Abstract
Study Objective:
Upon awakening from sleep, a fully awake brain state is not reestablished immediately, but the origin and physiological properties of the distinct brain state during the first min after awakening are unclear. To investigate whether neuronal firing immediately upon arousal is different from the remaining part of the waking episode, we recorded and analyzed the dynamics of cortical neuronal activity in the first 15 min after spontaneous awakenings in freely moving rats and mice.
Design:
Intracortical recordings of the local field potential and neuronal activity in freely-moving mice and rats.
Setting:
Basic sleep research laboratory.
Patients or Participants:
WKY adult male rats, C57BL/6 adult male mice.
Interventions:
N/A.
Measurements and Results:
In both species the average population spiking activity upon arousal was initially low, though substantial variability in the dynamics of firing activity was apparent between individual neurons. A distinct population of neurons was found that was virtually silent in the first min upon awakening. The overall lower population spiking initially after awakening was associated with the occurrence of brief periods of generalized neuronal silence (OFF periods), whose frequency peaked immediately after awakening and then progressively declined. OFF periods incidence upon awakening was independent of ongoing locomotor activity but was sensitive to immediate preceding sleep/wake history. Notably, in both rats and mice if sleep before a waking episode was enriched in rapid eye movement sleep, the incidence of OFF periods was initially higher as compared to those waking episodes preceded mainly by nonrapid eye movement sleep.
Conclusion:
We speculate that an intrusion of sleep-like patterns of cortical neuronal activity into the wake state immediately after awakening may account for some of the changes in the behavior and cognitive function typical of what is referred to as sleep inertia.
Citation:
Vyazovskiy VV, Cui N, Rodriguez AV, Funk C, Cirelli C, Tononi G. The dynamics of cortical neuronal activity in the first minutes after spontaneous awakening in rats and mice. SLEEP 2014;37(8):1337-1347.
Keywords: cortical neuronal activity, mice, rats, sleep, sleep inertia
INTRODUCTION
In most mammalian species, sleep periods do not last longer than 6-12 h and are followed by periods of consolidated wakefulness, which may last spontaneously for min to hours or days depending on species, age, time of day, light, ambient temperature, and many other factors.1–6 The exact mechanisms underlying spontaneous awakening from sleep are not yet clear, but likely involve both cortical and subcortical wake- and sleep-generating centers as well as depend on the circadian time and the levels of homeostatic sleep pressure.7 Several wake-promoting regions have been identified where neurons consistently increase firing at or close to the moment of awakening.8–10 Selective activation of those regions, located in the basal forebrain, hypothalamus, or in the brainstem, can lead to an arousal.11,12
Cortical activity in waking and sleep is characteristically different. Although the average firing activity seems to be lower during nonrapid eye movement (NREM) sleep as compared to waking,13 the main difference is in the overall pattern of population activity. Specifically, NREM sleep is characterized by brief periods of generalized suppression of cortical neuronal spiking activity (so-called OFF periods), which correspond to electroencephalographic (EEG) or local field potential (LFP) slow waves.14,15 The cellular counterpart of OFF periods is a down state of the slow oscillation, which is an intrinsically generated network phenomenon, characterized by membrane hyperpolarization and the virtual absence of spiking and synaptic activity.16–19 Although in vivo OFF periods and down states have been mostly investigated under anesthesia or during sleep, their occurrence has also been reported in awake animals.20–23
The awake state is not homogenous, but shows pronounced changes as reflected in many behavioral and physiological variables. From a behavioral point of view, we all know that the shift from a sleeping to an awake state is not abrupt, and we usually feel differently immediately upon waking. The initial waking period is usually characterized by reduced alertness, mild disorientation, and a distinct subjective feeling of “being not fully awake”—a state often referred to as sleep inertia.24,25 Notably, sleep inertia is not merely a subjective experience: in humans, the initial waking period is associated with differences in distinct EEG frequency bands and event-related potentials, altered cognitive performance in a variety of tasks, and characteristic changes in cerebral blood flow.26–32
Local changes in brain activity may account for a variety of mixed physiological and pathological states, including sleep inertia.33–35 However, the neurophysiologic underpinnings of the link between changes in brain activity and behavioral deficits initially after awakening are unknown. Moreover, it is yet unclear whether sleep inertia is simply an epiphenomenon of the carryover from a preceding sleep state or a beneficial state that ensures a gradual transition from sleep to waking. Most research on state dependency in the activity of cortical neurons has been performed either in head-fixed animals or during specific behaviors or learning paradigms. Much less is known about the neuronal correlates of early waking that occur immediately after a spontaneous arousal in unrestrained, freely moving animals. In this study, we set out to investigate cortical neuronal activity in the first min after spontaneous awakenings in laboratory rats and mice.
METHODS
Animals, Surgical Procedures, and Data Acquisition
Adult male WKY rats (total n = 8) and C57BL/6 mice (n = 5) were used. We chose to perform this study in both rats and mice for two reasons. First, it is important to investigate whether the changes in cortical activity that we expected to observe in the first min after awakening are consistent between different species. Second, we found that in rats most consolidated wake episodes occurred in the dark period, whereas in mice a sufficient number of waking episodes occurred also during the light phase (Figure 1). This gave us an opportunity to at least partially test to what extent the observed effects depend on time of day, long-term preceding sleep-wake history, and/or lighting conditions. Most analyses were performed in both species, whereas more detailed analysis on isolated neurons and local field potential (LFP)/electromyographic (EMG) activity was performed in rats only (see Results).
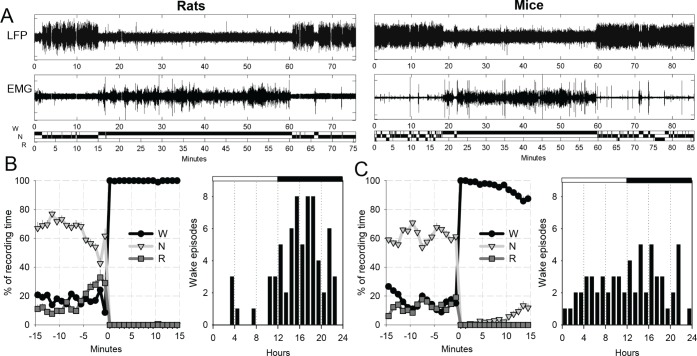
Figure 1.
(A) Local field potential (LFP) and electromyographic (EMG) traces and the hypnogram (Wake, NREM sleep, REM sleep, W, N, R) for one representative waking episode in one individual rat (left) and one individual mouse (right). (B) Left: Time course of NREM sleep, REM sleep and waking during the 15 min before awakening and in the first 15 min through the waking episode. The curves represent the average between all waking episodes contributing to the analyses presented in this study. Mean values (± standard error of the mean [SEM]). Right: Distribution of all waking episodes included in the analysis across 24 h in rats. (C) Same as in (B) for mice (n = 5).
All animals were housed individually in transparent Plexiglas cages. Lighting and temperature were kept constant (LD 12:12, light on at 10:00 am, 23 ± 1°C). Food and water were available ad libitum and replaced daily at 10:00 am. All procedures related to animal handling, surgery, and recording followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were in accordance with institutional guidelines. The animals were implanted with microwire arrays in the deep (V-VI) cortical layers of frontal cortex (rats: B: +1-2 mm, L: 2-3 mm, mice: B: +1-2 mm, L: 1-1.5 mm, confirmed by histology in a subset of animals) for LFP and neuronal activity recordings, as previously described.21 The ground and reference screw electrodes were placed above the cerebellum as previously discussed.13,21 Data acquisition and online spike sorting were performed with the Multichannel Neurophysiology Recording and Stimulation System (Tucker-Davis Technologies (TDT), Alachua, FL, USA). Spike data were collected continuously (25 kHz, 300 Hz - 5kHz), concomitantly with LFPs from the same electrodes (256 Hz, 0.1-100 Hz) and nuchal EMG (256 Hz, 10-100 Hz). Subsequent offline spike sorting was performed on rat data by principal component analysis followed by split-and-merge expectation maximization clustering algorithm as previously discussed.13,21
Scoring Vigilance States and Behavioral Analysis
Vigilance states were identified for consecutive 4-sec epochs. Signals were loaded with custom-written Matlab (The Math-Works, Inc., Natick, MA, USA) programs using standard TDT routines, and subsequently transformed into the European Data Format (EDF) with Neurotraces software (www.neurotraces.com). Sleep stages were scored offline by visual inspection of 4-sec epochs (SleepSign, Kissei Comtec, Nagano, Japan), where the LFP, EMG, and spike activity were displayed simultaneously. Waking was characterized by low voltage, high frequency LFP pattern, and high-amplitude, phasic EMG activity. Epochs of eating, drinking, and intense grooming were scored as artifacts to avoid contamination of multiunit activity (MUA). NREM sleep was characterized by the occurrence of high amplitude slow waves and low tonic EMG activity.13,36 During rapid eye movement (REM) sleep the cortical LFP was similar to that during waking, but only heart beats and occasional twitches were evident in the EMG signal.
Experimental Design
At least 1 w was allowed for recovery after surgery, and experiments were started only after the sleep/waking cycle had fully normalized, as evidenced by the entrainment of sleep and wake by the light/dark cycle and the homeostatic time course of NREM sleep LFP slow wave activity (SWA, 0.5-4.0 Hz). In each rat, several undisturbed nonconsecutive baseline days were selected for the analyses (4.0 ± 0.9 per animal), and in mice, one stable 24-h recording was selected. After vigilance state scoring, spontaneous uninterrupted waking episodes (> 15 min) preceded and followed by consolidated sleep periods (> 15 min) were automatically detected. Subsequently, all waking episodes were visually verified to exclude those with an intrusion of short sleep episodes into the beginning of waking episode or with more than 10% of all 4-s epochs scored as artifacts. A representative waking episode from a rat and a mouse is illustrated in Figure 1A. In rats, 68 waking episodes were identified and included in the final analysis (2.1 ± 0.2 (mean ± standard error of the mean [SEM]) episodes per animal per 24 h). In total, 8.5 ± 2.1 waking episodes per rat were used, which were on average 44.4 ± 1.6 min long (light period: 38.8 ± 2.1 min, dark period: 44.8 ± 1.9 min). In mice, 60 episodes in total (12.0 ± 0.9 per animal, light period: 5.4 ± 0.4/animal, dark period: 6.6 ± 0.6/animal) were included in the analyses, with a mean duration of 37.8 ± 3.2 min (light period: 31.8 ± 2.9 min, dark period: 42.3 ± 5.7 min).
The waking episodes selected for this analyses were preceded by a consolidated sleep period with minimal wake intrusions (see Results), and virtually no epochs were scored as sleep in the first 5 to 10 min, whereas occasional minor attempts to fall asleep later in the course of waking episode were apparent, especially in mice (Figure 1B and 1C, left). In rats, most episodes selected for the analyses occurred during the dark period (Figure 1B, right). Excluding those episodes that occurred during the light phase did not affect the results, and thus both dark and light wake episodes were included in all analyses. In mice, the episodes were evenly distributed across 24 h with only a slight predominance of waking episodes during the dark phase (Figure 1C, right).
Detection of OFF Periods
In naturally sleeping animals, extracellular recordings in the neocortex reveal periods of synchronous population silence of variable duration (OFF periods). Shorter and less frequent OFF periods also occur in waking, especially in a quiet state or after sleep deprivation. We hypothesized that during the first minutes after awakening the incidence of population OFF periods will be different from the rest of the waking episode.
The individual rats, in which offline spike sorting was performed, varied substantially with respect to the number of isolated neurons (average across recording days: min 4.3, max 15.8 neurons). In addition, there was a substantial variability in the firing rates between individual neurons—ranging from 0.17 Hz up to 44 Hz. We speculated therefore that the number of OFF periods might be overestimated in some animals and underestimated in others, merely because of the difference in the total population firing activity. To account for this variability, we developed a new approach for OFF period detection by using instantaneous firing rate of the population, defined as the sum of all spikes over a specified time window (window size, ws). A moving window was applied to the multiunit activity to obtain continuous population neuronal firing rate.
As a first step, we represent the instantaneous population (n neurons) firing rate as a matrix [(n*2-1) by (ws*2-1)], which is the multivariate convolution product of raw spike trains [n by ws] and a multivariate gaussian kernel [n by ws]. The spike trains are defined as time series S(t), where S(t) = [st-(ws-1)/2 st-(ws-1)/2+1 st-(ws-1)/2+2 … st+(ws-1)/2-1 st+(ws-1)/2]. The logic behind using a multivariate gaussian kernel as a moving window is that the spike data matrix at time t would take into account past firing and future firing of the neuronal population, and at the same time the current time t is emphasized by adding the most weight by positioning the peak of gaussian kernel at time t. The multivariate gaussian kernel is defined as:
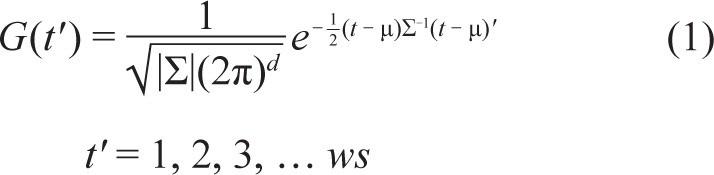 |
where μ is the mean, Σ is the covariance matrix, d is the dimension, 2 in this case (Figure S1A, supplemental material). We have performed the calculations for several different window sizes on a test data set, and selected 18 ms as a compromise taking the computation time into consideration. Correlation analyses performed on a representative subset of data using Kendall tau rank correlation test and Pearson correlation test revealed that transforming the data did not affect their statistical properties (Figure S2, supplemental material).
The instantaneous firing rate of the neuronal population, represented as a matrix, is calculated as:
For example, if the neuronal population consists of 14 neurons and the recording lasts 100 ms (Figure S1B), after the step of convolving the spike train with the gaussian kernel we obtain a continuous matrix [2*n+1 by 2*ws-1 by 100] representation of the population firing rates.
The next step is to perform size reduction, applying deconvolution twice from the expanded F(t) because of convolution to F′(t). Subsequently, mean of F′(t) (Figure S1C) was computed as:
where T is the total recording time of spike trains (Figure S1D).
An important aspect to consider is that because of large differences in the firing activity between individual neurons, the duration of their continuous nonspiking period varies significantly. To take into account the different contribution of neurons into the population OFF periods, we used F′ to estimate mean firing rate of each individual neuron and to give weightings to each neuron for their contribution in the population neuronal firing rate. This step is achieved by normalizing the spiking activity with the average group firing rates, as:
Note that even though neuron 7 is producing spikes, because its average firing rate is higher than other neurons, it contributes less toward an OFF period detection. However, neuron 9 has a slow firing rate (Figure S1B), therefore it contributes more to the calculation.
Subsequently, all resulting OFF periods < 30 ms were excluded, because based on the total firing rate of the population it was estimated that generalized silent periods < ∼30 ms are very likely to occur by chance. The resulting number of OFF periods was 6.4 ± 0.4 (mean ± SEM)/1 sec, and their average duration was 56.7 ± 3.1 ms. Subsequently, for the final analyses, we selected the top 20% longest OFF periods as previously,14 and for those animals/recording days where the resulting incidence of OFF periods was too low (< 1/sec) as a result of high population firing, all OFF periods (of at least 30 ms duration) were included. The resulting duration of OFF periods was 83.6 ± 19.7 ms, which corresponds closely to the average duration of ∼85 ms in our previous study,21 and they occurred on average 1.96 ± 0.2 times per second. To exclude the possibility that the results were biased by the criteria, used to detect OFF periods, we have calculated the time course of OFF periods across the waking episode based on two different methods—as previously14 and with the newly developed approach, as well as included all or only the top 20% of OFF periods for all animals. The main results were not affected by varying the criteria.
In mice, although a robust MUA was present, no clearly identifiable single units were visually detectable in most recording channels. Therefore, no spike sorting was performed and OFF periods were calculated based on the MUA (out of 16 recording channels, 7.4 ± 0.75 (mean ± SEM) channels per animal showed MUA with high signal-to-noise ratio and were included in the analysis). As in rats, individual recording channels in mice were highly variable with respect to the number of spikes and spike amplitude. To detect periods of local population silence, we first determined the noise level for each individual channel during the time periods when no spikes were observed (8.7 ± 0.4 μV [mean ± SEM]). Next, we concatenated all the spike occurrences and determined a threshold level at which the number of OFF periods (e.g., time periods when no spiking activity above the threshold is present) was maximal. To determine this threshold level, we progressively increased the threshold by multiplying the noise level by an integer number (1-10) and calculated the resulting number of OFF periods. As expected, at very low threshold levels only a few OFF periods were found, whereas increasing the threshold resulted in a progressively increasing number of OFF period followed by a decrease at high threshold values when consecutive OFF periods started to merge. For each animal a threshold value was determined where the number of OFF periods > 30 ms was maximal (on average 7.0 ± 0.9 (mean ± SEM) × noise level, corresponding to 62.8 ± 9.8 μV). Subsequently, as in rats, the analysis was performed on the 20% longest OFF periods, which had an average duration of 142.8 ± 12.2 ms. Because the amplitude of extracellular action potentials decreases exponentially with the distance from the recording tip of the electrode,37 by using a relatively high threshold we focused mostly on the OFF periods within a local network population, and minimized the potential influence of distant units or noise. However, varying the threshold within a broad range did not substantially affect the main results.
RESULTS
Overall Cortical Neuronal Firing in the Initial Waking
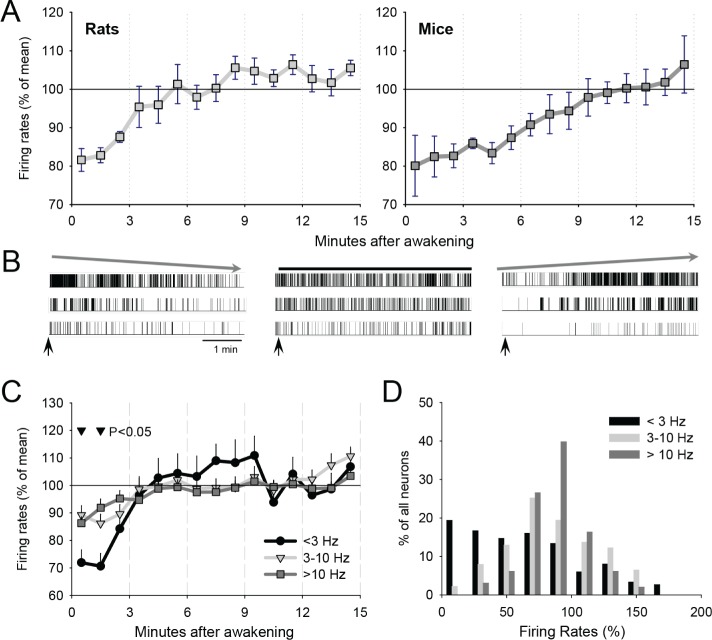
In both rats and mice it was possible to identify spontaneous prolonged waking episodes preceded and followed by consolidated sleep periods (Figure 1). As a first step, we investigated total population firing rates during waking upon arousal (rats: 12.0 ± 1.6 (mean ± SEM) neurons/animal, mice: 7.0 ± 0.9 channels/animal). In both rats and mice, neuronal firing activity was low initially, and reached average intraepisodic levels within the subsequent ∼5-10 min (Figure 2A). The changes in the firing rates within the first 15 min were highly significant in both species (factor “time after awakening,” rats: F(14,98) = 6.7, P < 0.0001; mice: F(14,56) = 5.4, P < 0.0001, analysis of variance [ANOVA] for repeated measures). The interaction “species” × “time after awakening” was not significant (F(14,165) = 0.69, P = 0.78). To investigate whether all neurons fire initially at a lower rate, or, depending on a firing phenotype some neurons may be affected more than others, we performed spike sorting on the data obtained in rats. Although the extracellular recording technique does not allow recording from a large number of neurons, and no spike-sorting algorithm guarantees an ideal separation of individual units, we focused on those neurons that appeared to be well isolated (6.8 ± 1.2 (mean ± SEM) per rat per episode). Visual inspection of the recording traces revealed a pronounced variability between individual neurons during the initial minutes after awakening. One subset of neurons (“leaders,” ∼20% of all recorded neurons) showed a transient brief elevation of firing activity immediately upon arousal, which dissipated in the next few minutes (Figure 2B, left). Another, substantial proportion of neurons (“laggards,” ∼60% of all neurons) showed a consistently slow or even absent spiking at the beginning of waking episode, which then built up gradually (Figure 2B, right). The remaining neurons fired at their average stable rate from the onset of waking episode, and did not show any significant intraepisodic fluctuations (Figure 2B, middle).
Figure 2.
(A) Mean firing rates (± standard error of the mean [SEM]) of the entire recorded neuronal population (see Methods) during the first 15 min after awakening in rats (left) and mice (right). (B) Raster plots of typical individual neuron spiking during the first 5 min after awakening in rats. Left: three neurons are shown, which showed initially high spiking activity, which declines progressively. Middle: three neurons, showing stable level of firing within the first 5 min after arousal. Right: Three individual neurons, which show initially low spiking activity, which increased substantially within the first several min after arousal. (C) Time course of neuronal firing rates, shown separately for slow-spiking (< 3 Hz), intermediate-spiking (3-10 Hz), and fast-spiking neurons (> 10 Hz). Mean values (± SEM) are shown. The triangles above the curves denote significantly lower relative firing activity of slow-spiking neurons (< 3 Hz) as compared to both 3-10 Hz and > 10 Hz populations. (D) Distribution of individual neurons as a function of the initial relative firing rates during the first 1 min after awakening shown separately for slow spiking, intermediate and fast spiking neurons (as in C). The neurons are pooled from all episodes in all rats.
Next, we examined whether neuronal firing phenotype contributed to the behavior of the neurons during the initial minutes after awakening. When we subdivided all neurons into slow spiking (< 3 Hz), intermediate spiking (3-10 Hz), and fast spiking units (> 10 Hz), we found that these groups exhibited a substantial difference in relative spiking activity initially after awakening. Specifically, the slow-spiking neurons fired even less during the first 1-2 min after arousal (Figure 2C), and it took longer for this population to reach their average intraepisodic firing rate, suggesting that they included many neurons of the “laggard” type. Indeed, during the first min of waking, a substantial proportion of slow-spiking neurons (42%) initially fired at a frequency below 50% of their average spiking activity, whereas only about 20% fired higher than average (Figure 2D). In contrast, the firing rate of most intermediate and fast spiking units during the first min after awakening clustered at approximately 100%.
The Dynamics of OFF Periods Upon Awakening
Because individual neurons have displayed a substantial variability in their firing activity during the first min of waking, we next hypothesized that the overall pattern of network population firing may be affected. As a measure of network activity we have chosen to compute OFF periods—brief generalized cessations of the activity across the entire recorded population. We have shown previously that OFF periods, which likely arise from neuronal hyperpolarization, increase in awake rats after sleep deprivation.14,21 Here we asked whether initial waking after awakening from physiological sleep is also associated with increased incidence of OFF periods.
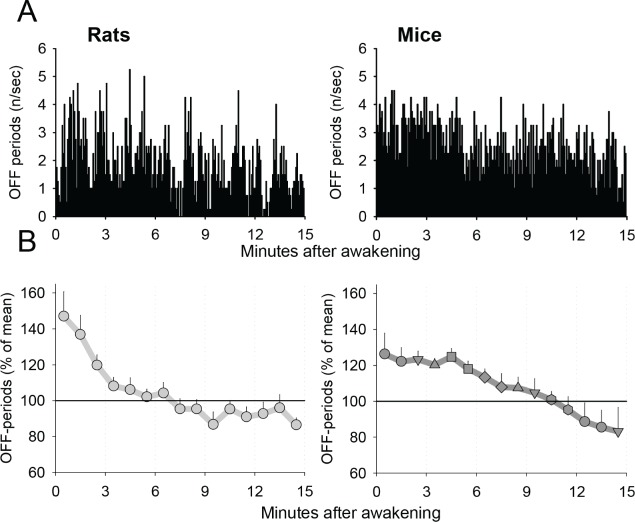
Visual inspection of the distribution of neuronal OFF periods in the first 15 minutes of waking episodes revealed that in both rats and mice, OFF periods were often more frequent initially after awakening, and declined thereafter (Figure 3A). On average, the initial incidence of OFF periods was approximately 150% of the average intraepisodic level in rats and about 125% in mice (Figure 3B). After the initially high values, the incidence of OFF periods in both species showed a progressive gradual decline, which was highly significant (factor “time after awakening,” rats: F(14,98) = 7.09, P < 0.01, mice: F(14,56) = 5.95, P < 0.0001, ANOVA for repeated measures). In rats, the average intraepisodic levels were reached within approximately 5 min. In contrast, in mice the initial levels of OFF periods were stable for approximately 5 min, and then showed a decrease, reaching average intraepisodic levels within approximately 10 min after awakening. Nevertheless, the interaction “species” × “time after awakening” did not reach significance (F(14,165) = 1.32, P = 0.2006). This time course was independent of specific criteria used to define OFF periods (see Methods).
Figure 3.
(A) A representative example of the time course of the incidence of brief periods of generalized neuronal silence (OFF periods) during the first 15 min after spontaneous awakening in one individual animal (left: rat, right: mouse). The bars represent the number of OFF periods per consecutive 1-sec bins. (B) Mean values of the incidence of OFF periods during the first 15 min after awakening (left: rats, 68 episodes; right: mice, 60 episodes). The values are first averaged between individual episodes within an animal prior to computing the mean values (± standard error of the mean [SEM]) between individual animals.
Preceding Sleep-Wake History Affects OFF Periods After Awakening
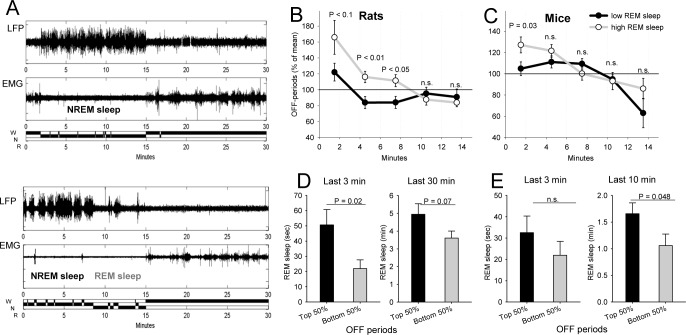
We noticed that the initial values of OFF periods were variable between individual waking episodes: in some cases, the initial values were high and showed a rapid decline, whereas in other episodes they were low from the very beginning. We hypothesized that cortical activity during initial waking may be determined by immediate preceding history, such as whether the animal woke up from NREM sleep or from REM sleep. To test this hypothesis, we first subdivided all waking episodes into those in which the preceding sleep mostly consisted of NREM sleep, and those enriched in REM sleep (Figure 4A). Computing the incidence of OFF periods during the corresponding subsequent waking episodes revealed only a modest nonsignificant relationship with preceding NREM sleep. Interestingly, NREM sleep SWA was also unrelated to subsequent incidence of OFF periods (P > 0.1). In contrast, preceding REM sleep appeared to have a significant role. Specifically, we found that neuronal OFF periods were substantially more frequent if an awakening followed a period rich with REM sleep (top 20% of all episodes) as compared to those cases when REM sleep was minimal (bottom 20% of the distribution) (Figure 4B). This effect was present in both rats and mice, albeit with some differences. Specifically, in rats the effects were significant irrespective of whether immediate (last 1-5 min) or longer term (30 min) preceding history was taken into account, whereas in mice only the amount of REM sleep in the preceding 10-20 min played a role. In addition, the effect in rats was stronger and longer lasting. To confirm this result, we next subdivided all waking episodes based on the initial (first 1-2 min) levels of OFF periods into those with high incidence (top 50% of the distribution) and low incidence (bottom 50%) and computed the corresponding preceding values of REM sleep (Figure 4D and 4E). We found that in rats the amount of REM sleep was significantly or at a tendency level higher before waking episodes with high incidence of OFF periods as compared to those waking episodes with a relatively low initial level of OFF periods. This was the case both for the immediate preceding history (3 min) and if an entire preceding 30 min interval was taken into account. In contrast, in mice, the effect was present for the last 10-20 min and declined when only immediate preceding history (last 3 min) was considered.
Figure 4.
(A) Representative transitions from sleep to waking (local field potential [LFP], electromyography [EMG], hypnogram) depicting a typical example of a case when sleep prior to awakening consisted mostly of nonrapid eye movement (NREM) state (top) or enriched in rapid eye movement (REM) sleep (bottom). The examples are taken from one representative rat. (B) Mean values (± standard error of the mean [SEM]) of the incidence of brief periods of generalized neuronal silence (OFF periods) during the first 15 min after awakening shown separately for waking episodes following sleep with high and low proportion of REM sleep in the preceding 30 min (top and bottom 20%; 15 and 14 episodes, respectively, contribute to this analysis). The values above the curves depict 3-min bins, where P-values (two-tailed paired t-test) were below 0.1. (C) Mean values of the incidence of OFF periods during the first 15 min after awakening shown separately for waking episodes following sleep with high and low proportion of REM sleep in the preceding 10 min (top and bottom 20%; 13 and 12 episodes, respectively, contribute to this analysis). The value above the curves depict 3-min bin, where the difference was significant (two-tailed paired t-test). (D) The amount of REM sleep (mean values ± SEM) in the last 3 min and 30 min before awakening, shown separately for waking episodes characterized by high initial levels of OFF periods (top 50%) and low levels of OFF periods (bottom 50%). (E) The amount of REM sleep (mean values ± SEM) in the last 3 min and 10 min before awakening, shown separately for the waking episodes characterized by high initial levels of OFF periods (top 50%) and low levels of OFF periods (bottom 50%).
To address the potential effects of the time of day or lighting conditions, we calculated the initial levels of OFF periods separately in the light and the dark period. This analysis was performed in mice only, as only in this species there were a sufficient number of prolonged waking episodes during the light period (on average light period: 5.2 episodes/animal, dark period: 6.0 episodes/animal, not significant). The absolute values for the incidence of OFF periods were similar (light: 2.5 ± 0.3 (mean ± SEM), dark: 2.6 ± 0.2/sec, not significant, paired t-test), but the modulation of OFF period incidence by preceding REM sleep was found in the dark period only. Specifically, the positive association between initial OFF periods and preceding amount of REM sleep was highly significant for the preceding 10 min (P = 0.004), 20 min (P = 0.007) and 30 min (P = 0.02), whereas only a trend was seen for more immediate preceding history (1 min: P = 0.078, 3 min: P = 0.047 and 5 min: P = 0.085). This was also the case for REM/total sleep time (not shown). In contrast, we found that during the light period there was no significant association between preceding amount of REM sleep and subsequent initial levels of OFF periods.
Ongoing Global Behavioral State and OFF Periods
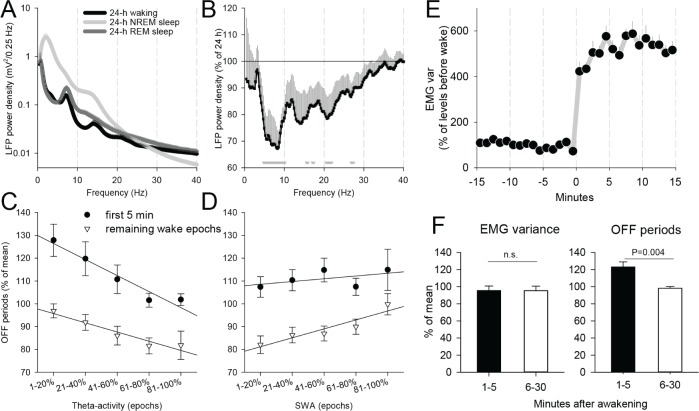
On average, waking EEG in rodents is characterized by a relatively low spectral power in the slow wave range and a distinct peak at “high” theta (6-9 Hz) frequency38,39 (Figure 5A). Because the most pronounced effect in terms of neuronal firing rates and OFF periods in rats was present in the first ∼5 min, we next investigated whether there was any systematic difference between the LFP power spectra in the first 5 min after awakening and the remaining part of the waking episode. Computing the corresponding LFP spectra revealed an ∼30% lower theta frequency power (6-9 Hz) within the first 5 min after awakening, as compared to the average level (Figure 5B). We hypothesized that different wake quality initially during the waking episode may be associated with the changes in cortical neuronal activity. To address this possibility, we subdivided all epochs during the first 5 min after awakening and during the remaining part of the waking episode into five 20% percentiles, corresponding to progressively increasing levels of theta activity in the LFP signal (Figure 5C). As a next step, we computed the incidence of the OFF periods for the corresponding epochs. The factor “theta percentile” in ANOVA was significant for the first 5 min (F(4,35) = 4.1, P = 0.008), and reached a tendency for the remaining part of the episode (F(4,35) = 2.5, P = 0.06). The correlation was negative: low values of theta activity were associated with higher incidence of OFF periods, and vice versa. However, irrespective of the level of theta activity, the incidence of OFF periods was invariably higher during the first 5 min after awakening (P < 0.05 for each of five percentiles, paired t-test). In other words, even during epochs with high theta power, presumably corresponding to active locomotion and exploratory behavior,36,40 the number of OFF periods in the first 5 min after awakening was higher than the intraepisodic level.
Figure 5.
(A) 24-h local field potential (LFP) power spectra in waking, nonrapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep in rats. Mean values (± standard error of the mean [SEM]). (B) Relative LFP spectrum (mean values ± SEM) during the first 5 min after arousal as % of average 24-h spectrum during waking (100%). Triangles below the curve depict those frequency bins in which electroencephalographic power during the first 5 min after awakening was significantly lower that the average 24-h power. (C) The relationship between theta-activity (6-9 Hz) and the incidence of brief periods of generalized neuronal silence (OFF periods), shown separately for the first 5 min after awakening and the remaining waking epochs. Mean values (± SEM) are plotted as a function of LFP theta power subdivided into five 20% percentiles from lowest to highest values. (D) The same as (C) for slow wave activity (SWA, 0.5-4 Hz). (E) The time course of electromyographic (EMG) variance during the last 15 min before a consolidated waking episode and the first 15 min after awakening. Mean values (± SEM, n = 8 rats). (F) Mean values of the incidence of OFF periods for epochs during the first 5 min after awakening and the remaining part of the episode, matched by the corresponding EMG values (bottom).
Next, we addressed whether there was an association between SWA (0.5-4 Hz) in the waking LFP signal (Figure 5D) and the incidence of OFF periods. This analysis revealed that these two variables were unrelated during the first 5 min after awakening (factor “percentile,” ANOVA, F(4,35) = 0.4, P = 0.79), whereas a significant positive association between the number of OFF periods and spectral power in the SWA range was found for the remaining part of the waking episode (F(4,35) = 3.1, P < 0.05), i.e., higher values of SWA corresponded to higher number of OFF periods. Thus, although the incidence of OFF periods overall declines later in the course of waking episode (Figure 3), those individual epochs, characterized by higher SWA are still likely to show more frequent OFF periods (Figure 5D). However, irrespective of the levels of SWA, the incidence of OFF periods was still significantly (P < 0.05) higher during the first 5 min, as compared to SWA-matched epochs during the remaining part of the waking episode, with an exception of the epochs where SWA was the highest (top percentile, P = 0.16). Because SWA (0.5-4 Hz) and “high” theta (6-9 Hz) frequency bands showed an opposite relationship with the incidence of OFF periods, we performed similar analyses for the LFP power in the “intermediate” 4-6 Hz frequency band. It was found that, unlike “high” theta-activity (6-9 Hz), “slow” theta-activity showed a positive association with OFF periods, although it did not reach statistical significance in the first 5 min, although it was significant for the remaining part of the waking episode (P = 0.01).
Finally, to investigate whether the observed changes in OFF periods are accounted for by the levels of locomotor activity, we investigated EMG variance across sleep-wake transitions. As expected, the EMG values abruptly increased several fold immediately after the awakening as compared to preceding sleep levels (Figure 5E). However, we noticed that during the first several min after awakening, the EMG levels were somewhat lower as compared to the remaining part of the waking episode. To address whether the initially higher incidence of OFF periods may simply reflect a quiet awake state, we applied an EMG-matching procedure. This was performed by finding the closest match in terms of the EMG value between each of the 4-sec epochs within the first 5 min after awakening and during the main part of the waking episode (5-30 min after awakening). In other words, for each low or high EMG epoch during the first 5 min there was a low or high EMG epoch, respectively, found later in the episode, such that the resulting average EMG levels were identical between the conditions. This analysis revealed that the incidence of OFF periods was still significantly higher during the first 5 min after awakening as compared to the values later in the waking episode, even if there was no difference in the corresponding EMG activity (Figure 5F).
DISCUSSION
In this study, we investigated the firing activity and population spiking patterns of neurons in the neocortex of rats and mice at the onset of spontaneous waking episodes. We found an overall lower firing rate of individual neurons and an increased number of silent periods (OFF periods) in the recorded population initially upon arousal, both of which gradually reached average waking levels within the next 5-10 minutes. Importantly, these effects were consistent between rats and mice, suggesting that these findings are valid for at least two rodent species.
Although the global population firing of cortical neurons was initially decreased, the individual cells were highly variable in their relative firing activity at the beginning of the wake episode. Specifically, a distinct population of slow-spiking neurons was found that was virtually silent in the first few min of waking. We hypothesize that this unusual firing phenotype may be unique to the first moments following an awakening. There are several possible reasons why this subset of neurons may be initially inactive upon waking, including differences in neuromodulatory signaling, increased inhibitory tone, or general depotentiation of synaptic strength during sleep. Waking from both REM and NREM sleep is associated with a surge of monoaminergic input onto the neocortex, particularly arising from noradrenergic neurons of the locus coeruleus.41,42 In contrast, cholinergic signaling is higher during both waking and REM sleep than in NREM sleep, as measured by in vivo microdialysis in the neocortex and hippocampus or by electrophysiological recordings from histologically identified cholinergic neurons in the basal forebrain.43–46 Because specific subtypes of cortical interneurons are differentially affected by noradrenaline and acetylcholine,47,48 it cannot be excluded that an increase in noradrenergic signaling during arousal could initiate a transient disruption in inhibitory/excitatory balance in favor of inhibition and thereby reduce firing of some excitatory cell populations. Such an inhibitory “reset” may be particularly important for quenching dream-related activity, thereby preventing hypnopompic hallucinations or other maladaptive hybrid behavioral states. Of course, from an evolutionary standpoint, a fast regaining of full-fledged awake state would be more adaptive. However, slow gradual arousal could have been favored given the neuroanatomical and neurochemical complexity of brain circuitry, especially in mammals with bigger brains.
The main finding of this study was that the altered patterns of activity at the single neuron level initially after awakening resulted in a higher incidence of population OFF periods. It was shown previously that such brief cessations of network activity are typical of NREM sleep13,49 or of waking after sleep deprivation.21 The novel finding that OFF periods are also frequent immediately upon awakening suggests an intriguing possibility that the changes in local network activities may diverge from global activity patterns during transitions between behavioral states. One possible explanation of this phenomenon is that the increase in OFF periods is simply a carryover of NREM-like activity into wake. However, if this were the case, OFF period incidence should be higher after NREM sleep and associated with increased SWA, whereas our findings revealed the opposite on both counts. Notably, the increased “leakage” of sleeplike activity after REM sleep awakenings we observed is also consistent with a recent human study that showed that, in the left anterior derivations, NREM sleep awakenings were associated with a prevalence of high-frequency EEG power as compared to REM sleep awakenings.50
Although no behavioral task has been implemented in the current study, and only undisturbed spontaneous waking episodes have been investigated, it is possible that the increased incidence of OFF periods immediately after awakening could also be associated with cognitive deficits typical for sleep inertia reported in humans.51 Numerous studies suggest that the brain state is the major determinant of how the brain receives and processes incoming information.14,52–55 For example, visual neurons in mice respond to a stimulus more strongly when mice run, as compared to when they are awake but immobile.56 One possibility is that the generalized low level of cortical neuronal activity, observed in early waking, is the key factor contributing to cognitive deficits. Yet another possibility is that the occurrence of OFF periods within specific cortical networks plays the major role. For example, it has been shown that the incidence of OFF periods in the frontal motor cortex in rats after sleep deprivation is associated with an impairment in a sugar pellet reaching task.21 We have also shown recently that the occurrence of OFF periods in awake rats resulted in a stronger and more synchronized response of cortical neurons after transcallosal electrical microstimulation.14 Thus, the occurrence of OFF periods immediately after awakening may affect fidelity of the representation and integration of sensory inputs, resulting in an overall performance decline. Our results therefore have an important methodological implication for studies in which the effects of preceding history are under scrutiny, as they can confound with state-dependent effects if the dynamics of sleep inertia are not taken into account.57
The relationship, or lack thereof, between features of the ongoing behavioral state and the incidence of OFF periods shed light on potential mechanisms and consequences of sleep inertia. First, LFP high theta activity, which is characteristic of active waking with exploratory behavior in rodents,21,36,40,58 was negatively associated with the incidence of OFF periods both initially during the waking episode, and throughout the rest of the wake period. However, even when theta activity was high, OFF periods after awakening were still above the average intraepisodic levels. It is possible that theta activity propagating from the hippocampus to the neocortex59 may be unable to entrain cortical neurons efficiently if they are undergoing OFF periods. Given the implication of theta activity in behavior, learning, and synaptic plasticity,60–65 it is possible that a disruption of the link between the cortex and the hippo-campus may underlie some of the cognitive deficits typical for sleep inertia. In contrast, slow theta activity (4-6 Hz) showed moderate positive association with the occurrence of OFF periods, although only in the latter part of the waking episode. Like SWA, low theta activity, which we speculate may be of cortical origin, has been shown both in humans and in rodents to be history- or experience-dependent, rather than merely state-dependent.21,36,40,58,66–68 Consistently, the increase in OFF periods was not associated with an increase in SWA in the first min after awakening, but a strong positive association between SWA and OFF periods emerged in the latter part of the episode.
Recent human studies showed an increase of low-frequency EEG power (1-9 Hz) initially after awakening,28,50 which was not the case in our study (Figure 5B). However, the recordings in humans were performed in a presumably identical quiet immo-bile state, which was not possible to obtain in freely moving animals. Another possible explanation for this discrepancy is that the recording technique we used is very sensitive to local changes in cortical activity within relatively small neuronal populations, which could often uncouple from the global state. Because scalp EEG recordings in humans highlight relatively global dynamics, caution is warranted in direct comparison of animal studies and human studies as well as low-frequency and high-frequency theta activity.
It is possible that OFF periods are mostly local at the beginning of the wake episode, and then become more global as waking progresses, resulting in the changes in a global brain state as reflected at the LFP level, in slow theta and slow waves. In other words, OFF periods may be qualitatively (or functionally) different in the first min after arousal and later during the wake episode, as sleep pressure increases. In the first case, they may be related to local and global neuromodulation,53,69 whereas later on, wake-dependent factors such as plastic changes70 or a need for cellular restoration15,71,72 could be the main driving force behind their occurrence.
Future studies can extend and strengthen the results reported here by recording more than one cortical location, which could potentially reveal regional differences in the dynamics of cortical activity immediately upon arousal, as has been done previously in humans using scalp EEG.50 Specifically, a hypothesis can be tested that average waking levels of cortical activity in primary sensory areas is re-established faster as compared to associative or motor regions. In addition, more detailed analysis of specific neuronal firing phenotypes or involvement of specific cortical layers is necessary to investigate the neurophysiologic underpinnings of specific changes in the network dynamics upon arousal. In the current study, spontaneous awakenings were investigated in baseline undisturbed conditions. An intriguing possibility remains that enforced awakening, e.g., by sensory stimulation73 or remote activation of arousal-promoting circuits using optogenetic tools,74–76 would lead to different results, such as a faster global arousal, as compared to spontaneous awakenings. Moreover, we did not investigate whether altered neural activity upon waking affected performance on a behavioral task, because engagement in a task immediately after arousal would have likely interfered with the spontaneous dynamics of the neuronal activity that we sought to characterize. Because sleep inertia in humans is traditionally defined based on behavior and subjective and objective measures of cognitive function, it will be important for future studies to determine whether altered neural activity interferes with behavior in the first minutes after awakening in rodents. Also, further studies should be performed following sleep deprivation to test the effects of physiologically increased sleep pressure, and in constant lighting conditions, to address the role of circadian factor, as homeostatic and circa-dian processes are both known to modulate the overall architecture and quality of vigilance states. Interestingly, we found in mice that the effects of REM sleep on a subsequent number of OFF periods were more pronounced during the dark period as compared to the light period. This intriguing observation suggests that not only immediate preceding state, but also sleep pressure, time of day, or lighting conditions affect the awake state quality upon arousal. However, caution is warranted in directly comparing the results obtained in polyphasic rodent species with humans, in which sleep is largely monophasic. Further carefully designed studies are necessary to address these limitations.
In summary, although there is substantial literature on electro-physiological, metabolic, or cognitive correlates of sleep inertia in humans, until now no animal studies have been performed addressing these topics. Our results showed for the first time, using continuous neuronal recordings from the cortex in mice and rats, that upon spontaneous arousal most neurons discharge initially at a slower rate, and it takes several minutes before neurons resume the levels of activity typical for waking. We speculate that an intrusion of sleeplike patterns of brain activity into early waking may account for some of the objectively measured behavioral manifestations of sleep inertia, as well as for the subjective feeling of “not being fully awake”, which is typical immediately after awakening. Additional insights can be provided by testing the behavioral consequences of these activity patterns in rodents and also by analyzing intracerebral recordings in human patients with drug-resistant epilepsy.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported by NIMH (R01MH099231 to Drs. Tononi and Cirelli). The authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
(A) 3-D Representation of the Gaussian kernel. The same window size (18 ms) and mean and standard deviation of Gaussian kernel are used throughout the study. (B) Raw spike trains from 14 neurons of a sample 100 ms recording in rats. (Sampling frequency = 500 Hz) (C) 3-D representation of deconvolved mean firing rates obtained using Gaussian kernel convolution method. Deconvolved mean firing rate preserves the original firing properties, and is subsequently used as the weighting matrix for normalizing the individual neuronal firing rates. (D) Summed population firing rates of normalized firing activity is shown in blue. OFF periods are detected by thresholding at 5% of maximum firing rates, shown in red. The detected OFF periods are then postprocessed to meet the duration criteria.
The distribution of instantaneous population firing rate (14 neurons) during a representative waking episode (50 min) before and after transforming the original data using Gaussian convolution method. (A) Mean population firing rates per 2ms time windows for the original data set plotted against transformed data (Kendall's rank correlation test, tau2ms = 0.2544, p2ms = 0; Pearson's correlation test, rho2ms = 0.2929, p2ms = 0). (B) Mean population firing rates per 20 ms time windows (tau20ms = 0.6870, p20ms = 0; rho20ms = 0.8196, p20ms = 0). (C,D) Mean population firing rates plotted for transformed data as a function of original values for 100 ms and 500 ms time windows respectively (tau100ms = 0.7526, p100ms = 0; rho100ms = 0.9033, p100ms = 0; tau500ms = 0.7996, p500ms = 0; rho500ms = 0.9388, p500ms = 0).
REFERENCES
- 1.Tobler I. Phylogeny of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W B Saunders; 2005. [Google Scholar]
- 2.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–71. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo CC, Chou T, Penzel T, et al. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci U S A. 2004;101:17545–8. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gall AJ, Joshi B, Best J, Florang VR, Doorn JA, Blumberg MS. Developmental emergence of power-law wake behavior depends upon the functional integrity of the locus coeruleus. Sleep. 2009;32:920–6. doi: 10.1093/sleep/32.7.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vyazovskiy VV, Tobler I. The temporal structure of behaviour and sleep homeostasis. PLoS One. 2012;7:e50677. doi: 10.1371/journal.pone.0050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porkka-Heiskanen T, Zitting KM, Wigren HK. Sleep, its regulation and possible mechanisms of sleep disturbances. Acta Physiol (Oxf) 2013;208:311–28. doi: 10.1111/apha.12134. [DOI] [PubMed] [Google Scholar]
- 7.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones BE. The role of noradrenergic locus coeruleus neurons and neighboring cholinergic neurons of the pontomesencephalic tegmentum in sleep-wake states. Prog Brain Res. 1991;88:533–43. doi: 10.1016/s0079-6123(08)63832-7. [DOI] [PubMed] [Google Scholar]
- 9.Fort P, Bassetti CL, Luppi PH. Alternating vigilance states: new insights regarding neuronal networks and mechanisms. Eur J Neurosci. 2009;29:1741–53. doi: 10.1111/j.1460-9568.2009.06722.x. [DOI] [PubMed] [Google Scholar]
- 10.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007:420–4. doi: 10.1038/nature06310. 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lecea L, Carter ME, Adamantidis A. Shining light on wakefulness and arousal. Biol Psychiatry. 2012;71:1046–52. doi: 10.1016/j.biopsych.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vyazovskiy VV, Olcese U, Lazimy YM, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyazovskiy VV, Olcese U, Cirelli C, Tononi G. Prolonged wakefulness alters neuronal responsiveness to local electrical stimulation of the neocortex in awake rats. J Sleep Res. 2012 Nov 21; doi: 10.1111/jsr.12009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14:443–51. doi: 10.1038/nrn3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 17.Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–34. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 19.Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents - EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–20. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–5. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci. 2010;30:4440–8. doi: 10.1523/JNEUROSCI.5062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hromadka T, Zador AM, DeWeese MR. Up states are rare in awake auditory cortex. J Neurophysiol. 2013;109:1989–95. doi: 10.1152/jn.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara M, De Gennaro L. The sleep inertia phenomenon during the sleep-wake transition: theoretical and operational issues. Aviat Space Environ Med. 2000;71:843–8. [PubMed] [Google Scholar]
- 25.Voss U. Changes in EEG pre and post awakening. Int Rev Neurobiol. 2010;93:23–56. doi: 10.1016/S0074-7742(10)93002-X. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara M, De Gennaro L, Bertini M. The effects of slow-wave sleep (SWS) deprivation and time of night on behavioral performance upon awakening. Physiol Behav. 1999;68:55–61. doi: 10.1016/s0031-9384(99)00150-x. [DOI] [PubMed] [Google Scholar]
- 27.Balkin TJ, Braun AR, Wesensten NJ, et al. The process of awakening: a PET study of regional brain activity patterns mediating the reestablishment of alertness and consciousness. Brain. 2002;125:2308–19. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara M, Curcio G, Fratello F, et al. The electroencephalographic substratum of the awakening. Behav Brain Res. 2006;167:237–44. doi: 10.1016/j.bbr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Groeger JA, Lo JC, Burns CG, Dijk DJ. Effects of sleep inertia after daytime naps vary with executive load and time of day. Behav Neurosci. 2011;125:252–60. doi: 10.1037/a0022692. [DOI] [PubMed] [Google Scholar]
- 30.Achermann P, Werth E, Dijk DJ, Borbely AA. Time course of sleep inertia after nighttime and daytime sleep episodes. Arch Ital Biol. 1995;134:109–19. [PubMed] [Google Scholar]
- 31.Signal TL, van den Berg MJ, Mulrine HM, Gander PH. Duration of sleep inertia after napping during simulated night work and in extended operations. Chronobiol Int. 2012;29:769–79. doi: 10.3109/07420528.2012.686547. [DOI] [PubMed] [Google Scholar]
- 32.Peter-Derex L, Perrin F, Petitjean T, Garcia-Larrea L, Bastuji H. Discriminating neurological from psychiatric hypersomnia using the forced awakening test. Neurophysiol Clin. 2013;43:171–9. doi: 10.1016/j.neucli.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Krueger JM, Tononi G. Local use-dependent sleep; synthesis of the new paradigm. Curr Top Med Chem. 2011;11:2490–2. doi: 10.2174/156802611797470330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahowald MW, Cramer Bornemann MA, Schenck CH. State dissociation, human behavior, and consciousness. Curr Top Med Chem. 2011;11:2392–402. doi: 10.2174/156802611797470277. [DOI] [PubMed] [Google Scholar]
- 35.Terzaghi M, Sartori I, Tassi L, et al. Dissociated local arousal states underlying essential clinical features of non-rapid eye movement arousal parasomnia: an intracerebral stereo-electroencephalographic study. J Sleep Res. 2012;21:502–6. doi: 10.1111/j.1365-2869.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 36.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–44. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–51. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 38.Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- 39.Vyazovskiy VV, Borbely AA, Tobler I. Interhemispheric sleep EEG asymmetry in the rat is enhanced by sleep deprivation. J Neurophysiol. 2002;88:2280–6. doi: 10.1152/jn.00304.2002. [DOI] [PubMed] [Google Scholar]
- 40.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–26. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–9. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marrosu F, Portas C, Mascia MS, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 46.Vanini G, Lydic R, Baghdoyan HA. GABA-to-ACh ratio in basal forebrain and cerebral cortex varies significantly during sleep. Sleep. 2012;35:1325–34. doi: 10.5665/sleep.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–76. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–8. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 49.Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–69. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marzano C, Ferrara M, Moroni F, De Gennaro L. Electroencephalographic sleep inertia of the awakening brain. Neuroscience. 2011;176:308–17. doi: 10.1016/j.neuroscience.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Hofer-Tinguely G, Achermann P, Landolt HP, et al. Sleep inertia: performance changes after sleep, rest and active waking. Brain Res Cogn Brain Res. 2005;22:323–31. doi: 10.1016/j.cogbrainres.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62:171–89. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 2013;16:1331–9. doi: 10.1038/nn.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–7. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zagha E, Casale AE, Sachdev RN, McGinley MJ, McCormick DA. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79:567–78. doi: 10.1016/j.neuron.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–9. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chauvette S, Seigneur J, Timofeev I. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75:1105–13. doi: 10.1016/j.neuron.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vyazovskiy VV, Tobler I. Handedness leads to interhemispheric EEG asymmetry during sleep in the rat. J Neurophysiol. 2008;99:969–75. doi: 10.1152/jn.01154.2007. [DOI] [PubMed] [Google Scholar]
- 59.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–97. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 61.Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–36. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–44. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 63.Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–4. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- 64.Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–8. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- 65.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–14. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landolt HP, Retey JV, Tonz K, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology. 2004;29:1933–9. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- 67.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 68.Hung CS, Sarasso S, Ferrarelli F, et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36:59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–8. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Hinard V, Mikhail C, Pradervand S, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–17. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maret S, Dorsaz S, Gurcel L, et al. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104:20090–5. doi: 10.1073/pnas.0710131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rolls A, Colas D, Adamantidis A, et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A. 2011;108:13305–10. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jego S, Glasgow SD, Herrera CG, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–43. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) 3-D Representation of the Gaussian kernel. The same window size (18 ms) and mean and standard deviation of Gaussian kernel are used throughout the study. (B) Raw spike trains from 14 neurons of a sample 100 ms recording in rats. (Sampling frequency = 500 Hz) (C) 3-D representation of deconvolved mean firing rates obtained using Gaussian kernel convolution method. Deconvolved mean firing rate preserves the original firing properties, and is subsequently used as the weighting matrix for normalizing the individual neuronal firing rates. (D) Summed population firing rates of normalized firing activity is shown in blue. OFF periods are detected by thresholding at 5% of maximum firing rates, shown in red. The detected OFF periods are then postprocessed to meet the duration criteria.
The distribution of instantaneous population firing rate (14 neurons) during a representative waking episode (50 min) before and after transforming the original data using Gaussian convolution method. (A) Mean population firing rates per 2ms time windows for the original data set plotted against transformed data (Kendall's rank correlation test, tau2ms = 0.2544, p2ms = 0; Pearson's correlation test, rho2ms = 0.2929, p2ms = 0). (B) Mean population firing rates per 20 ms time windows (tau20ms = 0.6870, p20ms = 0; rho20ms = 0.8196, p20ms = 0). (C,D) Mean population firing rates plotted for transformed data as a function of original values for 100 ms and 500 ms time windows respectively (tau100ms = 0.7526, p100ms = 0; rho100ms = 0.9033, p100ms = 0; tau500ms = 0.7996, p500ms = 0; rho500ms = 0.9388, p500ms = 0).