Abstract
BACKGROUND
The management of pediatric melanoma (PM) has largely been extrapolated from adult data. However, the behavior of PM appears to differ from its adult counterparts. Therefore, an international PM registry was created and analyzed.
METHODS
Twelve institutions contributed deidentified clinicopathologic and outcome data for patients diagnosed with PM from 1953 through 2008.
RESULTS
Overall survival (OS) data were reported for 365 patients with invasive PM who had adequate follow-up data. The mean age of the patients was 16 years (range 1 year-21 years). The 10-year OS rate, 80.6%, tended to vary by patient age: 100% for those aged birth to 10 years, 69.7% for those aged > 10 years to 15 years, and 79.5% for those aged > 15 years to 20 years (P =.147). Patients with melanomas measuring ≤ 1 mm had a favorable prognosis (10-year OS rate of 97%), whereas survival was lower but similar for patients with melanomas measuring > 1 mm to 2 mm, > 2 mm to 4 mm, and > 4 mm (70%, 78%, and 80%, respectively; P =.0077). Ulceration and lymph node metastasis were found to be correlated with worse survival (P =.022 and P =.017, respectively). The 10-year OS rate was 94.1% for patients with American Joint Committee on Cancer stage I disease, 79.6% for those with stage II disease, and 77.1% for patients with stage III disease (P <.001).
CONCLUSIONS
Tumor thickness, ulceration, lymph node status, and stage were found to be significant predictors of survival in patients with PM, similar to adult melanoma. There is a trend toward increased survival in children aged ≤ 10 years versus adolescents aged > 10 years. Further analyses are needed to probe for potential biological and behavioral differences in pediatric versus adult melanoma.
Keywords: melanoma, pediatric, sentinel lymph node biopsy, mitotic rate, survival
INTRODUCTION
Although pediatric melanoma (PM) is rare (occurring in approximately 1%-4% of melanomas), its incidence reportedly increased 46% from 1973 to 2001.1 Despite its low incidence (approximately 450 new patients aged < 20 years are diagnosed each year in the United States), melanoma is the most common solid tumor in those aged 15 years to 29 years.2,3 The rarity of PM and the challenge in differentiating PMs from pigmented atypical melanocytic neoplasms (PAMNs) can cause management delays, anxiety related to an uncertain diagnosis, and less-than-ideal outcomes.1,4-6 Recently, the expanding array of therapeutic options for patients with melanoma has provided an additional incentive to understand the behavior of PM.
The biology of nominally similar pathological lesions in pediatric and adult populations appears to differ.7,8 A variety of retrospective databases and clinical case reports, often based on single-institution experiences, have reported on PM, with varying results. Data are conflicting regarding the relative contributions of patient sex1,9-13 and lesion location.1,9,11 A variety of risk factors have also been examined. For example, inactivating mutations in the CDKN2A gene (encoding p16 and p14ARF) appear less common in patients with PM than in those with familial melanoma or in individuals with multiple primary melanomas.14,15 Many studies used age cutoff values (aged 10 years-15 years) as a proxy for prepubertal versus postpubertal melanoma. The larger, institutional-based, and population-based studies have demonstrated a higher prevalence of melanoma and decreased survival in adolescents versus children,5,7,14-18 but this is not the case in all studies.8,19
Melanomas in children frequently are diagnosed as having thicker Breslow depths than in adults. Some studies have demonstrated that thicker lesions are associated with decreased overall survival (OS), whereas others do not.19-21 Moreover, although some investigators have found survival outcomes are more favorable in younger patients than in adults with lesions of similar thickness,9 others suggest that, when controlled for thickness and sentinel lymph node (SLN) status, survival outcomes for children and adults are similar.22 Although PM has been reported to frequently metastasize to lymph nodes, the prognostic implications of such metastases require further clarification.9,23,24
Given the conflicting data in the literature from mostly small, single-center studies, we established an international registry in January 2006 to study the clinical behavior of PMs in a multicenter setting. We sought to identify prognostic factors associated with survival outcomes in patients with PM.
MATERIALS AND METHODS
Institutional Review Board approval was obtained at the 12 participating institutions for enrollment in the registry. An Excel spreadsheet/SQL (Structured Query Language) database was developed for Web entry of deidentified demographic and pathologic data for patients with PM who were aged ≤ 20 years. Patients were grouped into those aged ≤ 10 years and those aged > 10 years but ≤ 20 years as well as into 5-year age brackets. In addition, a group of 34 patients aged > 20 years to 23 years was entered into the database and retained for comparison, as recommended by our statisticians. The data were locked on October 31, 2008.
Pathologic, surgical, and follow-up data were collected for all lesions. For the category of mitosis, we used binary categorization (yes vs no, based on the presence of at least 1 mitosis per high-power field or per mm2), allowing outcomes from heterogeneous reports over the extended registry time frame as pathological evaluation methods changed over time. Central pathology review was not feasible for this initial effort. All staging was based on the sixth edition of the American Joint Committee on Cancer (AJCC) cancer staging manual because the data were planned for evaluation using this edition.25
Statistical Analysis
Differences in lesion frequency and specific descriptive information were compared using the chi-square test. The Kaplan-Meier method was used to generate survival curves, followed by univariate analysis for prognostic factors using linear trends and log-rank tests. Because the log-rank test compares the actual distribution of OS, younger patients potentially could have a better survival distribution by virtue of their age at the time of the melanoma diagnosis. A post hoc analysis employing the Fisher exact test was also used to evaluate survival outcomes and confirm the results of the log-rank test. We evaluated patients with 5 years and 10 years of follow-up and labeled them as dead or alive during the specified follow-up.
For nonsurvival outcomes, patients with in situ melanoma and invasive melanoma for whom there were no missing data elements were considered evaluable for each factor being investigated. For OS, only patients with invasive melanoma were considered. For analysis of disease-free survival (DFS), patients with stage IV disease at the time of diagnosis were further excluded.
RESULTS
Table 1 shows the distribution of PM by patient age. The T1 majority of the lesions were invasive melanoma, and the largest percentage of cases occurred in patients aged > 15 years to 20 years. There were 415 patients with invasive melanoma, but not all had complete data elements. The 32 patients with in situ melanoma were excluded from subsequent survival analysis, leaving a total of 365 patients with melanoma who were evaluable, with the years of diagnosis ranging from 1953 to 2008. The mean age of the patients was 16 years (standard deviation, 3.6 years) (range, 1 year-21 years). DFS was only analyzed for 351 patients with PM (excluding 14 patients with stage IV disease).
TABLE 1.
Distribution of Melanomas by Age
| Entire Patient Data Set |
Evaluable Patients |
|||
|---|---|---|---|---|
| Age, Years | In Situ (n = 39) |
Invasive (n = 415) |
In Situ (n = 32) |
Invasive (n = 365) |
| ≤5 | 0 | 8 | 0 | 6 |
| >5 to ≤10 | 4 | 21 | 2 | 19 |
| >10 to ≤15 | 12 | 107 | 10 | 98 |
| >15 to ≤20 | 21 | 252 | 19 | 222 |
| >20 | 2 | 27 | 1 | 20 |
Table 2 shows the characteristics of the patients with evaluable invasive melanoma stratified by age. Females predominated overall, as well as in the group aged > 10 years; males predominated among those aged ≤ 10 years. Mean thickness was significantly higher in children aged 10 years compared with those aged > ≤ 10 years and ≤ 20 years (2.66 mm vs 1.59 mm; P = .0041). There was a trend toward more mitoses in the younger age range compared with the other groups, although a large percentage of the cases were missing information. The majority of the lesions were not ulcerated. More ulceration was found in children aged ≤ 10 years of age compared with those aged > 10 years.
TABLE 2.
Prevalence and Tumor Characteristics in Patients With Invasive Melanomas
| Characteristic | All Patients | Patients Aged ≤10 Years |
Patients Aged >10 Years to ≤20 Years |
Patients Aged >20 Years |
|
|---|---|---|---|---|---|
| Year of diagnosis | Median (Range) |
2000 1953-2008 |
2001 1976-2007 |
2004 1953-2008 |
1999 1983-2006 |
| Sex, no. | Female | 210 (57.5%) | 9 (36.0%) | 185 (57.8%) | 16 (80.0%) |
| Male | 155 (42.5%) | 16 (64.0%) | 135 (42.2%) | 4 (20.0%) | |
| P = .04 | |||||
| Thickness | Mean (SD) | 1.63 (1.94) | 2.66 (2.06) | 1.59 (1.96) | 1.04 (0.72) |
| P = .0041 | |||||
| Clark level | I | 3 (1.0%) | 1 (6.3%) | 2 (0.7%) | 0 (0.0%) |
| II | 50 (16.2%) | 1 (6.3%) | 43 (15.6%) | 6 (35.3%) | |
| III | 79 (25.7%) | 1 (6.3%) | 77 (28.0%) | 1 (5.9%) | |
| IV | 159 (51.6%) | 8 (50.0%) | 141 (51.3%) | 10 (58.8%) | |
| V | 17 (5.5%) | 5 (31.3%) | 12 (4.4%) | 0 (0.0%) | |
| Unknown | 57 | 9 | 45 | 3 | |
| P = .0001 | |||||
| Mitosis | No | 122 (52.1%) | 6 (40.0%) | 104 (51.7%) | 12 (66.7%) |
| Yes | 112 (47.9%) | 9 (60.0%) | 97 (48.3%) | 6 (33.3%) | |
| Unknown | 131 | 10 | 119 | 2 | |
| P = NS | |||||
| Ulceration | No | 265 (87.8%) | 19 (79.2%) | 229 (88.4%) | 17 (89.5%) |
| Yes | 37 (12.3%) | 5 (20.8%) | 30 (11.6%) | 2 (10.5%) | |
| Unknown | 63 | 1 | 61 | 1 | |
| P = NS |
Abbreviations: NS, not significant; SD, standard deviation.
The positivity rate for SLN biopsy (SLNB) was 30%, noted in 55 of the 183 patients who underwent biopsy (of the 365 patients with invasive PM). The majority of SLNB-positive patients (41 patients; 75%) had only 1 SLNB-positive lymph node; 13 had 2 positive lymph nodes and 1 had 5 positive lymph nodes; 128 patients had no positive lymph nodes. Final lymph node and overall staging is shown in Table 3. The majority of patients (53.7%) presented with stage I disease. Furthermore, younger patients (those aged ≤ 10 years) were found to have higher-stage disease than the older cohorts (P = .0054).
TABLE 3.
Lymph Node Analyses and Staging for Invasive PM
| Parameter | All Patients | Patients Aged ≤10 Years |
Patients Aged >10 Years to ≤20 Years |
Patients Aged >20 Years |
|
|---|---|---|---|---|---|
| Lymph node evaluation |
Observation | 121 (36.6%) | 2 (8.7%) | 107 (37.0%) | 12 (63.2%) |
| No SLNB, LND+ | 30 (9.1%) | 1 (4.4%) | 27 (9.3%) | 2 (10.5%) | |
| SLNB− | 126 (38.1%) | 11 (47.8%) | 112 (38.8%) | 3 (15.8%) | |
| SLNB+, no LND | 5 (1.5%) | 0 (0.00%) | 4 (1.4%) | 1 (5.3%) | |
| SLNB+, LND− | 31 (9.4%) | 5 (21.7%) | 25 (8.7%) | 1 (5.3%) | |
| SLNB+, LND+ | 18 (5.4%) | 4 (17.4%) | 14 (4.8%) | 0 (0.0%) | |
| AJCC stage | I | 174 (53.7%) | 6 (25.0) | 153 (56.6%) | 15 (75.0%) |
| II | 67 (20.7%) | 7 (29.2) | 58 (20.7%) | 2 (10.0%) | |
| III | 75 (23.2%) | 10 (41.7%) | 62 (22.1%) | 3 (15.0%) | |
| IV | 8 (2.5%) | 1 (4.2) | 7 (2.5%) | 0 | |
| P = .0054 |
Abbreviations: +, positive; −, negative; AJCC, American Joint Committee on Cancer; LND, lymph node dissection, PM, pediatric melanoma; SLNB, sentinel lymph node biopsy.
OS data for patients with PM are shown in Table 4. The 10-year OS rate was 80.6%. Differences in OS across age groups were not found to be statistically significant (P = .1473, log-rank test). When the groups were condensed, the 10-year OS rate in those aged ≤ 10 years was not significantly different from that of patients aged > 10 years using either the log-rank test (P = .0856) or the Fisher exact test (P = .5019), even when those individuals aged > 20 years were removed. The 5-year OS rate for patients aged ≤ 10 years was 100% (16 of 16 patients) versus 81% (90 of 111 patients) for those aged > 10 years but was not statistically significantly different (P = .3572, Fisher exact test). The median follow-up was 3 years (range, 0.02 years-31 years). It is interesting to note that the survival analysis only included patients who had at least 5 years to 10 years of follow-up; few patients had ≥ 10 years of follow-up.
TABLE 4.
Overall Survival in Pediatric Patients With Invasive Melanoma
| Factor | Dead/No. | 5-Years OS, % | 10-Year OS, % |
|---|---|---|---|
| Overall | 31/365 | 88.9 | 80.6 |
| Age, y (P = .1473, log-rank test) | |||
| ≤5 | 0/6 | 100.0 | 100.0 |
| >5 to ≤10 | 0/19 | 100.0 | 100.0 |
| >10, to ≤15 | 11/98 | 81.4 | 69.7 |
| >15 to ≤20 | 20/222 | 88.9 | 79.5 |
| >20 to ≤21 | 0/20 | 100.0 | 100.0 |
| Sex (P = .9568, log-rank test) | |||
| Female | 16/210 | 89.2 | 80.8 |
| Male | 15/155 | 80.4 | 80.4 |
| Thickness, mm (P = .0077, log-rank test) | |||
| 0-1.0 | 2/147 | 97.0 | 97.0 |
| 1.01-2.0 | 9/84 | 87.2 | 70.1 |
| 2.01-4.0 | 6/71 | 84.1 | 77.6 |
| >4.0 | 4/25 | 80.1 | 80.1 |
| Ulceration (P = .0022, log-rank test) | |||
| No | 12/258 | 91.6 | 86.7 |
| Yes | 6/35 | 74.1 | 59.3 |
| Unknown | 3/34 | 91.9 | 76.6 |
| Mitoses (P = .1637, log-rank test) | |||
| No | 6/122 | 91.6 | 88.3 |
| Yes | 9/112 | 83.4 | 76.9 |
| Unknown | 16/131 | 90.2 | 77.6 |
| Lymph node status (P = .0170, log-rank test) | |||
| Observation | 9/121 | 90.8 | 84.6 |
| No SLNB, LND+ | 9/30 | 63.1 | 63.1 |
| SLNB− | 3/126 | 97.8 | 80.7 |
| SLNB+, no LND | 0/5 | 100.0 | NA |
| SLNB+, LND− | 2/31 | 76.0 | NA |
| SLNB+, LND+ | 1/18 | 88.9 | NA |
| Stage (AJCC 6th edition) (P<.0001, log-rank test) | |||
| I | 3/174 | 96.7 | 94.1 |
| II | 6/67 | 88.0 | 79.6 |
| III | 7/75 | 84.2 | 77.1 |
| IV | 4/8 | 40.0 | NA |
Abbreviations: +, positive; −, negative; AJCC, American Joint Committee on Cancer; LND, lymph node dissection; NA, not applicable; OS, overall survival; SLNB, sentinel lymph node biopsy.
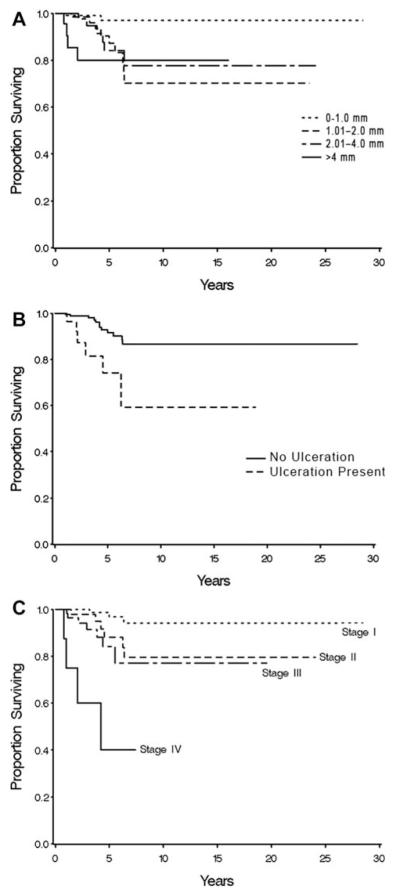
Other potential prognostic factors were analyzed for their impact on OS (Table 4). OS did not appear to differ by sex. Primary melanoma thickness was found to be significantly correlated with OS. OS was similar for all thicknesses of primary melanomas measuring > 1 mm (Table 4) (Fig. 1 Top). The presence of primary tumor ulceration also was found to be significantly correlated with OS (P = .0022) (Fig. 1 Middle). The presence or absence of mitoses did not appear to be correlated with OS. A positive lymph node dissection in the absence of SLNB was correlated with worse OS. Disease stage was found to be highly significant in predicting OS for patients with PM (Fig. 1 Bottom).
Figure 1.
Relation between overall survival and (Top) thickness and (Middle) ulceration are shown for patients with invasive primary pediatric melanoma. (Bottom) Stage of disease was found to be highly predictive of overall survival in these patients.
The 5-year and 10-year DFS rates were 79.7% and 71.5%, respectively, for the evaluable melanoma patients. As expected, DFS decreased with stage of disease: a 10-year DFS rate of 83.05% for patients with stage I disease, a 66.74% DFS rate for those with stage II disease, and a 63.30% DFS rate for patients with stage III disease (P = .0046, log-rank test). DFS did not appear to differ based on age, mitoses, sex, lymph node status, or ulceration (data not shown), but was found to be significantly decreased in patients whose primary tumors were thicker than 1 mm.
DISCUSSION
In this evaluation of a new international registry of patients with primary PM, AJCC stage of disease, thickness, and ulceration were found to be predictive of OS, similar to adult melanoma. However, children with primary lesions measuring > 1 mm in thickness and those with stage II or III disease had similar favorable survival outcomes. Despite the presence of high-risk features and an advanced stage of disease, children aged ≤ 10 years were not found to have worse survival outcomes compared with their adolescent counterparts aged > 10 years.
Lange et al published an analysis of PM from the National Cancer Data Base (as well as a comparison group aged 20 years-24 years).8,19 Several findings from the current study corroborate those from the analysis by Lange et al. Our results corroborated a higher frequency of invasive melanoma in adolescents aged > 10 years versus children aged ≤ 10 years. We confirmed a preponderance of PM with female sex overall, noting PM to be more frequent among males in the younger age group, whereas it was more frequent among females in the older age group.8 Furthermore, younger patients were more likely to have higher-stage disease at the time of diagnosis. In contrast to the findings of Lange et al, the results of the current analysis did not demonstrate decreased survival rates in patients aged ≤ 10 years. In fact, we found a trend toward improved survival in younger patients. We propose that, with increased numbers of patients, this survival advantage could be significant.
Although Lange et al found that primary tumor thickness was not related to survival outcomes in pediatric patients,8 we found thickness to be a significant predictor of survival. The effects of thickness on OS indicate a breakpoint at 1 mm, with worse outcomes for patients with lesions measuring > 1 mm. It is interesting to note that Lange et al used a cutoff value of 1.5 mm, which is similar to the pre-2002 AJCC staging system. The current study data suggest the breakpoint of 1 mm is now appropriate and consistent with the AJCC staging system after publication of the sixth edition. Although the effect of thickness may not be completely linear in relation to survival, the explanation for this is unclear. In general, patients in the current study aged < 10 years had thicker melanomas but, irrespective of their stage of disease at the time of presentation, their OS was 100%. The effect of thickness on OS appears to be driven by events in individuals aged 10 years to 20 years, in whom diminished prognosis was observed with thicker primary tumors. Further analysis of the effects of thickness as well as the association between puberty and melanoma behavior and prognosis will be important in the future.
Stage and ulceration were found to be highly significant predictors of OS among patients with PM. Among those with stage II and III disease, outcome appears to be driven by thickness and ulceration. However, the presence of mitotic activity in the primary tumor did not correlate with significant differences in survival. This lack of correlation may be related to the heterogeneous data from the 12 participating centers, in addition to a lack of standardization among pathologists for this factor before the formalization of mitotic rate assessment in the seventh edition of the AJCC staging manual. Central pathology review would enhance the precision of this prognostic factor and is planned in the future when funds are available.
Among the goals of the current study was the evaluation of the relationship between SLNB status and outcome, given prior reports of elevated percentages of positive SLNB results among younger patients with PM. The number of patients with SLN involvement is too small to reliably evaluate its impact on survival (there were only 3 deaths reported in 54 patients with SLN-positive PM). However, a positive lymph node dissection in the absence of SLNB was found to be correlated with poorer OS. This may reflect an earlier cohort of patients with worse outcomes. It is unclear from the medical records what percentage of patients underwent an elective lymph node dissection versus the percentage with clinically apparent disease. Patients with a negative SLNB had survival rates similar to those who were only observed (without undergoing SLNB or lymph node dissection). Despite relatively high rates of lymph node metastasis among children and adolescents with melanoma, the negative prognostic impact of lymph node metastases on survival appears to be less among children aged ≤ 10 years. However, we state this with caution while awaiting further data, because the overall correlation of lymph node metastases with adverse outcomes still predominates.
When evaluating cases of a relatively rare tumor over a long period of time, one often encounters changes in pathologic and surgical standards. For example, over the course of the registry time frame, there were marked changes in surgical techniques (including the introduction of SLNB), changes in routine pathologic assessment (introduction of the measurement of Breslow depth, Clark level, ulceration, and mitoses), and increased focus on early detection. It is possible that pathologic drift occurred over this time frame. In the future, we propose to institute a centralized review of the registry, which will allow us to determine whether pathologic drift has occurred26 and to impose a consistent pathologic scaling system, if necessary, to correct for it. Furthermore, with increased recruitment of study sites and prospective enrollment of subjects, we intend to increase the number of cases and enable greater homogeneity. In the current analysis, the sixth edition of the AJCC staging system was used and applied to all melanomas entered into the registry, which, in part, controls for or dampens pathologic drift. We have to accept that we cannot control for variations in the histological readings of these melanomas by individual dermatopathologists; however, this registry portrays a true cross-section from reliable institutions. Therefore, even without central pathology review, the current study is important in that we believe it represents realistic patient outcomes based on the evaluation and treatment of patients with PM by high-quality institutions.
Our original intention was to include PAMN data in the registry. Although we identified > 208 PAMNs in the database, the follow-up data on these lesions were poor, and we were unable to evaluate any of the survival outcomes for these patients. Because our database was locked, several of the individual institutions have further refined their institutional data and collected prospective data. The PAMN data currently are being evaluated for a separate publication that addresses the challenges of following such entities outside of cancer registries and the benefits of centralized pathology review for analyzing such lesions, for which follow-up and further analyses are essential. In some cases, lesions originally identified as PAMNs are found to be PM when they are followed for sufficient time periods.4 Given the challenges in distinguishing PAMNs from PM histologically, we plan to increase the number of PAMN cases and the length of follow-up, and institute central pathology review as well as investigate markers of metastatic potential in PAMNs.
We believe this registry provides a new platform for the evaluation and collection of prospective as well as retrospective data to enhance the understanding of the molecular differences between these lesions in adults and in children. Critical to this effort would be prospective tissue collection of PMs and PAMNs to ascertain their molecular characteristics and the molecular basis of any differences from nominally similar tumors in adults. Recent studies have evaluated molecular factors affecting the behavior of melanoma in different age groups. Differences have been found in microRNA expression for cell cycle, inflammation, and other pathways in melanomas based on age.2 Cutaneous melanoma in children and adolescents demonstrates gain of KIT, whereas BRAF mutations occur at a frequency similar to that noted in adults.27 Evaluation of tissue samples in our registry would allow us to define further the biologic behavior of PMs in comparison with adult melanoma. Given recent data suggesting a potential biologic sex difference in melanoma behavior,28,29 we would also like to measure the impact of puberty more precisely by determining Tanner stage; age at menarche; and, possibly, progesterone, testosterone, and estrogen status. To improve our understanding of melanoma biology and behavior in young adults and to provide accurate comparisons, we propose the inclusion of a cohort of adults aged 20 years to 29 years in future registry work.
Conclusions
To the best of our knowledge, this is the first summary of a new international effort to define the biology and outcomes of a PM registry and it demonstrates that whereas children aged ≤ 10 years with invasive melanoma present with more advanced disease, they also demonstrate a trend toward increased survival compared with older cohorts. Furthermore, we confirm that stage, thickness, ulceration, and lymph node status are significant predictors of OS among patients with PM. We anticipate that refinement of the international database, establishment of central pathology review, and increased institutional participation will better define the behavior of PM and improve our ability to care for patients with this disease. Future work is planned to improve the data collection, add prospective data entry for PAMNs, and potentially expand data collection into the young adult population.
Acknowledgments
FUNDING SUPPORT Formation of the registry was supported by the Pittsburgh Skin Cancer SPORE P50CA121973, provided by the National Cancer Institute; Eastern Cooperative Oncology Group (ECOG) U10 CA014548 grant to Case Western Reserve University and ECOG U10 CA39229 grant to the University of Pittsburgh; funding from MetroHealth Medical Center; and financial support for editorial assistance from AIM at Melanoma, the University of Pittsburgh, and MetroHealth Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
We thank Sharon Winters for data collection and Ashok Panneerselvam, PhD, and J. Sunil Rao, PhD for statistical support. We also thank AIM at Melanoma, the University of Pittsburgh, and MetroHealth Medical Center for their financial support for article development and Lisa A. Tushla, PhD, for editorial support.
Footnotes
CONFLICT OF INTEREST DISCLOSURES Dr. Averbook has offered expert testimony in the form of a written opinion in a melanoma case for the law firm of Jeffries, Kube, Forrest, and Monteleone. Dr. Curiel-Lewandrowski is a member of the board of DermSpectra, LLC, a total body imaging company, from whom she has received paid expenses to board and Scientific Advisory Committee meetings. She also acts as a consultant/advisor for MELA Sciences Inc; Medical Directions, LLC; and UpToDate and is a principal/stock owner of DermSpectra, LLC. In addition, Dr. Curiel-Lewandrowski has received grants from both the National Institutes of Health and the National Cancer Institute. Dr. Kirkwood has acted as a consultant and advisor for and received honoraria from GlaxoSmithKline, Novartis, Merck, Bristol-Myers Squibb, Celgene, and Vical and has received research funding from Prometheus Laboratories Inc. Dr. Messina has acted as a consultant/advisor for GlaxoSmithKline and Durect Corporation and was paid to give a lecture on atypical Spitz tumors at a meeting sponsored by Heme/Onc Today in February 2013. Dr. Sabel is a consultant/advisor for Merck Oncology. Dr. Sondak is a consultant/advisor for Merck and Navidea and has been a member of the Speakers’ Bureau for Merck. Dr. Testori is a consultant/advisor for Bristol-Myers Squibb, GlaxoSmithKline, and Amgen; has received honoraria from Bristol-Myers Squibb, GlaxoSmithKline, and Amgen; and has received other remuneration from OncoVision and IGEA. Dr. Zager is a member of the board of Delcath Systems and owns stock in the company and his institution has received funds from Delcath for his acting as a consultant and for Dr. Zager’s role in the development of educational presentations. Dr. Zager has also acted as a consultant for IGEA Inc.
Presented in part at the 2009 meeting of the American Society of Clinical Oncology; May 29-June 2, 2009; Orlando, FL and the American Academy of Pediatrics Section on Surgery Meeting; October 1-3, 2010; San Francisco, CA.
REFERENCES
- 1.Strouse JJ, Fears T, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 2.Jukic DM, Rao UN, Kelly L, et al. MicroRNA profiling analysis of differences between the melanoma of young adults and older adults. J Transl Med. 2010;8:27. doi: 10.1186/1479-5876-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleyer A, O’Leary M, Barr R, Ries LAG, editors. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. National Cancer Institute; Bethesda, MD: 2006. NIH Pub. No. 06-5767. [Google Scholar]
- 4.Mones JM, Ackerman AB. Melanomas in prepubescent children: review comprehensively, critique historically, criteria diagnostically, and course biologically. Am J Dermatopathol. 2003;25:223–238. doi: 10.1097/00000372-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Pappo AS. Melanoma in children and adolescents. Eur J Cancer. 2003;39:2651–2661. doi: 10.1016/j.ejca.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg NB. Update on malignant melanoma in children. Cutis. 2001;67:393–396. [PubMed] [Google Scholar]
- 7.Ferrari A, Bono A, Baldi M, et al. Does melanoma behave differently in younger children than in adults? A retrospective study of 33 cases of childhood melanoma from a single institution. Pediatrics. 2005;115:649–654. doi: 10.1542/peds.2004-0471. [DOI] [PubMed] [Google Scholar]
- 8.Lange JR, Palis BE, Chang DC, Soong SJ, Balch CM. Melanoma in children and teenagers: an analysis of patients from the National Cancer Data Base. J Clin Oncol. 2007;25:1363–1368. doi: 10.1200/JCO.2006.08.8310. [DOI] [PubMed] [Google Scholar]
- 9.Paradela S, Fonseca E, Pita-Fernandez S, et al. Prognostic factors in melanoma in children and adolescents: a clinicopathologic, single-centre study of 137 patients. Cancer. 2010;15:4334–4344. doi: 10.1002/cncr.25222. [DOI] [PubMed] [Google Scholar]
- 10.Saenz NC, Saenz-Badillos J, Busam K, LaQuaglia MP, Corbally M, Brady MS. Childhood melanoma survival. Cancer. 1999;85:750–754. doi: 10.1002/(sici)1097-0142(19990201)85:3<750::aid-cncr26>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Conti EM, Cercato MC, Gatta G, Ramazzotti V, Roscioni S, EUROCARE Working Group Childhood melanoma in Europe since 1978: a population-based survival study. Eur J Cancer. 2001;37:780–784. doi: 10.1016/s0959-8049(01)00006-5. [DOI] [PubMed] [Google Scholar]
- 12.Melnik MK, Urdaneta LF, Al-Jurf AS, Foucar E, Jochimsen PR, Soper RT. Malignant melanoma in childhood and adolescence. Am Surg. 1986;52:142–147. [PubMed] [Google Scholar]
- 13.Temple WJ, Mulloy RH, Alexander F, Marx LH, Jenkins M, Jerry LM. Childhood melanoma. J Pediatr Surg. 1991;26:135–137. doi: 10.1016/0022-3468(91)90893-x. [DOI] [PubMed] [Google Scholar]
- 14.Berg P, Wennberg AM, Tuominen R, et al. Germline CDKN2A mutations are rare in child and adolescent cutaneous melanoma. Melanoma Res. 2004;14:251–255. doi: 10.1097/01.cmr.0000131014.79262.bf. [DOI] [PubMed] [Google Scholar]
- 15.Greene MH. The genetics of hereditary melanoma and nevi: 1998 update. Cancer. 1999;86(suppl 11):2464–2477. doi: 10.1002/(sici)1097-0142(19991201)86:11+<2464::aid-cncr3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Sander B, Karlsson P, Rosdahl I, Westermark P, Boeryd B. Cutaneous malignant melanoma in Swedish children and teenagers 1973-1992: a clinicopathological study of 130 cases. Int J Cancer. 1999;80:646–651. doi: 10.1002/(sici)1097-0215(19990301)80:5<646::aid-ijc2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Berg P, Lindelof B. Differences in malignant melanoma between children and adolescents. A 35-year epidemiological study. Arch Dermatol. 1997;133:295–297. [PubMed] [Google Scholar]
- 18.Lewis KG. Trends in pediatric melanoma mortality in the United States, 1968 through 2004. Dermatol Surg. 2008;34:152–159. doi: 10.1111/j.1524-4725.2007.34032.x. [DOI] [PubMed] [Google Scholar]
- 19.Lange JR, Dunkel IF, Shaw HM, Sober AJ. Melanoma in children and teenagers. In: Balch CM, Houghton AN, Sober AJ, et al., editors. Cutaneous Melanoma. 5th ed Quality Medical Publishing, Inc; St. Louis, MO: 2009. pp. 351–361. [Google Scholar]
- 20.Gibbs P, Moore A, Robinson W, Walsh P, Golitz L, Gonzalez R. Pediatric melanoma: are recent advances in the management of adult melanoma relevant to the pediatric population. J Pediatr Hematol Oncol. 2000;22:428–432. doi: 10.1097/00043426-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Aldrink JH, Selim MA, Diesen DL, et al. Pediatric melanoma: a single-institution experience of 150 patients. J Pediatr Surg. 2009;44:1514–1521. doi: 10.1016/j.jpedsurg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Livestro DP, Kaine EM, Michaelson JS, et al. Melanoma in the young: differences and similarities with adult melanoma—a case-matched controlled analysis. Cancer. 2007;110:614–624. doi: 10.1002/cncr.22818. [DOI] [PubMed] [Google Scholar]
- 23.Moore-Olufemi S, Herzog C, Warneke C, et al. Outcomes in pediatric melanoma: comparing prepubertal to adolescent pediatric patients. Ann Surg. 2011;253:1211–1215. doi: 10.1097/SLA.0b013e318217e852. [DOI] [PubMed] [Google Scholar]
- 24.Mu E, Lange JR, Strouse JJ. Comparison of the use and results of sentinel lymph node biopsy in children and young adults with melanoma. Cancer. 2012;118:2700–2707. doi: 10.1002/cncr.26578. [DOI] [PubMed] [Google Scholar]
- 25.Thompson JA. The revised American Joint Committee on Cancer staging system for melanoma. Semin Oncol. 2002;29:361–369. doi: 10.1053/sonc.2002.34115. [DOI] [PubMed] [Google Scholar]
- 26.Levell NJ, Beattie CC, Shuster S, Greenberg DC. Melanoma epidemic: a midsummer night’s dream? Br J Dermatol. 2009;161:630–634. doi: 10.1111/j.1365-2133.2009.09299.x. [DOI] [PubMed] [Google Scholar]
- 27.Daniotti M, Ferrari A, Frigerio S, et al. Cutaneous melanoma in childhood and adolescence shows frequent loss of INK4A and gain of KIT. J Invest Dermatol. 2009;129:1759–1768. doi: 10.1038/jid.2008.422. [DOI] [PubMed] [Google Scholar]
- 28.Joosse A, de Vries E, Eckel R, et al. Munich Melanoma Group Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131:719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- 29.Joosse A, Collette S, Suciu S, et al. Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials. J Clin Oncol. 2012;30:2240–2247. doi: 10.1200/JCO.2011.38.0584. [DOI] [PubMed] [Google Scholar]