Abstract
A major problem after clinical autologous islet transplantation (AIT) is the difficulty in achieving insulin independence. To follow up on our demonstration in a murine model that high-mobility group box 1 (HMGB1) was released from islets and involved in early loss of transplanted islets, we tested the role of HMGB1 in clinical AIT. Serum HMGB1 levels from 15 AIT patients were significantly elevated during islet infusion (7.6 ± 1.2 ng/ml) and 24 h after infusion (8.0 ± 1.4 ng/ml) compared to admission levels (2.4 ± 0.6 ng/ml). The first elevation of HMGB1 was associated with islet damage, but the later elevation was not. The change in the HMGB1 level from admission to first peak (ΔHMGB1) was significantly higher in the AIT group (8.1 ± 1.1 ng/ml) than in the pancreatectomy-only control (2.2 ± 0.5 ng/ml) (p < 0.05). Circulating serum levels of soluble receptor for advanced glycation end products (sRAGE) were also elevated during islet infusion. In vitro studies demonstrated that damaged human islets released HMGB1 but not sRAGE. In terms of outcomes, the insulin-free group showed significantly lower ΔHMGB1 (5.2 ± 0.6 ng/ml) and higher ΔsRAGE (2.3 ± 0.6 ng/ml) than the insulin-dependent group (10.6 ± 1.9 ng/ml and 0.7 ± 0.2 ng/ml, respectively). The ΔHMGB1 correlated with the number of white blood cell, IP-10, EGF, and eotaxin. In conclusion, serum HMGB1 was elevated in AIT and could be associated with inflammatory reactions that deteriorate islet engraftment. Therefore, anti-HMGB1 therapy might be a candidate for further improving the outcomes of clinical AIT.
Keywords: Autologous islet transplantation (AIT), Islets, High-mobility group box 1 (HMGB1), Chronic pancreatitis, Pancreatectomy
INTRODUCTION
Autologous islet transplantation (AIT) is performed to prevent surgical diabetes after total or subtotal pancreatectomy (1,6,18,27,35). The leading indication for pancreatectomy is chronic pancreatitis with severe abdominal pain (3). While total pancreatectomy effectively reduces severe abdominal pain (6), it causes surgical diabetes (1). Therefore, intraportal AIT is a good option for preventing surgical diabetes after total pancreatectomy. However, previous reports have demonstrated that the insulin-independent rate after AIT was only 26% to 47%, despite the absence of auto- or allogeneic immunity (18). A higher rate of insulin independence after AIT would make this therapy more attractive for patients with chronic pancreatitis.
Recently, we reported that in a murine model, high-mobility group box 1 (HMGB1) protein was uniquely abundant within pancreatic islets, and damaged islets released HMGB1 (23). In this model, serum HMGB1 levels increased shortly after intraportal syngeneic islet transplantation, and HMGB1 stimulated production of inflammatory cytokines including interleukin-12 (IL-12) and interferon (IFN)-γ in concert with dendritic cells (DCs), natural killer (NK) T-cells, and neutrophils in the liver receiving syngeneic islets. Subsequently, these inflammatory cytokines accelerated the injury to transplanted islets (23). Furthermore, we recently reported that damaged human islets also released HMGB1 using in vitro studies (12).
Based on these findings, in the present study we hypothesized that i) serum HMGB1 levels would increase after AIT, ii) that release of HMGB1 would correlate as a marker of islet injury in AIT, and iii) increasing levels of HMGB1 would be inversely correlated to the outcomes of AIT.
MATERIALS AND METHODS
Patients
A total of 15 patients with chronic pancreatitis underwent total pancreatectomy with AIT, and 3 patients with chronic pancreatitis underwent total pancreatectomy without AIT (Px) from September 2010 to October 2011 at Baylor University Medical Center (Dallas, TX, USA) or the University of Leicester (Leicester, UK) (see Table 1). This study was approved by the institutional review board, and written informed consent was obtained from all patients included in this study.
Table 1.
Patient and Islet Characteristics of the AIT and Px Groups
| AIT | Px | p Value | |
|---|---|---|---|
| Gender (female/male) | 10:5 | 3:0 | ns |
| Age (years) | 41.5 ± 9.8 | 50.0 ± 13.1 | ns |
| BMI | 26.0 ± 5.8 | 19.9 ± 2.4 | ns |
| Etiology of pancreatitis | Idiopathic, 11; divisum, 2; alcohol, 1; autoimmune, 1 | Idiopathic, 2; alcohol, 1 | |
| Pretransplant HbA1c (%) | 5.8 ± 0.8 | 8.0 ± 0.7 | p < 0.05 |
| Total transplanted islets (IEQs) | 399,084.7 ± 211,782.5 | N/A | N/A |
| Transplanted islets (IEQs/kg) | 5,685.5 ± 2,907.8 | N/A | N/A |
| Tissue volume (ml) | 11.9 ± 7.0 | N/A | N/A |
| Purity (%) | 52.5 ± 18.0 | N/A | N/A |
| Viability (%) | 96.4 ± 1.2 | N/A | N/A |
AIT, autologous islet transplantation; Px, pancreatectomy without AIT; HbA1c, hemoglobin A1c (glycated); IEQs, islet equivalents. The data of age, body mass index (BMI), pretransplant HbA1c, total transplanted islets, transplanted islets/kg, tissue volume, purity, and viability are expressed as mean ± SD.
AIT
Immediately following total pancreatectomy, pancreata were delivered to the islet isolation facility at Baylor Research Institute (Dallas, TX) or the University of Leicester (Leicester, UK). Liberase MTF with thermolysin (Roche, Indianapolis, IN, USA) was infused into the main pancreatic duct. Pancreas digestion was performed using the modified Ricordi method (21,26). If the pellet volume was larger than approximately 20 ml, islets were purified using COBE 2991 cell processor (CaridianBCT, Inc., Lakewood, CO, USA) with continuous density gradient centrifugation (19). The final preparation of islets was assessed for its yield and purity using dithizone staining (2 mg/ml; Sigma-Aldrich, St. Louis, MO, USA). The islet yield was converted into a standard number of islet equivalents (IEQs), with the diameter standardizing to 150 μm. Islet viability in the final product was evaluated with fluorescein diacetate/propidium iodide (PI) staining (Sigma-Aldrich). The viability was calculated by averaging the viability of 50 islets.
Isolated islets were infused into the portal vein via the mesenteric vein with heparin (70 U/kg body weight; Hospira, Lake Forest, IL, USA) over 30 to 60 min while the patients were under general anesthesia. During islet infusion, portal vein pressure was measured with a pressure transducer (IntelliVue X2 Monitor, Philips Healthcare, Andover, MA, USA) intermittently. If portal vein pressure exceeded 20 mmHg, the infusion of islets was stopped until portal vein pressure decreased.
Serum Tests
Serum samples were obtained at admission, prior to islet infusion, during islet infusion, at the completion of islet infusion, and subsequently at 1 h, 3 h, 6 h, 24 h, 3 days, 5 days, and 7 days post-islet infusion. Serum samples were stored at −80°C until assayed.
The serum levels of HMGB1, C-peptide, proinsulin, and soluble receptor for glycation end products (sRAGE) were measured using HMGB1 ELISA kit II (Shino-test, Kanagawa, Japan) (37), C-peptide enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden), proinsulin ELISA kit (Mercodia), and Quantikine human soluble RAGE kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturers’ instructions. The levels of serum HMGB1, C-peptide, proinsulin, and sRAGE were expressed as mean ± SE.
Secretion of cytokines and chemokines was determined by measuring serum samples in a Luminex®200 (Millipore, Billerca, MA, USA) using xMAP technology (Luminex, Austin, TX, USA). The bead assay was performed according to the manufacturer’s instructions (11).
The secretory unit of islet transplant objects (SUITO) index was calculated by the following formula to access islet graft function: [fasting blood glucose (mg/dl) −63]/fasting C-peptide (ng/ml) × 1,500 (19). Hemoglobin A1c (HbA1c) was measured using high-pressure liquid chromatography (HPLC) by Medfusion (Lewisville, TX, USA).
In Vitro Studies
Two research-grade human pancreata from brain-dead donors were provided by a local organ procurement organization (Southwest Transplant Alliance, Dallas, TX, USA) for this study. Pancreata were procured using a standardized technique, with intraductal pancreatic preservation as previously described (20,21). Human islet isolation was conducted as previously described using the standard Ricordi technique with modifications introduced in the Edmonton protocol (18,21,22,26).
Isolated islets were cultured in Connaught Medical Research Laboratories medium (CMRL1066; Mediatech, Inc., Manassas, VA, USA) at 37°C in 95% air and 5% CO2 for 24 h after islet isolation. After 24 h of initial culture, islets were washed with culture medium and divided into three groups: control, cytokine treated, and hypoxia induced. In the control group, 100 handpicked islets were cultured at 37°C in 95% air and 5% CO2 for 48 h. In the cytokine-treated group, 100 handpicked islets were cultured at 37°C in 95% air and 5% CO2 for 48 h with tumor necrosis factor-α (TNF-α; 1,000 U/ml), IFN-γ (1,000 U/ml), and IL-1β (50 U/ml; all from R&D Systems). In the hypoxia-induced group, 100 handpicked islets were placed into modular incubator chambers (Billups-Rothenberg, Inc., Del Mar, CA, USA). The chambers were flushed with hypoxic (1% O2, 5% CO2, and 94% N2) gas, closed to maintain the hypoxic condition, and then put into an incubator at 37°C for 48 h.
After the normoxic and hypoxic culture, islets were lysed with 0.2% Triton X-100 (Sigma-Aldrich) and treated with a buffer containing 10 mM Tris-(hydroxymethyl) aminomethane hydrochloride (Sigma-Aldrich) and 5 mM ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich) to extract DNA. DNA aliquots of islets from each treatment group were measured using a dsDNA Assay Kit (Molecular Probes, Inc., Eugene, OR, USA). To compare the measured molecule amounts among the different samples, each amount of examined molecule was converted to μg DNA of total cultured islets (11). The quantity of proinsulin, C-peptide, HMGB1, and sRAGE in the culture medium was measured using identical kits used for the serum samples and normalized to the total μg DNA of cultured islets. The data were expressed as mean ± SD.
Islets from each group were also stained with Hoechst 33342 (HO342; Sigma-Aldrich) and PI (Sigma-Aldrich) for viability assays, as briefly described previously (28). For histological analysis, islets from each group were preserved in 10% formalin (Sigma-Aldrich), embedded in paraffin, and sectioned at 5 μm. Tissue sections were deparaffinized, and heat-mediated antigen retrieval was performed. The sections were stained immunohistochemically with rabbit anti-HMGB1 antibody (Abcam, Cambridge, UK), guinea pig anti-insulin antibody (Sigma-Aldrich), and 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) (11).
Statistical Analysis
The statistical significance of the serum and medium HMGB1, C-peptide, proinsulin, and sRAGE levels were determined by repeated measurements of ANOVA with Tukey’s post hoc tests for timeline. The statistical significance between AIT and Px, and between the insulin-dependent group and the insulin-free group, was determined by Student’s t test. The relationship between two variables was tested by Spearman correlation coefficient. To find independently associated cytokines or chemokines with ΔHMGB1, which is the difference between initial peak and baseline level at admission, multivariate linear regression analysis with stepwise method (criteria to enter p < 0.05 and to remove p > 0.1) was performed after univariate evaluations. Variables with p < 0.1 were entered for multivariate analysis after considering the cofounding factor. All statistical analyses were performed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA) or IBM SPSS Statistics Version 20 (IBM Corp., Armonk, NY, USA). Differences were considered significant when p < 0.05.
RESULTS
Patient and Islet Characteristics
The patient and islet characteristics of the 15 cases of AIT and 3 cases of Px are shown in Table 1. Pretransplant hemoglobin A1c (HbA1c) levels were significantly lower in the AIT group than in the Px group (p < 0.05). In 9 of 15 AIT patients, pretransplant HbA1c levels were less than 6.0%; in 6 of 15 AIT patients, pretransplant HbA1c levels were more than 6.0%.
Increase in Serum HMGB1 Levels After AIT and Association With Proinsulin, C-Peptide, and sRAGE
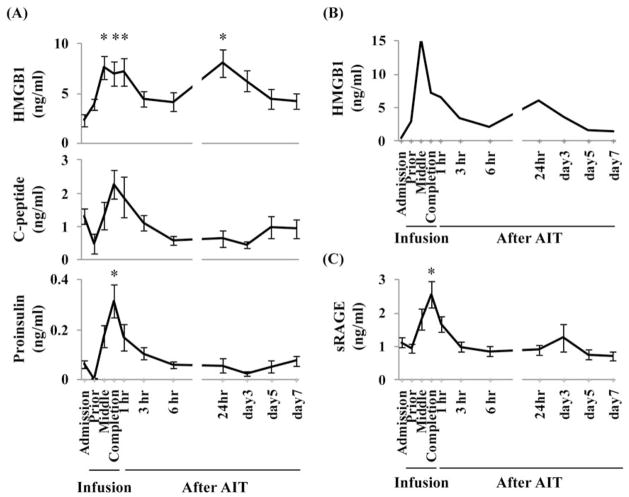
Serum HMGB1 was elevated at two distinct points after AIT: during islet infusion and 24 h after islet infusion. Serum HMGB1 levels at the middle of islet infusion (7.6 ± 1.2 ng/ml), at the completion of infusion (7.0 ± 1.2 ng/ml), 1 h after infusion (7.2 ± 1.4 ng/ml), and 24 h after infusion (8.0 ± 1.4 ng/ml) were significantly higher than levels at admission (2.4 ± 0.6 ng/ml) (p < 0.05) (Fig. 1A, upper panel).
Figure 1.
Elevation of high-mobility group box 1 (HMGB1) levels, and other serum levels, after autologous islet transplantation (AIT). Values for (A) HMGB1 (top), C-peptide (middle), and proinsulin (bottom) in 15 patients; data are expressed as mean ± SE. (B) HMGB1 serum levels after transplant with 100% purity islets. The curve is that of a single patient. (C) Soluble receptor for advanced glycation end products (sRAGE) in 15 patients; data are expressed as mean ± SE. *With repeated measurement of ANOVA, p < 0.05 compared to admission level.
Among the 15 cases of AIT, we experienced a unique case in which isolated islets showed approximately 100% purity without any purification. Interestingly, the serum samples from this patient showed the same two distinct points of elevation of serum HMGB1 (Fig. 1B). Serum HMGB1 levels at admission, at the middle of infusion, and 24 h after islet infusion were 0.5, 15.3, and 6.1 ng/ml, respectively. These results suggested that the serum HMGB1 was mainly released from islets and not from exocrine tissues, even if the HMGB1 was derived from transplanted tissues.
To assess the correlation of the islet damage and serum HMGB1 levels, we measured proinsulin and C-peptide levels. Proinsulin and C-peptide may be released by the damaged islets; therefore, we measured serum concentrations of proinsulin and C-peptide. The proinsulin levels and C-peptide levels decreased prior to islet infusion due to total pancreatectomy and then suddenly increased after islet infusion (Fig. 1A, lower and middle panels). The initial HMGB1 elevation coincided with the elevations of proinsulin and C-peptide. However, 24 h after islet infusion, HMGB1 was elevated, but proinsulin and C-peptide were not. This suggests that only the first elevation of HMGB1 was associated with islet damage.
Next, we examined whether sRAGE, an endogenous decoy receptor for RAGE ligands, was increased after AIT. Serum sRAGE levels were also elevated associated with AIT (Fig. 1C). The sRAGE level at the completion of infusion (2.6 ± 0.4 ng/ml) was significantly higher than that at admission (1.1 ± 0.1 ng/ml) (p < 0.05).
Taken collectively, HMGB1, proinsulin, C-peptide, and sRAGE were elevated during islet infusion; however, only HMGB1 was reelevated 24 h after islet infusion.
Comparison of HMGB1 Levels Between AIT and Px
To assess the correlation between elevation of HMGB1 levels and AIT, we compared the HMGB1 levels between AIT patients and Px patients. As chronic pancreatitis is an inflammatory disease, the pretransplant levels of serum HMGB1 at admission could be different from patient to patient based on disease severity. To minimize the influence of different basal HMGB1 levels due to different severity of disease, we calculated delta (Δ)HMGB1 levels between first peak level and admission level (ΔHMGB1). The ΔHMGB1 levels of the AIT group and Px group were 8.1 ± 1.1 and 2.2 ± 0.5 ng/ml, respectively (p < 0.05) (Fig. 2A). Furthermore, the ΔsRAGE levels of the AIT group and Px group were 1.5 ± 0.3 and 0.6 ± 0.4 ng/ml, respectively (not significant) (Fig. 2B).
Figure 2.
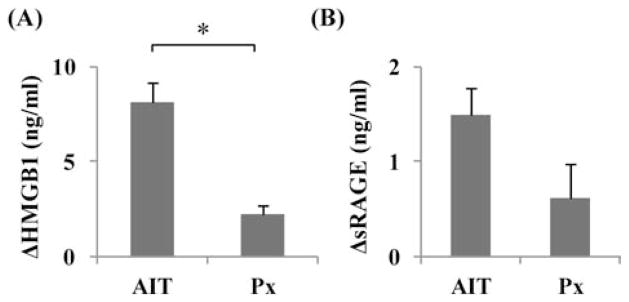
The change in high-mobility group box 1 (HMGB1; A) and soluble receptor for advanced glycation end products (sRAGE; B) between admission and first peak levels in the autologous islet transplantation (AIT) group (n = 15) and the pancreatectomy-only control (Px; n = 3). The data are expressed as mean ± SE. *With Student’s t test, p < 0.05.
In Vitro Islet Release of HMGB1, Proinsulin, C-Peptide, and sRAGE
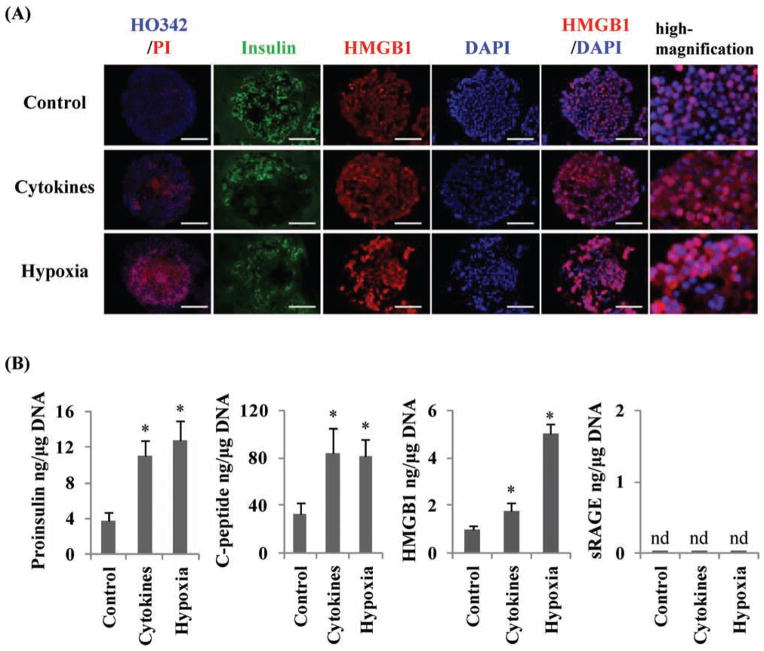
To determine whether damaged islets released HMGB1 and sRAGE in vitro, we tested three groups of cultured islets: control, cytokine treated, and hypoxia induced. A PI assay showed that islet cell death was increased with exposure to cytokines or hypoxia induced (Fig. 3A). In the control islets, HMGB1 stained mainly in the nucleus; however, in the cytokine- and hypoxia-induced damaged human islets, HMGB1 was present not only in the nucleus but also in the cytoplasm (Fig. 3A). Compared with the control group, the two groups with damaged islets (cytokine treated and hypoxia induced) released significantly higher levels of proinsulin, C-peptide, and HMGB1 (p < 0.05) (Fig, 3B). However, sRAGE was not detected in the culture medium in any of the three groups, despite the severity of islet damage (Fig. 3B). These in vitro studies indicated that the damaged human islets, themselves, released HMGB1 but did not release sRAGE.
Figure 3.
Release of high-mobility group box 1 (HMGB1), C-peptide, and proinsulin, but not soluble receptor for advanced glycation end products (sRAGE), by damaged human islets. (A) Control islets, cytokine-induced damaged islets, and hypoxia-induced damaged islets were stained with Hoechst33342 and propidium iodide (HO342/PI) (left), insulin (green), HMGB1 (red), and 4′,6-diamidino- 2-phenylindole (DAPI; blue). Scale bars: 100 μm. (B) ELISA measurements of the amount of released proinsulin, C-peptide, HMGB1, and sRAGE from the three groups of islets. The data are expressed as mean ± SD. *p < 0.05 compared to control. nd, not detected.
Correlations With Insulin-Free or Insulin-Dependent Outcome After AIT
In comparing outcomes after AIT, we wanted to avoid the influence of pretransplant glycemic control; we chose 9 of 15 patients whose pretransplant HbA1c levels were less than 6.0%. In this group, five of nine patients were insulin dependent and four of nine patients were insulin-free after AIT [median follow-up period: 8 months (range: 6–15 months)]. A patient became insulin independent immediately after AIT but resumed insulin therapy 4 months after AIT. We considered this patient as insulin dependent at this analysis. There were no significant differences in gender, age, or body mass index (BMI) between the two groups (Table 2). Pretransplant HbA1c levels were 5.5 ± 0.3% in the insulin-dependent group and 5.2 ± 0.3% in the insulin-free group (not significant) (Table 2). In addition, there were no significant differences in the total number of transplanted islets, transplanted islets/body weight, tissue volume, purity, or viability (Table 2).
Table 2.
Patient and Islet Characteristics of the Insulin-Dependent and Insulin-Free Groups
| Insulin Dependent | Insulin Free | p Value | |
|---|---|---|---|
| Gender (female/male) | 2:3 | 3:1 | ns |
| Age (years) | 48.2 ± 7.2 | 37.5 ± 10.0 | ns |
| BMI | 23.0 ± 3.3 | 26.3 ± 5.0 | ns |
| Etiology of pancreatitis | Idiopathic, 4; alcohol, 1 | Idiopathic, 3; divisum, 1 | |
| Pretransplant HbA1c (%) | 5.5 ± 0.3 | 5.2 ± 0.3 | ns |
| Total transplanted islets (IEQs) | 528,915.4 ± 310,822.9 | 349,367.0 ± 118,327.1 | ns |
| Transplanted islets (IEQs/kg) | 7,864.2 ± 4,162.4 | 5,173.8 ± 1,778.6 | ns |
| Tissue volume (ml) | 17.0 ± 6.8 | 13.0 ± 6.7 | ns |
| Purity (%) | 59.3 ± 12.8 | 51.5 ± 1.4 | ns |
| Viability (%) | 97.4 ± 1.1 | 96.2 ± 1.4 | ns |
HbA1c, hemoglobin A1c; IEQs, islet equivalents. The data of age, BMI, pretransplant HbA1c, total transplanted islets, transplanted islets/kg, tissue volume, purity, and viability were expressed as mean ± SD.
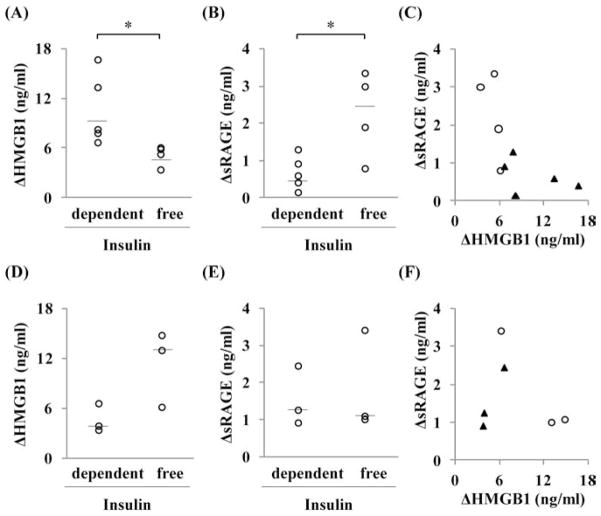
Significant differences were seen in the changes of serum HMGB1 and sRAGE levels between the insulin-dependent group and insulin-free group. The ΔHMGB1 levels of the insulin-dependent group and the insulin-free group were 10.6 ± 1.9 ng/ml and 5.2 ± 0.6 ng/ml, respectively (p < 0.05) (Fig. 4A). The ΔsRAGE levels of the insulin-dependent group and the insulin-free group were 0.7 ± 0.2 ng/ml and 2.3 ± 0.6 ng/ml, respectively (p < 0.05) (Fig. 4B). Most of the insulin-free patients had low ΔHMGB1 and high ΔsRAGE (Fig. 4C). Additionally, similar analysis was performed for patients who had over 6.0% of pretransplant HbA1c (n = 6) (Fig. 4D to F). There were no significant differences in ΔHMGB1 as well as in ΔsRAGE between the insulin-dependent and insulin-free groups.
Figure 4.
Differences between the insulin-dependent group and insulin-free group after autologous islet transplantation (AIT). Results from patients with pretransplant glycated hemoglobin (HbA1c) <6.0% (n = 9) and ≥6.0% (n = 6) are shown in A to C and D to F, respectively. (A and D) The changes in high-mobility group box 1 (HMGB1) between admission and first peak levels and (B and E) the change in soluble receptor for advanced glycation end products (sRAGE) between admission and peak levels. Individual data and median bar are shown. *With Student’s t test p < 0.05. (C and F) Correlation between ΔHMGB1, ΔsRAGE, and insulin dependency. The white circles show insulin-free patients (n = 4; n = 3), and the black triangles show insulin-dependent patients (n = 5; n = 3) in C and F.
To support the relationship between clinical outcome and ΔHMBG1 or ΔsRAGE, we also compared fasting C-peptide or SUITO index 6 months or later after AIT [median follow-up period: 9 months (range: 6–13 months)] with them (Table 3). Negative correlation with marginal significance level (p = 0.07) between ΔHMGB1 and SUITO index was found among six patients with pre-transplant HbA1c < 6.0%, although there were no correlations with statistical significance in any pairs for patients with over 6.0% of pretransplant HbA1c.
Table 3.
Correlation Between Fasting C-Peptide or SUITO Index and ΔHMGB1 or ΔsRAGE
| Patients | Variables | ΔHMGB1 | ΔsRAGE |
|---|---|---|---|
| Patients with pretransplant HbA1c < 6.0% (n = 6) | |||
| Fasting C-peptide | −0.348 | −0.087 | |
| SUITO index | −0.772* | 0.543 | |
| Patients with pretransplant HbA1c ≥ 6.0% (n = 6) | |||
| Fasting C-peptide | 0.118 | −0.677 | |
| SUITO index | 0.371 | 0.086 |
Spearman correlation coefficients are shown.
p = 0.07, showing marginally statistical significant level.
Other combinations showed no significant correlation. SUITO index, secretory unit of islet transplant objects index; HMGB1, high-mobility group box 1; sRAGE, soluble receptor for advanced glycation end products.
Correlation of ΔHMGB1 Levels and Other Inflammatory Factors
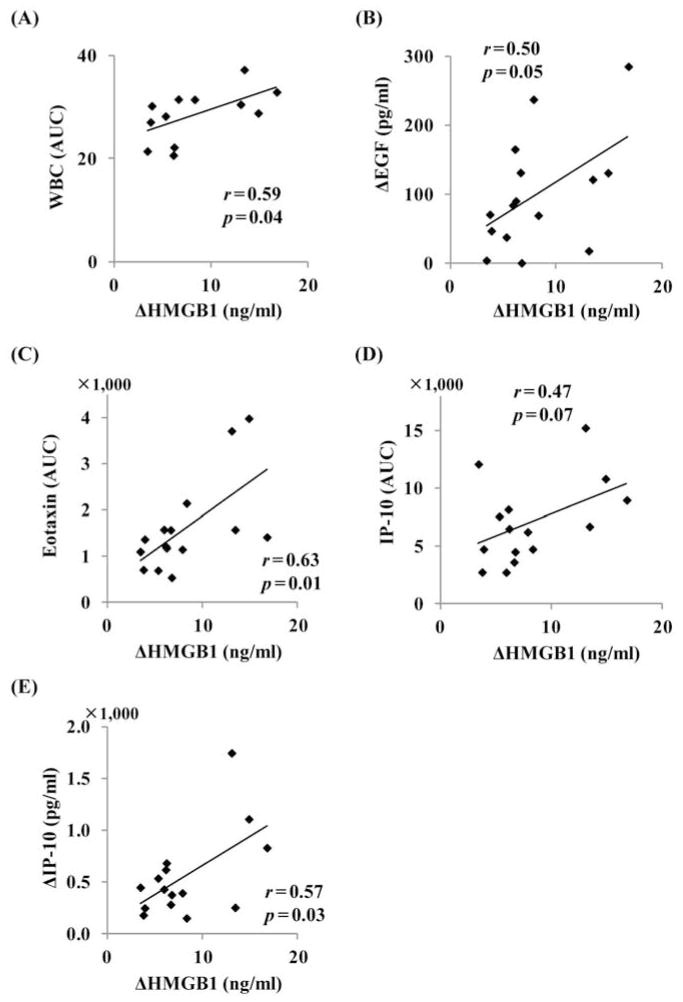
Finally, we evaluated the association between ΔHMGB1 and other inflammatory factors, including the number of white blood cells (WBCs), serum inflammatory cytokines, and chemokines. The serum concentrations of epidermal growth factor (EGF), granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), IL-10, IL-15, interleukin-1 receptor α (IL-1Rα), IL-6, IL-8, interferon inducible protein 10 [IP-10; also known as chemokine (C-X-C motif) ligand 10], monocyte chemoattractant protein 1 [MCP-1; also known as chemokine (C-C motif) ligand 2], and vascular endothelial growth factor (VEGF) showed a significant increase after AIT compared to that of admission (Fig. 5). The timing of the elevation of G-CSF, GM-CSF, IL-10, IL-15, IL-1Rα, IL-6, IL-8, IP-10, MCP-1, and VEGF was observed simultaneously or late to the first peak of serum HMGB1 (Fig. 5). The areas under the curve (AUC) of WBCs and cytokines or chemokines were calculated by the area under the number (WBCs) or the concentration (cytokines or chemokines) time curves. The changes (Δ) in cytokines or chemokines were calculated by the difference between peak levels and admission levels. In univariate regression analysis, AUC of WBCs (r = 0.59, p = 0.04) (Fig. 6A), ΔEGF (r = 0.50, p = 0.05) (Fig. 6B), AUC of eotaxin (r = 0.63, p = 0.01) (Fig. 6C), AUC of IP-10 (r = 0.47, p = 0.07) (Fig. 6D), and ΔIP-10 (r = 0.58, p < 0.05) (Fig. 6E) were identified as candidates of associated factors with ΔHMGB1 (p < 0.1 level). The other cytokines or chemokines did not have any significant correlations with ΔHMGB1 (Tables 4 and 5). We found significant correlation between AUC of IP-10 and ΔIP-10 (r = 0.725, p = 0.002) and excluded AUC of IP-10 for further multivariate analysis to avoid colinearity. Among the remaining four factors, AUC of eotaxin (standardized coefficient β = 0.658, p = 0.003) and ΔEGF (β = 0.601, p = 0.004) were extracted as independently associated variables with ΔHMGB1.
Figure 5.
Serum levels of inflammatory cytokines or chemokines after autologous islet transplantation (AIT). The serum concentrations of epidermal growth factor (EGF), eotaxin, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), interferon (IFN)-γ, interleukin (IL)-10, IL-12 subunit p40 (IL-12p40), IL-12p70, IL-15, IL-17, interleukin-1 receptor α (IL-1Rα), IL-6, IL-7, IL-8, interferon inducible protein 10 [IP-10; also known as chemokine (C-X-C motif) ligand 10; CXCL10], monocyte chemoattractant protein 1 [MCP-1; also known as chemokine (C-C motif) ligand 2; CCL2], macrophage inflammatory protein 1-β (MIP-1β; also known as CCL4), and vascular endothelial growth factor (VEGF) in 15 patients were measured. Data are expressed as mean ± SE. *With repeated measurements of ANOVA p < 0.05 compared to admission level.
Figure 6.
Association between changes in high-mobility group box 1 (HMGB1) levels and other inflammatory factors in univariate regression analysis. (A) Area under the curve (AUC) of white blood cells (WBCs), (B) change in epidermal growth factor (EGF), (C) AUC of eotaxin, (D) AUC of interferon-inducible protein-10 (IP-10), and (E) change in IP-10.
Table 4.
Coefficients Between ΔHMGB1 and ΔInflammatory Cytokines or Chemokines in Univariate Linear Regression Analysis
| Correlation Between ΔHMGB1 | Standardized Coefficient | p Value |
|---|---|---|
| ΔEotaxin | 0.153 | 0.585 |
| ΔG-CSF | −0.257 | 0.355 |
| ΔGM-CSF | 0.132 | 0.639 |
| ΔIFN-γ | 0.427 | 0.113 |
| ΔIL-10 | 0.175 | 0.534 |
| ΔIL-12p40 | 0.047 | 0.868 |
| ΔIL-12p70 | 0.437 | 0.103 |
| ΔIL-15 | −0.058 | 0.836 |
| ΔIL-17 | 0.371 | 0.173 |
| ΔIL-1Rα | −0.336 | 0.220 |
| ΔIL-6 | −0.053 | 0.852 |
| ΔIL-7 | 0.080 | 0.776 |
| ΔIL-8 | −0.081 | 0.775 |
| ΔMCP-1 | 0.045 | 0.874 |
| ΔMIP-1β | 0.333 | 0.226 |
| ΔVEGF | 0.041 | 0.886 |
| ΔWBCs | −0.093 | 0.774 |
Delta (Δ) was calculated by the difference between peak and admission levels. G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN, interferon; IL-10, interleukin 10; IL-1Rα, interleukin 1 receptor alpha; MCP-1, monocyte chemoattractant protein 1 [also known as chemokine (C-C motif) ligand 2; CCL2]; MIP-1β, macrophage inflammatory protein 1-beta (also known as CCL4); VEGF, vascular endothelial growth factor (VEGF); WBCs, white blood cells.
Table 5.
Coefficients Between ΔHMGB1 and AUC of Inflammatory Cytokines or Chemokines in Univariate Linear Regression Analysis
| Correlation Between ΔHMGB1 | Standardized Coefficient | p Value |
|---|---|---|
| AUC of EGF | 0.089 | 0.753 |
| AUC of G-CSF | −0.176 | 0.530 |
| AUC of GM-CSF | −0.138 | 0.623 |
| AUC of IFN-γ | 0.233 | 0.404 |
| AUC of IL-10 | 0.005 | 0.987 |
| AUC of IL-12p40 | 0.027 | 0.923 |
| AUC of IL-12p70 | 0.438 | 0.103 |
| AUC of IL-15 | 0.063 | 0.824 |
| AUC of IL-17 | 0.153 | 0.587 |
| AUC of IL-1Rα | −0.161 | 0.565 |
| AUC of IL-6 | −0.095 | 0.738 |
| AUC of IL-7 | 0.348 | 0.204 |
| AUC of IL-8 | −0.080 | 0.777 |
| AUC of IP-10 | 0.470 | 0.077 |
| AUC of MCP-1 | −0.202 | 0.471 |
| AUC of MIP-1β | 0.190 | 0.498 |
| AUC of VEGF | 0.251 | 0.368 |
The area under the curve (AUC) was calculated by the area under the concentration time curves. EGF, epidermal growth factor; IP-10, interferon inducible protein 10 [also known as chemokine (C-X-C motif) ligand 10; CXCL10].
DISCUSSION
The HMGB1 protein was initially found to be a DNA-binding protein that is present in almost all eukaryotic cells to stabilize nucleosome formation and acts as a nuclear factor that enhances gene transcription (25,31,32). HMGB1 plays a crucial role in response to tissue damage, indicating that HMGB1 is a prototype of the emerging damage-associated molecular pattern molecule (16,31,32,36).
Previously, we demonstrated that HMGB1 was highly expressed in islets, and damaged islets released HMGB1. Furthermore, the released HMGB1 stimulated dendritic cells (DCs) through the Toll-like receptor (TLR)-2 or RAGE to produce IL-12. Then NK T-cells were stimulated by IL-12, and the activated NK T-cells stimulated neutrophils to produce inflammatory cytokines in the liver receiving syngeneic islets in a murine model (23). The importance of HMGB1 in islet biology was shown by the inhibition of HMGB1, using treatment with neutralizing antibody or using TLR-2−/− or RAGE−/− mice as recipients, which prevented early islet graft loss and ameliorated receipt of a marginal number of syngeneic islets for streptozotocin-induced diabetic recipient mice (23). In addition, we demonstrated that damaged human islets also released HMGB1 in vitro (11,12). Based on this background, we hypothesized that serum HMGB1 levels would increase after AIT and that increasing levels of HMGB1 would be inversely correlated with the outcomes of AIT.
This is the first study to measure serum HMGB1 levels in clinical islet transplantation and describe its variation in AIT, which is a clinical situation without the confounding factors of autoimmunity, alloimmunity, and immunosuppression. We found two distinct elevations of HMGB1 in AIT patients. The maximum values of serum HMGB1 in the 15 AIT patients were 5.9 to 22.2 ng/ml, which are within the range of reported values for sepsis (36), stroke (24), and acute graft-versus-host disease (39). Only the first elevation coincided with C-peptide and pro-insulin elevations. Interestingly, the serum HMGB1 level was also elevated in the patient who received approximately 100% pure islets. The present study and our previous studies report that damaged islets release HMGB1 in mice and humans in vitro (10–12,23). Supportively, islet graft function evident by SUITO index 6 months or later after AIT was negatively correlated to early phase of ΔHMGB1 with marginally statistical significance, suggesting that HMBG1 can be a marker for islet damage in early engraftment. Taken collectively, the results suggest that the first peak of the serum HMGB1 represents a release mainly from damaged transplanted islets (not from exocrine tissue); however, the second elevation does not. HMGB1 is also known to be secreted by activated immune cells, including macrophages (9,36), dendritic cells (7), and natural killer cells (30). Therefore, the second elevation of HMGB1 may be related to release from activated immune cells in recipients.
The kinetics of HMGB1 has been studied in other surgical inflammatory conditions. Suda et al. (34) reported that serum HMGB1 levels in patients who received esophagectomy without complications at 1 day after surgery were between 0.0 and 6.0 ng/ml. Kohno et al. (13) reported that serum HMGB1 levels in patients who received thoracic aortic aneurysm repair were 5.4 ± 5.7 ng/ml immediately after the operation. These studies show that serum HMGB1 levels increase following major surgeries. Therefore, we compared the ΔHMGB1 levels between the AIT and the Px groups to examine whether elevation of HMGB1 was due mainly to the islet infusion or the surgery. The ΔHMGB1 was significantly higher in the AIT group compared to the Px group, suggesting that elevation of HMGB1 was mainly due to the islet infusion.
TLR-2, -4, -9, and RAGE are thought to be the receptors for HMGB1 (16). Soluble RAGE consists of several forms, including endogenous secretory RAGE (esRAGE), which is a spliced variant of RAGE, and a shedded form derived from cell-surface RAGE (sRAGE) (14,32). The esRAGE is thought to be the endogenous decoy receptor for the RAGE ligands, including HMGB1 (14,17,29,32,38). In the present study, we demonstrated that serum sRAGE levels were elevated during islet infusions (admission level, 1.1 ± 0.1 ng/ml; peak level, 2.6 ± 0.4 ng/ml) within a range reported for healthy persons and for intensive care unit patients with or without diabetes (2). It is known that RAGE is expressed at low levels in normal tissues but becomes upregulated at sites where its ligands accumulate (5). Our results suggest that the increased HMGB1 in the serum was functional for activating the RAGE pathway as RAGE ligands, and then sRAGE was upregulated.
sRAGE is widely considered to be an endogenous antagonist of RAGE activation, as recombinant sRAGE can prevent diabetic complications in experimental models (4,33). An inverse association between circulating concentrations of sRAGE and surrogate measures of atherosclerotic burden has also been described in cross-sectional studies in nondiabetic patients (8) and general population samples (15). A similar phenomenon was observed in the present study. A high ΔsRAGE was associated with insulin independence after AIT. In addition, we reported that RAGE-deficient streptozotocin-induced diabetic recipient mice were ameliorated after receiving a marginal number of syngeneic islets (23). Taken collectively, the present study suggests that RAGE is involved in the pathway of early islet graft loss in the AIT patients. Moreover, we found that sRAGE was not released from islets; therefore, it seems to be released from recipients. However, the islets in the in vitro model in the present study were isolated from healthy brain-dead donors, possibly leading to a different mechanism from islets in chronic pancreatitis patients. The cellular source and precise roles of sRAGE in AIT remain unclear.
At the conclusion of this study, we analyzed the correlation of the transplant outcomes and serum HMGB1 levels. Interestingly, we found significant differences in the ΔHMGB1 and ΔsRAGE between the insulin-dependent and the insulin-free groups even though the patient characteristics, the number of transplanted islets, and islet purity and viability were not significantly different between these two groups. Most of the insulin-free population had low ΔHMGB1 and high ΔsRAGE. Furthermore, the AUC of WBCs, ΔEGF, AUC of eotaxin, and ΔIP-10 were significantly associated with ΔHMGB1 in univariate analysis, and AUC of eotaxin and ΔEGF were in multivariate analysis. These results suggest that elevated HMGB1 from transplanted islets activates the inflammatory responses that result in poor clinical outcomes.
There were significant differences in ΔHMGB1 and ΔsRAGE between insulin-dependent and insulin-free groups for patients with less than 6.0% of preoperative HbA1c, although no differences were seen for patients with over 6.0% of pre-HbA1c. This finding suggests the pretransplant factors such as progressive inflammation in pancreas rather than islet damage triggered by ΔHMGB1 in early engraftment may have impact on clinical outcomes for the patients with deteriorating glycemic control. The present study includes a small cohort, which can cause insufficient power to detect statistical significance. We are continuing to measure HMGB1, sRAGE, and the other cytokines/chemokines to clarify islet damage in early engraftment and believe that the findings in the present study with autologous islet transplant can be applied to the improvement of allogeneic transplantation for type 1 diabetes.
In summary, serum HMGB1 and sRAGE were elevated after AIT. HMGB1 seemed to be released by damaged transplanted islets. Therefore, the serum HMGB1 levels might be one of the useful markers for detecting islet damage in clinical situations. Furthermore, a low ΔHMGB1 and a high ΔsRAGE were associated with insulin independence after AIT. These results suggest that HMGB1 might be a trigger of early inflammatory reactions and might cause graft loss in AIT patients. Also, inhibition of RAGE pathway by sRAGE also could be a treatment target for the improvement of islet engraftment in AIT. Therefore, anti-HMGB1 therapy including administration of sRAGE might be a candidate for further improving the outcomes of clinical AIT.
Acknowledgments
While we fully acknowledge Dr. Matsumoto’s contributions and tremendous expertise in this field, and while we in no way want to diminish his accomplishments, this work was conceived, executed, and disseminated by the Baylor Research Institute, and any further communication about this work should be directed to the Institute and its current members and not past members. This study was partially supported by grants from the National Institute of Diabetes and Digestive and Kidney Disease (1R21DK090513-02 to M.F.L.) and the Juvenile Diabetes Research Foundation (5-2011-372 to B.N. and 3-2011-447 to M.T.). The authors thank Ms. Sonnya Coultrup and Ms. Anne-Marie Brün for their technical support, the George and Fay Young Foundation for the financial support, and Ms. Cynthia Orticio for the professional editing.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Ahmad SA, Lowy AM, Wray CJ, D’Alessio D, Choe KA, James LE, Gelrud A, Matthews JB, Rilo HL. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 2.Arabi YM, Dehbi M, Rishu AH, Baturcam E, Kahoul SH, Brits RJ, Naidu B, Bouchama A. sRAGE in diabetic and non-diabetic critically ill patients: Effects of intensive insulin therapy. Crit Care. 2011;15:R203. doi: 10.1186/cc10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184–1197. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 4.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835. doi: 10.1161/01.cir.0000039325.03698.36. [DOI] [PubMed] [Google Scholar]
- 5.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect. 2004;6:1219–1225. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Clayton HA, Davies JE, Pollard CA, White SA, Musto PP, Dennison AR. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: The first 40 patients at the Leicester general hospital. Transplantation. 2003;76:92–98. doi: 10.1097/01.TP.0000054618.03927.70. [DOI] [PubMed] [Google Scholar]
- 7.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 8.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032–1037. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 9.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh T, Iwahashi S, Shimoda M, Chujo D, Takita M, Sorelle JA, Naziruddin B, Levy MF, Matsumoto S. High-mobility group box 1 expressions in hypoxia-induced damaged mouse islets. Transplant Proc. 2011;43:3156–3160. doi: 10.1016/j.transproceed.2011.09.100. [DOI] [PubMed] [Google Scholar]
- 11.Itoh T, Sugimoto K, Takita M, Shimoda M, Chujo D, SoRelle AJ, Naziruddin B, Levy MF, Matsumoto S. Low temperature condition prevent hypoxia-induced islet cell damage and HMGB1 release in a mouse model. Cell Transplant. 2012;21(7):1361–1370. doi: 10.3727/096368912X637514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itoh T, Takita M, SoRelle AJ, Shimoda M, Sugimoto K, Chujo D, Qin H, Naziruddin B, Levy MF, Matsumoto S. Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice. Cell Transplant. 2012;21(7):1371–1381. doi: 10.3727/096368912X640592. [DOI] [PubMed] [Google Scholar]
- 13.Kohno T, Anzai T, Shimizu H, Kaneko H, Sugano Y, Yamada S, Yoshikawa T, Ishizaka A, Yozu R, Ogawa S. Impact of serum high-mobility group box 1 protein elevation on oxygenation impairment after thoracic aortic aneurysm repair. Heart Vessels. 2011;26:306–312. doi: 10.1007/s00380-010-0056-6. [DOI] [PubMed] [Google Scholar]
- 14.Koyama H, Yamamoto H, Nishizawa Y. RAGE and soluble RAGE: Potential therapeutic targets for cardiovascular diseases. Mol Med. 2007;13:625–635. doi: 10.2119/2007-00087.Koyama. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsey JB, de Lemos JA, Cipollone F, Ayers CR, Rohatgi A, Morrow DA, Khera A, McGuire DK. Association between circulating soluble receptor for advanced glycation end products and atherosclerosis: Observations from the Dallas Heart Study. Diabetes Care. 2009;32:1218–1220. doi: 10.2337/dc09-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 17.Malherbe P, Richards JG, Gaillard H, Thompson A, Diener C, Schuler A, Huber G. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res. 1999;71:159–170. doi: 10.1016/s0169-328x(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S. Autologous islet cell transplantation to prevent surgical diabetes. J Diabetes. 2011;3:328–336. doi: 10.1111/j.1753-0407.2011.00128.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto S, Noguchi H, Naziruddin B, Onaca N, Jackson A, Nobuyo H, Okitsu T, Kobayashi N, Klintmalm G, Levy MF. Improvement of pancreatic islet cell isolation for transplantation. Proc Bayl Univ Med Cent. 2007;20:357–362. doi: 10.1080/08998280.2007.11928323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto S, Noguichi H, Shimoda M, Ikemoto T, Naziruddin B, Jackson A, Tamura Y, Olson G, Fujita Y, Chujo D, Takita M, Kobayashi N, Onaca N, Levy MF. Seven consecutive successful clinical islet isolations with pancreatic ductal injection. Cell Transplant. 2010;19:291–297. doi: 10.3727/096368909X481773. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto S, Okitsu T, Iwanaga Y, Noguchi H, Nagata H, Yonekawa Y, Yamada Y, Fukuda K, Shibata T, Kasai Y, Maekawa T, Wada H, Nakamura T, Tanaka K. Successful islet transplantation from nonheart beating donor pancreata using modified Ricordi islet isolation method. Transplantation. 2006;82:460–465. doi: 10.1097/01.tp.0000231710.37981.64. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto S, Rigley TH, Qualley SA, Kuroda Y, Reems JA, Stevens RB. Efficacy of the oxygen-charged static two-layer method for short-term pancreas preservation and islet isolation from nonhuman primate and human pancreata. Cell Transplant. 2002;11:769–777. [PubMed] [Google Scholar]
- 23.Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, Taniguchi M, Yasunami Y. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120:735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- 26.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 27.Robertson RP, Lanz KJ, Sutherland DE, Kendall DM. Prevention of diabetes for up to 13 years by autoislet transplantation after pancreatectomy for chronic pancreatitis. Diabetes. 2001;50:47–50. doi: 10.2337/diabetes.50.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Saldeen J. Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology. 2000;141:2003–2010. doi: 10.1210/endo.141.6.7523. [DOI] [PubMed] [Google Scholar]
- 29.Schlueter C, Hauke S, Flohr AM, Rogalla P, Bullerdiek J. Tissue-specific expression patterns of the RAGE receptor and its soluble forms—A result of regulated alternative splicing? Biochim Biophys Acta. 2003;1630:1–6. doi: 10.1016/j.bbaexp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 31.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 32.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 33.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Abraham E, Kitajima M, Ishizaka A. Serum concentrations of high-mobility group box chromosomal protein 1 before and after exposure to the surgical stress of thoracic esophagectomy: A predictor of clinical course after surgery? Dis Esophagus. 2006;19:5–9. doi: 10.1111/j.1442-2050.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet auto-transplantation in humans: Functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47:324–330. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 37.Yamada S, Maruyama I. HMGB1, a novel inflammatory cytokine. Clin Chim Acta. 2007;375:36–42. doi: 10.1016/j.cca.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yujiri T, Tagami K, Tanaka Y, Mitani N, Nakamura Y, Ariyoshi K, Ando T, Tanizawa Y. Increased serum levels of high-mobility group box 1 protein in patients who developed acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2010;85:366–367. doi: 10.1111/j.1600-0609.2010.01507.x. [DOI] [PubMed] [Google Scholar]