Abstract
Background
We explored whether spontaneous imaging tumor growth (estimated by the velocity of diametric expansion) and isocitrate dehydrogenase 1 (IDH1) mutation (estimated by IDH1 immunoexpression) were independent predictors of long-term outcomes of diffuse low-grade gliomas in adults.
Methods
One hundred thirty-one adult patients with newly diagnosed supratentorial diffuse low-grade gliomas were retrospectively studied.
Results
Isocitrate dehydrogenase 1 mutations were present in 107 patients. The mean spontaneous velocity of diametric expansion was 5.40 ± 5.46 mm/y. During follow-up (mean, 70 ± 54.7 mo), 56 patients presented a malignant transformation and 23 died. The median malignant progression-free survival and the overall survival were significantly longer in cases of slow velocity of diametric expansion (149 and 198 mo, respectively) than in cases of fast velocity of diametric expansion (46 and 82 mo; P < .001 and P < .001, respectively) and in cases with IDH1 mutation (100 and 198 mo, respectively) than in cases without IDH1 mutation (72 mo and not reached; P = .028 and P = .001, respectively). In multivariate analyses, spontaneous velocity of diametric expansion and IDH1 mutation were independent prognostic factors for malignant progression-free survival (P < .001; hazard ratio, 4.23; 95% CI, 1.81–9.40 and P = .019; hazard ratio, 2.39; 95% CI, 1.19–4.66, respectively) and for overall survival (P < .001; hazard ratio, 26.3; 95% CI, 5.42–185.2 and P = .007; hazard ratio, 17.89; 95% CI, 2.15–200.1, respectively).
Conclusions
The spontaneous velocity of diametric expansion and IDH1 mutation status are 2 independent prognostic values that should be obtained at the beginning of the management of diffuse low-grade gliomas in adults.
Keywords: isocitrate dehydrogenase 1, low-grade glioma, prognosis, velocity of tumor expansion
Supratentorial hemispheric diffuse low-grade gliomas (LGGs; World Health Organization [WHO] grade II)1 are a heterogeneous group of tumors with distinct clinical, histopathological, and molecular characteristics and prognosis. These tumors grow continuously and progress to a higher grade of malignancy, leading to neurological disability and death.2 Several risk factors allow prognostic refinements for LGGs: clinical parameters (age, preoperative neurological deficit, increased intracranial pressure, seizures), neuroimaging findings (tumor volume, tumor crossing the midline, contrast enhancement, relative cerebral blood volume on dynamic susceptibility contrast MRI), and histopathological and molecular findings (tumor subtype, TP53 mutation, 1p loss, 1p19q codeletion, proliferation rates).2–6 More recently, 2 additional prognostic factors have shown a strong and independent prognostic significance on overall survival for LGG: the isocitrate dehydrogenase (IDH) 1 gene mutation7–9 and the spontaneous tumor growth on imaging quantified by the velocity of diametric expansion (VDE) of the tumor.10–14 IDH1 mutation status has been shown to correlate independently with overall survival in gliomas whatever their histopathological grade.8,9,15 The spontaneous VDE has been shown to correlate independently with malignant progression-free survival and with overall survival.11,12 In addition, the spontaneous VDE has been shown to reflect the imaging, histopathological, and molecular risk factors for LGGs: LGGs with 1p19q codeletion grow slower and those with p53 overexpression grow faster than those without such abnormalities.12,16,17 By contrast, IDH1 mutation status has no significant impact on spontaneous VDE.12,17 Because IDH1 mutations occur at an early stage of the development of LGG9,18 and are not significantly involved in tumor growth,17 we hypothesized that spontaneous VDE and IDH1 mutation status are independent predictors of outcomes. The aim of the present retrospective study was to explore (i) whether IDH1 mutation status impacted the spontaneous VDE and (ii) whether the spontaneous VDE and IDH1 mutation status were independent predictors of long-term outcomes of supratentorial LGG in adults.
Methods
Selection Criteria
We searched the databases of a French glioma study group (Réseau d′Etude des Gliomes, Sainte-Anne Hospital, Nancy Hospital, Montpellier Hospital) for cases of adult patients with an LGG from 1992 to 2012. The following minimal criteria were required: (i) patients older than 15 years at histopathological diagnosis; (ii) histopathological diagnosis of WHO grade II glioma (gemistocytic histology excluded)1; (iii) supratentorial hemispheric location (gliomatosis cerebri excluded); (iv) available imaging follow-up before first-line oncological treatment (at least 2 MR examinations, minimum 6-wk interval) to estimate spontaneous VDE10,14; and (v) available IDH1 mutation status at histopathological diagnosis.
Procedures
The recorded variables gathered from clinical reports at histopathological diagnosis included sex (male vs female), age (<40 y vs ≥40 y), seizure (presence vs absence), neurological deficit (presence vs absence), increased intracranial pressure (presence vs absence), and Karnofsky performance status (>70% vs ≤70%). The recorded imaging characteristics gathered from MRI follow-up included main anatomic location of tumor (frontal vs temporal vs parietal vs insular vs occipital vs deep-seated), number of cerebral lobes involved (<2 vs ≥2), corpus callosum involvement (presence vs absence), tumor volume at diagnosis (<100 cc vs ≥100 cc), spontaneous VDE (<8 mm/y vs ≥8 mm/y), and contrast enhancement (presence with faint and patchy or nodular-like patterns vs absence according to Pallud et al.6). The recorded neuropathological characteristics included histopathological subtype (oligodendroglioma vs astrocytoma vs mixed glioma), oligodendroglial component (presence vs absence), proliferation rates (<5% vs ≥5%), 1p19q codeletion status (presence vs absence), TP53 mutation status (assessed by presence vs absence of overexpression of p53 protein), and IDH1 mutation status (presence vs absence) assessed by the research of IDH1 gene mutations in 77 cases (58.8%) and by the research restricted to IDH1 R132H mutation by immunohistochemistry in 54 cases (41.2%). The recorded therapeutic characteristics gathered from clinical reports included treatments (surgical resection, chemotherapy, and radiotherapy), extent of resection (biopsy vs partial removal with residual tumor ≥10 cc vs subtotal removal with residual tumor <10 cc vs total removal with no residual tumor, according to Berger et al.19), based on 3-month postoperative MRIs on T2-weighted or fluid attenuated inversion recovery sequences.19
Overall survival was defined as the time from histopathological diagnosis to death. Malignant progression-free survival was defined as the time from histopathological diagnosis to demonstration of evidence of malignant transformation or death. Malignant transformation was considered when new contrast enhancement appeared (nodular-like pattern) or progressed if originally present (progressive over time pattern) or when histologically proven.6 These intervals were censored at the date of last follow-up for survivors.
Estimation of the Individual Velocities of Diametric Expansion on Imaging
The spontaneous imaging tumor growth (ie, the VDE) was measured on serial MR images (various MR techniques, various MR machines) before first-line oncological treatment. The VDE was determined on T2-weighted and fluid attenuated inversion recovery sequences using a methodology detailed elsewhere.10,14 Tumor volumes were computed on digitized images or calculated using an ellipsoid approximation (volume = D1 × D2 × D3/2) after manual measurements of the 3 largest tumor diameters in 3 orthogonal planes (axial, coronal, and sagittal). The mean tumor diameter (MTD) was deduced from the tumor volume (V) through the formula: MTD = (2 × V)1/3. The VDE (ie, the glioma growth curve) was plotted as a function of MTD over time.
Statistical Analyses
Descriptive results are presented as means ± SD (median, range) for continuous data and percentages for categorical data. Analyses were tailored to address associations among clinical, imaging, histopathological, molecular, and treatment-related variables, spontaneous VDE, and outcomes. Univariate analyses were performed using χ2 or Fisher's exact tests for categorical variables and the unpaired t-test or Mann–Whitney rank-sum test for continuous variables, as appropriate. Kaplan–Meier analysis was performed for unadjusted survival curves, using log-rank tests to assess significance. Cox proportional hazards models were constructed, adjusting for predictors associated with mortality or malignant transformation in univariate analyses. Variables associated at the P < .2 level in unadjusted analysis were then entered into models, with the final model retaining only the variables significant at the P < .05 level. P < .05 was considered statistically significant. All statistical analyses were performed using JMP version 11.0.0 (SAS Institute).
Results
Patient and Tumor Characteristics
One hundred thirty-one patients (79 males, 52 females) fulfilled eligibility criteria. They were previously reported separately in 4 different series.12,17,20,21 The mean age at histological diagnosis was 38.0 ± 10.8 years (median, 38.0; range, 15–66), and the mean tumor volume at histological diagnosis was 65.0 ± 50.7 cc (median, 50.0; range, 5–220). Clinical, imaging, histopathological, molecular, and therapeutic findings are summarized in Table 1.
Table 1.
Patient and tumor characteristics
| Parameters |
n |
Spontaneous VDE (mm/y) |
IDH1 Mutation Status |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Range | P | IDH1 + | IDH1− | P | |||
| Clinical parameters | |||||||||
| Sex | 131 | .592 | .477 | ||||||
| Male | 79 | 5.2 ± 4.4 | 3.7 | 0–22.6 | 79.8% | 20.2 | |||
| Female | 52 | 5.7 ± 6.8 | 3.8 | 0–31.0 | 84.6 | 15.4 | |||
| Age | 131 | .887 | .031 | ||||||
| <40 | 75 | 5.8 ± 6.4 | 3.7 | 0–31.0 | 88.0 | 12.0 | |||
| ≥40 | 56 | 4.9 ± 4.0 | 3.9 | 0–19.2 | 73.2 | 18.3 | |||
| Increased intracranial pressure | 109 | .973 | .343 | ||||||
| Yes | 2 | 11.0 ± 15.8 | 11.0 | 0–31.0 | 50.0 | 50.0 | |||
| No | 107 | 5.0 ± 5.1 | 3.6 | 0–22.0 | 80.4 | 19.6 | |||
| Neurological deficit | 109 | .271 | .180 | ||||||
| Yes | 20 | 6.5 ± 7.0 | 4.6 | 0–27.9 | 90.0 | 10.0 | |||
| No | 89 | 4.8 ± 4.9 | 3.5 | 0–31.0 | 77.5 | 22.5 | |||
| Seizures | 109 | .156 | .365 | ||||||
| Yes | 78 | 5.6 ± 5.9 | 3.1 | 0–31.0 | 82.1 | 17.9 | |||
| No | 31 | 3.8 ± 3.2 | 3.8 | 0–15.0 | 74.2 | 25.8 | |||
| KPS | 131 | .005 | .114 | ||||||
| >70% | 125 | 5.1 ± 5.3 | 3.7 | 0–31.0 | 80.8 | 19.2 | |||
| ≤70% | 6 | 11.4 ± 6.6 | 9.9 | 4.7–22.0 | 100.0 | 0 | |||
| Imaging parameters | |||||||||
| Cerebral lobes involved | 118 | .119 | .054 | ||||||
| <2 | 79 | 4.8 ± 5.3 | 3.5 | 0–31.0 | 88.6 | 11.4 | |||
| ≥2 | 39 | 6.5 ± 5.7 | 4.7 | 0–22.6 | 74.4 | 25.6 | |||
| Corpus callosum involvement | 117 | .434 | .410 | ||||||
| No | 105 | 5.0 ± 5.3 | 3.7 | 1.1–31.0 | 84.8 | 15.2 | |||
| Yes | 12 | 7.6 ± 7.0 | 6.8 | 0–22.6 | 75.0 | 25.0 | |||
| Tumor location | 131 | .666 | .509 | ||||||
| Frontal | 62 | 5.2 ± 5.9 | 3.4 | 0–31.0 | 80.7 | 19.4 | |||
| Temporal | 22 | 6.7 ± 7.1 | 3.9 | 0–28.0 | 77.3 | 22.7 | |||
| Insular | 31 | 5.0 ± 3.5 | 4.7 | 0.4–14.2 | 80.7 | 19.3 | |||
| Parietal | 16 | 5.1 ± 4.1 | 3.7 | 0.6–16.9 | 93.8 | 6.2 | |||
| Contrast enhancement | 131 | .582 | .449 | ||||||
| No | 121 | 5.2 ± 5.0 | 3.8 | 0–31.0 | 81.0 | 19.0 | |||
| Yes | 10 | 7.5 ± 9.9 | 2.4 | 0–27.9 | 90.0 | 10.0 | |||
| Tumor volume | 131 | .778 | .589 | ||||||
| <100 cc | 104 | 5.2 ± 5.3 | 5.8 | 0–31.0 | 80.8 | 19.2 | |||
| ≥100 cc | 27 | 6.4 ± 6.1 | 3.6 | 0–22.6 | 85.2 | 14.8 | |||
| Spontaneous VDE | 131 | .898 | |||||||
| <8 mm/y | 108 | 81.5 | 18.5 | ||||||
| ≥8 mm/y | 23 | 82.6 | 17.4 | ||||||
| Histopathological and molecular parameters | |||||||||
| Histopathological diagnosis | 131 | .536 | .087 | ||||||
| Astrocytoma | 25 | 5.5 ± 5.5 | 4.0 | 0.4–27.9 | 72.0 | 28.0 | |||
| Oligodendroglioma | 71 | 5.3 ± 5.8 | 3.7 | 0–31.0 | 88.6 | 11.4 | |||
| Mixed glioma | 35 | 5.6 ± 4.9 | 4.6 | 0–22.9 | 75.0 | 25.0 | |||
| Oligodendroglial component | 131 | .526 | .222 | ||||||
| Yes | 106 | 5.4 ± 5.5 | 3.7 | 0–31.0 | 83.8 | 18.2 | |||
| No | 25 | 5.5 ± 5.5 | 4.0 | 0.4–27.9 | 72.0 | 28.0 | |||
| Proliferation rates | 115 | .888 | .914 | ||||||
| <5% | 70 | 5.4 ± 5.9 | 3.5 | 0–31.0 | 81.4 | 18.6 | |||
| ≥5% | 45 | 5.1 ± 4.7 | 3.7 | 0–22.0 | 82.2 | 17.8 | |||
| 1p19q Codeletion | 119 | .077 | .497 | ||||||
| Yes | 38 | 5.0 ± 6.0 | 3.1 | 0–31.0 | 79.0 | 20.1 | |||
| No | 81 | 5.6 ± 5.0 | 4.4 | 0–27.9 | 84.2 | 15.8 | |||
| p53 Overexpression | 125 | .202 | .250 | ||||||
| Yes | 65 | 5.8 ± 5.2 | 4.4 | 0–27.9 | 86.2 | 13.8 | |||
| No | 60 | 5.0 ± 5.6 | 3.5 | 0–31.0 | 78.3 | 21.7 | |||
| IDH1 mutation | 131 | .823 | |||||||
| Yes | 107 | 5.4 ± 5.6 | 3.8 | 0–31.0 | |||||
| No | 24 | 5.2 ± 5.0 | 3.8 | 0–19.2 | |||||
| First-line oncological treatments | |||||||||
| Surgical resection | 131 | .236 | .574 | ||||||
| Yes | 83 | 4.5 ± 4.2 | 3.6 | 0–31.0 | 83.1 | 16.9 | |||
| No | 48 | 6.8 ± 6.9 | 4.0 | 0.5–27.9 | 79.2 | 20.8 | |||
| Extent of surgical resection | 131 | .396 | .339 | ||||||
| Biopsy | 48 | 6.9 ± 7.7 | 4.0 | 0.5–27.9 | 79.2 | 20.8 | |||
| Partial removal | 16 | 4.4 ± 3.2 | 3.7 | 0–12.3 | 87.5 | 12.5 | |||
| Subtotal removal | 49 | 4.5 ± 3.1 | 4.0 | 0–15.0 | 83.7 | 16.3 | |||
| Total removal | 18 | 4.8 ± 7.1 | 3.2 | 0–31.0 | 77.8 | 22.2 | |||
| Radiation therapy | 131 | .467 | .158 | ||||||
| Yes | 23 | 6.7 ± 5.8 | 4.5 | 0.6–22.6 | 91.3 | 8.7 | |||
| No | 108 | 5.1 ± 5.4 | 3.6 | 0–31.0 | 79.6 | 20.4 | |||
| Chemotherapy | 131 | .040 | .099 | ||||||
| Yes | 14 | 3.7 ± 4.6 | 2.6 | 0.4–19.2 | 64.3 | 35.7 | |||
| No | 117 | 5.6 ± 5.5 | 4.0 | 0–31.0 | 83.8 | 16.2 | |||
Boldface indicates statistically significant.
IDH1 mutations were present in 107 cases (81.7%). Distribution of IDH1 mutation status by clinical, imaging, molecular, and therapeutic findings is summarized in Table 1. Codeletion of 1p19q was present in 38 (31.9%) of the 119 available cases. Codeletion of 1p19q was more frequent in tumors with an oligodendroglial component (40.9%) than in astrocytomas (0%; P < .001). Overexpression of p53 was present in 65 (52%) of the 125 available cases. In the same way, p53 overexpression was more frequent in both astrocytomas (73.9%) and oligoastrocytomas (60%) than in oligodendrogliomas (40.3%; P = .009).
Spontaneous Tumor Velocities of Diametric Expansion
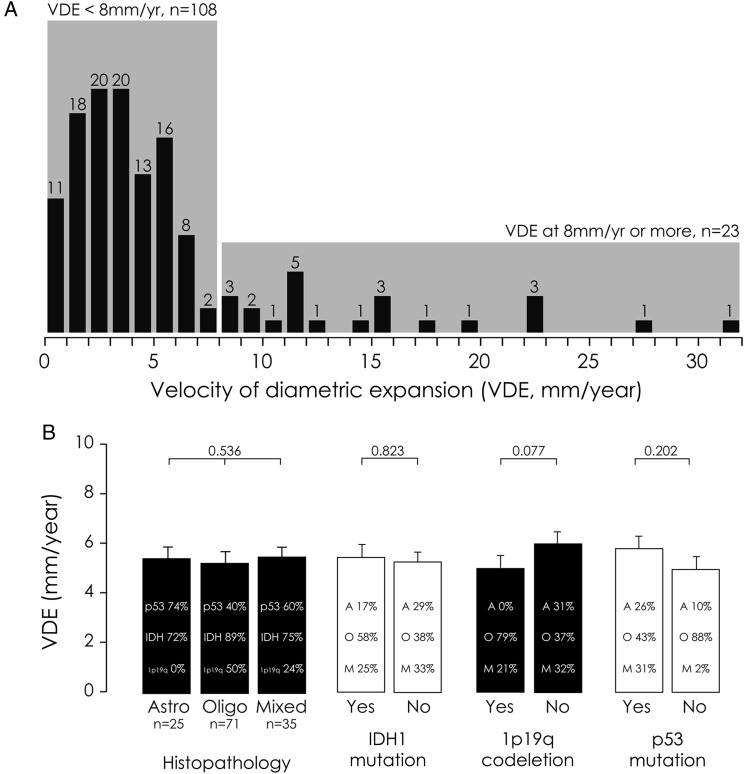
The mean number of MRIs performed before treatment was 3.1 ± 1.3 (median, 3.0; range, 2–7) per patient. The mean duration of repeated measurements before treatment was 20.2 ± 30.2 months (median, 9.6; range, 1.6–152.0). The MTD measurements were performed using the ellipsoid approximation in 117 cases (89.3%)12,17,20 and using the segmentation method in 14 cases (10.7%).21 The mean tumor spontaneous VDE before first oncological treatment was 5.40 ± 5.46 mm/y (median, 3.75 mm/y; range, 0–31.0 mm/y). When applying the cutoff previously described, 108 patients (82.4%, the slow VDE subgroup) presented a spontaneous VDE slower than 8 mm/y, and 23 patients (17.6%, the fast VDE subgroup) presented a spontaneous VDE equal to or faster than 8 mm/y (Fig. 1).11,12
Fig. 1.
(A) Distribution of the 131 patients by individual spontaneous VDEs (mm/y). (B) Molecular factors influencing the spontaneous VDE of LGGs.
Distribution of spontaneous VDE by clinical, imaging, molecular, and therapeutic findings is summarized in Table 1. Spontaneous VDE tended to be slower in tumors with 1p19q codeletion and tended to be faster in tumors with p53 overexpression without reaching significance (Fig. 1). Spontaneous VDE was not significantly different in tumors with IDH1 mutation (mean, 5.4 ± 5.6 mm/y; median, 3.8; range, 0–31.0) and without IDH1 mutation (mean, 5.2 ± 5.0 mm/y; median, 3.8; range, 0–19.2) (P = .823). Interestingly, there were significantly more tumors with a fast spontaneous VDE in the subgroup of patients who underwent only a biopsy (27.1%) than in the subgroup of patients who underwent a resection (12.1%; P = .032).
Velocity of Diametric Expansion, IDH1 Mutation Status, and Outcomes
The mean follow-up was 70 ± 54.7 months since histological diagnosis (median, 55; range, 3.6–262).
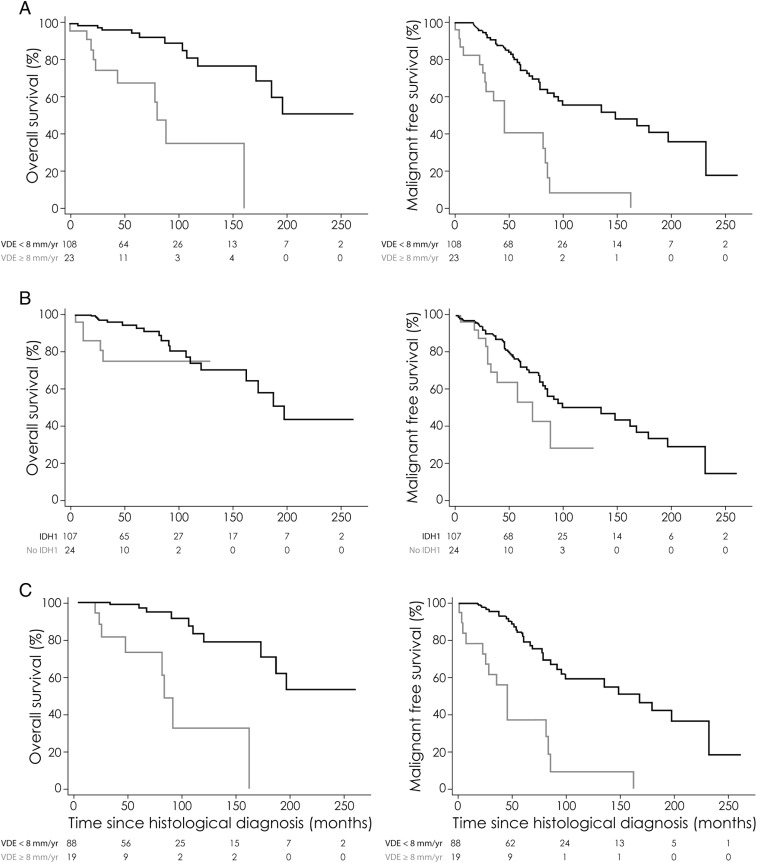
During the follow-up period, 56 patients (42.7%) presented with a malignant progression (histologically proven in 20 cases [35.7%] and suspected on imaging in the remaining 36 cases [64.3%] ) at a mean 63.8 ± 50.7 months (median, 51; range, 1.7–233). A malignant progression was observed in 17 of the 23 patients (73.9%) of the fast VDE subgroup at a mean 47.1 ± 42.2 months (median, 36; range, 1.7–163) and in 39 of the 108 patients (36.1%) of the slow VDE subgroup at a mean 71.2 ± 52.9 months (median, 58; range, 5–233). Malignant progression-free survival was significantly longer in the slow VDE subgroup (median, 149 mo; mean, 142) than in the fast VDE subgroup (median, 46 mo; mean, 56.2) (P < .001; Fig. 2). A malignant progression was observed in 45 of the 107 patients (42.1%) of the subgroup with IDH1 mutation at a mean 70.0 ± 53.6 months (median, 55; range, 1.7–233) and in 11 of the 24 patients (45.8%) of the subgroup without IDH1 mutation at a mean 38.4 ± 24.8 months (median, 30; range, 5–88.5). Malignant progression-free survival was significantly longer in the subgroup with IDH1 mutation (median, 100 mo; mean, 131.2) than in the subgroup without IDH1 mutation (median, 72 mo; mean, 60.6) (P = .028; Fig. 2). In the subgroup of 107 patients with IDH1 mutation, malignant progression-free survival remained significantly longer in the slow VDE subgroup (median, 169 mo; mean, 149.3) than in the fast VDE subgroup (median, 46 mo; mean, 54.4) (P < .001; Fig. 2). In the subgroup of 108 patients with a slow VDE, malignant progression-free survival remained significantly longer in the subgroup with IDH1 mutation (median, 169 mo; mean, 149.3) than in the subgroup without IDH1 mutation (median, 72 mo; mean, 53.0) (P = .009). When compiling the 2 parameters, malignant progression-free survival was significantly longer in the subgroup with IDH1 mutation and slow VDE (median, 169 mo; mean, 149.2) than in the subgroup with IDH1 mutation only or with slow VDE only (median, 58.3 mo; mean, 67.2) and in the subgroup without IDH1 mutation and with fast VDE (median, 46 mo; mean, 56.0) (P < .001).
Fig. 2.
Kaplan–Meier estimates of overall survival and malignant progression-free survival according to spontaneous VDE and IDH1 mutation status. (A) Overall survival and malignant progression-free survival according to VDE (cutoff at 8 mm/y) (n = 131). The unadjusted hazard ratio for death among patients harboring an LGG with a spontaneous VDE ≥8 mm/y compared with those harboring an LGG with a spontaneous VDE <8 mm/y was 6.61 (95% CI, 2.69–12.2; P < .001). The unadjusted hazard ratio for death or malignant progression among patients harboring an LGG with a spontaneous VDE ≥8 mm/y compared with those harboring an LGG with a spontaneous VDE <8 mm/y was 4.18 (95% CI, 2.30–7.35; P < .001). (B) Overall survival and malignant progression-free survival according to IDH1 mutation status (n = 131). The unadjusted hazard ratio for death among patients harboring an LGG without an IDH1 mutation compared with those harboring an LGG with an IDH1 mutation was 3.27 (95% CI, 1.03–8.84; P = .044). The unadjusted hazard ratio for death or malignant progression among patients harboring an LGG without an IDH1 mutation compared with those harboring an LGG with an IDH1 mutation was 2.05 (95% CI, 1.03–3.79; P = .042). (C) Overall survival and malignant progression-free survival according to VDE (cutoff at 8 mm/y) in the subgroup of patients with an IDH1 mutation (n = 107). The unadjusted hazard ratio for death among patients harboring an LGG with a spontaneous VDE ≥8 mm/y compared with those harboring an LGG with a spontaneous VDE <8 mm/y was 8.49 (95% CI, 3.01–24.4; P < .001). The unadjusted hazard ratio for death or malignant progression among patients harboring an LGG with a spontaneous VDE ≥8 mm/y compared with those harboring an LGG with a spontaneous VDE<8 mm/year was 5.16 (95% CI, 2.64–9.73; P < .001).
During the follow-up period, 23 patients (17.6%) died at a mean 77.3 ± 59.6 months (median, 111; range, 5–198). A death was observed in 10 of the 23 patients (43.5%) of the fast VDE subgroup at a mean 57.2 ± 48.1 months (median, 38; range, 5–163) and in 13 of the 108 patients (12.0%) of the slow VDE subgroup at a mean 92.8 ± 64.6 months (median, 91; range, 12–198). Overall survival was significantly longer in the slow VDE subgroup (median, 198 mo; mean, 166.1) than in the fast VDE subgroup (median, 82 mo; mean, 73.6) (P < .001; Fig. 2). Death was observed in 18 of the 107 patients (16.8%) of the subgroup with IDH1 mutation at a mean 94.0 ± 56.7 months (median, 87.5 mo; range, 20–198) and in 5 of the 24 patients (20.8%) of the subgroup without IDH1 mutation at a mean 17.4 ± 11.0 months (median, 12 mo; range, 5–30). Overall survival was significantly longer in the subgroup with IDH1 mutation (median, 198 mo; mean, 159.3) than in the subgroup without IDH1 mutation (median, not reached; mean, 27.0 mo) (P = .001; Fig. 2). In the subgroup of 107 patients with IDH1 mutation, overall survival remained significantly longer in the slow VDE subgroup (median not reached; mean, 172.9 mo) than in the fast VDE subgroup (median, 84 mo; mean, 96.3) (P < .001; Fig. 2). In the subgroup of 108 patients with a slow VDE, overall survival remained significantly longer in the subgroup with IDH1 mutation (median not reached; mean, 172.9 mo) than in the subgroup without IDH1 mutation (median not reached; mean, 27.8 mo) (P = .014). When compiling the 2 parameters, overall survival was significantly longer in the subgroup with IDH1 mutation and slow VDE (median not reached; mean, 172.9 mo) than in the subgroup with IDH1 mutation only or with slow VDE only (median, 92 mo; mean, 103.4) and in the subgroup without IDH1 mutation and with fast VDE (median not reached; mean, 22.3 mo) (P < .001).
In univariate analysis (Table 2), predictors of malignant progression-free survival were spontaneous VDE (P < .001), tumor volume (P = .022), IDH1 mutation status (P = .042), first-line resection (P = .018), and extent of surgical resection (P = .038). Predictors of overall survival were sex (P = .002), presence of seizures at diagnosis (P = .011), contrast enhancement (P = .001), tumor volume (P = .022), corpus callosum involvement (P = .042), spontaneous VDE (P < .001), 1p19q codeletion (P = .031), IDH1 mutation status (P = .044), first-line resection (P = .042), and extent of surgical resection (P = .031). In multivariate analysis (Table 2), independent prognostic factors for malignant progression-free survival were increased intracranial pressure (P = .024), parietal tumor location (P = .019), spontaneous VDE (P = .001), IDH1 mutation status (P = .019), surgical resection (P = .015), and extent of surgical resection (partial, P = .021; total, P = .025). Independent factors for overall survival were male sex (P = .001), tumor volume (P = .017), spontaneous VDE (P < .001), IDH1 mutation status (P = .007), and extent of surgical resection (subtotal, P = .038).
Table 2.
Survival analyses
| Parameters | Overall Survival |
Malignant Progression-free survival |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted Hazard Ratio (HR) |
Adjusted Hazard Ratio |
Unadjusted Hazard Ratio |
Adjusted Hazard Ratio |
|||||||||
| HR | 95% CI | P | HRa | 95% CI | P | HR | 95% CI | P | HRa | 95% CI | P | |
| Clinical parameters | ||||||||||||
| Sex | ||||||||||||
| Female | 1 (ref) | 1 (ref) | ||||||||||
| Male | 5.06 | 1.72–21.5 | .002 | 10.22 | 2.58–17.8 | .001 | 162 | 0.94–2.88 | .082 | 1.77 | 0.88–3.74 | .108 |
| Age | ||||||||||||
| <40 | 1 (ref) | 1 (ref) | ||||||||||
| ≥40 | 1.10 | 0.48–2.60 | .821 | 1.26 | 0.74–2.18 | .389 | ||||||
| Increased intracranial pressure | ||||||||||||
| No | 1 (ref) | 1 (ref) | ||||||||||
| Yes | 1.58 | 0.79–2.09 | .667 | 5.33 | 0.85–17.9 | .067 | 11.31 | 1.44–59.7 | .024 | |||
| Neurological deficit | ||||||||||||
| No | 1 (ref) | 1 (ref) | ||||||||||
| Yes | 1.03 | 0.26–5.18 | .964 | 1.25 | 0.56–2.52 | .562 | ||||||
| Seizures | ||||||||||||
| Yes | 1 (ref) | 1 (ref) | ||||||||||
| No | 4.13 | 1.40–11.6 | .011 | 3.69 | 0.56–20.4 | .161 | 1.12 | 0.58–2.38 | .752 | |||
| KPS | ||||||||||||
| >70 | 1 (ref) | 1 (ref) | ||||||||||
| ≥70 | 2.84 | 0.44–10.1 | .224 | 2.32 | 0.70–5.76 | .151 | 1.30 | 0.18–5.38 | .745 | |||
| Imaging parameters | ||||||||||||
| Cerebral lobes involved | ||||||||||||
| 1 | 1 (ref) | 1 (ref) | ||||||||||
| ≥2 | 1.99 | 0.84–4.7 | .113 | 2.31 | 0.37–16.1 | .373 | 1.36 | 0.73–2.42 | .314 | |||
| Corpus callosum involvement | ||||||||||||
| No | 1 (ref) | 1 (ref) | ||||||||||
| Yes | 4.69 | 1.07–14.6 | .042 | 1.35 | 0.19–7.83 | .743 | 2.03 | 0.61–5.02 | .220 | |||
| Anatomic location | ||||||||||||
| Frontal | 1 (ref) | 1 (ref) | ||||||||||
| Temporal | 1.63 | 0.57–4.32 | .352 | 1.94 | 0.93–3.83 | .074 | 2.05 | 0.63–5.95 | .215 | |||
| Parietal | 1.43 | 0.31–4.69 | .599 | 1.92 | 0.84–4.01 | .116 | 4.20 | 1.28–12.99 | .019 | |||
| Insular | 0.92 | 0.21–2.99 | .896 | 1.53 | 0.72–3.07 | .252 | 2.29 | 0.53–8.12 | .069 | |||
| Contrast enhancement | ||||||||||||
| No | 1 (ref) | 1 (ref) | ||||||||||
| Yes | 1.79 | 0.03–2.67 | .001 | 2.29 | 0.02–21.5 | .774 | 1.95 | 0.74–4.24 | .159 | 1.98 | 0.67–5.04 | .206 |
| Tumor volume, cm3 | ||||||||||||
| <100 | 1 (ref) | 1 (ref) | ||||||||||
| ≥100 | 2.44 | 1.15–4.72 | .0022 | 9.69 | 1.49–70.1 | .017 | 2.44 | 1.14–4.72 | .022 | 1.41 | 0.47–3.76 | .514 |
| VDE | ||||||||||||
| <8 mm/y | 1 (ref) | 1 (ref) | ||||||||||
| ≥8 mm/y | 6.61 | 2.69–12.2 | <.0001 | 26.3 | 5.42–185.2 | <.0001 | 4.18 | 2.30–7.35 | <.0001 | 4.23 | 1.81–9.40 | .001 |
| Histopathological parameters | ||||||||||||
| Histological subtype | ||||||||||||
| Astrocytoma | 1 (ref) | 1 (ref) | ||||||||||
| Oligodendroglioma | 0.94 | 0.31–4.09 | .926 | 0.92 | 0.45–2.05 | .819 | ||||||
| Mixed glioma | 0.88 | 0.22–4.35 | .873 | 1.09 | 0.48–2.67 | .826 | ||||||
| Oligodendroglial component | ||||||||||||
| Yes | 1 (ref) | 1 (ref) | ||||||||||
| No | 1.41 | 0.40–3.81 | .549 | 0.91 | 0.47–1.94 | .806 | ||||||
| Proliferation rates | ||||||||||||
| <5% | 1 (ref) | 1 (ref) | ||||||||||
| ≥5% | 1.28 | 0.53–3.27 | .577 | 1.13 | 0.62–2.02 | .684 | ||||||
| 1p19q Codeletion | ||||||||||||
| Yes | 1 (ref) | 1 (ref) | ||||||||||
| No | 3.91 | 1.11–24.7 | .031 | 2.92 | 0.35–60.9 | .353 | 1.50 | 0.80–2.87 | .220 | |||
| p53 Overexpression | ||||||||||||
| No | 1 (ref) | 1 (ref) | ||||||||||
| Yes | 2.42 | 0.95–7.41 | .062 | 1.38 | 0.26–11.32 | .720 | 1.33 | 0.77–2.34 | .307 | |||
| IDH1 expression | ||||||||||||
| Yes | 1 (ref) | 1 (ref) | ||||||||||
| No | 3.27 | 1.03–8.84 | .044 | 17.86 | 2.15–200.1 | .007 | 2.05 | 1.03–3.79 | .042 | 2.39 | 1.19–4.66 | .019 |
| Therapeutic parameters | ||||||||||||
| First-line surgery | ||||||||||||
| No | 1 (ref) | 1 (ref) | ||||||||||
| Yes | 0.41 | 0.16–0.97 | .042 | 2.05 | 0.57–7.83 | .270 | 0.53 | 0.31–0.90 | .018 | 0.40 | 0.20–0.83 | .015 |
| First-line surgery | ||||||||||||
| Biopsy | 1 (ref) | 1 (ref) | ||||||||||
| Partial removal | 0.18 | 0.01–0.88 | .031 | 0.21 | 0.04–1.52 | .088 | 0.44 | 0.15–1.05 | .067 | 0.27 | 0.07–0.83 | .021 |
| Subtotal removal | 0.59 | 0.21–1.47 | .265 | 0.22 | 0.05–0.91 | .038 | 0.64 | 0.34–1.14 | .127 | 0.51 | 0.24–1.11 | .091 |
| Total removal | 0.31 | 0.02–1.58 | .188 | 0.23 | 0.02–6.99 | .349 | 0.34 | 0.08–0.94 | .038 | 0.25 | 0.05–0.85 | .025 |
Boldface indicates statistically significant.
Discussion
We show for the first time in a retrospective exploratory dataset of 131 homogeneous cases that IDH1 mutation status represents an independent prognostic factor for LGG clinical outcome among adult patients, together with the spontaneous VDE that reflects the imaging tumor growth. Specifically, we find that they are associated, independently from each other, with better malignant progression-free survival and overall survival, whatever the clinical, imaging, histopathological, and molecular findings, including 1p19q codeletion and p53 overexpression. Thus, the determination of spontaneous VDE and IDH1 mutation status adds to the 1p19q codeletion status in determining outcomes of adult patients harboring an LGG.
In LGGs, IDH1 mutation proved to have significant impact on overall survival only,8,17,22–25 although with contradictory results,26,27 which may be explained by the heterogeneity of the histopathological LGG subgroup. Here, we confirm that IDH1 mutation impacts both malignant progression-free survival and overall survival of LGGs. However, the small number of observed deaths in the present study limits the observation. Further study with a longer follow-up period is needed. IDH1 mutation and 1p19q codeletion are independent favorable prognostic factors for LGGs, but previous studies showed that these molecular parameters are closely associated.15,17 Moreover, 1p19q codeletion status affects the imaging tumor growth, although IDH1 mutation does not. Thus, ignoring the effects of 1p19q codeletion, of IDH1 mutation status, and of spontaneous VDE, along with their interactions, while estimating their prognostic significance could lead to serious bias. We thus incorporated these parameters in the statistical modeling. In addition, we confirmed these results obtained on the whole series by performing complementary statistical analyses on the subgroup of LGGs with an IDH1 mutation (n = 107) and on the subgroup of LGGs with a slow VDE (n = 108).
In addition, we confirm that IDH1 mutation status does not significantly impact the spontaneous VDE, in accordance with previous reports12,17 and in accordance with the conflicting results of the effects of IDH1 mutation on glioma cell proliferation in experimental preparations.28–30 These findings reinforce the hypothesis that IDH mutations, because of their precocity, might be considered instead as a causative link between early cellular metabolism disturbances and the emergence of other driving molecular events in gliomagenesis15,17 in accordance with particular associations of gene mutations in brain tumors.31–33
The main limitation of the present retrospective study is the use of different techniques to assess IDH1 mutation status. The research of IDH1 gene mutations was the most frequent technique, performed in 77 cases (58.8%),17,21 and the research restricted to IDH1 R132H mutation by immunohistochemistry was performed in only 54 cases (41.2%).12,20 IDH2 mutations, present in only 2%–3% of gliomas,34 are unlikely to alter the present results. Of note, an IDH2 mutation was present in 3 cases of the subgroup of LGGs without IDH1 mutation (3.8%), 1 of them corresponding to the unique patient of this subgroup who is alive without malignant transformation at 130 months follow-up (see Fig. 2).17,21 Altogether, these findings should be interpreted with caution, given the inherent limitations arising from the retrospective study design and the possible bias resulting from the IDH mutation screening in this exploratory dataset.
Outcomes of LGGs vary considerably; the wide ranges of prognoses reflect the heterogeneity of LGGs and could be explained by the limitations of the histopathological diagnosis. Molecular markers, particularly 1p19q codeletion, p53 expression, and IDH1 mutation status, have improved diagnostic accuracy and refined prognosis. Although spontaneous VDE was shown to correlate with molecular markers, it represents an independent prognostic parameter for overall survival and for malignant progression-free survival and may help in predicting the natural history of LGGs.11,12 As the spontaneous VDE adds to IDH1 mutation status and to 1p19q codeletion status in determining outcomes, we propose that the spontaneous VDE could be integrated together with the other known prognostic parameters, including IDH1 mutation status, 1p19q codeletion status, and p53 overexpression, in a multidimensional approach to better predict the outcomes of an LGG at the individual level. Finally, the assessment of the prognostic significance of the spontaneous VDE is clinically relevant and would require prospective validation within the context of multicenter clinical trials.
As a practical consequence, the spontaneous VDE should be measured systematically10,12,14 at the beginning of the management of an LGG without delaying treatment and should be added to the other known risk parameters (Pignatti and Chang prognostic scores, contrast enhancement, astrocytoma subtype, 1p19q codeletion, p53 overexpression, IDH1 mutation)2,4–6,8 to adapt treatment and follow-up on an individual basis. Patients with a fast VDE should be considered as “high-risk,” with an increased risk of early malignant transformation, particularly in cases where only a biopsy has been performed due to the possible risk of undergrading. Because these LGGs share outcomes similar to those of malignant gliomas, treatment modalities should be selected accordingly, and a 3-month follow-up should be preferred.12
Funding
This work was supported by the Ligue Nationale contre le Cancer.
Acknowledgments
These physicians are greatly acknowledged (in alphabetical order): Georges Abi-Lahoud, Valérie Bernier, Françine Chassoux, Philippe Colin, Fabrice Chrétien, Frédéric Dhermain, Julien Domont, Denys Fontaine, Marc Frenay, Jacques Guyotat, Maria Koziak, Elisabeth Landre, Emmanuel Mandonnet, Michael Mann, Jean-François Meder, Charles Mellerio, Olivier Naggara, Karima Mokhtari, François Nataf, Catherine Oppenheim, Eduardo Parraga, Philippe Peruzzi, François-Xavier Roux, Raphaëlle Souillard-Scemama, Baris Turak. All authors have participated in the writing of this manuscript and all authors approve this final version.
Conflict of interest statement. None declared.
References
- 1.Louis DN, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. >Lyon, France: IARC Press; 2007. [Google Scholar]
- 2.Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO* Task Force. Eur J Neurol. 2010;17(9):1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 3.Bauman G, Lote K, Larson D, et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45(4):923–929. doi: 10.1016/s0360-3016(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 4.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 5.Chang EF, Smith JS, Chang SM, et al. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109(5):817–824. doi: 10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 6.Pallud J, Capelle L, Taillandier L, et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro-Oncol. 2009;11(2):176–182. doi: 10.1215/15228517-2008-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nobusawa S, Watanabe T, Kleihues P, et al. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 8.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 9.Ducray F, Idbaih A, Wang X-W, et al. Predictive and prognostic factors for gliomas. Expert Rev Anticancer Ther. 2011;11(5):781–789. doi: 10.1586/era.10.202. [DOI] [PubMed] [Google Scholar]
- 10.Pallud J, Taillandier L, Capelle L, et al. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma. Neurosurgery. 2012;71(3):729–740. doi: 10.1227/NEU.0b013e31826213de. [DOI] [PubMed] [Google Scholar]
- 11.Pallud J, Mandonnet E, Duffau H, et al. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol. 2006;60(3):380–383. doi: 10.1002/ana.20946. [DOI] [PubMed] [Google Scholar]
- 12.Pallud J, Blonski M, Mandonnet E, et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro-Oncol. 2013;15(5):595–606. doi: 10.1093/neuonc/nos331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandonnet E, Delattre J-Y, Tanguy M-L, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 14.Mandonnet E, Pallud J, Clatz O, et al. Computational modeling of the WHO grade II glioma dynamics: principles and applications to management paradigm. Neurosurg Rev. 2008;31(3):263–269. doi: 10.1007/s10143-008-0128-6. [DOI] [PubMed] [Google Scholar]
- 15.Labussière M, Idbaih A, Wang X-W, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74(23):1886–1890. doi: 10.1212/WNL.0b013e3181e1cf3a. [DOI] [PubMed] [Google Scholar]
- 16.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61(5):484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 17.Gozé C, Bezzina C, Gozé E, et al. 1p19q loss but not IDH1 mutations influences WHO grade II gliomas spontaneous growth. J Neurooncol. 2012;108(1):69–75. doi: 10.1007/s11060-012-0831-6. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174(4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Pallud J, Llitjos J-F, Dhermain F, et al. Dynamic imaging response following radiation therapy predicts long-term outcomes for diffuse low-grade gliomas. Neuro-Oncol. 2012;14(4):496–505. doi: 10.1093/neuonc/nos069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blonski M, Pallud J, Gozé C, et al. Neoadjuvant chemotherapy may optimize the extent of resection of World Health Organization grade II gliomas: a case series of 17 patients. J Neurooncol. 2013;113(2):267–275. doi: 10.1007/s11060-013-1106-6. [DOI] [PubMed] [Google Scholar]
- 22.Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120(6):719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 23.Leu S, Felten von S, Frank S, et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro-Oncol. 2013;15(4):469–479. doi: 10.1093/neuonc/nos317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Yin L, Li S, et al. Prognostic significance of IDH mutation in adult low-grade gliomas: a meta-analysis. J Neurooncol. 2013;113(2):277–284. doi: 10.1007/s11060-013-1107-5. [DOI] [PubMed] [Google Scholar]
- 25.Dahlrot RH, Kristensen BW, Hjelmborg J, et al. A population-based study of low-grade gliomas and mutated isocitrate dehydrogenase 1 (IDH1) J Neurooncol. 2013;114(3):309–317. doi: 10.1007/s11060-013-1186-3. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadi R, Stockhammer F, Becker N, et al. No prognostic value of IDH1 mutations in a series of 100 WHO grade II astrocytomas. J Neurooncol. 2012;109(1):15–22. doi: 10.1007/s11060-012-0863-y. [DOI] [PubMed] [Google Scholar]
- 27.Thon N, Eigenbrod S, Kreth S, et al. IDH1 mutations in grade II astrocytomas are associated with unfavorable progression-free survival and prolonged postrecurrence survival. Cancer. 2012;118(2):452–460. doi: 10.1002/cncr.26298. [DOI] [PubMed] [Google Scholar]
- 28.Jin G, Pirozzi CJ, Chen LH, et al. Mutant IDH1 is required for IDH1 mutated tumor cell growth. Oncotarget. 2012;3(8):774–782. doi: 10.18632/oncotarget.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Cui G, Chen M, et al. Expression of R132H mutational IDH1 in human U87 glioblastoma cells affects the SREBP1a pathway and induces cellular proliferation. J Mol Neurosci. 2013;50(1):165–171. doi: 10.1007/s12031-012-9890-6. [DOI] [PubMed] [Google Scholar]
- 30.Bralten LBC, Kloosterhof NK, Balvers R, et al. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69(3):455–463. doi: 10.1002/ana.22390. [DOI] [PubMed] [Google Scholar]
- 31.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333(6048):1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arita H, Narita Y, Takami H, et al. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013;126(6):939–941. doi: 10.1007/s00401-013-1203-9. [DOI] [PubMed] [Google Scholar]
- 33.Jiao Y, Killela PJ, Reitman ZJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–722. doi: 10.18632/oncotarget.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]