Abstract
Study Objectives:
To analyze the electroencephalographic (EEG) spectral content in untreated patients with restless legs syndrome (RLS) during the sleep onset period (SOP) and during the quiet wakefulness preceding sleep, in order to test the hypothesis that a state of hyperarousal might be present during the SOP with RLS.
Setting:
Sleep Research Centre.
Patients:
Twenty-seven untreated consecutive patients with RLS (mean age = 53.6 y), 11 untreated consecutive patients with primary insomnia (mean age = 58.9 y), and 14 normal controls (mean age = 50.3 y).
Methods:
SOP was defined as the 10-min period centered with the occurrence of the first sleep spindle in the EEG, and then subdivided into SOP-1 (period of 5 min before the first spindle) and SOP-2 (period of 5 min following). Leg movements occurring during SOP were counted and used as a covariate in the statistical analysis. Also, one period of 1 min of artifact-free quiet wakefulness after lights off was identified. EEG spectral analysis was run during these periods using the C3/A2 or C4/A1 channel.
Measurements and Results:
Increased EEG alpha and beta bands and/or beta/delta ratio in RLS versus normal controls, during both wakefulness preceding sleep and SOP (both parts SOP-1 and SOP-2) were found, which were, however, smaller than the increases found in patients with insomnia.
Conclusion:
The results of this study support the hypothesis of the presence of a state of hyperarousal in restless legs syndrome (RLS) during the sleep onset period. Treatment for RLS might need to take these findings into consideration.
Citation:
Ferri R, Cosentino FI, Manconi M, Rundo F, Bruni O, Zucconi M. Increased electroencephalographic high frequencies during the sleep onset period in patients with restless legs syndrome. SLEEP 2014;37(8):1375-1381.
Keywords: beta band, hyperarousal, insomnia, restless legs syndrome, sleep onset period, spectral EEG analysis
INTRODUCTION
Patients affected by restless legs syndrome (RLS) report, in approximately 70% of cases,1 insomnia characterized by difficulty falling asleep, because of the unpleasant sensations in the limbs and the urge to move them.2 The similarities between RLS and insomnia go beyond the simple initiating sleep problems and extend to more than three awakenings after sleep onset in approximately 60% of patients1 and, more in general, to increased sleep instability.3,4 Moreover, dopamine agonists, which greatly decrease unpleasant sensations and the urge to move the limbs at bedtime, seem to exert only a minor effect on sleep architecture and virtually no effect on nonrapid eye movement (NREM) sleep instability3 and arousals,5 at least in the acute period. This finding is in line with frequent reports in clinical practice of persisting insomnia in patients with RLS otherwise successfully treated with dopamine agonists and despite the complete resolution of sensory symptoms at bedtime and the great reduction of periodic limb movements during sleep (PLMS).6
The parallelism between RLS and insomnia is far from being sufficiently understood and several aspects need to be clarified. Among these aspects, the demonstration of a state of hyperarousal in RLS has never been specifically investigated. There is solid evidence, in the literature, that primary insomnia is associated with increased arousal level, as indicated by increased high-frequency electroencephalographic (EEG) activation, abnormal hormone secretion, increased metabolic activation (whole body and brain), and elevated heart rate and sympathetic nervous system activation during sleep. The term hyperarousal refers to this complex set of abnormalities.7,8 In particular, regarding sleep EEG spectral analysis, the most frequent finding in insomnia has been that of an increase in high-frequency bands, such as the beta band.9 On the contrary, previous data seem to show that in RLS sleep EEG changes might be associated essentially with the presence of leg movements (LMs) during sleep.10 Also, patients with insomnia can present frequent LMs during sleep11; however, this has not always been controlled for in insomnia studies because polysomnographic recordings have not always been performed and, when they have been carried out, often have not included tibialis anterior electromyographic (EMG) channels. However, insomnia associated with PLMS cannot be defined as primary insomnia because it satisfies the criteria for periodic limb movement disorder,12 even if a causal relationship between insomnia/ hypersomnia and PLMS has not been demonstrated.
For these reasons, we hypothesized that a state of hyper-arousal might be present at the time of the sleep onset period (SOP) in RLS, when its subjective clinical manifestations are maximal. Thus, the aim of this study was to analyze the EEG spectral content in untreated patients with RLS during SOP and during the quiet wakefulness preceding sleep and to compare the results with those obtained from normal controls and untreated patients with insomnia, while controlling for the presence of LMs.
SUBJECTS AND METHODS
Subjects
Twenty-seven untreated consecutive patients affected by idiopathic RLS were included in this study (mean age = 53.6 y, standard deviation [SD] = 14.90; 15 males and 12 females). The patients were recruited at the Sleep Research Centre of the Department of Neurology IC, Oasi Institute, Troina, Italy. In agreement with the International RLS Study Group,13 the minimum criteria accepted for the diagnosis of RLS were: (1) an urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the limbs; (2) the urge to move or unpleasant sensations that begin or worsen during periods of rest or inactivity such as lying or sitting; (3) the urge to move or unpleasant sensations that are partially or totally relieved by movement, such as walking or stretching; and (4) the urge to move or unpleasant sensations that are worse in the evening or night than during the day or only occur in the evening or night. Routine blood tests (including serum iron and ferritin) and neurophysiological investigation (electromyography [EMG] and electroneurography of the lower limbs) were also normal.
Also, 11 consecutive untreated subjects affected by chronic primary insomnia (mean age = 58.9 y, SD = 13.40; 5 males and 6 females), referred to the same sleep center, were included in the study. Diagnosis of primary insomnia was established by applying the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria.14 Patients with secondary insomnia, such as insomnia resulting from mental or medical disorders, inadequate sleep hygiene, or drugs or substance abuse, were excluded. Subjects with a history of RLS, according to the standard international criteria,13 or of other sleep disorders were excluded.
The sleep respiratory pattern of each patient (RLS and insomnia) was assessed by means of oral and nasal airflow (thermistor and/or nasal pressure cannula), thoracic and abdominal respiratory effort (strain gauge), and oxygen saturation (pulse oximetry), in a previous recording (within 1 w) or during this study recording; patients with an apnea/hypopnea index greater than 5 were not included. Neurological examination resulted unremarkable in all patients who were free of medication for at least 3 w before polysomnography.
Fourteen normal subjects (seven males and seven females, mean age = 50.3 y, SD = 15.83) were also included in the study and used as a control group. Control subjects were screened to exclude those with any current or prior symptoms suggestive of RLS, by using the same minimal criteria set by the International RLS Study Group for the diagnosis of RLS13 or of insomnia and had to be in general good health; they were excluded from the study if any of the following were present: diagnosis of any other significant sleep disorder(s), major mental illness including any indications of cognitive problems as determined by history, any history of neuroleptic-induced akathisia, or use of any neuroleptic agent in the past year. All controls were drug free.
This study was approved by the local ethics committee and all subjects provided informed consent before entering the study.
Polygraphic Sleep Recording
Each subject underwent a polysomnographic full night recording, after an adaptation night, carried out in a standard sound-attenuated (noise level to a maximum of 30 dB nHL) sleep laboratory. Subjects were not allowed to have beverages containing caffeine during the afternoon preceding the recording and were allowed to sleep until their spontaneous awakening in the morning.
The following parameters were included in the polysomnographic study: EEG (at least three channels, one frontal, one central, and one occipital, referred to the contralateral earlobe); electrooculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to A1), EMG of the submentalis muscle, EMG of the right and left tibialis anterior muscles (bipolar derivations with two electrodes placed 3 cm apart on the belly of the anterior tibialis muscle of each leg, impedance was kept less than 10 KΩ), and electrocardiography (ECG; one derivation). Sleep signals were sampled at 128 Hz and stored on hard disk in European data format.
Sleep stages were scored following standard criteria15 on 30-sec epochs by means of the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy). LMs during sleep were detected and marked.16
Spectral EEG analysis
For this study we defined SOP as the 10-min period centered with the occurrence of the first sleep spindle in the EEG, taken as indicator of sleep onset.17 SOP was then subdivided into SOP-1 (period of 5 min before the first spindle) and SOP-2 (period of 5 min following the appearance of the first sleep spindle). LMs occurring during SOP were counted. Moreover, we also identified one period of 1 min of artifact-free quiet wakefulness after lights off.
The EEG channel used for spectral analysis was C3/A2 or C4/A1 of all recordings; this derivation can be considered as a good representative of the whole scalp during SOP because it has been reported that, during the first 5 min of sleep, the time course of the different EEG bands is similar in frontal, central and occipital derivations.18
The signal, sampled at 128 Hz and digitally prefiltered at 0.1−35 Hz, was subdivided into 4-sec miniepochs, for a total of 165 miniepochs (15 during wakefulness, 75 during SOP-1, and 75 during SOP-2) per subject. Miniepochs containing muscle or other technical artifacts were carefully excluded from the analysis (on average 2.5 miniepochs per subject); all the remaining miniepochs of the 1-min wakefulness period and all artifact-free sleep miniepochs of SOP-1 and SOP-2 were used for the computation of power spectra. Power spectra were calculated for each miniepoch using the sleep analysis software Hypnolab 1.2, after Welch windowing, in order to minimize the truncation error and reduce spectral leakage by suppressing sidelobes,19 by means of the fast Fourier transform. The power spectrum was calculated for frequencies between 0.5 and 32 Hz with a frequency step of 0.25 Hz. The average absolute power for the different EEG bands (delta 0.5−3.75 Hz; theta 4.0−7.75 Hz; alpha 8−11.5 Hz; sigma 11.75−14.75 Hz; beta 15−32 Hz) and the ratio between the beta and delta bands were obtained for each condition: wakefulness (excluding sigma), SOP-1, and SOP-2.
Statistical Analysis
The comparison of age, number of LMs during SOP, sleep architecture parameters, and wake EEG spectral band power was carried out by analysis of variance (ANOVA), followed by post hoc comparisons (least significant difference test). Because of the different number of LMs found during SOP, comparisons between different subject groups were carried out using analysis of covariance (ANCOVA), with number of LMs used as a covariate. Also, ANCOVA was followed by post hoc comparisons. The chi-square test was used to compare the sex composition of the groups. Differences were considered as significant when they reached a P < 0.05 level. The data analysis software system STATISTICA (StatSoft, Inc. 2004, Tulsa, OK, version 6. www.statsoft.com) was used for statistical analysis.
RESULTS
The age of the three groups was not statistically different (F2,49 = 1.056, NS), as well as their sex composition (chi-square = 0.348, NS). On the contrary, the number of LMs during SOP was significantly different between the groups (F2,49 = 3.547, P < 0.035) with patients having RLS showing values (mean ± SD = 6.4 ± 8.85) significantly higher than both insomnia patients (mean ± SD = 1.4 ± 3.88, P < 0.042) and normal controls (mean ± SD = 1.4 ± 2.21, P < 0.03).
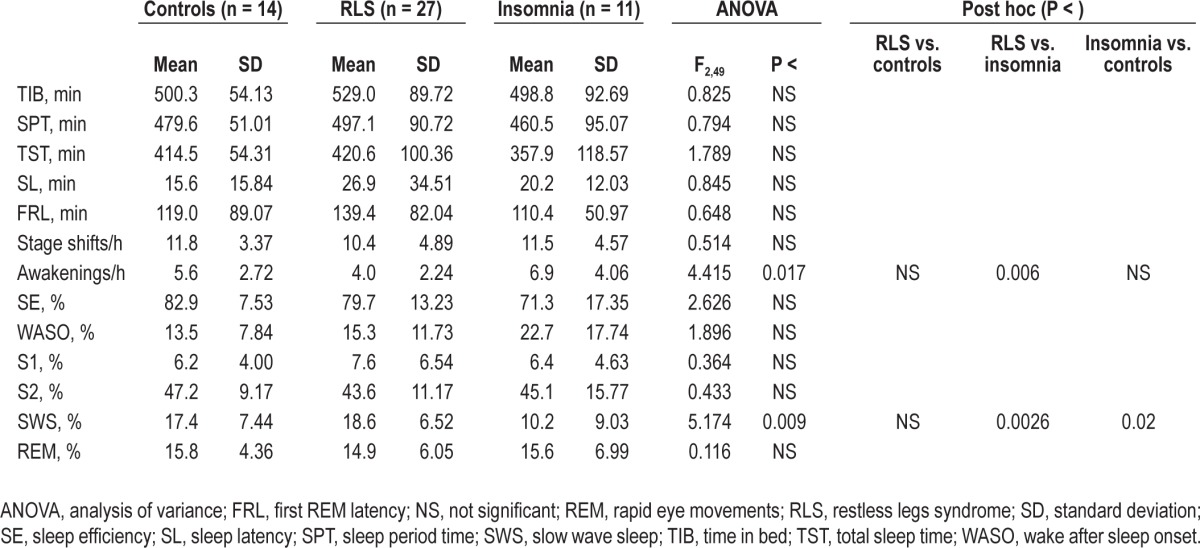
Table 1 reports the comparison between sleep architecture parameters in normal controls and in the two groups of patients. Even if some small differences between patients with RLS and normal controls can be seen in this table, none of the differences between these two groups was statistically significant; on the other side, patients with insomnia had a significantly higher number of awakenings and a lower percentage of slow wave sleep than patients with RLS.
Table 1.
Comparison between sleep architecture parameters in normal controls and in the two groups of patients
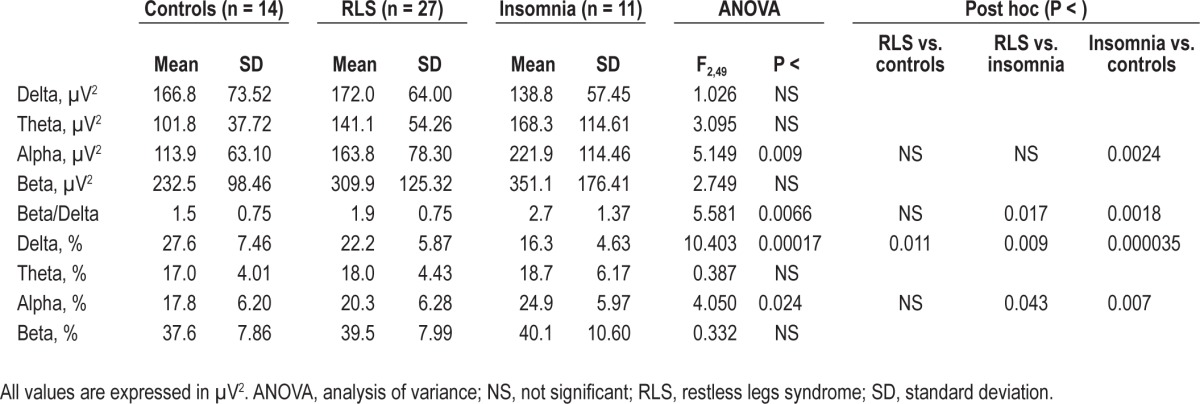
The comparison between the results of the spectral EEG analysis during quiet wakefulness preceding sleep in normal controls and in the two groups of patients is shown in Table 2. In this analysis, the theta, alpha, and beta band absolute power, as well as the beta/delta ratio, show the same incremental trend from normal controls, patients with RLS, and patients with insomnia; however, statistical significance was reached only for the alpha band and the beta/delta ratio. Delta band relative power values showed, conversely, a significant decrease from normal controls to patients with RLS and from these to patients with insomnia, whereas alpha band relative power showed the opposite significant trend.
Table 2.
Comparison between the results of the spectral electroencephalographic analysis during quiet wakefulness preceding sleep in normal controls and in the two groups of patients
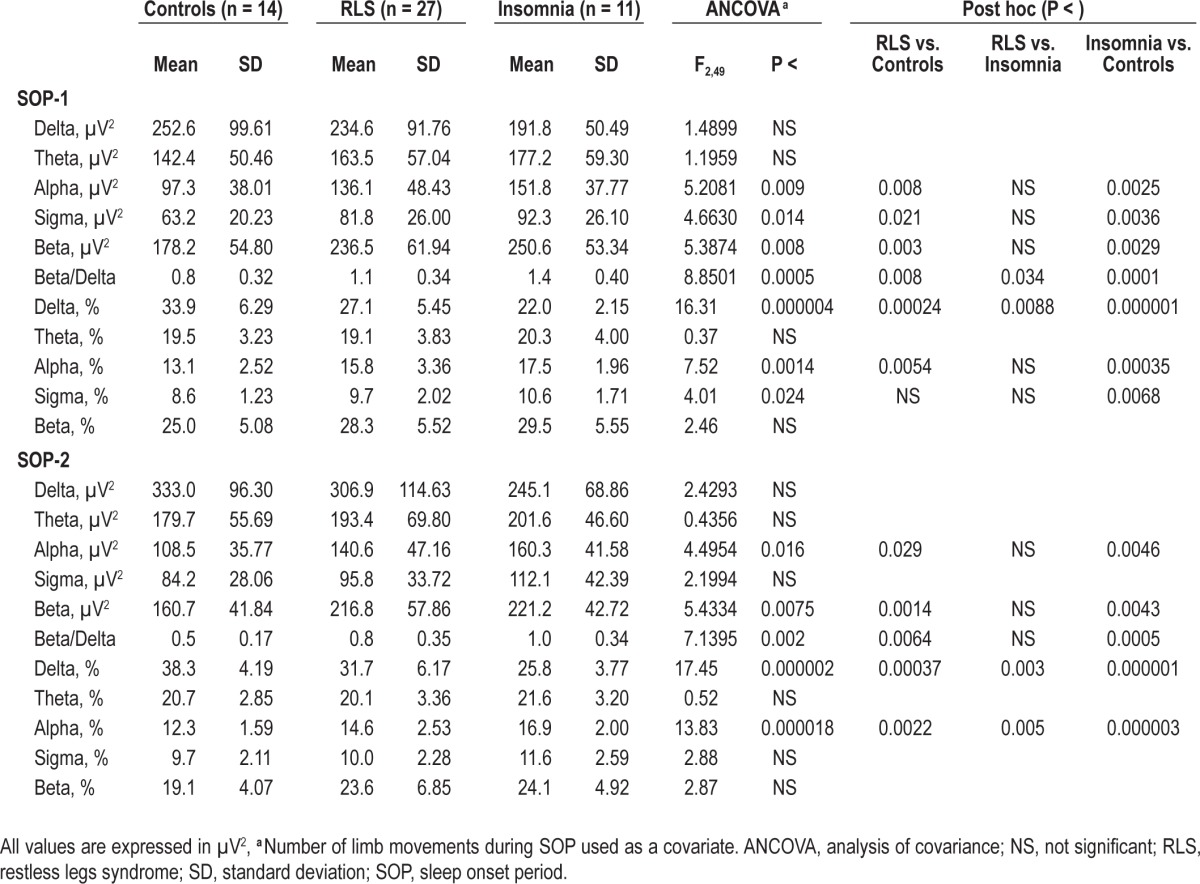
In Table 3, the comparison between the results of the spectral EEG analysis during SOP-1 and SOP-2 is shown. In this comparison, patients with RLS had significantly higher values than normal controls in the alpha, sigma, and beta bands and the beta/delta ratio, during SOP-1 and in the alpha and beta bands, and the beta/delta ratio, during SOP-2. The same comparisons between patients with RLS and insomnia did not disclose significant differences, with the exception of the beta/ delta ratio during SOP-1, which was higher in the insomnia group. Regarding relative power values, the delta band showed, similar to wakefulness, a significant decrease from normal controls to patients with RLS and from these patients to patients with insomnia, during both SOP-1 and SOP-2. The alpha band relative power showed a significant trend opposite to that of the delta band. In addition, the sigma band had a trend similar to that of the alpha band, reaching statistical significance during SOP-2.
Table 3.
Comparison between the results of the spectral electroencephalographic analysis during the sleep onset period in normal controls and in the two groups of patients
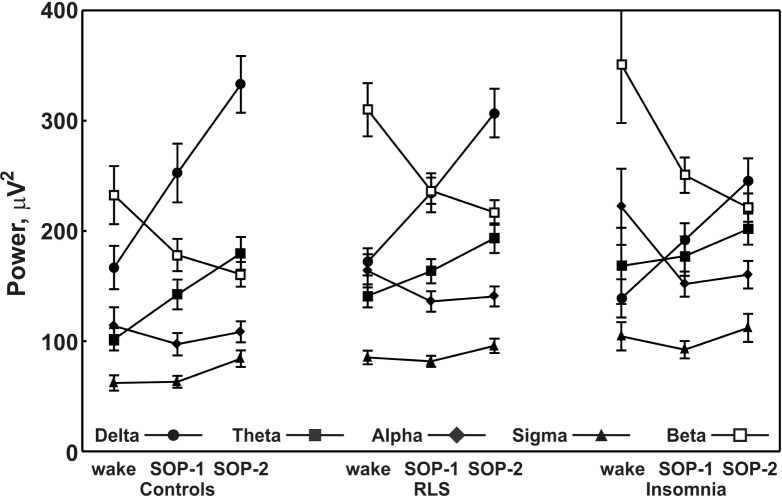
Finally, Figure 1 shows the changes observed in the power of each EEG frequency band from wakefulness to SOP-1 and then to SOP-2 in the three groups of subjects. It is clear from this figure that delta and theta bands show a progressive increase in all groups, whereas sigma only increases in SOP-2; alpha shows a clear decrease between wakefulness and SOP-1 and beta shows a progressive decrease. Overall, the trends are very similar in all groups.
Figure 1.
Changes in power of each electroencephalographic frequency band from wakefulness to sleep onset in the groups of subjects. RLS, restless legs syndrome; SOP, sleep onset period.
DISCUSSION
The results of this study seem to support our initial hypothesis of the presence of a state of hyperarousal in RLS during the SOP. This is confirmed by the general finding of increased EEG alpha and beta bands and/or beta/delta ratio in patients with RLS versus normal controls, during both wakefulness preceding sleep and SOP (both parts SOP-1 and SOP-2). The presence of increased EEG activity in the high-frequency range is probably not sufficient to demonstrate definitively the presence of hyperarousal in these patients. It would also be better to demonstrate the presence of other abnormalities such as abnormal hormone secretion, increased metabolic activation, and elevated heart rate and sympathetic nervous system activation during sleep.7,8 It is important to consider that enhanced nocturnal cortisol excretion has been reported earlier in RLS, demonstrating nocturnal hypothalamic-pituitary-adrenal system overactivity.20
It is remarkable that the findings in RLS and insomnia were somewhat similar, but also some differences existed, with patients with RLS generally showing intermediate values between normal controls (lowest) and insomnia (highest). It is also important to note that the results were not influenced by sleep latency, which was higher in patients with RLS than in those with insomnia; it is not surprising to find relatively mild polysomnographic abnormalities in patients with insomnia given their frequent sleep-state misperception.21 In RLS, this might reflect different intrinsic mechanisms that are not linked to the simple presence of LM-related arousals, which were significantly more frequent in patients with RLS, and an attempt was made to control for their presence, with the statistical analysis. It is also interesting to note that these results bear some resemblance with those of a recent study in which the EEG power of the beta band (18-29.75 Hz) was found to be significantly lower in sleep onset insomnia than in sleep maintenance insomnia.22 Thus, it is possible that hyperarousal is a stronger mechanism in sleep maintenance insomnia than in sleep onset insomnia; our patients with insomnia had both sleep onset and maintenance problems but sleep onset insomnia was predominant in our patients with RLS. We must acknowledge, in this respect, a limitation of our study because we did not collect a subjective report of sleep from the patients recruited.
The mechanisms supporting a hypothetical hyperarousal state during SOP in RLS, in addition to the presence of LMs, might be sought in different studies reported in the previous literature. First, a significant increase in NREM sleep instability has been reported in RLS,3 based on an increase in arousal-related transient events during sleep. This increase is not modified by the administration of dopamine agonists,3 which only decrease the number of LMs, but is significantly reduced by clonazepam,23 ineffective on LMs. It is interesting to note that a significantly enhanced nocturnal cortisol excretion has been reported in RLS, demonstrating nocturnal hypothalamic-pituitary-adrenal system overactivity.20 In the same study, nocturnal cortisol release showed a (weak but significant) positive correlation with the percentage of sleep stage 1 and a negative correlation with the percentage of sleep stage 2, but not with PLMS or visually scored arousals. Thus, it seems that in RLS there is an arousal system alteration that is largely independent from the occurrence of LMs, and also the results of the current EEG study support this view. As additional support for this finding, it has been suggested that RLS may benefit from cognitive behavioral therapy,24 which is thought (but not yet demonstrated) to act also by controlling the hyper-arousal state in insomnia.25,26
It is not known if this arousal system abnormality has the same circadian distribution as the sensory-motor symptoms and signs of RLS and it cannot be excluded that it can be detected all day long. Several reports have been published indicating the presence of altered (increased) central nervous system excitability in RLS, detected by means of transcranial magnetic stimulation that has been run during the daytime.27–29 However, it has also been reported that transcranial magnetic stimulation abnormalities show a circadian distribution pattern30 and that they can be reversed by dopamine agonist treatment.31–33 The focus of these studies has been intracortical inhibitory/excitatory mechanisms, but the arousal system has a more complex pathophysiology involving several subcortical structures34; additionally, an abnormal spinal excitability is believed to underline most of the RLS symptomatology.35,36 In this context, it is not trivial that an increased motor spinal cord excitability was believed to be at the basis of subclinical but significant changes in daytime muscle activation patterns during gait in patients with RLS.37
Although not universally accepted, the most frequently mentioned hypothesis for the mechanism of RLS concerns a dysfunction of the inhibitory descendent dopaminergic pathway projecting from hypothalamus (A11 area) to different gray matter spinal cord structures such as the ventral and dorsal horns, and the intermediolateral columns.35 The intermediolateral column contains the preganglional neurons of the sympathetic output and presents a high density of D2-like dopaminergic receptors. In RLS, a disinhibition of these autonomic spinal nuclei may enhance the sympathetic activity, which might sustain a state of hyperarousal also evident at the brain cortical level. If a hyperarousal state is present in RLS, this should be reflected also by changes in the autonomic system balance. It has been reported that spectral analysis of heart rate variability performed during epochs without LMs is not different from that of normal controls, but the heart rate response to the occurrence of LMs is enhanced in RLS, and treatment with a dopamine agonist was able to reduce such an exaggerated response.38 It is important to consider that, in the aforementioned study, LM-free epochs were most likely extracted from the second part of the night, when LMs are much less frequent than during the first part.39 If hyperarousal in RLS is under the same circadian modulation of RLS symptoms, it is possible that no differences were detected in heart rate variability because of the time of night from which the epochs were extracted. On the contrary, it is likely that LM-related heart rate changes were evaluated most often during the first part of the night, when LMs mostly occur. Differently from arousal-related transients, which were not modified by a dopamine agonist,3 LM-related heart rate changes might be modified by dopamine agonists because of their lower (subcortical) nature.
Finally, recent findings provide an additional support to the hypothesis of a hyperarousal state in RLS; thalamic glutamate/ glutamine has been found to be increased in RLS and related to disturbed sleep, but not to LMs.40 Also, our results, together with those on NREM sleep instability,3,23 point to a scarce relationship of LMs with arousals. Thus, in agreement with the views of Allen et al.,40 it is possible to hypothesize the existence of different system involvement in RLS and the glutamate system might be a better alternative to dopamine to explain increased arousals and sleep instability. The obvious consequence of this view is that a better treatment for RLS might need to take into consideration these findings that might constitute the rationale for a combined therapy for RLS, involving the dopamine and the glutamate systems.23,40
DISCLOSURE STATEMENT
This was not an industry supported study. This work was performed at the Sleep Research Centre, Department of Neurology IC, Oasi Institute (IRCCS), Troina, Italy. This work was partially supported by the Italian Ministry of Health (“Ricerca Corrente”). Dr. Ferri has consulted for Merck & Co., Sapio Life, and ATES Medica Device and has been a paid speaker for UCB Pharma. Dr. Manconi has participated in speaking engagements for Vifor Pharma. Dr. Bruni has consulted for Sapio Life. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Broman JE, Mallon L, Hetta J. Restless legs syndrome and its relationship with insomnia symptoms and daytime distress: epidemiological survey in Sweden. Psychiatry Clin Neurosci. 2008;62:472–5. doi: 10.1111/j.1440-1819.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferri R, Manconi M, Arico D, et al. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. Sleep. 2010;33:793–800. doi: 10.1093/sleep/33.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parrino L, Ferrillo F, Smerieri A, et al. Is insomnia a neurophysiological disorder? The role of sleep EEG microstructure. Brain Res Bull. 2004;63:377–83. doi: 10.1016/j.brainresbull.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Montplaisir J, Boucher S, Gosselin A, Poirier G, Lavigne G. Persistence of repetitive EEG arousals (K-alpha complexes) in RLS patients treated with L-DOPA. Sleep. 1996;19:196–9. doi: 10.1093/sleep/19.3.196. [DOI] [PubMed] [Google Scholar]
- 6.Manconi M, Ferri R, Zucconi M, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–7. doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Spiegelhalder K, Regen W, Feige B, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329–33. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Hornyak M, Feige B, Voderholzer U, Riemann D. Spectral analysis of sleep EEG in patients with restless legs syndrome. Clin Neurophysiol. 2005;116:1265–72. doi: 10.1016/j.clinph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Ferri R, Gschliesser V, Frauscher B, Poewe W, Hogl B. Periodic leg movements during sleep and periodic limb movement disorder in patients presenting with unexplained insomnia. Clin Neurophysiol. 2009;120:257–63. doi: 10.1016/j.clinph.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders, 2nd ed: Diagnostic and Coding Manual. [Google Scholar]
- 13.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision. [Google Scholar]
- 15.Rechtschaffen A, Kales A, editors. Washington: Washington Public Health service; US Government Printing Office; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 16.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.De GL, Ferrara M, Bertini M. The boundary between wakefulness and sleep: quantitative electroencephalographic changes during the sleep onset period. Neuroscience. 2001;107:1–11. doi: 10.1016/s0306-4522(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 18.Merica H, Fortune RD. A unique pattern of sleep structure is found to be identical at all cortical sites: a neurobiological interpretation. Cereb Cortex. 2003;13:1044–50. doi: 10.1093/cercor/13.10.1044. [DOI] [PubMed] [Google Scholar]
- 19.Press WH, Flannery BP, Teukolsky SA, Vetterling WT, editors. Cambridge: Press Syndicate of the University of Cambridge; 1989. Numerical recipes. The art of scientific computing. [Google Scholar]
- 20.Schilling C, Schredl M, Strobl P, Deuschle M. Restless legs syndrome: evidence for nocturnal hypothalamic-pituitary-adrenal system activation. Mov Disord. 2010;25:1047–52. doi: 10.1002/mds.23026. [DOI] [PubMed] [Google Scholar]
- 21.Manconi M, Ferri R, Sagrada C, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010;19:478–86. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 22.Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979–87. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 24.Hornyak M, Grossmann C, Kohnen R, et al. Cognitive behavioural group therapy to improve patients' strategies for coping with restless legs syndrome: a proof-of-concept trial. J Neurol Neurosurg Psychiatry. 2008;79:823–5. doi: 10.1136/jnnp.2007.138867. [DOI] [PubMed] [Google Scholar]
- 25.Vincent N, Walsh K. Hyperarousal, sleep scheduling, and time awake in bed as mediators of outcome in computerized cognitive-behavioral therapy (cCBT) for insomnia. Behav Res Ther. 2013;51:161–6. doi: 10.1016/j.brat.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Galuszko-Wegielnik M, Jakuszkowiak-Wojten K, Wiglusz MS, Cubala WJ, Landowski J. The efficacy of cognitive-behavioural therapy (CBT) as related to sleep quality and hyperarousal level in the treatment of primary insomnia. Psychiatr Danub. 2012;24:S51–5. [PubMed] [Google Scholar]
- 27.Tergau F, Wischer S, Paulus W. Motor system excitability in patients with restless legs syndrome. Neurology. 1999;52:1060–3. doi: 10.1212/wnl.52.5.1060. [DOI] [PubMed] [Google Scholar]
- 28.Scalise A, Pittaro-Cadore I, Golob EJ, Gigli GL. Absence of postexercise and delayed facilitation of motor cortex excitability in restless legs syndrome: evidence of altered cortical plasticity? Sleep. 2006;29:770–5. [PubMed] [Google Scholar]
- 29.Civardi C, Collini A, Monaco F, Cantello R. Applications of transcranial magnetic stimulation in sleep medicine. Sleep Med Rev. 2009;13:35–46. doi: 10.1016/j.smrv.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Gunduz A, Adatepe NU, Kiziltan ME, Karadeniz D, Uysal O. Circadian changes in cortical excitability in restless legs syndrome. J Neurol Sci. 2012;316:122–5. doi: 10.1016/j.jns.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Nardone R, Ausserer H, Bratti A, et al. Cabergoline reverses cortical hyperexcitability in patients with restless legs syndrome. Acta Neurol Scand. 2006;114:244–9. doi: 10.1111/j.1600-0404.2006.00669.x. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo V, Arico I, Mastroeni C, et al. Dopamine agonists restore cortical plasticity in patients with idiopathic restless legs syndrome. Mov Disord. 2009;24:710–5. doi: 10.1002/mds.22436. [DOI] [PubMed] [Google Scholar]
- 33.Scalise A, Pittaro-Cadore I, Janes F, Marinig R, Gigli GL. Changes of cortical excitability after dopaminergic treatment in restless legs syndrome. Sleep Med. 2010;11:75–81. doi: 10.1016/j.sleep.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Pfaff D, Ribeiro A, Matthews J, Kow LM. Concepts and mechanisms of generalized central nervous system arousal. Ann N Y Acad Sci. 2008;1129:11–25. doi: 10.1196/annals.1417.019. [DOI] [PubMed] [Google Scholar]
- 35.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 36.Ondo WG, He Y, Rajasekaran S, Le WD. Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov Disord. 2000;15:154–8. doi: 10.1002/1531-8257(200001)15:1<154::aid-mds1025>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Paci D, Lanuzza B, Cosentino FI, et al. Subclinical abnormal EMG activation of the gastrocnemii during gait analysis in restless legs syndrome: A preliminary report in 13 patients. Sleep Med. 2009;10:312–6. doi: 10.1016/j.sleep.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Manconi M, Ferri R, Zucconi M, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12:47–55. doi: 10.1016/j.sleep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 40.Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/ glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80:2028–34. doi: 10.1212/WNL.0b013e318294b3f6. [DOI] [PMC free article] [PubMed] [Google Scholar]