Abstract
Study Objectives:
To prospectively assess sleep reactivity as a diathesis of insomnia, and to delineate the interaction between this diathesis and naturalistic stress in the development of insomnia among normal sleepers.
Design:
Longitudinal.
Setting:
Community-based.
Participants:
2,316 adults from the Evolution of Pathways to Insomnia Cohort (EPIC) with no history of insomnia or depression (46.8 ± 13.2 y; 60% female).
Interventions:
None.
Measurements and Results:
Participants reported the number of stressful events they encountered at baseline (Time 1), as well as the level of cognitive intrusion they experienced in response to each stressor. Stressful events (OR = 1.13; P < 0.01) and stress-induced cognitive intrusion (OR = 1.61; P < 0.01) were significant predictors of risk for insomnia one year hence (Time 2). Intrusion mediated the effects of stressful events on risk for insomnia (P < 0.05). Trait sleep reactivity significantly increased risk for insomnia (OR = 1.78; P < 0.01). Further, sleep reactivity moderated the effects of stress-induced intrusion (P < 0.05), such that the risk for insomnia as a function of intrusion was significantly higher in individuals with high sleep reactivity. Trait sleep reactivity also constituted a significant risk for depression (OR = 1.67; P < 0.01) two years later (Time 3). Insomnia at Time 2 significantly mediated this effect (P < 0.05).
Conclusions:
This study suggests that premorbid sleep reactivity is a significant risk factor for incident insomnia, and that it triggers insomnia by exacerbating the effects of stress-induced intrusion. Sleep reactivity is also a precipitant of depression, as mediated by insomnia. These findings support the stress-diathesis model of insomnia, while highlighting sleep reactivity as an important diathesis.
Citation:
Drake CL, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. SLEEP 2014;37(8):1295-1304.
Keywords: stress, insomnia, sleep reactivity, depression, strain
INTRODUCTION
The significant individual differences in the risk for disease following stress exposure highlight the moderating influence of endogenous psychophysiological vulnerabilities.1 Presently, stress-diathesis theories constitute the most empirically robust etiological models of various forms of pathology, including depression, posttraumatic stress disorder, schizophrenia, congestive heart failure, and infectious disease.2–6 However, few studies have systematically examined the stress-diathesis hypothesis in the development of insomnia disorder. An important goal of current insomnia research is thus the identification of premorbid vulnerabilities, and the delineation of their interaction with environmental factors in triggering insomnia disorder. The validation of an inherent predisposition toward insomnia in an a priori defined subset of non-insomniacs would help elucidate the pathophysiology of this disorder, prompt the identification of premorbid phenotypes, and lay the groundwork for preventative care.
In contrast to sleep disturbance, which is a discrete report or polysomnographic (PSG) evidence of trouble sleeping, insomnia is a clinical disorder characterized by chronic difficulty in initiating or maintaining sleep that is associated with significant daytime impairment or distress. Further, while nearly half the U.S. population reports transient sleep disturbance, only about 4% to 22% meet diagnostic criteria for the syndrome of insomnia.7 In a recent longitudinal study, only 10% to 14% of individuals who reported sleep disturbance at baseline developed insomnia disorder at follow-up assessment a year later.8 Moreover, 63% of the same group showed significant remission of sleep disturbance. By contrast, almost 90% of individuals diagnosed with insomnia disorder remain symptomatic for as long as five years following onset, and only about 50% achieve remission after 10 years.9,10 Taken together, these studies offer converging epidemiological evidence for the distinction between transient sleep disturbance and insomnia disorder. A natural question thus arises: what distinguishes individuals who experience transient sleep disturbance from those who get mired in the chronic syndrome of insomnia?
Studies in healthy samples indicate substantial individual differences in the degree of sleep disruption following stress exposure. A subset of normal sleepers exhibits significantly higher stress related sleep disturbance than do other good sleepers. Notably, this group exhibits this response across a variety of stressors or challenges to the sleep system, including environmental factors such as a first night in a sleep laboratory, pharmacological challenges including caffeine administration, and circadian phase shifts.11,12 This “sleep reactivity,” i.e., the tendency to exhibit pronounced sleep disturbance in response to a sleep challenge, may constitute a premorbid vulnerability for future insomnia. In other words, individuals who typically respond to stress with a particularly strong albeit transient sleep response may harbor a predisposition for insomnia. Prior research suggests that for disorders characterized by the interaction between an environmental pathogen and a biological predisposition, variables associated with reactivity to that pathogen are likely candidates for the diathesis of that disorder.13 For instance, studies of normotensive subjects have identified an individual's blood pressure reactivity to a laboratory stressor as a reliable predictor of the development of hypertension.14 Similarly, studies of cardiac exercise stress tests in asymptomatic individuals show that reactivity predicts the long-term risk of cardiovascular disease and mortality.15,16
To serve as a diathesis for insomnia, sleep reactivity must potentiate the effects of stress exposure, exhibit within-person stability, and, finally, be predictive of incident insomnia. In our prior work examining this hypothesis, we developed and validated an instrument called the Ford Insomnia in Response to Stress Test (FIRST) to identify individuals with trait sleep reactivity.17 The FIRST comprises nine items which assess the likelihood of experiencing sleep disturbance in response to common environmental stressors. In a series of prior studies, we examined sleep reactivity using the FIRST in samples of healthy individuals with no current/past insomnia. Participants with high FIRST scores but comparable habitual sleep times as other good sleepers showed significantly greater PSG sleep disruption in response to their first night in the laboratory, as well as in response to a low dose (3 mg/kg) caffeine challenge.12,17 Importantly, despite significantly reduced sleep times during their first night in the laboratory, individuals with high FIRST scores showed evidence of heightened alertness (i.e., elevated multiple sleep latency test scores) as seen in insomniacs.18 Individuals with high FIRST scores may also exhibit sleep architectural abnormalities in response to stress. In a naturalistic study of school teachers by another research group, participants with high scores on the FIRST showed a greater number of arousals, more stage transitions, and a reduced proportion of REM sleep on at-home PSG recordings during self-reported “high stress” days in comparison to “low-stress” days.19 Together, these findings highlight both the interaction between sleep reactivity and stress exposure, as well as the trait-like within-person stability11 of this vulnerability. Finally, data from twin and sibling studies indicate that nearly 40% of the variance in sleep reactivity, as measured by the FIRST, is attributable to familial aggregation, indicating significant heritability.20,21
With regard to the role of sleep reactivity as a trait predisposition to insomnia, a recent study demonstrated that the development of chronic insomnia following hospitalization can be predicted by an individual's pre-hospitalization history of sleep disturbance.22 However, this study made no attempt to exclude individuals with a prior history of insomnia, or to measure sleep reactivity or other potential predisposing factors. Thus, the increased likelihood of chronic insomnia among patients with a past history of sleep disturbance may simply represent a recurrence of the disorder. A logical extension of this research is a naturalistic study of the interaction between sleep reactivity and “real-world” environmental stressors in a population with no history of insomnia. Such an approach will not only improve ecological validity, but also delineate the mechanisms by which premorbid sleep reactivity culminates in insomnia.
Naturalistic stress exposure is a well-established precipitant of insomnia.23 However, most prior insomnia studies operationalize stress as the sum total of negative life events, such as job loss, encountered during a particular period.24,25 As stress researchers from various disciplines have observed, stress is a dynamic, transactional process between the person and the environment, such that an individual's “response” to a stressor mediates its effects.2,26 As recent studies have emphasized, individuals with insomnia differ from controls not only in the number of stressors they report, but also in their cognitive appraisal of these stressors.27,28 Therefore, a valid assessment of stress requires measuring both the objective environmental stimulus as well as the individual's response to that stressor. Cognitive intrusion, or the level of perseverative, ego-dystonic ideation elicited by a stressor, has long been noted as a salient stress response in the etiology of sleep disturbance and insomnia.29 A small yet growing body of research suggests that cognitive intrusion is responsible for delayed sleep onset, increased wake time after sleep onset, and poor sleep quality.30–32 In a recent community-based study, levels of pre-sleep cognitive intrusion also distinguished between good sleepers and individuals with insomnia.33 Thus, to fully capture the interaction between stress and premorbid vulnerabilities, such as sleep reactivity, studies must examine both stress exposure (i.e., number of life events), as well as the response to those events, especially in the form of cognitive intrusion.
Finally, an intriguing question that any stress-diathesis model of insomnia must address regards its significant comorbidity with depression. Arguably, the most remarkable impact of insomnia on public health involves its role as a risk factor for a variety of psychological disorders. Epidemiological studies suggest that 35% to 70% of this population also meets criteria for mood disorders, anxiety disorders, schizophrenia, and substance abuse.34 However, the strongest of these associations, as consistently evidenced by over 40 years of research, underlies the comorbidity between insomnia and depression.35–37 In addition to the comorbidity between the disorders, data from longitudinal studies highlight the temporal precedence of insomnia in relation to depression.38 Given that insomnia typically precedes depression, it stands to reason that sleep reactivity leads to insomnia, which in turn precipitates depression. However, no study thus far has attempted to establish sleep reactivity as a trait vulnerability to depression, or to examine whether this effect is mediated through insomnia.
The present study sought to examine sleep reactivity as a trait vulnerability to insomnia and depression. We analyzed three waves of data from the Evolutions of Pathways to Insomnia Cohort (EPIC) study,39 a prospective investigation of a large community-based sample with no prior/current history of insomnia or depression at baseline. This design helped establish temporal relationships between risk factors, such as stress and trait sleep reactivity, and outcomes such as insomnia and depression. Specifically, participants completed psychometrically validated stress event inventories at baseline. Participants also reported the level of cognitive intrusion they experienced in response to each individually endorsed stress event. Premorbid levels of sleep reactivity were assessed using the FIRST. We hypothesized that: (1) higher levels of stress exposure and response would be associated with increased risk for insomnia; (2) sleep reactivity would moderate the relationship between stress and insomnia, such that individuals with higher sleep reactivity would exhibit a stronger association between stress and risk for insomnia; (3) higher sleep reactivity would be associated with increased risk for depression; (4) insomnia would act as a mediator between sleep reactivity and depression.
METHOD
Participants
Our data derive from the EPIC study, a 3-year NIMH-funded prospective investigation of a large community-based sample from southeastern Michigan.39 The third wave of data collection for the EPIC study is currently underway. For the initial assessment (Time 1), we mailed study invitation letters to a randomly generated list of individuals (n = 36,002) from a major HMO database. A total of 7,608 of these individuals completed a web-delivered eligibility survey which assessed for history of insomnia and depression; see Figure 1 for a detailed description of the flow of participants through the study. At the end of this survey, participants who met DSM-IV based diagnostic criteria40 for current/lifetime history of insomnia disorder and/or reported moderate levels of depression on an empirically validated instrument were excluded from the present study. The remaining survey takers (n = 4,869) were invited to participate in the study as soon as they completed the eligibility survey. The study invitation appeared on the subsequent webpage of the online portal, followed by the Time 1 questionnaire; thus, the eligibility survey and the Time 1 questionnaire were completed at the same assessment phase.
Figure 1.
Flow diagram depicting flow of participants through the study. †The third wave (Time 3) of data collection for this study is currently underway. As such, of the 2,892 participants who completed the Time 2 questionnaires, only 2,486 were eligible to complete the Time 3 questionnaires at the time of this report (based on the 1-year lag between data collection waves).
Of those survey takers who met inclusion criteria on the eligibility survey, 1,339 declined to participate (participation rate = 72.5%). Of the remaining 3,530 participants who completed the Time 1 questionnaire, 2,892 participants completed the Time 2 assessment one year later (retention rate: 81.9%). Finally, 2,486 of these participants were scheduled to participate in the Time 3 assessment at the time of this report; the others were ineligible owing to the stipulated one year gestation period between assessments. A total of 2,316 participants completed the Time 3 questionnaires (retention rate: 93.2%). This sample was predominantly white (66.6%) and female (60.0 %), with a mean age of 46.8 years (Table 1). The distributions of various demographic characteristics of this final sample were comparable to 2010 U.S. census data.39
Table 1.
Sample descriptive statistics

Procedure
To prospectively assess the relationship between stress and the incidence of insomnia, data were collected in three waves, each a year apart. Data collection for Time 1 began immediately after participants completed the eligibility survey, and was accomplished using web-administered electronic questionnaires. Study staff sent email reminders to each participant one-month prior to scheduled follow-up assessments. Each assessment took approximately 30 minutes to complete, and preliminary analyses revealed that nearly all participants responded appropriately to item content (96%).
Measures
Trait Sleep Reactivity
The Ford Insomnia Response to Stress (FIRST)12 is a validated measure of trait sleep reactivity, operationalized as a propensity for exaggerated sleep disruption in response to stressful events. The FIRST asks how likely (1 = not likely; 2 = somewhat likely; 3 = moderately likely; 4 = very likely) a respondent is to have difficulty sleeping in 9 distinct situations: before an important meeting the next day, after a stressful experience during the day, after a stressful experience in the evening, after getting bad news during the day, after watching a frightening movie or TV show, after having a bad day at work, after an argument, before having to speak in public, and before going on vacation the next day. Participants were asked to rate the likelihood of sleep disturbance even if they had not experienced the situation recently.
Insomnia
DSM-IV based diagnoses of insomnia disorder were established using the following questions: “have you experienced difficulty falling asleep?” “have you experienced difficulty staying asleep?” “have you experienced difficulty with non-refreshing sleep?” To meet diagnostic criteria, participants had to report experiencing one or more of the above symptoms ≥ 3 nights a week for a duration of a month or longer. Further, they had to endorse daytime impairment or distress as measured by the following question: “to what extent do you consider your sleep problems to interfere with your daily functioning?” Responses were coded on a 4-point Likert type scale ranging from 0 (“not at all”) to 4 (“very much”), such that participants who reported a score of 2 (somewhat) or higher met that particular criterion.
Depression
Participants completed the 16-item self-report version of the Quick Inventory of Depressive Symptomatology (QIDS) both at baseline and at follow-up 2 years later. The QIDS assesses the presence and persistence of DSM-IV based depressive symptomatology on a 4-point (0 to 3) Likert type scale. In a recent validation study, the QIDS exhibited excellent psychometric properties and concurrent validity.41 To minimize collinearity with insomnia, the sleep disturbance subscale of the QIDS was excluded from the overall scale. Total scores of 11 or greater on the QIDS are indicative of moderate levels of depression.41 Thus, to account for the elimination of the sleep subscale, on which a participant can score a maximum of 3 points, depression scores were dichotomized using a cutoff score of 8 in the present study.
Stress Exposure: Number of Events
We assessed stress exposure based on the revised Social Readjustment Rating Scale (SRRS-R), an empirically validated inventory of 52 stressful life events commonly reported by U.S. samples. The SRRS-R was recently normed in a nationally representative sample of over 3,000 adults and is widely recognized as the one of the most common stress measurement instruments.43,44 In the present study, participants reported whether or not they had experienced a particular life event in the past year. The total number of endorsed events served as our operationalization of stress exposure.
Stress-Response: Cognitive Intrusion
The impact of events scale (IES) is a 15-item questionnaire used to assess the psychological impact of stressful events along 2 dimensions: intrusion and avoidance.45 The present study employed the 8-item intrusion subscale of the IES, which measures the presence and pervasiveness of recurrent, intrusive ideation (e.g., “I thought about it when I didn't mean to” “other things kept making me think about it”) in response to a stressor on a 4-point Likert type scale. Specifically, participants completed this scale in response to each endorsed stressor on the SRRS-R. Further, question stems were modified to assess levels of intrusion in the “seven days following the event” to facilitate mediational analyses. Validation studies report good internal consistency (Cronbach α = 0.86) and excellent test-retest reliability (r = 0.94) for the intrusion subscale of the IES.46
Note that the avoidance subscale of the IES measures the extent of specific avoidance behaviors (e.g., “tried not to think about it” “I stayed away from reminders of it”). As such, the avoidance subscale is a more suitable measure of the coping construct than stress per se, and hence beyond the scope of the present report; see Sontag et al. for a detailed discussion of the distinction between stress and coping.26
Data Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows,47 unless otherwise noted (see mediation analyses below). Logistic regression analysis with maximum likelihood estimation (MLE) was used to assess risk for insomnia and depression at follow-up assessments. To test the assumptions of logistic regression, all continuous independent variables (IV) were examined for collinearity based on bivariate correlations as well as the standard errors of parameter estimates.48 Similarly, the large sample size (n = 2,316) and proportion of positive cases (e.g., n = 262 for insomnia at Time 2; > 10 cases for each estimated parameter, including the intercept) afforded sufficient variance for MLE.
Moderation
Moderation analyses were based on techniques outlined by Preacher et al.49 Specifically, we repeated aforesaid regression models with the product of the independent variable and the moderator added as a separate block. Interpretable moderation is said to occur if the product term acts as a statistically significant predictor. Further, to assist in the interpretation of coefficients, all covariates were standardized. Finally, to plot moderation effects, regression coefficients (B) were transformed from the logit curve scale to a probability scale using the formula:
 |
Mediation
For mediation analyses, we followed steps outlined by Fairchild and MacKinnon.50 Specifically, 3 separate regression analyses were conducted: the dependent variable (DV) was regressed on IV (Equation 1); the mediator (M) was regressed on the IV (Equation 2); and the DV was regressed on the M, while controlling for the IV (Equation 3),
where c represents the relation between the IV and the DV, a denotes the association between the IV and the M; c′ represents the relation between the IV and the DV adjusted for the effect of the mediator on the DV; b represents the relation between the DV and the M adjusted for the effects of the IV; e1, e2, and e3 denote unexplained variability; and the intercepts are i1, i2, and i3. The product of the a and b parameter estimates, ab, represents the mediated effect.
With regard to confidence intervals and significance testing, traditional methods are relatively underpowered and yield inaccurate confidence intervals given that mediated effects (the product of 2 distributions) do not follow a normal distribution.50 Accordingly, the confidence interval of the mediated effect was estimated via the PRODCLIN method in R-Mediation, a statistical computer package.51 This method does not assume a normal distribution, yields asymmetric confidence intervals (CI), and is thus more accurate than traditional significance tests.52,53 If the 95% CI for the mediated effect does not overlap zero, statistically significant mediation may be inferred.
RESULTS
Insomnia
Analyses of Time 2 data revealed 262 new cases of insomnia disorder, resulting in a one-year incidence rate of 9.1%. We first fit a logistic regression model predicting insomnia based on the following factors: age, gender, race, income, employment status, marital status, and education. All continuous demographic variables were normally distributed, with skewness and kurtosis within acceptable range.54 A test of this model with all predictors against a constant-only model was statistically significant (χ2 = 19.29; P < 0.01), indicating that our model reliably distinguished between participants with and without insomnia. Similarly, the Hosmer-Lemeshow test revealed that this model fit our data well (χ2 = 6.48; P = 0.59). Age (β = -0.02; OR = 0.99; 95% CI = 0.98–1.00; P < 0.01) and gender (β = 0.30; OR = 1.39; 95% CI = 1.01–1.83; P < 0.01) were significantly related to insomnia, such that female gender and younger age were associated with higher odds of developing insomnia disorder. None of the other demographic variables were significant predictors of insomnia. As such, all subsequent analyses controlled for age and gender.
Stress Exposure, Sleep Reactivity, and Insomnia
Next we assessed stress exposure and sleep reactivity at Time 1 as predictors of insomnia at Time 2. Logistic regression analyses revealed that our model fit the data well (χ2 = 127.77; P < 0.01); similarly, the Hosmer-Lemeshow did not suggest any cause for concern (χ2 = 6.67; P = 0.57). Stress exposure (β = 0.14; OR = 1.13; 95% CI = 1.07–1.20; P < 0.01) was significantly associated with insomnia, such that the odds of developing insomnia at Time 2 increased by 13% for every additional stressor endured at Time 1 (or by roughly 30% for a 1 SD increase in the stress exposure variable). Similarly, an increase of a single point on the sleep reactivity scale (β = 0.13; OR = 1.14; 95% CI = 1.11–1.17; P < 0.01) was associated with a 14% increase in the odds for developing insomnia, such that a 1 SD increase was associated with an almost 80% increase (OR = 1.78; 95% CI = 1.57–2.02; P < 0.01). Moderation analyses revealed that the interaction between stress exposure and sleep reactivity approached significance (β = 0.01; P = 0.06).
Cognitive Intrusion, Sleep Reactivity, and Insomnia
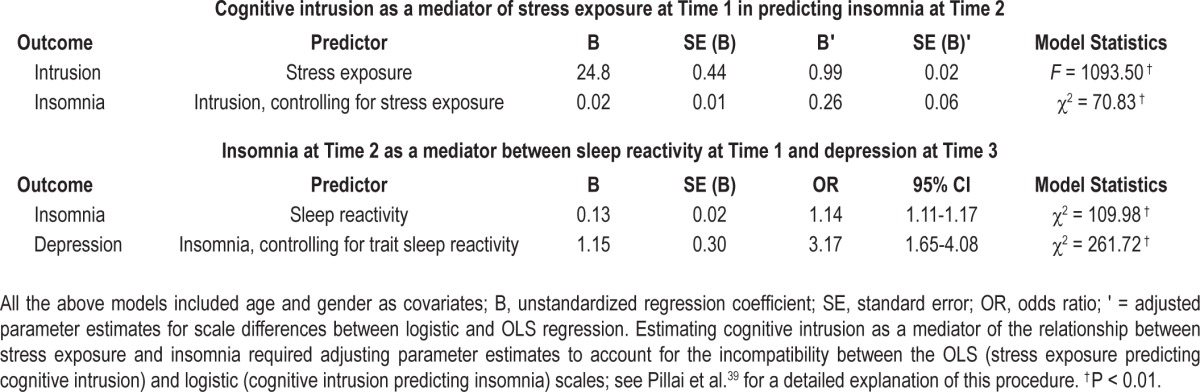
Descriptive statistics and visual inspection of histograms revealed significant positive skew in the cognitive intrusion variable (Table 1). Outliers > 3 standard deviations from the mean were eliminated. We then repeated the previous model (i.e., stress exposure predicting insomnia) with cognitive intrusion added as a predictor. Odds of developing insomnia increased by 2% for every 1-point increase on the intrusion scale (β = 0.02; OR = 1.02; 95% CI = 1.01–1.03; P < 0.01), whereas a 1-SD increase on the scale was associated with a 61% increase. Further, the association between stress exposure and risk for insomnia became nonsignificant (β = -0.05; P = 0.36). Post hoc tests revealed that cognitive intrusion mediated the association between stress exposure and insomnia, and the confidence interval (mediated effect: c′ = 0.09; 95% CI = 0.08–0.12) indicated that mediation was statistically significant (see Figure 2).
Figure 2.
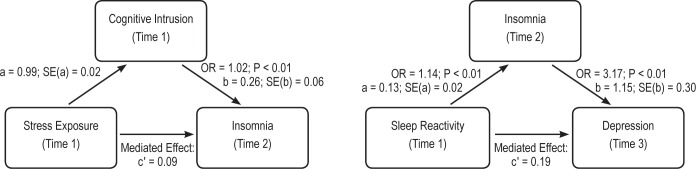
Insomnia as a mediator between sleep reactivity and depression. Mediated effects were estimated using the PRODCLIN method in R-Mediation.51 Both mediated effects were statistically significant at P < 0.05. a = association between the IV and the M. b = relation between the DV and M adjusted for the effects of the IV. c′ = relation between the IV and the DV adjusted for the effect of the mediator on the DV.
We then fit a logistic regression model with cognitive intrusion and sleep reactivity as predictors, and insomnia at Time 2 as the outcome. Fit indices suggested a reliable (χ2 = 101.13; P < 0.01) and well calibrated model (Hosmer-Lemeshow: χ2 = 9.17; P = 0.33). Cognitive intrusion and sleep reactivity remained significant independent predictors of insomnia. Further, there was a significant interaction (β = 0.02; χ2 = 3.96; P < 0.05) between sleep reactivity and cognitive intrusion, such that the relationship between cognitive intrusion and insomnia varied significantly as a function of sleep reactivity. Specifically, the odds of developing insomnia in response to cognitive intrusion were higher when sleep reactivity was high (see Figure 3).
Figure 3.
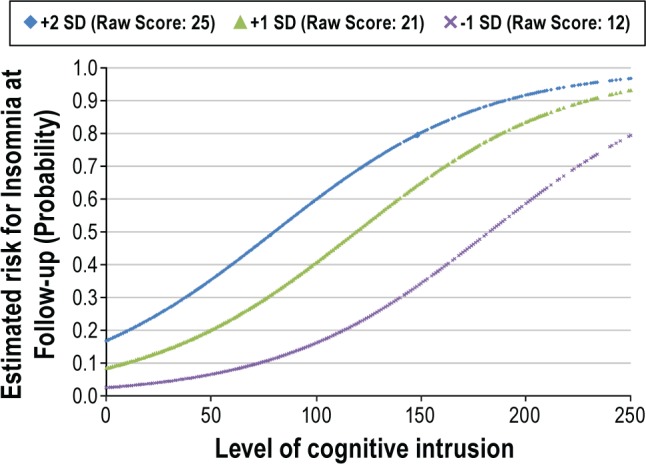
Incident insomnia as a function of cognitive intrusion at various levels of sleep reactivity: an interaction effect. Though plotted values were statistically (standard deviations) determined, descriptive statistics suggest that these values were also ecologically valid. Approximately 20% of the sample scored ≤ 12 on the FIRST, 50% scored between 14 and 18; 25% scored between 19 and 24; and 5% scored ≥ 25. SD = Standard deviation above/below the sample mean on the FIRST.
Depression
The two-year incidence rate of depression in the EPIC was 5.3 % (Time 3). As one of the aims of the study was to examine insomnia as a mediator of the effects of sleep reactivity on depression, we chose Time 3 depression as the primary endpoint of the following analyses. This strategy helped establish appropriate temporal precedence between the independent variable (baseline sleep reactivity), the mediator (Time 2 insomnia), and the outcome (Time 3 depression). We first assessed trait sleep reactivity as an independent predictor after controlling for Time 2 levels of depression, age, and gender. This model reliably distinguished between high and low levels of depression (χ2 = 245.85; P < 0.01) and fit the data well (Hosmer-Lemeshow: χ2 = 6.18; P = 0.63). Time 2, i.e., prior levels of depression were the strongest predictor of future depression (β = 2.33; OR = 11.52; 95% CI = 7.88–16.84; P < 0.01). Importantly, trait sleep reactivity was a significant independent predictor (β = 0.10; OR = 1.13; 95% CI = 1.08–1.18; P < 0.01) of depression at Time 3, such that the odds of a depression at Time 3 increased by 13% for a 1-point increase on the scale, and by nearly 70% for a 1-SD increase. Next, we examined Time 2 insomnia as a mediator of the relationship between trait sleep reactivity and Time 3 depression. Analyses proceeded as previously outlined. All individual models controlled for age, gender, and Time 2 depression; parameter estimates and omnibus model fit indices appear in Table 2. Time 2 insomnia was a significant predictor of depression at Time 3 after controlling for Time 2 depression (β = 1.15; OR = 3.17; 95% CI = 1.78–5.66; P < 0.01). Further, insomnia at Time 2 was a statistically significant (mediated effect: c′ = 0.19) mediator of the association between sleep reactivity and Time 3 depression (see Table 2, Figure 2). The confidence interval for the mediated effect (95% CI = 0.11–0.28) did not overlap zero.
Table 2.
Mediation analyses
DISCUSSION
The present study sought to prospectively assess the interaction between trait sleep reactivity and naturalistic stress in the development of insomnia in a cohort of healthy individuals with no current or prior history of this disorder. Consistent with our hypotheses, analyses revealed that stress exposure at baseline was a significant predictor of insomnia one year later. Further, the level of cognitive intrusion elicited by stress exposure mediated this effect. Importantly, a priori identified sleep reactivity moderated the effects of stress, such that individuals with heightened sleep reactivity exhibited a significantly higher risk for insomnia when exposed to stress. Finally, insomnia acted as a mediator between sleep reactivity and depression. Together, these findings offer strong empirical support for the stress-diathesis model of insomnia, while highlighting sleep reactivity as one such diathesis.
Sleep Reactivity as a Predisposing Factor
Stress-diathesis models of insomnia such as the Spielman 3P model55 are widely cited in the literature, and constitute the theoretical basis of current treatments such as cognitive-behavior therapy.56 However, despite their explanatory power, limited empirical support has emerged for these models. Cogent evidence for reliable predisposing factors of insomnia is rarer still.57 The present study offers data in support of sleep reactivity as a trait level risk factor for insomnia. Research from our lab and others suggests that this sleep-based vulnerability manifests in response to a diverse array of challenges to the sleep system, and that it exhibits significant trait-like stability in affected individuals.11 The present study provides insight into the mechanisms through which sleep reactivity translates into insomnia syndrome.
A critical finding of this study was that sleep reactivity moderated the risks conferred by stress-induced intrusion in the development of insomnia. In other words, the vulnerability of individuals who experienced comparable levels of cognitive intrusion varied as a function of trait sleep reactivity. Individuals with low levels of premorbid sleep reactivity appeared relatively protected from the effects of intrusion on insomnia. On the other hand, high levels of sleep reactivity were associated with a significantly lower stress-based threshold for insomnia. These results were particular noteworthy, given the long-term follow-up (1-2 years) between baseline and assessment in the present study. Further, this effect was robust even after controlling for other established risk factors, including gender.
This finding carries salient implications for future research and clinical practice. A recent well-controlled twin study estimated a 57% heritability for insomnia, thus highlighting the value of identifying and characterizing predisposing factors.58 However, given the significant symptom heterogeneity and comorbid disease in affected individuals, it is unlikely that this line of research will be fruitful among individuals with insomnia. Similarly, cost and time constraints often preclude the longitudinal assessment of a large and representative healthy sample. The present study offers a manageable target population on which to focus future work in the search for genetic predisposing factors. Further, the freely available and empirically validated FIRST scale provides a cost-effective and minimally invasive recruitment strategy to study the potential biological underpinnings of this trait. Future studies can also explore the presence and persistence in this group of other proposed vulnerabilities to insomnia disorder. Fernandez-Mendoza and colleagues found that trait levels of rumination (i.e., cognitive intrusion about past or future stressors) and neuroticism were significantly associated with FIRST scores in a sample of good sleepers, even after controlling for gender, depression, and anxiety.59 In another sample of good sleepers recruited from a university campus, FIRST scores were significantly correlated with a measure of dysfunctional beliefs and attitudes about sleep.60 Further research along these lines can shed light on the overlap between various cognitive, behavioral, and physiological vulnerabilities to insomnia disorder, thus integrating these largely disparate literatures.61,62
The study of individuals with premorbid sleep reactivity can also inform treatment. Nearly all behavioral treatments for insomnia involve a component of sleep hygiene education, a practice predicated on the assumption that individuals with insomnia engage in maladaptive behaviors to cope with sleep loss and disturbance.56 However, most studies suggest that good sleepers and insomniacs generally exhibit comparable sleep hygiene.33 Thus, the more important question is not whether sleep hygiene education is inherently effective, but for whom it may be a valid recourse. Our data suggest that individuals with trait sleep reactivity likely represent such a group. For instance, engagement in good sleep hygiene in response to acute sleep disturbance may represent an effective preventative strategy against insomnia disorder among individuals with high sleep reactivity who have not yet developed the disorder. Presently, little is known about the nature and prevalence of sleep hygiene during the prodromal stage of insomnia when sleep hygiene interventions are most likely to be effective. Addressing this gap in the literature may carry significant implications for more focused sleep health and awareness based outreach efforts.
Stress and Insomnia
Another noteworthy finding in our study was that the effects of stress exposure on insomnia were significantly mediated by cognitive intrusion. Further, though the interaction between stress exposure and sleep reactivity approached significance, cognitive intrusion acted as a robust mediator of the stress-insomnia relationship. Taken together, these findings suggest that stress events exert their effects through the responses they elicit, such that stress response is a more valid and meaningful assay of the stress construct than the simple frequency of stressors. Several recent reviews of the stress literature warn against the operationalization of stress as merely an objective life event/change, and specifically urge researchers to conduct more “tests of mediation” to improve our current understanding of the association between stress and pathology.2 Our findings highlight cognitive intrusion as an important response process variable in the transaction between stress exposure and outcome. A nuanced, multidimensional approach to stress assessment in future research59,63 will further enrich stress-diathesis models of insomnia and likely yield other meaningful targets for intervention.
Sleep Reactivity and Depression
Past research indicates that 60% to 90% of individuals with depression experience clinically significant levels of insomnia.64 Insomnia not only constitutes a significant risk factor for depression, but also exacerbates the severity and duration of existing depressive symptoms.65 Though the substantial comorbidity between depression and insomnia has received extensive research attention in the past decade, a comprehensive model of the risk factors driving this association has yet to emerge.66 Our results suggest that sleep reactivity may represent a common vulnerability to both disorders, and further that insomnia mediates the association between sleep reactivity and depression.
This finding is consistent with a growing body of research which indicates that depression and insomnia represent alternate manifestations of a shared or correlated set of vulnerabilities.67,68 The depression literature suggests that individuals with depression are significantly more likely to experience cognitive intrusion in the form of rumination and worry when confronted with stress than are healthy controls, and further that such cognitive intrusion is associated with sleep disturbance.69 Individuals with insomnia disorder also experience cognitive intrusion, though the focus of cognitive perseveration in this population is typically on the daytime impairments caused by sleep loss.70 Hence, it stands to reason that sleep-focused and mood-focused cognitive intrusion may represent correlated vulnerabilities for insomnia disorder and depression respectively. While sleep-specific intrusion may trigger and maintain insomnia disorder, a more diffuse ruminative style will more likely precipitate depression or depression comorbid with insomnia disorder. As noted earlier, Fernandez-Mendoza and colleagues59 found that trait levels of rumination were significantly associated with FIRST scores in a sample of good sleepers with no history of insomnia disorder. These findings offer preliminary support for sleep reactivity and cognitive intrusion as correlated vulnerabilities to insomnia disorder and depression.
Limitations
Our results should be interpreted in the context of certain methodological limitations. Despite a low initial participation rate, a comparison of the demographic features of our sample with nationally representative data including insomnia incidence rates27,71,72 suggested minimal selection or recruitment bias. Further, considering the longitudinal design of this study, we had a high retention rate. Secondly, self-report instruments can be vulnerable to recall bias. The absence of structured interviews by trained clinicians is another potential shortcoming, as are the threats to protocol validity inherent in web-based surveys. Despite these limitations, however, we believe the present study makes an important contribution to the field by systematically examining an important stress-diathesis model of the etiology of insomnia. Establishing the genetic underpinnings of diatheses of insomnia such as sleep reactivity could further enhance our current understanding of the pathophysiology of this debilitating disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by an NIMH Grant R01 MH082785 to Dr. Drake. Dr. Drake has served as consultant for Teva. He has received research support from Merck and Teva. He has served on speakers bureau for Jazz, Purdue, and Teva. Dr. Pillai reported no financial interest/conflict in this manuscript. Dr. Roth has served as consultant for Abbott, Accadia, AstraZenca, Aventis, AVER, Bayer, BMS, Cypress, Ferrer, Glaxo Smith Kline, Impax, Intec, Jazz, Johnson and Johnson, Merck, Neurocrine, Novartis, Proctor and Gamble, Pfizer, Purdue, Shire, Somaxon, and Transcept. He has received research support from Cephalon, Merck, and Transcept. He has served on speakers bureau for Purdue.
ACKNOWLEDGMENTS
The authors thank Catherine Jefferson, Joe Seto, Jacqueline Koshorek, Steven Kluck, and Heather Mengel for all their hard work in completing three waves of data collection. We are grateful to Ren Belcher for his thorough review of this manuscript.
Footnotes
A commentary on this article appears in this issue on page 1273.
REFERENCES
- 1.Rosenthal D. Genetic theory and abnormal behavior. New York, NY: McGraw-Hill; 1970. [Google Scholar]
- 2.Hammen C. Stress And depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 3.McKeever VM, Huff ME. A diathesis-stress model of posttraumatic stress disorder: Ecological, biological, and residual stress pathways. Rev Gen Psychol. 2003;7:237–50. [Google Scholar]
- 4.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–85. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 5.Ordovas JM, Corella D, Demissie S, et al. Dietary fat intake determines the effect of a common polymorphism in the hepatic lipase gene promoter on high-density lipoprotein metabolism: evidence of a strong dose effect in this gene-nutrient interaction in the Framingham Study. Circulation. 2002;106:2315–21. doi: 10.1161/01.cir.0000036597.52291.c9. [DOI] [PubMed] [Google Scholar]
- 6.Hill AV. The genomics and genetics of human infectious disease susceptibility. Annu Rev Genomics Hum Genet. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34:1161–71. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morin CM, Belanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 9.Jansson M, Linton SJ. The role of anxiety and depression in the development of insomnia: Cross-sectional and prospective analyses. Psychol Health. 2006;21:383–97. [Google Scholar]
- 10.Mendelson W. Long-term follow-up of chronic insomnia. Sleep. 1995;18:701. doi: 10.1093/sleep/18.8.698. [DOI] [PubMed] [Google Scholar]
- 11.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26:1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 12.Drake CL, Jefferson C, Roehrs T, Roth T. Stress-related sleep disturbance and polysomnographic response to caffeine. Sleep Med. 2006;7:567–72. doi: 10.1016/j.sleep.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–81. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 14.Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2006;47:391–5. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- 15.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–7. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 16.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 17.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 18.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen H, Kecklund G, D'Onofrio P, Nilsson J, Akerstedt T. Stress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepiness. J Sleep Res. 2013;22:50–7. doi: 10.1111/j.1365-2869.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 20.Drake CL, Friedman NP, Wright KP, Jr., Roth T. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34:1179–88. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake CL, Scofield H, Roth T. Vulnerability to insomnia: the role of familial aggregation. Sleep Med. 2008;9:297–302. doi: 10.1016/j.sleep.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths MF, Peerson A. Risk factors for chronic insomnia following hospitalization. J Adv Nurs. 2005;49:245–53. doi: 10.1111/j.1365-2648.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 23.Drake C, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: An overview. Depress Anxiety. 2003;18:176. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- 24.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43:439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Vollrath M, Wicki W, Angst J. The Zurich study. VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 26.Sontag LM, Graber JA. Coping with perceived peer stress: Gender-specific and common pathways to symptoms of psychopathology. Dev Psychol. 2010;46:1605–20. doi: 10.1037/a0020617. [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 29.Lichstein KL, Rosenthal TL. Insomniacs' perceptions of cognitive versus somatic determinants of sleep disturbance. J Abnorm Psychol. 1980;89:105–7. doi: 10.1037//0021-843x.89.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Carney CE, Edinger JD, Meyer B, Lindman L, Istre T. Symptom-focused rumination and sleep disturbance. Behav Sleep Med. 2006;4:228–41. doi: 10.1207/s15402010bsm0404_3. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen DK, Mehlsen MY, Christensen S, Zachariae R. Rumination-relationship with negative mood and sleep quality. Pers Individ Diff. 2003;34:1293–301. [Google Scholar]
- 32.Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med. 2009;71:771–5. doi: 10.1097/PSY.0b013e3181ae58e8. [DOI] [PubMed] [Google Scholar]
- 33.Gellis LA, Lichstein KL. Sleep hygiene practices of good and poor sleepers in the United States: an internet-based study. Behav Ther. 2009;40:1–9. doi: 10.1016/j.beth.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Germain A, Nofzinger E, Kupfer D. Arlington, Virginia: American Psychiatric Publishing, Inc; 2006. Mood disorders and sleep. The American Psychiatric Publishing textbook of mood disorders; pp. 717–37. [Google Scholar]
- 35.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9(Suppl1):S3–S9. doi: 10.1016/S1389-9457(08)70010-8. [DOI] [PubMed] [Google Scholar]
- 36.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 37.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 38.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Pillai V, Roth T, Mullins HM, Drake CL. Moderators and mediators of the relationship between stress and insomnia: stressor chronicity, cognitive intrusion, and coping. Sleep. 2014;37:1199–208. doi: 10.5665/sleep.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychological Association. Diagnostic and stastical manual of mental disorders. 4th ed. Washington, DC: American Psychological Association; 2000. [Google Scholar]
- 41.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 42.Hobson CJ, Kamen J, Szostek J, Nethercut CM, Tiedmann JW, Wojnarowicz S. Stressful life events: A revision and update of the Social Readjustment Rating Scale. Int J Stress Manag. 1998;5:1–23. [Google Scholar]
- 43.Hobson CJ, Delunas L. National norms and life-event frequencies for the revised Social Readjustment Rating Scale. Int J Stress Manag. 2001;8:299–314. [Google Scholar]
- 44.Scully JA, Tosi H, Banning K. Life event checklists: Revisiting the Social Readjustment Rating Scale after 30 years. Educ Psychol Meas. 2000;60:864–76. [Google Scholar]
- 45.Weiss DS. The Impact of Event Scale: Revised. In: Wilson JP, Tang CS, editors. Cross-cultural assessment of psychological trauma and PTSD. New York, NY US: Springer Science + Business Media; 2007. pp. 219–38. [Google Scholar]
- 46.Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry. 2002;180:205–9. doi: 10.1192/bjp.180.3.205. [DOI] [PubMed] [Google Scholar]
- 47.Armonk, NY: IBM Corp; 2010. IBM SPSS Statistics for Windows, Version 19.0. [Google Scholar]
- 48.Brand S, Gerber M, Pühse U, Holsboer-Trachsler E. Depression, hypomania, and dysfunctional sleep-related cognitions as mediators between stress and insomnia: The best advice is not always found on the pillow! Int J Stress Manag. 2010;17:114–34. [Google Scholar]
- 49.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;21:437–48. [Google Scholar]
- 50.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curran PJ, West SG, Finch JF. The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Methods. 1996;1:16–29. [Google Scholar]
- 55.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 56.Williams J, Roth A, Vatthauer K, McCrae CS. Cognitive behavioral treatment of insomnia. Chest. 2013;143:554–65. doi: 10.1378/chest.12-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drake C, Roth T. Predispostion in the evolution of insomnia: Evidence, potential mechanisms, and future directions. Sleep Med Clin. 2006;1:333–49. [Google Scholar]
- 58.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitiveemotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72:397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 60.Yang CM, Chou CP, Hsiao FC. The association of dysfunctional beliefs about sleep with vulnerability to stress-related sleep disturbance in young adults. Behav Sleep Med. 2011;9:86–91. doi: 10.1080/15402002.2011.557990. [DOI] [PubMed] [Google Scholar]
- 61.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 63.Morin CM, Bastien C, Savard J. Current status of cognitive-behavior therapy for insomnia: Evidence for treatment effectiveness and feasibility. In: Lichstein L, Perlis PL, editors. Treating sleep disorders: Principles and practice of behavioral sleep medicine. Hoboken, NJ: John Wiley & Sons; 2003. [Google Scholar]
- 64.Kloss JD, Szuba MP. Insomnia in psychiatric disorders. In: Szuba MP, Kloss JD, Dinges DF, editors. Insomnia: Principles and management. New York, NY: Cambridge University Press; 2003. pp. 43–70. [Google Scholar]
- 65.Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31:481–8. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry. 2011;70:912–9. doi: 10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Pillai V, Drake CL. Sleep and repetitive thought: The role of rumination and worry in sleep disturbance. In: Babson KA, Feldner MT, editors. Sleep and affect: assessment, theory, & clinical implications. Elsevier; 2014. [Google Scholar]
- 70.Carney CE, Harris AL, Falco A, Edinger JD. The relation between insomnia symptoms, mood, and rumination about insomnia symptoms. J Clin Sleep Med. 2013;9:567–75. doi: 10.5664/jcsm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 72.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13:346–53. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]



