Abstract
Novel treatment options, including targeted therapies, are needed for patients with medulloblastoma (MB), especially for those with high-risk or recurrent/relapsed disease. Four major molecular subgroups of MB have been identified, one of which is characterized by activation of the sonic hedgehog (SHH) pathway. Preclinical data suggest that inhibitors of the hedgehog (Hh) pathway could become valuable treatment options for patients with this subgroup of MB. Indeed, agents targeting the positive regulator of the pathway, smoothened (SMO), have demonstrated efficacy in a subset of patients with SHH MB. However, because of resistance and the presence of mutations downstream of SMO, not all patients with SHH MB respond to SMO inhibitors. The development of agents that target these resistance mechanisms and the potential for their combination with traditional chemotherapy and SHH inhibitors will be discussed. Due to its extensive molecular heterogeneity, the future of MB treatment is in personalized therapy, which may lead to improved efficacy and reduced toxicity. This will include the development of clinically available tests that can efficiently discern the SHH subgroup. The preliminary use of these tests in clinical trials is also discussed herein.
Keywords: expression profiling, hedgehog, medulloblastoma, targeted therapy
Molecular studies have identified pathways involved in the tumorigenesis of many cancers including medulloblastoma (MB), the most common malignant brain tumor in young children.1,2 The hedgehog (Hh) pathway was first implicated in MB when germline mutations in patched (PTCH) were detected in patients with Gorlin syndrome, a heritable condition associated with an increased risk of MB and certain other cancers including basal cell carcinoma (BCC).3,4 Since that discovery, profiling studies and other genetic analyses have confirmed the involvement of the Hh pathway in the pathogenesis of MB and BCC.5–10 In addition, inhibitors of the Hh pathway have demonstrated efficacy in MB and BCC.11–19 Vismodegib recently became the first US Food and Drug Administration (FDA)-approved Hh pathway inhibitor based on antitumor activity observed in a phase 2 study in participants with advanced BCC.13,20 Vismodegib and other agents targeting the Hh pathway are currently being tested in clinical trials in participants with Hh-activated MB.21 The outcome of these trials may change the landscape of MB treatment, resulting in a more personalized approach with therapies that offer improved efficacy and reduced toxicity.
Unmet Need in Medulloblastoma
There is a significant need for targeted therapies in the treatment of patients with MB, especially those with high-risk or recurrent/relapsed disease.1,22 Patients with high-risk disease, including those younger than aged 3–6 years or those with metastatic disease, large cell or anaplastic histology, or poorly resected tumors,1,22–24 have lower survival rates than patients with standard-risk disease; 5-year survival rates are 55%–76% and 70%–85%, respectively.1,25 There is no effective salvage treatment for patients with recurrent/relapsed disease, and the prognosis for these patients is poor.2,22,26
In addition, most survivors suffer from long-term toxicities associated with the current standard-of-care treatment, which includes surgery followed by craniospinal radiation and chemotherapy.2,22,25–27 In particular, craniospinal radiation-induced neurocognitive toxicities, which are inversely related to patient age, can be severe.22,24,25,27 For this reason, craniospinal radiation is not recommended for patients younger than aged 3–6 years.22,24,25 In these patients, postsurgical chemotherapy is suggested and usually includes high-dose chemotherapy with stem-cell rescue.22
Classification of Medulloblastoma: A Focus on the Sonic Hedgehog Molecular Subgroup
The 4 major histological variants of MB according to the World Health Organization include classical, desmoplastic/nodular, MB with extensive nodularity, and anaplastic/large cell,28 each of which is associated with distinct morphology. Patient age and prognosis have also been associated with the different variants.23,28 Recently, efforts to differentiate MB have shifted from histological to molecular classification. A consensus, based on gene expression profiling data from several independent laboratories,29–32 reports 4 molecular subgroups of MB33: wingless (WNT; group 1), sonic hedgehog (SHH; group 2), group 3 (v-myc avian myelocytomatosis viral oncogene homolog [MYC] amplified), and group 4. Patient demographics, histology, DNA copy-number aberrations, and prognosis generally differ between the 4 molecular subgroups; however, there is some overlap among the 4 groups. Additional characteristics of the WNT group, group 3, and group 4 are reported in the consensus paper.33
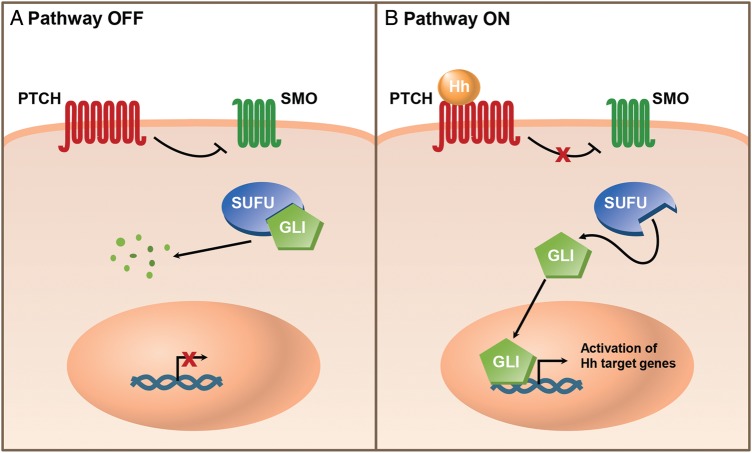
The SHH group is characterized by activated Hh pathway signaling.33 The Hh pathway is important for cell proliferation, differentiation, and survival during embryonic and fetal development9,34 and later, during postnatal development and adulthood, plays a role in bone development, stem cell maintenance, and maintenance and repair of some tissues.9,35,36 Hh signaling is initiated when 1 of 3 Hh ligands (SHH, Indian hedgehog, or desert hedgehog) binds to the transmembrane receptor PTCH, releasing its inhibition of the signal transducer smoothened (SMO).37 Activation of SMO initiates downstream signaling events, including release of glioma-associated oncogene (GLI) transcription factors from suppressor of fused (SUFU), a negative regulator of the pathway, allowing GLIs to translocate to the nucleus and induce expression of Hh pathway target genes (Fig. 1).37
Fig. 1.
The hedgehog signaling pathway. (A) In the absence of hedgehog (Hh) ligands, the transmembrane receptor patched (PTCH) inhibits entry of the G-protein-coupled receptor-like transmembrane signal transducer smoothened (SMO). Glioma-associated oncogene (GLI) transcription factors are sequestered in the cytoplasm in a complex containing the negative regulator suppressor of fused (SUFU). GLIs are processed into a transcriptional repressor form or degraded. (B) Hh signaling is activated when Hh binds to PTCH on the cell surface. Both molecules are then internalized (and PTCH is degraded), allowing SMO to activate downstream signaling events. The GLI transcription factors are released from SUFU and processed into their activating forms. Activated GLIs translocate into the nucleus and promote transcription of Hh pathway target genes.
Aberrant expression of Hh-target genes leads to excessive cell proliferation and tumorigenesis.9,35 SHH signaling, which normally stimulates the proliferation of lineage-restricted cerebellar granule neuron precursors (CGNPs) during cerebellar development,38–40 can lead to MB formation when aberrantly activated.40–42 Mutations in PTCH and SUFU have been identified in up to 17% and 10% of MBs, respectively.32,43–46 Amplification of GLI2 has also been identified in MB.47–50
In addition to aberrant SHH signaling, the SHH subgroup is sometimes characterized by v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog (MYCN) expression, gain of chromosome 3q, p53 mutations, and amplification of MYCN.8,33,51 The SHH subgroup is most prevalent in infants (< aged 4 years) and adolescents/adults (>aged 16 years) and less common in children aged 4–16 years (Fig. 2).8,33 This subgroup comprises all histological subtypes and does not often metastasize,8,33 with recurrences mainly occurring locally.52 Prognosis for patients with SHH-activated MB is intermediate.8,30,33
Fig. 2.
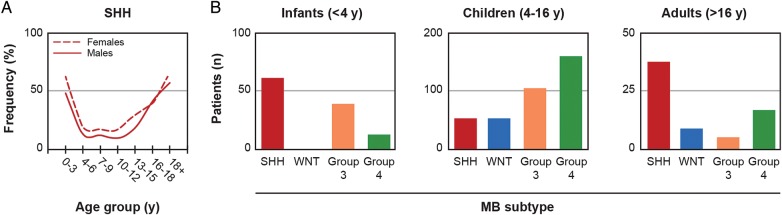
Frequency of the sonic hedgehog subgroup of medulloblastoma by age. (A) Frequency of the sonic hedgehog (SHH) subgroup of medulloblastoma (MB) in males and females by age group. (B) Incidences of the wingless (WNT), SHH, group 3, and group 4 subgroups of MB in infants, children, and adults. Figure adapted from Kool M, et al. Acta Neuropathol. 2012; 123(4): 473–484,8 under terms of the Creative Commons Attribution License.
Pediatric and adult patients with SHH-activated MB are distinct from one another in terms of genomic alterations, metastasis, and prognosis.47 A recent genomic sequencing analysis of adult and pediatric patients with SHH-activated MB identified age-dependent subgroups including those with PTCH1 mutations (across all age groups), SUFU mutations (infants), and SMO mutations (adults).49 Downstream amplifications of MYCN and GLI2 as well as mutations in p53 were identified in children between the ages of 4–17 years but were rarely observed in infants and adults.49 MBs with mutations in p53 have been associated with Li-Fraumeni syndrome and a worse overall prognosis.51 Overexpression of the chemokine receptor CXCR4 has been observed in young patients with desmoplastic histology.53 Data from a meta-analysis of 550 tumor samples from patients with MB (28% SHH) showed that metastasis was more common in infants (<aged 4 years, 17%) and children (aged 4–16 years, 22%) with SHH-activated MB than in adults.8 In another study of 66 SHH MBs, metastasis was found to be a marker for poor prognosis in adults but not in children.47 Survival data from the meta-analysis showed that 10-year overall survival (OS) in patients with SHH tumors is much higher in infants (77%) than in children (51%) and adults (34%).8 This may be due in part to the high percentage of infants with desmoplastic histology, which has been associated with a more favorable prognosis. In the analysis of 66 SHH MBs, children with desmoplastic histology were shown to have a better prognosis than children with classic histology; in contrast, desmoplastic histology was not prognostic for adults.47 Interestingly, adults with SHH MB carrying chromosomal alterations (2 gain, 10q deletion, 17q gain, 17p deletion) and/or GLI2 amplification have a much worse prognosis than children carrying the same genetic aberrations;47 however, the reasons for this difference are unclear and may relate to regimen intensity rather than chromosomal alterations.
Preclinical Evidence Supporting the Role of Hh Signaling in Medulloblastoma
Numerous preclinical studies have identified a role for the Hh pathway in the tumorigenesis of MB and further demonstrated that inhibition of the Hh pathway impedes tumor growth. Mouse models of MB, such as Ptch null, Ptch+/–p53–/–, and Ptch+/– hypermethylated in cancer 1 [Hic1]+/–, have been particularly useful for in vivo preclinical testing of Hh inhibitors because tumor cells cultured in vitro may differ from in vivo tumors with respect to biological characteristics and responses.54,55 In mouse models, treatment with inhibitors of SMO, the positive regulator of the Hh signaling pathway, leads to reduced tumor growth54,56–59 and increased survival.54,56–58
Hh pathway targets that may contribute to Hh-dependent MB tumorigenesis have been identified (Table 1). In MB cells and tumor models, SHH signaling has been shown to induce expression and/or stabilization of MYCN, miR-17/92, Snail1, CXCR4, Math1/Atoh1, cyclin D1, Sox2, Sox9, abnormal spindle-like microcephaly-associated, Bmi1, and Bcl2 in CGNPs and brain tumor initiating cells (BTICs), leading to increased proliferation and tumor growth.53,60–71 Inhibition or deletion of these targets reduces cell proliferation and tumor growth in MB models.53,64,66,72 In addition, several studies have shown that SHH-mediated metabolic programming increases MB tumor progression.73–75
Table 1.
Preclinical evidence supporting the role of hedgehog pathway targets in medulloblastoma tumorigenesis
| Hh Pathway Target | Effects of Activated Hh Signaling | Suspected Role in MB Tumorigenesis | Effects of Targeted Inhibition |
|---|---|---|---|
| MYCN60,61 | Increased expression in CGNPs | Induces miR-17/92 expression in CGNPs, promoting their proliferation | Anti–miR-17 and -19 reduced growth of MB tumor allografts (flank and brain) and prolonged survival in mice with intracranial transplants113 |
| Snail162 | Increased expression in GCPs, mouse MB models, and human MB-derived cell lines | Induces cell proliferation and transformation through induction of MYCN transcription | |
| CXCR453,72 | Induces cell surface localization and effector signaling in MB cells | Causes progrowth transcriptional response (increased expression of Math1/Atoh1 and cyclin D1) and increased tumor growth | CXCR4-specific antagonists, AMD3100 and AMD3465, inhibited growth of MB tumor xenografts |
| Sox264 | Increased expression in CGNPs and Sox2-positive MB in mouse models | Overexpression leads to proliferation | Deletion of Sox2 in primary CGNP cultures with constitutive SHH signaling led to decreased proliferation |
| Sox965 | Increased expression in SHH MB | Activation during embryogenesis leads to SHH-dependent MB; suppression during postnatal development promotes SHH-independent MB | |
| ASPM66 | Increased expression in CGNPs | Sustains the progenitor phenotype of CGNPs | ASPM knock-out led to decreased proliferative capacity and self-renewal |
| Bmi169–71 | Drives expression in BTICs | Contributes to SHH-mediated expansion of GCPs through direct regulation of the cyclin-dependent kinase inhibitor p21 Waf1/Cip1 and modulation of other cell cycle inhibitors and TGFβ pathway targets; positive feedback loop between Bmi1 and SHH in BTICs; may regulate the SHH pathway through transcriptional silencing of the E3 ubiquitin ligase Cullin3 | |
| Bcl267,68 | Increased expression | Increases proliferation and decreases apoptosis of SHH-induced MB | |
| Lipids73 | Lipid accumulation, increased lipogenic enzyme expression, and suppression of fatty acid oxidation | Fatty acid synthase-specific inhibitor C75 inhibited MB cell proliferation, promoted MB cell death, and prolonged survival in MB-bearing mice | |
| PPARγ74 | Increased expression in MB cells | Induces glycolysis | PPARγ-specific inhibitor, GW9662, blocked CGNP proliferation, drove MB cell death, and prolonged survival of mice with MBs (NeuroD2-SmoA1) |
Abbreviations: ASPM, abnormal spindle-like microencephaly-associated; Atoh1, atonal homolog 1; BTIC, brain tumor initiating cell; CGNP, cerebellar granule neuron progenitor; GCP, granule cell progenitor; MB, medulloblastoma; MYCN, v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog; PPARγ, peroxisome proliferator-activated receptor gamma; SHH, sonic hedgehog; Sox, sex determining region Y; TFGβ, transforming growth factor beta.
Signaling pathways that interact with Hh signaling in MB have also been identified (Table 2). Expression of components of the insulin growth factor (IGF), placental growth factor, phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR), WNT, and Notch pathways have been identified in SHH-treated CGNPs and SHH-mediated MBs.76–81 Activation of these pathways is required for and/or enhances SHH-induced MB formation.76,80–83 SHH signaling (proliferation) and IGF signaling (survival) also converge to regulate MYCN- and yes-associated protein (YAP)-mediated cell cycle control in CGNPs.84–86 Conversely, pituitary adenylate cyclase-activating polypeptide (PACAP)/cyclic adenosine monophosphate-dependent protein kinase A (PKA) signaling may antagonize Hh signaling in Hh-dependent MB.87,88
Table 2.
Signaling pathways shown to interact with the hedgehog pathway in preclinical models of medulloblastoma
| Signaling Pathway | Expression of Pathway Components in SHH-dependent MB Models | Suspected Role in MB Tumorigenesis |
|---|---|---|
| IGF |
|
|
| PlGF |
|
|
| AKT |
|
|
| WNT |
|
|
| Notch |
|
|
| PKA |
|
|
Abbreviations: CGNP, cerebellar granule neuron progenitor; Gli1, glioma-associated oncogene homolog 1; GNP, granule neuron progenitor; GSK3β, glycogen synthase kinase-3 beta; Hh, hedgehog; IGF, insulin-like growth factor; IRS1, insulin receptor substrate-1; MB, medulloblastoma; mTOR, mammalian target of rapamycin; MYCN, v-myc avian myelocytomatosis viral oncogene neuroblastoma derived homolog; Nrp1, neuropilin; PAC1, PACAP receptor 1; PACAP, pituitary adenylyl cyclase activating polypeptide; PlGF, placental growth factor; PKA, protein kinase A; SHH, sonic hedgehog; TSC, tuberous sclerosis complex; WNT, wingless; YAP, yes-associated protein.
Methods Being Employed to Identify SHH-activated Medulloblastoma and Their Use in the Clinic
Immunohistochemistry (IHC) and/or reverse-transcriptase polymerase chain reaction (RT-PCR) have been used to assess Hh pathway activity in patients with cancer based on GLI1 expression in hair, skin, and tumor biopsies.11,17,18,30,89,90 These methods have been used in several phase 1 trials of SMO inhibitors to determine Hh pathway activity and confirm targeted inhibition of the pathway.11,17,18 However, no IHC-based grouping of these tumors has been validated, and interobserver variability in identifying positive staining limits its clinical utility for patient stratification.5 Furthermore, due to cost and time constraints, these methods are not amenable for use as a patient preselection tool in large randomized trials.
Gene expression profiling methods in combination with IHC and/or RT-PCR have been used to identify MB subgroups in archival tumor samples.31,32 Several groups have used published profiling data to identify gene signatures specific to the different MB subgroups including the SHH subgroup.5–7 In addition, the assays developed have been optimized for use in the clinic; they are quicker, more cost-effective, and require less RNA than standard profiling techniques. Schwalbe et al identified an 8-gene SHH signature (BCHE, GLI1, ITIH2, MICAL1, PDLIM3, PTCH2, RAB33A, and SFRP1) for use in snap-frozen tumor samples.6 Northcott et al identified a 5-gene SHH signature (PDLIM3, EYA1, HHIP, ATOH1, and SFRP1) optimized for use in formalin-fixed paraffin-embedded (FFPE) tumor samples.5 At the American Association for Cancer Research annual meeting in 2012, Amakye et al presented the development of their unique 5-gene SHH signature (GLI1, SPHK1, SHROOM2, PDLIM3, and OTX2), which has also been optimized for use in FFPE tumor samples.7
The 5-gene SHH signature identified by Amakye et al received an investigational device exemption from the FDA for use in a clinical trial (NCT01708174; a phase 3 study of oral sonidegib (LDE225) vs temozolomide [TMZ] in participants with Hh pathway-activated relapsed MB; Novartis documentation on file), and to date it is the only signature that has shown an association with tumor response in participants treated with an Hh pathway inhibitor.91,92 Data presented at the Society for Neuro-Oncology Scientific Meeting in 2013 showed that, in 41 MB tumors from participents treated with sonidegib in 3 independent trials (adults with advanced solid tumors [NCT00880308], East Asian adults with advanced solid tumors [NCT01208831], and children with tumors potentially dependent on the Hh pathway [phase 1; NCT01125800]; Table 3), all who responded to sonidegib treatment were predicted to have Hh pathway-activated tumors using the 5-gene Hh signature assay.92 Three of 4 adults with Hh-activated MB responded (3 partial responses [PRs]) and 2 of 3 children with Hh-activated MB responded (2 complete responses [CRs]). Two participants (1 child, 1 adult) with Hh-activated MB had progressive disease. The reason for lack of response in these individuals is unknown but may be due to mutations downstream of SMO. The remaining 34 participants, who were predicted to have Hh pathway-nonactivated tumors, did not respond (5 stable disease, 28 progressive disease) or were not assessed (n = 1). In the phase 1 study in children with recurrent/refractory MB or other tumors potentially dependent on the Hh pathway (NCT01125800), only participants with MB responded.19 A phase 2 portion of the trial in pediatric and adult patients with recurrent or refractory MB has recently completed accrual (NCT01125800).19 The phase 3 trial in participants with relapsed Hh-activated MB described earlier is currently recruiting (NCT01708174).92 Participants will be treated with sonidegib or TMZ. Participants with TMZ-naive Hh-activated MB, as determined by the 5-gene signature assay mentioned above, who have relapsed following standard therapy will be eligible for randomization (2:1, sonidegib:TMZ). Participants who have previously been treated with TMZ or children < aged 6 years who are not candidates for or have declined radiotherapy will be eligible for a nonrandomized portion of the trial. The primary endpoint is overall response rate. Secondary endpoints include progression-free survival (PFS), duration of response (DoR), OS, safety, pharmacokinetics, and effects of sonidegib on Hh pathway biomarkers.
Table 3.
Clinical studies of hedgehog pathway inhibitors in participants with medulloblastoma
| Agent | Phase | Participant Population | Statusa | ClinicalTrials.gov |
|---|---|---|---|---|
| Sonidegib (LDE-225) | 1 | Adults with advanced solid tumors | Completed | NCT0088030818 |
| 1 | East Asian adults with advanced solid tumors | Active, not recruiting | NCT01208831 | |
| 1/2 | Children with recurrent/refractory MB or other tumors potentially dependent on the Hh pathway | Active, not recruiting | NCT0112580019 | |
| 3b | Patients with Hh pathway activated, relapsed MB | Recruiting | NCT0170817492 | |
| Vismodegib (GDC-0449) | 1 | Patients with refractory, locally advanced, or metastatic solid tumors | Completed | NCT0060772411,12 |
| 1 | Children with recurrent or refractory MB | Completed | NCT0082245816 | |
| 1/2c | Adults with recurrent, progressive, or refractory SHH-activated MB | Recruiting | NCT01601184 | |
| 2 | Adults with recurrent or refractory MB | Active, not recruiting | NCT0093948415 | |
| 2 | Children with recurrent or refractory MB | Active, not recruiting | NCT01239316 | |
| 2 | Children with newly diagnosed MB | Recruiting | NCT01878617 | |
| LY2940680 | 1 | Children with recurrent or refractory MB or rhabdomyosarcoma | Withdrawn due to poor accrual | NCT01697514 |
Abbreviations: Hh, hedgehog; MB, medulloblastoma; SHH, sonic hedgehog.
aStatus as of May 1, 2014.
bStudy will compare sonidegib versus temozolomide.
cStudy will evaluate treatment with vismodegib in combination with temozolomide versus temozolomide alone.
The only other Hh pathway inhibitor being tested specifically in participants with MB is the SMO inhibitor vismodegib (GDC-0449; Table 3). A PR was demonstrated in a participant with metastatic MB in the first-in-human phase 1 study of vismodegib (NCT00607724); this individual relapsed after 3 months.11,12 Among participants with treatment-refractory MB and SHH pathway activation (n = 7; determined by IHC) in a phase 1 study of vismodegib in children with recurrent or refractory MB (NCT00822458), 1 participant achieved a CR lasting <8 weeks; none of the participants with non-SHH tumors responded.16 A phase 2 study in adults with recurrent or refractory MB is ongoing (NCT00939484).15 Of the 32 participants enrolled, 20 were shown to have Hh-activated pathway as determined by IHC. Three participants (15%) with Hh-activated tumors had sustained responses. Six participants remained on treatment for >6.44 months and 3 were on therapy after 5.42, 9.34, and 13.61 months at the time the abstract was presented. Another ongoing phase 2 study in children with recurrent or refractory MB (NCT01239316) is investigating objective response rates based on Hh-activation status (as determined by pathological and genomic methods) and pharmacokinetics (primary endpoints); secondary endpoints include safety, DoR, and duration of PFS.
Currently, participants are being recruited for phase 1 and 2 studies investigating vismodegib in children (NCT01878617) or adults with MB (NCT01601184 [± TMZ]). In a phase 1/2 study in adults with recurrent, progressive, or refractory SHH-activated MB (NCT01601184), participants with SHH-activated MB (validated by IHC) will be treated with TMZ alone or in combination with vismodegib. The primary objectives of the phase 1 and 2 portions of the study are to determine the safety and 6-month PFS, respectively, of vismodegib in combination with TMZ. Secondary endpoints include 6-month objective response rate (overall), DoR, PFS, time to treatment failure, and safety. A phase 2 study in children with newly diagnosed MB is also recruiting (NCT01878617). Following surgery and craniospinal radiation, participants will be treated with a combination of chemotherapy and/or vismodegib based on clinical risk (low, standard, intermediate, or high) and molecular subtype (WNT, SHH, or non-WNT/non-SHH). Molecular subtype will be assessed by WNT and SHH biomarkers in tumor samples. Participant outcomes (PFS, OS) from this trial will be compared with those observed in a previous trial in participants with newly diagnosed MB, supratentorial primitive neuroectodermal tumors, or atypical teratoid rhabdoid tumors (NCT00085202). A summary of clinical studies evaluating Hh pathway inhibitors in participants with MB is provided in Table 3.
SHH-activated Tumors Downstream of SMO: Combating Resistance to SMO Inhibitors
Resistance to SMO inhibitors has been observed in preclinical MB mouse models56,59,93,94 and in at least 1 patient with MB in the clinic.12,93 The observed resistance has been attributed to acquired mutations in SMO (L225R, N223D, S391N, D338N, D477G [D473H in human SMO], G457S, and E518), amplification of GLI2, MYCN, and cyclin D1, upregulation of the IGF-1R-PI3K pathway (SUFU and PTEN are closely linked on chromosome 10), and upregulation of the adenosine triphosphate-binding cassette transporter p-glycoprotein substrate, which is a common mechanism of drug resistance in cancer cells.49,56,59,93,94 In addition, a truncated version of GLI1, identified in MB cells, was shown to regulate anchorage-independent growth and morphology and rendered MB cells resistant to the SMO inhibitors cyclopamine and vismodegib.95 As discussed earlier, results from preclinical studies suggest that the WNT pathway may interact with Hh signaling in MB78,79; cross talk between these pathways has also been described in gastrointestinal cancers in which Hh pathway activation was shown to suppress WNT signaling, leading to decreased proliferation of cancer cells.96–98 However, WNT pathway activation in MB as a result of treatment with SHH inhibitors has not been reported.
Potential mechanisms for overcoming resistance to SMO inhibitors in MB and other tumor types have been identified, including inhibitors that bind and inhibit mutant SMO. These novel SMO antagonists have binding sites that are distinct from those currently being investigated in the clinic and are capable of inhibiting both wild-type and mutant SMO.99 Active8, one small molecule inhibitor with activity against the SMO variant SMO-D473H (identified in the participant who relapsed after treatment with vismodegib93) was shown to inhibit Hh activity in MB mouse models and may provide additional activity in combination with different classes of SMO inhibitors to help prevent resistance.100
Inhibitors that target downstream components of the Hh pathway may also be useful for overcoming resistance to SMO inhibitors. Several inhibitors that target GLI have been identified in cellular screens.101–103 The small molecule inhibitors, GANT58 and GANT61, which primarily target nuclear GLI, were shown to inhibit in vitro tumor cell proliferation and block cell growth in a prostate xenograft model.101 In neuroblastoma cells, GANT61 treatment caused downregulation of GLI1, MYC, MYCN, and cyclin D1 and was shown to inhibit Hh signaling more effectively than SMO inhibitors.104 Moreover, in neuroblastoma xenografts, GANT61 enhanced the effects of chemotherapy, further reducing tumor growth. Four additional Hh pathway inhibitors (HPI 1–4), each with differential effects on GLI processing and stability, trafficking to the primary cilium, and ciliogenesis, were also identified.102 Two of the 4 HPIs were capable of blocking the proliferation of neural progenitors in CGNPs from Math1-Cre:SMOM2 mice and reduced expression of Gli1, Gli2, N-Myc, and cyclin D1 protein. Another novel compound, pyrrolo[3,2-c]quinoline-4-one derivative 12b, was shown to suppress stromal Gli1 mRNA expression and demonstrated antitumor activity in an MB mouse allograft.103 In addition to novel compounds, arsenic trioxide (ATO), an FDA-approved anticancer therapy, was also shown to inhibit GLI1 in GLI-dependent cancer cell lines and increase survival of MB mouse models.105 In a second study, treatment with ATO inhibited growth of Hh-driven MB models.106 This study suggested that ATO blocked Hh-induced ciliary accumulation of GLI2 and reduced GLI2 stability, whereas the previous study suggested that GLI1 was inhibited in the nucleus, independent of the primary cilia.105,106
Resistance to SMO inhibitors could also be overcome through activation or inhibition of other signaling pathways such as PKA and PI3K.59,87,94 Based on the observation that PACAP-dependent PKA signaling may antagonize Hh signaling in Hh-dependent primary MB tumorsphere cultures, its activation in tumor cells via activation of PACAP receptors may provide a SMO-independent mechanism to inhibit aberrant Hh signaling and thus inhibit MB growth.87 Conversely, inhibition of PI3K signaling in SMO inhibitor-resistant tumors or in combination with SMO inhibitors has been shown to inhibit MB growth.59,94 Treatment of vismodegib-resistant tumor models with the PI3K inhibitor GDC-0941 caused tumor growth inhibition.94 Similarly, inhibition of PI3K signaling using buparlisib (BKM120; another PI3K inhibitor) or BEZ235 (a dual PI3K/mTOR inhibitor) in combination with sonidegib delayed or inhibited the resistance observed in mouse MB models treated with sonidegib alone.59 Based on these preclinical data, a phase 1 study of sonidegib in combination with buparlisib in participants with advanced solid tumors is ongoing (NCT01576666). The p90 ribosomal S6 kinase inhibitor BI-D1870, which was identified in a screen against cells resistant to SHH inhibitors, was shown to induce apoptosis, restrict colony formation, and sensitize cells to chemotherapy.107 BI-D1870 showed activity in BTICs and in primary human samples. Age-based therapeutic combinations have also been suggested following the genomic sequencing analysis discussed above.49 Based on these results, combinations of SMO inhibitors with epigenetic modifiers or PI3K pathway inhibitors in adults and GLI inhibitors in children would be predicted to have improved activity.
Other agents that affect Hh signaling may provide benefit to patients with resistance to SMO inhibitors. The systemic antifungal itraconazole was identified in a screen as a potent inhibitor of Hh signaling.108 Itraconazole inhibited tumor growth in an MB allograft model by preventing the ciliary localization of SMO. Itraconazole was also shown to inhibit Hh signaling in SMO-mutant models.109 In MB cells and mice with intracranial drug-resistant SMO-D477G MB (or wild-type SMO), itraconazole alone or in combination with ATO inhibited tumor growth. Combination treatment led to greater inhibition and improved survival compared with treatment with either agent alone.109,110 These agents were efficacious in all reported drug-resistant SMO mutants and in wild-type SMO.110 A class of glucocorticoids (budesonide, ciclesonide) has also been shown to inhibit ciliary localization of SMO in Hh-responsive cells.111 Inhibition of SMO ciliary localization was coupled with inhibition of Hh signaling activity in cells with wild-type and mutant SMO (resistant to SMO inhibitors). Finally, botanicals are also being investigated as second-generation drugs that may benefit patients who have developed resistance to SMO inhibitors.112
Conclusions
Numerous preclinical and molecular profiling studies have implicated the Hh signaling pathway in the pathogenesis of MB. Several Hh pathway inhibitors have been developed and are being tested in the clinic. Two of these inhibitors, both targeting SMO, have shown efficacy in patients with Hh-activated MB. Other inhibitors that target different components of Hh signaling, including those that target SMO mutants, GLIs, and ciliary localization of GLI or SMO, are now being developed.
Regardless of the mode of Hh pathway inhibition, selection of patients with SHH-activated MB is critical for identifying those who would benefit from this therapy and preventing unnecessary potential toxicities associated with current standard-of-care therapies, especially in young children. Ongoing and future studies of Hh pathway inhibitors incorporating screening procedures that utilize IHC or gene signatures to identify SHH-activated MB will likely improve the way patients with MB are treated.
Funding
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Conflict of interest statement.: Mark W. Kieran has acted as a consultant/advisor for Novartis Pharmaceuticals Corporation and has received speaker honoraria and research funding from Novartis Pharmaceuticals Corporation.
Acknowledgments
The author thanks Jillian Brechbiel, PhD, and Karen Kaluza, PhD, for medical editorial assistance with this manuscript. Financial support for editorial assistance was provided by Novartis Pharmaceuticals Corporation.
References
- 1.Rossi A, Caracciolo V, Russo G, et al. Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res. 2008;14(4):971–976. doi: 10.1158/1078-0432.CCR-07-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samkari A, Hwang E, Packer RJ. Medulloblastoma/primitive neuroectodermal tumor and germ cell tumors: the uncommon but potentially curable primary brain tumors. Hematol Oncol Clin North Am. 2012;26(4):881–895. doi: 10.1016/j.hoc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Garrè ML, Cama A, Bagnasco F, et al. Medulloblastoma variants: age-dependent occurrence and relation to Gorlin syndrome—a new clinical perspective. Clin Cancer Res. 2009;15(7):2463–2471. doi: 10.1158/1078-0432.CCR-08-2023. [DOI] [PubMed] [Google Scholar]
- 4.Hahn H, Wicking C, Zaphiropoulos PG, et al. Mutations of the human homolog of drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85(6):841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 5.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. doi: 10.1007/s00401-011-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwalbe EC, Lindsey JC, Straughton D, et al. Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res. 2011;17(7):1883–1894. doi: 10.1158/1078-0432.CCR-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amakye D, Robinson D, Rose K, et al. The predictive value of a 5-gene signature as a patient pre-selection tool in medulloblastoma for hedgehog pathway inhibitor therapy [abstract 4818] Cancer Res. 2012;72(8 suppl 1) [Google Scholar]
- 8.Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, group 3, and group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805(2):181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Gailani MR, Stahle-Backdahl M, Leffell DJ, et al. The role of the human homologue of drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14(1):78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 11.Lorusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor GDC-0449 in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gajjar AJ, Gururangan S, Qaddoumi IA, et al. A prospective phase II study to determine the efficacy of GDC 0449 (vismodegib) in adults with recurrent medulloblastoma (MB):a Pediatric Brain Tumor Consortium study (PBTC 25B) [abstract 2035] J Clin Oncol. 2013;31(suppl) [Google Scholar]
- 16.Gajjar A, Stewart CF, Ellison DW, et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a Pediatric Brain Tumor Consortium study. Clin Cancer Res. 2013;19(22):6305–6312. doi: 10.1158/1078-0432.CCR-13-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jimeno A, Weiss GJ, Miller WH, Jr, et al. Phase I study of the hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19(10):2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodon J, Tawbi HA, Thomas AL, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20(7):1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 19.Kieran M, Geoerger B, Casanova M, et al. A phase 1/2 safety and preliminary efficacy study of sonidegib (LDE225), a hedgehog pathway inhibitor, in pediatric and adult patients with relapsed or refractory medulloblastoma and other solid tumors [abstract NO-068] Neuro Oncol. 2013;15(suppl 3) [Google Scholar]
- 20.Erivedge (vismodegib) [package insert] South San Francisco, CA: Genentech USA, Inc; 2012. [Google Scholar]
- 21.ClinicalTrials.gov. Available at http://www.clinicaltrials.gov . Accessed February 18, 2014.
- 22.Packer RJ, Cogen P, Vezina G, et al. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–250. doi: 10.1215/15228517-1-3-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011;13(6):669–679. doi: 10.1093/neuonc/nor025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 25.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65(11):1419–1424. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers V 2.2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf . Accessed February 18, 2014.
- 27.Bartlett F, Kortmann R, Saran F. Medulloblastoma. Clin Oncol (R Coll Radiol) 2013;25(1):36–45. doi: 10.1016/j.clon.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kool M, Koster J, Bunt J, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 33.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 35.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3(12):903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 36.Kimura H, Ng JM, Curran T. Transient inhibition of the hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13(3):249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22(1):103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 39.Wallace VA. Purkinje-cell-derived sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9(8):445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 40.Schuller U, Heine VM, Mao J, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14(2):123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han YG, Kim HJ, Dlugosz AA, et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15(9):1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 43.Parsons DW, Li M, Zhang X, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slade I, Murray A, Hanks S, et al. Heterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam Cancer. 2011;10(2):337–342. doi: 10.1007/s10689-010-9411-0. [DOI] [PubMed] [Google Scholar]
- 45.Zurawel RH, Allen C, Chiappa S, et al. Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer. 2000;27(1):44–51. doi: 10.1002/(sici)1098-2264(200001)27:1<44::aid-gcc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 46.Brugieres L, Remenieras A, Pierron G, et al. High frequency of germline SUFU mutations in children with desmoplastic/nodular medulloblastoma younger than 3 years of age. J Clin Oncol. 2012;30(17):2087–2093. doi: 10.1200/JCO.2011.38.7258. [DOI] [PubMed] [Google Scholar]
- 47.Northcott PA, Hielscher T, Dubuc A, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011;122(2):231–240. doi: 10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buczkowicz P, Ma J, Hawkins C. GLI2 is a potential therapeutic target in pediatric medulloblastoma. J Neuropathol Exp Neurol. 2011;70(6):430–437. doi: 10.1097/NEN.0b013e31821b94db. [DOI] [PubMed] [Google Scholar]
- 49.Kool M, Jones DT, Jager N, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Northcott PA, Shih DJ, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–2935. doi: 10.1200/JCO.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. doi: 10.1016/S1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sengupta R, Dubuc A, Ward S, et al. CXCR4 activation defines a new subgroup of sonic hedgehog-driven medulloblastoma. Cancer Res. 2012;72(1):122–132. doi: 10.1158/0008-5472.CAN-11-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6(3):229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Sasai K, Romer JT, Lee Y, et al. Shh pathway activity is down-regulated in cultured medulloblastoma cells: implications for preclinical studies. Cancer Res. 2006;66(8):4215–4222. doi: 10.1158/0008-5472.CAN-05-4505. [DOI] [PubMed] [Google Scholar]
- 56.Lee MJ, Hatton BA, Villavicencio EH, et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci USA. 2012;109(20):7859–7864. doi: 10.1073/pnas.1114718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohner A, Spilker ME, Lam JL, et al. Effective targeting of hedgehog signaling in a medulloblastoma model with PF-5274857, a potent and selective smoothened antagonist that penetrates the blood-brain barrier. Mol Cancer Ther. 2012;11(1):57–65. doi: 10.1158/1535-7163.MCT-11-0691. [DOI] [PubMed] [Google Scholar]
- 58.Bender MH, Hipskind PA, Capen AR, et al. Identification and characterization of a novel smoothened antagonist for the treatment of cancer with deregulated hedgehog signaling [abstract 2819] Cancer Res. 2011;71(8) [Google Scholar]
- 59.Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3 K pathway in medulloblastoma. Sci Transl Med. 2010;2(51):51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130(1):15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 61.Northcott PA, Fernandez-L A, Hagan JP, et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69(8):3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colvin Wanshura LE, Galvin KE, Ye H, et al. Sequential activation of Snail1 and N-myc modulates sonic hedgehog-induced transformation of neural cells. Cancer Res. 2011;71(15):5336–5345. doi: 10.1158/0008-5472.CAN-10-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briggs KJ, Corcoran-Schwartz IM, Zhang W, et al. Cooperation between the Hic1 and Ptch1 tumor suppressors in medulloblastoma. Genes Dev. 2008;22(6):770–785. doi: 10.1101/gad.1640908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahlfeld J, Favaro R, Pagella P, et al. Sox2 requirement in sonic hedgehog-associated medulloblastoma. Cancer Res. 2013;73(12):3796–3807. doi: 10.1158/0008-5472.CAN-13-0238. [DOI] [PubMed] [Google Scholar]
- 65.Swartling FJ, Savov V, Persson AI, et al. Distinct neural stem cell populations give rise to disparate brain tumors in response to N-MYC. Cancer Cell. 2012;21(5):601–613. doi: 10.1016/j.ccr.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia I, Crowther AJ, Gershon TR. ASPM, a target of sonic hedgehog, is a key element in cerebellar development and medulloblastoma pathogenesis [abstract MB-51] Neuro Oncol. 2012;14(suppl 1) [Google Scholar]
- 67.Bar EE, Chaudhry A, Eberhard CG. Hedgehog signaling promotes medulloblastoma survival via Bcl2 [abstract 4080] Proc Amer Assoc Cancer Res. 2006;7 [Google Scholar]
- 68.Fults D, McCall T, Pedone C. Apoptosis suppression by somatic cell transfer of bcl-2 promotes sonic hedgehog-dependent medulloblastoma formation in mice [abstract 3885]. Presented at: 98th Annual Meeting of the American Association for Cancer Research; Los Angeles, CA April 14–18, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Venugopal C, Manoranjan B, et al. Sonic hedgehog regulates Bmi1 in human medulloblastoma brain tumor-initiating cells. Oncogene. 2012;31(2):187–199. doi: 10.1038/onc.2011.232. [DOI] [PubMed] [Google Scholar]
- 70.Subkhankulova T, Zhang X, Leung C, et al. Bmi1 directly represses p21Waf1/Cip1 in Shh-induced proliferation of cerebellar granule cell progenitors. Mol Cell Neurosci. 2010;45(2):151–162. doi: 10.1016/j.mcn.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Manoranjan B, Hallett R, Venugopal C, et al. Bmi1 regulation of sonic hedgehog signaling through the E3 ubiquitin ligase Cullin3 in medulloblastoma stem cells [abstract MB-49] Neuro Oncol. 2012;14(suppl 1) [Google Scholar]
- 72.Yang L, Jackson E, Woerner BM, et al. Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Res. 2007;67(2):651–658. doi: 10.1158/0008-5472.CAN-06-2762. [DOI] [PubMed] [Google Scholar]
- 73.Bhatia B, Hsieh M, Kenney AM, et al. Mitogenic sonic hedgehog signaling drives E2F1-dependent lipogenesis in progenitor cells and medulloblastoma. Oncogene. 2011;30(4):410–422. doi: 10.1038/onc.2010.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhatia B, Potts CR, Guldal C, et al. Hedgehog-mediated regulation of PPARγ controls metabolic patterns in neural precursors and shh-driven medulloblastoma. Acta Neuropathol. 2012;123(4):587–600. doi: 10.1007/s00401-012-0968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malhotra A, Potts C, Fernandez-Lopez A, et al. Signaling network-based analyses of sonic hedgehog pathway components: predictions and possibilities [abstract CB-56] Neuro Oncol. 2012;14(suppl 6) [Google Scholar]
- 76.Rao G, Pedone CA, Del Valle L, et al. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23(36):6156–6162. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 77.Guldal C, Potts C, Rotenberry R, et al. HIF1 downstream of sonic hedgehog mitogenic and oncogenic signaling and brain development and medulloblastoma [abstract PL-12] Neuro Oncol. 2012;14(suppl 6) [Google Scholar]
- 78.Baryawno N, Sveinbjornsson B, Kogner P, et al. Medulloblastoma: a disease with disorganized developmental signaling cascades. Cell Cycle. 2010;9(13):2548–2554. doi: 10.4161/cc.9.13.12170. [DOI] [PubMed] [Google Scholar]
- 79.Dakubo GD, Mazerolle CJ, Wallace VA. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J Neurooncol. 2006;79(3):221–227. doi: 10.1007/s11060-006-9132-2. [DOI] [PubMed] [Google Scholar]
- 80.Snuderl M, Batista A, Kirkpatrick ND, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64(21):7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 82.Parathath SR, Mainwaring LA, Fernandez-L A, et al. Insulin receptor substrate 1 is an effector of sonic hedgehog mitogenic signaling in cerebellar neural precursors. Development. 2008;135(19):3291–3300. doi: 10.1242/dev.022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhatia B, Nahle Z, Kenney AM. Double trouble: when sonic hedgehog signaling meets TSC inactivation. Cell Cycle. 2010;9(3):456–459. doi: 10.4161/cc.9.3.10532. [DOI] [PubMed] [Google Scholar]
- 84.Mainwaring LA, Kenney AM. Divergent functions for eIF4E and S6 kinase by sonic hedgehog mitogenic signaling in the developing cerebellum. Oncogene. 2011;30(15):1784–1797. doi: 10.1038/onc.2010.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Browd SR, Kenney AM, Gottfried ON, et al. N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res. 2006;66(5):2666–2672. doi: 10.1158/0008-5472.CAN-05-2198. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez-L A, Squatrito M, Northcott P, et al. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31(15):1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen JR, Resnick DZ, Niewiadomski P, et al. Pituitary adenylyl cyclase activating polypeptide inhibits gli1 gene expression and proliferation in primary medulloblastoma derived tumorsphere cultures. BMC Cancer. 2010;10:676–683. doi: 10.1186/1471-2407-10-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicot A, Lelievre V, Tam J, et al. Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation. J Neurosci. 2002;22(21):9244–9254. doi: 10.1523/JNEUROSCI.22-21-09244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kadel E, DuPree K, Lee S, et al. Development of a clinical hedgehog antagonist qRT-PCR pharmacodynamic assay for Gli1 in hair follicle samples [abstract 3656]. Presented at: 99th Annual Meeting of the American Association for Cancer Research; April 12–16; San Diego, CA. 2008. [Google Scholar]
- 90.Rose K, Robinson D, Sharp T, et al. Gene expression analysis in human hair bulbs as a potential pharmacodynamic readout in the LDE225 clinical program [abstract 3092] J Clin Oncol. 2010;28 (15s ) [Google Scholar]
- 91.Amakye D, Robinson D, Rose K, et al. Development of a five-gene Hedgehog signature as a patient preselection tool for Hedgehog pathway-targeted therapy in medulloblastoma [abstract 0125] Neuro Oncol. 2013;15(suppl 1) [Google Scholar]
- 92.Kieran MW, Hargrave D, Wen PY, et al. A phase 3, multicenter, open-label, randomized, controlled study of the efficacy and safety of oral sonidegib (LDE225) versus temozolomide in patients with hedgehog pathway-activated relapsed medulloblastoma [abstract NO-069] Neuro Oncol. 2013;15(suppl 3) [Google Scholar]
- 93.Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326(5952):572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dijkgraaf GJ, Alicke B, Weinmann L, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71(2):435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 95.Cao X, Zhu H, Bigner D, et al. Gain-of-function tGLI1 transcription factor augments anchorage-independent growth of medulloblastoma cells and confers their resistance to SMO inhibitors [abstract 0039] Neuro Oncol. 2011;13(suppl 1) [Google Scholar]
- 96.He J, Sheng T, Stelter AA, et al. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J Biol Chem. 2006;281(47):35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 97.van den Brink GR, Bleuming SA, Hardwick JC, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36(3):277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 98.Akiyoshi T, Nakamura M, Koga K, et al. Gli1, downregulated in colorectal cancers, inhibits proliferation of colon cancer cells involving Wnt signalling activation. Gut. 2006;55(7):991–999. doi: 10.1136/gut.2005.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao H, Jin Q, Koo DI, et al. Small molecule antagonists in distinct binding modes inhibit drug-resistant mutant of smoothened. Chem Biol. 2011;18(4):432–437. doi: 10.1016/j.chembiol.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, Mook R, Lyerly HK, et al. Candidate hedgehog/smoothened inhibitors for pediatric medulloblastoma [abstract 0080] Neuro Oncol. 2011;13(suppl 1) [Google Scholar]
- 101.Lauth M, Bergstrom A, Shimokawa T, et al. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104(20):8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hyman JM, Firestone AJ, Heine VM, et al. Small-molecule inhibitors reveal multiple strategies for hedgehog pathway blockade. Proc Natl Acad Sci USA. 2009;106(33):14132–14137. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohashi T, Oguro Y, Tanaka T, et al. Discovery of pyrrolo[3,2-c]quinoline-4-one derivatives as novel hedgehog signaling inhibitors. Bioorg Med Chem. 2012;20(18):5496–5506. doi: 10.1016/j.bmc.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 104.Wickstrom M, Dyberg C, Shimokawa T, et al. Targeting the hedgehog signal transduction pathway at the level of GLI inhibits neuroblastoma cell growth in vitro and in vivo. Int J Cancer. 2013;132(7):1516–1524. doi: 10.1002/ijc.27820. [DOI] [PubMed] [Google Scholar]
- 105.Beauchamp EM, Ringer L, Bulut G, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121(1):148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J, Lee JJ, Kim J, et al. Arsenic antagonizes the hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci USA. 2010;107(30):13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pambid MR, Berns R, Hu K, et al. Overcoming resistance to sonic hedgehog inhibition by targeting p90 ribosomal S6 kinase for the management of medulloblastoma [abstract 0013] Neuro Oncol. 2013;15(suppl 1) doi: 10.1002/pbc.24675. [DOI] [PubMed] [Google Scholar]
- 108.Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aftab B, Kim J, Yang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to vismodegib [abstract 5644] Cancer Res. 2013;73(8 suppl 1) doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, Davidow L, Arvanites AC, et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem Biol. 2012;19(8):972–982. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drenkhahn SK, Jackson GA, Slusarz A, et al. Inhibition of Hedgehog/Gli signaling by botanicals: a review of compounds with potential hedgehog pathway inhibitory activities. Curr Cancer Drug Targets. 2013;13(5):580–595. doi: 10.2174/15680096113139990003. [DOI] [PubMed] [Google Scholar]
- 113.Murphy BL, Obad S, Bihannic L, et al. Silencing of the miR-17∼92 cluster family inhibits medulloblastoma progression. Cancer Res. 2013;73(23):7068–7078. doi: 10.1158/0008-5472.CAN-13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corcoran RB, Bachar Raveh T, Barakat MT, et al. Insulin-like growth factor 2 is required for progression to advanced medulloblastoma in patched1 heterozygous mice. Cancer Res. 2008;68(21):8788–8795. doi: 10.1158/0008-5472.CAN-08-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lelievre V, Seksenyan A, Nobuta H, et al. Disruption of the PACAP gene promotes medulloblastoma in ptc1 mutant mice. Dev Biol. 2008;313(1):359–370. doi: 10.1016/j.ydbio.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]