Abstract
Disease-specific induced pluripotent stem cells (iPSCs) provide an unprecedented opportunity to establish novel disease models and accelerate drug development using distinct tissue target cells generated from isogenic iPSC lines with and without disease-causing mutations. To realize the potential of iPSCs in modeling acquired diseases which are usually heterogeneous, we have generated multiple iPSC lines including two lines that are JAK2-wild-type and four lines homozygous for JAK2-V617F somatic mutation from a single polycythemia vera (PV) patient blood. In vitro differentiation of the same patient-derived iPSC lines have demonstrated the differential contributions of their parental hematopoietic clones to the abnormal erythropoiesis including the formation of endogenous erythroid colonies. This iPSC approach thus may provide unique and valuable insights into the genetic events responsible for disease development. To examine the potential of iPSCs in drug testing, we generated isogenic hematopoietic progenitors and erythroblasts from the same iPSC lines derived from PV patients and normal donors. Their response to three clinical JAK inhibitors, INCB018424 (Ruxolitinib), TG101348 (SAR302503), and the more recent CYT387 was evaluated. All three drugs similarly inhibited erythropoiesis from normal and PV iPSC lines containing the wild-type JAK2 genotype, as well as those containing a homozygous or heterozygous JAK2-V617F activating mutation that showed increased erythropoiesis without a JAK inhibitor. However, the JAK inhibitors had less inhibitory effect on the self-renewal of CD341 hematopoietic progenitors. The iPSC-mediated disease modeling thus underlies the ineffectiveness of the current JAK inhibitors and provides a modeling system to develop better targeted therapies for the JAK2 mutated hematopoiesis.
Keywords: Induced pluripotent stem cells, Hematopoietic progenitor cells, Erythropoiesis, Preclinical drug evaluation, Hematopoietic malignancies
Introduction
Disease-specific induced pluripotent stem cells (iPSCs) provide an unprecedented opportunity to establish novel human cell-based disease models and accelerate drug development [1-3]. In addition to the recent success of modeling inherited forms of blood disorders [4-9], progress in integration-free reprogramming of human blood cells through episomal vectors [10-12] has made it more feasible to develop iPSC models for studying acquired blood diseases such as myeloproliferative neoplasms (MPNs), aplastic anemia, myelodysplastic syndrome, paroxysmal nocturnal hemoglobinuria, and many forms of leukemia. Most of these diseases are heterogeneous and are caused by accumulation of somatic mutations in hematopoietic lineages. Distinct iPSC clones derived from each patient of an acquired disease may provide unique opportunities for understanding the sequential genetic events underlying disease development.
Patient-specific iPSCs can also have major contributions to therapeutic development by providing new platforms of drug screening and testing. Traditional drug screening and testing relies heavily on immortalized human cell lines or non-human cells. Primary cells from patients represent the most physiologically relevant cell source; however, they are typically a heterogeneous mixture of normal and diseased cells, and their availability is usually limited and difficult to sustain. As exemplified by myeloid cells in polycythemia vera (PV), these cells are composed of a mixture of clonal JAK2- V617F homozygous, heterozygous, and also by clonal JAK2 wild-type and normal polyclonal hematopoietic cells [13, 14], making the functional analyses of native cells challenging because of their genetic diversity.
Patient-iPSCs can be expanded without limits and redifferentiated back to the cell types most relevant to the disease and drug targets [3, 15]. In addition, the clonality of iPSCs that capture the exact genetic complement of a parental somatic cell makes it possible to determine the drug effects specific to the investigated genotype. The latter feature is particularly attractive as the primary patient samples are often phenotypically and genetically heterogeneous such as that in MPNs including PV, essential thrombocythemia (ET), and primary myelofibrosis [16-20]. Although patient-specific iPSCs have been used for drug testing and screening in genetic diseases [21-23], this approach has not been reported for acquired hematologic diseases associated with an array of somatic DNA changes and in some instances germline predisposing DNA alterations [24-28].
The acquired somatic mutation, IAK2-V617F, is associated with approximately 95% of PV patients and half of the ET and myelofibrosis patients [29-32]. We have established PV-specific iPSC lines from multiple patients with various allelic burdens of JAK2-V617F (Table 1) [33]. These iPSC lines, especially their hematopoietic progeny at various stages of lineage commitment and differentiation, provide a unique opportunity to examine the potential of drug testing for MPNs. JAK kinase inhibitors are the first targeted therapy for the MPN, with INCB018424 (Ruxolitinib) first approved by FDA in November 2011 for clinical use [34-37]. Several other JAK inhibitory drugs such as TG101348 (SAR302503) [38-40] and more recently CYT387 [41, 42] are being evaluated in clinical trials. However, the existing laboratory and clinical data show that INCB018424 and TG101348 do not suppress the mutated JAK2-V617F myeloid or erythroid cells selectively while blunting hematopoiesis from both wild-type and the mutated hematopoietic cells. Using the human iPSC-based in vitro hematopoietic differentiation model, we investigated the effects of INCB018424, TG101348, and CYT387 on hematopoietic cells differentiated from iPSC clones derived from normal and PV patients, some of them are from the same patient but with different JAK2 genotypes.
Table 1. PV-specific and control iPSC lines used in this study.
| iPSC clone | Donor | JAK2 WT allele |
JAK2 V617F allele |
|---|---|---|---|
| BC1 | Healthy donor | 2 | 0 |
| TNC1 | Sickle cell anemia | 2 | 0 |
| iPV183 | PV patient #1 | 1 | 1 |
| PVB1.11 | PV patient #2 | 2 | 0 |
| PVB1.15 | 2 | 0 | |
| PVB1.4 | 0 | 2 | |
| PVB1.18 | 0 | 2 |
A panel of iPSCs derived from healthy donors and PV patients were used for this study. In addition to a healthy donor- and a sickle cell anemia patient-derived iPSCs, two clones generated from a PV patient’s blood cells also contained only wild-type JAK2 alleles (WT/WT). iPSC clones with homozygous JAK2 V617F mutation (V617F/V617F) were derived from the same PV patient. iPSCs with heterozygous JAK2 (WT/V617F) were derived from a separate PV patient in a previous study [33].
Abbreviations: iPSC, induced pluripotent stem cell; PV, polycythemia vera.
Materials and Methods
Approvals of Using Human iPSCs and Primary Cells from Anonymous Donors
The experimental designs using human iPSCs were approved by the Institutional Stem Cell Research Oversight (ISCRO) committee in the Johns Hopkins University. Use of anonymous human samples for laboratory research was approved by the Institutional Review Board of the Johns Hopkins University and for UT001 patient samples at University of Utah, Salt Lake City.
Derivation and Expansion of Integration-Free iPSCs from Patient Blood Cells
Control iPSC lines BC1, TNC1, and one PV-iPSC line iPV183 were reported previously [10, 33]. For this study, we primarily used iPSC-derived from hematopoietic cells by nonintegrating method. The virus-free, integration-free iPSC derivation protocol was described in detail previously [11]. Briefly, 1 × 107 mononuclear cells from PV patient peripheral blood were cultured in serum-free medium (SFM) containing stem cell factor (SCF), interleukin-3 (IL-3), insulin-like growth factor-1, erythropoietin (EPO), and dexamethasone for 11 days before nucleofected with EBNA1-based episomal vectors pEB-C5 (addgene.org plasmid # 28213) and pEB-Tg (addgene.org plasmid# 28220) expressing Oct4, Sox2, Klf4, c-Myc, and Lin28 (in pEB-C5) and SV40 large T antigen.
A female patient (UT001) with typical PV and a high allele burden (99%) of JAK2-V617F, and approximately 1% of wild-type JAK2, was treated with pegylated interferon alpha (peg-IFNα). The JAK2-V617F allele burden decreased after the treatment. Peripheral blood mononuclear cells from this patient with a neutrophil JAK2-V617F allelic burden of 58% were used for derivation of iPSCs. JAK2-V617F genotyping was performed as previously described [43]. Human iPSC-like colonies were picked 14 days after nucleofection and expanded on Matrigel-coated plates with NutriStem medium (Stemgent, Cambridge, MA, http://www.stemgent.com) and were passaged with collagenase type IV (Sigma, St. Louis, MO, http://www.sigmaaldrich.com) or more recently cultured with the E8 medium (Life Technologies, Rockville, MD, http://www.lifetech.com) and passaged with Accutase (Sigma). Human iPSCs with and without somatic mutations were characterized by standard pluripotency and karyotyping assays [33]. For pluripotency marker staining, cells were grown on mouse embryonic fibroblast (MEF) feeder plates and fixed by 4% paraformaldehyde before primary antibody staining. Alexa Fluor 555-conjugated secondary antibodies (Life Technologies) were used for fluorescent imaging.
The use of immunodeficient mice for the teratoma formation assay was approved by the Animal Care and Use Committee at Johns Hopkins University. For teratoma assays, 5 × 106 undifferentiated iPSCs were harvested and injected intramuscularly into NOD/SCID IL2 receptor gamma chain knockout (NSG) mice with 200 μL of (1:1) diluted Matrigel (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) solution. Between 5 and 8 weeks after injection, tumors were harvested and sectioned for H&E staining.
Hematopoietic and Erythroid Differentiation of iPSCs
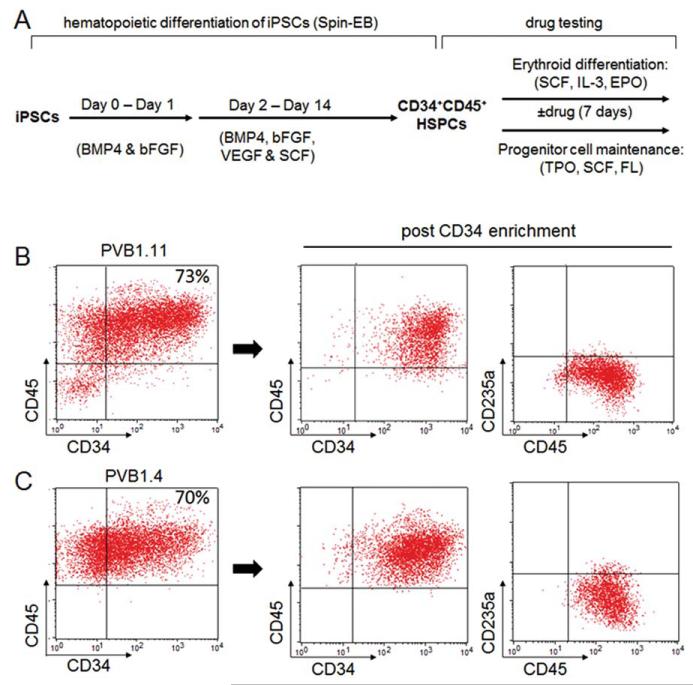
Human iPSCs were differentiated into hematopoietic cells following a spin-EB protocol (Fig. 2A) [33, 44]. Briefly, iPSCs were harvested by Accutase (Sigma) and plated into 96-well U bottom plates at density of 3,000–5,000 cells per well in a SFM containing bone morphogenetic protein 4 (BMP4) (10 ng/mL), basic fibroblast growth factor (bFGF) (10 ng/mL), vascular endothelial growth factor (VEGF) (10 ng/mL), and SCF (50 ng/mL). 50 microliter of fresh medium containing growth factors was added to the wells on day-2, −5, −8 and −11. On day 8, half of the medium was removed before addition of fresh medium. Cells resembling blood cell morphology can be observed after day 10. On day 14 of the spin-EB differentiation, many hematopoietic cells expressing CD45+ were released from embryoid bodies (EBs) into medium. Suspension cells (nearly all of them CD45+ positive, Fig. 2) were harvested and immature cells expressing the CD34 marker were enriched by positive selection through magnetic-activated cell sorting (MACS) cell separation (Miltenyi, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com) following manufacturer’s instruction.
Figure 2.
The in vitro hematopoietic differentiation-based disease modeling and drug testing. (A): A diagram of the spin-EB hematopoietic differentiation procedure and the following drug testing experiments using the iPSC-derived hematopoietic progenitors. iPSCs from both PV patients and controls were first differentiated into CD34+CD45+ hematopoietic progenitors using serum-free medium in the presence of bFGF, BMP4, VEGF, and SCF. The differentiated hematopoietic progenitors were then used to assess their responses to JAK inhibitors in both erythroid differentiation and progenitor self-renewal assays. (B): Flow cytometry analysis of the suspension cells harvested at the end of the 14-day spin-EB differentiation of PVB1.11 (JAK2 wild-type iPSC from a PV patient) showed high percentage of CD34+CD45+ cells. The CD34+CD45+ hematopoietic progenitor cells can be further enriched by CD34 positive selection. (C): Compared to PVB1.11, the JAK2 V617F-positive iPSC PVB1.4 (derived from the same patient as PVB1.11) showed comparable level of CD34+CD45+ hematopoietic cells at the end of the 14-day spin-EB differentiation. Abbreviations: BMP4, bone morphogenetic protein 4; bFGF, basic fibroblast growth factor; EPO, erythropoietin; FL, Flt-3 ligand; HSPCs, hematopoietic stem/progenitor cells; IL-3, interleukin 3; iPSCs, induced pluripotent stem cells; SCF, stem cell factor; TPO, thrombopoietin; VEGF, vascular endothelial growth factor.
For erythroid liquid culture, the purified CD45+CD34+ hematopoietic stem/progenitor cells (HSPCs) were plated in SFM containing 50 ng/mL SCF, 10 ng/mL IL-3, and EPO at various concentrations, ranging between 0 and 3 units/mL. The cells were cultured for 7 days before being harvested and stained by allophycocyanin (APC)-conjugated CD45 and phycoerythrin (PE)-conjugated CD235a antibodies (Life Technologies) and analyzed by flow cytometry using FACSCalibur (BD Biosciences).
For endogenous erythroid colony (EEC) forming assay, 1 × 104 cells were plated per dish into MethoCult H4534 Classic without EPO (Stem Cell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) for colony forming. Colonies were enumerated between day 12 and day 14.
Drug Treatment of iPSC-Derived Hematopoietic Cells
The CD45+CD34+ HSPCs generated from iPSCs were cultured in the SFM containing SCF (50 ng/mL), IL-3 (10 ng/mL), and EPO (0.2 unit/mL) for 7 days in the absence or presence of JAK inhibitor INCB018424, TG101348, or CYT387 (all from ChemieTek, Indianapolis, IN, http://www.chemietek.com). 1.5 × 105 cells were plated per well in 12-well culture plates in each culture condition. The fold of expansion was calculated after counting total live cell numbers by trypan blue exclusion and Countess Automated cell counter (Life Technologies) at the end of the 7-day culture. Cells were also stained by APC-conjugated CD45 and PE-conjugated CD235a antibodies (Life Technologies) and analyzed by flow cytometry using FACSCalibur (BD Biosciences).
For hematopoietic progenitor growth (or maintenance) assay, cells harvested at day-14 of iPSC differentiation were cultured in SFM containing thrombopoietin (TPO) (20 ng/mL), SCF (100 ng/mL), and Flt-3 ligand (FL) (100 ng/mL) for 7 days in the absence or presence of JAK inhibitors. 5 × 105 cells were plated in each culture condition. Fold of expansion was calculated after counting total live cell numbers at the end of 7-day culture. Cell surface expression of CD34 and CD45 were analyzed by flow cytometry. At the end of culture from dimethyl sulfoxide (DMSO) control condition, INCB018424 (250 nM), TG101348 (250 nM), or CYT387 (250 nM) treated conditions, 1 × 104 cells were plated into MethoCult H4034 medium (Stem Cell Technologies) for colony forming unit (CFU) assay. Colonies were numerated at day 14.
Quantitative Real-Time Polymerase Chain Reaction
Human iPSC-derived CD34+ cells enriched by MACS and the cells further cultured for 5 days in erythroid differentiation condition (SFM supplemented with 50 ng/mL SCF, 10 ng/mL IL-3, and 2 unit/mL EPO) or in progenitor expansion condition (SFM supplemented with 20 ng/mL TPO, 100 ng/mL SCF, and 100 ng/mL FL) were harvested for total RNA isolation using RNeasy isolation kit (Qiagen, Hilden, Germany, http://www1.qiagen.com). 0.5 microgram total RNA from each group was used for cDNA synthesis by SuperScript III first-strand synthesis kit (Life Technologies). TaqMan JAK2 assay (Hs.00234567_m1, Life Technologies) was used for quantitation of JAK2 expression by StepOne Plus Quantitative PCR instrument (Life Technologies). β-Actin was used as control.
Results
Generation of iPSCs with Distinct JAK2-V617F Allele Compositions from Blood Samples of the Same PV Patient
Using retroviral vectors and patient CD34+ cells, we have previously generated PV-specific iPSC line iPV183 containing heterozygous JAK2-V617F mutation (Table 1) [33]. Genotyping of the patient (PV patient 183 [45]) CD34+ progenitor cells, neutrophils, and burst forming unit-erythroid (BFU-e) cells had revealed 100% heterozygous JAK2-V617F genotype; therefore, all the iPSC clones obtained from this patient carry the heterozygous JAK2-V617F genotype. In order to generate integration free PV-specific iPSCs that have various JAK2 genotypes, we used a recently developed episomal vector-based technology [11] to reprogram blood cells from a female PV patient UT001. Genotyping of her neutrophils at the time of reprogramming revealed a 58% JAK2-V617F allele burden. After expansion in an erythroblast culture condition, patient peripheral blood unfractionated mononuclear cells were reprogrammed with episomal vectors [11]. JAK2-V617F allele burden analysis of the iPSCs showed that both JAK2 wild-type and JAK2-V617F homozygous cell lines were derived from this patient (Table 1). The iPSC derivation and expansion efficiency appeared normal as compared to those from normal and other types of blood samples. The iPSC lines with different JAK2 genotypes appeared normal at the rate similar to iPSCs derived from other normal cell types. Only the iPSC lines with a normal (cytogenetic) karyotype and validated pluripotency were used for this study (Fig. 1). Genotyping of the JAK2 gene also confirmed that iPSC lines from a healthy donor and a non-MPN (sickle cell anemia) patient [10] possess only the wild-type JAK2 allele, while the previously reported PV iPSC line iPV183 was heterozygous for JAK2-V617F as in the patient [33].
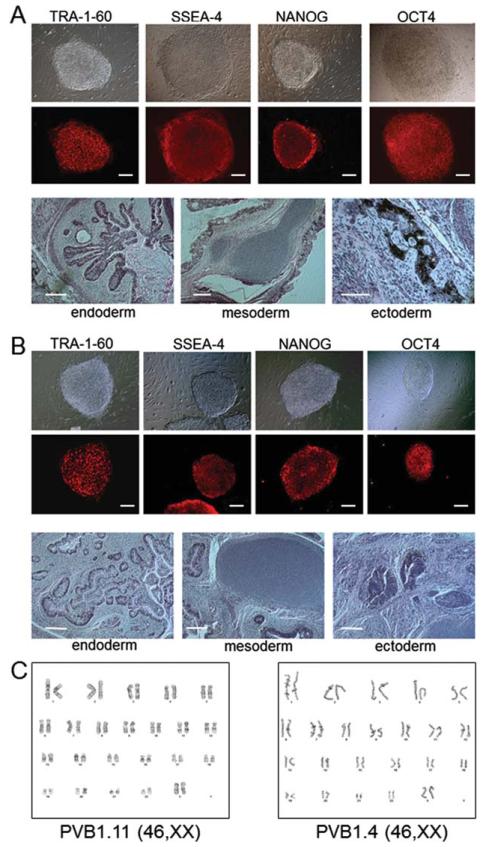
Figure 1.
Characterization of induced pluripotent stem cells (iPSCs) with various JAK2-V617F compositions derived from a single polycythemia vera (PV) patient. Representative images of undifferentiated phenotypes and in vivo pluripotency assays of JAK2 wild-type (PVB1.11, shown in (A)) and JAK2 V617F (PVB1.4, shown in (B)) PV-specific iPSCs. Colonies cultured on mouse embryonic fibroblasts were stained with antibodies against pluripotency-related cell surface markers TRA-1–60 and SSEA4. The fixed colonies were also stained with antibodies against human embryonic stem cell-specific transcription factors NANOG and OCT-4. Both types of cells were injected into immunedeficient NOD/SCID IL2 receptor gamma knockout (NSG) mice for teratomas formation. H&E staining of the teratomas showed the tissue types of all three embryonic germ layers endoderm, mesoderm, and ectoderm, demonstrating the pluripotency of iPSCs. (C): Karyotyping of both types of iPSCs showed normal karyotype. Scale bar = 200 μm.
Abnormal Erythropoiesis of Hematopoietic Progenitors Derived from PV-Specific JAK2-V617F iPSCs
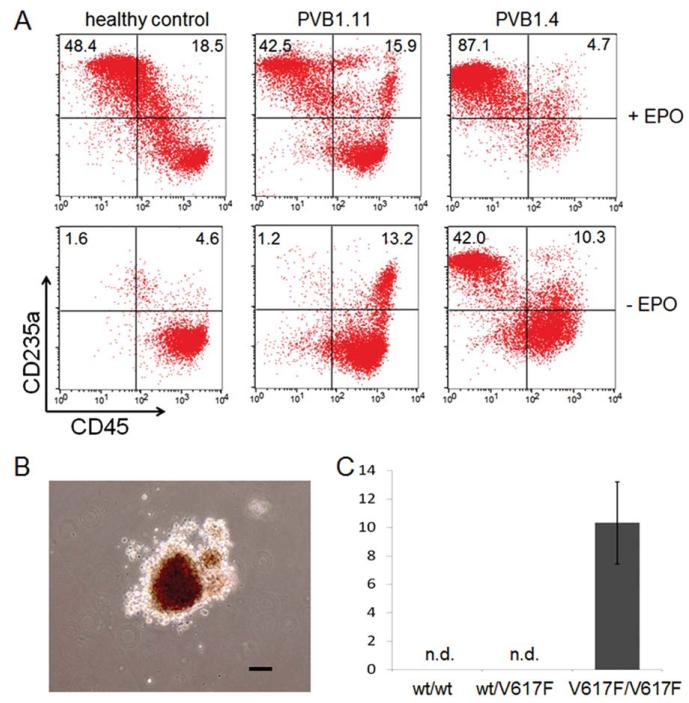
A major clinical feature of PV is the augmented erythropoiesis resulting in overproduction of red blood cells [17]. The panel of iPSCs with various JAK2 allele compositions generated from PV patients and controls provides an opportunity to study the association of the abnormal erythropoiesis as an effect of JAK2-V617F mutation. In order to assess their erythroid differentiation potential, the iPSCs were first differentiated to hematopoietic progenitor cells followed by an erythroid differentiation and expansion step (Fig. 2A) [33, 44]. In the first step, iPSCs were aggregated and cultured as EBs in a SFM supplemented with BMP4, bFGF, VEGF, and SCF. The levels of CD34+CD45+ hematopoietic progenitor cells generated at the end of the 14-day spin-EB differentiation were similar in all the iPSC lines used (Fig. 2B, 2C). The purified CD45+CD34+ HSPCs were then used for erythroid differentiation/expansion in SFM supplemented with SCF, IL-3, and EPO [33, 46]. Since EPO-independent erythropoiesis is a hallmark of PV, differentiation without exogenous EPO was also carried out in parallel to determine if PV-iPSC derived hematopoietic cells could also recapitulate the characteristic. After 7 days in these liquid culture conditions, the cells were enumerated and then analyzed by flow cytometry for their expression of erythroid-specific marker CD235a (Glycophorin A) and downregulation of pan-leukocyte marker CD45 (Fig. 3). All cell lines underwent erythroid differentiation in the presence of EPO, as indicated by the gain of CD235a and downregulation of CD45 (Fig. 3). The PV-specific iPSC line PVB1.4 which is homozygous for JAK2-V617F mutation underwent more extensive differentiation compared to JAK2-wild-type iPSCs (PVB1.11) derived from the same patient or from a healthy control (BC1). The enhanced erythropoiesis associated with JAK2-V617F was more apparent in the absence of EPO. More than 50% of the PVB1.4-derived cells gained the expression of CD235a after 7 days of culture when supplemented with only SCF and IL3 (Fig. 3A), recapitulating the EPO-independent erythropoiesis in liquid culture condition. These results using the iPSC lines derived from the same PV patient also recapitulated the differential contribution to the augmented erythropoiesis by individual hematopoietic clones from the patient.
Figure 3.
Elevated erythroid differentiation of hematopoietic progenitors generated from JAK2-V617F positive polycythemia vera (PV)-specific induced pluripotent stem cells (iPSCs). (A): iPSC-derived CD34+CD45+ progenitors were further differentiated to erythroblasts for 7 days in the presence or absence of EPO. Compared to wild-type JAK2 iPSCs derived from either healthy control (BC1) or PV patient (PVB1.11), the iPSCs with homozygous JAK2-V617F mutation (PVB1.4) displayed elevated erythroid differentiation based on flow analysis of erythroid-specific cell surface marker CD235a and pan-leukocyte marker CD45. (B): Hematopoietic progenitor cells derived from PVB1.4 formed endogenous erythroid colonies (EECs) in methylcellulose assay in the absence of EPO. (C): Only PV-iPSCs with homozygous JAK2-V617F showed the ability to form EECs. wt/wt: the data are an average of results from BC1 iPSC experiments (n = 3); wt/V617F: the data are an average of three experiments using iPV183 (n = 3); V617F/V617F: the data are an average of results from PVB1.4 and PVB1.18 (n = 3). Data are mean ± SD. n.d. = nondetectable. Scale bar = 100 μm. Abbreviation: EPO, erythropoietin.
EEC formation is a typical characteristic of PV [47]. To investigate the relationship between EEC formation and JAK2 allele composition, hematopoietic progenitor cells derived from PV-iPSCs and control iPSCs were plated in methylcellulose medium without EPO. After 14 days of incubation, typical erythroid colonies were formed from cells carrying homozygous JAK2-V617F (Fig. 3C), although the average colony size was smaller compared to those formed in the presence of EPO. In contrast, no EEC formation was observed in other groups that are either JAK2 wild-type or heterozygous for JAK2-V617F.
These results have demonstrated that iPSCs can be efficiently differentiated into CD34+CD45+ HSPCs which could be further differentiated into CD235a+ erythroid cells. The patient-specific iPSCs and the in vitro differentiation conditions not only provide experimental system for studying disease mechanism but also permit assessment of possible differential drug effects on these isogenic cell types at various developmental stages.
INCB018424, TG101348, and CYT387 Drugs Inhibit Erythroid Differentiation of JAK2 Wild-Type and V617F+ Cells
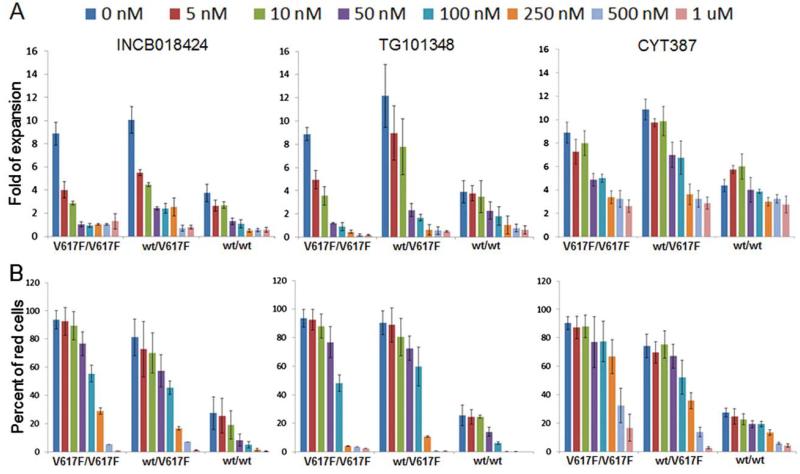
To assess the effects of candidate drugs on erythroblasts and on hematopoietic progenitors, iPSCs with various JAK2 allele compositions (Table 1) were first differentiated into CD34+CD45+ progenitor cells which were obtained by the same protocol (Fig. 2). The progenitor cells were then further cultured, in the presence or absence of drug, in conditions that favor either erythroid differentiation/expansion or HSPC growth/maintenance (Fig. 2A). For evaluation of drug effects on erythropoiesis, the sorted CD34+CD45+ cells were cultured for 7 days in SFM supplemented with SCF, IL-3, and EPO favoring erythroblast expansion and differentiation. Consistent with previous observations [33], the JAK2-V617F+ cells from PV iPSCs under such condition displayed a significant proliferative advantage over JAK2 wildtype cells (Fig. 4A). When JAK inhibitors INCB018424, TG101348, or CYT387 were added to the culture media at various dosages for the whole culture period, all three drugs inhibited cell proliferation in a dosage-dependent manner (Fig. 4A). Cell expansion was almost completely blocked by INCB018424 or TG101348 at doses ≥250 nM regardless of JAK2 mutation status. In comparison, cell proliferation was still observed with CYT387 treatment at doses ≥250 nM although at reduced level (Fig. 4A). To specifically assess the drug effect on erythroid (CD235a+CD45low) cells, we used fluorescence-activated cell sorting (FACS) to detect CD235a+ and CD45+ cells (Supporting Information Fig. S1). The percentage changes of the erythroid cells under each condition showed that at doses ≥250 nM, erythroid differentiation was significantly inhibited by INCB018424 and TG101348 and to a lesser degree by CYT387 (Fig. 4B). These results demonstrated the significant effect of JAK2 inhibitors on erythroblast proliferation, supporting the important role of JAK2 in erythropoiesis, and explained the clinical observation of anemia being one of the most common side effects in the clinical trials of JAK inhibitors INCB018424 and TG101348 [35, 37] while CYT387 has been reported to improve anemia conditions in some myelofibrosis patients [42].
Figure 4.
JAK inhibitors inhibit erythroid differentiation of hematopoietic progenitors generated from diversified induced pluripotent stem cell (iPSC) lines. (A): CD34+ cells generated from iPSCs were cultured in erythroid differentiation conditions for 7 days in the absence or presence of JAK inhibitors. The fold expansion was calculated after counting total cell numbers at the end of the 7-day culture. (B): Erythroid differentiation was measured by flow cytometry for CD235a expression. Percentages of CD235a+ cells at the end of 7-day culture in each culture condition were displayed as “Percent of red cells” on the y-axis. V617F/V617F: the data are an average of results from PVB1.4 and PVB1.18 (n = 2). WT/V617F: the data are an average of two independent experiments from iPV183 (n = 2). WT/WT: the data are an average of results from BC1, TNC1, and PVB1.15 in INCB018424 experiments (n = 3); BC1 and PVB1.11 in TG101348 and CYT387 experiments (n = 2). Data are mean ± SD. Abbreviation: wt, wild type.
The JAK Inhibitory Drugs Have Little Effect on Self-Renewal of CD34+ Progenitor Cells
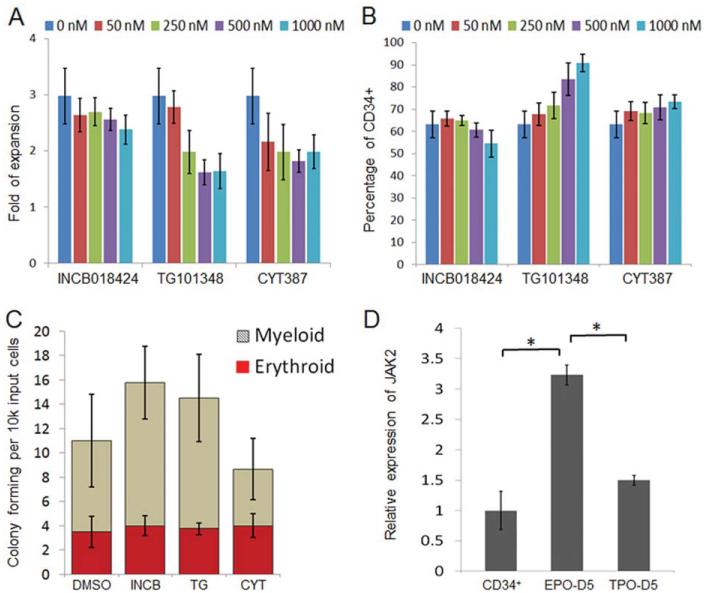
MPNs originate from long-lived malignant HSPCs [17, 20, 48, 49]; therefore, we investigated the effects of these JAK inhibitors on JAK2-V617F CD341 cells. Parallel to the erythroid differentiation, the CD34+CD45+ HSPCs derived from the same panel of PV-iPSCs were cultured in SFM containing SCF, TPO, and FL that favors HSPC self-renewal or maintenance [50]. After culture for 7 days, total cells were enumerated and the percentages of CD341 (also CD451) cells were measured by flow cytometry. Distinct from what was observed in the erythroid culture, the JAK inhibitors had little effect on progenitor cell expansion across a broad range of doses (Fig. 5A). Moreover, the cells in all conditions maintained comparable CD341 percentages (Fig. 5B). Although not statistically significant, slight increase of CD341 percentages were observed at higher doses of TG101348 and CYT387 where erythroid differentiation and expansion were markedly inhibited (Fig. 3). Similar results were obtained with JAK2 wild-type cells (Supporting Information Fig. S2). The colony forming ability of the cells treated at 250 nM was measured: no decrease of erythroid and myeloid progenitor cells was observed compared to the control (Fig. 5C). These data suggest that JAK2 inhibition does not affect HSPC growth while markedly inhibiting erythropoiesis from the same cell types with various JAK2 genotypes. The insensitivity of CD34+ cells to JAK inhibitors may be explained, at least partly, by the lower expression of JAK2 gene in CD34+ HSPCs than in erythroblasts as demonstrated by quantitative polymerase chain reaction analysis (Fig. 5D). The gene expression analysis demonstrated that although the JAK2 expression was significantly upregulated by EPO stimulation, it was much less by TPO or the TPO, SCF, and Flt3-ligand cytokine combination (Fig. 5D). Our data provide strong evidence that the current JAK2 inhibitors effectively inhibit erythropoietic differentiation from HSPCs of various JAK2 genotypes but had little effect on HSPCs, explaining the lack of reduction in JAK2-V617F mutated cells in patients after treatment.
Figure 5.
JAK inhibitors have little effect on self-renewal of CD34+ progenitor cells. Hematopoietic progenitor cells generated from spin-EB differentiation of PVB1.4 induced pluripotent stem cells (iPSCs) were cultured in serum-free medium supplemented with TPO, stem cell factor, and Flt3-L for 7 days. JAK2 inhibitors at indicated concentrations were added throughout the culture. (A): Total cell number in each condition was counted and divided by the input cell number to yield the fold of expansion (n = 2). (B): In the same experiments as shown in (A), cells were also analyzed by flow cytometry and the percentages of CD34+ cells were compared among all culture conditions. (C): Cells cultured with DMSO, 250 nM INCB018424, TG101348, or CYT387 were plated in methylcellulose medium for 14 days. Colonies with myeloid and erythroid morphology were numerated. (D): Purified CD34+ cells from day 14 of spin-EB differentiation of iPSCs, and the cells further differentiated for additional = days in either erythroid culture condition (EPO) or in progenitor culture condition (TPO) were harvested and analyzed by quantitative polymerase chain reaction for JAK2 expression. JAK2 expression in both cell types was first normalized by β-actin expression then normalized to that of the cells cultured in erythroid condition for 5 days (n = 3). Data are mean ± SD. *, p < .05. Abbreviations: DMSO, dimethyl sulfoxide; EPO, erythropoietin; TPO, thrombopoietin.
Discussion
The ability of reprogramming blood cell types not only allowed the generation of patient-specific iPSCs that carry blood-specific somatic mutations but also provides a means of generating distinct iPSC clones with diverse somatic mutations from a single patient. In this study, we have derived iPSCs with various JAK2-V617F allele compositions from the same patient. The generation of distinct iPSC clones with diverse somatic mutations demonstrates the genetic heterogeneity of the PV clones. Given the technical challenges of isolating and expanding individual HSC clones from patients, this iPSC approach may provide unique and valuable insights into the clonal evolution of PV. As red-cell overproduction is one of the major clinical features of PV, the same individualderived iPSC clones with different JAK2 genotypes may also provide an ideal system to elucidate the mechanisms of JAK2-V617F in the abnormal erythropoiesis. Our results have demonstrated the association of JAK2-V617F mutation and EPO-independent erythropoiesis and suggested that the EEC formation is an indicator of the presence of HSC clone(s) with homozygous JAK2-V617F mutation.
Although JAK2-V617F plays important roles in MPN pathology, it has been suggested that this somatic mutation is not the disease-initiating mutation and that identification of other genetic lesion(s) may be crucial for developing strategies to eliminate the disease-causing cells. One of the biggest challenges in MPN research is the identification of such pre-JAK2-V617F malignant clone(s) in the patients. We have derived two JAK2-wild-type iPSC clones from the UT001 patient who possessed high level of JAK2-V617F allelic burden in the blood. Due to the limitation of current knowledge and technology, it remains to be determined whether the JAK2-wild-type HSC clone(s) that gave rise to these iPSCs represent the normal or pre-JAK2-V617F polycythemic clones. The ability to clonally expand such cells through iPSC generation provides additional tools for future identification of such pre-JAK2-V617F malignant clones once a better assay becomes available.
A practical application for patient-iPSCs is drug testing and screening. Compared to most transformed animal or human cancer cell lines that have been frequently used in conventional drug development, patient-specific iPSCs retain their genomic configuration, therefore are more disease-relevant. Unlike the patient primary cells which are often difficult to obtain, iPSCs can be expanded extensively while maintaining karyotypes and differentiation capability. In addition, iPSC clones and their functional derivatives are genetically homogeneous, thus offering the opportunity to develop drugs targeting disease-causing cells. This is another advantage over primary samples, especially those from patients with acquired diseases, which are usually genetically heterogeneous. Moreover, as we have shown in this study, iPSCs can be sequentially differentiated to specific blood lineages, which facilitates the analysis of differential sensitivities to given drugs in isogenic cell types and at various developmental stages. Our study revealed that while the erythroblasts were sensitive to JAK inhibitors, CD34+ progenitors were much more resistant. These results also indicate that none of the tested drugs were selectively inhibitory to JAK2-V617F harboring cells, which corroborates with the clinical findings that the presence of JAK2-V617F mutation in patients does not significantly influence the therapeutic response to JAK inhibitors [34, 36]. The results have also shown that, in comparison to the previously used JAK inhibitors INCB018424 and TG101348, CYT387 inhibited erythroid differentiation to a lesser degree in the dose range tested. This is in agreement with the clinical trial results that patients treated with CYT387 are less likely transfusion-dependent than those treated with INCB018424 and TG101348 drugs [42]. The iPSC-based system described here may offer an in vitro model for development of new drugs that are selectively targeting the mutated cells.
With differentiation methods and screening technology continuously being improved, patient-specific iPSC lines will offer the advantage of identifying drugs specifically eradicating rare but potent malignant subclones such as those in the CD34+ HSPC compartment. This will be particularly beneficial in developing better targeted and efficient treatment for various acquired and inherited diseases with known and unknown mutations.
Summary
We have generated multiple patient-specific iPSC lines with various JAK2-V617F allele compositions from blood cells of a single PV patient. Directed in vitro differentiation of the iPSCs have shown that the JAK2-V617F containing cells underwent enhanced erythropoiesis and that the EEC formation was only observed in cells that are homozygous for JAK2-V617F mutation. Drug testing results using the hematopoietic progenitors and erythroblasts derived from a panel of PV-specific and control iPSCs have shown that three JAK inhibitors INCB018424, TG101348, and CYT387 similarly inhibited erythropoiesis from normal and PV iPSC lines. However, the JAK inhibitors had less inhibitory effect on the self-renewal of CD34+ hematopoietic progenitors. These results of using iPSC-mediated disease modeling underlie the ineffectiveness of the JAK inhibitors in eliminating the disease clones in PV patients. We suggest that this iPSC approach provides valuable system for understanding disease mechanisms and for discovering new drugs.
Supplementary Material
Acknowledgments
We thank Dr. Alison Moliterno for helpful discussions and Ophelia Rogers for assistance in JAK2 V617F genotyping. This work was supported in part by grants from Maryland Stem Cell Research Fund (2011-MSCRFE-0087 led by Z.Y. and 2009-MSCRFII-0047 led by L.C.) and by 1P01 CA108671-O1A2 Myelo-proliferative Disorders (MPD) Consortium project#1 (J.T.P.) and LLS 2012LLS000TRPren0071468 (J.T.P.).
Footnotes
Author Contributions
Z.Y.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; C.F.L., L.L., and S.N.D.: collection and/or assembly of data and data analysis and interpretation; C.H.: collection and/or assembly of data; X.H.: data analysis and interpretation; R.A.B.: data analysis and interpretation and manuscript writing; J.L.S.: provision of study material and manuscript writing; J.T.P.: provision of study material, data analysis and interpretation, and manuscript writing; L.C.: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Yamanaka S. Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Trounson A, Shepard KA, DeWitt ND. Human disease modeling with induced pluripotent stem cells. Curr Opin Genet Dev. 2012;22:509–516. doi: 10.1016/j.gde.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Ye Z, Chou BK, Cheng L. Promise and challenges of human iPSC-based hematologic disease modeling and treatment. Int J Hematol. 2012;95:601–609. doi: 10.1007/s12185-012-1095-9. [DOI] [PubMed] [Google Scholar]
- 4.Zou J, Sweeney CL, Chou BK, et al. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: Functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117:5561–5572. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou J, Mali P, Huang X, et al. Site-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell disease. Blood. 2011;118:4599–4608. doi: 10.1182/blood-2011-02-335554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebastiano V, Maeder ML, Angstman JF, et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells. 2011;29:1717–1726. doi: 10.1002/stem.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raya A, Rodríguez-Pizá I, Guenechea G, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller LU, Milsom MD, Harris CE, et al. Overcoming reprogramming resistance of Fanconi anemia cells. Blood. 2012;119:5449–5457. doi: 10.1182/blood-2012-02-408674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yung SK, Tilgner K, Ledran MH, et al. Brief report: Human pluripotent stem cell models of fanconi anemia deficiency reveal an important role for fanconi anemia proteins in cellular reprogramming and survival of hematopoietic progenitors. Stem Cells. 2013;31:1022–1029. doi: 10.1002/stem.1308. [DOI] [PubMed] [Google Scholar]
- 10.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowey SN, Huang X, Chou BK, et al. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat Protoc. 2012;7:2013–2021. doi: 10.1038/nprot.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, Yu J, Suknuntha K, et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kralovics R, Teo S, Li S, et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood. 2006;108:1377–1380. doi: 10.1182/blood-2005-11-009605. [DOI] [PubMed] [Google Scholar]
- 14.Nussenzveig RH, Swierczek SI, Jelinek J, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35:32–38. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman D. Toward clinical therapies utilizing hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–375. [PubMed] [Google Scholar]
- 17.Spivak JL. Polycythemia vera: Myths, mechanisms, and management. Blood. 2002;100:4272–4290. doi: 10.1182/blood-2001-12-0349. [DOI] [PubMed] [Google Scholar]
- 18.Skoda R. The genetic basis of myeloproliferative disorders. Hematol Am Soc Hematol Educ Program. 2007:1–10. doi: 10.1182/asheducation-2007.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Levine R, Gilliland D. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prchal JF, Prchal JT. Molecular basis for polycythemia. Curr Opin Hematol. 1999;6:100–109. doi: 10.1097/00062752-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, Ramirez CN, Kim H, et al. Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol. 2012;30:1244–1248. doi: 10.1038/nbt.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SM, Kim Y, Shim JS, et al. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57:2458–2468. doi: 10.1002/hep.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kralovics R, Stockton D, Prchal J. Clonal hematopoiesis in familial polycythemia vera suggests the involvement of multiple mutational events in the early pathogenesis of the disease. Blood. 2003;102:3793–3796. doi: 10.1182/blood-2003-03-0885. [DOI] [PubMed] [Google Scholar]
- 25.Landgren O, Goldin LR, Kristinsson SY, et al. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones A, Chase A, Silver R, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41:446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olcaydu D, Harutyunyan A, Jäger R, et al. A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41:450–454. doi: 10.1038/ng.341. [DOI] [PubMed] [Google Scholar]
- 28.Kilpivaara O, Mukherjee S, Schram A, et al. A germline JAK2 SNP is associated with predisposition to the development of JAK2(V617F)-positive myeloproliferative neoplasms. Nat Genet. 2009;41:455–459. doi: 10.1038/ng.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James C, Ugo V, Le Couédic J, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 30.Kralovics R, Passamonti F, Buser A, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 31.Levine R, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Z, Zhan H, Mali P, et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxo-litinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: Survival advantage in comparison to matched historical controls. Blood. 2012;120:1202–1209. doi: 10.1182/blood-2012-02-414631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 38.Geron I, Abrahamsson AE, Barroga CF, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer Cell. 2008;13:321–330. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Lasho TL, Tefferi A, Hood JD, et al. TG101348, a JAK2-selective antagonist, inhibits primary hematopoietic cells derived from myeloproliferative disorder patients with JAK2V617F, MPLW515K Or JAK2 Exon 12 mutations as well as mutation negative patients. Leukemia. 2008;22:1790–1792. doi: 10.1038/leu.2008.56. [DOI] [PubMed] [Google Scholar]
- 40.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardanani A, Lasho T, Smith G, et al. CYT387, a selective JAK1/JAK2 inhibitor: In vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23:1441–1445. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 42.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27:1322–1327. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moliterno A, Williams D, Rogers O, et al. Molecular mimicry in the chronic myeloproliferative disorders: Reciprocity between quantitative JAK2 V617F and Mpl expression. Blood. 2006;108:3913–3915. doi: 10.1182/blood-2006-03-008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng E, Davis R, Stanley E, et al. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 45.Moliterno A, Williams D, Rogers O, et al. Phenotypic variability within the JAK2 V617F-positive MPD: Roles of progenitor cell and neutrophil allele burdens. Exp Hematol. 2008;36:1480–1486. doi: 10.1016/j.exphem.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ugo V, Marzac C, Teyssandier I, et al. Multiple signaling pathways are involved in erythropoietin-independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol. 2004;32:179–187. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Prchal JF, Axelrad AA. Letter: Bone-marrow responses in polycythemia vera. N Engl J Med. 1974;290:1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 48.Jamieson CH, Gotlib J, Durocher JA, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci USA. 2006;103:6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishii T, Bruno E, Hoffman R, et al. Involvement of various hematopoietic-cell lineages by the JAK2V617F mutation in polycythemia vera. Blood. 2006;108:3128–3134. doi: 10.1182/blood-2006-04-017392. [DOI] [PubMed] [Google Scholar]
- 50.Novelli E, Cheng L, Yang Y, et al. Ex vivo culture of cord blood CD34+ cells expands progenitor cell numbers, preserves engraftment capacity in nonobese diabetic/severe combined immunodeficient mice, and enhances retroviral transduction efficiency. Hum Gene Ther. 1999;10:2927–2940. doi: 10.1089/10430349950016348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.