for obatoclax in AML was 0.18±0.07 μM, and for normal bone marrow was 0.44±0.22 μM.
Figure 5.
Obatoclax induces apoptosis in primary AML samples. A - Primary AML samples were treated with increasing concentrations of obatoclax and annexin V was measured in the CD34-positive compartment by flow cytometry after 24 h. Specific apoptosis was determined as described in the Materials and Methods. B – Primary AML samples were cultured with obatoclax as indicated and clonogenicity was determined as described in the Materials and Methods. C – The clonogenic potential of normal bone marrow samples treated with obatoclax was determined as above. D – Bcl-2 was immunoprecipitated from a primary AML sample treated with obatoclax and the presence of Bim was assessed by Western blot. Results are representative of three independent samples examined.
TABLE 1. Clinical data for patients.
| A. | |||||
|---|---|---|---|---|---|
| Patient # |
Source | Blast % |
FAB | Cytogenetics | Status |
| 1 | BM | 47 | RAEBT | 46,XX,del(5)(q13q33)[2],42-46,XX,−2,− 3,del(4)(q25q31.2),del(5)(q13q33),−6, −7,der(7;15)(q10;q10),+8,−12,−18,+20,+2-5mar[cp18] |
Relapsed Refractory |
| 2 | BM | 99 | UNK | Pseudodiploid metaphase 46,XY,− 3,add(12)(p13),+mar[1],Diploid male karyotype 46,XY[29] |
Relapsed Refractory |
| 3 | BM | 65 | M2 | Pseudodiploid clone 46,XX,t(6;9)(p23;q34)[19],Hyperdiploid metaphase 47,XX,t(6;9)(p23;q34),+16[1] |
New |
| 4 | BM | 88 | M4 | Pseudodiploid clone 46,XY,inv(16)(p13.1q22)[20] | New |
| 5 | BM | 86 | M1 | Hypodiploid clone 45,XX,−7[15],Diploid female karyotype 46,XX[5] |
Relapsed |
| 6 | BM | 83 | M1 | 42-44,Y,del(X)(p22.1),−3,add(4)(q35),− 5,del(7)(q22q34),add(12)(p13),−17, der(18)del(18)(p11.2)del(18)(q21.1),add(21)(p11.1),+0- 2mar[cp20] |
Relapsed |
| 7 | BM | 84 | UNK | Pseudodiploid clone 46,XY,inv(5)(p15.1q13)[12],Metaphases exhibiting random numerical and structural changes[2],Diploid male karyotype 46,XY[6] |
Relapsed Refractory |
| B. | |||||
|---|---|---|---|---|---|
| Patient # |
Source | Blast % |
FAB | Cytogenetics | Status |
| 1 | PB | 85 | M1 | Diploid female karyotype 46,XX[19 | New |
| 2 | PB | 20 | RAEBT | Pseudodiploid clone 46,XX,− 7,+mar[18],Metaphases exhibiting random changes involving chromosome 7[2] |
Primary Refractory during salvage |
| 3 | PB | 90 | M4 | Two hypodiploid clones 45,XX,t(3;3)(q21;q26),− 7[9],45,XX,inv(1)(p22q32),t(3;3)(q21;q26),−7[6] |
Relapsed Refractory |
| 4 | PB | 100 | M1 | Pseudodiploid clone 46,XY,t(12;17)(p13;q24)[20] | Relapsed Refractory |
| 5 | PB | 81 | M4 | Hypodiploid clone 45,XY,−7[11],Diploid male karyotype 46,XY[9] |
New |
| 6 | PB | 93 | M0 | 45,XX,del(1)(p13p31),− 7,t(11;19)(q23;p13.3),del(12)(p13)[5],45,idem,add (6)(q27)[2],45,XX,t(1;3)(p36.1;p21),t(5;6)(q11.2;q 11),−7,t(11;19)(q23;p13.3)[13] |
Relapsed Refractory |
| 7 | BM | 96 | M1 | Diploid male karyotype 46,XY[20] | New |
| 8 | PB | 62 | M5B | Pseudodiploid clone 46,X,idic(X)(p11.2)[4],Pseudodiploid metaphase 46,XX,t(1;4)(q42;q23)[1],Diploid female karyotype 46,XX[15] |
New |
| 9 | PB | 95 | M2 | Metaphases exhibiting random numerical and structural changes[4],Diploid male karyotype 46,XY[16] |
New |
| 10 | PB | 98 | UNK | 45,XY,t(3;3)(q21;q26.2),−7[11],43-45 XY,t(3;3)(q21;q26.2),del(4)(p14p16),−7,−8,−10,+0- 5mar[cp8] |
Refractory |
| 11 | PB | 73 | M4 | Diploid female karyotype 46,XX[20] | New |
| 12 | PB | 63 | M1 | Diploid male karyotype 46,XY[20] | New |
| 13 | BM | 81 | M4 | Diploid female karyotype 46,XX[20] | New |
| 14 | PB | 64 | Raising from MDS | Pseudodiploid clone 46,XX,t(5;7)(q34;p13)[17],Pseudodiploid metaphase 46,XX,t(1;8)(p22;q22)[1],Diploid female karyotype 46,XX[2] |
Relapse 2 |
(A) Samples used for colony assays.
(B) Samples used for in vitro assays.
UNK = unknown (outside diagnosis); BM = bone marrow; PB = peripheral blood; FAB = French-American-British; RAEBT = refractory anemia with excess of blasts in transformation.
Discussion
The development of BH3 mimetics has offered a novel therapeutic approach for the treatment of cancer (14). We have previously reported that the BH3 mimetic, ABT-737, efficiently induces cell death in AML cell lines and primary samples, and preferentially targets AML progenitor cells. ABT-737 binds with high affinity to Bcl-2, Bcl-xL, and Bcl-w, but not to Mcl-1 or A1, and as such it is ineffective in promoting cell death in cells that express Mcl-1 like OCI-AML3 cells. Albeit we observed that in certain cell contexts the expression of Mcl-1 can be diminished by pharmacological inhibition of the MAPK pathway, resulting in sensitization to apoptosis induction by ABT-737, we are concerned that in certain cancer cells Mcl-1 expression may be independent of MAPK and therefore cannot be downregulated by MAPK inhibitors. These cancer cell contexts will require the use of BH3 mimetics that can efficiently target Mcl-1 for therapeutic benefit. Additionally, from a mechanistic standpoint, investigating how direct Mcl-1 antagonism modulates apoptosis in leukemia cells, alone or in combination with other therapeutic approaches, is of utmost importance for the development of novel therapeutic approaches.
Here we report that obatoclax (GX070-15), a BH3 mimetic currently in clinical trials that displays a different binding affinity than that of ABT-737 to antiapoptotic Bcl-2 family members, is effective in inducing apoptosis in AML cell lines and primary samples. Like ABT-737, obatoclax induced apoptosis in a time- and dose-dependent manner, and apoptosis induction occurred at doses that reflected the affinity of this agent for Bcl-2 family proteins. Mechanistically, apoptosis induction by obatoclax was preceded by liberation of Bak from Mcl-1, dissociation of Bim from Bcl-2 and Mcl-1, and the formation of a complex of activated Bak and Bax. Interestingly, the activation of Bax and Bak by obatoclax has also been observed in peripheral blood mononuclear cells of patients with refractory CLL within a single agent Phase I trial. Unlike observed for ABT-737, apoptosis induced by obatoclax was diminished, but not abolished, in the absence of Bak/Bax suggesting that additional target(s) other than Bcl-2 contribute to the activation of the intrinsic pathway by this cycloprodigiosin derivative. In addition, we identified that obatoclax can abolish cell growth independently of apoptosis by inducing a S/G2 cell cycle block. This antiproliferative effect could be temporally separated from the proapoptotic effects of obatoclax in washout experiments suggesting that this agent has multiple targets. Nevertheless, the abolishment of clonogenicity of primary AML samples treated with low doses of obatoclax strongly suggests that the Bcl-2 independent targets of this agent may have clinical applicability. The mechanisms of these anti-proliferative effects of obatoclax require further studies which are outside of the scope of this manuscript.
From a therapeutic standpoint, we observed that obatoclax could potentiate the activity of AraC, and most interestingly, we found that this agent synergized with ABT-737 to induce apoptosis. These findings suggest that this agent may not only augment the clinical activity of traditional chemotherapy, but that it can potentiate the activity of other BH3 mimetics with different binding affinities/patterns. Interestingly, a recent report demonstrated that DNA damaging agents synergized with ABT-737 in killing of lung cancer cells via, in part, increased expression of Bim (15), suggesting that the observed synergy of obatoclax with ABT-737 may be mediated not just by liberation of Bak from Mcl-1, but amplified by the near complete release of Bim from Mcl-1. In addition, we observed that in primary samples obatoclax-induced apoptosis was associated with liberation of Bim from Bcl-2 suggesting the potential utility of this observation as a biomarker of activity in clinical trials with this agent. We propose that the combinatorial use of obatoclax with chemotherapy and/or ABT-737 may offer a superior therapeutic benefit for AML patients via modulation of the apoptotic rheostat.
Supplementary Material
Acknowledgments
This work is supported in part by grants from the NIH, CA16672, CA49639, CA55164
References
- (1).Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- (2).Fleischer A, Rebollo A, Ayllon V. BH3-only proteins: the lords of death. Arch Immunol Ther Exp (Warsz ) 2003;51:9–17. [PubMed] [Google Scholar]
- (3).Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- (4).Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- (5).Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- (6).Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- (7).Lickliter JD, Wood NJ, Johnson L, et al. HA14-1 selectively induces apoptosis in Bcl-2-overexpressing leukemia/lymphoma cells, and enhances cytarabine-induced cell death. Leukemia. 2003;17:2074–80. doi: 10.1038/sj.leu.2403102. [DOI] [PubMed] [Google Scholar]
- (8).Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Mdm2 and Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Samudio I, Konopleva M, Pelicano H, et al. A novel mechanism of action of methyl-2-cyano-3,12 dioxoolean-1,9 diene-28-oate (CDDO-Me): direct permeabilization of the inner mitochondrial membrane to inhibit electron transport and induce apoptosis. Mol Pharmacol. 2006;69:1182–93. doi: 10.1124/mol.105.018051. [DOI] [PubMed] [Google Scholar]
- (10).Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- (11).Borthakur G, O’Brien S, Favandi-Kashani F, Giles F, Schimmer AD, Viallet J, Kantarjian H. A phase I trial of the small moledule Pan-Bcl-2 family inhibitor obatoclax mesylate (GX15-070) administered by 24 hour infusion every 2 weeks to patients with myeloid malignancies and chronic lymphocytic leukemia (CLL) Blood 108 [ASH Annual Meeting Abstracts] 2006 Nov;108:2654. [Google Scholar]
- (12).Manero F, Gautier F, Gallenne T, et al. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–64. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- (13).Skommer J, Wlodkowic D, Matto M, Eray M, Pelkonen J. HA14-1, a small molecule Bcl-2 antagonist, induces apoptosis and modulates action of selected anticancer drugs in follicular lymphoma B cells. Leuk Res. 2006;30:322–31. doi: 10.1016/j.leukres.2005.08.022. [DOI] [PubMed] [Google Scholar]
- (14).Letai A. Restoring cancer’s death sentence. Cancer Cell. 2006;10:343–5. doi: 10.1016/j.ccr.2006.10.014. [DOI] [PubMed] [Google Scholar]
- (15).Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.