Abstract
Interspecies bacterial communication is mediated by autoinducer-2, whose synthesis depends on luxS. Due to the apparent universality of luxS (present in over 40 bacterial species), it may have an ancient origin; however, no direct evidence is currently available. We amplified luxS in bacteria isolated from 25 to 40 million year-old amber. Phylogenies and Principal Component Analyses (PCA) of luxS and the 16S rRNA gene from ancient and extant bacteria were constructed. Amber isolates exhibited unique 16S rRNA gene phylogenies, while the luxS phylogeny was very similar to that of extant Bacillus spp. This suggests that luxS may have been acquired by horizontal transfer millions of years ago. Molecular clocks of luxS suggest slow evolutionary rates, similar to those of the 16S rRNA gene and consistent with a conserved gene.
Keywords: Ancient bacteria, autoinducer-2, bacterial communication, luxS, quorum-sensing
INTRODUCTION
Interspecies bacterial communication, or quorum-sensing (QS), is mediated by autoinducer-2 (AI-2), a furanosyl borate diester (Schauder, Shokat et al. 2001). Synthesis of AI-2 depends on luxS, which product is S-ribosylhomocysteine lyase. luxS was first identified in Vibrio harveyi, Escherichia coli and Salmonella typhimurium and its expression has been associated with virulence in E. coli and Streptococcus pyogenes (DeLisa, Wu et al. 2001; Lyon, Madden et al. 2001), and biofilm formation in Bacillus cereus (Taga, Semmelhack et al. 2001; Xavier and Bassler 2005; Auger, Krin et al. 2006). More than 40 bacterial species harbor luxS and this apparent universality makes it attractive for evolutionary analyses (Bassler 1999; Surette, Miller et al. 1999; Winzer, Hardie et al. 2003; Rezzonico and Duffy 2008).
We propose that the evolution of QS mediated by luxS can be studied directly given that bacteria have been previously isolated from 25 to 40 million-year old amber. Amber isolates differ from present-day bacteria in their enzymatic and biochemical profiles, as well as their 16S rRNA gene phylogenies (Greenblatt, Davis et al. 1999). Most amber isolates are Bacillus spp., but Gram-positive cocci (Lambert, Cox et al. 1998; Greenblatt, Baum et al. 2004) and Gram-negative bacteria have been isolated as well, representing an opportunity to study QS in diverse ancient microorganisms (Jones, Jani et al. 2005; Auger, Krin et al. 2006; Rollins and Schuch 2010). In this study, we report luxS sequences in ancient microorganisms, reconstruct the phylogenies of luxS and the 16S rRNA gene from ancient and extant bacteria and calculated molecular clocks for both luxS and the 16S rRNA gene.
MATERIALS AND METHODS
Amber isolates: characterization and DNA extraction
All experiments were performed in a laminar flow cabinet, exclusive for amber bacteria. Amber bacteria were previously isolated by the Ambergene Corporation, under Class III aseptic protocols (Cano and Borucki 1995). Isolates were grown in Nutrient Broth, Brain Heart Infusion Broth or Trypticase Soy Broth supplemented with agar (1.5 % w/v) (Difco), and incubated for 24 to 72 h at 28 or 37 °C. Individual colonies were morphologically characterized by Gram-staining to confirm that the isolates corresponded to those previously reported by the Ambergene Corporation. Isolated colonies were picked and enriched in 1 mL of the broth in which growth was observed. DNA was extracted using the Fermentas GeneJet Genomic DNA Purification Kit following the manufacturer’s instructions. Extracted DNA was stained with GelStar Nucleic Acid Gel Stain (20 X) (Lonza, Rockland, ME, USA) and visualized in 0.7 % agarose gels. DNA quality and concentration were estimated using a NanoDrop® (ND-1000) spectrophotometer.
luxS and 16S rRNA gene amplification and sequencing
luxS primers were designed using Primer 3 (http://frodo.wi.mit.edu/) and checked for the formation of secondary structures (http://www.premierbiosoft.com/netprimer/index.html) (Table 1). Primers were designed from consensus sequences to increase the probability of amplification. Primers were designed for luxS present in Gram-positive and Gram-negative bacteria, since the phylogeny of luxS shows that bacteria cluster by groups (Lerat and Moran 2004). Primers for the amplification of the 16S rRNA gene were as described elsewhere (Amann, Ludwig et al. 1995; Turner, Pryer et al. 1999). Amplifications were performed at least three times in 10 µL per reaction as described previously (Patricio, Herbst et al. 2012), and included reactions without nucleic acids as negative controls. PCR conditions for luxS were: initial denaturation at 95 °C (2 min), followed by 35 cycles at 94 °C (45 s), annealing at 52 °C for (45 s), an extension at 72°C (45 s) and final extension at 72 °C (7 min). PCR conditions for the 16S rRNA gene consisted of an initial denaturation at 95 °C (3 min), followed by 35 cycles at 95 °C (30 s), annealing at 52 °C (30 s), an extension at 72 °C (30 s) and a final extension at 72 °C (10 min). Products were stained as described above, visualized in 1.0 % agarose gels and sequenced using an ABI 3130xl Genetic Analyzer.
Table 1.
Primers used in this study. Direction of the primer is represented by F-(Forward) or R-(Reverse). Primers were designed to amplify the luxS sequences of Gram-positive and Gram-negative bacteria. Accession Numbers for primer design are specified in the following column.
| Primer | Amplicon size (bp) |
Target | Reference | Accession numbers |
|---|---|---|---|---|
| F-GCCAAATAAACAAGCAATGA | 239 | luxS Gram-positive Bacillus spp. | This study | NC_014019.1 |
| R-TTGCAGCTGGAATTTCTGTA | NC_012472.1 | |||
| NC_000964.3 | ||||
| F-GGATTCATACGCTTGAGCA | 184 | luxS Gram-negative bacteria | This study | NC_000913.2 |
| R-TTCAACACATCTTCCATTGC | NC_003197.1 | |||
| NC_008800.1 | ||||
| NC_013971.1 | ||||
| NC_010554.1 | ||||
| F-CATATGATTATGTGGGGTCA | 180 | luxS Gram-positive cocci | This study | NC_008533.1 |
| R-TAAGATGAGTTTTGCCCATT | NC_004350.2 | |||
| NC_004668.1 | ||||
| F-AGAGTTTGATCCTGGCTCAG | 1398 | Universal 16S rRNA | Amann et. al., 1995 | |
| R-ACGGGCGGTGTGTRC | ||||
| R-GWATTACCGCGGCKGCTG | 511 | Universal 16S rRNA | Turner et. al., 1999 | |
Sequence alignments, phylogeny reconstruction and PCA analyses
The luxS and 16S rRNA gene sequences of 24 present-day bacteria were chosen according to previous studies (Lerat and Moran 2004), acquired from GenBank (Table 2) and added to a pool of 20 amber isolates that harbor luxS and for which the16S rRNA gene sequences were determined as well. Nucleotide sequences were aligned using ClustalW in MEGAv4.0 (Tamura, Dudley et al. 2007), keeping default parameters for multiple DNA alignment. Alignments were screened manually in Mesquite (Maddison and Maddison 2001) and exported as NEXUS files. The sequence alignment of luxS had 567 bp and the alignment of 16SrRNA had 1730 bp. Bayesian Markov chain Monte Carlo (MCMC) inference methods available in BEASTv1.7 (Drummond and Rambaut 2007; Drummond and Rambaut 2007) were used to reconstruct the phylogenies of the partial gene sequences. MCMC analyses included γ-distributed rate heterogeneity among sites + invariant sites and partition into codon positions (Drummond, Ho et al. 2007; Drummond and Rambaut 2007). Genealogy was estimated with the uncorrelated relaxed lognormal clock (Ho and Larson 2006) and using the Yule tree prior (Drummond, Ho et al. 2007). Two independent MCMC analyses were run for 10 million generations, sub-sampling every 1,000 generations. After a 10 % burn-in, the analyses were examined for convergence on Tracerv1.5 (Rambaut and Drummond 2003; Rambaut, Ho et al. 2009). Marginal posterior parameter means, the associated 95 % highest probability density intervals, and the effective sample size for each parameter were analyzed to assure statistically robust parameter estimates (Drummond, Nicholls et al. 2002). Summary trees were created with TreeAnnotator v1.6.0 (Rambaut and Drummond 2009) and edited in FigTree v1.3.1.
Table 2.
Extant bacteria in included in the phylogenetic and evolutionary analyses in the present study. Complete luxS and 16SrRNA gene sequences were acquired from GenBank. Accession Numbers are shown in the following column.
| Extant bacteria | Accession number |
|---|---|
| Deinococcus radiodurans R1 | AE000513.1 |
| Bifidobacterium longum NCC2705 | AE014295.3 |
| Neisseria meningitidis 8013 | NC_017501.1 |
| Campylobacter jejuni subsp. jejuni NCTC 11168 | AL111168.1 |
| Bacillus anthracis str. Ames | AE016879.1 |
| Bacillus cereus ATCC 14579 | AE016877.1 |
| Bacillus subtilis subsp. subtilis str. 168 | AL009126.3 |
| Bacillus megaterium QM B1551 | NC_014019.1 |
| Lactobacillus plantarum WCFS1 | AL935263.2 |
| Staphylococcus aureus subsp. aureus N315 | NC_002745.2 |
| Helicobacter pylori B8 | NC_014256.1 |
| Staphylococcus epidermidis ATCC 12228 | NC_004461.1 |
| Streptococcus mutans UA159 | NC_004350.2 |
| Streptococcus pneumoniae R6 | NC_003098.1 |
| Streptococcus pyogenes SSI-1 | NC_004606.1 |
| Enterococcus faecalis V583 | NC_004668.1 |
| Escherichia coli O26:H11 str. 11368 | NC_013361.1 |
| Salmonella enterica subsp. enterica serovar Typhi Ty2 | AE014613.1 |
| Vibrio cholerae O1 str. 2010EL-1786 | NC_016445.1 |
| Shigella flexneri 2002017 | NC_017328.1 |
| Vibrio fischeri ES114 | NC_006840.2 |
| Vibrio vulnificus MO6-24/O | CP002469.1 |
| Yersinia enterocolitica subsp. enterocolitica 8081 | NC_008800.1 |
| Yersinia pestis CO92 | NC_003143.1 |
| Erwinia amylovora CFBP1430 | NC_013961.1 |
| Borrelia burgdorferi B31 | NC_001318.1 |
Partial gene sequences were transformed to numbers (A=1; C=2; G=3; T=4; gaps=0) and were visualized as Principal Component Analysis (PCA) plots. PCA ordinations were calculated in Primer E Software v. 6 (Clarke and Gorley 2006).
Molecular clocks
The evolutionary divergence for chosen sequence pairs (ancient vs. extant) were calculated based on Ochman and Wilson molecular clock for SSU rRNA (0.1 × 10e−9 substitutions/site/year for eubacterial rDNA) (Ochman and Wilson 1987). Based on Masatoshi Nei's model of a phylogenetic test of the molecular clock and linearized trees (Ochman and Bergthorsson 1995). Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 5 (Tamura, Peterson et al. 2011). Trees were built for each ancient isolate against its closest modern ancestor(s). This was performed based on BLAST searches and using a high G+C outgroup (Streptomyces lavendulae). Results are similar to those from the Ochman and Wilson model. Molecular clocks for luxS were estimated similarly.
Luminescence assays
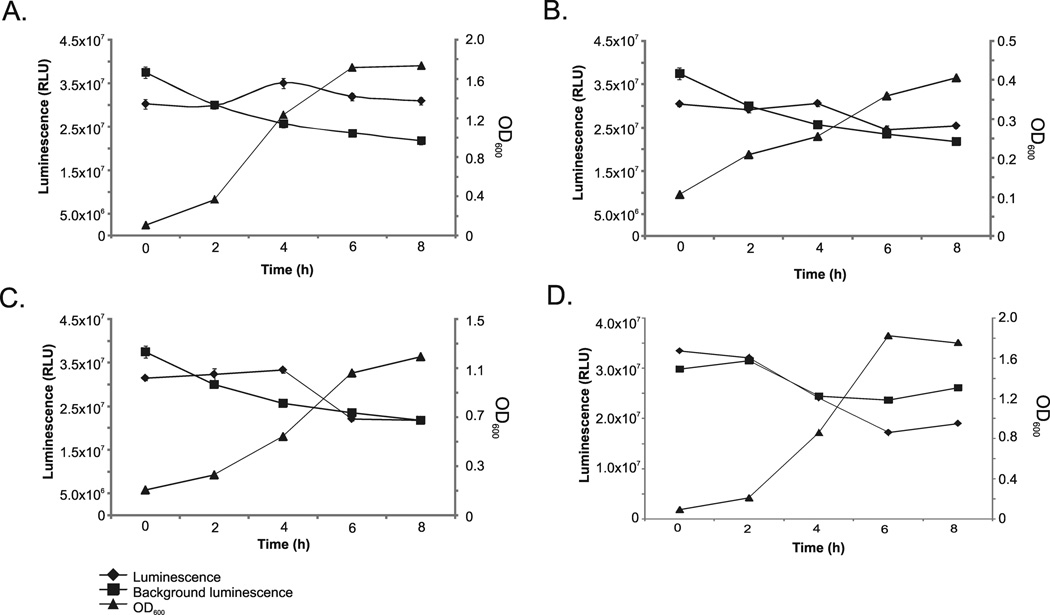
In order to evaluate the expression of luxS in the amber isolates, luminescence assays were performed using isolates 4_AG11AC10, 10_AG11AC13a and 16_AG11AC14 and V. harveyi BB170 as the reporter strain. Amber isolate 6_AG-11-AC-11 was used as negative control as it lacked luxS. The criteria for selection of the isolates for the assays included differences between the amplified region of the 16S rRNA gene and cell morphology. For these experiments, the growth curves of the amber isolates were determined by OD600 measurements of aliquots collected (in triplicate) every 2 h for up to 8 h. Aliquots were filtered and added to a final concentration of 10 % to the reporter strain (final OD600=0.1). Luminescence emitted by the reporter strain in the presence of the putative AI-2 was measured using a luminometer and is reported as Relative Light Units (RLU). Background luminescence, or the luminescence emitted by the reporter strain in the absence of bacterial filtrates was measured as well. Results are reported as plots of the luminescence emitted by the reporter strain in the presence of the supernatant of the amber isolates and OD600 measurements are shown as well (y-axis). The x-axis represents the timing of the response of V. harveyi BB170 after addition of the putative AI-2.
Statistical analyses
Oneway analysis of luminescence data was performed to test for difference between group means using jmp Pro 10 statistical analysis software (Statistical Discovery™, SAS Institute, Inc.).
RESULTS
Evolution and phylogeny of luxS
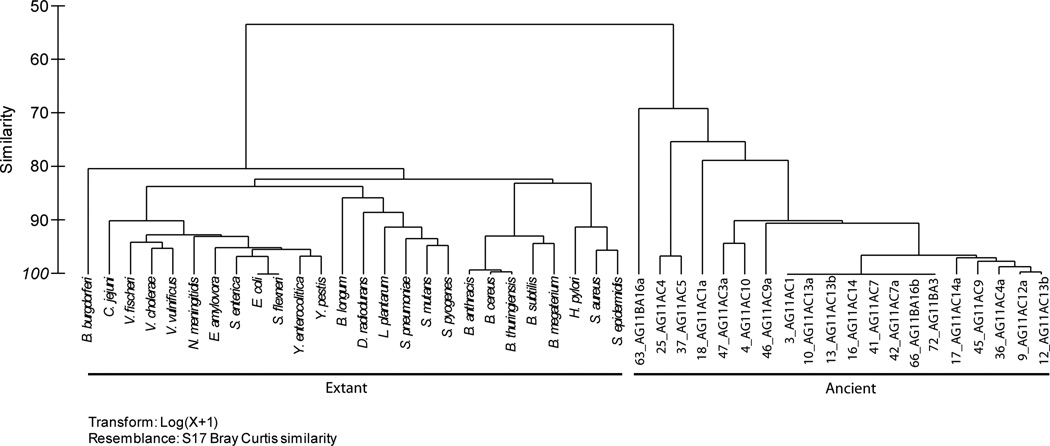
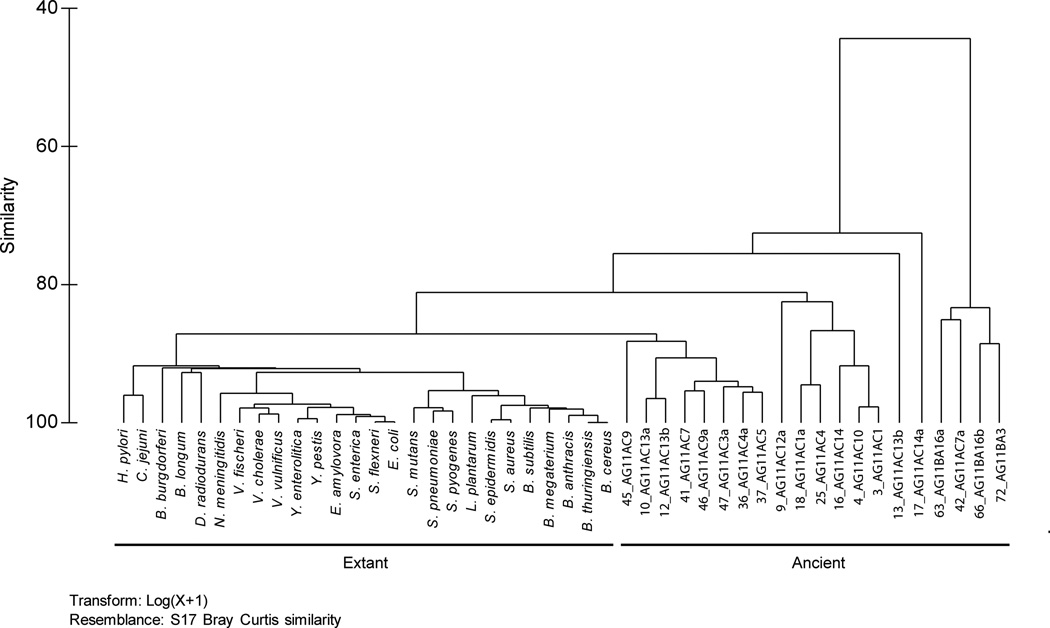
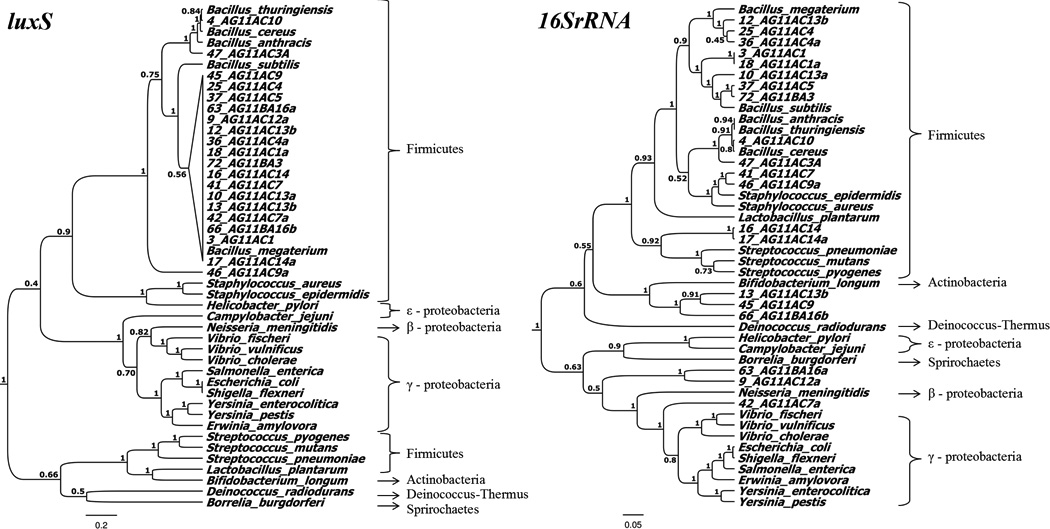
A total of 20 amber isolates were included in the present study (Table 3). luxS was not amplified in most of the Gram-negative isolates, with the exception of isolate 9_AG11AC12a. The tree topology of luxS in the present study is comparable to that reported previously (Lerat and Moran 2004). The amplified region of luxS clustered more closely to the luxS of Bacillus megaterium (Figure 1A). This was not the case, however, for the 16S rRNA phylogeny, where several amber isolates formed distinct branches and clustered with differing bacteria genera (Figure 1B). In the PCA plots, the luxS sequences of ancient and extant bacteria exhibited similarities of 60 to 80 % (Figure 2A). The 16S rRNA gene of ancient and extant bacteria exhibited similarities of 80 % (Figure 2B).
Table 3.
Amber isolates harboring luxS included in the present study. The negative control, or that lacked luxS is shown as well. The following columns show the possible corresponding present-day bacteria as determined by a BLAST search of the 16SrRNA gene, maximum identities (%) and e-values.
| Isolate | Amber | Age (My) | 16S rRNA gene BLAST hit | Max Identity (%) | e-value |
|---|---|---|---|---|---|
| 3_AG11AC1 | Dominican | 25–30 | Bacillus schakletonii | 99 | 0 |
| 4_AG11AC10 | Dominican | 25–30 | Bacillus cereus | 99 | 0 |
| 9_AG11AC12a | Dominican | 25–30 | Brevundimonas sp. | 99 | 0 |
| 10_AG11AC13a | Dominican | 25–30 | Bacillus safensis | 99 | 0 |
| 12_AG11AC13b | Dominican | 25–30 | Bacillus megaterium | 99 | 0 |
| 13_AG11AC13b | Dominican | 25–30 | Curtobacterium sp. | 100 | 0 |
| 16_AG11AC14 | Dominican | 25–30 | Paenibacillus alvei | 99 | 0 |
| 17_AG11AC14a | Dominican | 25–30 | Paenibacillus alvei | 99 | 0 |
| 18_AG11AC1a | Dominican | 25–30 | Bacillus schakletonii | 99 | 0 |
| 25_AG11AC4 | Dominican | 25–30 | Bacillus megaterium | 99 | 0 |
| 36_AG11AC4a | Dominican | 25–30 | Bacillus subtilis | 97 | 0 |
| 37_AG11AC5 | Dominican | 25–30 | Bacillus amyloliquefaciens | 98 | 0 |
| 41_AG11AC7 | Dominican | 25–30 | Staphylococcus sp. | 95 | 0 |
| 42_AG11AC7a | Dominican | 25–30 | Uncultured Pseudomonas sp. | 95 | 0 |
| 45_AG11AC9 | Dominican | 25–30 | Streptomyces sp. | 97 | 0 |
| 46_AG11AC9a | Dominican | 25–30 | Staphylococcus sp. | 94 | 0 |
| 47_AG11AC3a | Dominican | 25–30 | Bacillus cereus | 98 | 0 |
| 63_AG11BA16a | Baltic | 40 | Uncultured Brevudimonas sp. | 99 | 0 |
| 66_AG11BA16b | Baltic | 30 | Agrococcus jenensis | 99 | 0 |
| 72_AG11BA3 | Baltic | 30 | Bacillus amyloliquefaciens | 99 | 0 |
| Control | |||||
| 6_AG-11-AC-11 | Dominican | 25–30 | Bacillus thuringiensis | 99 | 0 |
Figure 1.
The y-axis shows the possible expression of luxS in bacteria isolated from amber by luminescence assays using Vibrio harveyi BB170 as the reporter strain. Optical densities were also measured (in triplicate) every 2h for up to 8h and standard deviations are represented by error bars. Isolates included (A) 4_AG11AC10, (B) 10_AG11AC13a, (C) 16_AG11AC14 and (D) 6_AG-11-AC-11 (Control). Luminescence produced by the reporter strain after the addition of the supernatant, and without it (background luminescence), was measured and is presented in Relative Light Units (RLU). The x-axis represents the timing of the Vibrio harveyi BB170 response after addition of the putative AI-2.
Figure 2.
Phylogeny of luxS (A) and the 16S rRNA gene (B) of amber and present-day bacteria.
The evolutionary rate or molecular clocks for luxS and 16S rRNA gene sequences were calculated. The criteria for selection of the isolates included identification at the species level by BLAST searches of the 16S rRNA gene partial sequences. The evolution rate of the16S rRNA gene of the amber isolates tested is shown in Table 4 and was estimated to be 14.5 to 30.3 million years. The results are consistent with the estimated age of the isolates (Table 1). In terms of luxS, it exhibited mean evolutionary rates ranging from 8.5 to 34.0 million years, which appear to be relatively similar to those values calculated for the 16S rRNA gene (Table 5).
Table 4.
Molecular clocks of the 16SrRNA gene for the amber isolates identified at the species level by BLAST searches of the 16S rRNA gene partial sequences. Time, in millions of years (MY), was calculated using the Takezaki et al. and Ochman-Wilson methods.
| Isolate ID | Molecular Clocks (MY) | BLAST Closest Match | ||
|---|---|---|---|---|
| Takezaki et al | Ochman-Wilson | Mean | ||
| 3_AG11AC1 | 27.5 | 29.8 | 28.7 | B. shacklestonii |
| 4_AG11AC10 | 17.0 | 23.6 | 20.3 | B. cereus |
| 10_AG11AC13a | 18.0 | 18.5 | 18.3 | B. safensis |
| 12_AG11AC13b | 18.5 | 24.3 | 21.4 | B. megaterium |
| 16_AG11AC14 | 23.0 | 28.2 | 25.6 | P. alvei |
| 17_AG11AC14a | 19.5 | 21.5 | 20.5 | P. alvei |
| 18_AG11AC1a | 26.5 | 34.0 | 30.3 | B. shacklestonii |
| 25_AG11AC4 | 13.5 | 15.5 | 14.5 | B. megaterium |
| 36_AG11AC4 | 22.5 | 27.2 | 24.9 | B. subtilis |
| 37_AG11AC5 | 21.5 | 25.5 | 23.5 | B. amyloliquifaciens |
| 47_AG11AC3a | 16.5 | 19.8 | 18.2 | B. cereus |
| 66_AG11BA16b | 24.0 | 23.8 | 23.9 | Agrococcus jenensis |
| 72_AG11BA3 | 20.5 | 26.8 | 23.7 | B. amyloliquefasciens |
Table 5.
Molecular clocks of luxS of chosen amber isolates in this study. Amber isolates were chosen as these were identified at the species level using the 16S rRNA gene partial sequences. Results show the number of substitutions, total bases used for the molecular clock analyses, K, Time (MYBP), r and the BLAST search closest match.
| Strain ID | No. Substitutions | Total Bases | K | Time (MYBP) | r | BLAST Closest Match |
|---|---|---|---|---|---|---|
| 3_AG11AC1 | 12 | 240 | 0.05 | 26.3 | 1.9E-09 | B. megaterium |
| 4_AG11AC10 | 4 | 236 | 0.02 | 23.2 | 7.3E-10 | B. thuringiensis |
| 10_AG11AC13a | 11 | 238 | 0.05 | 8.5 | 5.4E-09 | B. megaterium |
| 12_AG11AC13b | 11 | 239 | 0.05 | 22.1 | 2.1E-09 | B. megaterium |
| 16_AG11AC14 | 11 | 240 | 0.05 | 32.5 | 1.4E-09 | B. megaterium |
| 17_AG11AC14a | 12 | 241 | 0.05 | 21.5 | 2.3E-09 | B. megaterium |
| 18_AG11AC1a | 10 | 170 | 0.06 | 34.0 | 1.7E-09 | B. megaterium |
| 25_AG11AC4 | 7 | 144 | 0.05 | 18.7 | 2.6E-09 | B. megaterium |
| 36_AG11AC4 | 13 | 242 | 0.05 | 32.0 | 1.7E-09 | B. megaterium |
| 37_AG11AC5 | 7 | 154 | 0.05 | 25.5 | 1.8E-09 | B. megaterium |
| 47_AG11AC3A | 6 | 238 | 0.03 | 19.3 | 1.3E-09 | B. cereus |
| 66_AG11BA16b | 8 | 171 | 0.05 | 23.9 | 1.9E-09 | B. megaterium |
| 72_AG11BA3 | 11 | 239 | 0.05 | 23.7 | 1.9E-09 | B. megaterium |
| MEAN RATE | 0.05 | 23.9 | 2.1E-09 | |||
r(evolutionary rate) = K/Years
K = No. Substitutions/Total Bases
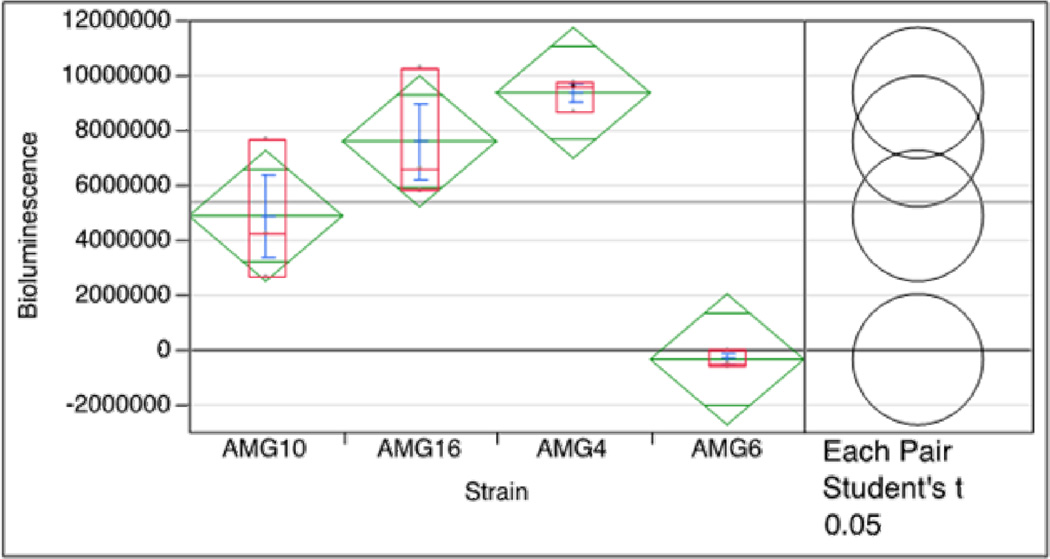
Luminescence in V. harveyi BB170 was induced when exposed to the supernatants of the amber bacteria tested. This was observed at 4 h in all the bacterial isolates tested which harbored luxS, and was not the case for the negative control tested. Luminescence values are shown in Figure 3, Panel A (isolate 4_AG11AC10), Panel B (isolate 10_AG11AC13a and Panel C (isolate 16_AG11AC14). Importantly, these values are statistically significant, as shown by the Oneway analysis of response (Figure 4). The overlapping circles for Each Pair Student’s t and Best Hsu’s MCB also indicate significant difference between the three strains and the control. Notably, the control did not emitted luminescence in any of the time points.
Figure 3.
Dendograms of the luxS (A) and the 16S rRNA gene (B) in ancient and present-day bacteria.
Figure 4.
Oneway analysis of response of the luminescence assays. All three strains (4, 10, and 16) show significantly greater luminescence response than the controls. The overlapping circles for Each Pair Student’s t and Best Hsu’s MCB also indicate significant difference between the three strains and the control.
DISCUSSION
Our results are the first to report the presence and evolutionary rate for genes involved in QS in ancient bacteria. The amplification of luxS in several of the amber isolates tested is neither contamination nor systematic errors of the PCR reactions. This was predicated by the differing 16S rRNA gene sequences among the isolates that were positive for luxS. Contamination would have been detected by looking at the similarities/differences in the16S rRNA sequences amplified from the amber isolates. Moreover, all three sets of luxS primers were tested in approximately 130 amber isolates, despite being Gram-positive or Gram-negative. If contamination of the primer sets would have occurred, luxS would have been amplified in all or most of the isolates tested. It should be noted that amber possesses preservative properties, representing an opportunity to isolate and extract suitable ancient DNA for analyses such as those performed in the present study (Cano 1996).
Most luxS sequences in the amber isolates were similar to the luxS sequences of extant Bacillus spp. when performing the BLAST search. This may be due to the unchanged region of amplified luxS region. This may not have been the case for most of the Gram-negative bacteria tested (except for isolate 9_AG11AC12a), which were negative for luxS. This may suggest that Gram-negative bacteria lacked luxS millions of years ago or that these harbored luxS sequences different from those of present-day bacteria. The presence of a luxS sequence similar to that of Bacillus spp. in an ancient Gram-negative isolate (isolate 9_AG11AC12a) is a matter of further research as this could suggest the horizontal transmission of the gene between Gram-positive and Gram-negative bacteria. Cross-contamination is a possibility that can be discarded as this isolate was identified as a Brevundimonas sp. by a BLAST search of the 16S rRNA gene sequence. Notably, the presence of a luxS sequence similar to that of Bacillus spp. in non-sporulating bacteria, such as those identified as Curtobacterium sp. (isolate 13_AG11AC13b) and Brevundimonas sp. (isolate 9_AG11AC12a), suggests a possible horizontal transmission of the gene as well (Urbanczyk, Furukawa et al. 2012). However, the possibility remains that the data presented are biased by the type of bacteria able to survive in amber and/or those that are cultivable. The lack of amplification of luxS in Gram-negative bacteria isolated from amber still leaves a gap in terms of the status of the gene in this bacterial group.
The luxS sequences corresponding to the amber bacteria accounted for the differences in the tree topologies of both genes considered. The reason is that the luxS sequences grouped with Bacillus spp., whereas the 16S rRNA sequences formed distinct clades in the phylogenetic tree. This suggests that luxS in the ancient bacteria tested may have been acquired by horizontal gene transfer mainly from Bacillus spp. Our data suggest that the lateral transmission of luxS took place at least 40 million years ago. Due to the similarity of the luxS tree topology to that corresponding to the 16S rRNA gene suggests that in extant bacteria, luxS may have been acquired mainly by vertical transmission (Lerat and Moran 2004; Sun, Daniel et al. 2004). The biological reasons and mechanisms of the horizontal transfer of luxS are a matter of further research, but this is a rare event in extant bacteria (Schauder, Shokat et al. 2001).
The relatively low mutation rate of luxS (similar to that of the 16S rRNA gene) may suggest that the gene has been conserved for millions of years and may have an important function in ancient microorganisms as well. Although this may be obvious, no data so far have directly shown that luxS has been conserved for millions of years. This, in turn, raises new questions about the possible role(s) of luxS in QS and metabolic processes in ancient bacteria. It is known that the primary role of LuxS resides in the Activated Methyl Cycle (AMC) and this remains to be addressed for ancient bacteria (Winzer, Hardie et al. 2003; Vendeville, Winzer et al. 2005; Xavier and Bassler 2005; Rezzonico and Duffy 2008).
luxS is a functional gene, as shown by the luminescence assays using the amber isolates tested. These data, although preliminary, open the opportunity to further determine the possible role of AI-2 in these unique isolates. Although it is known that luxS has an essential role in metabolic pathways, its role in other biological processes (e.g. virulence), as those shown with extant bacteria, warrant further studies. While experiments were performed using three amber isolates harboring luxS, results are still valuable as they provide insights of the expression of luxS. We are in the process of performing the luminescence experiments in more amber isolates.
CONCLUSIONS
The present study reported luxS sequences in 25 to 40 million year old bacteria, such as those identified as Bacillus schakletonii and B. aryabhattai, two extant bacterial species that had not been previously reported as carrying luxS. This opens the opportunity to study possible novel QS mechanisms. The amplified region of luxS may be at least 40 million years and that it has remained largely unchanged. Our data provide direct evidence of an ancient origin of a possible functional luxS. This in turn raises new questions on the specific role(s) of luxS in ancient microbes and if it is involved in the regulation of metabolism in amber bacteria.
ACKNOWLEDGEMENTS
We thank Karina Xavier and Jessica Thompson from the Instituto Gulbenkian de Ciencia for providing the reporter strains. The present study was partially financed by MBRS-RISE (NIH Grant Number 2R25GM061151-09). Sequencing was performed by Sylvia Planas and Dania Rodriguez at the Sequencing and Genotyping Facility of the University of Puerto Rico at Rio Piedras. We owe our thanks to Dashari Colon for the luminescence assays.
REFERENCES
- Amann RI, Ludwig W, et al. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59(1):143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger S, Krin E, et al. Autoinducer 2 affects biofilm formation by Bacillus cereus. Appl Environ Microbiol. 2006;72(1):937–941. doi: 10.1128/AEM.72.1.937-941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler BL. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol. 1999;2(6):582–587. doi: 10.1016/s1369-5274(99)00025-9. [DOI] [PubMed] [Google Scholar]
- Cano RJ. Analysing ancient DNA. Endeavour. 1996;20(4):162–167. doi: 10.1016/s0160-9327(96)10031-4. [DOI] [PubMed] [Google Scholar]
- Cano RJ, Borucki MK. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science. 1995;268(5213):1060–1064. doi: 10.1126/science.7538699. [DOI] [PubMed] [Google Scholar]
- Clarke KR, Gorley RN. PRIMER v6: User Manual/Tutorial. Plymouth: PRIMER-E; 2006. [Google Scholar]
- DeLisa MP, Wu CF, et al. DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli. J Bacteriol. 2001;183(18):5239–5247. doi: 10.1128/JB.183.18.5239-5247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A, Ho SY, et al. A Rough Guide to BEAST 1.4. 2007 Available from: < http://beast.bio.ed.ac.uk/>. [Google Scholar]
- Drummond A, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology. 2007;7(1):214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Nicholls GK, et al. Estimating Mutation Parameters, Population History and Genealogy Simultaneously From Temporally Spaced Sequence Data. Genetics. 2002;161(3):1307–1320. doi: 10.1093/genetics/161.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt CL, Baum J, et al. Micrococcus luteus -- survival in amber. Microb Ecol. 2004;48(1):120–127. doi: 10.1007/s00248-003-2016-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt CL, Davis A, et al. Diversity of Microorganisms Isolated from Amber. Microb Ecol. 1999;38(1):58–68. doi: 10.1007/s002489900153. [DOI] [PubMed] [Google Scholar]
- Ho SY, Larson G. Molecular clocks: when times are a-changin'. Trends Genet. 2006;22(2):79–83. doi: 10.1016/j.tig.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Jones MB, Jani R, et al. Inhibition of Bacillus anthracis growth and virulence-gene expression by inhibitors of quorum-sensing. J Infect Dis. 2005;191(11):1881–1888. doi: 10.1086/429696. [DOI] [PubMed] [Google Scholar]
- Lambert LH, Cox T, et al. Staphylococcus succinus sp. nov., isolated from Dominican amber. Int J Syst Bacteriol. 1998;48(Pt 2):511–518. doi: 10.1099/00207713-48-2-511. [DOI] [PubMed] [Google Scholar]
- Lerat E, Moran NA. The evolutionary history of quorum-sensing systems in bacteria. Mol Biol Evol. 2004;21(5):903–913. doi: 10.1093/molbev/msh097. [DOI] [PubMed] [Google Scholar]
- Lyon WR, Madden JC, et al. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol Microbiol. 2001;42(1):145–157. doi: 10.1046/j.1365-2958.2001.02616.x. [DOI] [PubMed] [Google Scholar]
- Maddison W, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2001 [Google Scholar]
- Ochman H, Bergthorsson U. Genome evolution in enteric bacteria. Curr Opin Genet Dev. 1995;5(6):734–738. doi: 10.1016/0959-437x(95)80005-p. [DOI] [PubMed] [Google Scholar]
- Ochman H, Wilson AC. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J Mol Evol. 1987;26(1–2):74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
- Patricio AR, Herbst LH, et al. Global phylogeography and evolution of chelonid fibropapilloma-associated herpesvirus. J Gen Virol. 2012;93(Pt 5):1035–1045. doi: 10.1099/vir.0.038950-0. [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond A. Tracer. 2003 [Google Scholar]
- Rambaut A, Drummond AJ. Tree Annotator v1.5.3: MCMC Output Analysis. 2009 Available at http://beast.bio.ed.ac.uk/TreeAnnotator. [Google Scholar]
- Rambaut A, Ho SY, et al. Accommodating the effect of ancient DNA damage on inferences of demographic histories. Mol Biol Evol. 2009;26(2):245–248. doi: 10.1093/molbev/msn256. [DOI] [PubMed] [Google Scholar]
- Rezzonico F, Duffy B. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiol. 2008;8:154. doi: 10.1186/1471-2180-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins SM, Schuch R. Crowd control: Bacillus anthracis and quorum sensing. Virulence. 2010;1(2):57–59. doi: 10.4161/viru.1.2.11051. [DOI] [PubMed] [Google Scholar]
- Schauder S, Shokat K, et al. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41(2):463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Daniel R, et al. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol Biol. 2004;4:36. doi: 10.1186/1471-2148-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surette MG, Miller MB, et al. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc Natl Acad Sci U S A. 1999;96(4):1639–1644. doi: 10.1073/pnas.96.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga ME, Semmelhack JL, et al. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol Microbiol. 2001;42(3):777–793. doi: 10.1046/j.1365-2958.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, et al. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Pryer KM, et al. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol. 1999;46(4):327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Urbanczyk H, Furukawa T, et al. Natural replacement of vertically inherited lux-rib genes of Photobacterium aquimaris by horizontally acquired homologues. Environmental Microbiology Reports. 2012;4(4):412–416. doi: 10.1111/j.1758-2229.2012.00355.x. [DOI] [PubMed] [Google Scholar]
- Vendeville A, Winzer K, et al. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3(5):383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- Winzer K, Hardie KR, et al. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. Adv Appl Microbiol. 2003;53:291–396. doi: 10.1016/s0065-2164(03)53009-x. [DOI] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-cell communication. Nature. 2005;437(7059):750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. Regulation of uptake and processing of the quorum-sensing autoinducer AI-2 in Escherichia coli. J Bacteriol. 2005;187(1):238–248. doi: 10.1128/JB.187.1.238-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]