Abstract
Background
We report a population-based study examining long-term outcomes for common pediatric CNS tumors comparing results from the UK with the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) data set and with the literature. No such international study has previously been reported.
Methods
Data between 1996 and 2005 from the UK National Registry of Childhood Tumours (NRCT) and the SEER registry were analyzed. We calculated actuarial survival at each time point from histological diagnosis, with death from any cause as the endpoint. Kaplan–Meier estimation and log-rank testing (Cox proportional hazards regression analysis) were used to calculate survival differences among tumor subtypes, adjusting for age at diagnosis.
Results
Population-based outcomes for each tumor type are presented. Overall age-adjusted survival, stratifying for histology (combining pilocytic astrocytoma, anaplastic astrocytoma, glioblastoma, primitive neuroectodermal tumor, medulloblastoma, and ependymoma), is significantly lower for NRCT than SEER (hazard ratio 0.71, P < .001) and at 1, 5, and 10 years. Both NRCT and SEER outcomes are worse than those reported from trials.
Conclusion
Analyzing data from comprehensive registries minimizes bias associated with trials and institutional studies. The reasons for the poorer outcomes in children treated in the UK are unclear. Likewise, the differences in outcomes between patients in trials and those not in trials need further investigation. We recommend that all children with CNS tumors be recruited into studies—even if these are observational studies. We also suggest that registries be suitably funded to publish independent outcome data (including morbidity) at both a national and an institutional level.
Keywords: neuro-oncology, pediatric, population, registry, survival
Central nervous system tumors are the most common solid tumors in children. In an era of increasing accountability, it is surprising that there is so little published literature comparing outcomes after surgery for CNS tumor.1 Regulatory bodies, peers, and patients alike expect objective evidence of performance2,3 so that they can make informed decisions—yet the CNS tumor literature is largely limited to individual or institutional case series or trials comparing different treatment strategies.
We recently reviewed early postoperative mortality for children with CNS tumors in the UK.4 The 30-day mortality of 2.7% was higher than that seen in most modern series (∼1%)—and some rare tumors in the UK series had 30-day mortality rates of 9%–13%. To further examine the outcome of children receiving surgical treatment for CNS tumors in the UK, we report here a population-based study looking at 1-, 5-, and 10-year survival for the most common histologically confirmed pediatric CNS tumor types. In addition, we compared these results from the UK with those published in the literature and those recorded in the USA by the Surveillance, Epidemiology, and End Results (SEER) data set. The SEER program is run by the National Cancer Institute and has evolved over time to collect data covering 28% of the US population.5 Treatment modalities are not recorded in the UK registry, and we have therefore not tried to analyze for other treatments (chemotherapy or radiotherapy). In effect, our study looks at the overall outcome of patients with a tissue diagnosis managed in the US (SEER) and compares this with the outcome of children managed in the UK.
Methods
National Registry of Childhood Tumours Data
Nearly all cases of malignant and nonmalignant CNS tumors diagnosed in children <15 years of age in the United Kingdom (England, Wales, Scotland, and Northern Ireland) are registered with the National Registry of Childhood Tumours (NRCT). The NRCT is the largest population-based registry of childhood cancers in the world.6
The topography and morphology of all neoplasms are coded using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) and classified using the third edition of the International Classification of Childhood Cancer (ICCC-3).7
We defined 1-, 5-, and 10-year survival at each time point from histological diagnosis as those patients remaining after those registered with any cause of death. In order to determine the time-specified postoperative survival for individual tumor categories, we examined data from the NRCT in the time period 1996–2005. Diagnoses included were those with ICD-O-3 behavior code 3 or higher, together with pilocytic astrocytoma (behavior code 1). Optic nerve glioma and unspecified glioma without histology were excluded, as these tumors had not necessarily undergone a surgical procedure. Only first tumors were analyzed. Only microscopically confirmed diagnoses were included.
Surveillance, Epidemiology, and End Results Data
We interrogated the SEER-18 database over a similar time period (1996–2005). Case selection was the same as for NRCT data (ie, children <15 y with a proven histological diagnosis). Site and histology codes were selected for each tumor subtype in order to make them comparable to the NRCT groupings.
During this period, SEER included only tumors coded as malignant as per the ICD-O-2 and ICD-10 criteria. Pilocytic astrocytoma was included because it was only downgraded to nonmalignant in ICD-O-3. Craniopharyngioma, choroid plexus papilloma, subependymal giant cell astrocytoma, dysembryoplastic neuroepithelial tumor, most gangliogliomas, and meningiomas have always been coded as nonmalignant, so there are no SEER data to make a comparison with UK cases. These tumors have therefore been excluded from this analysis.
Statistics
Data were collated and analyzed using SEERStat 6.6.2, Stata v10,8 and Microsoft Excel. Kaplan–Meier estimation was used to calculate actuarial survival from the date of diagnosis. Observed survival alone is presented, as it corresponds very closely to relative survival in children, since deaths due to competing risks are rare.9 Observed survival was calculated using the actuarial method.10 Differences in survival between patient subgroups were tested using the log-rank test. Cox proportional hazards regression analysis11 was used to model probability of survival in relation to tumor subtype and time epochs, adjusting for age and histology. As age at diagnosis is an independent risk factor for survival for most CNS tumors, separate analyses were undertaken for the whole cohort, for children aged <3 years and for those aged 3–14.
For glioblastoma we have also calculated the 2-year survival rates, as these are probably more relevant for this tumor subtype. As many studies (and virtually all trials) in the literature combine anaplastic astrocytomas and glioblastoma multiforme (GBM) into “high-grade gliomas,” we have also examined this combined group. Likewise, historically primitive neuroectodermal tumor (PNET) and medulloblastoma have been combined for analysis—we have therefore also calculated survival data for this group of patients both separately and combined.
Results
The present study has not looked at all CNS tumors and is limited to children under 15 years of age (in keeping with the NRCT data set). Between 1996 and 2005, a total of 1925 (NRCT) and 2411 (SEER) patients had CNS tumor surgery (Table 3). Overall actuarial survival rates for children aged <15 years at 1, 5, and 10 years were 84.4%, 69.5%, and 65.7%, respectively, for NRCT and 88.3%, 77.6%, and 76.1% for SEER. For children aged <3 years, the overall actuarial survival rates at 1, 5, and 10 years were 76.9%, 57.0%, and 51.4%, respectively, for NRCT and 81.1%, 70.6%, and 68.8% for SEER. For children aged 3–14 years, the overall actuarial survival rates at 1, 5, and 10 years were 86.5%, 72.9%, and 68.8%, respectively, for NRCT and 90.4%, 79.7%, and 78.2% for SEER.
Table 3.
1-, 5-, and 10-y postoperative survival for patients aged <15 y at diagnosis comparing SEER vs NRCT (1996–2005)
| Total (n) |
1-y Survival, % |
5-y Survival, % |
10-y Survival, % |
|||||
|---|---|---|---|---|---|---|---|---|
| SEER | NRCT | SEER | NRCT | SEER | NRCT | SEER | NRCT | |
| All ages | ||||||||
| Pilocytic astrocytoma | 1111 | 805 | 98.6 | 98.3 | 97.5 | 96.1 | 97.0 | 94.4 |
| Anaplastic astrocytoma | 123 | 95 | 57.7 | 51.6 | 30.1 | 18.9 | 28.5 | 17.9 |
| Glioblastoma | 121 | 142 | 44.6 | 35.9 | 22.3 | 8.5 | 20.7 | 7.0 |
| Medulloblastoma | 492 | 525 | 86.0 | 85.0 | 71.3 | 65.0 | 68.7 | 59.0 |
| PNET | 230 | 120 | 75.7 | 62.5 | 57.4 | 32.5 | 54.3 | 26.7 |
| Ependymoma | 334 | 238 | 93.1 | 89.5 | 72.5 | 64.7 | 69.8 | 56.7 |
| Overall | 2411 | 1925 | 88.3 | 84.4 | 77.6 | 69.5 | 76.1 | 65.7 |
| <3 y | ||||||||
| Pilocytic astrocytoma | 214 | 130 | 97.2 | 97.7 | 94.9 | 90.8 | 94.4 | 87.7 |
| Anaplastic astrocytoma | 20 | 12 | 70.0 | 50.0 | 65.0 | 41.7 | 65.0 | 41.7 |
| Glioblastoma | 17 | 11 | 35.3 | 45.5 | 35.3 | 27.3 | 35.3 | 27.3 |
| Medulloblastoma | 96 | 113 | 68.8 | 65.5 | 52.1 | 41.6 | 49.0 | 41.6 |
| PNET | 80 | 45 | 56.3 | 35.6 | 42.5 | 11.1 | 41.3 | 11.1 |
| Ependymoma | 117 | 101 | 87.2 | 88.1 | 66.7 | 56.4 | 62.4 | 48.5 |
| Overall | 544 | 412 | 81.1 | 76.9 | 70.6 | 57.0 | 68.8 | 54.1 |
| 3–14 y | ||||||||
| Pilocytic astrocytoma | 897 | 675 | 98.9 | 98.4 | 98.1 | 97.2 | 97.7 | 95.7 |
| Anaplastic astrocytoma | 103 | 83 | 55.3 | 51.8 | 23.3 | 15.7 | 21.4 | 14.5 |
| Glioblastoma | 104 | 131 | 46.2 | 35.1 | 20.2 | 6.9 | 18.3 | 5.3 |
| Medulloblastoma | 396 | 412 | 90.2 | 90.3 | 76.0 | 71.4 | 73.5 | 63.8 |
| PNET | 150 | 75 | 86.0 | 78.7 | 65.3 | 45.3 | 61.3 | 36.0 |
| Ependymoma | 217 | 137 | 96.3 | 90.5 | 75.6 | 70.8 | 73.7 | 62.8 |
| Overall | 1867 | 1513 | 90.4 | 86.5 | 79.7 | 72.9 | 78.2 | 68.8 |
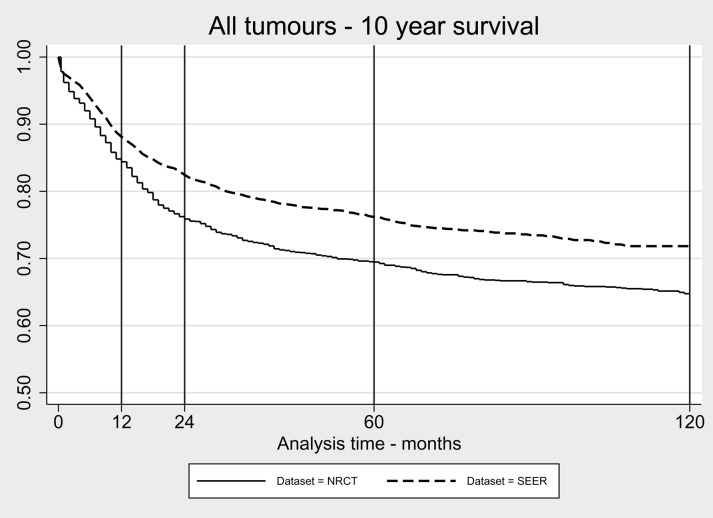
When combining all histologies (see Fig. 1), the SEER survival curve is significantly better (P < .001) than the NRCT curve, with a hazard ratio of 0.74 (95% confidence interval [CI]: 0.66–0.82) adjusting for age alone and 0.71 (95% CI: 0.64–0.80) adjusting for both age and histology (Table 4). This significant difference is maintained when analyzing this group split by age: <3 years, hazard ratio 0.69 (95% CI: 0.56–0.85), P < .001; and 3–14 years, hazard ratio 0.76 (95% CI: 0.67–0.87), P < .001.
Fig 1.
Kaplan–Meier survival curve for all CNS tumors analyzed in the present study over 10 years (SEER = 2411, NRCT = 1925).
Table 4.
Cox proportional hazards regression analysis for patients aged <15 y at diagnosis comparing SEER vs NRCT (all age-adjusted unless otherwise stated)
| Survival | Person Years at Risk | N Subjects | N Deaths | Hazard Ratio | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| All ages | |||||||
| Pilocytic astrocytoma | 10 y | 12934 | 1916 | 78 | 0.69 | 0.44–1.10 | .117 |
| Anaplastic astrocytoma | 10 y | 559 | 218 | 166 | 0.77 | 0.57–1.05 | .095 |
| Glioblastoma | 10 y | 426 | 263 | 228 | 0.72 | 0.55–0.93 | .013 |
| Medulloblastoma | 10 y | 5465 | 1017 | 369 | 0.80 | 0.65–0.99 | .036 |
| PNET | 10 y | 1355 | 350 | 193 | 0.51 | 0.38–0.67 | <.001 |
| Ependymoma | 10 y | 2986 | 572 | 204 | 0.85 | 0.64–1.12 | .240 |
| Overall | 10 y | 23725 | 4336 | 1238 | 0.74 | 0.66–0.82 | <.001 |
| Overall (age and histology adjusted) | 10 y | 25521 | 4336 | 1250 | 0.71 | 0.64–0.80 | <.001 |
| Anaplastic and GBM | 5 y | 783 | 481 | 387 | 0.70 | 0.57–0.85 | <.001 |
| PNET and medulloblastoma | 5 y | 4650 | 1367 | 504 | 0.75 | 0.63–0.89 | <.001 |
| <3 y | |||||||
| Pilocytic astrocytoma | 10 y | 2180 | 344 | 28 | 0.57 | 0.27–1.22 | .146 |
| Anaplastic astrocytoma | 10 y | 127 | 32 | 14 | 0.52 | 0.19–1.48 | .219 |
| Glioblastoma | 10 y | 68 | 28 | 19 | 1.07 | 0.43–2.67 | .883 |
| Medulloblastoma | 10 y | 827 | 209 | 115 | 0.80 | 0.55–1.16 | .234 |
| PNET | 10 y | 323 | 125 | 87 | 0.50 | 0.32–0.76 | <.001 |
| Ependymoma | 10 y | 1067 | 218 | 96 | 0.82 | 0.55–1.23 | .346 |
| Overall | 10 y | 4593 | 956 | 359 | 0.69 | 0.56–0.85 | <.001 |
| Anaplastic and GBM | 5 y | 139 | 60 | 33 | 0.75 | 0.38–1.48 | .400 |
| PNET and medulloblastoma | 5 y | 803 | 334 | 198 | 0.71 | 0.57–0.87 | <.001 |
| 3–14 y | |||||||
| Pilocytic astrocytoma | 10 y | 10754 | 1572 | 50 | 0.75 | 0.42–1.33 | .325 |
| Anaplastic astrocytoma | 10 y | 432 | 186 | 152 | 0.83 | 0.60–1.14 | .244 |
| Glioblastoma | 10 y | 358 | 235 | 209 | 0.68 | 0.51–0.89 | .006 |
| Medulloblastoma | 10 y | 4637 | 808 | 254 | 0.84 | 0.66–1.08 | .183 |
| PNET | 10 y | 1031 | 225 | 106 | 0.57 | 0.39–0.81 | .004 |
| Ependymoma | 10 y | 1920 | 354 | 108 | 0.86 | 0.59–1.26 | .446 |
| Overall | 10 y | 19132 | 3380 | 879 | 0.76 | 0.67–0.87 | <.001 |
| Anaplastic and GBM | 5 y | 644 | 421 | 354 | 0.69 | 0.52–0.91 | .009 |
| PNET and medulloblastoma | 5 y | 3848 | 1033 | 306 | 0.85 | 0.69–1.07 | .163 |
Combining all histologies, no statistical difference in survival was found between the 2 eras of diagnosis, 1996–2000 and 2001–2005, in either data set. Similarly, there was no statistical difference between ages of diagnosis for each tumor type in either data set, but we have not determined the age-specific incidence rates. Tables 1 and 2 compare the results from the present study with population-based and clinical trial studies in the literature. Table 3 gives the survival from each registry by tumor type, and Table 4 gives the hazard ratios for each tumor type.
Table 1.
Summary of literature reporting overall survival related to CNS tumors in population-based studies
| Tumor Type | Survival, % |
||
|---|---|---|---|
| Population-based9,12,20,32–42 | Present Study | ||
| All ages | Low-grade glioma (WHO grades I/II) | 5y 91–95 | |
| High-grade glioma (WHO grades III/IV) | 5y 21–28 | SEER 5y 26 | |
| NRCT 5y 13 | |||
| Ependymoma | 5y 52–83 | SEER 5y 73 | |
| 10y 55–60 | NRCT 5y 65 | ||
| SEER 10y 70 | |||
| NRCT 10y 57 | |||
| Embryonal (medulloblastoma, sPNET) | 5y 46–70 | SEER 5y 67 | |
| NRCT 5y 59 | |||
| Medulloblastoma | 5y 56–87 | SEER 5y 71 | |
| 10y 52–55 | NRCT 5y 65 | ||
| SEER 10y 69 | |||
| NRCT 10y 59 | |||
| sPNET | 5y 27 | SEER 5y 57 | |
| 10y 18–41 | NRCT 5y 33 | ||
| SEER 10y 54 | |||
| NRCT 10y 27 | |||
Abbreviation: WHO, World Health Organization.
Table 2.
Summary of literature reporting overall survival related to CNS tumors in clinical trials, split by ages <3 y and 3–14 y
| Tumor Type | Survival, % |
||
|---|---|---|---|
| Trials22,24,26,27,29,30,42–57 | Present Study | ||
| <3 y | Low-grade glioma (WHO grades I/II) | 5y 95 (1–3 y old, 95 <1 y old, 76) | |
| High-grade glioma (WHO grades III/IV) | 5y 31–66 | SEER 5y 51 | |
| 10y 38 | NRCT 5y 35 | ||
| SEER 10y 51 | |||
| NRCT 10y 35 | |||
| Ependymoma | 5y 63 | SEER 5y 67 | |
| NRCT 5y 56 | |||
| SEER 10y 62 | |||
| NRCT 10y 49 | |||
| Embryonal (medulloblastoma, sPNET) | SEER 5y 48 | ||
| NRCT 5y 33 | |||
| Medulloblastoma | 5y 43 | SEER 5y 52 | |
| NRCT 5y 42 | |||
| SEER 10y 49 | |||
| NRCT 10y 42 | |||
| sPNET | 5y 31 | SEER 5y 43 | |
| NRCT 5y 11 | |||
| SEER 10y 41 | |||
| NRCT 10y 11 | |||
| 3–14 y | Low-grade glioma (WHO grades I/II) | 5y 86 | |
| 10y 94 | |||
| High-grade glioma (WHO grades III/IV) | 2y 21 | SEER 2y 22 | |
| 5y 19 | NRCT 2y 13 | ||
| SEER 5y 22 | |||
| NRCT 5y 10 | |||
| Ependymoma | 5y 60–85 | SEER 5y 76 | |
| NRCT 5y 71 | |||
| SEER 10y 74 | |||
| NRCT 10y 63 | |||
| Embryonal (medulloblastoma, sPNET) | SEER 5y 73 | ||
| NRCT 5y 67 | |||
| Medulloblastoma | 5y 87 | SEER 5y 76 | |
| 10y 81 | NRCT 5y 71 | ||
| SEER 10y 74 | |||
| NRCT 10y 64 | |||
| sPNET | 5y 73 | SEER 5y 65 | |
| NRCT 5y 45 | |||
| SEER 10y 61 | |||
| NRCT 10y 36 | |||
Abbreviation: WHO, World Health Organization.
Discussion
This study compares the survival data from 2 well-recognized and validated registries—showing that for most pediatric CNS tumor types, survival at 1, 5, and 10 years was lower in the NRCT group than in the SEER group. The causes of this difference in outcome are unclear but might include: better access to health care, faster initial diagnosis, increased rates of resection by pediatric trained neurosurgeons, greater recruitment into clinical trials, types of adjuvant therapy, aggressiveness of management at relapse, and better supportive care.12 Certainly, the median interval between symptom onset and diagnosis in the United Kingdom is reported to be 3.3 months,13 which is longer than the interval reported from other countries, such as Germany (24 days)14 and Switzerland (60 days).15 Unfortunately, the NRCT does not yet record sufficient information on treatment received to allow for comparison between the 2 different health-care systems. However, it seems reasonable to assume that for the tumor types chosen, the rate of surgery is likely to be similar between the US and the UK, as most patients with these tumor types will present with symptoms due to mass effect.
The data from the present study indicate that patients in the UK with highly malignant tumors (high-grade gliomas and supratentorial PNETs [sPNETs] in particular) do disproportionately badly in comparison with other tumor types when comparing outcomes with the US. This likely reflects more aggressive surgery or adjuvant therapy either at the time of diagnosis or at relapse.
Children with CNS tumors remain at significantly increased risk of death compared with the general population well beyond 5 years after diagnosis.16–18 In fact, comparing an age- and sex-matched (US) population, the mortality rate of children who have been treated for a CNS tumor beyond 5 years from time of diagnosis is almost 13 times higher. The time epoch of highest risk is 5–9 years from diagnosis.19 Reulen et al17 have shown that there is a persistence of elevated risk of mortality beyond 25 years in survivors of childhood cancer relative to the general population. Causes include second primary neoplasms and cardiorespiratory disease caused by the effects of adjuvant chemoradiotherapy. This study confirms the need for long-term surveillance but interestingly (Table 3) shows only a modest drop-off in overall survival for SEER between 5 and 10 years—but a larger drop-off in the UK (1.5% vs 3.8%, respectively).
As far as we are aware, this is the first study to compare in detail population-based long-term survival from specific surgically treated pediatric CNS tumors. In addition, due to the large numbers of patients recruited to these registries, this study adds significantly to our understanding of patient survival for most common pediatric CNS tumors at a population-based level and may provide a reference point for outcome that is more relevant to daily pediatric neuro-oncological practice.
Comparisons With the Literature
Reviewing the literature shows that studies fall into 3 broad categories: population-based, trial data, and institutional series. Most of the former articles are aimed at looking at overall trends in incidence and outcome—often covering all pediatric malignancies and grouping CNS tumors in ways of limited clinical relevance. From such studies it is therefore usually not possible to predict 1-, 5-, and 10-year survival for specific CNS tumor types.
A systematic review of the literature on clinical trial results for each tumor type was outside the scope of this study. Nevertheless, comparison of the outcomes from the present study with those from relevant contemporaneous trials highlights the substantial differences in survival reported for patients treated as part of a trial and unselected populations treated at the discretion of the clinician. This difference in survival is almost certainly underscored by this study, as many of the patients within both SEER and NRCT will have been in clinical trials, presumably improving the overall survival for the cohort. Part of the difference in survival between trial and nontrial patients can almost certainly be explained by the strict entry requirements of most trials, but more research is required in both health-care settings in order to understand this more fully. Efforts to close this gap could be an effective way of improving the overall outcome for children with CNS tumors.
For some time there has been evidence of poor survival in the UK for both children and young adults with CNS tumors.9 Recently, Solheim et al20 reported that the Norwegian CNS tumor survival rates in children (1988–2008) were 90%–91% at 1 year and 78%–79% at 5 years. The Solheim paper is the only other population-based study limited to operated cases in the literature that we are aware of, but it does not give sufficient breakdown by tumor type to allow direct comparison.
In this study we have specifically looked at the most common pediatric CNS tumor types. For each specific tumor studied, the literature suggests that outcome is independently related to the extent of surgical resection and evidence of dissemination at time of diagnosis. Likewise, young age (<3 y) is usually associated with a worse outcome, although this may in part reflect the avoidance of radiotherapy in this age group. Age is the only variable that we can stratify for using the present data, and this age-related finding is confirmed in this study in both health-care settings (see Table 3).
Presently most research is aimed at better understanding the biology of the various tumor types so that clinical trials can be tailored to lower the burden of treatment and toxicity in low-risk patients while improving survival in high-risk patients. Long-term survival and, perhaps more importantly, quality of life are now recognized as the aims of treatment. With this in mind, we would strongly recommend that national registries, in collaboration with neuro-oncologists, develop an internationally agreed-upon extended data set, to include staging, treatments received, and morbidity.
Specific Tumor Types
Pilocytic astrocytomas
These are the most common pediatric CNS tumors and are generally treated by surgery alone, with very good long-term survival rates being seen in patients in whom resection of 95% or more has been achieved.21 It is therefore surprising to see a hazard ratio of 0.69 (95% CI: 0.44–1.10) for children treated in NRCT compared with SEER regions. One possible explanation for this difference, which it is not possible to explore with these data sets, is that there were a greater proportion of children in the SEER data set for whom a more complete resection was achieved.
High-grade gliomas
Survival from high-grade gliomas has changed little in the past 20–25 years.22 It is becoming increasingly clear that the biology of these tumors in children is different than that in adults,23 and tumors in young children are different from those in older children. In particular, there is evidence of superior survival in children <3 years with high-grade glioma (5-y overall survival 66.3%).24 This is borne out in the present study with significantly more children <3 years with anaplastic astrocytoma and GBM surviving to 5 and 10 years compared with older children. Nonetheless, in the literature, overall long-term survival remains dismal, with 5-year figures ranging from 17% to 40% in anaplastic astrocytoma and 5% to 29% in GBM.23
Ependymoma
Good evidence exists of the impact of gross total resection on outcome; however, controversy still exists regarding the degree of neurological dysfunction that should be accepted in pursuit of this aim. Investigation of the role of postoperative radiotherapy (including protons) and chemotherapy continues, as does the hunt for potential biological markers to guide treatment.25 A recent report looking at children in the US recruited to a phase II preirradiation chemotherapy trial (1995–1999) showed an overall 5-year survival of 71% ± 6%.26 This is in keeping with most trial and review articles, which quote 5-year survival in the range of 60%–85% (see Table 2) and 10-year survival on the order of 50%. Merchant et al27 reported a 93% 5-year survival after gross total removal, as opposed to 52.4% after sub- or near-total removal, and overall 5-year survival of 85% for their total cohort of ependymoma patients.
In the present study (Table 3), there was again a trend for better results in the SEER group. As a potential reflection of the common brainstem involvement, the 1-year survival for both SEER (93.1%) and NRCT (89.5%) is lower than that for other tumors in which surgery is initially considered to be the most important form of treatment. Interestingly and particularly evident in children <3 years is the drop-off in survival between 1 and 5 years in the UK (88.1% to 56.4%). It would be of great interest to know the rate of complete resections in this cohort.
Medulloblastoma/primitive neuroectodermal tumors
Medulloblastomas, located in the posterior fossa, are the most common type of malignant CNS tumor seen in children. Recurrence, usually within 2 years of diagnosis, remains a major problem. At the time of recurrence, despite second surgery, chemotherapy, and autologous stem cell rescue, the prognosis remains very poor and most patients die within 1 year.28 For medulloblastomas, the SEER data set (Table 3) again shows better survival. The 1-year survival rate for SEER and NRCT (86% and 85%, respectively) are very similar, but the NRCT rate is lower at 5 years and beyond. Perhaps even more striking is the difference in 5-year survival between both the SEER (71%) and NRCT (65%) data sets and figures of well over 80% reported in clinical trials.29 This lower survival at 5 years and beyond in the NRCT group is particularly pronounced in the <3-year age group (65.5% at 1 y and 41.6% at 5 y) and may reflect less aggressive treatment at time of presentation or recurrence.
Children with PNET fared particularly badly in the UK, with only 11.1% and 45.3% of children aged <3 years and 3–14 years, respectively, surviving to 5 years. Geyer et al30 report a 5-year overall survival of 31% in infants with sPNET in a US clinical trial. The difference with the SEER data (42.5% and 65.3% for the respective age groups) may in part reflect differences in diagnostic practice between the US and UK (see below).
Solheim et al,20 in a Norwegian population-based study, reported the 5-year survival of combined PNET/medulloblastoma as 65% in low-volume regions (but somewhat surprisingly 42% in the high-volume region). The 65% figure is similar to the 67% for SEER in the present study.
Study Limitations
The comparability of results between different time periods can be impaired by changes in cancer coding.9 We have tried to limit the impact of this by comparing the same periods and including the same tumor types in both data sets. Another limitation is intraobserver variability amongst histopathologists, causing skewed distributions of histopathological subgroups, and their subsequent analyses. This is best exemplified by PNETs, which make up 9.5% of the SEER cohort that we have analyzed but only 6% of the NRCT cohort, while GBM makes up 5% and 7%, respectively. However, while this might be used to question the reliability of comparing SEER with NRCT data for specific histological tumor types, it does not explain the significant differences seen in outcome for the whole cohort of patients between the 2 health-care settings.
Although there might be geographical variation in survival, SEER data remain the best source of population-based survival in the US. All hospitals in SEER areas are required to submit data on reportable neoplasms to the central SEER registry in their area/state. SEER registries are assessed annually by the National Cancer Institute for completeness, and a 98% ascertainment is expected. The population covered by SEER is comparable to the general US population with regard to measures of poverty and education but is more urban and has a higher proportion of foreign-born persons than the general US population.31
Conclusions
This study adds to our understanding of the survival of children with pediatric CNS tumors. Being population based, the results also give more reliable estimates of survival than can be ascertained from the literature. The gap in survival exposed by this study between most children treated in trials and those receiving care outside of such studies needs addressing by health-care providers—perhaps by delivering these treatments in a limited number of accredited centers. We would recommend strongly that all children with CNS tumors who are not entered into a clinical trial be registered in an observational study.
This study also highlights the need for continued systematic national data collection, with increased input and use by those involved in treating pediatric neuro-oncology patients. We urge health-care purchasers to suitably fund national registries so as to allow comparison of outcomes between institutions and between countries.
Lastly, this study exposes worrying differences in outcomes for children diagnosed and treated in the UK compared with those treated in the US (SEER), although this study is unable to shed light on the causes for these differences.
Funding
The Childhood Cancer Research Group has received funding from the Department of Health, the National Cancer Intelligence Network, the Scottish Government, and Children with Cancer, UK. The views expressed here are those of the authors and not necessarily of any of these funding bodies.
Acknowledgments
We thank the following for presentations: the Society of British Neurological Surgeons at its September 2012 meeting in Leeds, and the American Association of Neurological Surgeons at its April 2013 meeting in New Orleans. Study design: Paul Chumas and Charles Stiller; data acquisition and interrogation: Charles Stiller, Ryan Mathew, Roddy O'Kane, and Roger Parslow; literature search: Susan Picton, Ryan Mathew, and Roddy O'Kane; writing article: Ryan Mathew, Paul Chumas, Tom Kenny, and Charles Stiller; reviewing article: all authors.
Conflict of interest statement. None declared.
References
- 1.Shaw K, Cassel CK, Black C, et al. Shared medical regulation in a time of increasing calls for accountability and transparency: comparison of recertification in the United States, Canada, and the United Kingdom. JAMA. 2009;302(18):2008–2014. doi: 10.1001/jama.2009.1620. [DOI] [PubMed] [Google Scholar]
- 2.Maintenance of Certification: Competencies and Criteria. Available at http://www.abms.org/maintenance_of_certification/MOC_competencies.aspx . Accessed June 25, 2012.
- 3.Good Medical Practice. Available at http://www.gmc-uk.org/Good_Medical_Practice_English_0910.pdf_48904554.pdf . Accessed June 25, 2012.
- 4.O'Kane R, Mathew R, Kenny T, et al. United Kingdom 30-day mortality rates after surgery for pediatric central nervous system tumors. J Neurosurg Pediatr. 2013;12(3):227–234. doi: 10.3171/2013.5.PEDS12514. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. Available at http://seer.cancer.gov/csr/1975_2008/sections.html . Accessed June 25, 2012.
- 6.Stiller CA. Methods. In: Stiller CA, editor. Childhood Cancer in Britain: Incidence, Survival, Mortality. Oxford: Oxford University Press; 2007. pp. 7–21. [Google Scholar]
- 7.Steliarova-Foucher E, Stiller C, Lacour B, et al. International Classification of Childhood Cancer, third edition. Cancer. 2005;103(7):1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 8.Acock AC. A Gentle Introduction to Stata. Texas: College Station, Stata Press; 2010. [Google Scholar]
- 9.Gatta G, Zigon G, Capocaccia R, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer. 2009;45(6):992–1005. doi: 10.1016/j.ejca.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Hakulinen T, Abeywickrama KH. A computer program package for relative survival analysis. Comput Programs Biomed. 1985;19(2–3):197–207. doi: 10.1016/0010-468x(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 11.Cox DR. Regression models and life tables. J Roy Stat Soc B. 1972;34(2):187–200. [Google Scholar]
- 12.Peris-Bonet R, Martinez-Garcia C, Lacour B, et al. Childhood central nervous system tumours—incidence and survival in Europe (1978–1997): report from Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2064–2080. doi: 10.1016/j.ejca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Wilne S, Collier J, Kennedy C, et al. Progression from first symptom to diagnosis in childhood brain tumours. Eur J Pediatr. 2012;171(1):87–93. doi: 10.1007/s00431-011-1485-7. [DOI] [PubMed] [Google Scholar]
- 14.Reulecke BC, Erker CG, Fiedler BJ, et al. Brain tumors in children: initial symptoms and their influence on the time span between symptom onset and diagnosis. J Child Neurol. 2008;23(2):178–183. doi: 10.1177/0883073807308692. [DOI] [PubMed] [Google Scholar]
- 15.Dobrovoljac M, Hengartner H, Boltshauser E, et al. Delay in the diagnosis of paediatric brain tumours. Eur J Pediatr. 2002;161(12):663–667. doi: 10.1007/s00431-002-1088-4. [DOI] [PubMed] [Google Scholar]
- 16.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304(2):172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong GT, Pan Z, Ness KK, et al. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28(7):1224–1231. doi: 10.1200/JCO.2009.24.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14(4):298–303. doi: 10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solheim O, Salvesen O, Cappelen J, et al. The impact of provider surgical volumes on survival in children with primary tumors of the central nervous system—a population-based study. Acta Neurochir (Wien) 2011;153(6):1219–1229. doi: 10.1007/s00701-011-0967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nejat F, El Khashab M, Rutka JT. Initial management of childhood brain tumors: neurosurgical considerations. J Child Neurol. 2008;23(10):1136–1148. doi: 10.1177/0883073808321768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald TJ, Arenson EB, Ater J, et al. Phase II study of high-dose chemotherapy before radiation in children with newly diagnosed high-grade astrocytoma: final analysis of Children's Cancer Group Study 9933. Cancer. 2005;104(12):2862–2871. doi: 10.1002/cncr.21593. [DOI] [PubMed] [Google Scholar]
- 23.Pollack IF, Finkelstein SD, Woods J, et al. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346(6):420–427. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 24.Sanders RP, Kocak M, Burger PC, et al. High-grade astrocytoma in very young children. Pediatr Blood Cancer. 2007;49(7):888–893. doi: 10.1002/pbc.21272. [DOI] [PubMed] [Google Scholar]
- 25.Kilday JP, Mitra B, Domerg C, et al. Copy number gain of 1q25 predicts poor progression-free survival for pediatric intracranial ependymomas and enables patient risk stratification: a prospective European clinical trial cohort analysis on behalf of the Children's Cancer Leukaemia Group (CCLG), Societe Francaise d'Oncologie Pediatrique (SFOP), and International Society for Pediatric Oncology (SIOP) Clin Cancer Res. 2012;18(7):2001–2011. doi: 10.1158/1078-0432.CCR-11-2489. [DOI] [PubMed] [Google Scholar]
- 26.Garvin JH, Jr., Selch MT, Holmes E, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children's Cancer Group protocol 9942: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59(7):1183–1189. doi: 10.1002/pbc.24274. [DOI] [PubMed] [Google Scholar]
- 27.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modha A, Vassilyadi M, George A, et al. Medulloblastoma in children—the Ottawa experience. Childs Nerv Syst. 2000;16(6):341–350. doi: 10.1007/s003810050529. [DOI] [PubMed] [Google Scholar]
- 29.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 30.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 31.SEER. SEER Characteristics. Available at http://www.seer.cancer.gov/registries/characteristics.html . Accessed June 25, 2012.
- 32.Baade PD, Youlden DR, Valery PC, et al. Population-based survival estimates for childhood cancer in Australia during the period 1997–2006. Br J Cancer. 2010;103(11):1663–1670. doi: 10.1038/sj.bjc.6605985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stack M, Walsh PM, Comber H, et al. Childhood cancer in Ireland: a population-based study. Arch Dis Child. 2007;92(10):890–897. doi: 10.1136/adc.2005.087544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peris-Bonet R, Salmeron D, Martinez-Beneito MA, et al. Childhood cancer incidence and survival in Spain. Ann Oncol. 2010;21(Suppl 3):iii103–iii110. doi: 10.1093/annonc/mdq092. [DOI] [PubMed] [Google Scholar]
- 35.Desandes E, Berger C, Tron I, et al. Childhood cancer survival in France, 1990–1999. Eur J Cancer. 2008;44(2):205–215. doi: 10.1016/j.ejca.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellison LF, De P, Mery LS, et al. Canadian cancer statistics at a glance: cancer in children. CMAJ. 2009;180(4):422–424. doi: 10.1503/cmaj.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellison LF, Pogany L, Mery LS. Childhood and adolescent cancer survival: a period analysis of data from the Canadian Cancer Registry. Eur J Cancer. 2007;43(13):1967–1975. doi: 10.1016/j.ejca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez D, Cheung MC, Housri N, et al. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005) J Surg Res. 2009;156(2):340–351. doi: 10.1016/j.jss.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh PM, Byrne J, Capra M, et al. Childhood cancer survival in Ireland: temporal, regional and deprivation-related patterns. Eur J Cancer. 2011;47(12):1852–1862. doi: 10.1016/j.ejca.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Lannering B, Sandstrom PE, Holm S, et al. Classification, incidence and survival analyses of children with CNS tumours diagnosed in Sweden 1984–2005. Acta Paediatr. 2009;98(10):1620–1627. doi: 10.1111/j.1651-2227.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 42.Stokland T, Liu JF, Ironside JW, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702) Neuro Oncol. 2010;12(12):1257–1268. doi: 10.1093/neuonc/noq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187–3193. doi: 10.1200/JCO.2011.39.8719. [DOI] [PubMed] [Google Scholar]
- 44.Kuhl J, Muller HL, Berthold F, et al. Preradiation chemotherapy of children and young adults with malignant brain tumors: results of the German pilot trial HIT'88/'89. Klin Padiatr. 1998;210(4):227–233. doi: 10.1055/s-2008-1043883. [DOI] [PubMed] [Google Scholar]
- 45.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 46.Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21(8):1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 47.Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group phase I/II study. J Clin Oncol. 2012;30(21):2648–2653. doi: 10.1200/JCO.2011.40.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chastagner P, Kalifa C, Doz F, et al. Outcome of children treated with preradiation chemotherapy for a high-grade glioma: results of a French Society of Pediatric Oncology (SFOP) pilot study. Pediatr Blood Cancer. 2007;49(6):803–807. doi: 10.1002/pbc.21051. [DOI] [PubMed] [Google Scholar]
- 51.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol. 2011;13(3):317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chintagumpala M, Hassall T, Palmer S, et al. A pilot study of risk-adapted radiotherapy and chemotherapy in patients with supratentorial PNET. Neuro Oncol. 2009;11(1):33–40. doi: 10.1215/15228517-2008-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961. Neuro Oncol. 2013;15(1):97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundy RG, Wilne SH, Robinson KJ, et al. Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46(1):120–133. doi: 10.1016/j.ejca.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Wolff JE, Driever PH, Erdlenbruch B, et al. Intensive chemotherapy improves survival in pediatric high-grade glioma after gross total resection: results of the HIT-GBM-C protocol. Cancer. 2010;116(3):705–712. doi: 10.1002/cncr.24730. [DOI] [PubMed] [Google Scholar]
- 56.Batra V, Sands SA, Holmes E, et al. Long-term survival of children less than six years of age enrolled on the CCG-945 phase III trial for newly-diagnosed high-grade glioma: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2014;61(1):151–157. doi: 10.1002/pbc.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8(8):696–705. doi: 10.1016/S1470-2045(07)70208-5. [DOI] [PubMed] [Google Scholar]