Abstract
Nine anthocyanins (1–9) from the edible fruits of Eugenia brasiliensis were identified by HPLC-PDA and LC-MS, and seven of these are described for the first time in this Brazilian fruit. Two of the major anthocyanins, delphinidin (8) and cyanidin (9), were studied for their inhibitory activity against chemokine interleukin-8 (IL-8) production before and after cigarette smoke extract (CSE) treatment of cells. In non-treated cells the amount of IL-8 was unchanged following treatment with cyanidin and delphinidin in concentrations 0.1–10 M. Both delphinidin (8) and cyanidin (9) decreased the production of IL-8 in treated cells, at 1 M and 10 M, respectively. Delphinidin (8) demonstrated IL-8 inhibition in the CSE treated cells in a dose-dependent manner.
Keywords: Eugenia brasiliensis, Myrtaceae, anthocyanins, cyanidin, delphinidin, IL-8, COPD
1. Introduction
In addition to their role as plant secondary metabolites, anthocyanins have gained attention as functional pigments in food colorants (He & Giusti, 2010). Interest in anthocyanins has intensified in recent years because of their possible health benefits as dietary antioxidants (Leiris J. & Martin, 2008). Numerous studies have indicated that they might be positively implicated in human health (Lila, 2004). Anthocyanins have a wide range of reported biological activity, including anti-inflammatory activity (Longo & Vasapollo, 2006), risk reduction for coronary heart disease (Basu, Rhone & Lyons, 2010), vasoprotective effects (Bell & Gochenaur, 2006), cytotoxicity (Shin et al., 2009), antidiabetic effects (Nizamutdinova et al., 2009), and prevention of adipocyte dysfunction that leads to obesity (Wei et al., 2011). Some of the positive effects of anthocyanins could be related to their potent antioxidant activity as demonstrated through in vitro and in vivo studies (Tsoyi et al., 2008).
Chronic obstructive pulmonary disease (COPD), a major cause of death and disability, is expected to be the third leading cause of death worldwide by 2020 (Murray & Lopez, 1997). It is characterized by progressive airflow obstruction and airway inflammation, with the major cause being cigarette smoking (Ling et al., 2011). Alveolar macrophage numbers are increased in COPD patients, and play a key role in the pathogenesis of COPD through the secretion of pro-inflammatory mediators such as chemokines and cytokines (Barnes, 2003). Of particular importance is the neutrophil chemoattractant interleukin-8 (IL-8) (De Diego et al., 2011). Cigarette smoke extract (CSE) contains high levels of oxygen free radicals, nitric oxide, and organic compounds that cause oxidative stress (Pryor, Prier & Church, 1983). In vitro studies of human alveolar macrophages have shown that oxidative stress caused by acute CSE exposure increases the release of IL-8 (Walters et al., 2005). Currently, little progress has been made toward developing effective therapies for COPD and although treatments can improve symptoms, their effects are limited and they have not reduced disease progression (Calverley et al., 2007). It has been noted that since COPD is considered steroid-resistant nonsteroidal anti-inflammatories that target chemokine pathways are needed as new therapies (Biswas, McClure, Jimenez, Megson & Rahman, 2005). Currently, a variety of nonsteroidal anti-inflammatory drugs are used to treat chronic inflammatory diseases, but these drugs have notable side effects (Brueggemann, Mani, Mackie, Cribbs & Byron, 2010). Thus, phytochemicals that can be used as natural chemopreventive agents, for example, those found in fruits and vegetables, are an attractive alternative (Halvorsen et al., 2006).
Several researchers have pointed out in the last years the role of diet in the prevention of COPD (Keranis et al., 2010). Epidemiological studies have shown that fruit and vegetable consumption is inversely related to incidence of a number of diseases, including cancer and COPD (Stan, Kar, Stoner & Singh, 2008). Gauliard et al. (2008) have found that a fraction from raspberry juice enriched in anthocyanins may be beneficial to COPD treatment. We hypothesize that phytochemicals, like anthocyanins, may be useful for the treatment of COPD. Along this line, in a patent from our laboratory, we proposed the two anthocyanins, cyanidin-3-glucoside and delphinidin-3-glucoside, as promising novel therapies for COPD (D’Armiento, Reynertson, Kennelly & Wallace, 2008). As part of our ongoing study of antioxidant compounds from tropical fruits with therapeutic effects for COPD E. brasiliensis was investigated (Dastmalchi, Flores, Petrova, Pedraza-Penalosa & Kennelly; Flores, 2011; Reynertson et al., 2006). The focus of this study is to identify anthocyanins in this plant and to explore their potential benefits for the treatment of COPD.
Eugenia brasiliensis Lamarck, commonly known as “grumixama” or Brazilian cherry, is a tree from the coastal Brazilian forests that belongs to the genus Eugenia in the Myrtaceae family. It is one of the largest genera of the Myrtaceae family and comprises around 350 species (Fischer, Limberger, Henriques & Moreno, 2005). Several Eugenia species are cultivated for their edible fruits and others are used in folk medicine. Traditionally, the leaves, fruits, and bark wood of E. brasiliensis are astringent, diuretic and taken as a treatment for rheumatism (Revilla, 2002). Pietrovski et al. (2008) reported the inhibition of ear oedema of the hydroalcoholic extract, fractions, and compounds isolated from E. brasiliensis in response to topical application of croton oil on the mouse ear.
The fruits of E. brasiliensis are purple and red in color, characteristic of fruits rich in anthocyanins. We previously reported the presence of cyanidin-3-glucoside and delphinidin-3-glucoside in this plant (Reynertson, Yang, Jiang, Basile & Kennelly, 2008). However the anthocyanin composition of E. brasiliensis has not been further reported in the literature. To test the potential therapeutical effect of the major anthocyanins identified on COPD they were evaluated for IL-8 inhibitory activity in human small airway epithelial (SAE) cells before and after treatment with CSE.
2. Materials and methods
2.1. General experimental procedures
Sephadex LH-20 (25–100 μm) (Pharmacia Fine Chemicals, Piscataway, NJ, USA) was used for column chromatography. TLC analyses were carried out on RP-18 F254 plates (200–270 μm layer thickness, EMD Chemicals Inc., Gibbstown, NJ, USA), with compounds visualized by spraying with a vanillin solution (1.0 g of vanillin in 10 ml of concentrated H2SO4 and 90 ml of EtOH). Solvents for chromatography, HPLC-grade MeOH, formic acid and acetonitrile were obtained from J.T. Baker (Phillipsburg, NJ, USA). GR-grade MeOH, ethyl acetate, and n-butanol were supplied by VWR Inc. (Bridgeport, PA, USA). Ultrapure water was prepared using a Millipore Milli-RO 12 plus system (Millipore Corp., Bedford, MA, USA).
Trolox was purchased from Sigma Chemical-Aldrich (St. Louis, MO, USA). 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulphonate) diammonium salt (ABTS) was obtained from TCI-Ace (Tokyo, Japan). Delphinidin-3-glucoside, cyanidin-3-glucoside, cyanidin-3-arabinoside, malvidin-3-glucoside, delphinidin, and cyanidin were supplied by Chromadex (Irvine, CA, USA).
2.2. Plant material
Fruits of E. brasiliensis were collected at the Fruit and Spice Park (Homestead, FL, USA). Fruits were frozen and shipped by overnight courier on dry ice to the laboratory, where they were kept in cold (−20 °C) dark storage until processed. Voucher specimens were prepared, identified, and deposited at the Steere Herbarium of The New York Botanical Garden (Bronx, NY, USA).
2.3. Extraction solvent preparation
The effect of solvent variation on total anthocyanin extraction was optimized through the variation of three parameters: type of organic solvent; type of acid; and amount of acid used. A mixture of organic solvent/water/acid (70:29:1, v/v/v) was considered for a screening, using MeOH and EtOH as organic solvents and trifluoroacetic acid and formic acid as acids. Acid concentrations tested include 0, 1, 5, and 10 volume parts.
2.4. Sample extraction
Eugenia brasiliensis anthocyanins were extracted three times with EtOH/water/formic acid (70:25:5, v/v/v) mixture at room temperature with a blender for 5 min per extraction, and the combined extract was dried in vacuo. The extract was suspended in water and sequentially partitioned three times with ethyl acetate, and then n-butanol. The combined ethyl acetate and n-butanol partitions were dried in vacuo, and analyzed by HPLC.
2.5. Fractionation
The n-butanol partition, enriched with anthocyanins, was fractionated over a Sephadex LH-20 column using MeOH (0.1 % formic acid) as eluent and 39 fractions were collected. These fractions were combined into seven on the basis of the RP-18 TLC (70:30 H2O, 5% formic acid/acetonitrile) analysis. All fractions were analyzed by HPLC and tested in the ABTS assay. Fraction 4, containing anthocyanins, was analyzed by LC-TOF, and nine anthocyanins were identified.
2.6. HPLC-PDA
A Waters (Milford, MA, USA) liquid chromatography system equipped with a 2695 Separation Module and a 2996 photodiode-array detector (PAD) coupled to the Waters Empower (version 5.0) for data acquisition and processing was used. Separation was carried out on a 250 × 4.6 mm, 4 μm Synergi Hydro-RP 80A column (Torrance, CA, USA). The elution solvents A (1% aqueous formic acid solution) and B (acetonitrile) were applied as follows: flow rate, 1 mL/min; isocratic 95% B for 10 min, from 95–90% over 6 min, from 90–85 % over 10 min and from 85–75 % over 15 min. The composition was then changed to initial condition in 5 min, and maintained for 10 min. Anthocyanins were detected by monitoring the elution at 520 nm.
2.7. LC-MS analyses of anthocyanins
High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) was performed using a LCT premier XE TOF mass spectrometer (Waters, Manifold, MA, USA) equipped with an ESI interface and controlled by MassLynx V4.1 software. Mass spectra were acquired in both the positive and negative mode over the range m/z 100–1000. The capillary voltages were set at 3000 V (positive mode) and 2800 V (negative mode), respectively, and the cone voltage was 20 V. Nitrogen gas was used for both the nebulizer and in desolvation. The desolvation and cone gas flow rates were 600 and 20L/h, respectively. The desolvation temperature was 400°C, and the source temperature was 120 °C. The analytical column used was a 250 × 4.6 mm, 4 μm Synergi Hydro-RP 80A column (Torrance, CA, USA). The same elution solvent and method as the one described above for HPLC-PDA were applied.
2.8. ABTS assay
The antioxidant activity of the ethyl acetate and n-butanol partitions and of the fractions were measured by the ABTS•+ scavenging assay (Re et al., 1999). A Molecular Devices Versamax microplate reader (Sunnyvale, CA, USA) was used. This assay is based on the formation of the free radical cation ABTS•+ by reacting of ABTS aqueous solution (7mM) with K2S2O8 (2.45 mM, final concentration) at ambient temperature in the dark for 12–16 h. Before use, this solution was diluted with EtOH to an absorbance of 0.700 0.020 at 734 nm. In a final volume of 200 L, the reaction mixture compromised 198 L of ABTS•+ solution and 2 L of the sample at different concentrations. Absorbances at 734 nm were measured at 5 min intervals during 40 min. Similarly, the reaction mixture of standard group was obtained by mixing 198 L of ABTS•+ solution and 2 L of Trolox. ABTS scavenging ability was expressed as the Trolox equivalent antioxidant capacity (TEAC, mmol Trolox/g of the sample) at different time intervals. Quercetin was used as positive control.
2.9. IL-8 Immunoassay
Human SAE cells were cultured according to supplier instructions (Lonza, Walkersville, MD, USA) and maintained in a controlled atmosphere of air-5% CO2 at 37°C. 80 % confluent SAE cells at passages 2 to 5 were used for experiments. CSE was prepared using a modified protocol (Laurent, Janoff & Kagan, 1983). Briefly, a Barnet vacuum pump operating at constant flow was used to draw the smoke of one 3R4F research grade cigarette (University of Kentucky, Lexington, KY, USA) through 25 mL of Dulbecco’s phosphate-buffered saline. This solution (100% CSE) was adjusted to pH 7.4, filtered, diluted with small airway growth medium to a final concentration of 5%, and added to the cells immediately.
Cells were treated with 5% CSE or pure compounds or pretreated with pure compounds 1 h prior to 5% CSE exposure. Cell viability was assessed following CSE exposure using the alamarBlue kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s specifications. After 24 h, measurement of human IL-8 in cell culture supernatants were performed by ELISA (R&D Systems Inc., Minneapolis, MN, USA).
2.10. Statistical analysis
Data are expressed as means values ± 95 % confidence interval. Analysis of variance was performed by one-way analysis of variance (ANOVA) with significant differences between means determined by the Student’s t-test. JMP Statistics software package version 8 was used for statistical analyses (SAS Institute Inc., NC).
3. Results and discussion
We identified nine anthocyanins from E. brasiliensis, and two major anthocyanins constituents found in this specie were tested for their IL-8 inhibition in SAE cells before and after treatment with CSE.
3.1. Extraction of anthocyanins
The extraction solvent for anthocyanins was optimized to enhance the possibility to detect anthocyanins present in small amounts. The total anthocyanin content was approximated by the total summation of peak areas observed at 520 nm during HPLC-UV analysis to optimize detection of these compounds.
The extraction optimization was done using MeOH and EtOH as organic solvents and trifluoroacetic acid and formic acid as acids in a mixture of organic solvent/water/acid (70:29:1, v/v/v). The combination of EtOH and formic acid with water provided the significant greatest total peak area at 520 nm (Fig. 1). The pH of the extraction solvent is a factor in providing a suitable extraction condition for anthocyanins. Extraction of E. brasiliensis fruit pulp with 5 and 10% formic acid concentrations, yielded the highest levels of anthocyanins (Fig. 1). Since no significant difference was found in the total anthocyanin area when 5 or 10 % of formic acid were added to the solvent or in the number of anthocyanin peaks, 5 % was selected as the acid concentration. Therefore, the pulp of the E. brasiliensis was extracted with EtOH/water/formic acid (70:25:5, v/v/v), and the resulting extract was suspended in water and partitioned with ethyl acetate and n-butanol. The ethyl acetate and n-butanol partitions were analysed by HPLC. The anthocyanin-rich n-butanol partition was separated over Sephadex LH-20 to afford seven fractions. Each fractions was analyzed by HPLC and evaluated for their antioxidant activity.
Fig. 1.
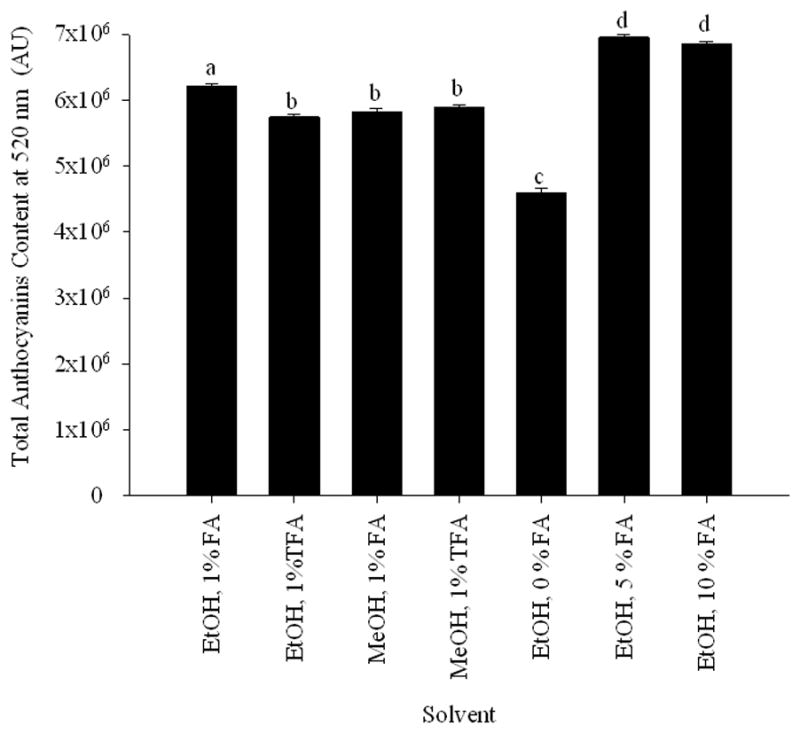
Total anthocyanin content at 520 nm of E. brasiliensis vs. solvent system used for extraction. Based on these results a mixture of EtOH/water/formic acid (70:25:5, v/v/v) was selected for the extraction of E. brasiliensis. Data are presented as mean values ± 95% confidence limits (n = 3). Bars with the same lower case letters (a–d) are not significantly (P > 0.05) different.
3.2. HPLC-PDA
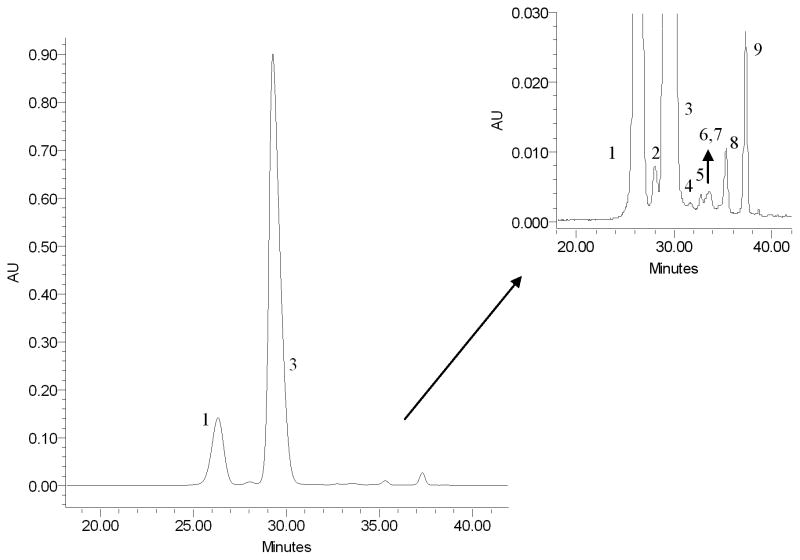
The seven fractions obtained from E. brasiliensis were analyzed by HPLC-PDA and monitored at 520 nm. Fraction 4 proved to be enriched in anthocyanins (Fig. 2) and was therefore selected for compositional analysis.
Fig. 2.
HPLC chromatogram of fraction 4 at 520 nm.
3.3. ABTS•+ antioxidant activity
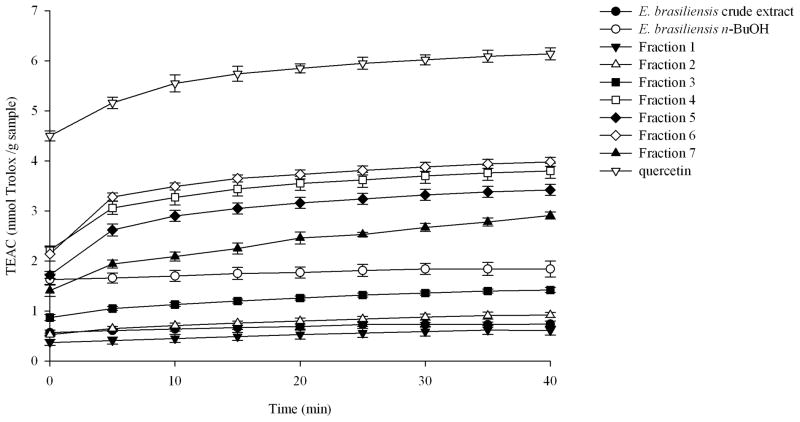
Since oxidative stress is associated with the processes in the pathogenesis of COPD it is proposed that drugs with antioxidant effect will provide effective disease therapies (D’Armiento, Reynertson, Kennelly, Wallace, 2008). In order to determinate the antioxidant capacity of the crude extract, the n-butanol partition, and fractions 1–7 of E. brasiliensis the ABTS•+ assay was used (Fig. 3). As the free radical scavenging activity was monitored over time, the slow-acting antioxidants could be observed. Due to their contribution to the scavenging activity, the order of activity among the samples changed during the assay reaching their highest activity at 40 min. The n-butanol partition showed significantly higher activity than the crude extract, which is in accordance with its higher concentration of active components. All the fractions demonstrated a wide range of ABTS•+ scavenging activities. They exerted an increase in their activity over time; however, the order of activity was kept the same from 0 to 40 min. Except for fraction 1 all the fractions showed significantly higher ABTS inhibitory activity than the crude extract. When the activity was compared with that of the n-butanol partition fractions 1–3 exerted significantly lower activity whereas the activity of fractions 4–7 was significantly higher. Fraction 4 and 6 showed the highest antioxidant activity. With fraction 4 being enriched in anthocyanins it shows the high contribution of these compounds to the antioxidant capacity of the Eugenia brasiliensis fruits.
Fig. 3.
ABTS·+ scavenging activity of E. brasiliensis crude extract, n-butanol partition, and fractions 1–7. Values are expressed as means 95% confidence intervals (n=8) of Trolox equivalent antioxidant capacity (TEAC) (milimoles of Trolox per gram of dry extract).
3.4. Identification of anthocyanins
Fraction 4 was analyzed by LC-TOF. Anthocyanins were identified by their elution order, UV/vis, co-injection with standard compounds, and MS characteristics as compared with reported data in the literature. The identified anthocyanins are represented in Table 1. Compounds 1 and 2 are major constituents that correspond to 12.2 and 76.5 % respectively of the total anthocyanin content, based on peak area at 520nm, followed by compound 9, 6.1 %, and compound 8, 3.4 %, whereas most of the remaining anthocyanins had a percentage area of less than 1.5 %. Within a reversed-phase HPLC, anthocyanins follow a general retention order based on the degree of polarity of the molecular structure, primarily affected by the anthocyanidin and secondly by the number and type of attached glycosides. The anthocyanins elute before their corresponding aglycones. Anthocyanins that differ by only the anthocyanidin would follow this elution series (from shortest to longest retention time): delphinidin, cyanidin, peonidin, and malvidin. For different glycosides groups with the same anthocyanidin content (cyanidin and delphinidin in our study), the elution order was galactoside, glucoside, arabinoside, and xyloside.
Table 1.
LC-MS data for E. brasiliensis fraction 4
| compound numbera | retention time (min) | area (%) | [MS]+ (m/z) | MS/MS (m/z) | identification |
|---|---|---|---|---|---|
| 1 | 26.32 | 12.23 | 465 | 303 | delphinidin-3-glucoside |
| 2 | 28.04 | 1.03 | 449 | 287 | cyanidin-3-galactoside |
| 3 | 29.27 | 76.54 | 449 | 287 | cyanidin-3-glucoside |
| 4 | 31.91 | 0.08 | 419 | 287 | cyanidin-3-arabinoside |
| 5 | 32.71 | 0.20 | 435 | 303 | delphinidin-3-pentoside |
| 6 | 33.63 | 0.42 | 419 | 287 | cyanidin-3-xyloside |
| 7 | 493 | 331 | malvidin-3-glucoside | ||
| 8 | 35.32 | 3.36 | 303 | delphinidin | |
| 9 | 37.31 | 6.14 | 287 | cyanidin |
Compounds numbers and retention times refers to the numbers given in Fig. 2
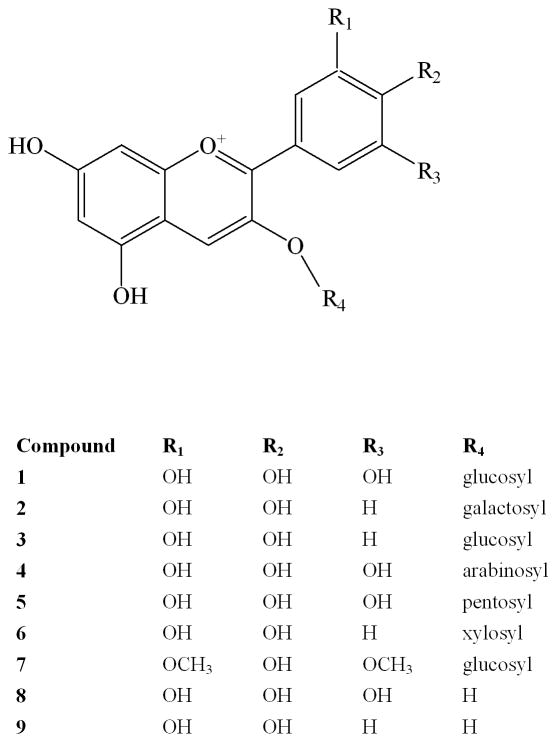
The MS/MS profile of compounds 1, 3, 4, 7, 8, and 9 correspond to delphinidin-3-glucoside, cyanidin-3-glucoside, cyanidin-3-arabinoside, malvidin-3-glucoside, delphinidin, and cyanidin, respectively. Their identification was confirmed by the coinjection of the standard. Both delphinidin-3-glucoside and cyanidin-3-glucoside have been previously found in our lab as constituents of this plant (Reynertson, Yang, Jiang, Basile & Kennelly, 2008). Compound 2 has the same mass spectrometry profile as compound 3 but a different retention time. Using the above rules for anthocyanin identification, compound 2 was determined to be cyanidin-3-galactoside. Compound 5, associated with delphinidin-3-pentoside, showed a molecular ion M+ at m/z 435, with a fragmentation ion at m/z 303 resulting from the loss of a pentose moiety [M+-132]. The mass spectrometry fragmentation pattern of compound 6 (M+ m/z 419; MS/MS 287) suggests that it contains a cyanidin linked to a pentoside. Following the rules above, compound 6 was identified as cyanidin-3-xyloside. Fig. 4 shows the structures of compounds 1–9.
Fig. 4.
Chemical structures of anthocyanins identified in E. brasiliensis. delphinidin-3-glucoside (1), cyanidin-3-galactoside (2), cyanidin-3-glucoside (3), cyanidin-3-arabinoside (4), delphinidin-3-pentoside (5), cyanidin-3-xyloside (6), malvidin-3-glucoside (7), delphinidin (8), and cyanidin (9).
3.5. IL-8 inhibition
In order to assess the potential effect of anthocyanins in COPD, delphinidin (8) and cyanidin (9) were tested for their ability to inhibit IL-8 activity in SAE cells untreated and treated with CSE.
The ability of delphinidin-3-glucoside (1) and cyanidin-3-glucoside (3) to decrease the production of IL-8 in SAE cells treated with CSE was reported before from our laboratory (D’Armiento, Reynertson, Kennelly & Wallace, 2008). Both anthocyanins were more effective at blocking IL-8 production in untreated SAE cells than catechin. Delphinidin-3-glucoside (1) showed higher efficiency than catechin at blocking cigarette-induced inflammation.
First, the effect of the anthocyanins on SAE cell viability was examined at various concentrations. The treatment of anthocyanins (0.1–10 M, for 24 h) did not show any significant cytotoxic effect in the present experiments (data not shown).
In non-treated SAE cells, IL-8 levels were unchanged following the addition of delphinidin (8) and cyanidin (9) in concentrations 0.1–10 M (Fig. 5).
Fig. 5.
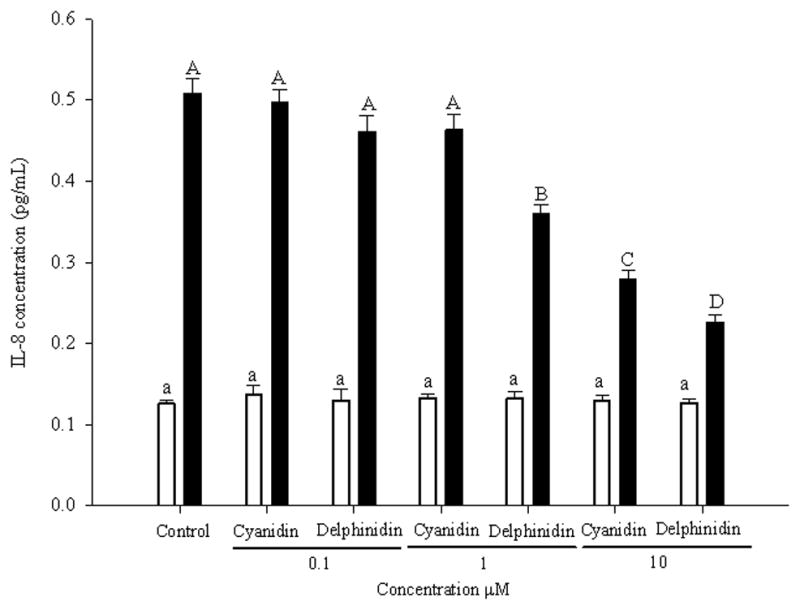
Dose-response of cyanidin and delphinidin at 0.1, 1, and 10 μM on the expression of IL-8 in SAE cells untreated (open bars) and treated (bold bars) with CSE. Data are presented as mean values ± 95% confidence limits (n = 3). Open bars with the same lower case letters (a) and bold bars with the same upper case letters (A–C) are not significantly (P > 0.05) different.
When the control cells were exposed to CSE, the amount of IL-8 increased by threefold. The addition of delphinidin (8) and cyanidin (9) reduced the production of IL-8 (Fig. 5). In the case of cyanidin (9) the significant inhibitory effect was observed at 10 M whereas delphinidin (8) was able to significantly decrease the production of IL-8 at 1M. The inhibitory effect of delphinidin (8) demonstrated a dose-response effect (Fig. 5). Delphinidin (8) displayed significantly higher activity than cyanidin (9) in the inhibition of IL-8 production. Delphinidin (8) has shown higher activity than cyanidin (9) when it was evaluated in different assays. Azevedo et al. (2010) have reported higher antioxidant activity of delphinidin (8) than cyanidin (9) when it was tested in the DPPH and in the FRAP assays. The COX-2 inhibitory activity of delphinidin (8) has been described to be higher than the inhibitory activity of cyanidin (9) (Hou, Yanagita, Uto, Masuzaki & Fujii, 2005). Delphinidin (8) structure has a vicinal trihydroxyl group (pyrogallol) in the B-ring whereas cyanidin (9) structure has an ortho-dihydroxyl group (catechol) in the B-ring. These structural features are likely to induce a significant different bioactivity.
Before these compounds can be translated into effective therapies for patients, issues of bioavailability need to be carefully addressed. Recent studies on anthocyanins in humans have reported that although certain anthocyanins are absorbed rapidly from the stomach and the intestine (Talavera et al., 2005), most of them are poorly bioavailable, reaching the plasma in small concentrations after oral administration in human (Prior & Wu, 2006). For the treatment of COPD it has been proposed that the development of improved inhaled delivery techniques would allow clinically relevant concentrations of antioxidants to be deposited in the lung while avoiding the first pass metabolism that occurs during systemic absorption (Zhu, Chen & Li, 2000).
4. Conclusions
In this article we have identified nine anthocyanins from E. brasiliensis, seven of them are reported for the first time in this plant. Recently, increased attention has been given to the possible health benefits of these compounds in preventing chronic and degenerative diseases incluiding heart disease and cancer (Lim et al., 2011; Ziberna et al., 2010). The great abundance of this fruit in the southeast Brazil could make its fruits a promising anthocyanin-rich crop. On the other hand the major anthocyanins of E. brasiliensis, delphinidin-3-glucoside (1), cyanidin-3-glucoside (3), delphinidin (8), and cyanidin (9), demonstrated inhibitory effect of the inflammation secondary to smoke exposure. These compounds could provide a novel therapeutic role for these compounds in COPD. As part of our ongoing study of antioxidant compounds with therapeutic effects for COPD from tropical fruits we are further investigating these lead compounds and we are planning our experiments to use them in animal studies.
Highlights.
Nine anthocyanins (1–9) were characterized from Eugenia brasiliensis edible fruit.
Seven of them are reported for the first time in this plant.
Delphinidin (8) and cyanidin (9), major constituents, were evaluated for their IL-8 inhibition in cells treated with CSE.
Both anthocyanins were able to decrease IL-8 expression.
Acknowledgments
Support for this study was provided by NIH-NHLBI grant 5SC1HL096016, NIH-NCCAM grant F31AT00801, and by the Spanish Ministry of Science and Innovation postdoctoral fellowship (G.F.). The authors thank the staff of the Fruit and Spice Park (Homestead, FL, USA). The authors also thank Dr. Chunhui Ma, Lehman College (CUNY) for his help in the LC-MS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azevedo J, Fernandes I, Faria A, Oliveira J, Fernandes A, de Freitas Victor, Mateus N. Antioxidant properties of anthocyanidins, anthocyanidin-3-glucosides and respective portisins. Food Chemistry. 2010;119(2):518–523. [Google Scholar]
- Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annual Review of Medicine. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutrition Reviews. 2010;68(3):168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DR, Gochenaur K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. Journal Applied Physiology. 2006;100(4):1164–1170. doi: 10.1152/japplphysiol.00626.2005. [DOI] [PubMed] [Google Scholar]
- Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxidant Redox Signal. 2005;7(1–2):32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Mani BK, Mackie AR, Cribbs LL, Byron KL. Novel Actions of Nonsteroidal Anti-Inflammatory Drugs on Vascular Ion Channels: Accounting for Cardiovascular Side Effects and Identifying New Therapeutic Applications. Molecular and Cellular Pharmacology. 2010;2(1):15–19. [PMC free article] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- D’Armiento J, Reynertson K, Kennelly EJ, Wallace A. Bioactive depside and anthocyanin compounds, compositions, and methods of use. WO 2008016593 A2008016592 2020080207 PCT Int Appl. 2008
- Dastmalchi K, Flores G, Petrova V, Pedraza-Penalosa P, Kennelly EJ. Edible neotropical blueberries: antioxidant and compositional fingerprint analysis. Journal of Agricultural and Food Chemistry. 2011;59(7):3020–3026. doi: 10.1021/jf200367j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Diego Damia A, Cortijo Gimeno J, Selma Ferrer MJ, Leon Fabregas M, Almudever Folch P, Milara Paya J. A study of the Effect of Proinflammatory Cytokines on the Epithelial Cells of Smokers, with or without COPD. Archivos de Bronconeumologia. 2011;47 (9):447–453. doi: 10.1016/j.arbres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Fischer DCH, Limberger RP, Henriques AT, Moreno PRH. Essential oils from leaves of two Eugenia brasiliensis specimens from southeastern Brazil. Journal of Essential Oil Research. 2005;17(1):499–500. [Google Scholar]
- Flores G, Dastmalchi K, Dabo AJ, Whalen K, Pedraza-Peñalosa P, Foronjy RF, D’Armiento JM, Kennelly EJ. Antioxidants of therapeutic relevance in COPD from the neotropical blueberry Anthopterus wardii. Food Chemistry. 2011 doi: 10.1016/j.foodchem.2011.08.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauliard B, Grieve D, Wilson R, Crozier A, Jenkins C, Mullen WD, Lean M. The effects of dietary phenolic compounds on cytokine and antioxidant production by A549 cells. J Med Food. 2008;11:382–384. doi: 10.1089/jmf.2007.593. [DOI] [PubMed] [Google Scholar]
- Halvorsen BL, Carlsen MH, Phillips KM, Bohn SK, Holte K, Jacobs DR, Jr, Blomhoff R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. American Journal of Clinical Nutrition. 2006;84(1):95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annual Revision of Food and Science Technology. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Hou DX, Yanagita T, Uto T, Masuzaki S, Fujii M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure-activity relationship and molecular mechanisms involved. Biochemical Pharmacology. 2005;70(3):417–425. doi: 10.1016/j.bcp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Keranis E, Makris D, Rodopoulou P, Martinou H, Papamakarios G, Daniil Z, Zintzaras E, Gourgoulianis KI. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. European Respiratory Journal. 2010;36(4):774–780. doi: 10.1183/09031936.00113809. [DOI] [PubMed] [Google Scholar]
- Kuskoski EM, Vega JM, Rios JJ, Fett\ R, Troncoso AM, Asuero AG. Characterization of anthocyanins from the fruits of baguaçu (Eugenia umbelliflora Berg) Journal of Agricultural and Food Chemistry. 2003;51(18):5450–5454. doi: 10.1021/jf030014z. [DOI] [PubMed] [Google Scholar]
- Laurent P, Janoff A, Kagan HM. Cigarette smoke blocks cross-linking of elastin in vitro. American Revision of Respiratory Disease. 1983;127(2):189–192. doi: 10.1164/arrd.1983.127.2.189. [DOI] [PubMed] [Google Scholar]
- Leiris JBF, Martin C. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. Journal of Nutrition. 2008;138:747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- Lila MA. Anthocyanins and Human Health: An In Vitro Investigative Approach. Journal of Biomedicine Biotechnology. 2004;2004(5):306–313. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SH, McDonough JE, Gosselink JV, Elliott WM, Hayashi S, Hogg JC, Van Eeden SF. Patterns of retention of particulate matter in lung tissues of COPD: potential role in disease progression. Chest. 2011 doi: 10.1378/chest.10-2281. in press. [DOI] [PubMed] [Google Scholar]
- Lim TG, Kwon JY, Kim J, Song NR, Lee KM, Heo YS, Lee HJ, Lee KW. Cyanidin-3-glucoside suppresses B[a]PDE-induced cyclooxygenase-2 expression by directly inhibiting Fyn kinase activity. Biochemical Pharmacology. 2011;82(2):167–174. doi: 10.1016/j.bcp.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Longo L, Vasapollo G. Anthocynanins as potential antioxidant and anti-inflammatory agents. Recent Progress in Medicinal Plants. 2006;14(27):31–57. [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Nizamutdinova IT, Jin YC, Chung JI, Shin SC, Lee SJ, Seo HG, Lee JH, Chang KC, Kim HJ. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Molecualr Nutrition and Food Research. 2009;53(11):1419–1429. doi: 10.1002/mnfr.200800526. [DOI] [PubMed] [Google Scholar]
- Pietrovski EF, Magina MD, Gomig F, Pietrovski CF, Micke GA, Barcellos M, Pizzolatti MG, Cabrini DA, Brighente IM, Otuki MF. Topical anti-inflammatory activity of Eugenia brasiliensis Lam (Myrtaceae) leaves. Journal of Pharmacy Pharmacology. 2008;60(4):479–487. doi: 10.1211/jpp.60.4.0011. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Research. 2006;40(10):1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environmental Health Perspectives. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Revilla J. Plantas úteis da bacia amazônica. Rio de Janeiro: Inpa; 2002. [Google Scholar]
- Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, D’Armiento J, Weinstein IB, Kennelly EJ. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora) Journal of Natural Products. 2006;69(8):1228–1230. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- Reynertson KA, Yang H, Jiang B, Basile MJ, Kennelly EJ. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chemistry. 2008;109(4):883–890. doi: 10.1016/j.foodchem.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DY, Lee WS, Lu JN, Kang MH, Ryu CH, Kim GY, Kang HS, Shin SC, Choi YH. Induction of apoptosis in human colon cancer HCT-116 cells by anthocyanins through suppression of Akt and activation of p38-MAPK. International Journal of Oncology. 2009;35(6):1499–1504. doi: 10.3892/ijo_00000469. [DOI] [PubMed] [Google Scholar]
- Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. Journal of Cellular Biochemistry. 2008;104(1):339–356. doi: 10.1002/jcb.21623. [DOI] [PubMed] [Google Scholar]
- Talavera S, Felgines C, Texier O, Besson C, Gil-Izquierdo A, Lamaison JL, Remesy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. Journal of Agricultural and Food Chemistry. 2005;53(10):3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Shim HJ, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. Protective effect of anthocyanins from black soybean seed coats on UVB-induced apoptotic cell death in vitro and in vivo. Journal of Agricultural and Food Chemistry. 2008;56(22):10600–10605. doi: 10.1021/jf802112c. [DOI] [PubMed] [Google Scholar]
- Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Molecular Pharmacology. 2005;68(5):1343–1353. doi: 10.1124/mol.105.012591. [DOI] [PubMed] [Google Scholar]
- Wei X, Wang D, Yang Y, Xia M, Li D, Li G, Zhu Y, Xiao Y, Ling W. Cyanidin-3-O-beta-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. Journal of Science and Food Agriculture. 2011;91(6):1006–1013. doi: 10.1002/jsfa.4275. [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen Y, Li RC. Oral absorption and bioavailability of tea catechins. Planta Medica. 2000;66(5):444–447. doi: 10.1055/s-2000-8599. [DOI] [PubMed] [Google Scholar]
- Ziberna L, Lunder M, Moze S, Vanzo A, Tramer F, Passamonti S, Drevensek G. Acute cardioprotective and cardiotoxic effects of bilberry anthocyanins in ischemia-reperfusion injury: beyond concentration-dependent antioxidant activity. Cardiovascular Toxicology. 2010;10(4):283–294. doi: 10.1007/s12012-010-9091-x. [DOI] [PubMed] [Google Scholar]