Abstract
Background
The aim of this study was to prospectively examine the effects of hearing loss and posterior fossa syndrome (PFS), in addition to age at diagnosis and disease risk status, on change in intellectual and academic outcomes following diagnosis and treatment in a large sample of medulloblastoma patients.
Methods
Data from at least 2 cognitive and academic assessments were available from 165 patients (ages 3–21 years) treated with surgery, risk-adapted craniospinal irradiation, and 4 courses of chemotherapy with stem cell support. Patients underwent serial evaluation of cognitive and academic functioning from baseline up to 5 years post diagnosis.
Results
Serious hearing loss, PFS, younger age at diagnosis, and high-risk status were all significant risk factors for decline in intellectual and academic skills. Serious hearing loss and PFS independently predicted below-average estimated mean intellectual ability at 5 years post diagnosis. Patients with high-risk medulloblastoma and young age at diagnosis (<7 years) exhibited the largest drop in mean scores for intellectual and academic outcomes.
Conclusions
Despite a significant decline over time, intellectual and academic outcomes remained within the average range at 5 years post diagnosis for the majority of patients. Future studies should determine if scores remain within the average range at time points further out from treatment. Patients at heightened risk should be closely monitored and provided with recommendations for appropriate interventions.
Keywords: academic, cognitive, hearing, medulloblastoma, posterior fossa syndrome
Survivors of pediatric brain tumors are at risk for disease and treatment-related cognitive and academic declines.1,2 Medulloblastoma, the most common malignant pediatric brain tumor, commonly occurs in the posterior fossa region and is usually diagnosed before the age of 10 years.3,4 Five-year survival rates for patients with medulloblastoma are 70%–85% with a treatment protocol that includes surgical resection, craniospinal irradiation, and chemotherapy.5 Younger age at diagnosis and higher doses of radiation are associated with worse cognitive and academic outcomes.6–10 Thus, contemporary protocols designed to limit radiation exposure use risk-adapted radiation dosing and a reduced clinical target volume (CTV) for the boost irradiation to the primary tumor site. The current study presents results from one of the first clinical protocols to reduce the CTV to the tumor bed to 1.0 cm for all patients.
Clinical treatment-related events also have the potential to impact changes in cognitive and academic outcomes. Hearing loss can occur in patients treated with the platinum-based compound cisplatin because of its ototoxic effects, especially in children.11 Platinum-induced hearing loss is typically bilateral, symmetrical, permanent, and sensorineural in nature.12–14 Hearing loss is also a potential adverse outcome from cranial irradiation, which can damage any auditory structures from the external ear to the higher auditory pathways that are within the radiation fields. Thus, hearing loss can present as conductive, sensorineural, mixed, or retrocochlear in nature.15
Cisplatin is a platinum-based chemotherapy that is effective against medulloblastoma16 and is typically included as an adjuvant in protocol-based treatment that includes surgery and cranial irradiation. Patients receiving combined cranial irradiation and platinum-based chemotherapy experience greater hearing loss compared with patients receiving irradiation alone.17,18 Thus, medulloblastoma patients are at high risk for hearing loss. To date, no study has examined the longitudinal impact of hearing loss on changes in cognitive and academic outcomes in medulloblastoma patients.
Posterior fossa syndrome (PFS), considered a consequence of surgery in the posterior fossa region, occurs in up to 29% of medulloblastoma patients.19,20 PFS is variable in presentation but typically includes diminished speech or mutism and is often accompanied by ataxia, hypotonia, emotional lability, and other neurocognitive sequelae.21 Recently, PFS was associated with worse cognitive outcomes at 12-months post diagnosis in medulloblastoma patients.22 Few studies have examined the longitudinal impact of PFS on changes in cognitive and academic outcomes in medulloblastoma patients.
The present study aims to estimate changes in intellectual ability and academic outcomes (reading and math) during the 5 years following diagnosis of medulloblastoma. Hearing loss, PFS, age at diagnosis, and disease risk status (high vs average) were included in models that examined changes in cognition associated with treatment-related factors. We hypothesized that children with serious hearing loss (ie, loss serious enough to require hearing aids) would exhibit a steeper decline in intellectual and academic outcomes compared with children without serious hearing loss. Similarly, we postulated that children who developed PFS, compared with children who did not, would exhibit a steeper decline in intellectual and academic outcomes. Finally, as predicted by the literature, we expected that children who were younger at the time of diagnosis and who received more cranial radiation due to high-risk status would exhibit a steeper decline in intellectual and academic outcomes compared with patients older at diagnosis who received less radiation due to average-risk status.
Materials and Methods
Patient Population
Participants comprised 318 patients, aged 3–21 years at diagnosis, with histologically confirmed medulloblastoma who enrolled in an ongoing institutional review board-approved multisite clinical trial for patients newly diagnosed with an embryonal brain tumor. Participants provided informed consent at one of 9 participating institutions. All institutions followed the same protocol-driven medical treatment. After resection, participants were classified as having average-risk medulloblastoma (≤1.5 cm2 residual tumor and no metastatic disease) or high-risk medulloblastoma (>1.5 cm2 residual disease and/or metastatic disease localized to the neuraxis) according to a modified Chang staging system.23 Participants were treated with postsurgical, risk-adapted craniospinal irradiation (CSI) that was initiated within 31 days of surgery. Participants with high-risk disease status (HR) received CSI (M0–1, 36 Gy; M2–3, 36–39.6 Gy) and supplemental boost irradiation to the tumor bed using conformal treatment methods (total dose 55.8 Gy). When appropriate, local sites of metastasis received supplemental irradiation (total dose 50.4–54 Gy). Participants with average risk disease status (AR) received 23.4 Gy CSI and supplemental boost conformal irradiation to the tumor bed to 55.8 Gy. The CTV to the tumor bed was 1.0 cm for all participants. Following radiation therapy, at ∼12 weeks post-treatment initiation, all participants received 4 cycles of high-dose chemotherapy (cyclophosphamide, cisplatin, and vincristine) with stem cell support. Each cycle was 28 days in duration, and the cisplatin dose level was 75 mg/m2 to a cumulative prescribed dose of 300 mg/m2. Amifostine was used to reduce the ototoxic effect of therapy and was administered at a dosage of 600 mg/m2 in a 1 min intravenous infusion immediately preceding and again 3 hours into each of the 4 courses of cisplatin. Participants were followed medically every 3 months for 2 years and every 6 months after completion of treatment.
Serial audiometric evaluations were completed as standard of care in this protocol and typically consisted of evaluations at baseline (occurring within 2 weeks of initiation of radiation therapy), prior to each high-dose cisplatin chemotherapy cycle, and at regular intervals following diagnosis. The present study utilized available data from the most recent audiogram conducted, which was at ∼24-months post diagnosis for the majority of participants (mean [M ]= 23.26, SD = 8.57, median = 24, range 6–72 months post diagnosis).
Under protocol guidelines, all patients were to undergo serial assessments of cognitive and academic functioning spanning from baseline (after surgical resection and within 2 weeks of initiation of radiation therapy) up to 5 years following treatment. Of the 318 participants enrolled at the time of the current analyses, 55 were excluded due to having only one valid cognitive or academic assessment, and 98 were excluded because they had no cognitive or academic assessments. Reasons for having no cognitive or academic assessments included enrollment at a site that did not participate in the testing (n = 19), medical status restricting assessment or progressive disease (n = 32), lack of English fluency (n = 14), no informed consent (n = 11), scheduling conflicts (n = 7), data not received from site or assessment not scheduled (n = 4), patients/parents refused testing (n = 7), patient moved (n = 1), and patients ineligible (due to comorbid psychological issues, blindness, and mutism; n = 3). The current sample included 165 participants from 8 collaborative sites who provided data from at least 2 assessment time points over the course of study participation. Given the interest in modeling change over time, the current study did not require that all participants complete a baseline assessment. Due to the ongoing nature of this study, 52.12% of the participants aged through all 5 years of the protocol at the time of data analysis. The majority of participants (81.21%) aged through at least 3 years of the protocol. The representativeness of the study sample was examined and described below.
Cognitive and Academic Assessment
Assessments were conducted at baseline, 1, 3, and 5 years post diagnosis at all participating institutions. At St. Jude Children's Research Hospital, participants were evaluated at baseline and annually from the time of diagnosis. To be included in the current study, participants completed protocol-driven assessments of cognitive and academic functioning using the Woodcock-Johnson Tests of Cognitive Abilities Third Edition (WJIII-Cognitive Abilities)24 and the Woodcock Johnson Tests of Achievement Third Edition (WJIII Tests of Achievement)25 at 2 or more time points. These batteries were chosen because they could be administered to a broad age range (2 years through 90 years) and thus were suitable for the entire participant population. They were also available in an Australian-specific version. Age-adjusted standardized scores were used in the analyses and had a population mean of 100 and a standard deviation of 15. The WJIII-Cognitive Abilities and WJIII Tests of Achievement were typically administered on 2 separate but consecutive days. Collectively, the 165 participants completed 552 assessments of WJIII-Cognitive Abilities and 530 assessments of WJIII Tests of Achievement between zero and 5 years from diagnosis (median = 3 assessments per patient; range = 2–6). Of the participants who finished all 5 years of the protocol, 53 completed an evaluation at 5 years post diagnosis.
General Intellectual Ability
The WJIII-Cognitive Abilities, General Intellectual Ability (GIA) composite was used to assess overall intellectual ability. This is comparable to the full-scale IQ score, both of which provide a measure of broad intellect or “g.” The GIA score was obtained by administering the Standard Battery, which consists of the following 7 subtests: Verbal Comprehension, Visual-Auditory Learning, Spatial Relations, Sound Blending, Concept Formation, Visual Matching, and Numbers Reversed.
Academic Abilities
The WJIII Tests of Achievement, Broad Reading and Broad Math composites were used to assess reading and math ability, respectively. The WJIII Tests of Achievement have high-criterion validity correlations with several widely used tests of achievement.25 The Broad Reading composite was obtained by administering the following subtests to participants 5.10 years or older: Letter-Word Identification, Reading Fluency, and Passage Comprehension. The Broad Math composite was obtained by administering the following subtests to participants 5.6 years or older: Calculation, Math Fluency, and Applied Problems. Ten patients were too young to complete academic testing at baseline; however, these patients were evaluated and included in the analyses at subsequent time points.
Audiological Assessment
A variety of hearing evaluation methods were used to assess hearing depending on the participant's age, development, cognition, and cooperation. These included tympanometry, assessment of pure-tone air conduction thresholds and pure-tone bone conduction thresholds, auditory brainstem response, auditory steady-state response, and/or distortion-product otoacoustic emissions measurements. Audiometric data from all institutions were reviewed and assigned an ototoxicity grade by a St. Jude clinical research audiologist based on the Chang Ototoxicity Grading Scale (Table 1).26 A Chang grade ≥2b was considered serious hearing loss requiring the use of hearing aids. Thus, this score was used as a cutoff for considering those with serious hearing loss versus those without serious hearing loss.
Table 1.
Chang ototoxicity grading scale
| Grade | Sensorineural hearing threshold (dB HL) bone conduction or air conduction with normal tympanogram |
|---|---|
| 0 | ≤20 dB at 1, 2, and 4 kHz |
| 1a | ≥40 dB at any freq 6–12 kHz |
| 1b | >20 and <40 dB at 4 kHz |
| 2a | ≥40 dB at 4 kHz and above |
| 2b | >20 and <40 dB at any freq below 4 kHz |
| 3 | ≥40 dB at 2 or 3 kHz and above |
| 4 | ≥40 dB at 1 kHz and above |
Statistical Analysis
Descriptive analyses of demographic and clinical variables were used to characterize the sample. Linear mixed-effect models (LMM) using PROC MIXED procedure in SAS Release 9.2 (SAS Institute) were fitted to examine the effect of each factor on the average rate of change over time (slope) and the differences in estimates of slope between groups. LMM is a rigorous technique based on maximum likelihood and can accommodate variations in the number and timing of measurements provided by each participant. Given the interest in modeling change over time, all participants providing data from 2 or more assessment time points were included for analysis. Before any analyses were completed, extensive visual inspection and spline smoothing of the scatter plots were completed. A linear change was assumed in all models because no deviations from linearity were apparent. All analyses performed were 2-tailed, and a significance threshold alpha level of P = .05 was used.
Variables
Time from diagnosis (ie, years), serious hearing loss (yes/no), PFS (yes/no), age at diagnosis, and risk status (high risk [HR] vs average risk [AR]) were included as explanatory variables in separate LMMs. In addition, age at diagnosis was dichotomized at 7 years for comparison with previous studies (<7 vs ≥7).8 This is considered an important age for learning because a critical transition from learning to read to reading to learn occurs around this time (or during the third grade).27 If diagnosis and treatment disrupt the early focus on learning to read, we might expect patients to have more difficulty with subsequent academic achievement. The GIA composite score, Broad Reading composite score, and Broad Math composite score were all taken as outcomes in separate analyses.
First, simple models were employed to estimate average change over time (slope) for each outcome variable. Second, separate unadjusted models were fitted to examine the effects on slope by the treatment-related factors: hearing loss, PFS, age at diagnosis, and risk status. Finally, a model was used to investigate the effects of the age at diagnosis and risk status on slope simultaneously.
Results
Demographic and Clinical Comparisons
Demographic and clinical characteristics of participants are listed in Table 2. Of the participants included in this study, 21.82% experienced serious hearing loss, 19.39% had PFS, and most had AR (75.15%). Mean age at diagnosis was 9.88 years (SD = 4.0; median = 9.12). Comparisons between participants from the source population who were included versus participants who were excluded due to missing data indicated no statistical differences in sex, race, or percentage with serious hearing loss. However, the nonparticipants (n = 153) had a significantly lower mean age at diagnosis (M = 8.25; SD = 3.56) and comprised a higher percentage of patients with HR (35.29%) than the participants included in the study.
Table 2.
Characteristics of participants compared with nonparticipants
| Variable | Excluded (n = 153) | Included (n = 165) | P value |
|---|---|---|---|
| Hearing, % (n) | |||
| <2b | 83.69 (118) | 78.18 (129) | .22b |
| ≥2b | 16.31 (23) | 21.82 (36) | |
| PFS, % (n) | |||
| No | 71.90 (110) | 80.61 (133) | .07b |
| Yes | 28.10 (43) | 19.39 (32) | |
| Age at diagnosis, M (SD) | 8.25 (3.56) | 9.88 (4.04) | .00a |
| <7 years, % (n) | 41.83 (64) | 27.27 (45) | |
| ≥7 years, % (n) | 58.17 (89) | 72.72 (120) | |
| Risk, % (n) | |||
| Average | 64.71 (99) | 75.15 (124) | .04b |
| High | 35.29 (54) | 24.85 (41) | |
| Sex, % (n) | |||
| Female | 35.29 (54) | 37.58 (62) | .67b |
| Male | 64.71 (99) | 62.42 (103) | |
| Race, % (n) | |||
| Indigenous Australian | 0.00 (0) | 0.61 (1) | .41c |
| Asian | 6.54 (10) | 4.85 (8) | |
| Asian and white | 0.65 (1) | 0 (0) | |
| Black | 6.54 (10) | 8.48 (14) | |
| Black and white | 0.00 (0) | 1.21 (2) | |
| Multiple race (NOS) | 0.65 (1) | 0.00 (0) | |
| Other | 3.92 (6) | 4.24 (7) | |
| Pacific Islander | 1.31 (2) | 0.61 (1) | |
| Unknown | 4.58 (7) | 1.21 (2) | |
| White | 75.82 (116) | 78.79 (130) | |
Abbreviations: NOS, not otherwise specified.
a2-sample t test.
bPearson chi-square test.
cFisher's exact test.
Multivariate Modeling
Without taking into account risk factors, LMMs suggested statistically significant declines in reading (−0.67 points/y; P < .01; 95% CI, −1.10 to −0.23) and math ability (−0.99 points/y; P < .01; 95% CI, −1.45 to −0.53); GIA did not decline significantly (−0.35 points/y; P = .18; 95% CI, −0.86 to 0.16). The mean estimated outcomes at 5 years post diagnosis remained within the average range (standard scores = 90–110) for all outcomes. The main effect of sex was explored with no significant effect, and thus sex was removed from further modeling.
Clinical Risk Factors
Hearing Loss Status
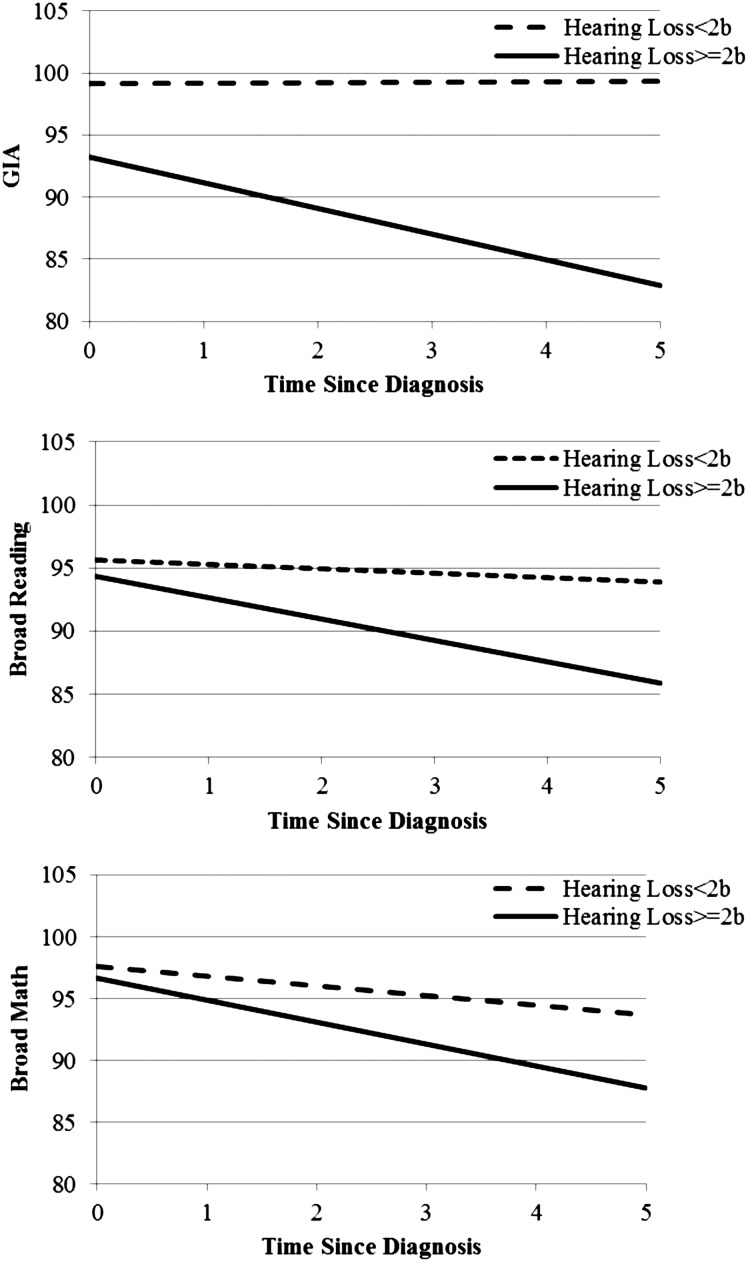
See Table 3 for model statistics. LMMs suggested that participants with serious hearing loss (≥2b on Chang scale) exhibited a significant decline in GIA (−2.07 points/y; P < .01), but individuals without serious hearing loss did not (0.04 points/y; P = .89). The 2 groups differed significantly (P < .01). Participants with serious hearing loss exhibited a significant decline in reading ability (−1.69 points/y; P < .01), but those without serious hearing loss did not (−0.35 points/y; P = .14). The 2 groups differed significantly (P = .01). Participants with and without serious hearing loss exhibited significant declines in math ability (−1.78 points/y; P < .01and −0.78 points/y; P < .01, respectively), but there was no significant difference between groups (P = .09). Fig. 1 indicates that participants with serious hearing loss had mean estimated GIA that dropped below average by 4 years post diagnosis.
Table 3.
Parameter estimates of models with hearing loss and posterior fossa syndrome
| Outcome | N (Obs) | Hearing Loss |
PFS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | P value | Effect | Estimate | SE | P value | ||
| GIA | 153 (552) | Intercept | 95.32 | 3.37 | <.01 | Intercept | 81.34 | 3.60 | <.01 |
| Hearing | 3.80 | 3.77 | .32 | PFS | 20.07 | 3.92 | <.01 | ||
| Time | −2.07 | 0.57 | <.01 | Time | −0.15 | 0.66 | .82 | ||
| Time*Hearing | 2.11 | 0.63 | <.01 | Time*PFS | −0.13 | 0.72 | .86 | ||
| Reading | 148 (530) | Intercept | 96.04 | 2.70 | <.01 | Intercept | 89.38 | 3.02 | <.01 |
| Hearing | −0.04 | 3.03 | .99 | PFS | 8.24 | 3.28 | .01 | ||
| Time | −1.69 | 0.47 | <.01 | Time | −1.75 | 0.56 | <.01 | ||
| Time*Hearing | 1.34 | 0.53 | .01 | Time*PFS | 1.34 | 0.61 | .03 | ||
| Math | 145 (528) | Intercept | 98.45 | 2.71 | <.01 | Intercept | 92.15 | 3.07 | <.01 |
| Hearing | −0.05 | 3.05 | .99 | PFS | 7.56 | 3.34 | .03 | ||
| Time | −1.78 | 0.52 | <.01 | Time | −1.21 | 0.60 | <.05 | ||
| Time*Hearing | 1.00 | 0.58 | .09 | Time*PFS | 0.29 | 0.65 | .66 | ||
Abbreviations: GIA, general intellectual ability; Hearing, serious hearing loss; N, number of patients; Obs, observations; SE, standard error; Time, time since diagnosis; PFS, posterior fossa syndrome.
Fig. 1.
Estimated intellectual and academic outcomes by hearing loss.
Given the findings for participants with serious hearing loss, additional analyses were run to determine if those with serious hearing loss were also more likely to have other identified risk factors. Fisher's exact test indicated no significant difference in risk status between participants with and without serious hearing loss. A 2-sample t test indicated no significant difference in age at diagnosis between participants with and without serious hearing loss.
Posterior Fossa Syndrome Status
See Table 3 for model statistics. LMMs suggested that participants with PFS exhibited a significant decline in reading ability (−1.75 points/y; P < .01), but participants without PFS did not (−0.41 points/y; P = .08). The 2 groups differed significantly (P = .03). No significant differences were detected between participants with and without PFS in change in GIA (P = .86) or math ability (P = .66). As indicated by the intercept estimates in Table 3, the estimated mean score for GIA was below average at baseline. However, only 33% of the participants with PFS provided assessment data at baseline due to medical status restricting participation in many cases.
Risk Status
LMMs suggested that HR participants exhibited a significant decline in GIA (−2.32 points/y; P < .01), whereas AR participants did not (0.19 points/y; P = .50); the groups differed significantly (P < .01). Similarly, HR participants exhibited a significant decline in reading ability (−1.48 points/y; P= ≤.01), whereas AR participants did not (−0.41 points/y; P = .10); the groups differed significantly (P = .04). HR and AR participants exhibited significant declines in math ability (−1.98 points/y; P= ≤.01 and −0.70 points/y; P = .01, respectively). HR participants demonstrated a significantly steeper decline (P = .02).
Age at Diagnosis
Model fitting suggested that age at diagnosis had a significant effect on the rate of decline in GIA (0.27 points/y; P < .01), reading (0.29 points/y; P < .01), and math abilities (0.19 points/y; P < .01). Next, age at diagnosis was dichotomized at 7 years (<7 vs ≥7). Results suggested participants <7 years at diagnosis exhibited a significant decline in GIA (−2.14 points/y; P < .01), but participants ≥7 years at diagnosis did not (0.52 points/y; P = .09), although they showed a trend toward increasing GIA. The groups differed significantly (P < .01). Similarly, participants <7 years at diagnosis exhibited a significant decline in reading ability (−2.63 points/y; P < .01), whereas participants ≥7 years at diagnosis did not (−0.20 points/y; P = .39). The groups differed significantly (P < .01). Participants <7 years and ≥7 years at diagnosis exhibited significant declines in math ability (−1.12 points/y; P = .04 and −0.96/points/y; P = .01, respectively). The group difference was not significant (P = .79).
Risk and Age at Diagnosis
LMMs were fitted to examine the effect on change by risk status and dichotomized age at diagnosis (<7 vs ≥7 years) simultaneously. Results were similar to those reported above. There was a significant difference between participants <7 versus ≥7 years at diagnosis in GIA (P < .01), and reading (P < .01) but not math ability, (P = .81). There was a significant difference between HR versus AR participants in GIA (P < .01), reading (P = .03), and math ability (P = .02). Table 4 provides the estimates of intercept and slopes.
Table 4.
Estimated intercepts and slopes of intellectual ability and academic outcomes by age and risk groups
| <7 years average risk (n = 58) |
<7 years high risk (n = 19) |
≥7 years average risk (n = 66) |
≥7 years high risk (n = 22) |
|||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Intercept+ (points) | Slope (points/y) | Intercept+ (points) | Slope (points/y) | Intercept+ (points) | Slope (points/y) | Intercept+ (points) | Slope (points/y) |
| GIA | 97.64 | −1.54** | 104.91 | −3.96** | 95.80 | 1.03** | 103.07 | −1.40** |
| Reading | 100.43 | −2.38** | 102.77 | −3.42** | 94.88 | 0.05 | 97.22 | −0.99* |
| Math | 97.80 | −0.81 | 99.75 | −2.08** | 98.02 | −0.67* | 99.97 | −1.94** |
Abbreviations: GIA, general intellectual ability; n, number of patients.
Note: +Expected population average = 100 (Standard Deviation = 15); *P < .05, **P < .01.
For the younger (<7 y at diagnosis) HR participants, the declines in GIA (−3.96 points/y; P < .01), reading (−3.42 points/y; P < .01), and math ability (−2.08 points/y; P < .01) were significant. For the younger AR participants, the declines in GIA (−1.54 points/y; P < .01) and reading ability (−2.38 points/y; P < .01) were significant. The decline in math ability was not (−0.81 points/y; P = .15).
For the older (≥7 y at diagnosis) HR participants, the declines in GIA (−1.40 points/y; P < .01), reading (−0.99 points/y; P = .03), and math ability (−1.94 points/y; P < .01) were significant. For the older AR participants, GIA increased significantly (1.03 points/y; P < .01), math decreased significantly (−0.67 points/y; P = .02); however, change in reading ability was not significant (0.05 points/y; P = .85).
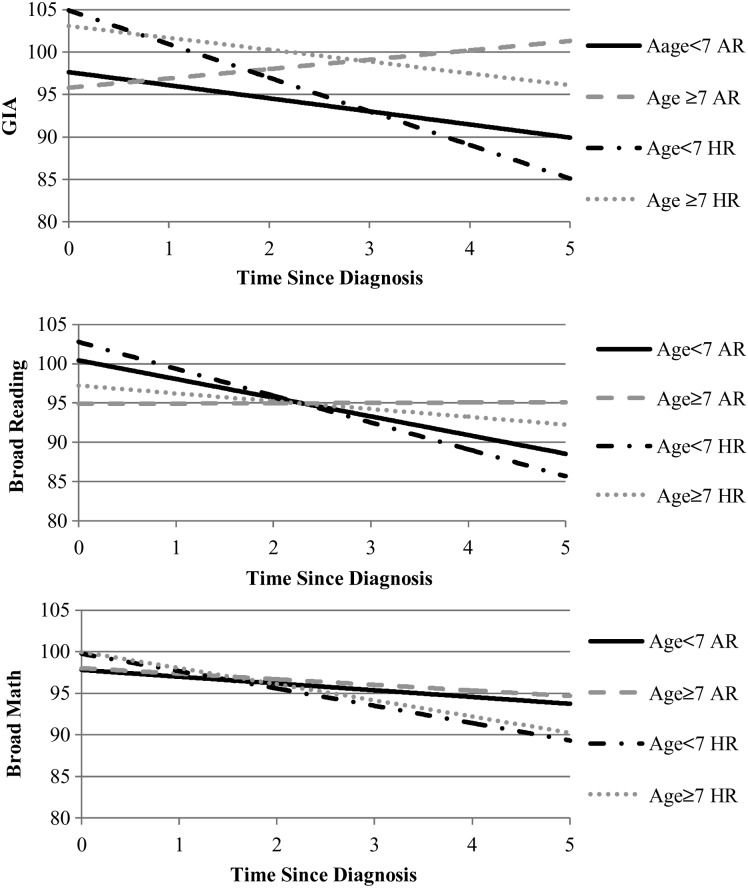
Examination of the intercept and slope estimates in Table 4 reveals that, although many participants exhibited significant declines over time, most participants had mean scores that were estimated to remain within the average range at 5 years post diagnosis. Notably, younger, HR participants were estimated to exhibit the largest drop in mean scores for GIA, reading, and math ability at 5 years post diagnosis (19.8 points, 17.1 points, and 10.4 points, respectively) with mean estimated GIA and reading ability approaching below average by 5 years post diagnosis (Fig. 2). Young AR participants had the second-largest estimated drop in estimated mean scores for GIA and reading ability (7.7 points and 11.9 points, respectively).
Fig. 2.
Estimated intellectual and academic outcomes by risk and age at diagnosis.
Discussion
The current study prospectively examined clinical risk factors for change in intellectual and academic outcomes over 5 years post diagnosis in pediatric patients treated for medulloblastoma with risk-adapted therapy that included a reduced CTV (1.0 cm) for the boost irradiation to the tumor bed for all patients. Our study contributes uniquely to the literature in being the first to examine associations between serious hearing loss and change in intellectual and academic outcomes in medulloblastoma patients. Patients with hearing loss serious enough to require hearing aids demonstrated significant declines in intellectual and academic outcomes, with mean intellectual ability estimated to drop below average by 4 years post diagnosis. This is not surprising, given the literature indicating that unilateral or mild bilateral hearing loss can have detrimental effects on a child's speech, language, and academic achievement.28–30 Our findings are consistent with those from a retrospective study of neuroblastoma patients who suffered hearing loss after platinum-based chemotherapy and were subsequently found to be at risk for academic problems.31
Our findings confirm that hearing loss is an additional risk for intellectual and academic decline following treatment for medulloblastoma. Regular and consistent hearing tests are imperative for detecting hearing loss as early as possible. When hearing loss occurs, emphasis should be placed on the importance of using hearing aids, particularly with patients who are at heightened risk for declines in intellectual and academic outcomes due to other identified risk factors (eg, young age at diagnosis and high-risk disease). The current study did not have data to examine the adherence rate of hearing aid use; however, persistent hearing difficulties have been documented in a large number of children, even when using a hearing aid regularly.32 Therefore, caregivers of patients with hearing loss should also ensure that all school professionals working with the patient understand the impact of hearing loss on educational demands.
An additional clinical risk factor, PFS, was associated with a significant decline in reading ability but not intellectual or math ability. Only a third of participants with PFS provided assessment data at baseline due to medical status restricting participation in many cases; however, estimated baseline mean scores were below average for intellectual ability. Intellectual ability was estimated to remain below average, and reading ability was estimated to drop below average by 3 years post diagnosis. Our findings confirm that patients with PFS are at high risk for poor cognitive and academic outcomes. More detailed study is needed to further elucidate the specific impairments in this group of patients.
Consistent with the literature,6–10 younger age at diagnosis and high-risk disease (eg, higher doses of radiation) predicted declines in intellectual and academic outcomes; however, estimated mean scores remained within the average range for most participants. Those who were younger at diagnosis (<7 years) and higher risk had estimated intellectual and reading ability mean scores that approached below average by 5 years post diagnosis. Younger age at diagnosis was the stronger and more consistent predictor of larger declines in intellectual and reading ability.
Our findings indicate that reading ability may be particularly vulnerable to young age at diagnosis, as suggested by prior pediatric brain tumor studies.8,33,34 The language skills that support these emerging reading skills might also be vulnerable following treatment.35 Studies propose that posterior fossa-directed boost irradiation at young ages may disrupt the normal patterns of brain development that underlie the acquisition of reading skills.8,33 Despite the smaller CTV (1.0 cm) for the boost irradiation in the present study, our findings also lend support for this hypothesis. Future neuroimaging studies are needed to confirm altered patterns of brain activation during language and reading-related tasks in patients who are younger at the time of diagnosis.
Although this large study of participants treated on the same clinical protocol provides important findings about intellectual and academic outcomes of medulloblastoma patients, limitations must be acknowledged. First, participants who were excluded due to missing data had a lower mean age at diagnosis, and more were high-risk patients. Although we found that younger age at diagnosis and higher risk predicted declines in intellectual and academic outcomes, our results may underestimate the true severity of the declines in the population given the missing data. Second, only 33% of the patients with PFS provided baseline data due to medical complications restricting participation in testing. Thus, results from this population must be interpreted with caution. More focused study of patients with PFS is needed. Third, a common issue with clinical studies is the lack of a comparison group. Since our study did not have a comparison, we had to rely on normative data provided by the psychometric measures that were standardized on large, representative samples.
In summary, serious hearing loss, PFS, younger age at diagnosis, and high-risk disease are significant clinical risk factors for decline in intellectual and academic outcomes in medulloblastoma patients. However, our results also suggest that intellectual and academic functioning remain within the average range at 5 years post diagnosis for the majority of patients. Future studies need to provide assessments at points further out from diagnosis to determine if the decline in skills continues beyond 5 years post diagnosis. Thus, our results further highlight the importance of continued monitoring of patients' cognitive functioning after treatment for pediatric medulloblastoma.
Funding
This work was supported in part by the National Cancer Institute through a Cancer Center Support (CORE) grant (P30-CA21765), the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), and by the American Lebanese Syrian Associated Charities (ALSAC).
Conflicts of interest statement. None declared.
References
- 1.Robinson KE, Kuttesch JF, Champion JE, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–531. doi: 10.1002/pbc.22568. [DOI] [PubMed] [Google Scholar]
- 2.Robinson KE, Fraley CE, Pearson MM, et al. Neurocognitive late effects of pediatric brain tumors of the posterior fossa: a quantitative review. J Int Neuropsychol Soc. 2013;19(1):44–53. doi: 10.1017/S1355617712000987. [DOI] [PubMed] [Google Scholar]
- 3.Gurney JG, Severson RK, Davis S, et al. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75(8):2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Northcott PA, Jones DT, Kool M, et al. Medulloblastomics: the end of the beginning. Nat Rev Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 6.Mulhern RK, Kepner JL, Thomas PR, et al. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 7.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19(2):472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- 8.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 10.Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol. 2001;19(8):3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 11.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23(34):8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 12.Blakley BW, Myers SF. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg. 1993;109(3 Pt 1):385–391. doi: 10.1177/019459989310900302. [DOI] [PubMed] [Google Scholar]
- 13.Higby DJ, Wallace HJ, Jr., Albert D, et al. Diamminodichloroplatinum in the chemotherapy of testicular tumors. J Urol. 1974;112(1):100–104. doi: 10.1016/s0022-5347(17)59652-4. [DOI] [PubMed] [Google Scholar]
- 14.Waters GS, Ahmad M, Katsarkas A, et al. Ototoxicity due to cis-diamminedichloroplatinum in the treatment of ovarian cancer: influence of dosage and schedule of administration. Ear Hear. 1991;12(2):91–102. doi: 10.1097/00003446-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Jereczek-Fossa BA, Zarowski A, Milani F, et al. Radiotherapy-induced ear toxicity. Cancer Treat Rev. 2003;29(5):417–430. doi: 10.1016/s0305-7372(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 16.Gajjar A, Kuhl J, Epelman S, et al. Chemotherapy of medulloblastoma. Childs Nerv Syst. 1999;15(10):554–562. doi: 10.1007/s003810050543. [DOI] [PubMed] [Google Scholar]
- 17.Low WK, Toh ST, Wee J, et al. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. J Clin Oncol. 2006;24(12):1904–1909. doi: 10.1200/JCO.2005.05.0096. [DOI] [PubMed] [Google Scholar]
- 18.Warrier R, Chauhan A, Davluri M, et al. Cisplatin and cranial irradiation-related hearing loss in children. Ochsner J. 2012;12(3):191–196. [PMC free article] [PubMed] [Google Scholar]
- 19.De Smet HJ, Baillieux H, Catsman-Berrevoets C, et al. Postoperative motor speech production in children with the syndrome of ‘cerebellar’ mutism and subsequent dysarthria: a critical review of the literature. Eur J Paediatr Neurol. 2007;11(4):193–207. doi: 10.1016/j.ejpn.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Korah MP, Esiashvili N, Mazewski CM, et al. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77(1):106–112. doi: 10.1016/j.ijrobp.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 21.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006;105(6_suppl):444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 22.Palmer SL, Hassall T, Evankovich K, et al. Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro Oncol. 2010;12(12):1311–1317. doi: 10.1093/neuonc/noq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 24.Woodcock R, McGraw K, Mather N. Woodcock-Johnson Third Edition, Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 25.Woodcock R, McGraw K, Mather N. Woodcock-Johnson Third Edition, Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 26.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28(10):1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 27.Chall JS. Stages of reading development. 2nd ed. Fort Worth, TX: Harcourt Brace; 1996. [Google Scholar]
- 28.Holstrum WJ, Gaffney M, Gravel JS, et al. Early intervention for children with unilateral and mild bilateral degrees of hearing loss. Trends Amplif. 2008;12(1):35–41. doi: 10.1177/1084713807312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuppler K, Lewis M, Evans AK. A review of unilateral hearing loss and academic performance: is it time to reassess traditional dogmata? Int J Pediatr Otorhinolaryngol. 2013;77(5):617–622. doi: 10.1016/j.ijporl.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Tharpe AM. Unilateral and mild bilateral hearing loss in children: past and current perspectives. Trends Amplif. 2008;12(1):7–15. doi: 10.1177/1084713807304668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics. 2007;120(5):e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 32.Russ SA, Kenney MK, Kogan MD. Hearing difficulties in children with special health care needs. J Dev Behav Pediatr. 2013;34(7):478–485. doi: 10.1097/DBP.0b013e3182a39878. [DOI] [PubMed] [Google Scholar]
- 33.Conklin HM, Li C, Xiong X, et al. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol. 2008;26(24):3965–3970. doi: 10.1200/JCO.2007.15.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 35.Lewis F, Murdoch B. Language skills following risk-adapted treatment for medulloblastoma. Dev Neurorehabil. 2010;13(3):217–224. doi: 10.3109/17518421003733856. [DOI] [PubMed] [Google Scholar]