Abstract
The ability of the human immune system to repel infections is drastically diminished with age. Elderly individuals are more susceptible to new threats and are less able to control endogenous infections. The thymus, which is the sole source of new T cells, has been proposed as a target for regenerative efforts to improve immune competence, as thymic activity is dramatically reduced after puberty. In this review, we review the role of the thymus in the maintenance of T cell homeostasis throughout life and contrast the differences in mice and humans. We propose that in humans, lack of thymic T cell generation does not explain a decline in T cell receptor diversity nor would thymic rejuvenation restore diversity. Initial studies using next generation sequencing are beginning to establish lower boundaries of T cell receptor diversity. With increasing sequencing depth and the development of new statistical models, we are now in the position to test this model and to assess the impact of age on T cell diversity and clonality.
Keywords: Aging, T cells, Thymus, Immunosenescence, T cell receptor repertoire
Highlights
-
•
Mechanisms of T cell homeostasis with age are different in mouse and men.
-
•
T cell generation in the human adult is derived from peripheral T cell proliferation.
-
•
Peripheral fitness selection during proliferation causes unevenness in the repertoire.
-
•
Thymus rejuvenation may restore lymphopenia but not repertoire evenness.
-
•
Next generation sequencing will provide information on repertoire diversity with age.
1. Introduction
A decline in regenerative capacity is one of the hallmarks of aging. Cells from the immune system are not exempted from this general failure. However, aging of the immune system does not appear to be a random process; different lineages are affected in distinct ways. Not only does the pool of hematopoietic stem cells decline, the remaining stem cells in circulation may have already acquired lineage commitment and therefore are not fully pluripotent. The partial differentiation results in the preferential production of myeloid cells at the expense of the lymphoid lineages (Beerman et al., 2010, Pang et al., 2011, Wang et al., 2012). As a consequence, the ability to generate new B cells declines with age (Guerrettaz et al., 2008, Kogut et al., 2012). The story is even more complicated for T cells, where thymic activity develops into a true bottleneck. It is currently unclear whether a decline in thymocyte precursor cells contributes to thymic dysfunction. However, the major culprit appears to be the thymic epithelial cells which degenerate (Taub and Longo, 2005). Thymic involution is a complicated process that begins early in life and is clearly evident after puberty. Although linked to aging, it cannot be considered a typical age-associated degenerative process (Linton and Dorshkind, 2004). However, thymic involution has obvious implications for T cell homeostasis and has therefore been alleged to contribute to the immunosenescent features that compromise the adaptive immune system in the elderly. In support of this notion, thymectomy during early childhood caused changes in T cell population similar to those in the elderly, such as reduced CD4 and CD8 T cell numbers, in particular of naïve T cells (Halnon et al., 2005, Prelog et al., 2009, Sauce et al., 2009, van Gent et al., 2011, Zlamy and Prelog, 2009). Perturbations in the T cell repertoire were particularly evident in the subgroup of individuals who acquired an infection with cytomegalovirus (Sauce et al., 2009).
With progressive age, the ability to mount an adaptive response clearly declines (Weng, 2006). This is most evident from the impaired and insufficiently protective generation of immune memory after vaccination (Gagliardi et al., 2012, Jefferson et al., 2005, Levin, 2012, Nichol et al., 2007). In addition, the ability to control endogenous infections such as herpes zoster declines (Levin, 2012). Elderly individuals also have increased susceptibility to viral infections, particularly newly arising infectious organisms such as the virus causing severe acute respiratory syndrome or West Nile fever cause increased morbidity and mortality (Jean et al., 2007, Nikolich-Zugich et al., 2012, Peiris et al., 2003). The current prevailing paradigm proposes that the shrinkage of compartment size and diminution in T and B cell receptor diversity, due to defective generation of T cells and B cells, eventually compromises the ability of the immune system to respond to a universe of antigens.
2. Thymic function in the adult
About three decades ago, Steinmann et al. published the paper on the histological changes of the thymus with aging and described a steady loss in thymic epithelial space after puberty (Steinmann et al., 1985). While it is an unequivocal that thymic function declines with age, it has been debated how much and for how long some residual activity can be found. Several studies have described islands of active epithelial thymic tissue in the aged thymus, but the overall thymic architecture is not maintained and it is unclear whether these small islands are of any functional importance (Flores et al., 1999, Jamieson et al., 1999, Taub and Longo, 2005). This question is important in two settings: first, does the aging thymus contribute to the naïve T cell repertoire under steady state condition? Second, does the aging thymus have renewal capacity to rebuild a T cell repertoire in situations where the existing repertoire has been wiped out and the compartment has at least been partially emptied, such as with chemotherapy or bone marrow transplantation. T cell receptor excision circles (TRECs), byproduct of T cell receptor alpha chain rearrangements, are frequently used to assess the frequency of recent thymic emigrants. Although several studies have shown that these TRECs exponentially decline with age, some low level of TRECs can still be detected even in the very elderly (Douek et al., 1998, Naylor et al., 2005, Poulin et al., 1999). Mathematical modeling has suggested that the decline is fully explained by partial cell loss and compensation by homeostatic proliferation, and thymic T cell generation does not have to be entered into the model to explain the persistence of TRECs (Hazenberg et al., 2003). TREC frequencies are therefore consistent with the model that thymic T cell production in the adult is quantitatively irrelevant. Conversely, while T cell reconstitution after intensive chemotherapy is incomplete even in young adults (Mackall et al., 1995), several studies have emphasized renewed thymic activity. However, in most studies, thymic activity could be demonstrated only in a diminishing subset of individuals older than 40 to 50 years (Castermans et al., 2011, Hakim et al., 2005). We have come to similar conclusions from studies of patients with rheumatoid arthritis who were treated with the anti-CD52 antibody Campath 1H and therefore did not receive cytotoxic agents that could have diminished thymic epithelial function (Jendro et al., 1995). These patients, when older than 50 years were not able to rebuild a diverse repertoire. Thymic activity can be augmented by cytokine treatment. Treatment of lymphopenic patients with IL-7 showed increased homeostatic expansion of naïve CD4 and CD8 T cells and generation of new T cells. The observed increase of T cell receptor repertoire diversity was likely due to a combination of thymic activity and peripheral naïve T cell expansion (Sportes et al., 2008).
3. T cell homeostasis — mice versus men
Most initial studies on T cell homeostasis have relied on the mouse model, where some degree of thymic activity is needed to maintain a naïve T cell repertoire and a severe contraction in size and diversity is observed with age (Linton and Dorshkind, 2004). Thymectomy in neonatal mice seriously impairs the development of the immune system; most mice die within four months from progressive wasting and diarrhea (Metcalf, 1960, Parrott et al., 1966). Thymectomy in adult mice reduced the population of lymphocytes. Rejection of skin transplants in these mice was still intact, but these studies were done only few months after thymectomy at which the repertoire may not have been sufficiently contracted (Miller, 1965). Indeed, holes in the repertoire have been identified in aged mice and implicated in defective T cell responses toward the influenza virus (Yager et al., 2008).
Already early studies by Mackall and Gress emphasized differences in T cell homeostasis of mice and men (Mackall and Gress, 1997). More recent data modeling T cell subset numbers and TREC frequencies support the conclusion that the T cell kinetics in humans are fundamentally different and that results from mouse models cannot be easily extrapolated to humans (den Braber et al., 2012). In humans, T cell homeostasis depends on the renewal capacity of the existing pool of naïve CD4 and CD8 T cells (Fig. 1 ). As a consequence of turnover, TREC concentrations decline with age in humans while staying constant in the mouse. For human CD4 naïve T cells, this system is very efficient in maintaining a sizable naïve compartment. However, there is a larger loss in naïve CD8 T cells, for which the reason is not known (Czesnikiewicz-Guzik et al., 2008). Since T cell homeostatic proliferation occurs in tissue niches driven by the recognition of self-antigen and homeostatic cytokines, it is possible that this niche effect is different for CD4 and CD8 T cells. It is far less likely that imbalanced thymic production accounts for the larger depletion of naïve CD8 T cells.
Fig. 1.
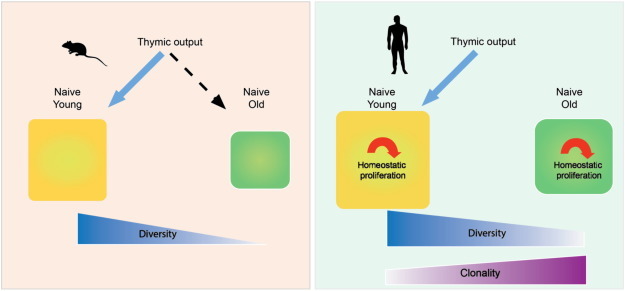
Mechanisms regulating the T cell repertoire with age.
The schematic diagram contrasts the effect of age on the naïve T cell repertoire in mice and humans. In mice, the repertoire is dependent on thymic influx throughout life, and decreasing thymic activity leads to a decreased size of the naïve compartment with contracted diversity. In contrast, the human repertoire is maintained through homeostatic proliferation of existing T cells and only modestly contracts. However, uneven proliferation and peripheral fitness selection can cause increasing clonality.
4. In silico simulation of the effect of thymic loss on T cell diversity
In addition to compartment size, T cell homeostasis is associated with maintaining a high diversity of T cell receptors expressed by naïve CD4 and CD8 T cells. Since T cell receptor rearrangement in the thymus is the only means to generate diversity, the ability of homeostatic proliferation to maintain the established diversity is of critical importance for immune function in the elderly. To address this question, we have used a stochastic agent-based in silico simulation of T cell homeostasis during aging (Johnson et al., 2012). We accounted for the carrying capacity in the number of cells, the relative division rate per cell per day, the death rate per cell per day, the initial clonal size in terms of number of cells, the initial thymic influx per day and the thymic recombination bias. Our simulations estimated the total number of different T cell receptor β chains in the naïve T cell compartment and the Simpson diversity index. In this model, homeostatic proliferation was highly efficient; a diverse repertoire was maintained for 50 + years after the thymic production of new T cells and the influx of new T cell receptors had completely ceased. Even if the compartment size was contracted by 50 or 95%, i.e., modeling an extreme shrinkage with age, the contraction in diversity was minimal. A repertoire contraction was only observed in cases of uneven homeostatic proliferation and peripheral fitness selection. The contraction could be severe and occur within a short time window, when a clonal progeny acquires cumulative changes in growth behavior. The associated clonal expansion within the naïve compartment could eventually lead to a severe compression in diversity.
5. Current estimates of repertoire diversity and their limitations
To assess the validity of these predictions, strategies have to be developed to sequence the T cell receptor repertoire in sufficient depth in strictly purified naive CD4 and CD8 T cells. Initial estimates by Arstila and colleagues were based on sequencing only a few hundred T cell receptor β chains sharing one particular VB–JB combination; their estimates were in the order of about 1 million different TCRB genes (Arstila et al., 1999). Recently, next-generation sequencing by the Robins' and Warren's group established a lower boundary of 3 to 4 million TCRB genes (Robins et al., 2009, Warren et al., 2011). These studies tried to extrapolate from a small sample of a few million cells to the global T cell compartment that may encompass up to 1012 T cells. It is unclear at this time whether the model used to do this extrapolation is valid or whether it underestimates infrequent events and the true repertoire is more diverse. Sufficient sequencing depth in these models is of particular importance when clonal expansions are frequent, otherwise, diversity will be severely underestimated. Clonal expansions may represent contaminating memory cells that masquerade as naïve T cells. Alternatively, the expansion may occur within the naïve compartment as a result of uneven homeostatic proliferation. In our previous studies, we had not seen a difference in TCRB gene diversity in naive CD4 T cells between the age of 20 and 65 years, while TREC frequencies declined. This observation is consistent with our in silico modeling that thymic function is not needed to maintain diversity in humans (Johnson et al., 2012, Naylor et al., 2005). We had, however, noted the sudden emergence of increased clonality after the age of 70 years. In parallel, the number of T cells proliferating in vivo under steady state conditions increased. In these initial studies, many of the expanded T cell clones were also found in the memory compartment, suggesting that clonal expansions of memory T cells assume a naïve phenotype and are therefore presumably able to compete for the resources in the niches that sustain naïve cells. Since these studies relied on CDR3 hybridization experiments with a small number of sequences, sequencing depth was not sufficient to detect rare TCR sequences. Therefore, we were not able to assess whether these clonal expansions have a negative impact on overall diversity. With increasing sequencing depth using next generation sequencing, we have also identified clonal expansions in the naïve compartment that were not detectable in the memory compartment (unpublished observation).
6. Future perspectives
Next-generation sequencing now provides the tools to sequence the T cell receptor repertoire in great depth and address the question of how diverse the repertoire truly is and whether thymic function is necessary to maintain sufficient diversity to recognize the universe of potential antigens. The challenge in these studies is not the technical component of next-generation sequencing but rather the statistical design and analysis. Rigorous statistical analysis will be required to properly assess infrequent events and to extrapolate the data from relatively small sampling to the entire T cell compartment, particularly if the repertoire of naïve T cells also contains clonally expanded cells. We predict that thymic involution alone is not sufficient to lead to repertoire contraction. However, advancing age may be associated with uneven proliferation due to peripheral fitness selection, leading to clonal expansions of naïve CD4 and CD8 T cells (Fig. 2 ). If this selection is excessive in either number or size, repertoire diversity would be impacted. Since these clones have been selected for fitness and survival, they would out-compete any new thymic immigrants, and thymic rejuvenation would not be able to restore diversity. A similar effect could be seen with clonal expansions induced by the recognition of exogenous antigens if these clones assume a semi-naïve phenotype and compete for the same tissue niche (Johnson et al., 2014). This may be of particular relevance for cytomegalovirus infection, where memory inflation and phenotypic conversion in the CD8 compartment are frequent (O'Hara et al., 2012). Interestingly, latent infection with murine cytomegalovirus has been shown to induce similar changes in T cell subset composition in mice as observed in humans. More importantly, latent infection was associated with a defective naïve CD8 T cells to unrelated viruses (Cicin-Sain et al., 2012, Smithey et al., 2012). The finding of clonal expansion of naïve T cells may also provide an explanation for the increased incidence of some autoimmune diseases in the elderly (Goronzy and Weyand, 2001, Goronzy and Weyand, 2012): T cells clonally expanded in the naïve compartment are selected on self-antigens. Therefore, these clonally expanded autoreactive cells could be the underlying mechanism for the frequent asymptomatic autoantibody production in otherwise healthy elderly, and these cells may even cause symptomatic autoimmune disease in some elderly patients (Goronzy and Weyand, 2005, Goronzy et al., 2010).
Fig. 2.
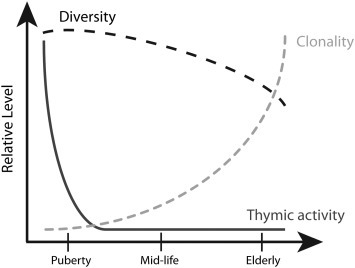
The temporal relationship between thymic involution, T cell repertoire contraction and T cell clonal expansion in humans.
The graph illustrates the model that repertoire contraction is temporally unrelated to thymic involution. Thymic activity rapidly declines in early life and is minimal throughout adult life under steady state conditions. The T cell receptor repertoire remains highly diverse independent of thymic activity, and diversity only shows moderate contraction, which may be accelerated in the elderly. One of the hallmarks of aging is the emergences of increasing clonal expansions within the naïve repertoire.
Competing financial interests
The authors declare no competing financial interests.
Acknowledgments
This work was supported by National Institutes of Health grants U19 AI090019, R01 AG015043, U19 AI057266 (JJG), and R01 AI044142, R01 AR042527 and P01 HL058000 (CMW). Q.Q. is supported by an AFAR Postdoctoral Fellowship in Aging Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Section Editor: Daniela Frasca
References
- Arstila T.P., Casrouge A., Baron V., Even J., Kanellopoulos J., Kourilsky P. A direct estimate of the human alpha beta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., Rossi D.J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castermans E., Hannon M., Dutrieux J., Humblet-Baron S., Seidel L., Cheynier R., Willems E., Gothot A., Vanbellinghen J.F., Geenen V., Sandmaier B.M., Storb R., Beguin Y., Baron F. Thymic recovery after allogeneic hematopoietic cell transplantation with non-myeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96:298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicin-Sain L., Brien J.D., Uhrlaub J.L., Drabig A., Marandu T.F., Nikolich-Zugich J. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8:e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M., Lee W.W., Cui D., Hiruma Y., Lamar D.L., Yang Z.Z., Ouslander J.G., Weyand C.M., Goronzy J.J. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braber I., Mugwagwa T., Vrisekoop N., Westera L., Mogling R., de Boer A.B., Willems N., Schrijver E.H., Spierenburg G., Gaiser K., Mul E., Otto S.A., Ruiter A.F., Ackermans M.T., Miedema F., Borghans J.A., de Boer R.J., Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Douek D.C., McFarland R.D., Keiser P.H., Gage E.A., Massey J.M., Haynes B.F., Polis M.A., Haase A.T., Feinberg M.B., Sullivan J.L., Jamieson B.D., Zack J.A., Picker L.J., Koup R.A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Flores K.G., Li J., Sempowski G.D., Haynes B.F., Hale L.P. Analysis of the human thymic perivascular space during aging. J. Clin. Invest. 1999;104:1031–1039. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi A.M., Gomes Silva B.N., Torloni M.R., Soares B.G. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst. Rev. 2012;10:CD008858. doi: 10.1002/14651858.CD008858.pub2. [DOI] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001;22:251–255. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. Rheumatoid arthritis. Immunol. Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- Goronzy J.J., Weyand C.M. Immune aging and autoimmunity. Cell. Mol. Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J.J., Shao L., Weyand C.M. Immune aging and rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrettaz L.M., Johnson S.A., Cambier J.C. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim F.T., Memon S.A., Cepeda R., Jones E.C., Chow C.K., Kasten-Sportes C., Odom J., Vance B.A., Christensen B.L., Mackall C.L., Gress R.E. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Invest. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halnon N.J., Jamieson B., Plunkett M., Kitchen C.M., Pham T., Krogstad P. Thymic function and impaired maintenance of peripheral T cell populations in children with congenital heart disease and surgical thymectomy. Pediatr. Res. 2005;57:42–48. doi: 10.1203/01.PDR.0000147735.19342.DE. [DOI] [PubMed] [Google Scholar]
- Hazenberg M.D., Borghans J.A., de Boer R.J., Miedema F. Thymic output: a bad TREC record. Nat. Immunol. 2003;4:97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- Jamieson B.D., Douek D.C., Killian S., Hultin L.E., Scripture-Adams D.D., Giorgi J.V., Marelli D., Koup R.A., Zack J.A. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- Jean C.M., Honarmand S., Louie J.K., Glaser C.A. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg. Infect. Dis. 2007;13:1918–1920. doi: 10.3201/eid1312.061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson T., Rivetti D., Rivetti A., Rudin M., Di Pietrantonj C., Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- Jendro M.C., Ganten T., Matteson E.L., Weyand C.M., Goronzy J.J. Emergence of oligoclonal T cell populations following therapeutic T cell depletion in rheumatoid arthritis. Arthritis Rheum. 1995;38:1242–1251. doi: 10.1002/art.1780380912. [DOI] [PubMed] [Google Scholar]
- Johnson P.L., Yates A.J., Goronzy J.J., Antia R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc. Natl. Acad. Sci. U. S. A. 2012;109:21432–21437. doi: 10.1073/pnas.1209283110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.L., Goronzy J.J., Antia R. A population biological approach to understanding the maintenance and loss of the T cell repertoire during aging. Immunology. 2014 Jan 10 doi: 10.1111/imm.12244. [Epub ahead of print]. PMID: 24405293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut I., Scholz J.L., Cancro M.P., Cambier J.C. B cell maintenance and function in aging. Semin. Immunol. 2012;24:342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Levin M.J. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr. Opin. Immunol. 2012;24:494–500. doi: 10.1016/j.coi.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Linton P.J., Dorshkind K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Mackall C.L., Gress R.E. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol. Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Mackall C.L., Fleisher T.A., Brown M.R., Andrich M.P., Chen C.C., Feuerstein I.M., Horowitz M.E., Magrath I.T., Shad A.T., Steinberg S.M. Age, thymopoiesis, and CD4 + T-lymphocyte regeneration after intensive chemotherapy. N. Engl. J. Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The effect of thymectomy on the lymphoid tissues of the mouse. Br. J. Haematol. 1960;6:324–333. doi: 10.1111/j.1365-2141.1960.tb06248.x. [DOI] [PubMed] [Google Scholar]
- Miller J.F. Effect of thymectomy in adult mice on immunological responsiveness. Nature. 1965;208:1337–1338. doi: 10.1038/2081337a0. [DOI] [PubMed] [Google Scholar]
- Naylor K., Li G., Vallejo A.N., Lee W.W., Koetz K., Bryl E., Witkowski J., Fulbright J., Weyand C.M., Goronzy J.J. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Nichol K.L., Nordin J.D., Nelson D.B., Mullooly J.P., Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J., Li G., Uhrlaub J.L., Renkema K.R., Smithey M.J. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin. Immunol. 2012;24:356–364. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara G.A., Welten S.P., Klenerman P., Arens R. Memory T cell inflation: understanding cause and effect. Trends Immunol. 2012;33:84–90. doi: 10.1016/j.it.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Pang W.W., Price E.A., Sahoo D., Beerman I., Maloney W.J., Rossi D.J., Schrier S.L., Weissman I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott D.V., De Sousa M.A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J. Exp. Med. 1966;123:191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J.F., Viswanathan M.N., Harris J.M., Komanduri K.V., Wieder E., Ringuette N., Jenkins M., McCune J.M., Sekaly R.P. Direct evidence for thymic function in adult humans. J. Exp. Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelog M., Keller M., Geiger R., Brandstatter A., Wurzner R., Schweigmann U., Zlamy M., Zimmerhackl L.B., Grubeck-Loebenstein B. Thymectomy in early childhood: significant alterations of the CD4(+)CD45RA(+)CD62L(+) T cell compartment in later life. Clin. Immunol. 2009;130:123–132. doi: 10.1016/j.clim.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Robins H.S., Campregher P.V., Srivastava S.K., Wacher A., Turtle C.J., Kahsai O., Riddell S.R., Warren E.H., Carlson C.S. Comprehensive assessment of T-cell receptor beta-chain diversity in alpha beta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauce D., Larsen M., Fastenackels S., Duperrier A., Keller M., Grubeck-Loebenstein B., Ferrand C., Debre P., Sidi D., Appay V. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 2009;119:3070–3078. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithey M.J., Li G., Venturi V., Davenport M.P., Nikolich-Zugich J. Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J. Immunol. 2012;189:5356–5366. doi: 10.4049/jimmunol.1201867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportes C., Hakim F.T., Memon S.A., Zhang H., Chua K.S., Brown M.R., Fleisher T.A., Krumlauf M.C., Babb R.R., Chow C.K., Fry T.J., Engels J., Buffet R., Morre M., Amato R.J., Venzon D.J., Korngold R., Pecora A., Gress R.E., Mackall C.L. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann G.G., Klaus B., Muller-Hermelink H.K. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand. J. Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Taub D.D., Longo D.L. Insights into thymic aging and regeneration. Immunol. Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- van Gent R., Schadenberg A.W., Otto S.A., Nievelstein R.A., Sieswerda G.T., Haas F., Miedema F., Tesselaar K., Jansen N.J., Borghans J.A. Long-term restoration of the human T-cell compartment after thymectomy during infancy: a role for thymic regeneration? Blood. 2011;118:627–634. doi: 10.1182/blood-2011-03-341396. [DOI] [PubMed] [Google Scholar]
- Wang J., Sun Q., Morita Y., Jiang H., Gross A., Lechel A., Hildner K., Guachalla L.M., Gompf A., Hartmann D., Schambach A., Wuestefeld T., Dauch D., Schrezenmeier H., Hofmann W.K., Nakauchi H., Ju Z., Kestler H.A., Zender L., Rudolph K.L. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Warren R.L., Freeman J.D., Zeng T., Choe G., Munro S., Moore R., Webb J.R., Holt R.A. Exhaustive T-cell repertoire sequencing of human peripheral blood samples reveals signatures of antigen selection and a directly measured repertoire size of at least 1 million clonotypes. Genome Res. 2011;21:790–797. doi: 10.1101/gr.115428.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N.P. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager E.J., Ahmed M., Lanzer K., Randall T.D., Woodland D.L., Blackman M.A. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlamy M., Prelog M. Thymectomy in early childhood: a model for premature T cell immunosenescence? Rejuvenation Res. 2009;12:249–258. doi: 10.1089/rej.2009.0864. [DOI] [PubMed] [Google Scholar]


