Abstract
Chemicals used experimentally to evoke itch elicit activity in diverse subpopulations of cutaneous pruriceptive neurons, all of which also respond to painful stimuli. However, itch is distinct from pain: it evokes different behaviors, such as scratching, and originates from the skin or certain mucosae but not from muscle, joints or viscera. New insights regarding the neurons that mediate the sensation of itch have been gained from experiments in which gene expression has been manipulated in different types of pruriceptive neurons as well as from comparisons between psychophysical measurements of itch and the neuronal discharges and other properties of peripheral and central pruriceptive neurons.
Introduction
Itch is defined as the sensation that causes the desire to scratch, and it can be induced by mechanical, thermal and chemical stimuli. We do not understand the biological and evolutionary advantages of scratching an itch. But we do know that chronic or severe acute itch causes needless suffering that is often difficult to alleviate1. If we better understood the sensory neuronal conditions that are unique to itch - the particular neurons involved and their molecular and functional properties - we might be able to devise better methods for the selective prevention and treatment of itch.
In this review we focus on itch-related studies in humans, monkeys and mice and specifically on the activity of peripheral sensory neurons and neurons of the spinothalamic tract that carry information about pruritic chemical stimuli from the spinal cord to the thalamus – a major sensory gateway to the cerebral cortex. Specifically, we focus on the types of sensory neurons that encode itchy (pruritic) chemical stimuli. We ask whether they exhibit unique molecular markers that could be used for identification and characterization, targeted cell ablation or manipulation of their physiological properties. Finally we consider the endogenous neural mechanisms that act to suppress or enhance the transmission of pruritic information in the spinal or medullary dorsal horn.
There is a surprising diversity in the capacities of sensory neurons to respond to different types of pruritic chemicals. However, it appears that both peripheral and central pruriceptive neurons are subsets of a larger population of neurons that respond to noxious stimuli, raising a fundamental question: what information is used by the brain to decode neuronal activity in populations of neurons as ‘itch’ rather than ‘pain’? One way to address this issue is to assess the input that populations of peripheral sensory neurons provide to the neuronal cells in the spinal or medullary dorsal horn, that is, to the populations of interneurons that modulate sensory transmission and the projection neurons that transmit the information to the brain.
In this overview we summarize current knowledge of the neuronal mechanisms underlying the sensation of itch in humans or itch-like behaviors in animals in response to pruritic chemicals applied to the skin. We refer readers interested in additional information on the mechanisms of itch, its peripheral mediators, and its treatment in the clinic, to additional reviews on these topics2–4.
Responses to pruritic agents
The neural mechanisms of itch have been investigated by directly relating measurements of itch in humans, or itch-like behavior in animals, to the responses of sensory neurons to the same set of stimuli. Usually, these studies use chemical agents that elicit sustained itch or pain in humans and induce behaviors thought to represent the presence of itch in animals. These chemicals are also applied to isolated cells in vitro to study, for example, mechanisms of transduction.
Histamine has been the ‘gold standard’ pruritic agent in many experimental studies of itch5–7. However, most types of pruritic disorders are poorly treated with antihistamines8, and the experimental use of histamine-independent pruritogens may therefore help to unravel causes of pathological itch. Some non-histaminergic agents such as cowhage, bovine adrenal medulla 8–22 peptide (BAM8–22), a proteolytically cleaved product of proenkephalin A and β-alanine, have been validated as having pruritic properties in both human and animal studies 7,9–17 (Fig. 1).
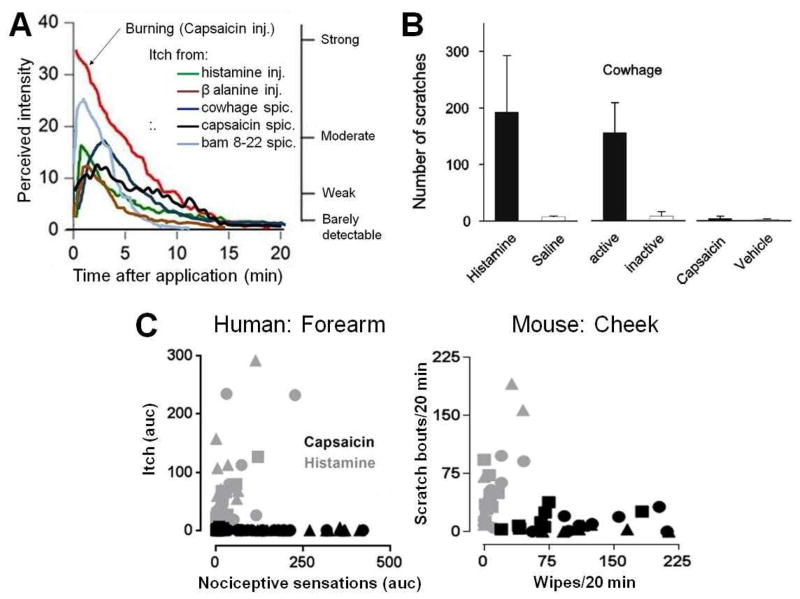
Figure 1. Sensory responses to pruritic or painful stimuli.
Humans, monkeys, and mice respond differently to chemical agents that are predominantly pruritic than they do to those that are painful. A. Graph shows mean ratings of perceived intensity of itch by humans in response to injection (inj.) of histamine and to various chemical agents that evoked a histamine-independent itch, including cowhage, β–alanine, BAM8-22 and capsaicin (the latter two each delivered by means of a heat-inactivated cowhage spicule). These are compared with the perceived intensity of burning elicited by a painful injection of capsaicin. Subjects moved a cursor along a labeled magnitude scale from “no sensation” to “strongest imaginable sensation of any kind”. Four of the descriptor labels are shown on the right vertical axis in correspondence with the numerical ratings of perceived intensity indicated on the left vertical axis. B. Graph shows the scratching response of monkeys to some of the same agents. They scratched in response to application of cowhage spicules or injection of histamine solution but not in response to application of inactive spicules, saline, or injection of capsaicin or its vehicle. C. Mice, like humans, respond differently to injections of histamine (gray) and capsaicin (black). In the left panel, for each of 15 humans, the area under the intensity-time rating curve (auc) for itch was plotted against the auc for nociceptive sensations (pricking/stinging or burning, which every was judged greater at the time of a given rating) in response to histamine or capsaicin at a given dose injected into the forearm (from experiments described in REF. 7). For histamine and capsaicin: squares = 0.1 μg in 10 μl, circles = 1.0, triangles = 10. In the right panel, for each of 8–10 mice the number of bouts of scratching/20 min was plotted against the number of wipes/20 min directed toward the site of injection in the cheek of a given dose of histamine or capsaicin. Histamine: squares = 10 μg in 10 μl, circles = 20, triangles = 50; capsaicin: squares = 1 μg in 10 μl, circles = 10, triangles = 40 (modified from fig. 5, REF 15). Panel a is modified, with permission from REFS 7, 9, 12 and 13. Panel b is reproduced, with permission, from REF 14. Panel C is modified, with permission from REFs 7 and 15.
These and other pruritogens can be applied in similar manner and at similar concentrations in psychophysical experiments in humans and in behavioral and neuronal studies in animals, enabling direct comparisons between species. One caveat is that the site-directed responses used as behavioral indicators of itch in animals, such as scratching or biting, or indicators of pain, such as wiping or licking, produce tactile and nociceptive sensory stimulation that can modulate the sensations that the experimenter wishes to measure. These site-directed responses are therefore indirect indicators of sensation. By contrast, sensations in humans and neuronal electrophysiological activity in neurons are recorded in the absence of itch- or pain-altering site-directed stimulation. By identifying the magnitude and time course of different qualities of sensation in humans it has been possible to define and characterize the sensory information that is represented by the electrophysiologically recorded responses of sensory neurons that encode pruritic and algesic chemicals.
When humans judged and directly compared the perceived intensities of itch and the nociceptive sensory qualities of pricking and/or stinging and burning on a single scale of sensory magnitude (that is, a labeled magnitude scale), the itch in response to histamine and to non-histaminergic pruritic agents (such as native cowhage spicules, β-alanine, and capsaicin delivered by a single spicule) was similar in magnitude and time course7,9–13 (Fig. 1A). The itch induced by each pruritogen was typically accompanied by slightly weaker and shorter lasting nociceptive sensations of pricking and/or stinging, and burning. The itch and nociceptive sensations were positively correlated in magnitude. In contrast to the sensory effects of pruritogens, an intradermal injection of capsaicin elicited pain described as pricking and/or stinging and burning, but resulted in virtually no itch7,19. Analogously, different types of behavior are elicited when agents rated by humans as more pruritic than nociceptive (or vice versa) are applied to the skin of a monkey14 (Fig. 1B) or a mouse15–17 (Fig. 1C). These findings show that the qualitatively different behaviors evoked in animals by stimuli that humans describe as primarily itchy or painful help to validate the association of a neural mechanism in animals with one or the other of these sensory qualities.
Diversity of pruriceptive nociceptors
Electrophysiological in vivo recordings from peripheral sensory neurons in humans, monkeys, or mice indicate that pruritic chemicals applied to the skin elicit action potentials in a subset of nociceptors14,18–23. These pruriceptive nociceptive neurons (or pruriceptors) and the nociceptive neurons that do not respond to pruritic chemicals (non-pruriceptive nociceptors) can be further sub-classified according to their responsiveness to noxious mechanical, thermal or chemical stimuli. For example, mechanosensitive nociceptive afferents or MSAs that respond to noxious mechanical stimuli are distinguished from those that are mechano-insensitive (MIAs). The axons of nociceptive neurons can be unmyelinated and slowly conducting (known as “C-fibers”, such as the C-fiber mechanoheat sensitive nociceptors (CMHs)) or thinly myelinated and faster conducting (known as “A-fibers”, such as the A-fiber mechanoheat nociceptors (AMHs)).
Non-pruriceptive nociceptors
There is no evidence for the existence of peripheral sensory neurons that are itch-specific, that is, responsive only to pruritic but not to algesic stimuli. However, non-pruriceptive nociceptive neurons have been identified that are unresponsive to pruritic chemicals. These include high-threshold nociceptors with C- or A-fibers that are activated only by noxious mechanical stimuli, heat or capsaicin14,18–24. In humans, for example, some of the MIAs supplying the skin include C-fibers with nociceptors that are insensitive to both cowhage and histamine but are activated by noxious heat and/or capsaicin. The discharge rates of these neurons, which could not be directly determined due to a low signal to noise ratio, have been assessed indirectly by counting the number of “activation periods” following stimulus application. The activation periods display a time course similar to the sensations of pain reported19,26. MIAs have also been identified in monkey (C- and A-fibers) 27,84 and mice (C-fibers) 28 but there are fewer tests of their responses to pruritogens. Thus, non-pruriceptive nociceptors appear mainly to contribute to pain signaling; nevertheless, a possible role for these neurons in itch has not been ruled out.
Pruriceptors responsive to histamine
Histamine activates subsets of MIAs such as those with C-fiber nociceptors that are responsive to heat or to capsaicin 6,19 and, less effectively, mechanosensitive nociceptors (MSAs) with A- or C-fibers (AMs and CMs). The MSAs include some that are also responsive to heat (MHs or, more specifically, CMHs and AMHs)14,18,21–23,29. In vivo recordings of neural activity evoked by histamine have been obtained from peripheral nerve fibers in monkeys and humans and from dorsal root ganglion (DRG) neurons in mice. The neuronal responses of C-fiber MIAs in humans6,19, CMH’s in mice23, and CMHs and AMs in monkey14,22 exhibit temporal profiles that generally match the time course of itch in humans6,7,11, suggesting that activity in these afferents may contribute to histamine-induced itch. However, the histamine-induced activity in CMH’s is weak14. Taking advantage of the fact that C-fiber MIAs have larger cutaneous receptive fields than CMH’s 24, it was found that after blocking a cutaneous nerve with a local anesthetic, histamine itch and pain to electrical but not mechanical stimuli could be elicited from an area adjacent to the completely anesthetized region of skin presumably by activating C-fiber MIAs that originated from neighboring, unblocked nerves. It was concluded that C-fiber MIAs alone may be sufficient to mediate histamine- induced itch25. Currently, it is not possible to selectively block conduction in MIAs to investigate the role of mechanosensitive pruriceptor in histamine-induced itch.
In mice, the cutaneous sensory neurons that respond to histamine include, but are not limited to29,30, those that express MrgprA3 (Mas-related G-protein coupled receptor member A3), the receptor for chloroquine, an anti-malarial drug that is linked to pruritus in malarial patients with dark skin and to itch-like behavior in mice29,31. Sensory neurons that are responsive to chloroquine are also activated by, and express the corresponding receptors for, histamine (the ligand for the H1 receptor), BAM8–22 (the ligand for the receptor MrgprC11), SLIGRL-NH2 (a peptide agonist for protease activating receptor 2 (PAR2), but which evokes itch behavior by activating MrgprC11)32, capsaicin (which acts on transient receptor potential vanilloid 1 (TRPV1))29 and serotonin (Fig. 2). Each receptor may also be linked to different effector molecules by distinct signaling pathways33,34. For example, histamine can signal through TRPV135, and chloroquine and BAM 8-22 can signal via separate pathways to activate transient receptor potential cation channel ankyrin1 (TRPA1) 36,37.
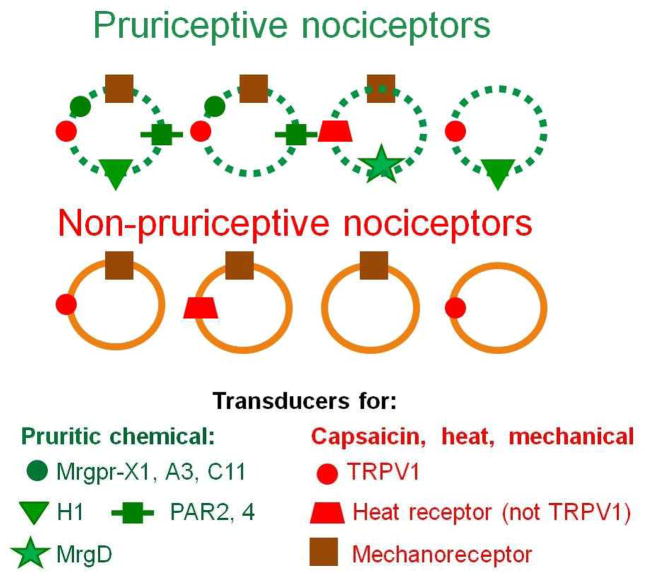
Figure 2. Pruriceptive neurons are a subset of nociceptive neurons.
The circles symbolize subtypes of nociceptors that express receptors for different types of stimuli in their axonal terminals in the skin. The hypothetical combinations of receptors are based on published recordings of the capacities of different types of stimuli to activate primary, cutaneous sensory neurons when delivered either to their peripheral receptive fields in vivo (in mouse, monkey or human) or to their dissociated, cultured, cell bodies, in vivo (mouse only). There are two main types of nociceptors: those that respond to pruritic chemicals and those that do not (pruriceptive and non-pruriceptive nociceptors, respectively). Both types of nociceptor also respond to one or more noxious stimuli (mechanical or heat stimuli or capsaicin injection) that elicit pain and not itch. The symbols illustrate some of the receptors expressed by each subtype. In mice, some pruriceptive nociceptors have been shown to express the receptor for chloroquine, Mas-related G-protein coupled receptor member A3 (MrgprA3), and the receptor for bovine adrenal medulla 8–22 peptide (BAM8-22), MrgprC11. Primates have been shown to express the receptor for either of these ligands (MrgprX1) in a subset of nociceptors. The transduction of protease contained in cowhage is poorly understood but is hypothesized to involve protease-activated receptor 2 (PAR2) and/or PAR4. Other receptors shown are for histamine (H1), β-alanine (MrgprD), capsaicin, noxious mechanical stimuli (mechanoreceptors), and heat that is trandsuced either by transient receptor potential vanilloid 1 (TRPV1)), or by a mechanism independent of TRPV1. For clarity, other combinations of the receptors shown or other markers associated with nociceptive/pruriceptive transduction (such as TRPA1,TRPM8 and P2X3) or intracellular signaling are omitted.
TRPV1 and TRPA1 are ion channels that can also be directly activated by algesic chemicals: for example, TRPV1 can be activated by capsaicin and TRPA1 is primarily activated by electrophilic irritants such as allyl isothiocyanate. The DRG neurons that express TRPA1 are mostly a subpopulation of those expressing TRPV138,39 and both channels play roles in neuronal excitation, hypersensitivity and the release of inflammatory neuropeptides40
Only 5% of DRG neurons express MrgprA3 and these are restricted to small diameter neurons that innervate the stratum granulosum of the epidermis, providing one biological reason for itch arising from skin and not from deeper tissues29. By expressing a green fluorescent protein (GFP) in MrgprA3-expressing neurons, the cell bodies of these rare neurons were identified not only in dissociated cells in vitro but also in situ in the DRG of an intact mouse29. This enabled experimenters to position, in vivo, an electrode at the cell body of MrgprA3-expressing neurons and to record neuronal activity evoked by stimuli applied to the neuron’s cutaneous receptive field. Most or all MrgprA3-expressing neurons responded with sustained action potential activity to histamine and to other pruritogens previously shown29,31 to activate the cell bodies of these neurons in vitro. The MrgprA3-expressing neurons also responded to mechanical and noxious heat stimuli and to the (painful) intradermal injection of capsaicin. A selective ablation of MrgprA3-expressing neurons by diphtheria toxin reduced itch behavior in response to histamine and certain other pruritogens, but it did not affect itch produced by β-alanine (the agonist for MrgprD, a receptor expressed in another neuronal population13) or the responses to painful stimuli29. These findings suggest that activity in MrgprA3-expressing neurons activates an afferent pathway that signals itch and not pain and that itch is not exclusively mediated by these MrgprA3-expressing neurons.
Using an activity-dependent silencing strategy (that is, application of a charged sodium channel blocker, QX314, together with a specific pruritogen), a recent study found that histamine-induced scratching is also mediated by MrgprA3-negative neurons. 30. There is no single molecular marker available to genetically label this MrgprA3-negative, histamine sensitive population. Therefore, an intersectional strategy (for example, using a combination of two recombinase systems to express a transgene in overlapping cell types defined by two driver genes)41 may be required to allow the genetic manipulation of MrgprA3 negative neurons.
Pruriceptors responsive to other pruritogens
Virtually all CMH nociceptors in monkey and human, about half of the CMHs tested in the mouse and a subset of MSAs with A-fibers (AM and AMHs) tested in monkey respond to native cowhage spicules14.20–23, which elicit a histamine independent itch9,10. The mean peak discharge rates of these MSAs in the monkey are typically greater in response to cowhage than to histamine injection14. When compression block of a peripheral nerve was used to eliminate conduction in A- but not C-fibers, cowhage-evoked itch and nociceptive sensations were reduced or eliminated in some trials but remained unaffected in others. These findings provide evidence that both A- and C-fiber MSAs can mediate cowhage-evoked sensations and support the idea that the relative contribution of each fiber type depends on which one happens to be activated at a skin test site.22
The cellular mechanisms by which cowhage elicits action potentials in MSAs are largely unexplored. The active pruritic agent in cowhage spicules, a cysteine protease, might activate receptors directly on the sensory neuron such as PAR2 and/or PAR442. For example, SLIGRL-NH2, the peptide ligand of PAR2 that elicits a histamine-independent itch in mice, can activate dissociated DRG neurons presumably via PAR2 or MrgprC1132. In addition, or alternatively, keratinocytes possess many chemosensitive receptors and could be a source of neuroactive pruritic mediators that might activate pruriceptive neurons. For example, SLIGRL-induced activation of PAR2 receptors on keratinocytes causes these epithelial cells to release thymic stromal lymphopoietin (TSLP), a cytokine that plays a role in atopic dermatitis; TSLP, in turn, can directly activate a subset of TRPA1-positive cutaneous sensory neurons to elicit itch-like behavior43.
In primates, MrgprX1 shares sequence homology with members of the mouse MrgprA and MrgprC subfamilies, is restricted to small diameter DRG neurons, and is activated by both chloroquine and BAM8–2231. In addition to cowhage, other chemical agents such as BAM8-22 or β-alanine that elicit a histamine independent itch in humans12,13 can activate one or more subsets of CMHs as tested in mice,13,29 or monkeys44. Some of these CMHs, when electrophysiologically recorded in vivo, are also responsive to histamine whereas others are not. For example, in mice most MrgprA3-expressing CMH neurons responded to histamine, cowhage and BAM 8-22 (although not to β-alanine) applied to their receptive fields (Fig. 2)29. By contrast, another subset of CMH neurons, identified by the expression of GFP under the control of the MrgprD promoter, responded to β-alanine but not to histamine13. However, this subset comprised only about 40% of the total number of MrgprD-GFP neurons. The β-alanine unresponsive MrgprD-GFP neurons were mechanosensitive but heat unresponsive nociceptors. None of the MrgprD expressing neurons responded to capsaicin or to other pruritic chemicals tested (Fig. 2)13.
MrgprD-expressing neurons, like those expressing MrgprA3, supply only the epidermis of the mouse29,45, consistent with a role in itch, which originates specifically from skin. Cellular studies in mice have indicated that MrgprD-expressing neurons bind isolectin-B4 (IB4), do not produce neuropeptides and do not express MrgprA3 or TRPV129,45. Those that respond to heat may do so via a TRPV1-independent mechanism28,46 (Fig. 2). Deletion of the MrgprD gene in mice abolished behavioral responses to β-alanine (without affecting responses to histamine)13. However, mice with MrgprD-expressing neurons ablated also exhibited decreased behavioral sensitivity to noxious mechanical stimuli47. Thus, more information is required to determine whether β-alanine sensitive and insensitive types of MrgprD neurons mediate itch and pain or both sensations.
In summary, there is diversity in the capacities of primary, cutaneous nociceptive sensory neurons in mouse and primate to signal mechanical, thermal and chemical noxious stimuli. Some of these neurons are pruriceptive – that is, they have the additional capability of signaling the presence of one or more types of chemicals that elicit a sustained itch – whereas others (non-pruriceptive neurons) do not.
Pruriceptive projections to the brain
Pruriceptive nociceptive primary sensory neurons – like non-pruriceptive nociceptive neurons from the skin of the body (or the head) - terminate in the spinal (or medullary) dorsal horn where they provide input to interneurons and to projection neurons whose axons project to the brainstem or forebrain. The only projection neurons in primate that have been characterized for their electrophysiological responses to pruritic chemicals are those that project from the spinal cord to the thalamus, the spinothalamic tract (STT) neurons. The axons of these and other projection neurons from the spinal cord ascend within the anterolateral funiculus, the transection of which impairs itch as well as pain and temperature sensations48,49.
In vivo electrophysiological recordings in the monkey were obtained from STT neurons responsive to noxious thermal, chemical and/or mechanical stimuli50–52. Approximately two-thirds of these nociceptive neurons were non-pruriceptive. The other third was pruriceptive and responded either to histamine or to cowhage spicules but individual neurons rarely responded to both of these pruritic agents51,52. Most of these pruriceptive STT neurons responded with greater discharge rates to heat and/or capsaicin than to either pruritic agent. The types of primary afferents delivering the input, directly and polysynaptically via interneurons, to these STT neurons could not be determined in these in vivo studies. Nevertheless, in Fig 3 we summarize our current understanding of the functional properties of nociceptors in mouse and primate and STT neurons in primate and present a hypothetical scheme for the peripheral input to the pruriceptive and non-pruriceptive nociceptive STT neurons in monkey.
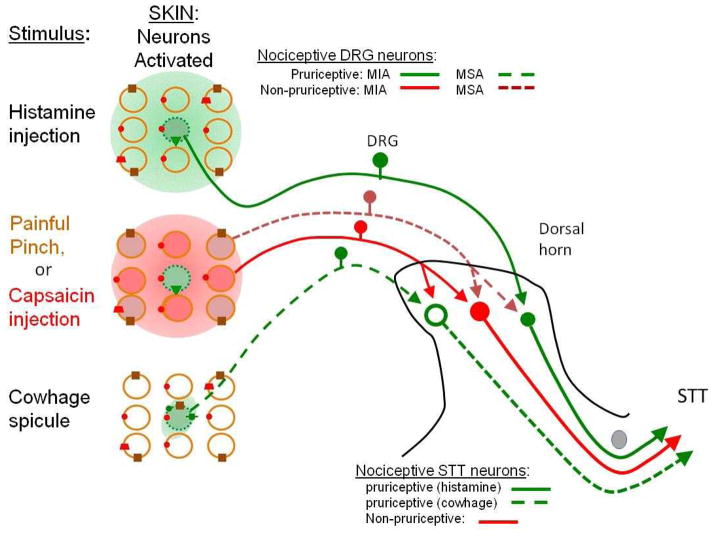
Figure 3. Hypothesized cutaneous input to pruriceptive and non-pruriceptive spinothalamic tract neurons in primate.
On the left of the figure, different types of stimuli are presented to the same set of 9 nociceptors and the nociceptors that they activate in each case are shown. Shaded circles indicate activated nociceptors and empty circles indicate unresponsive nociceptors. The presence of particular transduction mechanisms in each neuron are as shown. Non-pruriceptive nociceptors include those that respond only to noxious mechanical stimuli (brown) and mechanically insensitive nociceptors expressing transient receptor potential vanilloid 1 (TRPV1) (red). The nociceptors depicted in green respond to one or more pruritic chemicals but also to one or more noxious stimuli. On the right, the schematic shows how these different types of peripheral sensory neurons might provide input to three types of nociceptive spinothalamic tract (STT) neurons, based in part on information obtained in the monkey. All three types of STT neurons respond to noxious heat, capsaicin and/or mechanical stimuli but one pathway (red) is non-pruriceptive whereas the other two are pruriceptive (green). The pruriceptive neurons consist of two populations: STTs that are more responsive to cowhage than to histamine (green dashed line) and thus presumably receive a dominant input from C- and A-mechanosensitive afferent (MSA) neurons and STTs that are more responsive to histamine than to cowhage (green solid line) and receive a dominant pruriceptive input from histamine responsive mechanically insensitive afferent (MIA) neurons. The unknown synaptic mechanisms by which each type of afferent fiber contacts the STT neurons are indicated with arrows: these including a direct monosynaptic input and, via one or multiple interneurons, polysynaptic input (not shown). Action potential activity in the STT neurons is also subject to modulation by excitatory and inhibitory interneurons (not shown) as described in Fig. 5. The image also shows the relatively greater spatial spread of a noxious stimulus or pruritogen when it is applied by injection than when it is applied by a spicule (as illustrated by the size of the shaded area on the left).
Matching pruriceptive neuron activity to itch sensation
One criterion for the encoding of pruritic information by both pruriceptors and pruriceptive STT neurons is that their responses to a pruritic chemical should match the time course of the sensation of itch: that is, the onset of the response should coincide with the beginning of the itch and the response should reach a peak and decline at approximately the same time as the itch. Assuming that humans and monkeys experience the same itch in response to histamine or to cowhage this criterion is supported by the findings that there is a correspondence in the time course of mean sensory ratings of itch by humans7,9 and mean discharges of peripheral and STT pruriceptive neurons as electrophysiologically recorded in the monkey14,22,52 (Fig. 4). Specifically, for both the delivery of cowhage spicules and the intradermal injection of histamine, the time course of itch in humans matches the time course of the mean discharges of mechanosensitive A- and C-peripheral neurons and STT neurons recorded in the monkey.
Figure 4. Pruriceptive neuronal activity matches the time course of itch sensation.
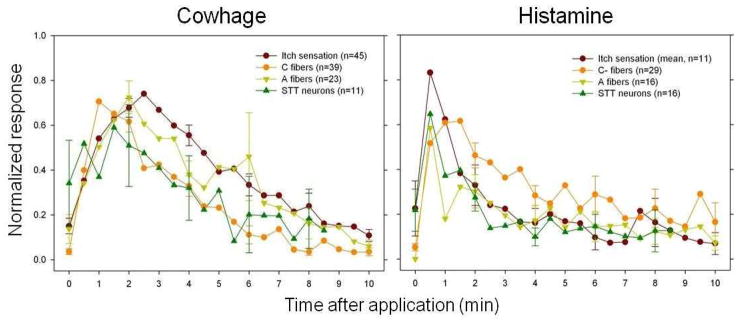
The mean perceived intensity of itch in humans and the mean discharge rates of primary afferents and spinothalamic tract neurons recorded in the monkey are shown (n = number of human subjects or number of nerve fibers or STT neurons tested). The responses of pruriceptors – that is, mechanoheat sensitive C-fibers and mechanosensitive A-fiber nociceptors – to cowhage and histamine are compared with the responses of pruriceptive spinothalamic tract (STT) neurons to the same stimuli. The responses of each neuron and each subject were normalized to the peak value obtained for that subject or neuron for a given pruritic agent (baseline activity for each STT is subtracted from the responses). Assuming that similar sensations are present in monkeys and humans, the finding that itch begins with the onset of activity in these neurons, reaches a peak magnitude and declines in approximate correspondence with the activity suggests that the itch is mediated at least in part by the activity of these neurons. Data obtained from REFS 7, 9, 14, 22 and 52.
Nevertheless, there are caveats and issues to resolve. First, it is not known whether the discharges in these neurons mediate itch alone or whether they also convey the minor nociceptive sensations that accompany the itch elicited by a pruritic chemical, which typically display a slightly lesser magnitude and shorter time course7,9. Second, potential input to STT neurons from other types of pruriceptive nerve fibers needs to be evaluated. These include peripheral neurons responsive to β-alanine or BAM8-22 (an important questions is whether these neurons activate only cowhage-responsive STT neurons) and the MIAs with C fibers that are responsive to histamine. The recorded discharges of the latter require quantitative analyses, but it is thought that they are likely to make an important contribution to histamine-induced itch6,19. In addition, there is indirect psychophysical evidence for an “anti-pruritic” effect of a histamine injection that is possibly attributable to the activation of nociceptive afferents that in turn may act centrally to limit the histamine induced activity in pruritic nociceptive afferents53. That is, not all pruriceptive neurons may act to mediate itch; some may act to reduce itch. Third, there is presently no means of molecularly identifying and selectively activating or deleting a particular type of STT neuron in primate (or mouse) in order to link a particular ascending pathway to behavior.
Modulation of pruriceptive information
Spinal interneurons as well as input from descending pathways, such as those that originate in the brainstem, exert excitatory and inhibitory effects on the excitability of projection neurons54, thereby potentially modulating transmission of pruriceptive information in the dorsal horn. For example, the removal the homeobox gene Tlx3 in select spinal neurons during development 85 or the deletion of testicular receptor 4 (TR4), an orphan nuclear receptor55, leads to a loss of excitatory interneurons in the superficial dorsal horn and a significant attenuation of both itch and pain behavior.
Recent findings have revealed several candidate neurotransmitters that may mediate transmission of pruritic information in the dorsal horn. Gastrin releasing peptide (GRP) and its receptor (GRPR) are promising candidates for an itch-specific peptide transmitter and receptor in primary and secondary neurons, respectively. Ablation of a subset of dorsal horn neurons expressing GRPR in mice eliminated scratching in response to a variety of chemical pruritogens56,57. However, a recent study found that natriuretic peptide B (Nppb), rather than GRP, may be the itch specific neurotransmitter expressed in pruriceptive MrgprA DRG neurons58. In addition, this study found that GRP is not expressed by DRG neurons but rather by secondary dorsal horn neurons that express the Nppb receptor (Npra) and presumably release GRP to activate GRPR expressing tertiary neurons. Additional experiments are needed to prove that pruritogens cause DRG neurons to release Nppb peptide in order to activate Npra in dorsal horn neurons, to determine what types of neurons the Npra-expressing neurons are and to examine whether Npra is also expressed in DRG neurons mediating inflammatory pain (as previously found in the rat59). In addition to GRP and Nppb, glutamate and substance P probably have a role in the spinal transmission of pruriceptive input: histamine-induced scratching was completely blocked by an α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR)/kainite receptor antagonist, but unaffected by a GRP antagonist or a combination of GRP-, neurokinin 1 (NK1)- and AMPAR/kainite receptor antagonists 60. In contrast, chloroquine- induced, histamine-independent scratching behavior was abolished by a combination of GRP-, NK1- and AMPAR/kainite receptor antagonists60.
Although some interneurons in mouse appear to be crucial for the transmission of chemically evoked itch from the skin others act to prevent spontaneous itch or to reduce stimulus-evoked itch. Indeed, behavioral signs of enhanced stimulus-evoked and spontaneous itch occur after a knock out of the vesicular glutamate transporter 2 (VGLUT2) in nociceptors, which results in reduced glutamate activation of pain mediating neurons that normally act to reduce itch transmission in the CNS61.62. Similarly, a loss, in the superficial dorsal horn, of itch inhibiting interneurons that express the transcription factor Bhlhb5 (basic helix–loop–helix domain-containing, class B5) 63,64 increases itch-related behavior (Fig. 5A).
Figure 5. Models of modulation of itch transmission in the dorsal horn.
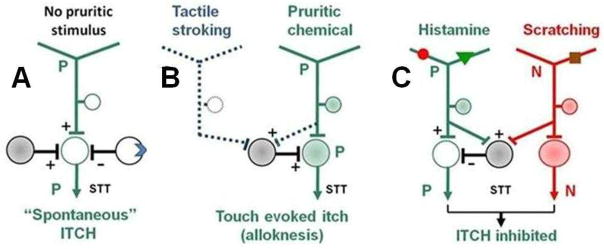
A: The activity of pruriceptive (green) spinothalamic tract (STT) neurons is influenced by opposing inputs from excitatory and inhibitory interneurons. Here, the excitatory interneuron remains active (filled circle), whereas the inhibitory interneuron is either silenced (open circle), for example, by activation of an opiate receptor (blue symbol) by intrathecal morphine67 or absent as a result of elimination of inhibitory interneurons such as those expressing the transcription factor Bhlhb163. This results in the generation of ‘spontaneous’ itch sensations. B: Pruriceptive afferents selectively sensitize (dashed line) interneurons that receive input from low-threshold mechanoreceptors. As a result a pruriceptive STT neuron now exhibits enhanced responses to innocuous tactile stimuli (to induce tactile alloknesis). C: Simultaneous activity in histamine responsive pruriceptive neurons and activity generated by scratching in non-pruriceptive (red) mechanosensitive nociceptors, activate an interneuron that inhibits a pruriceptive - STT neuron (modified from an “and-gate” model 76). All STT neurons are also subject to modulation by suprasegmental descending pathways that are not shown. For simplicity, both the neuropeptides and their receptors that are thought to be involved in the itch circuit are not included.
Opiates also modulate the transmission of pain and itch in the dorsal horn. Intrathecally administered morphine produces analgesia in humans and animals but, as a side effect, can elicit itch and scratching behavior65,66. Electrophysiological recordings from nociceptive trigeminothalamic tract neurons in the medullar dorsal horn in the rat provided a correlate to the analgesic and pruritic effects of intrathecally delivered morphine: morphine excited and increased the responses of pruriceptive neurons to a chemical pruritogen, whereas it inhibited the non-pruriceptive neurons that selectively transmitted signals elicited by painful stimuli67. It was deemed likely that morphine indirectly affected these projection neurons by acting on opiate receptors on primary afferent terminals or on spinal interneurons. For example, there may be an inhibition of interneurons that suppress pruriceptive transmission (Fig. 5A). However, a recent study provides evidence that morphine induced analgesia and itch are mediated in parallel by different isoforms of mu-opioid receptor 1 (MOR1) and MOR1D, respectively, and that MOR1D, by heterodimerizing with GRPR, activates the GRPR-expressing pruriceptive neurons68.
Pruritic and algesic chemical stimuli can induce persistent enhanced mechanically evoked itch or pain within the region of application and in the skin surrounding the locus (known as primary and secondary dysesthesias, respectively). In the skin surrounding a local site of pruritogen application, itch is sometimes evoked by innocuous tactile stroking of the skin (alloknesis), and/or there may be enhanced mechanically evoked pricking pain (hyperalgesia) or itch (hyperknesis)7,9 (Fig. 6). One or more of these abnormal, unpleasant states or “dysesthesias” may outlast the itch, sometimes by an hour or longer. Similarly, an intradermal injection of capsaicin produces mechanically evoked hyperalgesia and sometimes also hyperknesis7 that can long outlast the chemically evoked pain but, in contrast to the effects of a pruritogen, produces allodynia (pain or tenderness to innocuous stroking) rather than alloknesis7,69.
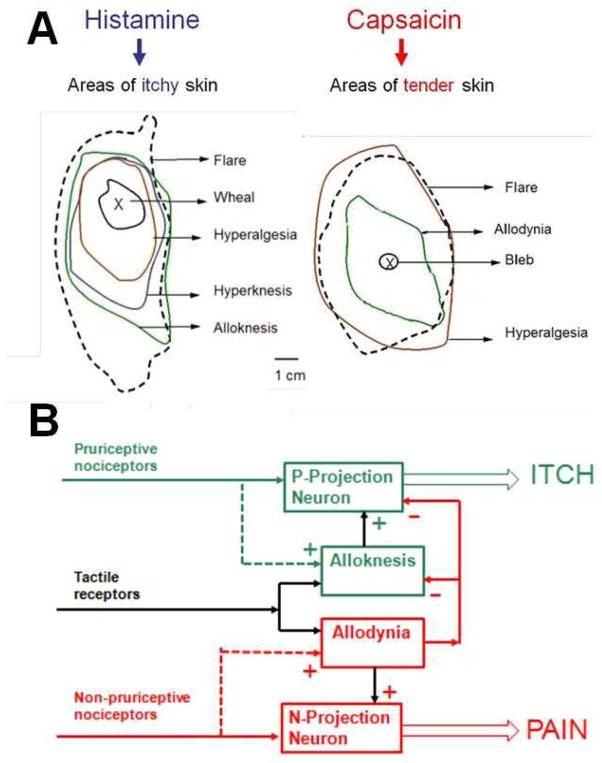
Figure 6. Secondary dysesthesias in response to pruritic or algesic chemicals.
A. The images show, for a human subject, the typical areas of skin reactions and hypersensitivities to mechanical stimulation surrounding the site of intradermal injection of either histamine or capsaicin. The chemical stimuli elicit a neurogenic flare and areas of increased pain and itch in response to punctuate mechanical stimuli. These responses include hyperalgesia, an enhanced pricking pain to a normally painful von-Frey filament (200 μm diameter, 200 mN bending force) and hyperknesis (enhanced pricking evoked itch to a von-Frey filament of 50 μm and 20mN)7. Histamine can evoke alloknesis but not allodynia (that is, it can evoke itch, but not pain or tenderness, in response to gently stroking the skin) whereas the reverse is true after capsaicin. In addition, the area of allodynia is “anti-pruritic” – that is, itch cannot be elicited by injection of histamine74. B. The schematic shows that chemosensitive nociceptive primary afferents differ in their central projections and capacities to selectively enhance (sensitize, shown by dashed lines and + symbols) the responses of a projection neuron to certain types of input (here tactile receptors are used as an example). Pruriceptive primary afferents, such as those responsive to histamine enhance the responses of some but not all pruriceptive (P) STT neurons. Non-pruriceptive nociceptive primary afferents that respond to capsaicin are capable of sensitizing some, though not all, non-pruriceptive (N) STT neurons thereby contributing to tactile allodynia. The presence of allodynia blocks (indicated by the - symbol) the occurrence of itch and alloknesis.
Some pruriceptive and non-pruriceptive projection neurons in the spinal dorsal horn become sensitized (in a process known as central sensitization) after pruritic or algesic chemical stimulation, thereby providing a possible substrate for mechanical secondary dysesthesias. After an injection of histamine, a minority of histamine-responsive STT neurons in the monkey exhibit increased responses to mechanical stroking or to punctuate indentation of the skin with a probe that evokes pricking pain in humans50,52. That is, these neurons become sensitized, and such sensitization, if maintained chronically, might contribute to the itch evoked by painful stimuli in patients with atopic dermatitis70. Similarly, many non-pruriceptive nociceptive STT neurons in monkey become sensitized to mechanical stimuli after an injection of capsaicin50,71. Although there are studies of the cellular mechanisms of central sensitization72, it remains to be discovered what neural circuitry (Fig. 5B) might account for the fact that secondary dysesthesias induced by a pruritic (or algesic) stimulus typically occur in response to mechanical stimuli and not to other types of stimuli such as to heat. A puzzle for those attempting to understand the neural mechanisms of the mechanical dysesthesias induced by a pruritic chemical is that after capsaicin injection the incidence and magnitude of sensitization are greater in both non-pruriceptive and pruriceptive STT neurons50. Thus, whether enhanced mechanically-evoked activity in these two populations of neurons will result, for example, in alloknesis or allodynia may be determined by a suprasegmental decoding mechanism.
Itch can be selectively suppressed by noxious stimulation or by a state of hyperalgesia or allodynia. In the presence of certain types of chronic pain, histamine applied to neuropathic hyperalgesic skin can elicit pain instead of itch73. Similarly, itch is suppressed within an area of allodynia surrounding the site of an injection of capsaicin74 (Fig. 6).
Ongoing noxious/painful stimulation can sometimes suppress an on ongoing experimentally produced itch75. In monkeys, the responses of pruriceptive STT neurons to histamine are suppressed when the skin is scratched76 (Fig. 5C). However, in the same neurons capsaicin-induced responses were increased by scratching. This may be due to the action of excitatory interneurons receiving input from non-pruriceptive, capsaicin sensitive, mechanically insensitive nociceptors. In mice, responses of dorsal horn neurons to pruritogens are decreased by scratching and this effect is prevented by the application of antagonists of the inhibitory neurotransmitters glycine and γ-aminobutyric acid (GABA) that are released from interneurons77. However, pruriceptive dorsal horn neurons in mice, like the STT neurons in monkey, are normally excited by noxious stimuli – therefore, the neural circuitry governing the distinction between itch and pain does not occur in the dorsal horn and is likely located in the brain.
How is itch decoded?
As described above, neurons that respond to pruritic stimuli are a subgroup of nociceptive neurons. This raises the issue of how information contained in the activity of these neurons might be used by the brain to produce the specific sensation of itch. In the following section we review some of the models that have been proposed to address this problem.
Specificity and population models
The classical notion of specificity is that there are peripheral sensory neurons that are activated solely by pruritic stimuli (but not by other stimuli such as those that evoke pain) causing activation of a “‘labeled line” pathway in the CNS that produces itch. But although there are peripheral neurons with receptors for specific chemical pruritogens, these neurons also respond to noxious stimuli that evoke pain (Fig. 7A). This raises the following question: would the selective activation of a certain type of pruriceptive neuron such as those expressing the MrgprA3 receptor, elicit itch or pain? To address this question, a genetically modified mouse in which TRPV1 is expressed only in MrgprA3-positive neurons was generated29. In these mice, capsaicin injection (normally painful) into the cheek evoked scratching (indicative of itch) and not wiping (indicative of pain) presumably because only pruriceptive MrgprA3 neurons were activated and the non-pruriceptive neurons that mediate pain could no longer respond to capsaicin (Fig. 7B). The findings suggest one mechanism by which the brain might decode itch from pain. The selective activation of these neurons -even by a noxious stimulus- elicits itch and it is the action potentials in these neurons and not the stimulus itself that are linked to itch (consistent with Muller’s doctrine of specific nerve energies).
Figure 7. Representing itch versus pain in the responses of nociceptive neurons.
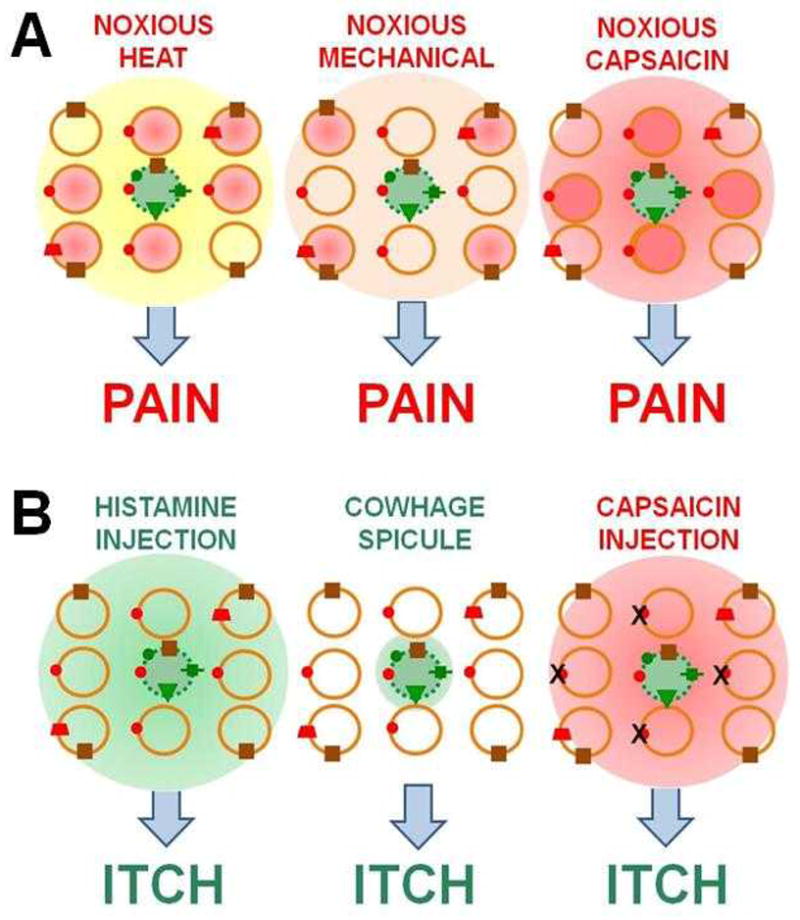
A: The matrix of circles represents a population of neurons exposed to noxious stimulation produced by heat (yellow), mechanical indentation (brown) or capsaicin injection (red). Responsive and non-responsive neurons are respectively shaded in or open. In this example, the pruriceptive neuron (green dashed circle) expresses MrgprA3 (in mouse). Both pruriceptive (green circles) and non-pruriceptive (orange circles) neurons respond to noxious stimuli B: Selective activation of the pruriceptive neuron by histamine, by a cowhage spicule, and by an injection of capsaicin (the latter in a mouse in which the capsaicin receptor has been knocked out29. The schematic illustrates how the sensation of itch may be derived from both the specific types of neurons activated and the number and distribution of neurons activated as predicted by the ”specificity”, “population”, “intensity”, and “spatial contrast” models of itch.
However, action potentials in these pruriceptive neurons are not always sufficient for itch to occur. In wild type mice (and presumably in humans), noxious stimuli such as capsaicin injection, or noxious heat or mechanical stimuli, typically elicit pain rather than itch, possibly because of the simultaneous activation of non-pruriceptive and pruriceptive nociceptors. Supporting evidence for this concept was obtained from transgenic mice in which the glutamate transporter, VGLUT2 was deleted from most nociceptive neurons62. This deletion resulted in spontaneous itch, a decrease in pain behavior, and a loss of the inhibition of itch by pain such that an intradermal injection of capsaicin (normally painful) evoked itch- and not pain behavior. An analogous result was obtained after silencing putative pain mediating neurons thought to express both TRPV1 and TRPA1 (respective receptors for capsaicin and allyl isothiocyanate)30. Specifically, after injecting the skin with a charged sodium channel blocker that selectively silenced neurons expressing either TRPA1 or TRPV1 a subsequent injection of either capsaicin or allyl isothiocyanate evoked itch- and not the usual pain behavior. The itch was thought to result from the activation of TRPA1 or TRPV1 in pruriceptive neurons without the activation of the hypothesized pain mediating neurons that express both receptors30. The implication of these collective findings is that under normal conditions (in wild type mice) sufficient activation of pain mediating neurons somehow prevents or masks the effects of simultaneous activity in pruriceptive neurons and pain without itch results. This would be consistent with a “population theory” of the neural code upon which itch is based78. Thus, whether an itch or pain sensation occurs depends on the relative activity in neurons constituting two labeled lines, one for itch and one for pain such that a sufficiently greater activity in pruriceptive versus non-pruriceptive nociceptive (and perhaps also non-nociceptive) would result in itch. The neural mechanisms by which the brain assesses the relative number and discharge rates of different populations of neurons are, however, unknown.
Intensity and temporal pattern models
There is insufficient support for the idea that itch and pain are mediated solely by weaker activation and stronger activation, respectively, of the same types of nociceptors or that they are distinguished by differences in the temporal pattern of activation79. When itch was elicited in humans by a train of electrical pulses delivered to the skin, alterations in the pulse frequency (and presumably the discharge rate in pruriceptive cutaneous neurons) altered only the degree of itch and did not elicit pain; similarly, changes in the temporal pattern of stimulation did not turn itch to pain or vice versa80,81. The responses of STT neurons to both pruritic agents and capsaicin are bursting in nature52. Although the interburst interval is shorter in response to capsaicin, which might provide a temporal code for pain (vs. itch), this might instead reflect differences in sensory intensity as capsaicin is likely to be perceived as producing a more intense sensation by humans7. However, pruritogens elicit much lower rates of discharge than moderately painful stimuli (such as heat and capsaicin) in peripheral and STT neurons52 and the idea that discharge rates and perhaps the number of activated nociceptive neurons (and perhaps low-threshold neurons as well) have a role in a central neural mechanism that compares activity in different populations of neurons to produce itch has not been ruled out.
Spatial models
There is little evidence that the particular spatial pattern of nerve impulse activity in nociceptive neurons determines whether itch or pain occurs79. Instead, it has been proposed that itch may occur when there is a “spatial contrast” between the activity of one or a few nociceptive cutaneous nerve fibers (even if non-pruriceptive) and the absence of activity in neighboring nociceptive fibers supplying the same and surrounding region of skin20,82. Thus, a cowhage spicule, or a spicule containing only capsaicin11 probably activates only a few CMH nociceptive nerve endings whereas a pinch, a hot probe, or an injection of capsaicin applied to the skin would activate a larger and spatially coherent population of the same and/or other types of nociceptors (Fig. 7). Similarly, histamine activated a larger population of CMH nociceptors (and at greater discharge rates) in the rat when preceded by an application of bradykinin, a sensitizing algogen; and, when preceded by bradykinin in humans, histamine elicited burning instead of itch 83. Thus, it might be argued that if a small population of non-pruriceptive nociceptors - similar in size to the MrgprA3-expressing neuronal population- could be selectively activated it might elicit an itch- and not pain behavior in mice. In that case, a labeled line might not be essential. However, some observations don’t seem to fit with this notion of spatial contrast. For example, humans do not rate itch as decreasing in magnitude in relation to pain with an increase in the dose of histamine or with an increase in the spatial area of skin exposed to histamine when it is injected vs. applied by a single spicule7,11 despite the fact that such increases might be expected to activate a greater number of pruriceptors.
Conclusions and future directions
The generation of chemically evoked itch begins with action potential activity in a subset of peripheral cutaneous nociceptors (pruriceptors) and the concurrent activity of a subpopulation of pruriceptive STT nociceptive neurons that convey pruritic stimulus information to the brain. In primate, discharges in these neurons correlate with the time course of itch in humans. In a genetically engineered mouse, selective activation of a class of pruriceptors elicits itch-like and not pain-like behavior. Pruriceptive neurons readily respond to noxious stimuli that elicit pain, a feature that they share with non-pruriceptive peripheral and STT nociceptive neurons. These pruriceptive and non-pruriceptive neurons are therefore regarded as subclasses of nociceptive neurons. Itch may therefore result from activity in the pruriceptive neurons in the absence of sufficient activity in non-pruriceptive neurons. Itch transmission can be enhanced or reduced in the spinal or medullary dorsal horn. The neural circuitry hypothesized to evaluate the relative activity in pruriceptive and non-pruriceptive neuronal populations and to decode itch from pain is unknown, but likely resides in suprasegmental regions of the brain.
To further our understanding of the neural activity associated with itch, it will be useful to record, in vivo, from projection neurons and their neuronal targets in the midbrain, thalamus and cortex. In addition, cell specific labeling and selective activation of different types of pruriceptive and non-pruriceptive peripheral or central projection neurons are needed to unravel the neuronal mechanism of itch. This might be accomplished in transgenic animals expressing a receptor that could be quantitatively activated, for example, by an optical stimulus, to generate different rates and patterns of action potential activity. One might be able to manipulate the number of neurons activated and the patterns of their discharge to assess the role of the number of afferents and their discharge rates in eliciting itch versus pain behavior. This approach would provide experimental tests of the different models of the neuronal basis for itch.
There is also a need to determine the neural circuitry responsible for different types of central sensitization leading to alloknesis and hyperknesis versus allodynia and hyperalgesia and why secondary dysesthesias typically occur in response to mechanical but not to other types of stimuli.
Ultimately, more electrophysiological experiments in the monkey and psychophysical experiments in humans are needed to help translate the findings of behavioral and molecular studies in rodent to experimental itch and itch disorders in humans. These studies will also be useful to differentiate further the neuronal mechanisms underlying the sensation of pain and itch in human.
Glossary terms
- Central sensitization
An enhanced responsiveness of nociceptive neurons in the central nervous system to normal input from peripheral sensory neurons
- Cutaneous receptive field
an area of skin within which a stimulus activates a sensory neuron, for example, by evoking action potentials
- GRPR
Gastrin-releasing peptide receptor
- Labeled line pathway
A pathway serving a particular sensory quality, such as itch, such that if selectively activated, regardless of the type of activating stimulus, will elicit that type of sensation
- Nociceptor
A high-threshold peripheral receptor or sensory neuron that transduces and encodes noxious stimuli
- Pruriceptor
a nociceptor that responds to one or more pruritic chemicals
- Noxious stimulus
one that is overtly or potentially damaging to normal tissues and/or a stimulus that is normally associated with pain pain-like (nociceptive) sensations, such as pricking/stinging or burning that are unpleasant but may or may not hurt
- TRPA1
Transient receptor potential cation channel, member A1, receptor primarily for electrophilic environmental irritants such as allyl isothiocyanate, a pungent ingredient in mustard oil
- TRPV1
transient receptor potential cation channel subfamily V member 1, the receptor for capsaicin
Contributor Information
Robert H. LaMotte, Email: Robert.LaMotte@yale.edu.
Xinzhong Dong, Email: xdong2@jhmi.edu.
Matthias Ringkamp, Email: PLATELET@jhmi.edu.
References
- 1.Chen SC. Pruritus. Dermatol Clin. 2012;30:309–321. doi: 10.1016/j.det.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuraishi Y. Potential new therapeutic targets for pathological pruritus. Biol Pharm Bull. 2013;36(8):1228–34. doi: 10.1248/bpb.b13-00343. [DOI] [PubMed] [Google Scholar]
- 4.Steinhoff M, Cevikbas F, Ikoma A, Berger TG. Pruritus: management algorithms and experimental therapies. Semin Cutan Med Surg. 30:127–137. doi: 10.1016/j.sder.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hägermark O, Strandberg K, Grönneberg R. Effects of histamine receptor antagonists on histamine-induced responses in human skin. Acta Derm Venereol. 1979;59:297–300. [PubMed] [Google Scholar]
- 6.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–2494. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522–1555. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 9.LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101:1430–1443. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johanek LM, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7567. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, et al. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32:14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johanek LM, et al. A role for polymodal C-fiber afferents in non-histaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and μ-opioid modulation in mice. Acta Derm Venereol. 2010;90:575–581. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- 17.LaMotte RH, Shimada SG, Sikand P. Mouse models of acute, chemical itch and pain in humans. Exp Dermatol. 2011;20:778–782. doi: 10.1111/j.1600-0625.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol. 1991;66:307–315. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- 19.Schmelz M, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. Using microneurography to record neuronal activity from unmyelinated C fibers in human, this paper showed that some mechanoinsensitive C fiber are pruriceptive and respond to histamine though they also respond to a wide range of chemicals including algogens; others are non-pruriceptive and respond to algogens though not to histamine. [DOI] [PubMed] [Google Scholar]
- 20.Namer B, et al. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. Using microneurography in humans, this study found that histamine and cowhage active two separate, non-overlapping populations of unmyelinated C-fibers, i.e. mechanoinsensitive C-fibers and mechanosensitive C fibers. These findings suggest histaminergic and non-histaminergic itch are mediated through separate pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringkamp M, Borzan J, Schaefer K, Hartke TV, Meyer RA. Activation of polymodal nociceptors in monkey by punctate chemical stimulation with histamine and capsaicin. Soc Neurosci Abstr. 2010;584:6. [Google Scholar]
- 22.Ringkamp M, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. The findings of this study demonstrate that activity in A fibers are involved in mediating cowhage-induced itch and nociceptive sensations in humans and that cowhage spicules activate mechanosensitive A fiber nociceptors in the monkey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, Nie H, Gu Q, Sikand P, LaMotte RH. In-vivo responses of cutaneous C-mechanosensitive neurons in mouse to punctate chemical stimuli that elicit itch and nociceptive sensations in humans. J Neurophysiol. 2011;107:357–363. doi: 10.1152/jn.00801.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt R, et al. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schley M, et al. Mechano-insensitive nociceptors are sufficient to induce histamine-induced itch. Acta Derm Venereol. 2013;93:394–399. doi: 10.2340/00015555-1513. [DOI] [PubMed] [Google Scholar]
- 26.Schmelz M, Schmidt R, Handwerker HO, Torebjörk HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–571. doi: 10.1093/brain/123.3.560. [DOI] [PubMed] [Google Scholar]
- 27.Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- 28.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han L, et al. A subpopulation of nociceptors specifically linked to itch. Nature Neurosci. 2012;16:174–182. doi: 10.1038/nn.3289. The results of this study, together with those of [31] demonstrate a type of primary sensory neuron that expresses receptors for multiple pruritogens and that specifically mediates itch-like behavior in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberson DP, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–8. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Q, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:135313–135365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imamachi N, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffry J, Kim S, Chen ZF. Itch signaling in the nervous system. Physiology (Bethesda) 2011;26:286–292. doi: 10.1152/physiol.00007.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim WS, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SR, et al. TRPA1 is required for histamine-independent Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013 May 29;33(22):9283–94. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 39.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 40.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2012;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 2010;477:183–213. doi: 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed] [Google Scholar]
- 42.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease - a ligand of protease activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson SR, et al. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell. 2013 doi: 10.1016/j.cell.2013.08.057. http://dx.doi.org/10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed]
- 44.Ringkamp M, et al. A subclass of cutaneous polymodal nociceptive C fiber afferents in non human primates responds to β-alanine. Soc Neurosci Abstr. 2013;556.07 [Google Scholar]
- 45.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Rau KK, et al. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cavanaugh DJ, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bickford RG. Experiments relating to the itch sensation, its peripheral mechanism and central pathways. Clin Sci. 1938;3:377–86. [Google Scholar]
- 49.Hyndman OR, Wolkin J. Anterior cordotomy: further observations on the physiologic results and optimum manner of performance. Arch Neurol Psychiatry. 1943;50:129–148. [Google Scholar]
- 50.Simone DA, et al. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 51.Davidson S, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson S, et al. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. With the use of antidromic stimulation at the thalamus to identify spinothalamic dorsal horn neurons in monkeys, this study, together with [51] found that separate populations of nociceptive dorsal horn neurons in primate are activated by histamine and cowhage spicules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atanassoff PG, et al. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens Mot Res. 1999;16:291–298. doi: 10.1080/08990229970357. [DOI] [PubMed] [Google Scholar]
- 54.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–24. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 57.Sun YG, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. These findings, along with those of [56], support the conclusion that neurons in the dorsal horn that express the receptor for gastrin-releasing peptide are necessary for itch-like behavior in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang FX, et al. Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J Neurosci. 2010;30:10927–10938. doi: 10.1523/JNEUROSCI.0657-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akiyama T, Tominaga M, Takamori K, Carstens MI, Carstens E. Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain. 2013 Sept. doi: 10.1016/j.pain.2013.09.011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagerstrom MC, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. The findings of this study (together with [61]) support the conclusion that neurons expressing VGLUT2 exert an inhibitory action on itch transmission in the mouse. Mice lacking VGLUT2 in most nociceptors exhibit enhanced itch-like behavior and exhibit itch- rather than pain-like behavior in response to capsaicin injection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross SE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. These authors discovered that a specific population of inhibitory interneuron in the spinal cord acts to suppress itch-like behavior in the mouse; the loss of these neurons results in a pathological increase in itch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Curr Opin Neurobiol. 2011;21(6):880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Kam PC, Tan KH. Pruritus--itching for a cause and relief? Anaesthesia. 1996;51:1133–1138. doi: 10.1111/j.1365-2044.1996.tb15050.x. [DOI] [PubMed] [Google Scholar]
- 66.Ko MC, Naughton NN. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moser HR, Giesler GJ., Jr Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci. 2013;33:6093–6101. doi: 10.1523/JNEUROSCI.0216-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu XY, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.LaMotte RH, Shain CN, Simone DA, Tsai EFP. Neurogenic hyperalgesia psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 70.Ikoma A, et al. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- 71.Simone DA, et al. Neurogenic hyperalgesia: Central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- 72.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 73.Baron R, Schwarz K, Kleinert A, Schattschneider J, Wasner G. Histamine induced itch converts into pain in neuropathic hyperalgesia. Neuroreport. 2001;12:3475–3478. doi: 10.1097/00001756-200111160-00020. [DOI] [PubMed] [Google Scholar]
- 74.Brull SJ, Atanassoff PG, Silverman DG, Zhang J, LaMotte RH. Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens Mot Res. 1999;16:299–303. doi: 10.1080/08990229970366. [DOI] [PubMed] [Google Scholar]
- 75.Yosipovitch G, Fast K, Bernhard JD. Noxious heat and scratching decrease histamine-induced itch and skin blood flow. J Invest Dermatol. 2005;125:1268–1272. doi: 10.1111/j.0022-202X.2005.23942.x. [DOI] [PubMed] [Google Scholar]
- 76.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6:e22665–22669. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma Q. Population coding of somatic sensations. Neurosci Bull. 2012;28:91–99. doi: 10.1007/s12264-012-1201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- 80.Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Electrically evoked itch in humans. Pain. 2005;113:148–154. doi: 10.1016/j.pain.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol. 1982;79:368–373. doi: 10.1111/1523-1747.ep12529734. [DOI] [PubMed] [Google Scholar]
- 82.Namer B, Reeh P. Scratching an itch. Nat Neurosci. 2013;16:117–118. doi: 10.1038/nn.3316. [DOI] [PubMed] [Google Scholar]
- 83.Koppert W, Reeh PW, Handwerker HO. Conditioning of histamine by bradykinin alters responses of rat nociceptors and human itch sensation. Neurosci Lett. 1993;152:117–120. doi: 10.1016/0304-3940(93)90497-9. [DOI] [PubMed] [Google Scholar]
- 84.Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci. 2001;21:4460–4468. doi: 10.1523/JNEUROSCI.21-12-04460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, et al. Ontogeny of excitatory spinal neurons processing distinct somatic sensory modalities. J Neurosci. 2013;33:14738–14748. doi: 10.1523/JNEUROSCI.5512-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]