Abstract
Study Objective:
To investigate respiratory cycle-related electroencephalographic changes (RCREC) in healthy children and in children with sleep disordered breathing (SDB) during scored event-free (SEF) breathing periods of sleep.
Design:
Interventional case-control repeated measurements design.
Setting:
Paediatric sleep laboratory in a hospital setting.
Participants:
Forty children with SDB and 40 healthy, age- and sex-matched children.
Interventions:
Adenotonsillectomy in children with SDB and no intervention in controls.
Measurements and Results:
Overnight polysomnography; electroencephalography (EEG) power variations within SEF respiratory cycles in the overall and frequency band-specific EEG within stage 2 nonrapid eye movement (NREM) sleep, slow wave sleep (SWS), and rapid eye movement (REM) sleep. Within both groups there was a decrease in EEG power during inspiration compared to expiration across all sleep stages. Compared to controls, RCREC in children with SDB in the overall EEG were significantly higher during REM and frequency band specific RCRECs were higher in the theta band of stage 2 and REM sleep, alpha band of SWS and REM sleep, and sigma band of REM sleep. This between-group difference was not significant postadenotonsillectomy.
Conclusion:
The presence of nonrandom respiratory cycle-related electroencephalographic changes (RCREC) in both healthy children and in children with sleep disordered breathing (SDB) during NREM and REM sleep has been demonstrated. The RCREC values were higher in children with SDB, predominantly in REM sleep and this difference reduced after adenotonsillectomy.
Citation:
Immanuel SA, Pamula Y, Kohler M, Martin J, Kennedy D, Saint DA, Baumert M. Respiratory cycle-related electroencephalographic changes during sleep in healthy children and in children with sleep disordered breathing. SLEEP 2014;37(8):1353-1361.
Keywords: adenotonsillectomy, children, electroencephalogram, respiratory cycle, sleep disordered breathing, sleep stage
INTRODUCTION
Childhood sleep disordered breathing (SDB) is characterized by an increased work of breathing, restless night sleep, and excessive daytime sleepiness and has been associated with cognitive impairment, behavioral disturbances, and early cardiovascular changes that may predispose to an increased risk of developing cardiovascular diseases.1 Compared to normal controls, children with SDB have elevated arousal thresholds and impaired upper airway responses to respiratory stimuli,2,3 and present a pattern of partial obstructive hypoventilation in the absence of significant oxygen desaturation or arousals.4,5 Importantly, obstructions in children with SDB are not always terminated with cortical arousal as indicated by high-frequency changes in EEG.6 Cortical activation in children may occur, but be too subtle to be visually scored. Thus, obtaining markers that reflect brain activation associated with sleep related respiratory loads is still a challenge in clinical practice. Studies have investigated changes in EEG spectra and arousal patterns associated with central and/or obstructive apneas in children,7–9 but such approaches still remain linked to visually identified and scored respiratory events with polysomnography (PSG). Alternative approaches to evaluate neural function in children with SDB that do not rely on standard PSG EEG criteria include respiratory-related evoked potentials10,11 and autonomic responses to arousals.12,13
A different analytical approach toward the quantification of SDB-related EEG changes was developed by Chervin et al.14 It is based on measuring subtle changes in cortical activity that occurs phase-locked with respiration, termed respiratory cycle related EEG changes (RCREC). In the initial study, notable differences in average EEG spectral powers linked to the phases of the respiratory cycle were observed in a child with SDB and these differences were reported to diminish after adenotonsillectomy.14 In a subsequent study, similar observations were made on a small group of children with SDB, in whom RCREC during nonapneic sleep was reduced after adenotonsillectomy and the change was correlated with improvement in daytime sleepiness between baseline and follow-up.15 Chervin et al. speculated that RCREC may represent numerous micro-arousals in response to labored breathing that is well known to occur in children with SDB, who hypoventilate for most of the sleep periods outside of traditionally scored periods of apnea/ hypopnea and arousals. However, the physiological significance of the RCREC is not yet clearly understood. Subsequent studies by Chervin et al. in adult patients and children have also shown that pretreatment RCREC correlates with daytime sleepiness and predicts postoperative improvement in sleepiness.16–18
In this current study, we sought to corroborate on the findings by Chervin et al. in a substantial cohort of normal children and children with predominately mild SDB. We investigated sleep periods free of scored apnea/hypopneas, arousals, or artifacts over the entire night duration to find (1) if frequency and sleep stage-specific RCREC exists in normal children and to quantify reproducibility, (2) if the magnitude of RCREC in children with SDB differs significantly from that of healthy children, and (3) if so, whether that difference diminished after the children with SDB underwent adenotonsillectomy.
METHODS
Subjects
This study was approved by the Women's and Children's Health Network Human Research Ethics Committee, South Australia, with parental consent and child assent obtained from all participants. Participants were 40 children aged 3.25-12.9 y, with a history of frequent snoring, awaiting adenotonsillectomy for suspected SDB, and a matched group of 40 nonsnoring healthy controls. Both groups underwent overnight PSG to evaluate sleep and breathing parameters. Children with SDB had two PSGs; one before and one after surgical intervention (adenotonsillectomy), whereas control children had two PSGs at the same time points. It was ensured that participants had not undergone previous ear, nose, throat, or craniofacial surgery, had a medical condition (other than SDB) associated with hypoxia or sleep fragmentation, or were taking medication known to affect sleep or cardiorespiratory physiology.
Overnight PSG
Overnight PSG was conducted for all children if they were well on the night of testing and free of sedation, sleep deprivation, or any recent illness including respiratory infection. Overnight PSG began close to each child's usual bedtime and a parent was present throughout the procedure. The S-Series Sleepwatch System (Compumedics, Australia) was used to continuously record: electroencephalogram (EEG; C3-A2 and C4-A1), left and right electrooculogram (EOG), heart rate by electrocardiogram (ECG), submental and diaphragmatic electromyogram (EMG) with skin surface electrodes, leg movements by piezoelectric motion detection, oronasal airflow by thermistor and nasal pressure, respiratory movements of the chest and abdominal wall using uncalibrated respiratory inductive plethysmography (RIP), arterial oxygen saturation (SpO2) by pulse oximetry (Nellcor N595 with a 3-sec averaging time) and transcutaneous CO2, using a heated (43°C) transcutaneous electrode (TINA, Radiometer Pacific, Melbourne, Vic., Australia). Each child was monitored continuously overnight via infrared camera and by a pediatric sleep technician who also documented observations of sleep behavior, including the presence or absence of snoring. A repeat study was performed on average 28.0 ± 5.0 w later (range, 19–42 w) in controls and 32.4 ± 6.7 w later (range, 23–55 w) in children with SDB. Sleep stages were scored visually in 30-sec epochs according to the standardized EEG, EOG, and EMG criteria of Rechtschaffen and Kales.19 Movement time (> 50 % of an epoch obscured by movement artifact) was scored as a separate category and was not included in either sleep or wake time. Obstructive events were scored according to standard guidelines recommended for paediatric sleep studies.20 The total number of obstructive apneas, mixed apneas, and obstructive hypopneas divided by the total sleep time and expressed as the number of events per hour of sleep yielded the obstructive apnea/hypopnea index (OAHI). The saturation of peripheral oxygen (SpO2) desaturation index represents the number of ≥ 3% oxygen desaturations per hour of sleep. The body mass index (BMI) z-scores were calculated using the height and weight of the children measured on the night of PSG along with established growth charts corrected for age and sex.21
Data Analysis
Respiratory data from the rib cage (RC) RIP channel and EEG data from C3-A2/C4-A1were extracted from PSG data using the programming library libRASCH and analyzed using custom-written algorithms developed with the signal processing toolbox in MATLAB (The Mathworks Inc., Natick, MA). In the current study, stage 2 nonrapid eye movement (NREM) sleep, slow wave sleep (SWS; stages 3 + 4) and rapid eye movement (REM) sleep were considered. Sleep stage 1 was omitted from analysis because of insufficient amount of time spent in that stage. To analyze nonapneic breathing cycles, artifact-free uninterrupted sleep durations were retained by excluding all portions of data falling within durations identified and scored by the sleep technician as body movements or abnormal cardio-respiratory events (e.g., apnea, hypopnea, arousals). Retained periods of sleep are referred to as scored event-free periods (SEF) throughout this study. Within each sleep stage, the RC signal during retained SEF 30- sec epochs were extracted and used for further analysis.
Details of RCREC computation have been previously published.22 Briefly, each respiratory cycle was divided into four segments: early/late inspiration (Ins1, Ins2) and early/ late expiration (Exp1, Exp2) based on expiratory and inspiratory onsets and their midpoints located on thoracic excursion signal. The average EEG power was calculated for each respiratory segment. To obtain normalized segmental EEG power, power in each segment was divided by the EEG power of the whole respiratory cycle and a value of 1 subtracted from this ratio. Both segmental powers and normalized segmental powers were averaged across all SEF respiratory cycles within a given sleep stage. The maximum difference between the averaged normalized segmental powers within the four respiratory phases was defined as RCREC. The aforementioned steps were performed on the overall EEG and band-pass filtered EEG, which was obtained using fifth-order elliptic digital filters corresponding to delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), and beta (15–30 Hz) frequencies. Thus, in each subject, mean segmental powers, normalized mean segmental powers and RCREC were computed from both overall EEG and frequency band specific EEG within each sleep stage.
To test whether frequency band and sleep stage-specific RCREC values in normal children reflect nonspurious phase-locked EEG cycling, we generated phase-randomized EEG surrogates using the EEG data from the baseline overnight PSG. The Fourier transform approach was used to generate surrogates that retain the power spectrum of the original EEG, by transforming the original data into the frequency domain using fast Fourier transform (FFT), randomizing the phases, and converting back into time domain using the inverse FFT.23
To compare the RCREC approach with conventional EEG analysis, we performed power spectrum analysis of EEG in 30-sec SEF epochs with a nonparametric FFT algorithm based on the Welch method with a Hamming window and 50% overlap between segments. The spectral power in different frequency bands was estimated with respect to the overall spectral power of the EEG and expressed as delta power, theta power, alpha power, sigma power, and beta power percentages, respectively.
Statistical Analysis
Data were analyzed using the statistical software SPSS (IBM, Boston, Mass: International Business Machines Corp,USA) version 18 and the Prism (GraphPad Software Inc., San Diego CA) version 5.01 for Windows (Microsoft, Washington, USA). Normality of data distribution was tested using the Kolmogrov-Smirnov tests. Student t-test and one-way analysis of variance (ANOVA) were used to compare demographic data and PSG results between groups. To test for within-group sleep stage-specific respiratory cycle-related changes in mean EEG power in normal children as well as those with SDB, we performed one-way ANOVA and post hoc multiple comparisons using Tukey method. To test for differences between RCRECs computed using original and phase-randomized surrogate EEG, we used two-way ANOVA followed by Bonferroni multiple comparison tests. Reproducibility of RCREC was investigated based on the repeat PSG of normal children, using intra-class correlation reliability analysis with a two-way mixed model. To test for differences between normal children and children with SDB in the overall and frequency band-specific RCREC within each sleep stage, we performed two-way ANOVA. Post hoc analysis was performed using Bonferroni multiple comparison tests. To validate significant between-group differences in RCREC values, a two-way ANOVA test with controls versus SDB as one factor and baseline versus follow-up as the other factor was used to test for interaction effects. To test for frequency band-specific differences in conventionally estimated EEG power spectra, we used two-way ANOVA. Associations between RCREC and subject demographics and PSG results were determined using Pearson or Spearman correlation analysis as appropriate. All data were normal distributed, presented as mean ± standard deviation, and P < 0.05 was considered statistically significant unless stated otherwise.
RESULTS
Subject Characteristics and PSG Results
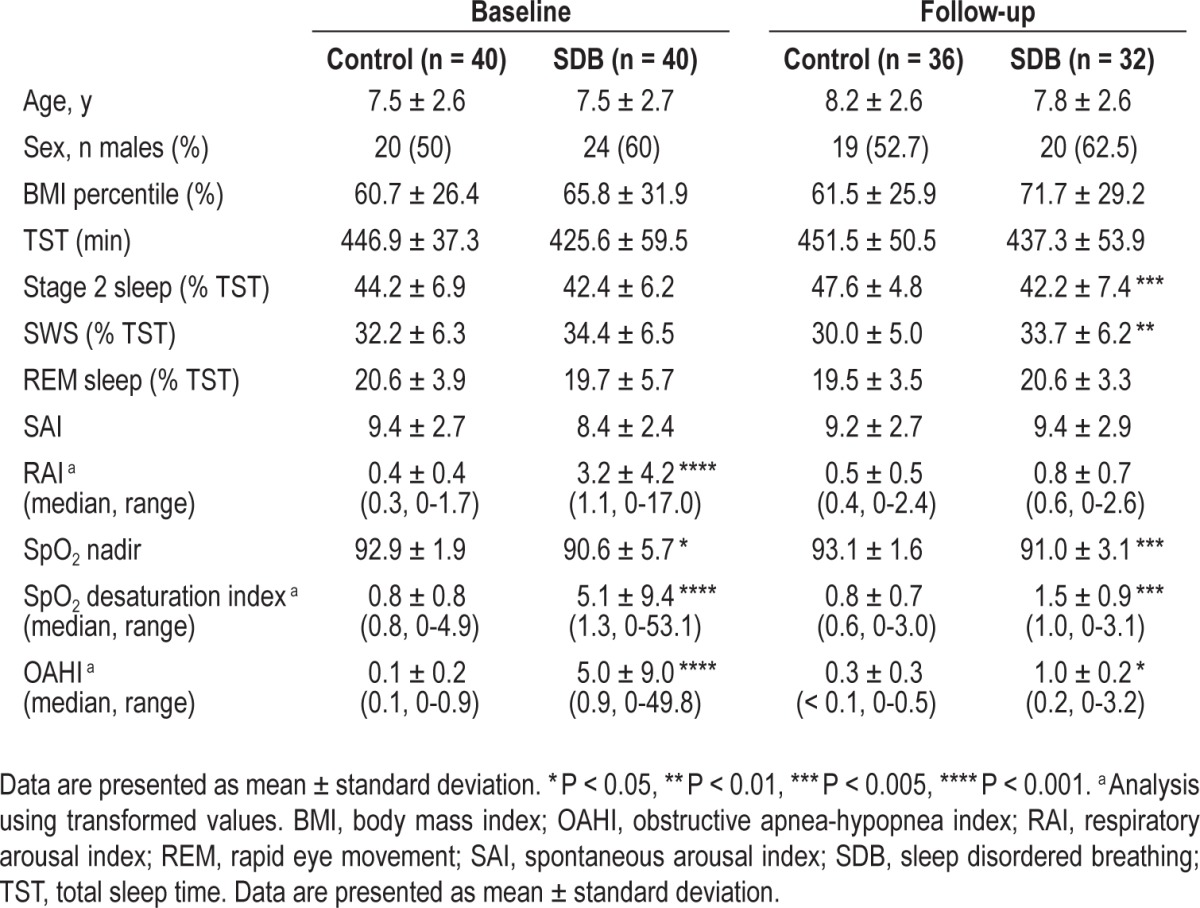
Results of overnight PSG have been reported earlier.24–26 Briefly, baseline PSG confirmed the presence of respiratory abnormalities in the children with SDB, who had a significantly higher OAHI, elevated respiratory arousals, increased frequency of SpO2 desaturations, and a significantly lower mean SpO2 nadir compared to controls. Overall, the degree of SDB can be considered mild to moderate (OAHI: 5.0 ± 9.0). There were no significant differences between groups with respect to sleep architecture in the baseline PSG (Table 1). Follow-up PSG data suitable for analysis were available for 36 of the 40 normal children and 32 of the 40 children with SDB. Following adenotonsillectomy, there was a marked reduction in OAHI, SpO2 desaturation frequency, and respiratory arousal rate in the SDB group. However, on average, those children still had a small but significantly higher OAHI and SpO2 desaturation frequency and lower SpO2 nadir compared to normal children (Table 1).
Table 1.
Subject demographics, sleep and respiratory parameters for the baseline and follow-up polysomnography
RCREC in Normal Children
Example traces of raw EEG and EEG power averaged over four respiratory phases along with the thoracic RIP signal are shown in Figure 1. Although no clearly discernible phase-locked EEG pattern is visible, averaging of EEG power for all artifact-free nonobstructed respiratory cycles throughout the night resulted in statistically significant respiratory cycle-related changes in EEG power.
Figure 1.
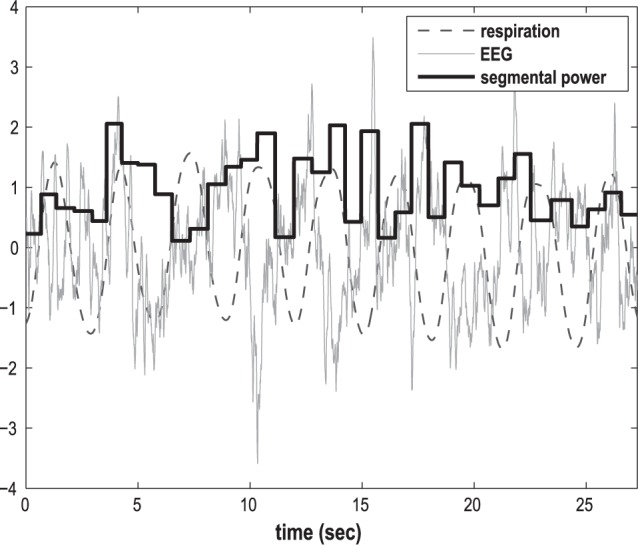
Example plot of raw electroencephalograph (EEG) and EEG power segmented into four respiratory phases during nine nonapneic respiratory cycles in a control subject during stage 2 sleep.
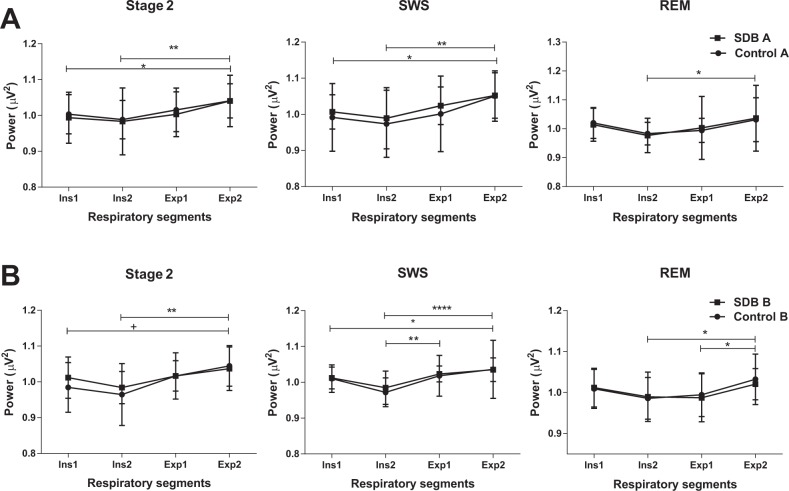
A significant effect of the respiratory phase on mean segmental EEG power was observed in stage 2, SWS and REM sleep. Within the control group, one-way ANOVA between the four mean segmental EEG powers, averaged over all subjects, showed significant respiratory cycle effect in stage 2, SWS, and REM sleep (Table 2). A trend of EEG power to decrease during inspiration, followed by an increase toward the late expiratory segment, was observed. Multiple comparisons between the segmental EEG powers showed a significant difference between late inspiration (Ins2) and late expiration (Exp2). This observation was consistent in all three stages and in both groups at baseline and follow-up studies as shown in Figures 2A and 2B. There were further significant intersegmental differences, which were less consistent than the Ins2 versus Exp2 difference, but support the overall finding of inspiratory fall and an expiratory rise in total EEG power (Figure 2).
Table 2.
Within-group one-way analysis of respiratory cycle related changes in overall electroencephalograph power within stage 2, SWS, and REM sleep in control and sleep disordered breathing groups at baseline and follow-up polysomnography
Figure 2.
Mean electroencephalograph power of the four respiratory segments (Ins1, Ins2, Exp1, and Exp2) averaged across subjects within the control and sleep disordered breathing (SDB) group (A) at baseline (B) at follow-up polysomnography (* P < 0.05, ** P < 0.01, **** P < 0.001). REM, rapid eye movement sleep; SWS, slow wave sleep.
In the control group, RCREC values derived from real EEG data were significantly higher than those from the phase-randomized EEG surrogates, in all stages of sleep (Figure 3: ANOVA group effect: stage 2: P < 0.0001; SWS: P < 0.0001; REM: P < 0.0001). Post hoc comparison with respect to EEG frequency bands demonstrated higher than random RCREC values for delta, theta, alpha, and sigma frequency bands in any sleep stage, except sigma in REM sleep. Results for alpha were less consistent during SWS and REM sleep. Beta RCREC values were higher than random only in SWS and not in stage 2 and REM.
Figure 3.
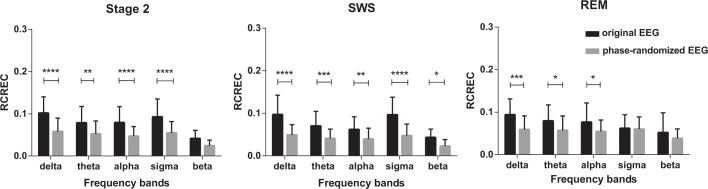
Comparison of respiratory cycle-related electroencepholographic changes (RCREC) between original electroencephalograph (EEG) and its phase-randomized surrogate in delta, theta, alpha, sigma, and beta frequency bands with respect to sleep stage within the control group at baseline PSG (* P < 0.05, ** P < 0.01, *** P < 0.005, **** P < 0.001). REM, rapid eye movement sleep; SWS, slow wave sleep.
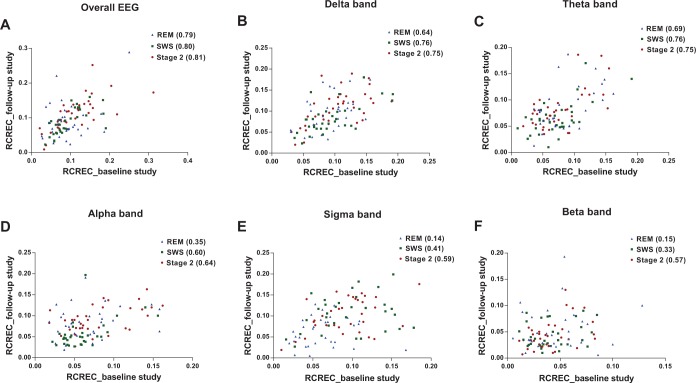
In a two-way ANOVA comparing baseline and repeated PSG of normal children, none of the sleep stages (2, SWS, REM) showed significant difference in RCREC. To test for reproducibility, we performed intraclass correlations between the two PSGs, on the overall and frequency band-specific RCRECs within each sleep stage. Scatterplots of RCREC measure between baseline and the follow-up PSG along with the correlation coefficients are shown in Figure 4. Based on the associations observed, overall RCREC showed relatively high reproducibility (intraclass correlation coefficient in stage 2: 0.81; SWS: 0.80; REM: 0.79) (Figure 4). Reproducibility of frequency band-specific RCRECs was slightly weaker than for overall EEG in delta (intraclass correlation coefficient: stage 2: 0.75; SWS: 0.76; REM: 0.64) and theta bands (intraclass correlation coefficient: stage 2: 0.75; SWS: 0.76; REM: 0.69) (Figure 4). RCREC measured in the alpha, sigma, and beta frequency ranges showed poorer reproducibility, particularly during REM sleep. (Intraclass correlation coefficients: alpha - stage 2: 0.64; SWS: 0.6; REM: 0.35; sigma - stage 2: 0.59; SWS: 0.41; REM: 0.14; beta - stage 2: 0.57; SWS: 0.33; REM: 0.15).
Figure 4.
Scatterplots of respiratory cycle-related electroencephalographic changes (RCREC) between baseline and follow-up polysomnography within the control group in (A) overall EEG, and (B) delta, (C) theta, (D) alpha, (E) sigma, and (F) beta frequency bands. The intraclass correlation coefficients of each band within each sleep stage are shown. REM, rapid eye movement sleep; SWS, slow wave sleep.
RCREC in Children with SDB
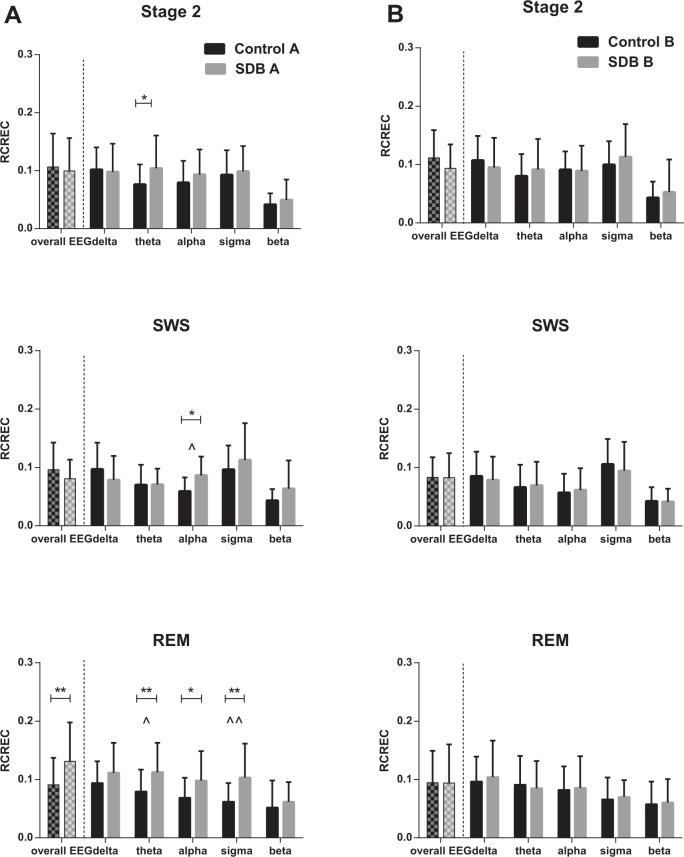
Similar to the control group, a within-group one-way ANOVA showed significant respiratory cycle- related changes in EEG power in all stages of sleep in the SDB group (Table 2). Post hoc multiple comparisons showed an inspiratory decrease in power, followed by an increasing trend toward the late expiratory segment (Figure 2). The magnitude of RCREC in overall EEG and in frequency band- specific EEG was compared within each sleep stage, between children with SDB and the controls (Figure 5). RCREC values of overall EEG were higher in the SDB group during REM sleep (Figure 5A). Two-way ANOVA of frequency band-specific RCRECs in each sleep stage showed a significant group difference in all three stages of sleep (ANOVA group effect: stage 2: P = 0.01; SWS: P = 0.04; REM: P < 0.0001). Post hoc multiple comparisons demonstrated significantly higher RCREC values in the SDB group in the theta band of stage 2 and REM sleep, alpha band of SWS and REM sleep, and sigma band of REM sleep. The RCREC values in the delta band tended to be higher in the SDB group during REM sleep, but the difference did not reach statistical significance (P = 0.051) (Figure 5A).
Figure 5.
Comparison of respiratory cycle-related electroencephalographic changes (RCREC) between control and sleep disordered breathing (SDB) groups in the overall electroencephalograph and in delta, theta, alpha, sigma, and beta frequency bands with respect to sleep stage (A) at baseline (B) at follow-up PSG (* P < 0.05, ** P < 0.01 using two-way analysis of variance). ∧ indicates significant interaction effect between baseline and follow-up studies in frequency band-specific RCREC (∧ P < 0.05, ∧∧ P < 0.01). REM, rapid eye movement sleep; SWS, slow wave sleep.
Following adenotonsillectomy the RCREC levels of children with SDB in both the overall EEG and in frequency-specific bands were no longer statistically different from those of normal children (Figure 5B) (ANOVA group effect: stage 2: P = 0.4; SWS: P = 0.6; REM: P = 0.6)]. A two-way ANOVA to test group-by-time interaction effects on those frequency band-specific RCREC values that were significantly higher in the SDB group at baseline study (theta band of stage 2 and REM sleep, alpha band of SWS and REM sleep, and sigma band of REM sleep) showed significant interaction effects in alpha band of SWS (P = 0.04) and theta (P = 0.01) and sigma (P = 0.008) bands of REM sleep (Figure 5A). Repeated measures two-way ANOVA of children with SDB confirmed a significant decrease in RCREC of total EEG power in REM sleep following surgery (P = 0.001). Paired comparisons between their RCREC during REM in each frequency band before and after surgery showed a significant decrease in theta RCREC (P = 0.01) and sigma RCREC (P = 0.01) post-intervention.
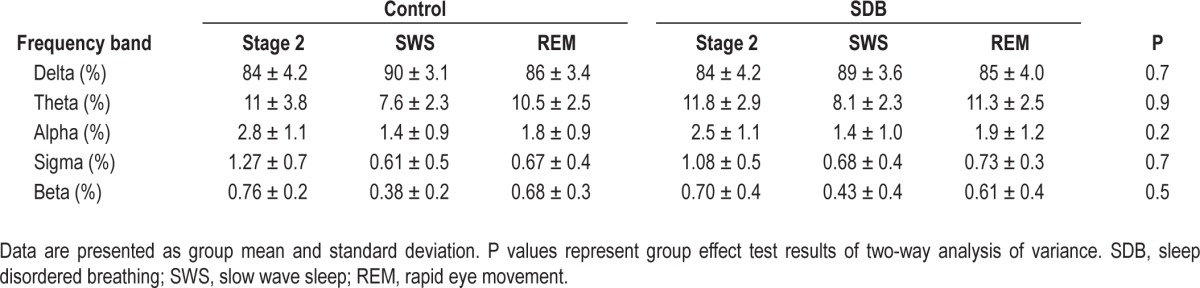
Results of conventional power spectral analysis of EEG showed no significant differences between children with SDB and controls (Table 3).
Table 3.
Relative electroencephalograph power across standard frequency bands for different sleep stages in controls and children with sleep disordered breathing
Clinical Correlates of RCREC
No significant correlations between frequency band-specific RCREC values and age or BMI were observed, and the measures were not significantly different between the two sexes in any of the sleep stages or in either group of children. Within the SDB group, the RCREC of overall EEG, but not the frequency band-specific RCRECs, was found to have a weak yet significant association with the spontaneous arousal index (SAI) in all three sleep stages (ss2: r = 0.35 SWS: r = 0.35 REM: r = 0.36, all P < 0.05). Also, the RCREC of total EEG power showed a weak positive correlation with BMI in the SDB group in REM sleep (r = 0.353, P < 0.05) but not in the NREM stages of sleep. None of the RCREC measures correlated with the OAHI in any of the sleep stages.
DISCUSSION
The main findings of our study were: (1) significant, nonrandom variations in EEG power phase-locked to event-free breathing cycles in healthy children as well as in children with SDB, where the mean EEG power tended to be lower during inspiration than expiration; (2) sleep stage-specific increases in RCREC of theta, alpha, and sigma power in children with SDB compared to normal children; and (3) normalization of RCREC values in SDB children postadenotonsillectomy.
Our study confirms the existence of RCREC in normal children and those with SDB that has been previously reported in a small group of children by Chervin et al.14,15 Our data also confirm the reduction in RCREC to normal levels following adenotonsillectomy. Chervin et al. reported elevated RCREC in five children with SDB compared to five normal children in both delta and theta power and a significant correlation between theta RCREC and apnea-hypopnea index (AHI). By using a considerably larger data set, we were able to extend on earlier reports significantly. We incorporated a sleep stage-specific analysis of the RCREC phenomenon and confirmed significantly higher theta RCREC in stage 2 and REM sleep, and in addition observed higher alpha RCREC in SWS and REM; all of these changes normalized after surgery. However, we were not able to confirm elevated RCREC in the delta band or significant associations between RCREC measures and OAHI. These discrepancies could be attributed to methodological differences between the studies as we have analyzed the entire night sleep data, within a given sleep stage, on a larger cohort of 40 control children, none of whom underwent any surgery, and on 40 children with predominantly mild to moderate SDB (OAHI: 5.0 ± 9.0). The earlier study by Chervin et al.15 has looked at the first 3 h of night sleep, taking no sleep stage effects into account, on a group of five controls (four children underwent adenotonsillectomy and one underwent hernia repair) and five children with SDB with a similarly wide range of obstructive apnea index (OAI) and AHI indices [OAI: 2.7 to 38.2 (11.9 ± 14.8) and AHI: 4 to 55.6 (17.3 ± 21)].
Previous studies on RCREC relied largely on observational data. To validate methodological aspects of the RCREC approach we performed surrogate analyses with phase-randomized EEG and were able to demonstrate that the RCREC phenomena are present also in normal children. Importantly, they cannot be solely attributed to random effects, which yielded significantly lower RCREC values of approximately 0.05 (i.e., REM: 0.06 ± 0.03 in delta bands, 0.05 ± 0.03 in theta bands and 0.05 ± 0.02 in alpha bands). Reproducibility assessed by intraclass correlation analysis was better in RCREC derived from the overall EEG when compared to those derived from frequency band-specific EEGs. Among frequency band-specific EEGs, reproducibility varied across sleep stages with highest reproducibility observed in delta and theta power during SWS and poorest reproducibility in sigma power during REM sleep. Unless a clear physiological rationale for frequency-specific RCREC analysis is established, its estimation based on total EEG power may be more reliable and avoid spurious results.
RCREC in nonapneic sleep have been shown in healthy adults27 and in patients with obstructive sleep apnea syndrome,18 providing mounting evidence for RCREC. Its physiological basis, however, in particular during sleep, remains largely unknown. EEG changes linked to respiration have been demonstrated in awake subjects with an increase in EEG power during inspiration, which is opposite to what has been observed during sleep.28 Increased rhythmic fluctuations in intracranial pressure during sleep have been shown to affect EEG.29 Chervin et al. showed a relation between esophageal pressure and sleep RCREC, suggestive of a possible hemodynamic explanation.30 In awake adults, the electrical activity of the brain has been shown to respond to hemodynamic changes in the brain and carotid pressure swings.31,32 Further, correlated slow fluctuations in EEG, respiration, and cerebral blood flow-dependent functional magnetic resonance imaging signals have been demonstrated previously.33 Differences in respiratory phase-related EEG changes were shown between nasal and mouth breathing in awake adults.34,35 It has been suggested that these EEG changes during wakefulness are linked to the olfactory sampling.36 In our study, RCREC of overall EEG power within the SDB group showed a weak positive association with the SAI, suggestive of a link between respiratory-modulated cortical activity and the spontaneous arousal process. In support of neural mechanisms involved in generating increased RCREC, cortical processing of respiratory-related information was measured in children with OSA, as respiratory-related evoked potentials during sleep and their responses were found to be blunted compared to those in control children.11 Cortical processing of airway occlusion-related afferents were also found abnormal in awake adults with severe obstructive sleep apnea syndrome.37 Indicative of neural pathways mediating RCREC, left versus right nostril breathing was shown to have a significant effect on spatial EEG activity.38
Findings from our study extend previous evidence15 that, compared to controls, RCREC values are higher in children with SDB during SEF periods, though the differences in our study were not consistent in all sleep stages and in every frequency band of the EEG. Chervin et al. speculated that higher RCREC values in children with SDB may reflect repetitive micro-arousals that translate into neurological consequences and daytime sleepiness, although the evidence was solely based on correlation analyses. In our data, associations between RCREC and OAHI indices did not reach statistical significance as had been previously demonstrated.15 This might be attributed to the fact that our study cohort comprised children with mostly mild SDB.
Interestingly, significantly elevated RCREC values were observed in children with SDB almost exclusively in REM sleep. From a physiological point of view, REM sleep is characterized by cortical activation that is distinctly different from all other stages sleep. This may indicate alterations in neural processes in SDB children. However, in particular REM sleep results in respiratory instability in children with SDB, which may modulate RCREC via hemodynamic pathways.
In conclusion, our study demonstrated the presence of nonrandom RCREC in both healthy children and in children with SDB during NREM and REM sleep. The RCREC values were higher in children with SDB, predominantly in REM sleep and this difference reduced after adenotonsillectomy. A better understanding of the mechanisms implicated in the RCREC phenomenon is crucial for establishing its clinical utility for diagnostics of SDB.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Baumert holds a fellowship from the Australian Research Council and this project was partly supported by grant ARC DP 110102049, DP 0663345 and NH&MRC project grant 250369. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.O'Brien LM, Gozal D. Behavioural and neurocognitive implications of snoring and obstructive sleep apnoea in children: facts and theory. Pediatr Respir Rev. 2002;3:3–9. doi: 10.1053/prrv.2002.0177. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol. 1998;84:1926–36. doi: 10.1152/jappl.1998.84.6.1926. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57:99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CL, D'andrea L, Haddad GG. Adult criteria for obstructive sleep apnea do not identify children with serious obstruction. Am J Respir Crit Care Med. 1992;146:1231–4. doi: 10.1164/ajrccm/146.5_Pt_1.1231. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 6.Grigg-Damberger M, Gozal D, Marcus CL, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–40. [PubMed] [Google Scholar]
- 7.Bandla HP, Gozal D. Dynamic changes in EEG spectra during obstructive apnea in children. Pediatr Pulmonol. 2000;29:359–65. doi: 10.1002/(sici)1099-0496(200005)29:5<359::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Yang JS, Nicholas CL, Nixon GM, et al. EEG spectral analysis of apnoeic events confirms visual scoring in childhood sleep disordered breathing. Sleep Breath. 2012;16:491–7. doi: 10.1007/s11325-011-0530-0. [DOI] [PubMed] [Google Scholar]
- 9.McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J Appl Physiol. 1996;81:2651–7. doi: 10.1152/jappl.1996.81.6.2651. [DOI] [PubMed] [Google Scholar]
- 10.Melendres MC, Marcus CL, Abi-Raad RF, Trescher WH, Lutz JM, Colrain I. Respiratory-related evoked potentials during sleep in children. Sleep. 2008;31:55–61. doi: 10.1093/sleep/31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Colrain IM, Melendres MC, et al. Cortical processing of respiratory afferent stimuli during sleep in children with the obstructive sleep apnea syndrome. Sleep. 2008;31:403–10. doi: 10.1093/sleep/31.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53:580–8. doi: 10.1203/01.PDR.0000057206.14698.47. [DOI] [PubMed] [Google Scholar]
- 13.Pillar G, Bar A, Shlitner A, Schnall R, Shefy J, Lavie P. Autonomic arousal index: an automated detection based on peripheral arterial tonometry. Sleep. 2002;25:543–9. [PubMed] [Google Scholar]
- 14.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Method for detection of respiratory cycle-related EEG changes in sleep-disordered breathing. Sleep. 2004;27:110–5. doi: 10.1093/sleep/27.1.110. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Correlates of respiratory cycle-related EEG changes in children with sleep-disordered breathing. Sleep. 2004;27:116–22. doi: 10.1093/sleep/27.1.116. [DOI] [PubMed] [Google Scholar]
- 16.Chervin RD, Burns JW, Subotic NS, et al. Respiratory cycle-related EEG changes (RCREC) predict neurobehavioral outcomes in childhood OSA. Sleep. 2004;27:A99. (Abstract Suppl) [Google Scholar]
- 17.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs. other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 18.Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171:652–8. doi: 10.1164/rccm.200408-1056OC. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Washington, DC: U.S. Government Printing Office, U.S. Public Health Service; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 20.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 22.Chervin RD, Shelgikar AV, Burns JW. Respiratory cycle-related EEG changes: response to CPAP. Sleep. 2012;35:203–9. doi: 10.5665/sleep.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theiler J, Eubank S, Longtin A, Galdrikian B, Doyne Farmer J. Testing for nonlinearity in time series: the method of surrogate data. Physica D: Nonlinear Phenomena. 1992;58:77–94. [Google Scholar]
- 24.Kohler MJ, Lushington K, van den Heuvel CJ, Martin J, Pamula Y, Kennedy D. Adenotonsillectomy and neurocognitive deficits in children with sleep disordered breathing. PloS One. 2009;4:e7343. doi: 10.1371/journal.pone.0007343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumert M, Kohler M, Kabir M, et al. Altered cardio-respiratory response to spontaneous cortical arousals in children with upper airway obstruction. Sleep Med. 2011;12:230–8. doi: 10.1016/j.sleep.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Immanuel SA, Pamula Y, Kohler M, et al. Respiratory timing and variability during sleep in children with sleep-disordered breathing. J Appl Physiol. 2012;113:1635–42. doi: 10.1152/japplphysiol.00756.2012. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Molina G, Bialas P. Respiratory-cycle related analysis of the EEG-spectrum during sleep: A healthy population study. Proceedings of the 35th Annual International Conference of the IEEE in Engineering in Medicine and Biology Society (EMBC); 2013 July 3-7; Osaka, Japan. pp. 1940–1943. http://dx.doi.org/10.1109/EMBC.2013.6609928. [DOI] [PubMed] [Google Scholar]
- 28.Bušek P, Kemlink D. The influence of the respiratory cycle on the EEG. Physiol Res. 2005;54:327–33. [PubMed] [Google Scholar]
- 29.Cooper R, Hulme A. Changes of the EEG, intracranial pressure and other variables during sleep in patients with intracranial lesions. Electroencephalogr Clin Neurophysiol. 1969;27:12–22. doi: 10.1016/0013-4694(69)90104-7. [DOI] [PubMed] [Google Scholar]
- 30.Chervin RD, Malhotra RK, Burns JW. Respiratory cycle-related EEG changes during sleep reflect esophageal pressures. Sleep. 2008;31:1713–20. doi: 10.1093/sleep/31.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanhatalo S, Tallgren P, Becker C, et al. Scalp-recorded slow EEG responses generated in response to hemodynamic changes in the human brain. Clin Neurophysiol. 2003;114:1744–54. doi: 10.1016/s1388-2457(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 32.Walker BB, Walker JM. Phase relations between carotid pressure and ongoing electrocortical activity. Int J Psychophysiol. 1983;1:65–73. doi: 10.1016/0167-8760(83)90025-9. [DOI] [PubMed] [Google Scholar]
- 33.Yuan H, Zotev V, Phillips R, Bodurka J. Correlated slow fluctuations in respiration, EEG, and BOLD fMRI. NeuroImage. 2013;79:81–93. doi: 10.1016/j.neuroimage.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 34.Lorig TS, Schwartz GE, Herman KB, Lane RD. Brain and odor: II. EEG activity during nose and mouth breathing. Psychobiology. 1988;16:285–7. [Google Scholar]
- 35.Bell IR, Kline JP, Schwartz GE, Peterson JM. Quantitative EEG patterns during nose versus mouth inhalation of filtered room air in young adults with and without self-reported chemical odor intolerances. Int J Psychophysiol. 1998;28:23–35. doi: 10.1016/s0167-8760(97)00069-x. [DOI] [PubMed] [Google Scholar]
- 36.Haehner A, Gruenewald G, Dibenedetto M, Hummel T. Responses to olfactory and intranasal trigeminal stimuli: relation to the respiratory cycle. Neuroscience. 2011;175:178–83. doi: 10.1016/j.neuroscience.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Donzel-Raynaud C, Redolfi S, Arnulf I, Similowski T, Straus C. Abnormal respiratory-related evoked potentials in untreated awake patients with severe obstructive sleep apnoea syndrome. Clin Physiol Funct Imaging. 2009;29:10–7. doi: 10.1111/j.1475-097X.2008.00830.x. [DOI] [PubMed] [Google Scholar]
- 38.Stančák A, Jr, Kuna M. EEG changes during forced alternate nostril breathing. Int J Psychophysiol. 1994;18:75–9. doi: 10.1016/0167-8760(84)90017-5. [DOI] [PubMed] [Google Scholar]