Abstract
Study Objectives:
Obstructive sleep apnea (OSA) diagnosis using simplified methods such as portable sleep monitoring (PM) is only recommended in patients with a high pretest probability. The aim is to determine the diagnostic efficacy, consequent therapeutic decision-making, and costs of OSA diagnosis using polysomnography (PSG) versus three consecutive studies of PM in patients with mild to moderate suspicion of sleep apnea or with comorbidity that can mask OSA symptoms.
Design and Setting:
Randomized, blinded, crossover study of 3 nights of PM (3N-PM) versus PSG. The diagnostic efficacy was evaluated with receiver operating characteristic (ROC) curves. Therapeutic decisions to assess concordance between the two different approaches were performed by sleep physicians and respiratory physicians (staff and residents) using agreement level and kappa coefficient. The costs of each diagnostic strategy were considered.
Patients and Results:
Fifty-six patients were selected. Epworth Sleepiness Scale was 10.1 (5.3) points. Bland-Altman plot for apnea-hypopnea index (AHI) showed good agreement. ROC curves showed the best area under the curve in patients with PSG AHI ≥ 5 [0.955 (confidence interval = 0.862–0.993)]. For a PSG AHI ≥ 5, a PM AHI of 5 would effectively exclude and confirm OSA diagnosis. For a PSG AHI ≥ 15, a PM AHI ≥ 22 would confirm and PM AHI < 7 would exclude OSA. The best agreement of therapeutic decisions was achieved by the sleep medicine specialists (81.8%). The best cost-diagnostic efficacy was obtained by the 3N-PM.
Conclusions:
Three consecutive nights of portable monitoring at home evaluated by a qualified sleep specialist is useful for the management of patients without high pretest probability of obstructive sleep apnea or with comorbidities.
Clinical Trial Registration:
http://www.clinicaltrials.gov, registration number: NCT01820156
Citation:
Guerrero A, Embid C, Isetta V, Farre R, Duran-Cantolla J, Parra O, Barbé F, Montserrat JM, Masa JF. Management of sleep apnea without high pretest probability or with comorbidities by three nights of portable sleep monitoring. SLEEP 2014;37(8):1363-1373.
Keywords: obstructive sleep apnea, polysomnography, home respiratory polygraphy, comorbidities, CPAP
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder with a high prevalence,1,2 associated with significant morbidity and mortality3–7 and increased use of healthcare resources. OSA treatment reduces the health-related consequences.8–11 Standard laboratory polysomnography (PSG) is considered the gold standard for the diagnosis of OSA. The evaluation of patients with a clinical suspicion of OSA is usually undertaken in reference centers by physicians specialized in sleep medicine. In addition to being expensive, this procedure requires considerable technical expertise and is of limited availability in many areas. There currently is a large number of patients requiring a sleep test, resulting in considerable economic, social, and health problems.
Various strategies have been implemented to tackle this issue. One of these is the performance of type 3 portable sleep monitoring system at home (PM).12–20 However, this simplified procedure has only been demonstrated as being useful in patients with a high pretest probability of OSA.12,14,18,20 In patients without high pretest probability it is not recommended, mainly because of a lack of analysis of sleep efficiency and the number of arousals,21,22 variability in the apnea-hypopnea index (AHI),15,16 or even the first-night effect.23 Moreover, PM is also not recommended in disorders such as insomnia, fibromyalgia, anxiety, and depression, as well as clinical diseases that can mask OSA symptoms.
Because this group of patients currently comprises a large number of subjects, its management represents a huge challenge that could lead to important social, health, and economic repercussions. Therefore, a potential valuable approach to make a diagnosis in this population could be the use of simplified devices over 3 consecutive nights.
HYPOTHESIS AND OBJECTIVE
We hypothesize that performing at least three consecutive home studies with a simplified system could be as useful as a full PSG in establishing a cost-efficient diagnosis and reaching a decision about treatment in patients without high pretest possibility of OSA or with associated comorbidity. We carried out a randomized, blinded, crossover study of 3 nights of PM (3N-PM) versus standard laboratory PSG to determine the diagnostic efficacy, therapeutic decision-making, and the cost of the two procedures in this group of patients.
METHODS
Study Subjects
The study population consisted of subjects who were referred to the sleep unit of the Clínic Hospital, Barcelona, Spain, or the San Pedro de Alcantara Hospital, Cáceres, Spain, with a mild-moderate clinical suspicion of OSA or with significant comorbidity that induced frequent symptoms which mimicked those of OSA or could reduce sleep time.
The inclusion criteria were: (1) patients with snoring and/ or some observed apneas during sleep; (2) Epworth Sleepiness Scale less than 1524,25; (3) subjects with significant comorbidity with secondary daily symptoms (see Table 1)25; and (4) age between 18 and 75 y.
Table 1.
Significant comorbidities (comorbidities with significant every day symptoms)

The exclusion criteria were as follows: (1) high suspicion of sleep apnea (heavy snoring, breathing pauses, and somnolence that makes social life or working difficult, without any other causes of hypersomnia); (2) diagnosis of OSA; (3) heart disease that substantially impairs daily activities or with ejection fraction (EF) < 30%, resistant systemic hypertension,26 disabling stroke, unstable pulmonary disease; (4) suspicion of nonapneic sleep disorders such as narcolepsy, rapid eye movement behavior disorders, and restless leg syndrome; (5) psychophysical disability that would impede the application of the type 3 portable sleep monitoring system; and (6) lack of informed consent for the protocol approved by the ethics committees of the two centers.
Protocol
We compared 3N-PM with laboratory-standard PSG in randomized patients who met the inclusion and exclusion criteria. The subjects were studied on a random basis for 1 night in a sleep laboratory (standard PSG) and for 3 consecutive nights with PM at home. After the first test had started, the second test was scheduled to begin within the following 2 days. PSG and PM were scored separately and the technicians and physicians were blinded to any identifying information about the patients, as well as to any previous results (Figure 1 shows the design of the study).
Figure 1.
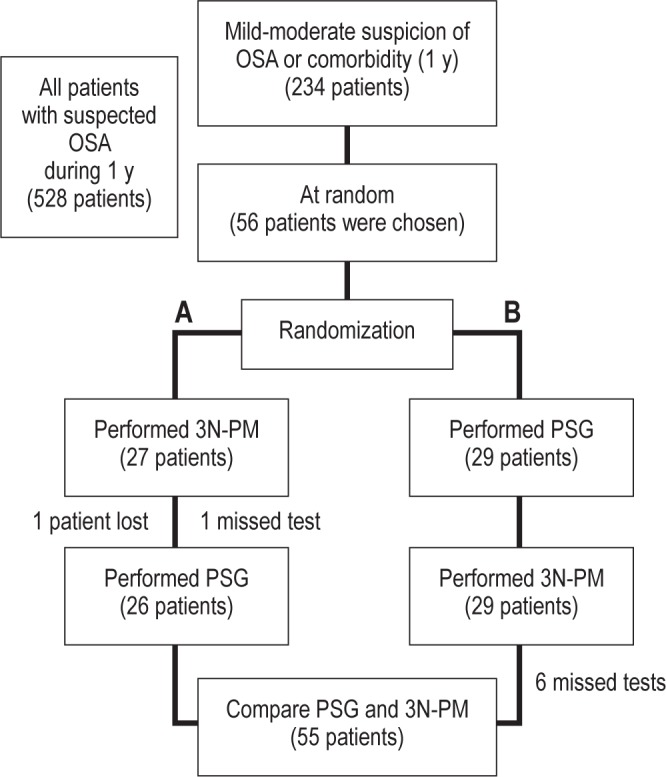
Flow chart of the patients during the study. In branch A, 27 patients we subjected to the 3 nights of type 3 portable monitoring at home (3N-PM) first. One patient was lost (during the 3 nights the oxymeter signal was not recorded), and one study was missed (during the first night the oxymeter signal was not recorded). The polysomnography (PSG) was then performed in 26 patients. In branch B, 29 patients received PSG first. When the 3N-PM were performed in this group, six studies were invalidated: two studies during night 1 (one recording of less than 180 min and another with no recording); one, during night 2 (no recording); and three during night 3 (two recording of less than 180 min and another unperformed by the patient).
Measurements
Clinical Data
Sex, age, weight, height, body mass index (BMI), waist-hip ratio, neck circumference, systolic and diastolic blood pressure, alcohol intake measured as ≤ 60 g/day or > 60 g/day, and tobacco consumption.
Significant Comorbidity
Cardiovascular, metabolic, or lung diseases, as well as diagnosis of insomnia, anxiety, depression, fibromyalgia, chronic fatigue syndrome, or psychiatric treatment.
Symptoms Related to OSA
Episodes of nocturnal choking, nocturia, morning headache, or morning tiredness. These data were collected in four degrees of intensity (never, sometimes, frequently, and always). Sleepiness was measured by the Epworth Sleepiness Scale and the American Sleep Disorders Association (ASDA) Sleepiness Scale.24,25,27
Other Questionnaires
(1) (Functional Outcomes of Sleep Questionnaire (FOSQ), which assesses the effect of excessive sleepiness on everyday multiple activities.28 (2) Euroqol-5D (EQ-5D), which is a standardized instrument to use as a measure of health outcome.29
Sleep Studies
Standard laboratory PSG (Somté PSG, Compumedics Limited 2006, Abbotsford, Victoria, Australia) was performed according to the technical specifications of the American Academy of Sleep Medicine (AASM).30 The recorded variables were electroencephalogram (EEG), with derivations F4-M1, C4-M1, and O2-M1; electro-oculogram (EOG); chin electromyogram (EMG); leg EMG; and electrocardiogram (ECG). Respiratory variables measured by linearized nasal pressure prongs and oronasal thermal flow waveform; respiratory effort signals measured by inductive bands that recorded ribcage and abdominal movements; oxygen saturation; body position and snoring. A type 3 portable sleep apnea testing was used to perform PM (Sleep&Go, Bitmed, Sibel S.A., Barcelona, Spain).31 Recorded variables were: flow measured by linearized nasal pressure prongs, thermal flow, body position, rib-cage and abdominal movements measured by inductive bands, and oxygen saturation. After a detailed explanation of the use of the PM device (setup and withdrawal) in the hospital setting, it was taken home and returned by the patient after three home studies.
A valid PSG or PM should have at least 180 recorded min. Moreover, a valid PM had to have at least 3 h of flow or bands and oximetry measurements for scoring. The PM time considered for scoring (PM scoring time) was defined as recording time minus the erratic breathing periods according to previously validated criteria.17 Mean values were obtained for the 3 nights of PM. If one study was considered not to be valid, this was removed and the mean values were obtained from the other 2 nights with PM. If two studies in the same patient were considered not to be valid, the patient was excluded.
The PSG and PM were scored manually, separately, and blinded by independent technicians. Sleep staging was performed using the standardized AASM criteria.30 The respiratory variables obtained from PM and PSG were scored according to the AASM criteria30: apnea was defined as a decrease in the peak signal excursion of ≈90% from the preevent baseline with a duration of ≥ 10 sec; and hypopnea was defined as a discernible reduction in the amplitude of the airflow signal (≈30% of preevent baseline) of at least 10 sec of duration, associated with an arousal and/or ≥ 3% oxygen desaturation from the preevent baseline. For PM, the apnea had the same definition and hypopnea was defined as a discernible reduction in the amplitude of the airflow signal (≈30% of pre-event baseline) of at least 10 sec of duration, associated with ≥ 3% oxygen desaturation.
Making the Therapeutic Decision
In addition to the real treatment decision of the physicians from the two sleep units, the other 15 reviewers (five sleep medicine specialists; five respiratory physicians; and five respiratory resident physicians who had been trained for at least 3 mo in a sleep laboratory) from the other five sleep laboratories in Spain, via a website showing clinical data from the patients and data from the sleep studies, took the therapeutic decisions. The increasing prevalence of OSA has led to establish networks between sleep reference and primary care centers. In our area, a respiratory physician with knowledge in sleep medicine acts as a link between both. Therefore, to improve management of the sleep apnea patients, we wanted to evaluate the therapeutic decision made among the different levels implicated. The reviewers chose one of two options: (1) continuous positive airway pressure (CPAP) treatment; (2) no CPAP treatment/ other therapeutic measures. Each patient was presented twice (with PSG or PM information), blinded and nonconsecutively. The criteria for recommending CPAP were an AHI between 5 and 30 events/h with significant symptoms or consequences or an AHI ≥ 30 events/h, taking less into account symptoms or consequences, according to the Spanish Sleep Network guidelines.32 Additionally, a simulation based on AASM criteria (CPAP would be recommended for both PSG and PM if the AHI score was ≥ 15 or between 5 and 15 with significant symptoms or consequences) was performed.33
Statistics
It was estimated that, after accepting an alpha risk of 0.05 and beta risk of 0.2 in a two-sided test, 55 subjects were needed for a minimum difference in AHI of 7.0 units to be recognized as statistically significant.19 The standard deviation was assumed to be 18 units.19 A dropout rate of 5% was anticipated.
Outcomes to be Studied and Statistical Analysis
The means of the data obtained from the 3N-PM were compared with PSG data. Student t-test and Bland-Altman plots were used to determine the agreement between the AHI measurements obtained by PSG and PM. Analysis of variance was used to assess the variability between each of the nights of PM. The efficacy of the diagnostic test was evaluated using sensitivity and specificity; positive and negative likelihood ratios [LR (+) and LR (−)]; and the percentage of patients with positive and negative diagnosis. The posttest probability of obtaining a true positive when the test was positive or negative was calculated, based on the pretest probability (prevalence of the disease) and the positive and negative LRs. Receiver operating characteristic (ROC) curves were plotted for the mean of the 3N-PM with the different polysomnographic AHI cutoff points (≥ 5, ≥ 10, ≥ 15, ≥ 30) for OSA diagnosis to determine the best ROC curve based on area under the curve (AUC) measurements.
Making the Therapeutic Decision
The reviewers were grouped as sleep medicine specialists, respiratory medicine physicians and resident physicians. A median of the therapeutic decisions of each group was obtained. Agreement level (100 minus the sum of false positives and negatives) and Cohen kappa coefficient was used to determine the agreement between therapeutic decisions. All the analyses were developed with the Statistical Package for Social Sciences (SPSS, 21.0 for Windows; SPSS Inc., Chicago, IL).
Cost Analysis
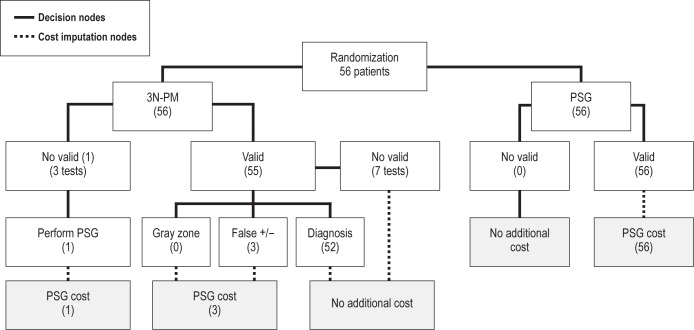
We considered the costs of each diagnostic strategy in an analysis of two equally effective alternatives.19 Figure 2 shows the flowchart used to calculate the two strategies' costs per patient for equal diagnostic efficacy.
Figure 2.
Costs of the each diagnostic strategy. The costs of each diagnostic strategy were considered in an analysis of two equally effective alternatives. In the 3 nights of type 3 portable monitoring at home (3N-PM) branch, only one patient was lost and had to undergo PSG to reach a diagnosis. Fifty-five patients were considered as having valid recordings, although seven missed tests were found in a single night, which did not influence the final diagnosis. In the polysomnography (PSG) branch all 56 tests were valid. For PSG, the test cost for equal diagnostic efficacy was the sum of the test cost and the cost of repeated PSGs because of no valid recordings. For PM, the test cost for equal diagnostic efficacy was the sum of the test cost and the following: the cost of PSG for one patient with no valid PM recordings, and the cost of three patients with false-positive and false-negative results (False +/−).
Test, Patient, and Total Costs for PSG and PM
The estimated test costs of PSG and PM were obtained from the financial department from the two hospitals involved. These costs included the following: personnel involved, equipment depreciation, and expendable material. In the case of PM, personnel costs included the time spent for patient's training on how to use the portable device, for device setup, cleaning, and data downloading, and for scoring three tests as well as reviewing and interpreting them. Patient costs were estimated according to the average cost for each patient of traveling from home to hospital and back by taxi. We considered a round trip to the hospital in the case of PSG and two round trips to pick up and return the PM device. To estimate the costs per patient, the test and patient costs were divided by the number of patients with a valid recording. To obtain the total costs of PSG and PM, we added the test and patient costs.
Test, Patient, and Total Costs for Equal Diagnostic Efficacy for PSG and PM
For PSG, the test cost for equal diagnostic efficacy was the sum of the test cost and the cost of repeated PSGs because of invalid recordings. For PM, the test cost for equal diagnostic efficacy was the sum of the test cost and the following: the cost of PSG for patients with invalid PM recordings; the costs of PSG in patients with indeterminate diagnostic results (“gray zone”) and false-positive and false-negative results. To calculate the patient costs for equal diagnostic efficacy, we also considered the transport costs because of repetition of tests. To calculate the costs per patient, these costs were divided by the number of patients who completed the trial. Total PSG and PM costs for equal diagnostic efficacy were obtained by adding up the test and patient costs.
RESULTS
General Data
Of 528 patients evaluated at the sleep laboratory during 1 y, 234 patients met the inclusion criteria, and of these 56 were selected at random and then the protocol was randomized (Figure 1). The 56 PSGs performed were valid. With the portable monitoring, 48 patients (85.7%) had three valid tests; 7 patients (12.5%) had two valid tests, and only in 1 patient (1.8%) were all tests invalid.
The clinical characteristics and symptoms of the patients are shown in Table 2. The Epworth Sleepiness Scale had a mean ± standard deviation (SD) of 10.1 (5.3) points. The ASDA sleepiness scale showed that 47 patients (83.9%) had mild sleepiness; 7 patients (12.5%), moderate sleepiness; and 2 patients (3.6%), severe sleepiness. Table 3 shows the health status measured by the EQ-5D descriptive system. Greater frequencies were found for problems in the dimension of pain/discomfort in 59.0% and in the dimension of anxiety/ depression in 57.2%. The visual analog scale (EQ-VAS) had a mean ± SD of 63.0 (22.6); the EQ-5D index, a mean ± SD of 0.6942 (0.2789); and the FOSQ questionnaire, a mean ± SD of 86.9 (24.3).
Table 2.
Clinical and anthropometric characteristics
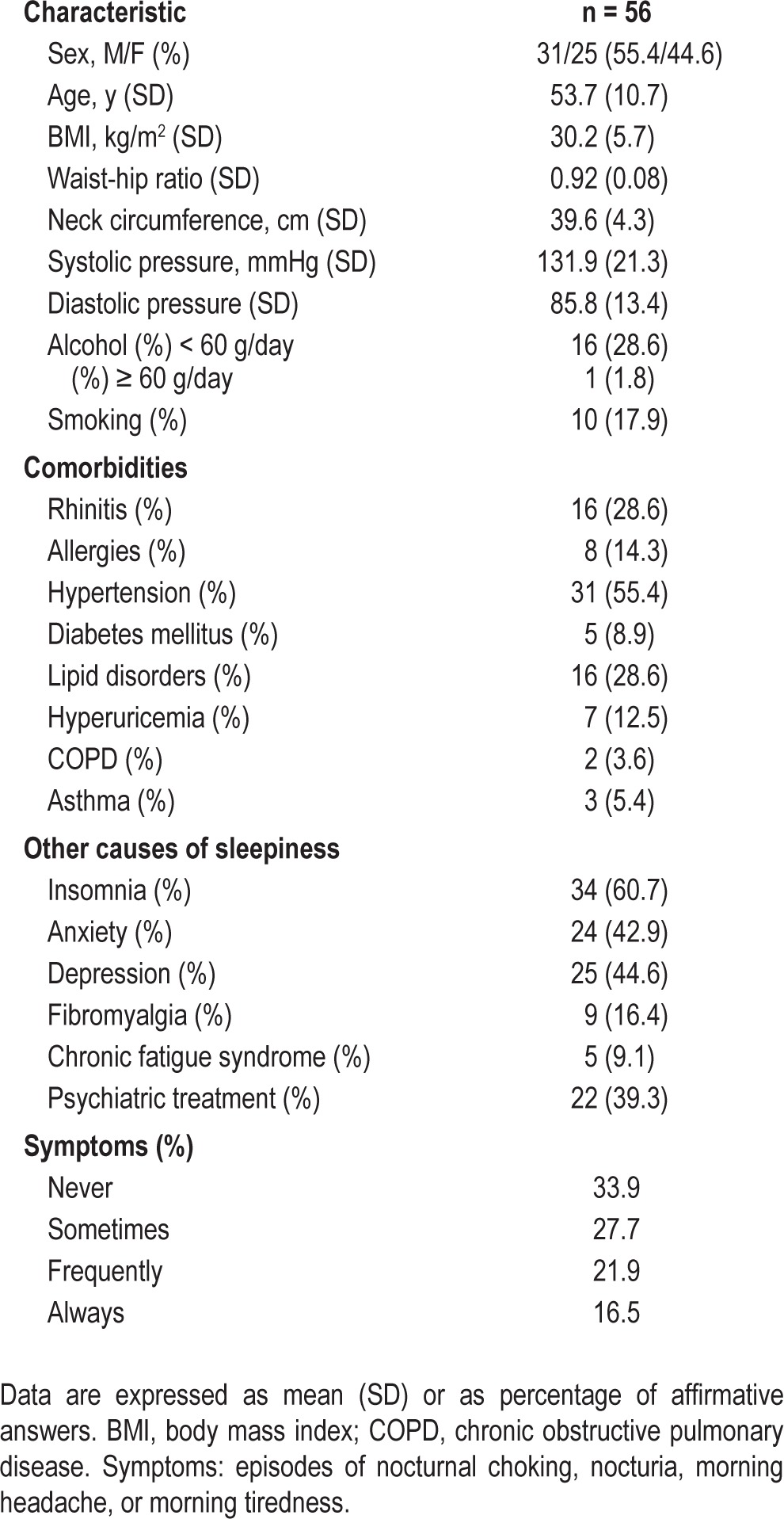
Table 3.
Euroqol 5D descriptive system

Diagnosis
Data from the sleep studies can be seen in Table 4. PSG and 3N-PM showed no statistically significant differences in the scoring time (PM) versus sleep time (PSG), body in back position, desaturation index 3%, and the percentage of the night with arterial oxygen saturation below 90%. Recording time, subjective sleep time, AHI, and AHI in back (supine posture) were statistically different (P < 0.05). After comparing nights 1, 2, and 3 and the mean of PM, no statistically significant differences were found between each night in the different parameters.
Table 4.
Comparison of the data obtained from polysomnography and 3 nights of type 3 portable monitoring at home
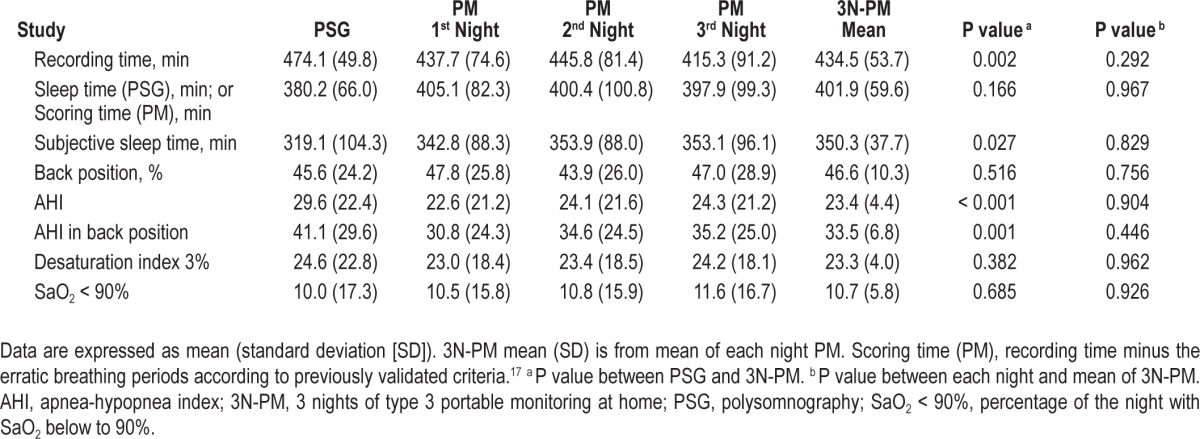
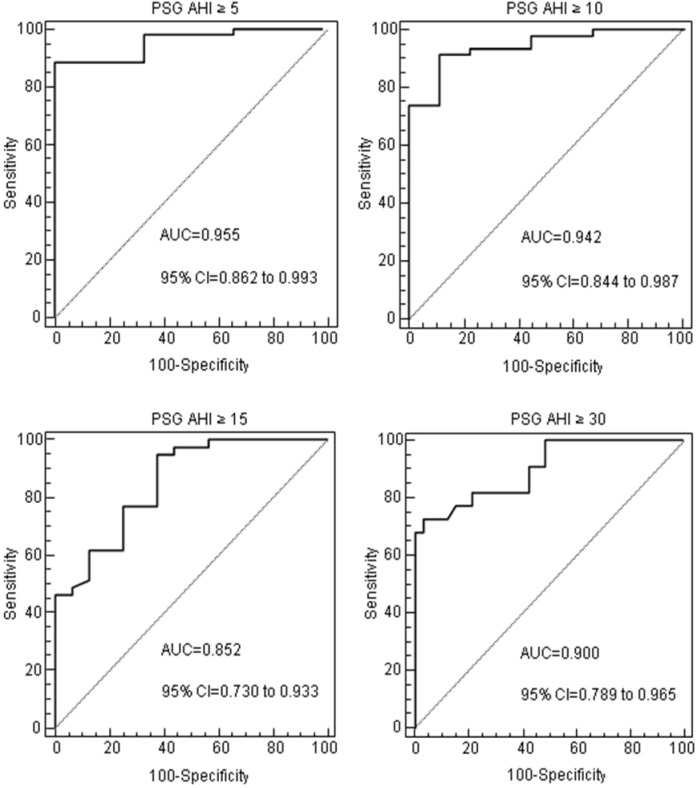
Bland-Altman plots (Figure 3) for AHI between PSG and 3N-PM show good agreement, especially in the lower AHI values. ROC curves for the 3N-PM with the different polysomnographic AHI cutoff points for OSA diagnosis are shown in Figure 4. At different cutoff points of ≥ 5, ≥ 10, ≥ 15, and ≥ 30, the best AUC was in patients with AHI ≥ 5, [0.955 (95% confidence interval [CI] = 0.862–0.993)]. All the AUCs were statistically significant at different cutoff points (P < 0.001).
Figure 3.
Bland-Altman plots. Bland-Altman plots. Mean of polysomnography (PSG) and 3 nights of type 3 portable monitoring at home (3N-PM) apnea-hypopnea index (AHI) versus difference in AHI between PSG and 3N-PM. Central lines represent mean values whereas upper and lower lines represent the agreement limits (mean ± 1.96 standard deviation of the differences).
Figure 4.
Receiver operating characteristic curves. Receiver operating characteristic (ROC) curves for the mean of the 3 nights of type 3 portable monitoring at home based on the four polysomnographic cutoff points of obstructive sleep apnea (≥ 5, ≥ 10, ≥ 15, and ≥ 30). AHI, apnea-hypopnea index; AUC, area under the curve; CI, confidence interval; PSG, polysomnography.
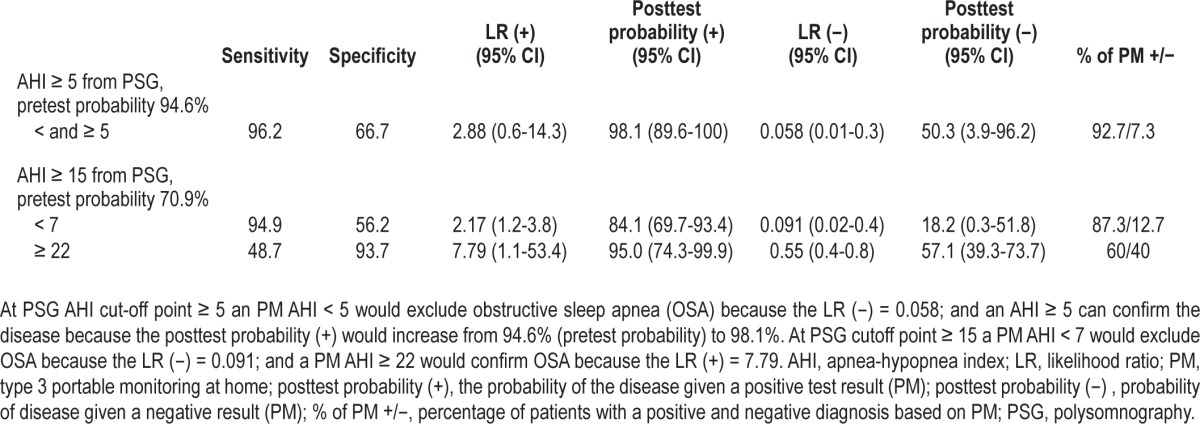
The diagnostic efficacy of PM was evaluated at PSG AHI cutoff point ≥ 5 and ≥ 15, because these were the best and worst AUCs, respectively. As shown in Table 5, at PSG AHI cutoff point ≥ 5 the prevalence of the disease (pretest probability) was 94.6%. A PM AHI < 5 would effectively exclude OSA diagnosis because the LR (−) was 0.058 and the posttest probability would decrease to 50.3%; a PM AHI ≥ 5 would confirm OSA because the positive posttest probability would increase to 98.1%. For a PSG AHI cutoff point ≥ 15, the prevalence (pretest probability) was 70.9%. The PM AHI cutoff point for confirming OSA would be ≥ 22 because the LR (+) was 7.79 and the posttest probability would increase to 95.0 %; a PM AHI < 7 would effectively exclude OSA because the LR (−) was 0.091 and the posttest probability would decrease to 18.2%.
Table 5.
Exclusion and confirming 3 nights of type 3 portable monitoring at home cutoff points with polysomnographic apnea-hypopnea index cutoff points ≥ 5 and ≥ 15
Making the Therapeutic Decision
The best concordance of the therapeutic decisions comparing PSG with 3N-PM was achieved by the sleep medicine specialists, as they had good strength of agreement with a level of 81.8% (95% CI = 69.7–89.8) and a kappa = 0.657 (SD = 0.096); whereas the respiratory medicine physicians had an agreement level of 72.7% (95% CI = 59.8–82.7) and kappa = 0.486 (SD = 0.089); and the resident physicians had an agreement level of 67.3% (95% CI = 54.1–78.2) and kappa = 0.427 (SD = 0.087). The main discrepancy in the therapeutic decision-making in the 55 patients was the disagreement of the sleep medicine specialists in 10 cases (18.2%). The main cause of this discrepancy was the differences in the AHI (recommendation of CPAP when evaluated by PSG [AHI ≥ 30], but not when evaluated by 3N-PM [AHI < 30]). The respiratory physicians disagreed in 15 cases (27.3%), again because of differences in the AHI, as well as discrepancies between symptoms and a low AHI. Finally, the residents had disagreements in 18 patients (32.7%), mainly for the two reasons mentioned previously, but also because of the presence of comorbidities. Interobserver variability between therapeutic decision-making by sleep medicine specialists had a kappa = 0.615 for PSG and kappa = 0.521 for 3N-PM; by respiratory medicine physicians, for PSG kappa = 0.517 and 3N-PM kappa = 0.474; and by resident physicians, PSG kappa = 0.579 and 3N-PM kappa = 0.422.
Simulation Following AASM Criteria
The results, based on the simulation with AASM criteria, were: the sleep medicine specialist therapeutic decisions based on 3N-PM, compared with PSG, had an agreement level of 81.8% (95% CI = 69.7–89.8); the respiratory medicine physicians therapeutic decisions had an agreement level of 81.8% (95% CI = 69.7–89.8); and the resident's therapeutic decisions had an agreement level of 72.7% (95% CI = 59.8–82.7).
These values were similar to the ones found following Spanish guidelines for recommend CPAP therapy; however, the agreement level of the decisions made by the respiratory medicine physicians was the same as the agreement level of the decisions made by the sleep medicine specialists.
Cost Analysis
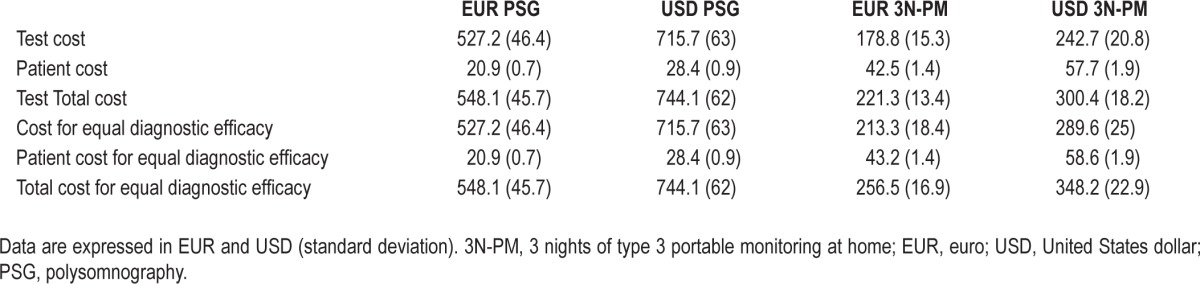
Table 6 shows the costs per patient of PSG and PM. The cost of PM was a third of the PSG cost. After adding the cost of patients transport, PM was still less than half as costly as PSG. To estimate the cost of PM for the same diagnostic efficacy of PSG, we considered the cutoff point of AHI ≥ 5, because this is considered the cutoff for the diagnosis of OSA. As shown in Figure 2, no patients fell in the “gray zone” because the best PM AHI cutoff points for effectively excluding and confirming OSA is 5. Three patients were in the zone of false-positive and false-negative tests. The PSG cost for equal diagnostic efficacy did not vary because of the absence of invalid results and consequent repetitions of tests. To achieve the same diagnostic efficacy as PSG, the 3-night PM strategy reached a cost of EUR 256.5 (16.9) [USD 348.2 (22.9)], still much lower than the PSG cost of EUR 548.1 (45.7) [USD 744.1 (62.0)].
Table 6.
Cost per patient of polysomnography and 3 nights of type 3 portable monitoring at home
DISCUSSION
The results of our study suggest that 3N-PM is useful for confirming or excluding the diagnosis of OSA in patients with a low pretest probability of sleep apnea or with comorbidities that can mimic or mask the symptoms of OSA or could impede an adequate sleep time. Specifically, on the basis of a PSG AHI cutoff ≥ 5, an AHI of 5 from 3N-PM would effectively exclude and confirm an OSA diagnosis. At this cutoff point the pretest probability is high (94.6%) and the potential increase to posttest probability is small (98.1%). Nonetheless, we considered this increase interesting because the CI is smaller, attaining 100%. This favorable increase in posttest is achieved by losing only 7% of the population (in 7% of patients a diagnosis cannot be made by portable monitoring at home [Table 5]). For a PSG AHI cutoff point ≥ 15, a 3N-PM AHI < 7 could exclude the disease and an AHI ≥ 22 would confirm the OSA diagnosis.
As mentioned previously, the prevalence of the disease has led to the development of various alternative techniques to diagnose OSA in patients with suspicion of OSA, such as single-channel devices or even oximetry.34 Overnight pulse oximetry has been used but it is not currently recommended because of the existence of multiple false negatives, especially in young patients, as well as false positives in patients with comorbidities and a lack of standardization in interpretation. Single-channel devices only analyze the airflow and there is a lack of information about electrophysiological variables and, more particularly, the respiratory effort.35 None of these devices have been recommended by the AASM for diagnosis. Type 3 devices, which record airflow, respiratory effort, and oxygen saturation, have been recommended by the AASM for unattended PM, but only in patients with high pretest suspicion of OSA. Patients with low suspicion, significant comorbidities, or other suspected sleep disorders are excluded.36
Several randomized studies comparing PM with PSG in all types of patients with suspicion of OSA have shown that the difference in mean AHI is slightly higher with PSG than PM.12,15,19,37 Masa et al.,19 in a large, multicenter, randomized study, showed that PM scoring produced lower AHIs than PSG (AHI difference -7.0); at the PSG cutoff point ≥ 5, an AHI < 5 from PM had a LR (−) of 0.07 and the pretest probability decreased from 90% to 39%; a PM AHI ≥ 10 had a LR (+) of 6.25 and the pretest probability increased from 90% to 98%. The most important finding of this study is its endorsement of the AASM recommendation. Other studies, albeit excluding patients with comorbidities, have been performed and produced similar outcomes.16,38 Regarding the AHI differences, these may be caused by factors such as hypopneas associated with arousals but without oxyhemoglobin desaturation (more frequent in mild-to-moderate patients),39 sleep time or variability of respiratory events. In contrast, as our study shows, the greater duration of subjective sleep time at home may lead to increased sleep quality and stability, resulting in fewer respiratory events. However, in our study no differences were found between sleep time (PSG) and scoring time (PM), probably because the PM analysis was performed according to a previous study in which we estimated sleep time on the basis of breathing patterns.17
Our study, as mentioned previously, deals with a different and specific question, the management of patients with mild or moderate OSA suspicion or with comorbidities. We believe that this is an important point because currently this population is probably more prevalent than severe patients, because of the various factors mentioned.40,41 We consider that our proposal represents a new step: a PM undertaken several times over a few days. Our data show that, in this population, performing three consecutive studies combined with management by a sleep physician achieves results similar to those described by other studies considering only high suspicion of OSA. Although there were no differences between each of the 3 nights in the PM obtained from a single night, we believe that 3 nights offer greater confidence and reliability, which is essential, especially in therapeutic decision-making. Furthermore, an analysis of only the first night of PM produced three failed tests (5.5%); in the ROC curves the AUCs for the cutoff points ≥ 5, ≥ 10, and ≥ 15 (0.966, 0.935, and 0.856, respectively) were similar to 3N-PM; however, the AUC for the cutoff point ≥ 30 was better with 3N-PM (0.900) than with the first night of PM (0.855).
There have been other studies that performed more than 1 night of testing, but the methodology was different and it did not deal with our specific population. Ayappa et al.15 evaluated 102 subjects. The PM covered 2 nights and the data were cumulative from these nights of recording, although a PSG was performed simultaneously with a respiratory polygraph. The simultaneous PSG and respiratory polygraph showed a slightly greater difference in AHI with the PSG of 0.5/h (95% CI: -1.0–2.0/h). The PSG versus PM showed a greater difference in AHI greater with the PSG, 4.1/h (95% CI: 0.8–7.3/h). Reichert et al.42 evaluated 45 patients for 1 night with simultaneous PSG and respiratory polygraphy and for 3 nights with respiratory polygraphy at home within 7 days (NovaSom QSG,” NovaSom, Inc. Maryland, United States). A clinical cutoff of AHI equal to 15 was used to compare in-laboratory PSG and an average respiratory polygraphy at home throughout all the nights. They found a sensitivity of 91% and a specificity of 83%. However, in this case the population of OSA suspicion was not selected. Other authors have performed studies that excluded patients with comorbidity.16,38 Therefore, the novelty of our study is the population studied, as well as the fact that the studies were performed at home.
The population of patients that we studied was with low-moderate pretest probability of OSA, so that PSG AHI cutoff point ≥ 5 had a prevalence of 94.6%; AHI cutoff point of ≥ 10 the prevalence decreases to 83.6%; AHI ≥ 15, prevalence of 70.9; and in patients with AHI ≥ 30 the prevalence was 40%. We believe that detecting typical high-probability groups of patients can be in some ways easy. However, patients with low-moderate-pretest possibility, especially if they have associated disease symptoms, can be turbid and mixed from those coming from the comorbidities. Because the symptoms are problematic to interpret in this type of patient, we consider the difficulty to interpret a simplified sleep study if it is performed just during 1 night. With more than 1 night of data we believe that the interpretation would be more accurate. However, our results should be taken with caution when considering a population with a lower prevalence of disease or reporting poor sleep time during PM.
Making the Therapeutic Decision
Only a few studies have evaluated the therapeutic decision-making by comparing in-laboratory full PSG versus PM. Parra et al.,12 in their work with 89 patients, considered the therapeutic decision of use or nonuse of CPAP on the basis of the following criteria: (1) documented OSA (AHI > 10) in the presence of clear clinical impairment; and (2) an AHI > 30, even with moderate clinical impairment. They achieved an 89% of agreement level in therapeutic decisions. Eighty-four percent of patients with a PSG diagnosis had OSA (mean AHI 34.3), whereas 82% of patients had a diagnosis of PM (mean AHI 31.8). Hernández et al.13 evaluated in 88 patients the agreement in choice of treatment made by a sleep physician in a reference center (PSG) and by a respiratory physician trained in sleep medicine in a nonreference center using respiratory polygraphy. The choice of treatment was: (1) no diagnosis of OSA, the patient is discharged from the hospital; (2) mild OSA, conservative treatment and clinical control; (3) moderate to severe OSA, begin CPAP treatment; and (4) other sleep disorders, or a need for full-night PSG. The clinical therapeutic decision taken with respiratory polygraphy agrees with the one taken with PSG in most cases. The main discordances in the choice of treatment were caused by discrepancies in AHI with mild symptoms, or no symptoms other than snoring. Masa et al.20 performed a large multicenter, randomized, blinded, clinical trial using PM and PSG as a gold standard in patients with suspected OSA. The same criteria as ours were used to recommend CPAP. They show a sensitivity of 73%, a specificity 77%, LR (+) of 3.53, LR (−) of 0.32, and an agreement level of 76%. Patients with higher PM AHI scores (≥ 30; 41% of the total sample) had a sensitivity of 94%, a specificity of 44%, and the agreement level was 91%. Masa et al. concluded that the PM-based therapeutic decision was adequate when the AHI was high. We have reached similar conclusions, but with a different population.
In our study, the therapeutic decision was made by sleep medicine specialists, respiratory medicine specialists, and resident physicians, choosing one of two options: CPAP treatment or no CPAP treatment/other therapeutic measures. The therapeutic decisions were more consistent when were made by sleep specialists (agreement level of 81.8% and kappa = 0.657) than by respiratory physicians (agreement level of 72.7% and kappa = 0.486) or residents (agreement level of 67.3% and kappa = 0.427). These findings are consistent with the AASM recommendations about the use of type 3 devices, which specify that a clinical evaluation should be made by a specialist in sleep medicine. However, our patient population had no high pretest probability of OSA or presented comorbidity.
A recent randomized, controlled study43 compared an ambulatory primary care-based management strategy versus standard care in patients who were screened for moderate to severe OSA. Sleep medicine specialists tended to provide more conservative measures or other types of treatment than non-specialists (28% versus 3%). In our study, the percentage of conservative measures recommended by a sleep specialist was 76.4%, compared with 61.8% by respiratory medicine physicians and 54.5% by residents. This is probably because the sleep medicine specialist tends to recommend non-CPAP treatment as the first option in young patients or those with few symptoms. We believe that the role of the sleep physician is crucial, especially in the population considered in our study.
Making the AASM guidelines simulation, the respiratory medicine physicians and the residents showed better results than using Spanish Sleep guidelines, probably because the possible lack of experience in interpreting the relevancy of clinical symptoms and consequences in the population with AHI scores between 15 and 30 was replaced with AASM guidelines because this could avoid variability. Additionally, this analysis was a simulation, and all patients with 3N-PM AHI greater than 15 (e.g., 15.5) were automatically recommended for CPAP treatment. However, during the study with the Spanish protocol, some patients with AHI values slightly above 30 (for example, 31) and moderate clinical symptoms or comorbidities could be recommended against CPAP treatment.
Costs
Few papers have addressed this topic but a clear possibility of potential cost-saving in this population has been demonstrated.12,14,19,44,45 In addition to the study by Masa et al.,19 (PSG, EUR 577; PM, EUR 333), Parra et al.12 showed that PM is three times more cost effective than in-laboratory full PSG. In our cost analysis we found that 3N-PM is less costly than full PSG for equal diagnostic efficacy [PSG, EUR 548.1 (45.7) USD 744.1 (62.0); 3N-PM, EUR 256.5 (16.9) USD 348.2 (22.9)]. These results are similar to those found in previous studies but it needs to be stressed once again that in our study we used only the type of patient for whom full PSG is normally recommended.
CONCLUSIONS
The value of our study lies in the fact that, to our knowledge, there are no previous clinical trials with portable sleep monitoring system at home that only include patients with low-moderate suspicion of OSA or comorbidity taking place during 3 consecutive nights; this offers a more reliable interpretation of the results. Therefore, in view of our results, 3N-PM in conjunction with a comprehensive evaluation by an experienced sleep medicine physician could be recommended in patients without high pretest probability of OSA or with coexisting causes of sleepiness.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was performed in Pulmonary Service, Clínic Hospital, UB-IDIBAPS, Barcelona and in the Hospital San Pedro de Alcantara, Caceres. Spain. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
Dr. Montserrat and Dr. Masa contributed equally to this work.
ABBREVIATIONS
- 3N-PM
three nights of type 3 portable monitoring at home
- 95% CI
confidence interval 95%
- AASM
American Academy Sleep Medicine
- AHI
apnea-hypopnea index
- ASDA
American Sleep Disorders Association
- AUC
area under the curve
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- EF
ejection fraction
- EQ-5D
Euroqol 5D
- EQ-VAS
Euroqol–Visual analogic scale
- EUR
euro (currency)
- FOSQ
Functional Outcomes of Sleep Questionnaire
- LR
likelihood ratio
- OSA
obstructive sleep apnea
- PM
type 3 portable monitoring at home
- PSG
polysomnography
- REM
rapid eye movement
- ROC
receptor operator characteristic
- SaO2
saturation level of oxygen in hemoglobin
- SaO2 < 90%
percentage of the night with SaO2 below to 90%
- SD
standard deviation
- USD
United States dollar
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apneahypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 3.Gasa M, Salord N, Fortuna AM, et al. Obstructive sleep apnoea and metabolic impairment in severe obesity. Eur Respir J. 2011;38:1089–091. doi: 10.1183/09031936.00198810. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186:909–16. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 5.Hedner J, Grote L, Bonsignore M, et al. The European Sleep Apnoea Database (ESADA)—Report from 22 European sleep laboratories. Eur Respir J. 2011;38:635–42. doi: 10.1183/09031936.00046710. [DOI] [PubMed] [Google Scholar]
- 6.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–4. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between Obstructive Sleep Apnea and Cancer Incidence in a Large Multicenter Spanish Cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Rio F, Alonso-Fernandez A, Armada E, et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168:1328–35. doi: 10.1016/j.ijcard.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: Continuous Positive Airway Pressure Improves Insulin Resistance in Patients with Sleep Apnea without Diabetes. Ann Am Thorac Soc. 2013;10:115–20. doi: 10.1513/AnnalsATS.201209-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra O, Garcia-Esclasans N, Montserrat JM, et al. Should patients with sleep apnoea/hypopnoea syndrome be diagnosed and managed on the basis of home sleep studies? Eur Respir J. 1997;10:1720–4. doi: 10.1183/09031936.97.10081720. [DOI] [PubMed] [Google Scholar]
- 13.Hernández L, Torrella M, Roger N, et al. Management of sleep apnea: concordance between nonreference and reference centers. Chest. 2007;132:1853–7. doi: 10.1378/chest.07-0250. [DOI] [PubMed] [Google Scholar]
- 14.Alonso Alvarez ML, Teran SJ, Cordero GJ, et al. [Reliability of home respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome: analysis of costs] Arch Bronconeumol. 2008;44:22–8. [PubMed] [Google Scholar]
- 15.Ayappa I, Norman RG, Seelall V, Rapoport DM. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med. 2008;4:26–37. [PMC free article] [PubMed] [Google Scholar]
- 16.Santos-Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–36. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrero A, Embid C, Farre R, et al. Sleep breathing flow characteristics as a sign for the detection of wakefulness in patients with sleep apnea. Respiration. 2010;80:495–9. doi: 10.1159/000264656. [DOI] [PubMed] [Google Scholar]
- 18.Kuna ST. Portable-monitor testing: an alternative strategy for managing patients with obstructive sleep apnea. Respir Care. 2010;55:1196–215. [PubMed] [Google Scholar]
- 19.Masa JF, Corral J, Pereira R, et al. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax. 2011;66:567–73. doi: 10.1136/thx.2010.152272. [DOI] [PubMed] [Google Scholar]
- 20.Masa JF, Corral J, Pereira R, et al. Therapeutic decision-making for sleep apnea and hypopnea syndrome using home respiratory polygraphy: a large multicentric study. Am J Respir Crit Care Med. 2011;184:946–71. doi: 10.1164/rccm.201103-0428OC. [DOI] [PubMed] [Google Scholar]
- 21.Punjabi NM, Aurora RN, Patil SP. Home sleep testing for obstructive sleep apnea: one night is enough! Chest. 2013;143:291–4. doi: 10.1378/chest.12-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohsenin V. Portable monitoring for obstructive sleep apnea: the horse is out of the barn-avoiding pitfalls. Am J Med. 2013;126:e1–3. doi: 10.1016/j.amjmed.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18:463–9. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest. 1993;103:30–6. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Lloberes P, Duran-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143–56. doi: 10.1016/j.arbres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, De BG, Dominiczak A, et al. [ESH/ESC 2007 Guidelines for the management of arterial hypertension] Rev Esp Cardiol. 2007;60:968–94. doi: 10.1157/13109650. [DOI] [PubMed] [Google Scholar]
- 27.American Sleep Disorders Association. Rochester, MN: American Sleep Disorders Association; 1997. International classification of sleep disorders, revised: Diagnostic and coding manual. [Google Scholar]
- 28.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 29.Herdman M, Badia X, Berra S. [EuroQol-5D: a simple alternative for measuring health-related quality of life in primary care] Aten Primaria. 2001;28:425–30. doi: 10.1016/S0212-6567(01)70406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2012. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. www.aasmnet.org. [Google Scholar]
- 31.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 32.Grupo Español de Sueño (GES) Consenso Nacional sobre el sindrome de apneas-hipopneas del sueno. Arch Bronconeumol. 2005;41:3–110. [Google Scholar]
- 33.Iber C, Ancoli-Israel S, Chesson AL, Jr., Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 34.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 36.Chiner E, Andreu AL, Sancho-Chust JN, Sanchez-de-la-Torre A, Barbe F. The use of ambulatory strategies for the diagnosis and treatment of obstructive sleep apnea in adults. Expert Rev Respir Med. 2013;7:259–73. doi: 10.1586/ers.13.19. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Diaz E, Quintana-Gallego E, Ruiz A, et al. Respiratory polygraphy with actigraphy in the diagnosis of sleep apnea-hypopnea syndrome. Chest. 2007;131:725–32. doi: 10.1378/chest.06-1604. [DOI] [PubMed] [Google Scholar]
- 38.Campbell AJ, Neill AM. Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res. 2011;20:207–13. doi: 10.1111/j.1365-2869.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 39.Nerfeldt P, Aoki F, Friberg D. Polygraphy vs. polysomnography: missing osas in symptomatic snorers-a reminder for clinicians. Sleep Breath. 2013 doi: 10.1007/s11325-013-0884-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Bixler EO, Vgontzas AN, Ten HT, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 41.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 42.Reichert JA, Bloch DA, Cundiff E, Votteri BA. Comparison of the NovaSom QSG, a new sleep apnea home-diagnostic system, and polysomnography. Sleep Med. 2003;4:213–8. doi: 10.1016/s1389-9457(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 43.Chai-Coetzer CL, Antic NA, Rowland LS, et al. Primary care vs specialist sleep center management of obstructive sleep apnea and daytime sleepiness and quality of life: a randomized trial. JAMA. 2013;309:997–1004. doi: 10.1001/jama.2013.1823. [DOI] [PubMed] [Google Scholar]
- 44.Simon S, Collop N. Latest advances in sleep medicine: obstructive sleep apnea. Chest. 2012;142:1645–51. doi: 10.1378/chest.12-2391. [DOI] [PubMed] [Google Scholar]
- 45.Usmani ZA, Chai-Coetzer CL, Antic NA, McEvoy RD. Obstructive sleep apnoea in adults. Postgrad Med J. 2013;89:148–56. doi: 10.1136/postgradmedj-2012-131340. [DOI] [PubMed] [Google Scholar]