Abstract
Study Objective:
To assess the short-term efficacy of a video-based cognitive behavioral therapy for insomnia (CBT-I) as compared to a professionally administered CBT-I and to a no-treatment group.
Design:
Randomized controlled trial.
Setting:
Radio-oncology department of a public hospital affiliated with Université Laval (CHU de Québec).
Participants:
Two hundred forty-two women with breast cancer who had received radiation therapy in the past 18 mo and who had insomnia symptoms or were using hypnotic medications were randomized to: (1) professionally administered CBT-I (PCBT-I; n = 81); (2) video-based CBT-I (VCBT-I; n = 80); and (3) no treatment (CTL; n = 81).
Interventions:
PCBT-I composed of six weekly, individual sessions of approximately 50 min; VCBT-I composed of a 60-min animated video + six booklets.
Measurement and Results:
Insomnia Severity Index (ISI) total score and sleep parameters derived from a daily sleep diary and actigraphy, collected at pretreatment and posttreatment. PCBT-I and VCBT-I were associated with significantly greater sleep improvements, assessed subjectively, as compared to CTL. However, relative to VCBT-I, PCBT-I was associated with significantly greater improvements of insomnia severity, early morning awakenings, depression, fatigue, and dysfunctional beliefs about sleep. The remission rates of insomnia (ISI < 8) were significantly greater in PCBT-I as compared to VCBT-I (71.3% versus 44.3%, P < 0.005).
Conclusions:
A self-administered cognitive behavioral therapy for insomnia (CBT-I) using a video format appears to be a valuable treatment option, but face-to-face sessions remain the optimal format for administering CBT-I efficaciously in patients with breast cancer. Self-help interventions for insomnia may constitute an appropriate entry level as part of a stepped care model.
Trial Registration:
ClinicalTrials.gov Identifier: NCT00674830.
Citation:
Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. SLEEP 2014;37(8):1305-1314.
Keywords: cancer, cognitive behavioral therapy, insomnia, low intensity treatment, RCT, self-help treatment, stepped care, video
INTRODUCTION
Insomnia is among the most common symptoms reported by cancer patients with a prevalence rate varying from 30% to 60%.1–3 Cognitive behavioral therapy (CBT) is considered to be the treatment of choice for insomnia4 and its efficacy is well established for primary insomnia (with no comorbidity).5–7 An increasing number of studies have supported the efficacy of cognitive behavioral therapy for insomnia (CBT-I) in patients with cancer. Overall, results have been quite consistent in showing that CBT-I is associated not only with improved subjective sleep but also with a reduction of psychological distress and improved quality of life.8–14
Despite accumulating evidence supporting its efficacy when administered face-to-face, the accessibility to CBT-I remains extremely limited. Only a few cancer centers have mental health professionals formally trained in the psychological management of insomnia. In addition, the time and human resources required to administer CBT-I in person preclude its implementation as part of routine cancer care. Currently, most patients do not receive any treatment for their condition or are prescribed only hypnotic medications despite their side effects, risks, and limitations.15
Clearly, alternative treatment delivery models are needed in order to increase access to CBT-I. Self-administered CBT-I is efficacious in the context of primary insomnia and insomnia comorbid with psychological disorders.16 In the specific context of cancer, a small-scale randomized controlled trial (RCT) of 28 cancer survivors showed that a web-based treatment was associated with greater sleep improvements than a waiting-list control condition.17 Although these preliminary findings are encouraging, further research is warranted in order to assess to what extent a self-help form of CBT-I is as efficacious as a standard professionally administered format and would represent a viable alternative for patients with cancer.
This RCT compared the short-term efficacy of a video-based CBT-I (VCBT-I) to a professionally administered format (PCBT-I) and to a no-treatment condition (CTL) in women with breast cancer. The video-based intervention was found to be feasible and to lead to promising treatment effects in a pilot study with patients with breast cancer.18 It was hypothesized that both treatment conditions would be associated with greater sleep improvements than CTL, but PCBT-I was expected to produce greater benefits than VCBT-I on some variables. However, because of the paucity of comparable studies, we were unable to speculate on which variables differences would be obtained. The study also aimed at comparing remission rates of insomnia across groups.
METHOD
Participants
Eligibility Criteria
Inclusion criteria: (1) radiation therapy for a nonmetastatic breast cancer within the past 18 mo; (2) insomnia symptoms as defined by a score ≥ 8 on the Insomnia Severity Index (ISI)19 or utilization of a psychotropic medication as a sleep aid ≥ 2 nights in the past 2 w; (3) age between 18 and 75 y old; and (4) able to readily read and understand French. Exclusion criteria: (1) severe cognitive impairments (e.g., diagnosis of Parkinson disease or a score ≤ 23 on the Mini-Mental State Examination (MMSE)20; (2) severe psychiatric disorder as assessed using the Structured Clinical Interview for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) (SCID21; e.g., severe major depression) or reported by the patient (e.g., bipolar disorder); (3) diagnosis of a sleep disorder other than insomnia (e.g., sleep apnea); (4) nightshift work in the past 3 mo or in the next 18 mo; (5) received psychotherapy specifically for insomnia; and (6) severe visual, hearing, or language defects.
Recruitment
The participants were recruited between April 2008 and October 2011. Patients meeting the study's initial inclusion criteria were approached by a research assistant at the radio-oncology department of L'Hôtel-Dieu de Québec (CHU de Québec). The assistant briefly introduced the study, explained the screening procedure and further assessed patients' eligibility. Eligible and consenting patients were then asked to read and sign the informed consent form and were invited to a clinical interview. Patients with no insomnia symptoms at that time but otherwise eligible for the study were asked to participate in a phone screening procedure, using the ISI, administered every 2 to 4 mo until 18 mo postradiation therapy. Patients who had developed insomnia symptoms or reported using hypnotic medications at that time were then invited to a clinical interview. This study was approved by the research ethics committee of CHU de Québec.
Among the 1,817 patients approached at the clinic, 36 refused to complete the ISI screening and 135 were excluded (Figure 1). Reasons for exclusion were: sleep disorder other than insomnia (n = 51); severe psychiatric disorder (n = 38); night shift work (n = 15); distant metastasis (n = 15); specific psychotherapy for insomnia received in the past (n = 8); diagnosis of Parkinson disease, Alzheimer disease, or another type of dementia (n = 6); insufficient French literacy (n = 5); and too long a period since the end of radiotherapy treatment (n = 5). (The total exceeds 100% because some patients met more than one exclusion criterion.) Among the eligible women (n = 1,646), 819 had insomnia symptoms (ISI ≥ 8 or hypnotic medication ≥ 2 nights in the past 2 w) and 827 did not, but were otherwise eligible and were therefore offered the screening procedure.
Figure 1.
Participants' flow chart. CBT-I, cognitive behavioral therapy for insomnia; ISI, Insomnia Severity Index; RCT, randomized controlled trial.
Among the 819 patients who had insomnia symptoms, 182 accepted to participate in the RCT (participation rate = 22.2%). Of the 827 patients who did not have insomnia symptoms but were eligible for the screening procedure, 528 accepted the screening and were assessed over the phone up to six times until 18 mo postradiotherapy, of whom 78 became eligible and accepted to participate in the RCT (participation rate = 17.7% over up to six screenings) for a total of 260 participants.
The most common reasons for declining participation (combining initial recruitment and the phone screening procedure) were: no subjective sleep complaint (n = 514); lack of interest (n = 194); study judged too burdensome (n = 171); unable to come to the research center (n = 162); the wish to stop thinking about cancer (n = 30); no reason reported (n = 26); or refusal of the randomization procedure (n = 4). Of the 260 patients who accepted to participate in the RCT, 18 were excluded following the clinical interview at the research center, leaving 242 participants who were randomized in the study. Reasons for exclusion at this stage were: ISI total score < 8 or insufficient hypnotic medication use when reassessed at the interview (n = 9); substance use disorder (n = 4); cognitive impairments (n = 2); suicidal thoughts (n = 2); and sleep disorder other than insomnia (n = 1).
Thirty-eight patients dropped out of the study following randomization, 11 in the PCBT-I group, 23 in the VCBT-I group, and four in the CTL group. Reasons for discontinuation were: exceeded the allocated time for the completion of follow-ups (n = 8); study judged too burdensome (n = 8); lack of interest (n = 7); no longer had a subjective sleep complaint (n = 6); refusal to pursue completion of sleep diary (n = 3); dissatisfaction with the treatment (n = 3); worsening of the physical condition/death (n = 2); or no reason provided (n = 1).
Comparisons Between Completers and Noncompleters
Comparisons between completers (n = 203) and noncompleters (n = 39) were performed on demographic, medical, and main outcome variables at baseline. Patients who dropped out at posttreatment were more likely: (1) to be in the VCBT-I condition (61.5% of the noncompleters versus 27.6% of completers), χ2(2) = 19.28, P < 0.001; (2) to have a high school education or less (55.6% versus 29.2%), χ2(2) = 9.62, P = 0.008; (3) to have a psychiatric disorder (any type; 71.8% versus 49.8%, P = 0.01), an anxiety disorder (59.0% versus 34.0%, P = 0.003) or a mood disorder (18.0% versus 5.9%, P = 0.01); (4) to report anxiolytic medication use (35.9% versus 20.7%, P = 0.04); and (5) to obtain a higher baseline ISI score (mean = 16.2 versus 13.9, t(240) = 9.52, P = 0.002).
Sample Size Justification and Power Analyses
To estimate the sample size needed for adequately powered comparisons between the active treatment conditions and the control condition, expected pretreatment to posttreatment effect sizes (ES) on the main sleep variables (ISI total score; total wake time (TWT) and sleep efficiency (SE) from the sleep diary) were computed using data from our previous RCT.13 ES ranging from 0.60 to 0.70, corresponding to large effects,22 were observed. Power computations using G*Power 3.1.523 showed that 80 participants per group (total of 240) were necessary to detect these effect sizes, using standard power conditions (two-tailed 5% alpha, 80% power, 1:1:1 allocation).
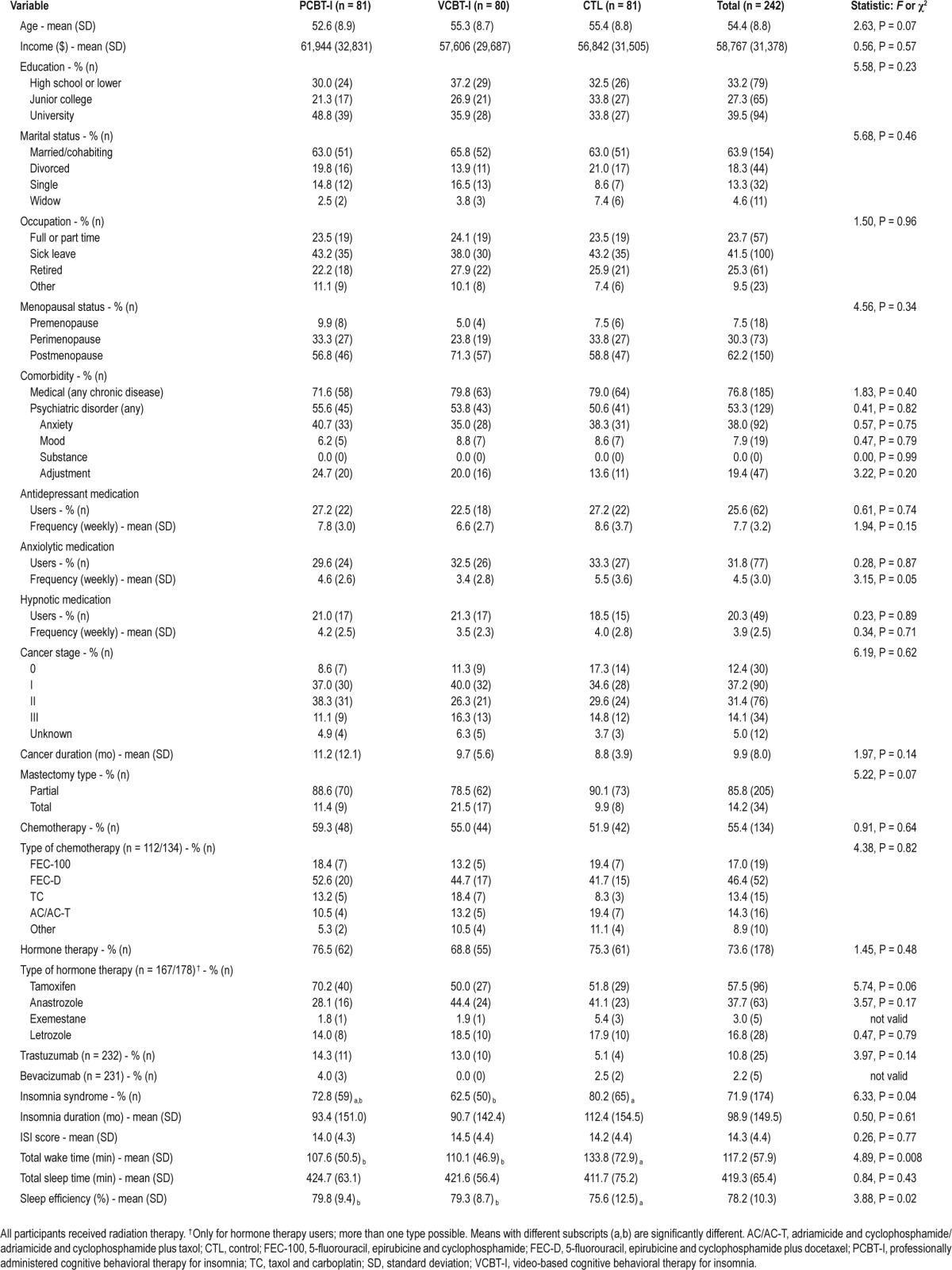
Participants' Characteristics
At pretreatment, the average ISI score was 14.3 and hypnotic medications were used by 14.9% of the sample (Table 1). About half of the participants (55%) received chemotherapy before radiation therapy. No between-group differences were found at baseline on any demographic or medical variable. However, the CTL group reported a significantly lower SE (P = 0.02) and a greater TWT (P = 0.008) on the sleep diary as compared to the two other groups. In addition, a larger proportion of participants met the diagnostic criteria for an insomnia disorder in the CTL than in the VCBT-I group (P = 0.01).
Table 1.
Participants' characteristics at baseline
Study Design
This study was a three-group RCT with an allocation ratio of 1:1:1: (1) professionally administered CBT-I (PCBT-I; n = 81); (2) video-based CBT-I (VCBT-I; n = 80); and (3) control group (CTL; no CBT-I; n = 81). Participants were assessed at pretreatment and posttreatment, as well as at 3-, 6-, and 12-mo follow-ups. The current report presents only posttreatment data because the follow-up data are still being analyzed.
Randomization and Allocation Concealment
The randomization sequence was prepared by our biostatistician using a random permuted-block procedure (SAS 9.3 PROC PLAN, SAS Institute Inc.) with block sizes varying from 6 to 12. The allocation sequence was concealed in opaque, sealed envelopes that were numbered in advance and opened sequentially. All research assistants and clinicians were blind to the group allocation sequence. The envelopes also contained the information as to whether patients had to wear the actigraphic recorder at pretreatment and posttreatment and at 6-mo follow-up. A third of the participants were randomly selected within each condition to do so (total of 79 patients).
PROCEDURE
Pretreatment Assessment
At recruitment, patients were given or mailed (phone screening) a battery of self-report scales and a 2-w daily sleep diary that they had to complete at home. A clinical interview (90 min) at the research center was then scheduled within 2 w with a graduate student in psychology. The interviewer first completed any missing data with the patients and then administered the MMSE, the SCID, and the Insomnia Interview Schedule (IIS).24 After their eligibility was confirmed, participants were informed of their group assignment.
Randomly selected participants were given an actigraph and instructed to wear it for seven consecutive 24-h periods and to complete an additional 7-day sleep diary prior beginning any intervention. In order to decrease the possibility of a contamination bias, participants of the two treatment groups signed an agreement not to distribute the treatment material to anyone. Participants assigned to VCBT-I were given the treatment material (DVD and booklets) or were mailed the material upon reception of the actigraph 1 week later.
Intervention Phase
The duration of the treatment phase was 6 w. CBT for insomnia was multimodal and combined behavioral (i.e., stimulus control therapy, sleep restriction), cognitive (i.e., cognitive restructuring), and educational (i.e., sleep hygiene) strategies. Participants of the two CBT groups received the same series of six booklets (13-21 pages) explaining CBT strategies for insomnia using practical examples. A different theme was covered for each of the 6 w of treatment (Week 1: Basic sleep facts; Week 2: Stimulus control and sleep restriction; Week 3: Cognitive restructuring; Week 4: Dysfunctional beliefs about sleep; Week 5: Sleep hygiene; and Week 6: Evaluation and maintenance of treatment gains).
PCBT-I
These participants received six weekly, individual treatment sessions of approximately 50 min. A similar treatment protocol, based on clinical procedures developed by Morin24 and slightly adapted for the cancer population, has been found to effectively treat chronic insomnia in patients with breast cancer.12,13 Seventy-four participants (91.4%) received all six treatment sessions. CBT-I sessions were administered by certified psychologists and PhD students in clinical psychology with significant experience.
VCBT-I
These participants received a self-help treatment package composed of a 60-min video (DVD format) and six booklets.18 The video has an animated cartoon format and presents Professor Morpheus and four patients with cancer participating in a group CBT for insomnia, each representing a different situation in terms of age groups, cancer sites, and types of sleep difficulties. The content covered in the video is the same as in the face-to-face sessions. More details about the video-based intervention can be found elsewhere.18 Each week, participants had to first watch a video segment (5-20 min each) and then read a booklet. They were informed that they could call a licensed psychologist to get further information on the treatment strategies proposed, but only seven participants (8.8%) did. Adherence data were available on 54 participants (i.e., VCBT-I completers): 100% reported to have read the six booklets and 89.8% watched all six video segments. In addition, 96%, 81%, and 94% reported that they at least “moderately” put into practice the behavioral, cognitive, and sleep hygiene recommendations, respectively.
Control Group
These participants did not receive CBT-I.
Description of the Treatment Content
Stimulus control therapy aims at reassociating the bed and the bed environment with sleep, and establishing a regular sleep-wake rhythm by following a specific set of guidelines such as “Go to bed only when you feel sleepy” and “When unable to fall asleep within 15 to 20 minutes, get out of bed, leave the bedroom and return to bed only when sleepy.” Sleep restriction aims at curtailing the time in bed to the actual sleep time, thereby resulting in more consolidated and efficient sleep. Time in bed is progressively increased as SE improves during treatment. Cognitive restructuring strategies consist of modifying dysfunctional thoughts and attitudes about sleep, sleep difficulties, and the impact of these sleep difficulties on patients' daily functioning (e.g., “I need 8 hours of sleep to function well during the day”; “If I do not sleep well, my cancer will come back”). This was done by guiding the patient to identify maladaptive sleep cognitions, challenging their validity, and reframing them into more adaptive substitutes. Sleep hygiene education included information about the effect of environmental factors and health behaviors on sleep and about the appropriate usage of hypnotic medications.
Other Information on the Treatment Phase
Participants of the three groups were allowed to initiate or continue any psychosocial or pharmacological services normally offered, including psychosocial services and psycho-tropic medications. No significant difference was found between the three conditions on the percentage of patients who consulted a healthcare professional during the treatment phase (PCBT-I = 81.4%; VCBT-I = 87.0%; CTL = 73.0%), χ2(2) = 4.01, P = 0.13.
Patients were instructed to continue completing a daily sleep diary throughout treatment for clinical purposes. Those interested in reducing their use of sleep-promoting medications were advised to consult their physician or pharmacist for the development of a withdrawal program, although some information was offered during treatment on why and how to stop their medication if they wished to do so.
Assessment of Treatment Integrity
All face-to-face sessions were audiorecorded in order to assess the extent to which the treatment protocol was followed by the therapist. The first six to 12 sessions of each therapist were systematically listened to by a senior therapist (licensed psychologist) and brief written reports on the quality of the session and possible improvements were provided. Then, assessments were conducted as needed, for example, for more difficult sessions or for patients with particular problems. Overall, 18.4% of the treatment sessions were assessed.
Posttreatment Assessment
At posttreatment, participants were asked to complete the same self-report scales and another 2 w of sleep diary (+ 7 days of actigraphy for selected participants). After the completed measures were received, another phone interview was conducted to supply any missing data and administer the IIS.
Participants received $40 (Canadian) for each time assessment completed (i.e., questionnaires and clinical interview), $50 if they also wore the actigraph.
MEASURES
All French–Canadian versions of measures used have been empirically validated or developed by the authors of the original version.
Main Outcome Measures
Insomnia Severity Index (ISI)24,25
A seven-item questionnaire evaluating insomnia severity. A score of 8 or higher is used to detect clinically significant insomnia, whereas a score of 15 or higher indicates the possible presence of an insomnia disorder.19 The ISI was validated in patients with cancer.19
Daily Sleep Diary
To provide subjective estimates of sleep onset latency (SOL), wake after sleep onset (WASO), early morning awakening (EMA), TWT, total sleep time (TST), and SE.
Insomnia Interview Schedule (IIS)24
A semistructured interview to diagnose the presence of an insomnia syndrome defined as follows: subjective complaint of sleep difficulties, SOL or WASO greater than 30 min, ≥ 3 nights/w, duration ≥ 1 mo, associated with impaired daytime functioning or marked distress or using a hypnotic medication ≥ 3 nights/w for ≥ 1 mo.
Secondary Dependent Variables
Multidimensional Fatigue Inventory (MFI)26
The French–Canadian version of the Multidimensional Fatigue Inventory27 contains 15 items (rated on a scale from 0 to 4). The average individual score of fatigue was used in this study.
Hospital Anxiety and Depression Scale(HADS)28,29
A 14-item questionnaire rated on a scale from 0 to 3 and divided into two subscales: depression (HADS-D: seven items) and anxiety (HADS-A: seven items).
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)30
A 13-item questionnaire rated on a scale from 0 to 3. Scores are transformed to give a score ranging from 0 to 100. Only the global quality of life score (item 13) was used for the purpose of this study.
Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS)– Abbreviated Version31
Composed of 16 items assessing to what extent the person endorses erroneous beliefs about sleep (e.g., unrealistic sleep expectations) on an 11-point Likert scale ranging from 0 (strongly disagree) to 10 (strongly agree).
Actigraphy
The Actiwatch-64® (Philips Respironics, Andover, MA) is a small, waterproof, nonintrusive actigraphy device that is worn on the wrist and is similar in size and appearance to a wristwatch. By calculating orientation and movement, the Actiwatch records sleep-wake activity and provides an objective measure of the same sleep parameters as the sleep diary (SOL, WASO, TWT, SE, TST), in addition to number of wake bouts, number of sleep bouts, number of naps, and naps duration. Each trace was scored manually, visually on screen using 30-sec epochs. Periods of rest (sleep), nap, and activity, as well as artefacts, were scored independently by two graduate students. Analyses of inter-rater agreement revealed excellent intraclass correlations (ICC, varying between 0.93 and 0.99) for the main sleep parameters (i.e., SOL, WASO, TST, SE, number of awakenings, number and duration of naps). Any disagreement in scoring was then discussed until a consensus was attained. Data from sleep diary were used to help score naps, lights out/lights on, and periods when the actigraph was removed.
Statistical Analyses
All data were double-entered, and missing or aberrant data were verified for maximal integrity. Analyses for the main hypotheses were performed using an intent-to-treat approach. No data imputation was performed.
Demographics, health-related data, psychiatric diagnoses, medication use, cancer characteristics and treatments were investigated as potential covariates.32 No variable was found to meet our criterion (i.e., correlations > 0.30 with main outcomes), hence no covariate was included in the models. To investigate changes on study variables within and between conditions, 3 (Groups) × 2 (Time: Pre and Post) split-plot linear (for continuous outcomes) and generalized (for binary outcomes) mixed model analyses were completed to test Group, Time, and Interaction effects (decomposed using simple effects when significant). Effect sizes (Cohen's d) were computed as the ratio of raw change and the root mean square error (RMSE) of the model. All analyses were performed using SAS 9.3 (SAS Institute Inc.)33 with a standard two-tailed 5% alpha level and Bonferroni correction for post hoc comparisons.
RESULTS
Treatment Effects on Sleep Measures
Significant Group × Time interactions were found on all subjective sleep variables except EMA (P = 0.06) and TST (P = 0.40; Table 2). Simple effects revealed that VCBT-I was associated with significantly greater sleep improvements than CTL, except for EMA and TST. There was no significant difference between PCBT-I and VCBT-I on SOL (P < 0.13), WASO (P < 0.24), TWT (P < 0.07) and SE (P < 0.13). However, PCBT-I was associated with a significantly greater reduction of ISI scores (P < 0.03; Figure 2) and EMA (P < 0.04). Moreover, except for TST, effect sizes of time effects were consistently greater in PCBT-I (from 0.66 to 1.84) than in VCBT-I (from 0.50 to 1.40), which were greater than in CTL (from 0.23 to 0.69).
Table 2.
Mean scores obtained on sleep diary variables by each group at pretreatment and posttreatment and effects obtained
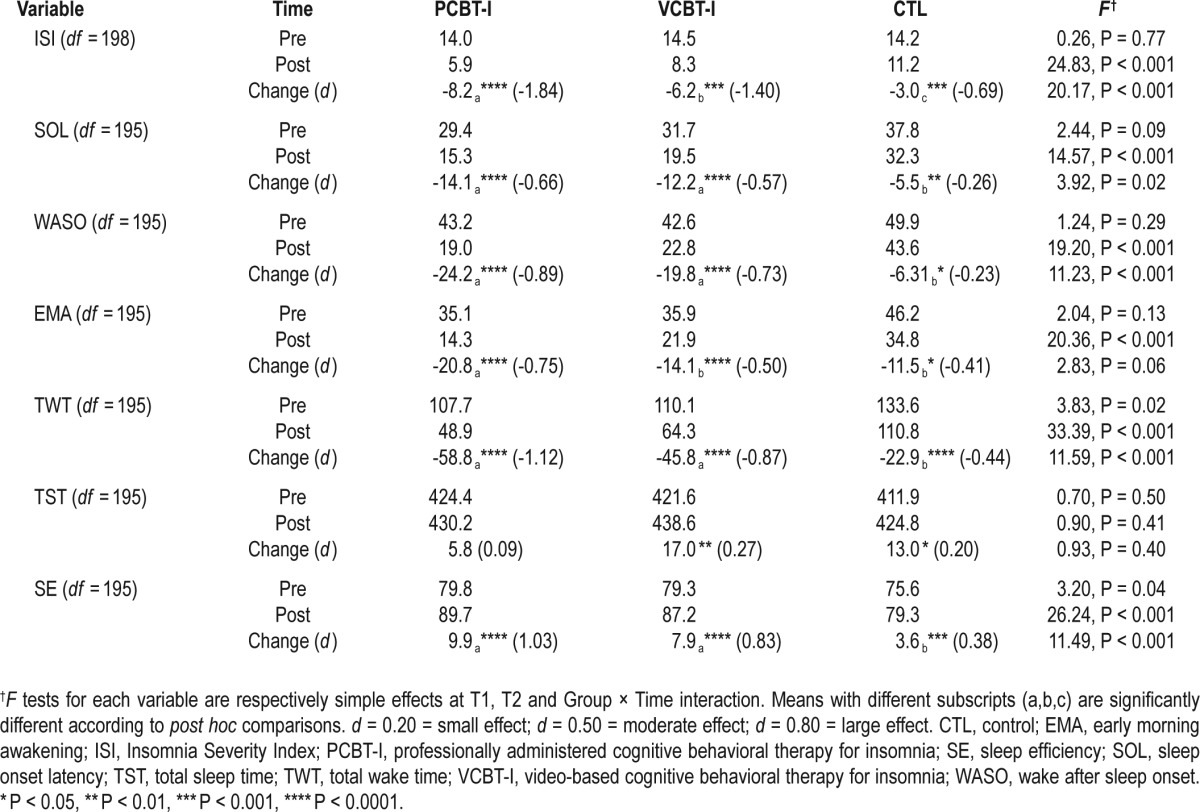
Figure 2.
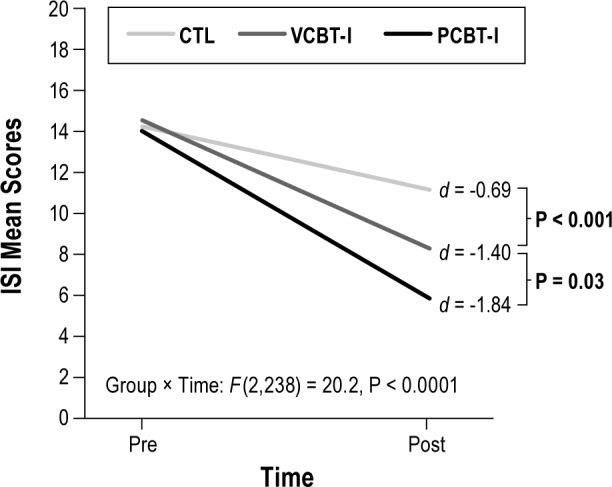
Between-groups differences on pretreatment versus posttreatment Insomnia Severity Index total scores. CTL, control; PCBT-I, professionally administered cognitive behavioral therapy for insomnia; VCBT-I, video-based cognitive behavioral therapy for insomnia.
No significant Group × Time interaction was found on any actigraphy parameter, except on TST, F(2,52) = 3.21, P = 0.04, for which a significant reduction was found from pretreatment to posttreatment in PCBT-I participants only, t(52) = -2.08, P = 0.05.
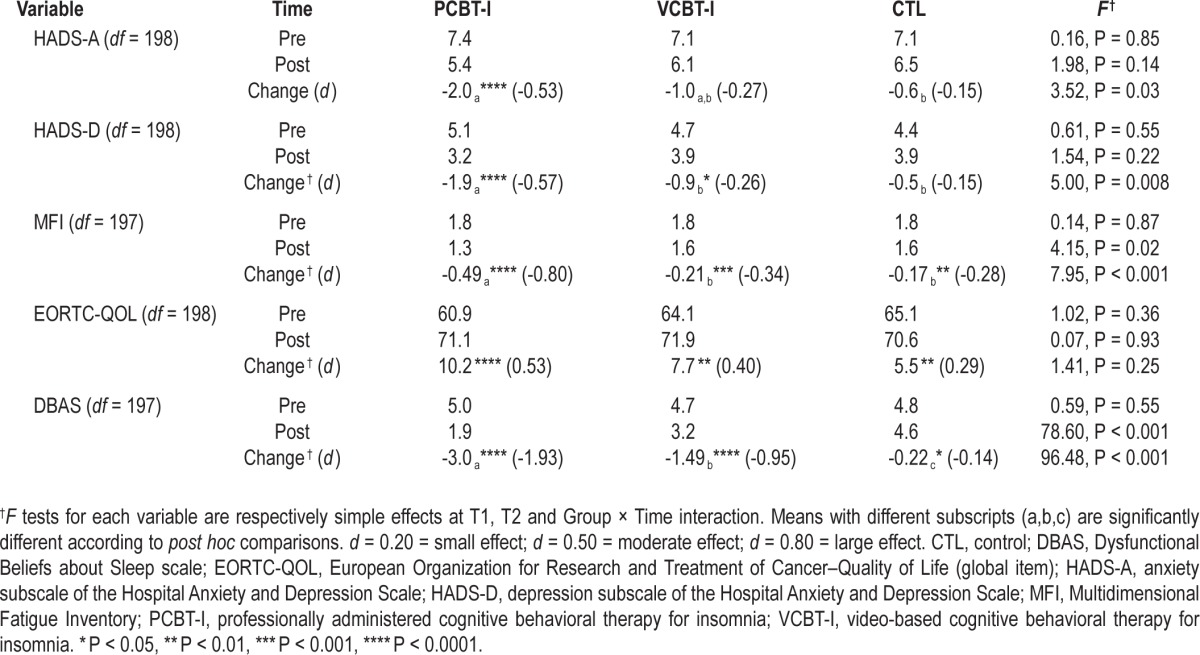
Treatment Effects on Secondary Variables
Significant Group × Time interactions were found on all secondary variables except quality of life, which significantly improved over time in all three conditions (Table 3). Simple effects indicated that depression, fatigue, and dysfunctional beliefs about sleep scores were significantly more improved in patients in the PCBT-I group than in the VCBT-I group. Although the VCBT-I group showed larger decreases in dysfunctional beliefs about sleep than the CTL group at post-treatment, no significant differences were found between these two groups on reductions of fatigue and depression. A different pattern of findings was found for anxiety: PCBT-I was associated with significantly greater reductions than CTL but there was no significant difference between VCBT-I and the other two groups. Again, for all variables, larger effect sizes for time effects were found in the PCBT-I group (from 0.53 to 1.93) as compared to the VCBT-I group (from 0.26 to 0.95), which were larger than those obtained in the CTL group (from 0.14 to 0.29).
Table 3.
Mean scores obtained on secondary variables by each group at pretreatment and posttreatment and effects obtained
Remission Rates by Group
After adjusting for baseline scores, the remission rate based on the ISI total score (< 8) significantly differed between the three conditions, F(2,197) = 12.46, P < 0.001. The proportion of remitted patients was significantly larger in the PCBT-I group (71.3%) than in the VCBT-I group (44.3%), t(197) = 2.85, P = 0.005, which was significantly greater than in the CTL group (25.7%), t(197) = 2.08, P = 0.04. After adjusting for baseline status, the IIS remission rate (absence of an insomnia syndrome) was also found to be significantly different across groups at posttreatment, F(2,193) = 5.68, P = 0.004. It was significantly larger in PCBT-I (77.7%) and VCBT-I (68.2%) groups compared to the CTL group (49.6%), t(193) = 3.31, P < 0.01 and t(193) = 1.96, P = 0.05, respectively, but the difference between PCBT-I and VCBT-I groups was not significant, t(193) = 1.13, P = 0.26.
DISCUSSION
This study evaluated the efficacy of VCBT-I as compared to a standard CBT-I administered by a professional (PCBT-I) and to a no-treatment condition (CTL). As hypothesized, compared to CTL, both PCBT-I and VCBT-I were associated with significantly greater improvements of all subjective sleep variables, except EMA (for VCBT-I only) and TST, as well as a larger reduction of dysfunctional beliefs about sleep. However, although PCBT-I participants showed significantly greater decreases than CTL on anxiety, depression, and fatigue scores, no significant differences were found between VCBT-I and CTL on these outcomes. Moreover, PCBT-I was significantly more efficacious than VCBT-I in reducing ISI scores, EMA, depression, fatigue, and dysfunctional beliefs about sleep. Accordingly, effect sizes of pretreatment to posttreatment changes were consistently larger in PCBT-I than in VCBT-I, which were greater than in CTL. PCBT-I and VCBT-I were also associated with significantly larger remission rates of insomnia than CTL, with, again, an advantage for PCBT-I.
These findings are consistent with prior research supporting the efficacy of: therapist-led CBT-I in patients with cancer11,13 (including the lack of effect on actigraphic data11,34–36), self-administered CBT-I in individuals with no comorbidity when compared to a no-treatment or a waiting list control condition,37,38 and self-administered CBT-I compared to a professionally administered format in individuals with no cancer.39,40 Although studies have generally found no statistically significant differences between these two formats of administration, a recent meta-analysis revealed consistently smaller treatment effects for self-help forms.16 These differences appear to be clinically significant.40
The superiority of interventions offering some professional guidance may be explained by specific factors. When treated by a professional therapist, patients are guided on how to implement strategies and are provided corrective feedback when problems arise. This may also help patients generalize the use of the learned strategies to related symptomatology (e.g., depression, anxiety, fatigue, dysfunctional beliefs about sleep), as suggested by the current findings. However, nonspecific factors can also play a role. Indeed, therapist support may reinforce patients' perceived self-efficacy, which may increase their adherence to the treatment strategies and their expectancies for improvement. In addition, the two treatment conditions importantly differed in terms of their intensity, with the PCBT-I patient group being five times more exposed to treatment (60 min video versus 6 × 50 min sessions).
Strengths of this study include the use of a large sample size, recruited directly at the clinic, and few exclusion criteria, thus increasing the generalization of the findings, as well as the assessment of treatment integrity. However, the findings' generalization is limited by a relatively low participation rate. This is mainly explained by the large number of eligible patients (n = 514) who declined participation because they thought they did not have insomnia severe enough to warrant treatment despite reporting clinical levels of insomnia symptoms. In addition, although participants had to sign an agreement not to share the treatment material with anyone, the risk of contamination was not completely eliminated.
The differential dropout rate obtained across groups (13.6% in PCBT-I versus 28.8% in VCBT-I) is also worth discussing. Given that noncompleters were less educated and had more psychiatric comorbidity, variables that have been found to be associated with a lower likelihood to benefit from a psychological treatment for insomnia,41 the greater dropout rate found in the VCBT-I patient group may have overestimated treatment effects in that condition. Also, it is possible that only the most motivated individuals remained in the treatment, thus also possibly inflating treatment effects. However, this finding should probably be interpreted more as an indication that a self-administered intervention is not a suitable treatment format for everyone.
In summary, results of this study suggest that, although a self-help treatment is a highly valuable alternative, face-to-face therapy remains the optimal format to administer efficaciously CBT for insomnia in patients with cancer. An alternative option to increase the efficacy of the self-administered treatment would be to add some professional guidance during the intervention. Indeed, there is evidence that the addition of therapist support to low intensity interventions maximizes their efficacy.37,42,43 Phone calls planned in advance would be particularly relevant given the low number of participants in the VCBT-I group who elected to call for assistance in this study.
Alternatively, we think it is probably more appropriate to integrate the use of minimal interventions as part of a stepped care model of care where the self-administered treatment constitutes the entry level, which will be followed, if necessary (if the patient is still symptomatic at posttreatment), by a professionally administered treatment. The entry level of a stepped care model is a minimal intervention in terms of therapeutic contact, but which has the potential to be efficacious for a significant proportion of the patients,44,45 a condition that is clearly met by our video-based intervention. A stepped care model has recently been suggested as a way of increasing the accessibility to CBT-I,46 but studies are needed to assess its feasibility and utility.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was funded by the Canadian Breast Cancer Research Alliance (grant #017738) and a research scientist award from the Fonds de la recherche en santé du Québec to the first author. This study was conducted at the Cancer Research Center, Université Laval, Québec, Québec, Canada. Dr. Morin is on the Advisory boards of Merck, Valeant, and Novartis; has received research support from Novartis; and is on the Speakers Bureau of Valeant. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors sincerely thank the patients for their participation in this study and Fred Sengmueller for revising the manuscript. We also acknowledge the contribution of the following people for their involvement in the recruitment, assessment and treatment of the study participants: Virginie Audet-Croteau, Emmanuelle Bastille-Denis, Marie-Solange Bernatchez, Rosée Bruneau-Bhérer, Genevieve Dionne, Aude Caplette-Gingras, Lucie Casault, Joanne Castonguay, Caroline Desautels, Anne-Josée Guimond, Stéphanie Hamel, Catherine Marcotte, Louis-Philippe Marion, Joanie Mercier, Mylène Ross-Plourde, Véronique Roy, Sophie Ruel, Eugénie Simard, Valérie Tremblay, Claudia Trudel-Fitzgerald, and Julie Villa.
ABBREVIATIONS
- CBT
cognitive behavioral therapy
- CBT-I
cognitive behavioral therapy for insomnia
- CTL
control/no-treatment condition
- DBAS-16
Dysfunctional Beliefs and Attitudes about Sleep Scale–Abbreviated version
- EORTC QLQ-C30
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire
- ES
effect size
- HADS
Hospital Anxiety and Depression Scale
- HADS-A
Hospital Anxiety and Depression Scale–anxiety subscale
- HADS-D
Hospital Anxiety and Depression Scale– depression subscale
- IIS
Insomnia Interview Schedule
- ISI
Insomnia Severity Index
- MFI
Multidimensional Fatigue Inventory
- MMSE
Mini-Mental State Examination
- PCBT-I
professionally administered cognitive behavioral therapy for insomnia
- RCT
randomized controlled trial
- SCID
Structured Clinical Interview for DSM-IV
- VCBT-I
video-based cognitive behavioral therapy for insomnia
Footnotes
A commentary on this article appears in this issue on page 1277.
REFERENCES
- 1.Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27:5233–9. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 2.Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580–6. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 3.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–8. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health. National Institutes of Health State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 5.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 6.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 7.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18:634–46. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 9.Davidson JR, Waisberg JL, Brundage MD, MacLean AW. Nonpharmacologic group treatment of insomnia: a preliminary study with cancer survivors. Psychooncology. 2001;10:389–97. doi: 10.1002/pon.525. [DOI] [PubMed] [Google Scholar]
- 10.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34:E51–9. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 11.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–8. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 12.Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol. 2003;71:189–200. [PubMed] [Google Scholar]
- 13.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083–95. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 14.Simeit R, Deck R, Conta-Marx B. Sleep management training for cancer patients with insomnia. Support Care Cancer. 2004;12:176–83. doi: 10.1007/s00520-004-0594-5. [DOI] [PubMed] [Google Scholar]
- 15.Casault L, Savard J, Ivers H, Savard MH, Simard S. Utilization of hypnotic medication in the context of cancer: predictors and frequency of use. Support Care Cancer. 2012;20:1203–10. doi: 10.1007/s00520-011-1199-4. [DOI] [PubMed] [Google Scholar]
- 16.van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13:61–71. doi: 10.1016/j.smrv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21:695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savard J, Villa J, Simard S, Ivers H, Morin CM. Feasibility of a self-help treatment for insomnia comorbid with cancer. Psychooncology. 2011;20:1013–9. doi: 10.1002/pon.1818. [DOI] [PubMed] [Google Scholar]
- 19.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14:429–41. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hilesdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 23.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 24.Morin CM. Insomnia: psychological assessment and management. New York: The Guilford Press; 1993. [Google Scholar]
- 25.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 26.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 27.Fillion L, Gélinas C, Simard S, Savard J, Gagnon P. Validation evidence for the French Canadian adaptation of the Multidimensional Fatigue Inventory as a measure of cancer-related fatigue. Cancer Nurs. 2003;26:143–54. doi: 10.1097/00002820-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 29.Savard J, Laberge B, Gauthier JG, Ivers H, Bergeron MG. Evaluating anxiety and depression in HIV-infected patients. J Pers Assess. 1998;71:349–67. doi: 10.1207/s15327752jpa7103_5. [DOI] [PubMed] [Google Scholar]
- 30.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): Validation of a brief version (DBAS-16) Sleep. 2007;30:1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frigon J-Y, Laurencelle L. Analysis of covariance: A proposed algorithm. Educ Psychol Meas. 1993;53:1–18. [Google Scholar]
- 33.SAS Institute Inc. SAS/STAT 9.3 User's Guide. Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- 34.Berger AM, Kuhn BR, Farr LA, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol. 2009;27:6033–40. doi: 10.1200/JCO.2008.20.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging. 2002;17:288–98. [PubMed] [Google Scholar]
- 37.Mimeault V, Morin CM. Self-help treatment for insomnia: bibliotherapy with and without professional guidance. J Consult Clin Psychol. 1999;67:511–9. doi: 10.1037//0022-006x.67.4.511. [DOI] [PubMed] [Google Scholar]
- 38.Morin CM, Beaulieu-Bonneau S, LeBlanc M, Savard J. Self-help treatment for insomnia: a randomized controlled trial. Sleep. 2005;28:1319–27. doi: 10.1093/sleep/28.10.1319. [DOI] [PubMed] [Google Scholar]
- 39.Bastien CH, Morin CM, Ouellet MC, Blais FC, Bouchard S. Cognitive-behavioral therapy for insomnia: comparison of individual therapy, group therapy, and telephone consultations. J Consult Clin Psychol. 2004;72:653–9. doi: 10.1037/0022-006X.72.4.653. [DOI] [PubMed] [Google Scholar]
- 40.Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive-behavioural interventions for insomnia in recovering alcoholics. Br J Addict. 2004;99:1121–32. doi: 10.1111/j.1360-0443.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 41.Vincent N, Walsh K, Lewycky S. Determinants of success for computerized cognitive behavior therapy: examination of an insomnia program. Behav Sleep Med. 2013;11:328–42. doi: 10.1080/15402002.2012.700662. [DOI] [PubMed] [Google Scholar]
- 42.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;10:54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 43.Cavanagh K. Turn on, tune in and (don't) drop out: engagement, adherence, attrition, and alliance with internet-based interventions. In: Bennett-Levy J, Richards DA, Farrand P, et al., editors. Low intensity CBT interventions. New York: Oxford University Press; 2010. pp. 227–33. [Google Scholar]
- 44.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. Br J Psychiatry. 2005;186:11–7. doi: 10.1192/bjp.186.1.11. [DOI] [PubMed] [Google Scholar]
- 45.Newman MG. Recommendations for a cost-offset model of psychotherapy allocation using generalized anxiety disorder as an example. J Consult Clin Psychol. 2000;68:549–55. [PubMed] [Google Scholar]
- 46.Espie CA. Stepped care: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549–58. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]



