Abstract
Background
The biological functions of regulatory factor (RF)X1, a transcription factor, are not known. Since the RFX1 gene is often epigenetically silenced and clusters of differentiation (CD)44 proteins that regulate cancer cell biology are increased in human glioblastomas, we designed this study to determine whether RFX1 could regulate CD44 expression in glioblastoma.
Methods
Regulatory factor X1 was overexpressed in 4 human glioblastoma cell lines. CD44 expression and cell proliferation, apoptosis, and invasion were assayed under in vitro conditions. In vivo growth of human glioblastoma xenografts was determined in mice. The expression of RFX1 and CD44 in human glioblastoma tissues was quantified.
Results
A putative RFX1 binding sequence existed in the first exon of the human CD44 gene. The transcription activity of the DNA fragment containing this putative sequence was decreased in cells overexpressing RFX1. Regulatory factor X1 bound to the CD44 gene in glioblastoma cells. It reduced CD44 expression and activated Akt and extracellular signal-regulated kinase, signaling molecules downstream of CD44 to regulate cell proliferation and survival. Overexpression of RFX1 inhibited the survival, proliferation, and transwell invasion of glioblastoma cells and in vivo growth of human glioblastoma xenografts. CD44 overexpression reversed RFX1 effects on cell proliferation. Finally, CD44 protein levels were inversely correlated with RFX1 protein levels in human glioblastoma tissues.
Conclusions
These results suggest that RFX1 directly regulates CD44 expression. This mechanism may contribute to RFX1's effects on proliferation, survival, and invasion of glioblastoma cells. Our results provide initial evidence that RFX1 may be an important target/regulator of the malignancy of glioblastoma.
Keywords: CD44, glioblastoma, regulatory factor X1
Regulatory factor (RF)X proteins are unique transcription factors that contain a highly conserved 76-amino-acid DNA binding domain.1 This domain binds the X-box consensus sequence in promoter regions to regulate gene expression. Seven RFX proteins have been identified in mammals.2 Each of them may have different functions. For example, RFX5 has been shown to play an important role in regulating the expression of the major histocompatibility complex class II genes.3 RFX5 knockout results in bare lymphocyte syndrome in mice.4 Knockout of the RFX3 gene causes severe ciliopathies that can result in diabetes and left-right asymmetry specification.5,6 However, the biological functions of RFX1, the prototype of the RFX family, are not well known. RFX1 downregulates the proto-oncogene c-myc.7 The RFX1 gene is often epigenetically silenced due to hypermethylation in human glioblastomas, the most common and most deadly primary human brain tumors.8 RFX1 expression in the esophageal epithelium is decreased gradually, with the pathology changed from normal epithelium to esophageal adenocarcinoma.9 We recently discovered that RFX1 directly downregulates transforming growth factor (TGF)β2 in human SH-SY5Y cells, a neuroblastoma cell line. RFX1 expression was inversely correlated with TGFβ2 in human neuroblastoma tissues.10 TGFβ2 plays an important role in cell proliferation and survival.11 These results suggest that RFX1 is an important molecule that participates in the regulation of human cancer biology.
Clusters of differentiation (CD)44 is a cell surface protein that works as a receptor for hyaluronan and other ligands, such as collagens.12–15 Although there is only one human CD44 gene, multiple CD44 splicing variants have been found.12,13,15 Activated CD44 can interact with growth factor receptors and the leukemia-associated Rho-guanine nucleotide exchange factor to activate Akt for cell survival, mitogen-activated protein kinase for cell proliferation, and calcium/calmodulin-dependent protein kinase type II to activate cytoskeleton for cell migration and invasion.12,13,15 CD44 has been found in most cell types under normal conditions.14 However, increased CD44 has been shown to play a critical role in the biology of many human cancers, including breast cancer and head and neck squamous cell carcinoma.12,15 A recent study has shown that CD44 is upregulated in human glioblastoma tissues.16
To determine whether RFX1 can regulate CD44 expression, we searched for a putative RFX1 binding site in the CD44 gene. We identified an excellent putative RFX1 binding site in the first exon of the CD44 gene. Thus, we hypothesized that RFX1 might directly regulate CD44 expression and that this regulation could lead to the regulation of proliferation, survival, migration, and invasion of tumor cells. In this study, we experimentally tested these hypotheses and found that RFX1 directly regulates CD44 expression to inhibit glioblastoma malignant functions.
Materials and Methods
Cell Culture
Four glioblastoma cell lines were used in this study. U87 cells were cultured in Minimum Essential Medium Eagle (MEM) supplemented with 1 mM sodium pyruvate, 1% nonessential amino acids, 0.15% sodium bicarbonate, and 10% fetal bovine serum (FBS); T98G cells were cultured in MEM with 10% FBS; A172 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/L glucose and supplemented with 10% FBS; U251 cells were cultured in Roswell Park Memorial Institute medium with 5% FBS. The 293FT cells were maintained in DMEM supplemented with 10% FBS, 0.1 mM nonessential amino acids, 6 mM l-glutamine, 1 mM sodium pyruvate, and 500 μg/mL geneticin. These cells were cultured at 37°C in 5% CO2/95% O2.
Generation of RFX1-Overexpressing Cells
Cells overexpressing RFX1 were generated by retrovirus transduction as we described previously.10 Briefly, the recombinant retrovirus was made by cotransfecting the pOZ-RFX1 or pOZ-N with pGag-pol and pEnv in 293FT cells. The culture media that contained the retrovirus were used to transduce the glioblastoma cells. Forty-eight hours later, cells were sorted by the magnetic Dynabeads M-450 (Invitrogen) coated with interleukin-2R antibody (Millipore). After one round of positive and another round of negative sorting, the cells without beads were used for the study. Retrovirus produced with pOZ-N was used to prepare control cells. Retrovirus generated with pOZ-RFX1 was used to prepare cells overexpressing RFX1.
Chromatin Immunoprecipitation Assay
To determine whether RFX1 directly binds to the CD44 gene, a chromatin immunoprecipitation (ChIP) assay was conducted according to the protocol of Magna ChIP G (Millipore). Approximately 2 × 106 control cells or cells overexpressing RFX1 were used per ChIP assay. The DNA fragments were incubated with 2 μg E-16 goat anti-RFX1 antibody (Santa Cruz Biotechnology). The primers used for PCR amplification were: F1, 5′-GAAGAAAGCCAGTGCGTCTC-3′ and R1, 5′-TGCTCTGCTGAGGCTGTAAA-3′. Two microliters of ChIP DNA was used as the PCR templates and the results were normalized by 1% input for each sample.
Luciferase Activity Assays
To determine whether the transcription activity of the CD44 gene was affected by RFX1, DNA fragments containing the putative RFX1 binding site (+89 to +101) were amplified in the 4 glioblastoma cell lines with the primers: F2, 5′-CCCTATGACAGGCCATCAGT-3′ and R2, 5′-TCGGAAGTTGGCTGCAG-3′. DNA fragments were sequenced to confirm the inclusion of the binding site in the gene of these cell lines. Then the DNA oligos Up1, D1, Up2 (containing the RFX1 binding site indicated by italics), and D2 were synthesized. Their sequences were as follows: Up1, 5′-CCGGGAGCGGGAGAAGAAAGCCAGTGCGTCTCTGGGCGCAGGGGCCAGTGGGGCTCGGA GGCACAGGCACCCCGCGACACTCCAGGTTCCCCG-3′; D1, 5′-pho GTGGGTCGGGGAACCTGGAGTGT CGCGGGGTGCCTGTGCCTCCGAGCCCCACTGGCCCCTGCGCCCAGAGACGCACTGGCTTTCTTCTCCCGCTC-3′; Up2, 5′-phoACCCACGTCCCTGGCAGCCCCGATTATTTACAGCCTCAGCAGAGCACGGGGCGGG GGCAGAGGGGCCCGCCCGGGAGGGCTGCTACTTCTTAAAAA-3′; and D2, 5′-GATCTTTTTAAGAAGTA GCAGCCCTCCCGGGCGGGCCCCTCTGCCCCCGCCCCGTGCTCTGCTGAGGCTGTAAATAATCGGGGCTGCCAGGGAC-3′. The italicized bases in the sequences indicate the sticky ends formed by annealing and used for the ligation. Up1 and D1, Up2 and D2 were annealed separately and then ligated together into the vector pGL3-Luc (digested by Xmal and BglII) to generate pGL3-CD44, which contained the CD44 gene fragment from −7 to +183. To prepare pGL3-Mu, which lacked the RFX1 binding site, the DNA oligos Mu-Up1, Mu-D1, Mu-Up2, and Mu-D2 were used. Their sequences were as follows: Mu-Up1, 5′-CCGGGAGCGGGAGAAGAAAGCCAGTGCGTCT CTGGGCGCAGGGGCCAGTGGGGCTCGGAGGCACAGGCACCCCGCGACACTCCAGGT-3′; Mu-D1, 5′-pho CGGGGAACCTGGAGTGTCGCGGGGTGCCTGTGCCTCCGAGCCCCACTGGCCCCTGCGCCCAGAGA CGCACTGGCTTTCTTCTCCCGCTC-3′; Mu-Up2, 5′-pho TCCCCGATTATTTACAGCCTCAGCAGAGCACG GGGCGGGGGCAGAGGGGCCCGCCCGGGAGGGCTGCTACTTCTTAAAAA-3′; and Mu-D2, 5′-GATCTTTTTAAGAAGTAGCAGCCCTCCCGGGCGGGCCCCTCTGCCCCCGCCCCGTGCTCTGCTGAGGCTGTAAATAAT-3′. The plasmids were sequenced to confirm the accuracy of the DNA fragments before the luciferase activity assay. The Renilla-luciferase expression plasmid was cotransfected as an internal control. Forty-eight hours after transfection, luciferase activity was measured by a Promega dual-luciferase reporter assay as we did before.10,17
Quantitative Real-time PCR
Total RNA was purified with the RNeasy Micro Kit (Qiagen); cDNA was synthesized and quantitative real-time PCR analysis was performed by the ABI Real-Time PCR System. PCR of the human 18S rRNA was carried out for each sample as an internal control. Two pairs of primers, CD44-F1, 5′-TGAGCCTGGCGCAGATC-3′ and CD44-R1, 5′-CTCCGTCCGAGAGATGCTGTAG-3′ or CD44-F2, 5′-TCAAGAAGGTGGAGCAAACACA-3′ and CD44-R2, 5′-AATCAAAGCCAAGGCCAAGAG-3′, which were located on the 5′ or 3′ end of the transcripts, respectively, were used for quantifying the CD44 transcription.
Western Blotting
Cells were lysed with M-PER Mammalian Protein Extraction Reagent (Promega) containing a protease inhibitor cocktail (Sigma) and PhosSTOP phosphatase inhibitor (Roche Applied Science). Thirty micrograms of protein per sample were subjected to western blotting assay using the following primary antibodies: RFX1 (E-16) (catalog #sc-10651, Santa Cruz Biotechnology), CD44 (#3570S, Cell Signaling Technology), phospho–extracellular signal-regulated kinase (ERK; #4377S, Cell Signaling), ERK (#sc-93, Santa Cruz), phospho-Akt (#4058, Cell Signaling), Akt (#9272S, Cell Signaling), glyceraldehyde-3-phosphate dehydrogenase (#G9545, Sigma), and β-actin (I-19, #sc-1616, Santa Cruz). Western blotting was performed as we did before.10
Cell Proliferation Assay
Retrovirus-transduced glioblastoma cells were plated at 1 × 104 cells per well in 6-well plates. The cells were collected every 48 h for 6 subsequent days and counted with a hemocytometer to generate a growth curve.
Cell Apoptosis Assay
To evaluate the effect of RFX1 on glioma cell death, the transduced cells were cultured for 48 h, washed with cold phosphate buffered saline, harvested, and stained with annexin V–phycoerythrin and 7-aminoactinomycin D (BD Biosciences) according to the manufacturer's instructions. Cell samples were analyzed on a FACScan (Becton Dickinson) to determine apoptotic cell fractions.
Cell Invasion Assay
The effect of RFX1 on cell invasion was assessed by a transwell invasion assay as we described before.18 Retrovirus-transduced glioblastoma cells (1 × 105) were suspended in 300 μL 0.1% FBS medium and added to the upper chamber of the wells. The lower chamber contained 600 μL of 10% FBS medium. The plate was kept in air with 5% CO2 for 6 h at 37°C. The cells on the upper membrane surface were then mechanically removed. The cells that had migrated to the lower side of the collagen IV–coated membrane were fixed and stained with 0.1% crystal violet. Migrated cells were counted in 5 randomly chosen fields under a microscope, and the average number of these cells per field was calculated.
In vivo Growth of Human Glioblastoma Xenografts
We used an intracranial glioblastoma xenograft model to test the effects of RFX1 on in vivo tumor growth. RFX1-overexpressing or control U87 cells (3 × 105) were stereotactically implanted into the striata of nude mice (n = 6 for each cell type). Four weeks later, the animals were sacrificed. Their brains were removed, fixed, and cut into 2-mm-thick coronal slices. One 5-µm-thick sections were cut from each side of the slice and stained with hematoxylin and eosin. The tumor area in each brain section was quantified using National Institutes of Health Image 1.60. The tumor volume in each brain slice was calculated (2 mm × the average of tumor areas from the 2 sections, in mm2). The total tumor volume in each mouse was derived by adding together the tumor volumes in each brain slice.
Immunohistochemistry
Coronal 5-µm-thick sections of brains containing the xenografts were subjected to immunohistochemical staining for RFX1 and CD44 as we described before.19 The primary antibodies for RFX1 and CD44 were the same as used for western blotting—the secondary antibodies were donkey anti-goat IgG conjugated with NL637 (#NL002, R&D Systems) and donkey anti-mouse IgG conjugated with NL493 (#NL009, R&D Systems), respectively. The immunostaining of RFX1 and CD44 was observed and photographed under a fluorescence microscope.
CD44 Rescue Assay
Lentivirus encoding the standard isoform of CD44 (RefSeq: NM_001001391) was made by packaging the CD44 lentiviral plasmid pReceiver-LV105 (GeneCopoeia) according to the protocol of the Lenti-Pac HIV Expression Packaging Kit (GeneCopoeia). Used as a control was the eGFP lentivirus encoding eGFP protein. U251 cells were first transduced with retrovirus to prepare the control cells or cells overexpressing RFX1. These cells were then transduced the next day by the lentivirus encoding CD44. Forty-eight hours later, these cells were used to generate growth curves.
Studies of Human Glioblastoma Tissues
Frozen human glioblastoma tissues from the Biorepository and Tissue Research Facility at the University of Virginia, Charlottesville, were sonicated on ice in a lysis buffer (200 mM mannitol and 80 mM HEPES [4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid], pH 7.4) containing protease inhibitor cocktail. The samples were centrifuged at 13 000 g for 15 min at 4°C. The supernatants were used for western blotting of RFX1, CD44, and β-actin. The results of RFX1 and CD44 were normalized by the data of β-actin in the same sample.
Statistical Analysis
Statistical analysis was performed using SigmaStat software. When appropriate, a t-test, 1-way ANOVA, or 2-way repeated-measures ANOVA (for growth curves) was performed. A linear regression was performed between the data of RFX1 and CD44 after they were normalized by the corresponding β-actin result. The results were presented as means ± SD. P ≤ .05 was considered significant.
Results
RFX1 Directly Downregulated CD44 Expression in Glioblastoma Cells
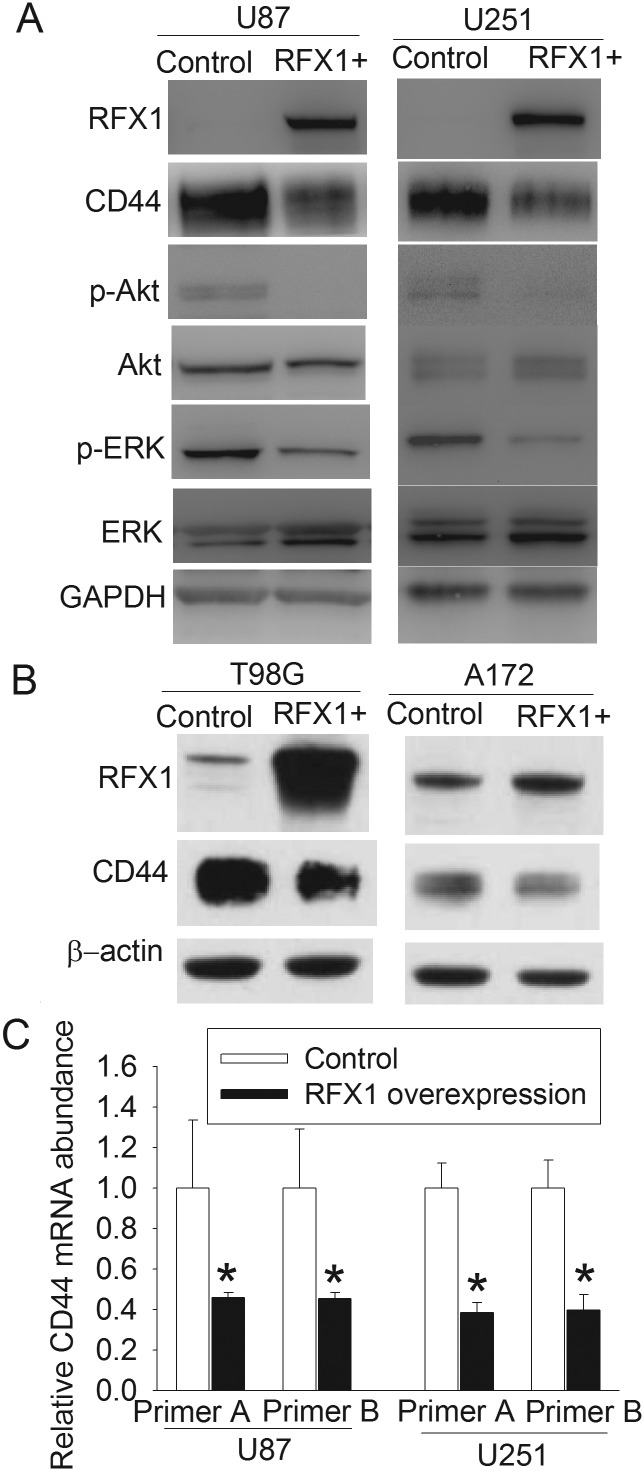
Glioblastoma U87 and U251 cells did not express detectable amounts of RFX1 proteins (Fig. 1A). A low level of RFX1 was expressed in the T98G and A172 glioblastoma cells (Fig. 1B). When U87 and U251 cells were made to express abundant RFX1 after transfection with pOZ-RFX1, their expression of CD44 mRNA was significantly reduced (Fig. 1C). Consistent with these mRNA findings, RFX1 overexpression also reduced CD44 proteins in all of the 4 cell lines (Fig. 1A and B). Most CD44 proteins in these cells had molecular weights of 85–90 kDa. In addition, RFX1 overexpression decreased the expression of phospho-Akt and phospho-ERK but not the total amount of Akt and ERK (Fig. 1A). These results are consistent with published findings that Akt and ERK are signaling molecules downstream of CD44.15,16
Fig. 1.
RFX1 overexpression reduced CD44 expression and activation of downstream molecules. Human glioblastoma cells were transduced with retrovirus carrying the human RFX1 DNA coding sequence or control retrovirus to prepare cells stably overexpressing RFX1 or control cells. (A) Expression of RFX1, CD44, and the downstream signaling molecules of CD44 in U87 and U251 cells were determined by western blot. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Expression of RFX1 and CD44 in T98G and A172 cells were determined by western blotting. (C) CD44 mRNA expression was detected by real-time PCR. Two different sets of primers were used. Results are mean ± SD (n = 3). *P < .05 compared with control cells. RFX+: RFX1 overexpression.
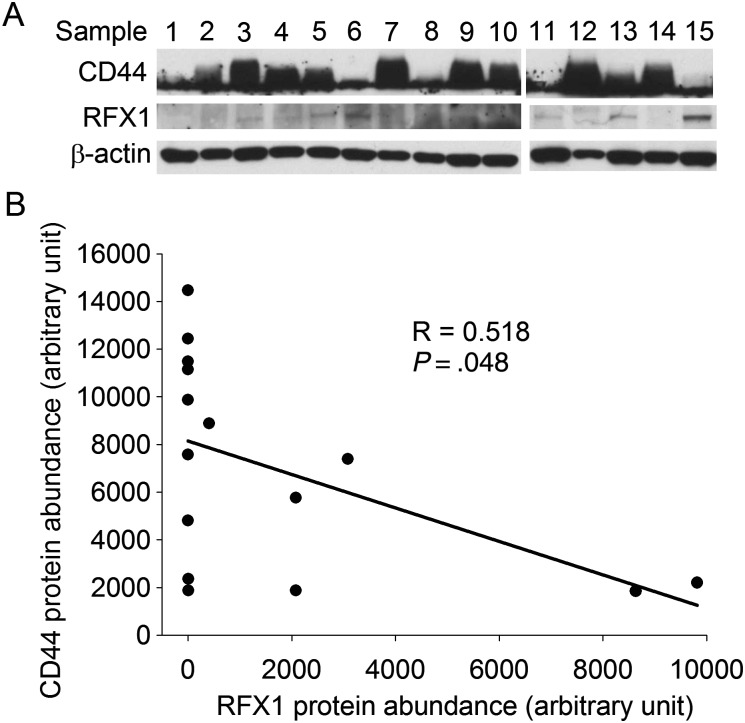
Consistent with the findings from glioblastoma cell lines, a significant reverse correlation between RFX1 and CD44 protein levels existed in the human glioblastoma tissues (Fig. 2).
Fig. 2.
Inverse correlation between the levels of RFX1 and CD44 proteins in human glioblastoma. Human glioblastoma tissues were subjected to western blotting of RFX1 and CD44. (A) Gel images of western blotting. (B) Plot of the negative linear relationship between the levels of RFX1 and CD44 proteins.
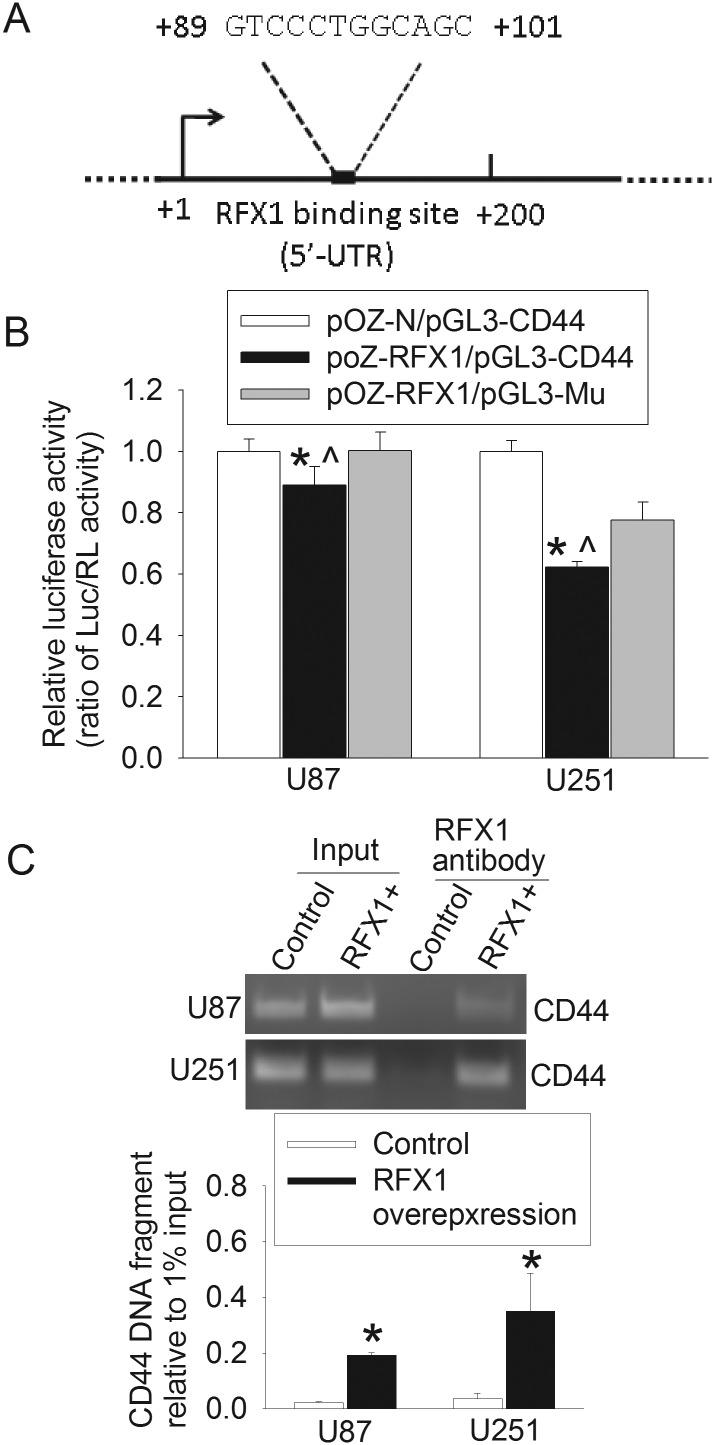
An excellent putative RFX1 binding site was identified in the first exon of the CD44 gene (Fig. 3A). This exon is upstream of any introns in the gene. The putative site was at +89 to +101 downstream from the transcription start site in the human CD44 gene. When a CD44 gene fragment that contained this binding site was transfected into U87 and U251 cells, luciferase activity was significantly reduced by overexpression of RFX1. This RFX1 effect was not observed when the RFX1 binding site was deleted from the CD44 gene fragment (Fig. 3B). The CD44 DNA fragments prepared from U87 and U251 cells overexpressing RFX1 were precipitated by an anti-RFX1 antibody. The amount precipitated in the cells overexpressing RFX1 was much higher than that in cells without RFX1 overexpression (Fig. 3C). These results suggest that RFX1 directly regulates CD44 expression in the glioblastoma cells.
Fig. 3.
RFX1 directly regulated CD44 expression. (A) Location of the consensus binding sequence for RFX1 in the human CD44 gene. (B) Control cells or cells overexpressing RFX1 were transfected with plasmids containing the coding sequence for luciferase whose expression was regulated by a CD44 DNA fragment that contained the putative RFX1 binding sequence (pGL3-CD44) or did not contain the sequence (pGl3-Mu). The luciferase (Luc) results were normalized by the corresponding Renilla luciferase (RL) data. Results are mean ± SD (n = 6). *P < .05 compared with cells expressing normal amount of RFX1; ^P < .05 compared with cells overexpressing RFX1 and the mutated CD44 DNA fragment. (C) Chromatin immunoprecipitation results. The precipitates were amplified by PCR with a pair of primers for CD44 at around the putative RFX1 binding sequence. Primers designed for other locations of the CD44 gene that appeared to be potential RFX1 binding sites did not resolve any PCR bands. Results are mean ± SD (n = 3). *P < .05 compared with control cells. RFX1+: RFX1 overexpression.
RFX1 Overexpression Inhibited the Proliferation, Survival, and Invasion of Glioblastoma Cells as well as the In vivo Growth of Human Glioblastoma Xenografts
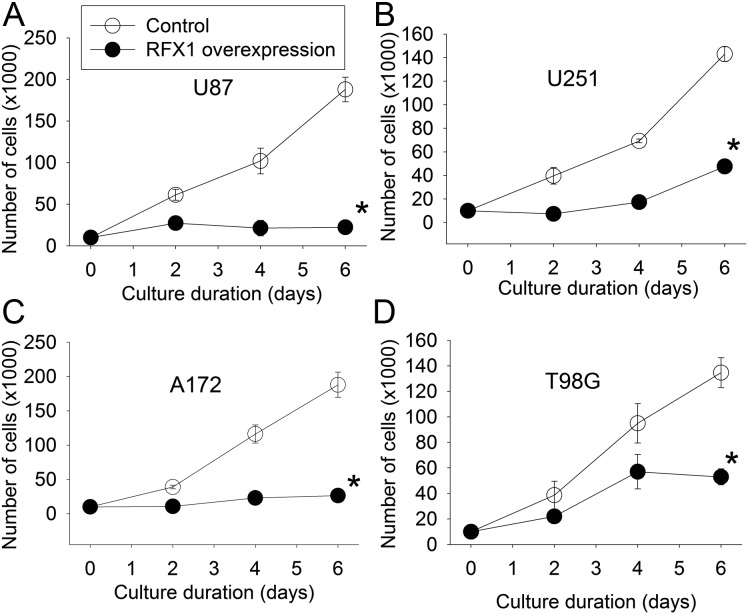
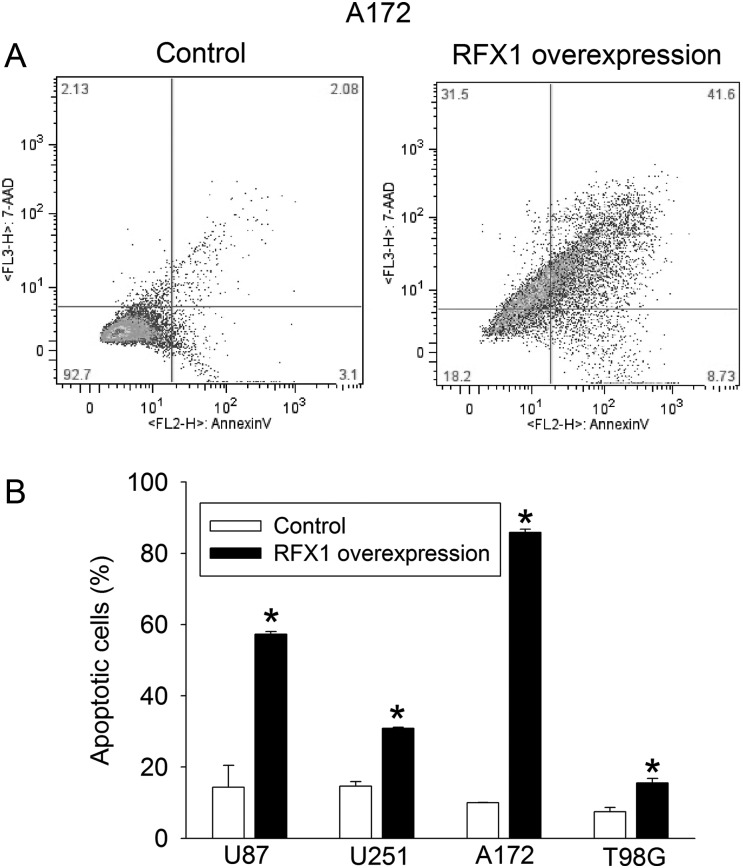
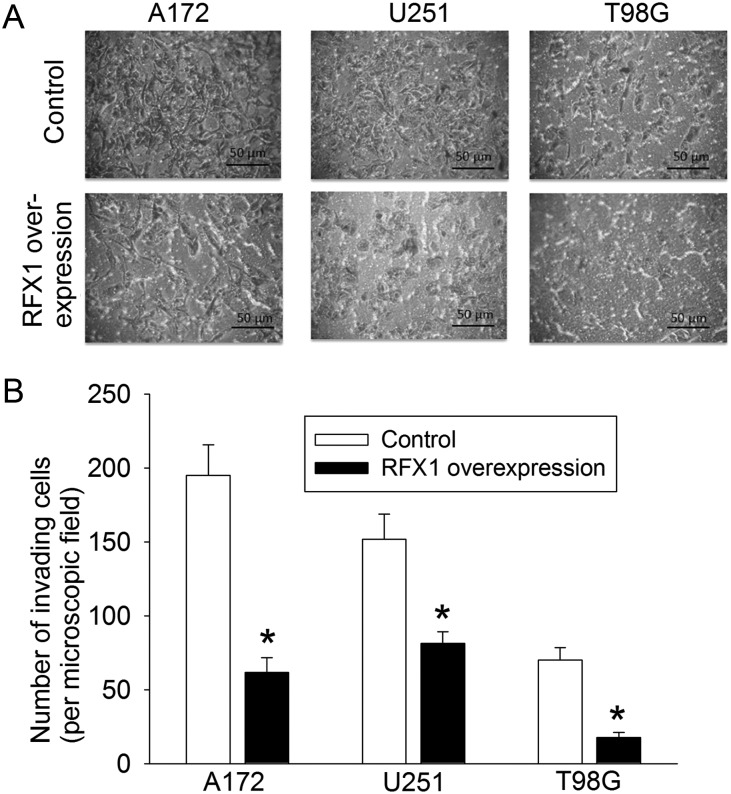
RFX1 overexpression in U87, U251, A172, and T98G cells—4 glioblastoma cell lines—significantly inhibited the increase of cultured cell numbers; values of F(1,4) were 219 for U87, 1135 for U251, 261 for A172, and 80 for T98G; all Ps < .001 compared with control cells by 2-way repeated-measures ANOVA (Fig. 4). RFX1 overexpression also increased the number of apoptotic cells in the cultures (Fig. 5). The number of cells migrating to the lower chamber in the transwell invasion assay was significantly reduced by RFX1 overexpression in the U251, A172, and T98G cells (Fig. 6). Finally, we injected U87 cells with or without RFX1 overexpression into the brains of CD1 nude mice. Two mice in the group receiving cells without RFX1 overexpression died before the preset observation time (4 wk postinjection). Both mice had large brain tumors and presumably died due to the mass effect of these tumors. Of the mice that survived till the end of the 4-week observation time, tumor volumes were significantly smaller in those injected with U87 cells overexpressing RFX1 than those in mice with control U87 cells (Fig. 7A and B). Immunohistochemistry study confirmed that those cells that were made to overexpress RFX1 in vitro also expressed a higher level of RFX1 and a lower level of CD44 under in vivo conditions (Fig. 7C). These results suggest that RFX1 exerts tumor suppressive effects on glioblastoma.
Fig. 4.
RFX1 reduced cell numbers in culture. Human glioblastoma cells were transduced with retrovirus carrying the human RFX1 DNA coding sequence or control retrovirus to prepare cells stably overexpressing RFX1 or control cells. (A–D) Growth curves of U87, U251, A172, and T98G cells. Results are mean ± SD (n = 3). *P < .05 compared with the growth curve of control cells.
Fig. 5.
RFX1 increased apoptotic cells. Human glioblastoma cells were transduced with retrovirus carrying the human RFX1 DNA coding sequence or control retrovirus to prepare cells stably overexpressing RFX1 or control cells. The apoptotic cells were determined by flow cytometry at 48 h after being plated. (A) Representatives of sorted A172 cells by flow cytometry. The x-axis and y-axis are signal intensity of annexin V and 7-aminoactinomycin D (7AAD), respectively. (B) Quantitative results. Results are mean ± SD (n = 3). *P < .05 compared with control cells.
Fig. 6.
RFX1 reduced the migration of human glioblastoma cells. The migration and invasion were assessed by the transwell invasion assay. The membrane in the lower chamber was fixed and stained with 0.1% crystal violet. (A) Representative images (200× magnifications). Scale bar = 50 µm. (B) Quantitative results. Results are mean ± SD (n = 3). *P < .05 compared with control cells.
Fig. 7.
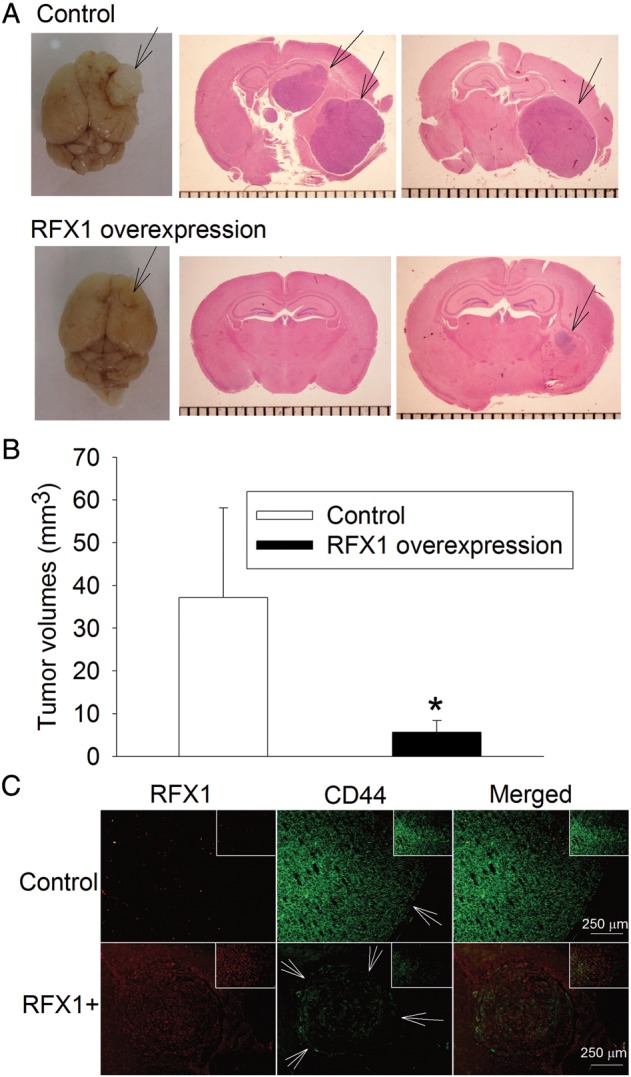
RFX1 reduced tumor volumes formed by U87 cells. U87 cells were transduced with retrovirus carrying the human RFX1 DNA coding sequence or control retrovirus to prepare cells stably overexpressing RFX1 or control cells. These cells (3 × 105) were injected into 5-week-old CD1 nude mouse brains. The brains were harvested 4 weeks after the tumor cell implantation for hematoxylin and eosin staining to calculate the tumor volumes. (A) Representative photos of the brains and images of brain sections. Arrows indicate the tumor. (B) Quantitative data. Results are mean ± SD (n = 4–6). *P < .05 compared with control cells. (C) Immunohistochemical staining of RFX1 (red) and CD44 (green). Arrows indicate the tumor. Scale bar = 250 µm. The inserts in each panel are 200× views of the tumor tissues. RFX1+: RFX1 overexpression.
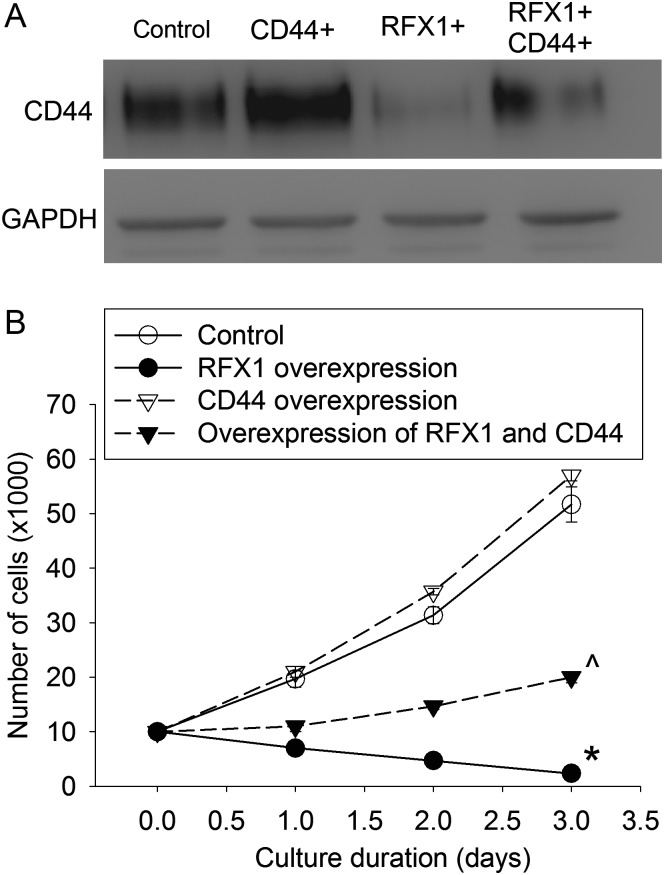
CD44 Expression Attenuated RFX1-induced Inhibition of Proliferation of Glioblastoma Cells
To determine whether CD44 mediated the effects of RFX1 on glioblastoma cells, we transfected U251 cells with lentivirus encoding CD44 standard isoform. This transfection increased CD44 proteins in the cells with or without RFX1 overexpression. This increase significantly attenuated the inhibition of cell proliferation by RFX1, F(1,4) = 564; P < .001 (Fig. 8). These data suggest that CD44 can reverse the effects of RFX1 on glioblastoma cells.
Fig. 8.
CD44 reversed RFX1 effects on U251 cells. U251 cells were transduced with retrovirus carrying the human RFX1 DNA coding sequence or control retrovirus to prepare cells stably overexpressing RFX1 or control cells. They were then transfected with lentivirus containing CD44 coding sequence. (A) Cells were harvested 2 days after the lentiviral transfection for western blotting. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Growth curves. Cells were used in the assay 2 days after the lentiviral transfection. Results are mean ± SD (n = 3). *P < .05 compared with the growth curve of control cells; ^P < .05 compared with the growth curve of cells overexpressing RFX1 only. CD44+: CD44 overexpression, RFX1+: RFX1 overexpression.
Discussion
A recent study has shown that RFX1 expression in the esophageal epithelium is decreased gradually with transformation of the tissues from normal esophageal epithelium to esophageal adenocarcinoma.9 Also, the RFX1 gene in human glioblastoma tissues and cells is hypermethylated. This methylation epigenetically silences this gene.8 These results show that RFX1 expression is decreased in tumor tissues and suggest that RFX1 plays a role in cancer biology. Our current study showed that RFX1 overexpression decreased human glioblastoma cell survival, proliferation, and invasion as well as in vivo growth of human glioblastoma xenografts. These findings provide initial and solid evidence that RFX1 indeed is a tumor suppressive transcription factor in vivo and in vitro.
Our results showed that the increase of cell number over time in glioblastoma cell cultures was significantly reduced in cells overexpressing RFX1. This effect can be due to decreased cell proliferation and/or increased cell death. Increased cell death via apoptosis was prominent in all 4 types of glioblastoma cells overexpressing RFX1. This increased cell death may be the major contributing factor for the reduction of cell number increase in culture because the ranking order of the inhibition of cell number increase was consistent with the ranking order of the degree of cell apoptosis among the 4 glioblastoma cell lines. However, RFX1 overexpression in U87 cells and A172 cells almost totally inhibited the increase of these cells in culture. The degree of cell apoptosis was different between these 2 cells. These results suggest that RFX1 also inhibits the proliferation of glioblastoma cells. Consistent with this suggestion, our previous study clearly shows that RFX1 inhibits the proliferation of the human neuroblastoma SH-SY5Y cells.10
A major finding of our study is that RFX1 directly regulates CD44 expression. This finding is supported by decreased CD44 mRNA and protein expression in cells overexpressing RFX1, the reverse correlation between RFX1 and CD44 protein levels in human glioblastoma tissues, and the existence of a putative RFX1 binding sequence in the CD44 gene. This sequence can bind with RFX1, and this binding can alter the transcription activity of the gene as shown by the ChIP and luciferase activity assays. Although the effect of RFX1 on the CD44 promoter activity assayed by luciferase activity in the U87 cells appeared to be small, this effect should be biologically significant because RFX1 overexpression reduced CD44 protein expression and inhibited the survival and proliferation of these cells and their in vivo growth. In addition, the size of the effect in the luciferase activity assay can be affected by many factors, such as the degree of RFX1 overexpression in these cells.
CD44 is a plasma membrane protein known to play an important role in tumor cell biology.12,13,15 It can interact and bind with growth factor receptors to activate multiple intracellular pathways, such as Akt and ERK, to promote tumor cell survival and growth.12,13,15 Our results suggest that inhibition of CD44 expression contributes to RFX1 effects on glioblastoma cell biology because CD44 overexpression reversed RFX1-induced inhibition of cell number increase in the U251 cells. Also, RFX1 overexpression inhibited the activation of Akt and ERK phosphorylation, molecular events downstream of CD44.
In addition to CD44, RFX1 has been found to transcriptionally silence a few genes that are involved in cell proliferation and transformation. These genes include the proto-oncogene c-myc,7 proliferating cell nuclear antigen (PCNA),20 TGFβ2,10 and fibroblast growth factor (FGF)1.21 PCNA is an essential factor for eukaryotic DNA replication and repair.22 TGFβ2 is an important regulator of cell proliferation and transformation.11 FGF1 enhances cell proliferation.21 Thus, decreased RFX1 expression may increase the expression of c-myc, PCNA, and TGFβ2, which may increase cell proliferation and transformation. Consistent with this possibility, our recent study showed that overexpression of RFX1 reduced TGFβ2 and proliferation of human SH-SY5Y cells.10 RFX1 downregulation increases neurosphere formation in human glioblastoma cells.21 The contribution of these factors to RFX1 effects on glioblastoma cell biology is not determined in the current study. However, TGFβ2 and FGF1 may share downstream signaling pathways with CD44 because CD44 is known to interact with growth factor receptors to activate intracellular signaling molecules for enhancing cell proliferation and survival.12,13,15
Glioblastoma multiforme (GBM) is a deadly disease that is currently incurable. It is the most aggressive brain tumor and is resistant to chemotherapy and radiotherapy. Patients with GBM have a median survival of <1 year, and <3% live longer than 5 years.23,24 A recent study suggests that CD44 increase in GBM cells may be an important contributor to their resistance to chemotherapy.16 Our study clearly showed that RFX1 inhibits CD44 expression. Thus, our study has identified a potential target/intervention to improve the response of these tumors to chemotherapy. Our study has also revealed a target to alter the cell biology of GBM per se. Since hypermethylation is indicated as a mechanism for the silencing of the RFX1 gene in GBM cells,8 demethylation of the gene might be an approach to inhibit the growth, survival, and invasion of GBM cells.
In summary, we have shown that RFX1 directly inhibits CD44 expression in GBM. This inhibition may contribute to the inhibition of RFX1 on the proliferation, survival, and invasion of GBM cells. Our study therefore identifies RFX1 as a new tumor suppressive gene and transcription factor that acts by regulating CD44 in glioblastoma.
Funding
This study was supported by a grant from Voices Against Brain Tumor (Investigator-initiated Project to Z.Z.); by grants (R01 GM065211 and R01 GM098308 to Z.Z.; R01 NS045209 and R01 CA134843 to R.A.) from the National Institutes of Health; by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z.Z.); by a grant-in-aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z.Z.); and by the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville.
Acknowledgments
Research design: C.F., Y.Z., R.A., and Z.Z.; performing research and initial data analysis: C.F., Y.Z., J.Y., and J.L.; final data analysis: C.F., Y.Z., and Z.Z.; and manuscript preparation: C.F., R.A., and Z.Z.
Conflict of interest statement. None declared.
References
- 1.Emery P, Durand B, Mach B, et al. RFX proteins, a novel family of DNA binding proteins conserved in the eukaryotic kingdom. Nucleic Acids Res. 1996;24:803–807. doi: 10.1093/nar/24.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aftab S, Semenec L, Chu JS, et al. Identification and characterization of novel human tissue-specific RFX transcription factors. BMC Evol Biol. 2008;8:226. doi: 10.1186/1471-2148-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villard J, Peretti M, Masternak K, et al. A functionally essential domain of RFX5 mediates activation of major histocompatibility complex class II promoters by promoting cooperative binding between RFX and NF-Y. Mol Cell Biol. 2000;20(10):3364–3376. doi: 10.1128/mcb.20.10.3364-3376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clausen BE, Waldburger JM, Schwenk F, et al. Residual MHC class II expression on mature dendritic cells and activated B cells in RFX5-deficient mice. Immunity. 1998;8(2):143–155. doi: 10.1016/s1074-7613(00)80467-7. [DOI] [PubMed] [Google Scholar]
- 5.Ait-Lounis A, Baas D, Barras E, et al. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes. 2007;56(4):950–959. doi: 10.2337/db06-1187. [DOI] [PubMed] [Google Scholar]
- 6.Bonnafe E, Touka M, Ait-Lounis A, et al. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol. 2004;24(10):4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Smith L, Johnson MR, et al. Activation of protein kinase C induces nuclear translocation of RFX1 and down-regulates c-myc via an intron 1 X box in undifferentiated leukemia HL-60 cells. J Biol Chem. 2000;275(41):32227–32233. doi: 10.1074/jbc.M002645200. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi Y, Ueda M, Kawase T, et al. Identification of an epigenetically silenced gene, RFX1, in human glioma cells using restriction landmark genomic scanning. Oncogene. 2004;23(47):7772–7779. doi: 10.1038/sj.onc.1208058. [DOI] [PubMed] [Google Scholar]
- 9.Watts JA, Zhang C, Klein-Szanto AJ, et al. Study of FoxA pioneer factor at silent genes reveals Rfx-repressed enhancer at Cdx2 and a potential indicator of esophageal adenocarcinoma development. PLoS Genet. 2011;7(9):e1002277. doi: 10.1371/journal.pgen.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng C, Zuo Z. Regulatory factor X1-induced down-regulation of transforming growth factor beta 2 transcription in human neuroblastoma cells. J Biol Chem. 2012;287(27):22730–22739. doi: 10.1074/jbc.M111.338590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 12.Bourguignon LY, Singleton PA, Zhu H, et al. Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor beta receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277(42):39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 13.Toole BP. Hyaluronan-CD44 interactions in cancer: paradoxes and possibilities. Clin Cancer Res. 2009;15(24):7462–7468. doi: 10.1158/1078-0432.CCR-09-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 15.Wang SJ, Bourguignon LY. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am J Pathol. 2011;178(3):956–963. doi: 10.1016/j.ajpath.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70(6):2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma K, Zheng S, Zuo Z. The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J Biol Chem. 2006;281(30):21250–21255. doi: 10.1074/jbc.M600521200. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Guessous F, Zhang Y, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69(19):7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Li L, Lin D, et al. Isoflurane induces learning impairment that is mediated by interleukin 1beta in rodents. PLoS One. 2012;7(12):e51431. doi: 10.1371/journal.pone.0051431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu M, Lee BH, Mathews MB. Involvement of RFX1 protein in the regulation of the human proliferating cell nuclear antigen promoter. J Biol Chem. 1999;274(22):15433–15439. doi: 10.1074/jbc.274.22.15433. [DOI] [PubMed] [Google Scholar]
- 21.Hsu YC, Liao WC, Kao CY, et al. Regulation of FGF1 gene promoter through transcription factor RFX1. J Biol Chem. 2010;285(18):13885–13895. doi: 10.1074/jbc.M109.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivji KK, Kenny MK, Wood RD. Proliferating cell nuclear antigen is required for DNA excision repair. Cell. 1992;69(2):367–374. doi: 10.1016/0092-8674(92)90416-a. [DOI] [PubMed] [Google Scholar]
- 23.Giese A, Bjerkvig R, Berens ME, et al. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 24.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]