Abstract
We have previously demonstrated that PDGF-BB enhances proliferation of C2 myoblasts. This has led us to examine whether the mitogenic influence of PDGF-BB in the C2 model correlates with modulation of specific steps associated with myogenic differentiation. C2 myoblasts transiting through these differentiation specific steps were monitored via immunocytochemistry. We show that the influence of PDGF on enhancing cell proliferation correlates with a delay in the emergence of cells positive for sarcomeric myosin. We further monitored the influence of PDGF-BB on differentiation steps preceding the emergence of myosin+ cells. We demonstrate that mononucleated C2 cells first express MyoD (MyoD+/myogenin− cells) and subsequently, myogenin. Cells negative for both MyoD and myogenin (the phenotype preceding the MyoD+ state) were present at all times in culture and comprised the majority, if not all, of the cells which responded mitogenically to PDGF. Additionally, the frequency of the MyoD+/myogenin+ cell phenotype was reduced in cultures receiving PDGF, suggesting that PDGF can modulate the transition of the cells into the myogenin+ state. We determined that many of the myogenin+ cells subsequently become MEF2A+ and this phenomenon is not influenced by PDGF-BB. FGF-2 also enhanced the proliferation of C2 myoblasts and suppressed the appearance of the myogenin+ cells, but did not influence the subsequent transition into the MEF2A+ state. The study raises the possibility that PDGF-BB and FGF-2 might delay the transition of the C2 cells into the MyoD+/myogenin+ state by depressing a paracrine signal that enhances differentiation.
Keywords: PDGF, FGF, C2 myoblasts, myogenic regulatory factors, myogenic enhancer factor 2, cyclin A, desmin p21
INTRODUCTION
During development, growth and regeneration of skeletal muscle, myogenic precursor cells proliferate, withdraw from the cell cycle, and differentiate. Studies on isolated myoblasts have identified various growth factors which can modulate proliferation and differentiation of myoblasts maintained in cell culture (reviewed in Olson, 1992, 1993; Grounds and Yablonka-Reuveni, 1993). The role of the fibroblast growth factors (FGFs) during myogenesis has been extensively analyzed, beginning with the studies of Gospodarowicz et al. (1976). Several members of the FGF family have been shown to promote proliferation of cultured myoblasts and various studies have also suggested that FGFs can suppress myogenic differentiation independently of their effect on cell proliferation (Clegg et al., 1987; Hannon et al., 1996; reviewed in Olson, 1992; Olson, 1993).
In contrast to the extensive analyses of the influences of FGFs there are few studies regarding the effect of platelet-derived growth factor (PDGF) on myogenesis. Our studies have previously demonstrated that PDGF enhances proliferation of mouse-derived C2 myoblasts and of adult-type chicken myoblasts. This enhanced proliferation was inferred from the increased 3H-thymidine incorporation detected when PDGF was present (Yablonka-Reuveni et al., 1990; Yablonka-Reuveni and Seifert, 1993; Yablonka-Reuveni, 1995a). For both C2 and chicken myoblasts. PDGF-BB supported more extensive proliferation than PDGF-AB while PDGF-AA did not enhance proliferation. In accordance with the PDGF receptor subunit model (Seifert et al., 1989; reviewed in Heldin, 1992; Claesson-Welsh, 1994), the binding and mitogenic influences of the three PDGF isoforms seen in our studies suggested that C2 and chicken myoblasts express high levels of PDGF receptor beta-subunits and low levels of alpha-subunits. Additionally, we demonstrated that FGF-2 (bFGF) can enhance 3H-thymidine incorporation by C2 cells to levels comparable to those supported by PDGF-BB (Yablonka-Reuveni, 1995a). Independently of our studies, Jin and colleagues have provided evidence for a role of PDGF-BB during myogenesis of rat-derived L6J1 myoblasts and human fetal myoblasts (Jin et al., 1991; 1993). Subsequent studies with clonally-derived turkey and porcine myoblasts (McFarland et al., 1993; Cook et al., 1993; Cook et al., 1995) have provided additional support for the mitogenic role of PDGF-BB during myogenesis in cell culture.
The investigation described in this paper was conducted in order to analyze in detail the influence of PDGF-BB on the myogenesis of C2 cells. We first were interested to determine the effect of the growth factor on proliferation by direct quantification of the dividing cells. This approach, combined with immunocytochemistry. allow the monitoring of which subpopulations of cells were proliferating and which cells were expressing specific proteins related to myoblast proliferation and differentiation. Previous investigations on the mitogenic effect of PDGF which relied on measuring levels of 3H-thymidine incorporation did not enable direct cellular analysis.
Second, we were interested in analyzing the influences of PDGF-BB on the expression of the myogenic regulatory factors MyoD and myogenin as the C2 cells transit from proliferation to differentiation. MyoD and myogenin together with Myf5 and MRF4 comprise a family of myogenic transcription factors. These four myogenic regulatory factors (MRFs) are thought to be involved in myogenic determination during early embryogenesis and in facilitating normal muscle histogenesis (reviewed in Megency and Rudnicki, 1995; Cossu et al., 1996a; Molkentin and Olson, 1996). Expression of the MRFs has also been detected at the mRNA and/or protein level in cultures of myoblasts isolated from developing and postnatal muscle (Hinterberger et al., 1991; Smith et al., 1993; Smith et al., 1994; Yablonka-Reuveni and Rivera 1994). This expression by cells which have already acquired a myogenic fate likely reflects the role of the myogenic factors in regulating the progression from proliferation to myogenic differentiation (reviewed in Olson 1992; Olson 1993; Weintraub 1993). In cultures of rodent myoblasts, MyoD expression precedes myogenin expression (Hinterberger et al., 1991; Smith et al., 1994; Yablonka-Reuveni and Rivera 1994) and it has been documented that MyoD is first seen in proliferating myoblasts (Tapscott et al., 1988; Yablonka-Reuveni and Rivera 1994). Nevertheless, several studies of myogenic lines suggested that MyoD, when present in its active form, eventually leads to withdrawal of myoblasts from the cell cycle (Sorrentino et al., 1990; Thorburn et al., 1993; Halevy et al., 1995). Myogenin expression has been thought to mark an early phase of differentiation (Wright et al, 1989) and can begin prior to the expression of differentiation-specific structural proteins such as sarcomeric myosin (Wright et at., 1989; Yablonka-Reuveni and Rivera, 1994). In many cell culture studies the influences of mitogens or growth/differentiation promoting media on MRF expression have been analyzed by measuring levels of mRNA (Vaidya et al., 1989; Salminen et al., 1991; Li et al., 1994). Although such an approach gives an overall view of MRF expression, the cells are not necessarily homogenous with respect to their specific phenotype when collected for mRNA analysis. In the present study we have analyzed MRF protein expression in C2 cultures via immunocytochemistry. This direct cell analysis allows quantification of the actual number of cells expressing MyoD or myogenin.
We show in the present study that PDGF-BB enhances the number of C2 myoblasts by keeping the cells cycling for a longer time. Most if not all of the cells responding to the mitogenic effect of the growth factor fail to show the distinct nuclear expression of MyoD or myogenin when analyzed via immunocytochemistry. These mitogenically responsive cells are presumably in an early phase of the myogenic program, one preceding MyoD expression. Using the same approach we show that FGF-2 also enhances the proliferation of the ‘pre-MyoD’ cells. Additionally, we show that neither growth factor actually blocks the transition of C2 cells through the MyoD — myogenin expression program. The study indicates that the transition to the MyoD+/myogenin+ state is less accelerated in the presence of the mitogens. Our investigation at the single cell levels has additionally documented that the myogenin+ cells express the protein MEF2A prior to the expression of sarcomeric myosin. MEF2A is a memeber of the myocyte enhancer binding factor 2 (MEF2) family of transcription factors which are thought to act cooperatively with the MRF family during myoblast differentiation and the activation of muscle genes (Molkentin et al., 1995; 1996; Molkentin and Olson, 1996). Once C2 cells become myogenin+, their transition into the MEF2A state is not influenced by the absence or presence of PDGF-BB or FGF-2. Collectively, the direct cell analysis approach employed in the present study provides a more detailed insight into phenotypic transitions and influences of PDGF and FGF during myogenesis.
MATERIALS AND METHODS
Cell Culture
C2 cells, originally isolated from adult mouse skeletal muscle by Yaffe and Saxel (1977), were used throughout the study. The stock of C2 cells used for the studies has been previously described by us (Yablonka-Reuveni et al., 1990; Yablonka-Reuveni, 1995a) and was initially provided by Dr. D. Yaffe. Medium used to propagate cells (proliferation medium) consisted of MEM (GIBCO Laboratories, Grand Island, NY) containing 20% fetal calf serum (FCS, HyClone Laboratories, Logan, UT), 0.5% chicken embryo extract (prepared as described in Yablonka-Reuveni, 1995b), penicillin and streptomycin at 105 U per liter each. Cells were maintained at 37.5°C in humidified air containing 5% CO2, using tissue culture plates precoated with 2% gelatin. Cells were plated sparsely, grown to 50–60% confluence, passaged by treating with 0.12% trypsin (GIBCO Laboratories) for 30 minutes at 37°C, and seeded for specific experiments as described below. For all experiments described in this paper, the cells harvested for subculturing into the experimental plates represent the first passage upon removal from liquid nitrogen storage. We paid special attention to this issue, as we and others (Florini et al., 1991) have observed that there is a variation in rates of growth and differentiation with the increasing number of passages after thawing.
For all experiments (except when analyzing clones derived from single C2 cells), trypsinized cells were subcultured on gelatin-coated 35-mm dishes, using 1.5 ml proliferation medium. Cultures were initiated a density of 2.4 × 104 to 4.8 × 104 cells per plate (‘low density’) or at 4-fold higher (‘high density’). Following 24 hours in proliferation medium, cultures were rinsed with warm MEM and medium was changed to MEM containing 2% FCS ± growth factors. Medium and growth factors were replaced every 24 hours. At the indicated time, cultures were fixed for immunofluorescence as described below. PDGF-BB (human recombinant, produced in yeast) was added at 20 ng/ml; this highly purified PDGF-BB was kindly provided by Dr. C. E. Hart (ZymoGenetics Incorporation, Seattle, WA) or by Dr. R. Seifert (University of Washington). FGF-2 (human recombinant produced in yeast) was added at 2 ng/ml; this highly purified FGF-2 was kindly provided by Dr. S. Hauschka (University of Washington). FCS used for the 2% FCS medium was heat inactivated at 56°C for 30 minutes. Note that our original C2 studies were conducted with FCS which was not heat inactivated (Yablonka-Reuveni et al., 1990) but in subsequent studies we have used heat inactivated FCS (Yablonka-Reuveni, 1995a).
For clonal cultures we used 60 mm tissue culture plates precoated with gelatin as for the above mass cultures. Cultures were initiated with 50 to 100 cells per plate using 3 ml of proliferation medium. Following 5 days in proliferation medium, cultures were rinsed with warm MEM and received MEM containing heat inactivated 2% FCS. This 2% FCS medium (± PDGF-BB, 20 ng/ml) was replenished on day 6 and clonal cultures were collected on days 7 and 8.
Labeling Cells with 5-bromo-2′-deoxyuridine
In some experiments cultures were incubated with 5-bromo-2′-deoxyuridine (BrdU) to label S-phase cells. In these instances BrdU was added to the culture medium at a concentration of 10 µm for the last 2 hours of incubation prior to collecting plates for immunocytochemistry. Plates were than processed as described in the next section to visualize BrdU+ cells.
Immunofluorescence and Cell Quantification
I. Primary antibodies
Reactivity of the C2 cells with various antibodies was analyzed via single and double immunofluorescence as described in the next section. The following primary antibodies diluted in TBS-NGS (0.05 M Tris, 0.15 M NaCl, 1% normal goat serum, pH 7.4) were used:
(a) Antibodies against myogenic regulatory proteins
Anti-myogenin
A mouse monoclonal antibody against rodent myogenin (mAb F5D, hybridoma supernatant form) was developed and provided by Dr. W. Wright, University of Texas, Dallas. The use of this antibody has been described by us in a previous study (Yablonka-Reuveni and Rivera, 1994). In C2 cultures the anti-myogenin distinctly highlights nuclei of some of the mononucleatcd cells and nuclei in all myotubes.
Anti-MyoD (monoclonal Ab)
A mouse monoclonal antibody against murine MyoD (mAb 5.8A, hybridoma supernatant) was developed and provided by Drs. P. Houghton and P. Dias (St. Jude Children’s Research Hospital, Memphis) (Dias et al., 1992). Double immunofluorescence of C2 cells using this mAb and the polyclonal antibody against MyoD described below showed that the two antibodies have the same staining pattern.
Anti-MyoD (polyclonal Ab)
A rabbit polyclonal antibody against rodent MyoD was prepared and provided by Dr. S. Alemá (Inst. of Cell Biology, CNR, Rome, Italy). We described in a previous study the use and additional characterization of this antibody (Yablonka-Reuveni and Rivera, 1994). In C2 cultures the anti-MyoD distinctly highlights nuclei of some of the mononucleated cells; nuclei in newly formed, small myotubes also react with the antibody but staining is often below detection levels in many of the larger myotubes.
Anti-Myf5
A rabbit polyclonal antibody against Myf5 was developed and provided by Dr. S. Konieczny (Purdue University, West Lafayette). Depending on the medium conditions, C2 cells show various levels of Myf5 expression when analyzed by immunocytochemistry (see further discussion in results). The production of this antibody and its immunoreactivity with mouse primary myoblasts was described by Smith et al. (1993).
Anti-MEF2A
A rabbit polyclonal antibody against MEF2A was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). In C2 cultures the anti-MEF2A stains nuclei of some mononucleated cells and all nuclei within myotubes. The use of this antibody for detecting MEF2A in extracts of C2 cultures via immunoblotting was described recently (Molkentin et al., 1996).
(b) Antibodies against cystoskeletal muscle proteins
Anti-myosin
A mouse monoclonal antibody specific for all forms of sarcomeric myosin (mAb MF20, hybridoma supernatant form) was originally developed by Bader et al (1982).
Anti-desmin
A mouse monoclonal antibody against desmin (mAb D3, hybridoma supernatant form) was originally developed by Danto and Fischman (1984). Although originally made against chicken desmin, studies of other laboratories (Allen et al., 1991) and of our laboratory (unpublished) documented the specificity of the antibody for rodent desmin as well.
The antibodies against myosin and desmin were obtained from the Developmental Studies Hybridoma Bank. In C2 cultures both antibodies recognize the cytoplasm of some of the mononucleated cells and of all myotubes but cells positive for desmin appear during earlier days in culture compared to cells positive for myosin (see further details under Results).
(c) Antibodies against cell cycle parameters for detecting proliferating cells
Anti-cyclin A
A rabbit polyclonal antibody against cyclin A was developed and kindly provided by Drs. J. Roberts and E. Firpo (Fred Hutchinson Cancer Research Center, Seattle). The antibody was prepared as described by Koff et al. (1991). In C2 cultures this antibody recognizes the nuclei of some of the mononucleated cells.
Anti-BrdU
A fluorescein-labeled monoclonal antibody against BrdU was purchased from Boehringer-Mannheim (Indianapolis, IN). This directly labeled antibody was used in experiments where cells were costained for myosin and BrdU.
II. Single and double immunofluorescence
Cultures were rinsed with MEM at room temperature, fixed for 10 minutes at 4°C with ice-cold 100% methanol and air dried at room temperature for about 10 minutes. Cultures were then kept at 4°C in sterile Tris-buffered saline containing normal goat serum (TBS-NGS; 0.05 M Tris, 0.15 M NaCl, 1% normal goat serum, pH 7.4) to block non specific antibody binding. Following a minimum of 24 hours in TBS-NGS cultures were rinsed (3x) with Tris-buffered saline containing Tween 20 (TBS-T20; 0.05 M Tris, 0.15 M NaCl, 0.05% Tween 20, pH 7.4). For reaction with single antibodies cultures were incubated with the primary antibody (listed above) for 1 hour at room temperature followed by an overnight at 4°C. Cultures were then rinsed (3x) with TBS-T20 and incubated at room temperature for 1–2 hours with fluorescently-tagged secondary antibody diluted 1:100 with the blocking buffer TBS-NGS. The secondary antibodies were either fluorescein-labeled rabbit anti-mouse IgG (to detect reactivity with the primary monoclonal antibodies) or rhodamine-labeled goat anti-rabbit IgG (to detect reactivity with the primary polyclonal antibodies). These secondary antibodies were obtained from Organon-Technika, Cappel (Downington, PA). Following reaction with the secondary antibodies cultures were again rinsed with TBS-T20 and mounted in VECTASHIELD mounting medium from Vector Laboratories (Burlingame, CA).
Double immunofluorescence was conducted for analzying the coexpression of different proteins. In these instances, one rabbit polyclonal antibody and one mouse monoclonal antibody were used. The two primary antibodies were added to the cultures together and subsequently the two appropriate secondary antibodies were added together under the same conditions as for single antibody staining. For the MyoD / myogenin analyses the reactivity with the polyclonal antibody against MyoD was visualized with a fluorescein-conjugated donkey anti-rabbit IgG (diluted 1:75) obtained from Jackson ImmunoResearch Laboratories (West Grove, PA); the reactivity with the monoclonal antibody against myogenin was visualized with a rhodamine-conjugated goat anti-mouse IgG (diluted 1:100) obtained from Organon-Technika, Cappel.
Double immunofluorescence was also performed for monitoring proliferating (BrdU+) cells along with differentiating cells (myosin+). Cultures used for the BrdU / myosin analysis were first blocked with TBS-NGS as described above. Prior to addition of the antibodies cells were treated with 2N HC1 and subsequently rinsed with a neutralizing buffer according to the instructions of the company. Cultures were then simultaneously exposed to the fluorescein-conjugated anti-BrdU and the anti-myosin antibodies for 1 hour at room temperature. Reactivity of the anti-myosin antibody was subsequently visualized with rhodamine-labeled goat anti-mouse IgG presented to the cultures for 1 hour at room temperature.
III. Counterstaining of nuclei in mass and clonal cultures and cell counting
Total number of cells in the low and high density cultures were analyzed in arbitrary microscopic fields by monitoring the number of nuclei. To accurately monitor nuclei, cells were counterstained (following the immunostaining process) with 4,6-diamidino-2-phenylindole (DAPI, 1 µg/ml) and visualized with Hoescht filters as previously described (Hartley et al., 1991). Total number of cells includes the number of nuclei in myotubes when present. The same microscopic fields were used for quantification of total cells and immunostained cells. Unless otherwise noted (i.e., Figure 1), microscopic analyses were made using a 40x objective and 10 to 25 microscopic fields were analyzed per plate depending on the culture density, using at least duplicate plates (see specific experiments for details). While experiments were repeated several times, the standard deviations shown are based on duplicates within an individual experiment. Clonal cultures were also counterstained with DAPI and individual clones were identified with the DAPI optics using 16x objective. Clones were further analyzed with a 40x objective to determine their degree of reactivity with the antibodies. In the experiments involving immunostaining with anti-BrdU, the nuclei were monitored without any further counterstain; the HC1 / borate treatment for visualizing BrdU (which interfered with the DAPI staining) enhanced the distinct appearance of the nuclei and no further staining was required. In all experiments observations were made with a Zeiss microscope equipped for epifluorescence, and Kodak EL 135 film (400 ASA) was used for photography.
FIGURE 1.
Quantification of total cell numbers, the number of BrdU+ cells and the number of myosin+ cells when C2 myoblasts are maintained in 2% FCS ±PDGF. ‘Lower density’ cultures (lower panels) were initiated at 2.4 × 104 cells/plate and ‘higher density’ cultures (upper panels) were initiated at 4 fold higher density. Cultures were initiated in the serum-rich, proliferation medium and 24 hours later switched into medium consisting of 2% FCS ±PDGF which was replaced daily; BrdU was administrated to the culture medium during the last 2 hours before fixation. Cultures were doubly labeled with antibodies against BrdU and myosin. Total cell numbers reflect the total number of nuclei including nuclei in myotubes (when myotubes are present). The number of myosin+ cells also reflects the number of nuclei in both mononucleated cells and myotubes. The height of the y-axis used for presenting the number of myosin+ cells in the higher and the lower density cultures was chosen in order to maintain the same ratio used for graphing the total cell numbers. Each analysis is based on the average of two parallel plates analyzing five microscopic fields per plate using a 25x objective. Note that in all other experiments we used a 40x objective counting at least ten microscopic fields; the area of one microspic field observed with the 25x objectives is about 2.5 fold larger than the area of a field observed with a 40x objective.
RESULTS
Proliferation of C2 Myoblasts in the Absence and Presence of PDGF
Analysis of cell proliferation was conducted with parallel cultures initiated at two different densities. Lower density cultures were initiated at 2.4 × 104 per 35-mm plate and higher density cultures were intiated at a 4-fold higher density. These two cell densities were analyzed in view of our earlier findings which suggested that the kinetics of proliferation and mitogenic response to PDGF (determined by 3H-thymidine incorporation) varies for this range of cell densities (Yablonka-Reuveni et al., 1990; Yablonka-Reuveni, 1995a). Quantification of the degree of cell proliferation was performed in the current study by analyzing the total number of cells and by monitoring the frequency of S-phase cells via labeling with BrdU during different days in culture (Figure 1). Quantification of total cell numbers is summarized in panels A and E in Figure 1. While PDGF enhances the number of cells in both the low and high density cultures, its mitogenic influence is more immediate in the higher density cultures. In the high density cultures PDGF-effect on increasing the total cell numbers is already apparant by the second day in culture. In the the low density cultures proliferation is maintained at a similar rate for the first two days regardless of the absence or presence of PDGF and only by the third day in culture the mitogenic influence of PDGF becomes apparent.
The same culture plates used for total cell counts were also analyzed for the number of cells which were positive for BrdU following a pulse with the drug during the last 2 hours in culture prior to fixing the plates. A summary of the frequencies of BrdU+ cells at the different time points is shown in panels B and F in Figure 1. PDGF promotes increased frequency of BrdU+ cells in both the low and high density cultures. However, PDGF influence on the frequency of BrdU+ cells is far more robust in the high density cultures. In the absence of PDGF, the frequency of the BrdU+ cells in the high density culture is already below 10% within the initial 24 hours following the transition into the 2% FCS medium. In parallel high denisty cultures receiving PDGF the frequency of the BudR+ cells is nearly 40% by these initial 24 hours (panel B). In the low density cultures the initial frequency of the BrdU+ cells is high regardless the absence or presence of PDGF and the control and PDGF-treated cultures show a similar pattern of decline in the frequency of BrdU+ cells over the days in culture (panel F). It is important to note that the quantification of BrdU+ cells in the above study reflects only those cells that synthesized DNA during the 2-hour labeling with BrdU. Because we were interested in analyzing both proliferation and differentiation (see next paragraph) a more prolonged exposure to BrdU was avoided as BrdU can interfere with the progression of myogenesis (Bischoff and Holtzer, 1970; Tapscott et al., 1989).
The BrdU-labeled cultures were also analyzed (via double immunocytochemistry) for the number of myosin+ cells; this co-analysis was performed to provide a measure for the ‘myogenicity’ of the cultures. The quantification of the myosin+ cells (shown in panels C and G in Figure 1) reflects the number of nuclei in mononucleated cells positive for myosin as well as the number of nuclei in myotubes. The low density cultures begin to show myosin+ cells by day 3 while in the high density cultures, myosin+ cells are already detectable on day 2. Comparison of the numbers of myosin+ cells with the total cell numbers indicates that the myosin+ cells accumulate at a more rapid rate in the control cultures compared to the PDGF-treated cultures. This analysis of the frequency of myosin+ cells is summarized in panels D and H in Figure 1. In accordance with earlier studies on myogenesis, regardless of cell density and the absence or presence of PDGF, we did not detect proliferating cells (BrdU+) which were also positive for myosin. Collectively, the data have suggested that PDGF enhances proliferation of the C2 cells and that the transition of this cells into the myosin+ state is delayed.
Dual Expression of the Proteins MyoD and Myogenin by C2 Cells Maintained in the Absence or Presence of PDGF-BB
Employing double immunofluorescence with antibodies against MyoD and myogenin we set out to analyze whether the influence of PDGF on enhancing proliferation (and apparently suppressing the appearance of myosin+ cells) is potentially linked to influences on the transition of the cells through the MyoD — myogenin program. Dual immunostaining allowed us to differentiate between MyoD+ cells which already advanced to the myogenin+ slate (MyoD+/myogenin+), MyoD+ cells which have not yet achieved the myogenin+ state (MyoD+/myogenin−), and myogenin+ cells which were no longer positive for MyoD (MyoD−/myogenin+). For each microscopic field analyzed, we monitored total cell numbers (based on nuclei in single and multi-nucleated cells), the number of nuclei in myotubes, the total number of mononucleated cells positive for MyoD or myogenin, and the specific number of cells which are doubly or singly labeled for MyoD and myogenin. Table I provides a summary of the number of cells with the different phenotypic combinations analyzed. The final column in Table I summarizes the total number of cells which have entered the MyoD — myogenin expression program at the different time points. These total ‘myogenic phenotype’ cells consist of the mononucleated cells which are MyoD+/myogenin−, MyoD+/myogenin+, MyoD−/myogenin+ and cells fused into myotubes. The data presented in Table I are further summarized in Figure 2 where the distribution of each of the different cell phenotypes is expressed as percent of the total cells. Examples of stainability (via double immunofluorescence) with the polyclonal antibody against MyoD and the monoclonal antibody against myogenin are shown in Figure 3.
TABLE I.
Distribution of Cell Phenotypes in C2 Cultures Reacted via Double Immunofluorescence with Antibodies Against MyoD and Myogenin
| Averaged Per Ten Fieldsa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days in 2% FCS |
Growth Factor Added |
Total Number of Cells |
Number of Cells Fused into Myotubes |
Mononucleated Cells
Only |
Total Number of Cells with Myogenic Phenotypeb |
||||
| All MyoD+ | All Myog+ | MyoD+/Myog− | MyoD+/Myog+ | MyoD−/Myog+ | |||||
| 1 | None PDGF |
1049.2 ± 135.2 980.7 ± 103.2 |
0.5 ± 0.5 0 |
107.2 ± 14.2 100.7 ± 17.7 |
28 ± 6 18.5 ± 4 |
79.2 ± 8.2 82.2 ± 13.7 |
28 ± 6 18 ± 4.5 |
0 0.5 ± 0.5 |
107.7 ± 14.7 100.7 ± 17.7 |
| 2 | None PDGF |
1074.5 ± 73.5 2314.5 ± 224.5 |
41 ± 29 6 ± 2 |
191.5 ± 0.5 228.5 ± 2.5 |
170 ± 6 180 ± 13 |
75.5 ± 8.5 115.5 ± 6.5 |
116 ± 9 113 ± 9 |
54 ± 3 67 ± 4 |
286.5 ± 26.5 301.5 ± 4.5 |
| 3 | None PDGF |
1224.5 ± 85.5 2932.5 ± 119.5 |
80.5 ± 20.5 40.5 ± 2.5 |
276 ± 41 324 ± 24 |
246 ± 18 275 ± 65 |
71.5 ± 13.5 139.5 ± 8.5 |
204.5 ± 27.5 184.5 ± 15.5 |
41.5 ± 9.5 90.5 ± 47.5 |
398 ± 1 455 ± 76 |
| 4 | None PDGF |
1292.5 ± 2.5 3468.5 ± 151.5 |
263.5 ± 15.5 143.5 ± 29.5 |
139 ± 45 402.5 ± 46.5 |
178 ± 6 402.5 ± 42.5 |
58 ± 23 202.5 ± 45.5 |
81 ± 22 200 ± 1 |
97 ± 16 202.5 ± 43.5 |
499.5 ± 13.5 751.5 ± 23.5 |
Cultures were initiated at higher cell density in proliferation medium and 24 hours later switched to 2% FCS ( ± PDGF-BB). Results shown for days 2 through 5 under all categories reflect the actual numbers scored for ten microscopic fields which were further averaged for duplicate plates. Scores for day 1 were obtained by analyzing twenty fields per plate and numbers were then averaged per ten fields using results of duplicate plates. Additional fields were analyzed for day 1 in order to more reliably measure the myogenin+ cells which were infrequent in the day 1 plates. Microscopic fields were randomly selected and counted using 40x objective.
The total number of cells with a ‘myogenic phenotype’ is the sum of the number of cells under: MyoD+/myogenin−, MyoD+/myogenin+. MyoD−/myogenin+, and cells fused into myobtubes.
FIGURE 2.
Frequency of mononucleated cells positive for MyoD and/or myogenin and of cells fused into myotubes in C2 cultures initiated at ‘higher density’ and reacted via double immunofluorescene with antibodies against MyoD and myogenin. Frequencies are based on the data summarized in Table I. Note that except for the graphs summarizing the frequency of nuclei in myotubes and the frequency of cells with a myogenic phenotype (panels D and H) all other graphs demonstrating frequency of cells represent mononucleated cells only.
FIGURE 3.
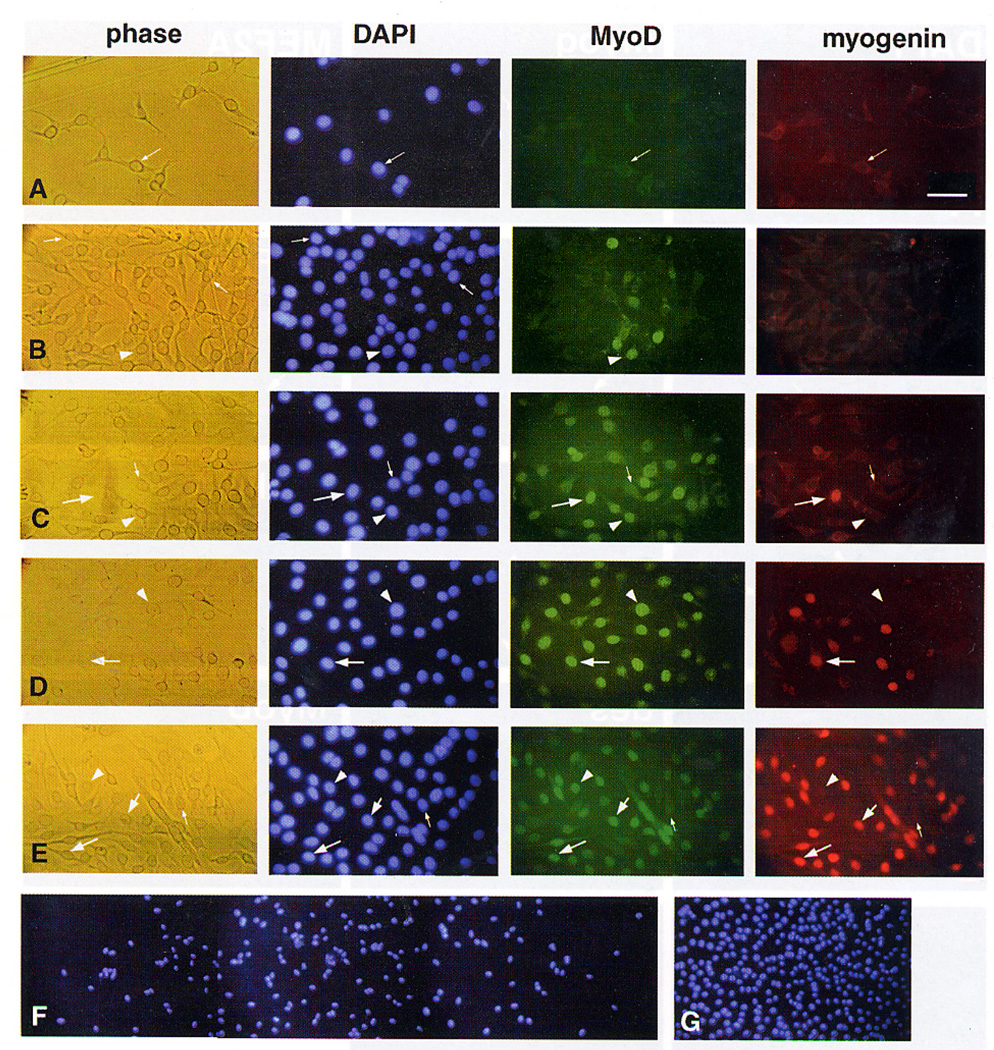
Micrographs demonstrating large clones doubly stained with antibodies against MyoD and myogenin along with DAPI stain of the nuclei (A–E) and an over view of a large clone at lower magnification (F–G). Clones were initiated in proliferation medium and switched following 5 days into medium consisting of 2% FCS (±PDGF). Reactivity with the anti-MyoD polyclonal antibody was visualized with a fluorescein-conjugated secondary antibody and reactivity with the anti-myogenin monoclonal antibody was visualized with a rhodamine-conjugated secondary antibody. (A) Micrographs of the outer region of a large clone whose center is already positive for MyoD and myogenin; culture was maintained for 2 days in the 2% FCS medium (+PDGF). (B–D) Micrographs of central regions of different large clones; cultures were maintained for 2 days in the 2% FCS medium (PDGF was present in B and C and absence in D). (E) Micrographs of the central region of a large clone showing small myotubes; the culture was maintained for 3 days in the 2% FCS medium in the presence of PDGF. (F–G) Lower magnification micrographs of a large clone shown to provide an overview of the density of the cells in (F) the more peripheral region of the clone and (G) the more central region of the clone; culture was maintained for 2 days in the 2% FCS medium with PDGF; nearly all cells in the region shown in panel G were found positive for MyoD, in contrast most cells in the periphery of the region shown in panel F were negative for MyoD. Arrowheads in parallel micrographs mark the position of the cells positive for MyoD but negative for myogenin, larger arrows mark the position of cells positive for both MyoD and myogenin and smaller arrows mark the position of cells negative for both MyoD and myogenin. Micrographs in panels A–E were taken with a 40x objective; bar, 57 µm. Micrographs in panels F–G were taken with a 16x objective; bar 146 µm.
Data in Table I show that initially, by the first day in 2% FCS (±PDGF), the majority of the MyoD+ cells were negative for myogenin. In subsequent days, regardless of the absence or presence of PDGF, three cell phenotypes were identifiable; MyoD+/myogenin−, MyoD+/myogenin+, MyoD−/myogenin+. By the first day in culture the frequencies of the MyoD+/myogenin− and the MyoD+/myogenin+ cells were not influenced by the presence or absence of PDGF (panels E and F in Figure 2). Over time in culture (days 2 and 3), the frequency of the MyoD+/myogenin+ was higher in cultures maintained without PDGF (panel F in Figure 2). Furthermore, in these cultures maintained without PDGF there was an increase in the frequency of cells fused into myotubes on culture day 4, correlating to the decrease in the frequency of the mononucleated, MyoD+/myogenin+ cells in the same cultures (panels D and F in Figure 2). The maximum % fusion recorded in the present experiment is just over 20% (panel D in Figure 2). In subsequent days, similar cultures routinely reach a differentiation level of 60–70% (i.e., frequency of nuclei in mononucleated and multinucleated cells expressing sarcomeric myosin); however, parallel PDGF-treated cultures become so dense that an accurate analysis of the MyoD±/myogenin± cells becomes problematic. The frequency of all ‘myogenic phenotype’ cells (mononucleated cells which are MyoD+/myogenin−, MyoD+/myogenin+, MyoD−/myogenin+, and nuclei in myotubes (Figure 2, panel H)) provides an overall measure which indicates that the transition through the MyoD — myogenin program is more accelerated in the absence of PDGF. In cultures lacking PDGF, the frequency of ‘myogenic phenotype’ cells increased by 4 fold between days 1 and 4 while in cultures which received PDGF the frequency of ‘myogenic phenotype’ cells increased only by 2 fold. At day 1 the frequency of such myogenic phenotype cells was about 10% regardless the absence or presence of PDGF. It is the group of cells with the MyoD+/myogenin+ phenotype, along with the group of the cells fused into myotubes, that primarily contribute to the enhanced frequency of the ‘myogenic phenotype’ cells in the control cultures.
We also conducted experiments where the influences of PDGF-BB and FGF-2 were compared under culture conditions similar to those used in the above study. With the exception that FGF supported a higher number of cells than PDGF (see additional studies in Tables III and IV), the transition of the cells through the MyoD — myogenin expression program and the influence on the frequencies of the different cell phenotypes (mononucleated cells which are MyoD+/myogenin−, MyoD+/myogenin+, MyoD−/myogenin+, and nuclei in myotubes), was similar for both growth factors. The reduced frequency of ‘myogenic phenotype’ cells in cultures maintained with PDGF-BB or FGF-2 became apparent in the lower density cultures later than in the higher density cultures. In some experiments initiated at low density the frequency of the cells transiting through the MyoD — myogenin program was significantly reduced by day 3 in the growth factor-treated cultures (see Table III). In other experiments this reduced frequency became more apparent on the fourth day (for example see Table IV). Routinely, in all experiments with low density cultures, there were very few if any cells fused into myotubes by culture day 3 but the levels of myogenin + cells varied to some degree between experiments (details in Tables below). Taken together, the experiments with the lower density cultures indicate that there is a correlation between the time that myogenin+ cells become more abundant and the time when the frequency of ‘myogenic phenotype’ cells begin to differ for cultures receiving growth factors versus the cultures which do not. The timing of this increase in myogenin+ cells differs slightly for different experiments and might be related to the composition of the initial cells harvested for specific experiments.
Dual Expression of MyoD and Myogenin Proteins in C2 Clones
We analyzed individual C2 clones in order to gain further insight into the transition of the C2 cells through the various compartments of MyoD — myogenin protein expression. As each clone is derived from one cell founder, analysis of multiple clones can also assist in determining whether the myogenic potential of the C2 cells is similar for all cells. Clonal cultures were maintained for 5 days in routine proliferation medium to allow for clones to develop under optimal conditions, then switched into the medium containing 2% FCS (±PDGF) for two or three additional days. Table II shows the distribution of clones by size and staining with MyoD and myogenin following two or three days in 2% FCS in the absence or presence of PDGF. In general, positive cells were present almost exclusively in larger clones. Furthermore, when clones contained MyoD and/or myogenin positive cells, the positive cells were generally concentrated in the central parts of the clone while the periphery consisted primarily of negative cells. Figure 3 summarizes examples of stainability of the clones with the two antibodies and the distribution of positive and negative cells within the larger clones. Judging by the gradient of MyoD+ and/or myogenin+ cells from the center to the periphery in clones of the two time points analyzed, we concluded that cells are first positive for MyoD and subsequently coexpress myogenin. Cells positive only for myogenin were rare but identifiable in some clones (data not shown). By the third day in 2% FCS medium (±PDGF) some of the clones also demonstrated myotubes. Myotubes at this clonal stage were rather small and the myonuclei were positive for both MyoD and myogenin (see Figure 3, panel E).
TABLE II.
Distribution of C2 Clones by Size and Staining with Antibodies against MyoD and Myogenina
| In 2% FCS |
In 2% FCS &
PDGF |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 days |
3 days |
2 days |
3 days |
|||||||||
| Cloneb No. |
Clonec Size |
MyoD+d Cells |
Myogenin+ Cells |
Clone Size |
MyoD+ Cells |
Myogenin+ Cells |
Clone Size |
MyoD+ Cells |
Myogenin+ Cells |
Clone Size |
MyoD+ Cells |
Myogenin+ Cells |
| 1 | + | 0 | 0 | ++ | 0 | 0 | ++ | 0 | 0 | ++ | 0 | 0 |
| 2 | + | 0 | 0 | ++ | 0 | 0 | ++ | 0 | 0 | ++ | 0 | 0 |
| 3 | + | 0 | 0 | +++ | 0 | 0 | ++ | 0 | 0 | ++ | 1 | 0 |
| 4 | + | 0 | 0 | +++ | 0 | 0 | ++ | 0 | 0 | ++ | 3 | 1 |
| 5 | + | 0 | 0 | +++ | 0 | 0 | ++ | 0 | 0 | +++ | 0 | 0 |
| 6 | + | 0 | 0 | +++ | 1 | 0 | +++ | 0 | 0 | +++ | 0 | 0 |
| 7 | ++ | 0 | 0 | +++ | 2 | 1 | +++ | 0 | 0 | +++ | 0 | 0 |
| 8 | ++ | 0 | 0 | +++ | 3 | 3 | +++ | 0 | 0 | +++ | 0 | 0 |
| 9 | ++ | 0 | 0 | ++++ | 0 | 1 | +++ | 0 | 0 | +++ | 1 | 0 |
| 10 | ++ | 0 | 0 | ++++ | 1 | 1 | +++ | 0 | 0 | +++ | 3 | 0 |
| 11 | ++ | 0 | 0 | ++++ | 1 | 2 | +++ | 1 | 0 | +++ | 4 | 1 |
| 12 | +++ | 0 | 0 | ++++ | 1 | 2 | ++++ | 0 | 0 | ++++ | 0 | 0 |
| 13 | +++ | 0 | 0 | ++++ | 2 | 2 | ++++ | 0 | 0 | ++++ | 1 | 0 |
| 14 | +++ | 0 | 0 | ++++ | 2 | 4 | ++++ | 0 | 0 | ++++ | 1 | 1 |
| 15 | +++ | 0 | 0 | ++++ | 3 | 1 | ++++ | 0 | 0 | ++++ | 2 | 0 |
| 16 | +++ | 0 | 0 | ++++ | 3 | 1 | ++++ | 0 | 0 | ++++ | 2 | 0 |
| 17 | +++ | 0 | 0 | ++++ | 3 | 3 | ++++ | 0 | 0 | ++++ | 2 | 1 |
| 18 | +++ | 0 | 0 | ++++ | 3 | 3 | ++++ | 0 | 0 | ++++ | 2 | 1 |
| 19 | +++ | 0 | 0 | ++++ | 3 | 3 | ++++ | 0 | 0 | ++++ | 2 | 1 |
| 20 | ++++ | 0 | 0 | ++++ | 3 | 3 | ++++ | 0 | 0 | ++++ | 2 | 2 |
| 21 | ++++ | 1 | 0 | ++++ | 3 | 5 | ++++ | 1 | 0 | ++++ | 2 | 2 |
| 22 | ++++ | 1 | 0 | ++++ | 4 | 3 | ++++ | 1 | 0 | ++++ | 2 | 2 |
| 23 | ++++ | 1 | 1 | ++++ | 4 | 3 | ++++ | 2 | 1 | ++++ | 3 | 1 |
| 24 | ++++ | 2 | 1 | ++++ | 4 | 3 | ++++ | 2 | 1 | ++++ | 3 | 2 |
| 25 | ++++ | 3 | 1 | ++++ | 4 | 4 | ++++ | 2 | 1 | ++++ | 3 | 2 |
| 26 | ++++ | 3 | 2 | ++++ | 4 | 4 | ++++ | 3 | 1 | ++++ | 3 | 3 |
| 27 | ++++ | 4 | 2 | ++++ | 5 | 1 | ++++ | 3 | 1 | ++++ | 4 | 4 |
| 28 | ++++ | 4 | 2 | ++++ | 5 | 2 | ++++ | 3 | 3 | ++++ | 5 | 3 |
| 29 | ++++ | 5 | 3 | ++++ | 5 | 3 | ++++ | 4 | 3 | ++++ | 5 | 3 |
| 30 | ++++ | 5 | 3 | ++++ | 5 | 5 | ++++ | 5 | 1 | ++++ | 5 | 3 |
Clonal cultures were maintained for 5 days in proliferation medium containing 20% FCS and 0.5% chicken embryo extract, then switched into the serum-poor medium containing 2% FCS ± PDGF for 2 or 3 additional days.
Clones are arranged in the table by their size; within each size group clones are arranged first by the proportion of MyoD+ cells and second by the proportion of myogenin+ cells.
Clone size was determined based on examining the clone using a 16x objective. “+” represents clones which occupy less than 1 microscopic field. “++” represents clones which occupy about 1 microscopic field. “+++” represents clones ranging in size from more than 1 microscopic field up to 2 fields. “++++” represents clones which occupy more than 2 microscopic fields. Sparse peripheral cells were not included in this sizing method.
Numbers under MyoD+ cells and myogenin+ cells are based on visual estimates of positive cells within a clone using a 0 to 5 scale where 0 reflects no positives and 5 reflects over 80% positives.
In Table II we assigned quantitative measures to the degree of MyoD and myogenin positive cells present in each clone based on estimating the proportion of positive cells present (for further details see foot notes in the Table). Data obtained with clonal cultures maintained in 2% FCS without PDGF (shown on the left half of Table II) demonstrate that MyoD — myogenin protein expression was pronounced primarily in larger clones (marked as ‘++++’ in the Table) and that the number of such clones increased with time in culture. The analysis of parallel clonal cultures maintained with PDGF (shown on the right half of Table II) demonstrates a higher number of larger clones (i.e., clones marked as ‘++++’) at the earlier time point examined. In clonal cultures maintained for 2 days in the 2%-FCS medium we detected 11 such large clones in the absence of PDGF versus 19 large clones when PDGF was present (for each groups a total of 30 clones was examined). However, a significant number of these larger clones in the cultures receiving PDGF for 2 days were negative for MyoD or myogenin compared to the cultures lacking PDGF; in the PDGF-treated cultures 9 clones out of the 19 large clones were negative for MyoD and myogenin whereas in the cultures lacking PDGF only 1 clone out of the 11 large clones was negative. In the subsequent time point (day 3 in 2% FCS), the proportion of the larger clones increased in the cultures lacking PDGF (21 out of a total of 30 clones) resembling somewhat the number of the larger clones in the culture receiving PDGF (19 out of a total of 30 clones). However, at this later day all large clones in cultures lacking PDGF were positive for myogenin, but in the cultures receiving PDGF one of the larger clones was still negative for both MyoD and myogenin and three more larger clones were positive only for MyoD. The data with both day 2 clones and day 3 clones suggest that the progression through the MyoD — myogenin program is slower in the presence of PDGF. Collectively, the clonal study indicates that the majority, if not all, of the clonable cells are myogenic, and upon attaining a specific clone size the progeny of the clone founders express the two characteristic myogenic regulatory factors. PDGF can generate more, larger clones at an earlier time but the transition of these larger clones into the MyoD — myogenin program is slower than seen in the absence of PDGF. The finding that PDGF-treated clones are also able to enter the MyoD — myogenin program indicates that PDGF influences proliferation of cells with myogenic potentialities (i.e., cells that are able to produce progeny with myogenic characteristics) and does not merely enhance proliferation of ‘defective’ C2 cells which are not able to give rise to differentiated myoblasts (the term ‘diferentiation-detective’ cells was introduced by Hauschka and colleagues in reference to cells which derived from a myogenic line but fail to differentiate (Lim and Hauschka, 1984)).
Phenotypic Transitions of Myogenin+ Cells
The appearance of myogenin+ cells precedes the appearance of myosin+ cells by at least 1 day when C2 cells are maintained in the 2% FCS medium (for example see data in Table III). This lag in the emergence of myosin+ cells compared to myogenin+ cells is not influenced by the addition of PDGF (Table III) or by the initial cell density of the cultures (data not shown). Subsequently, using double immunostaining with anti-myogenin / anti-MEF2A or anti-myogenin / anti-myosin, we noted that nearly all MEF2A+ cells were also positive for myogenin but cells became positive for MEF2A only after they became positive for myogenin. Likewise, nearly all sarcomeric myosin+ cells were positive for MEF2A but the number of MEF2A+ cells is higher than myosin+ cells, indicating that the myogenin+ cells become positive for MEF2A prior to becoming positive for myosin (data not shown, examples of immunostaining with the anti-MEF2A in combinations with anti-myogenin or anti-myosin are shown in panels A and B in Figure 4). We further analyzed the possibility that the transition of the myogenin+ cells into the MEF2A+ state might be influenced by PDGF-BB or FGF-2. This potential influence of the mitogen was examined especially in view of a recent study of C2 cultures which suggested that the myogenin+ cells acquire the post-mitotic/myosin+ state only after becoming positive for the cell cycle inhibitor p21 (Andrés and Walsh, 1996). Hence, it was possible that the initial phenotypic transitions of the myogenin+ cells might potentially be influenced by growth factors. The analysis was conducted with cultures initialed at the lower density, capturing the initial transition of the cells into the different phenotypic states analyzed. Parallel cultures were examined for total cell numbers or for the presence of myogenin±/MEF2A± cells. Results of the study are summarized in Table III along with the number of MyoD+ cells and the number of myosin+ cells determined on parallel cultures. The data indicate that by day 3 the frequency of myogenin+ cells is reduced in cultures receiving either PDGF or FGF compared to the control cultures (the frequencies of myogenin+ cells are 15.3% for cultures that did not receive any mitogen, 8.7% for PDGF-treated cultures, and 8.6% for FGF-treated cultures). However, the frequency of the myogenin+ cells which transited into the MEF2A+ state is similar for all treatment groups (about 50 to 54% of the myogenin+ cells), suggesting that the transition of the myogenin+ cells into the MEF2A+ state is not influenced by PDGF-BB or FGF-2.
TABLE III.
Distribution of Cell in C2 Cultures Doubly Stained with Antibodies Against Myogenin and MEF2A
| Averaged Per Ten Fieldsa |
|||||||
|---|---|---|---|---|---|---|---|
| Days in 2% FCS |
Growth Factor Added |
Total Number of Cells |
MyoD+ Cells | Myogenin/MEF2A |
Myosin+ Cells |
||
| Myogenin+ Cells | MEF2A+ Cells | Myog+/MRF2A+ Cells |
|||||
| 1 | None | 177.4 ± 4 | 18.2 ± 0.7 | 4 | 0 | 0 | - |
| PDGF | 201 ± 19 | 6.7 ± 0.7 | 0 | 0 | 0 | - | |
| FGF | 253 ± 14 | 6 ± 1 | 0 | 0 | 0 | - | |
| 2 | None | 410.5 ± 20.5 | 25.5 ± 10.5 | 14.2 ± 7.7 | 0b | 0 | - |
| PDGF | 433 ± 67 | 44.2 ± 15.7 | 21.2 ± 3.2 | 2.5 ± 0.5 | 2.5 ± 1.5 | - | |
| FGF | 643 ± 3 | 56 ± 6 | 10.5 ± 2.5 | 4.2 ± 2.2 | 2.5 ± 1.5 | - | |
| 3 | None | 730 ± 47 | 110.5 ± 11.5(15.1%)c | 111.7 ± 6.7(15.3%)c | 63.3 ± 4.8(8.9%)c | 60.2 ± 4.7(53.9%)d | 28.2 ± 7.7 |
| PDGF | 1342 ± 111 | 168 ± 12(10.4%) | 116 ± 3.2(8.7%) | 62.3 ± 2.3(4.4%) | 62.0 ± 2.5(53.4%) | 50.5 ± 7.5 | |
| FGF | 1868 ± 232 | 195 ± 41(12.5%) | 164 ± 16.5(8.6%) | 81.8 ± 1.3(4.5%) | 81.2 ± 1.7(49.5%) | 32.5 ± 3 | |
Cultures were initiated at 4.8 × 104 cells per plate in proliferation medium and 24 hours later the medium was replaced with the 2% FCS medium ( ± growth factors). Only infrequent cells fused into myotubes by day 3 (total of 0–5 nuclei within myotubes per ten fields). Total cell numbers and the numbers of MyoD+ cells were examined using the same plates; ten fields per plate were analyzed for day 3 plates; more fields were analyzed for day 1 and day 2 plates so that minimum of 500 cells were counted. The analysis of myogenin ± /MEF2A ± cells or of myosin+ cells was done by scoring twenty fields per plate for all time points (day 2 results for the analysis of myogenin ± /MEF2A ± were further confirmed by scoring 100 myogenin+ cells). All results shown were eventually averaged per ten fields per plate using duplicate plates.
Analyzing 100 myogenin+ cells we found infrequent myogenin+/MEF2A+ cells as in the growth factor-treated cultures.
Numbers in parentheses show the percent cells with the specific phenotype out of total cells.
Numbers in parentheses show the percent of Myog+/MFF2A+ cells out of all myogenin+ cells.
FIGURE 4.
Micrographs demonstrating C2 cultures which were immunoreacted with various combinations of mouse monoclonal and rabbit polyclonal antibodies and counter stained with DAPI. Panel A — reactivity with a monoclonal antibody against myogenin and a polyclonal antibody against MEF2A. Panel B — reactivity with a monoclonal antibody against myosin and a polyclonal antibody against MEF2A. Panel C — reactivity with a monoclonal antibody against MyoD and a polyclonal antibody against cyclin A. Panel D — reactivity with a monoclonal antibody against desmin and a polyclonal antibody against MyoD. Panel E — reactivity with a polyclonal antibody against Myf5. Reactivity with the monoclonal antibodies was visualized with a fluorescein-labeled secondary antibody and reactivity with the polyclonal antibodies was visualized with a rhodamine-labeled secondary antibody. Cultures were initiated in proliferation medium and switched following 24 hours into medium consisting of 2% PCS in MEM (±PDGF). The different panels represent cultures fixed following 1 to 4 days in culture. Arrows in parallel DAPI and immunofluorescent micrographs in panels A through D mark the position of cells positive for both antibodies examined while arrowheads in these panels mark the position of cells which are positive for only one of the two antibodies examined. Arrows and arrowheads in the parallel micrographs in panel E mark the position of cells whose cytoplasm or nuclei, respectively, is immunopositive for the antibody tested. Micrographs in all panels were taken with a 40x objective.
Expression of Cyclin A by MyoD+ or Myogenin+ Cells
As discussed above, studies from various laboratories have recognized that MyoD expression can be first detected in proliferating cells (Tapscott et al., 1988; Yablonka-Reuveni and Rivera, 1994). However, it has been also recognized that the active form of MyoD can influence additional regulators of the cell cycle and induce cell cycle arrest and terminal withdrawal of myoblasts from the cell cycle (Sorrentino et al., 1990; Thorburn et al., 1993; Halevy et al., 1995; Skapek et al., 1995). Hence, the MyoD+ cells identified in the current study by immunocytochemistry may include both proliferating and differentiating cells. We therefore were interested in determining the proliferative status of the MyoD+ cells which we traced by immunocytochemistry. If the MyoD+ cells are capable of proliferation they could be differentially affected by various mitogens. To further examine the status of the MyoD+ cells we have analyzed their co-expression of cyclin A and compared it to the expression of cyclin A by cells which have not yet entered the MyoD — myogenin program. Active complexes of cyclin A are required for DNA replication as well as for mitosis and cyclin A is expressed during the cell cycle from the S phase throughout the G2/M transition (Minshull et al., 1990; Girard et al., 1991; Walker and Maller, 1991; Pagano et al., 1992). Hence, the expression of cyclin A facilitates the monitoring of cycling cells and has been previously used as a tool to trace cell proliferation (Cardoso et al., 1993; Paterlini et al., 1995).
Results of the cyclin A/MyoD analysis are shown in Tables IVa and IVb for control (i.e., cultures maintained in 2% FCS only) and cultures receiving PDGF-BB or FGF-2; cultures were initiated at the lower cell density. Micrographs demonstrating double immunofluorescence with the polyclonal antibody against cyclin A and the monoclonal antibody against MyoD are included in Figure 4 (panel C). The co-expression of cyclin A by the myogenin+ cells was analyzed on parallel cultures and the results are also included in the Tables IVa and IVb. Cultures were initiated at lower density to allow the capturing of the onset of transition into the MyoD+ state when no myogenin+ cells are present and the comparison of this stage with a later time point where there is an increased number of MyoD+ cells (and some myogenin+ cells are also present). Table IVa provides an overview of the cultures in terms of total cell numbers and the number of cells positive for MyoD, myogenin or cyclin A. The transition into the myogenin+ state in the experiment summarized in Table IVa is somewhat slower than that described in Table III and the day 3 cultures shown in Table IVa have not reached yet the stage where they demonstrate an increased frequency of myogenin+ cells in the absence of the growth factors. Regardless, the study demonstrates that for each treatment group, the frequency of the cyclin A+ cells progressed differently between days 1 and 3 (we have reproduced these cyclin A results in several independent experiments). Table IVb further summarizes the distribution of the MyoD+ and myogenin+ cells in respect to their co-expression of the cyclin A protein. Initially the number of MyoD+ cells is low, making the statistical analysis of cells which are MyoD+/cyclin A+ problematic. Nevertheless the data suggest that many of the MyoD+ cells are also positive for cyclin A at this stage. By culture day 3 only a small number of MyoD+ cells is positive for cyclin A regardless the absence or presence of growth factors. We obtained similar results regarding the co-expression of MyoD and cyclin A when C2 cells were maintained in the high-serum, proliferation medium which contains 20% FCS and 0.5% chicken embryo extract; by the first day in culture, 44% of the MyoD+ cells (9 MyoD+ cells out of 410 cells total) were doubly stained for cyclin A; by the third culture day, only about 10% of MyoD+ cells (177 MyoD+ cells out of 3339 cells total) were doubly stained for cyclin A. In the later time point, when myogenin+ cells emerge, we noted that some of these cells are also positive for cyclin A (Table IVb). In agreement with the report of Andrés and Walsh (1996) these cells may represent residual myogenin+ cells which are still capable of proliferation. An earlier study by Maley et al. (1994) also noted the expression of myogenin protein by proliferating myoblasts in primary cultures prepared from adult mouse skeletal muscle. It is noteworthy that the detection of S-phase cells via the incorporation of BrdU was avoided in our study because the dual detection of MyoD protein and BrdU incorporation via immunocytochemistry is technically problematic (the acid treatment of the cultures which is required for immunodetection of BrdU+ cells drastically reduces the immunostain of the antibodies against the MRFs). Also, attempts to detect S-phase cells via the expression of cyclin E, which marks the G1/S entry (Koff et al., 1991) were not productive as the expression of cyclin E seems to be very transient in our culture model.
TABLE IV.
| a Distribution of Cells
Positive for MyoD, Myogenin, or Cyclin A in C2 Cultures | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number of Cells Per Ten
Fieldsa |
||||||||
| Days in 2% FCS |
Growth Factor Added |
Total Number of Cells |
MyoD+ cells |
Myogenin+ cells |
Cyclin A+ cells |
|||
| Number | % of Total Cells |
Number | % of Total Cells |
Number | % of Total Cells |
|||
| 1 | None | 216.5 ± 6.5 | 4 ± 2.2 | 1.9 ± 1.1 | - | - | 30.0 ± 3.8 | 13.8 ± 1.3 |
| PDGF | 318.2 ± 9.2 | 5.5 ± 0 | 1.7 ± 0.0 | - | - | 68.2 ± 6.2 | 21.8 ± 2.6 | |
| FGF | 395 ± 28.2 | 7 ± 1.5 | 1.7 ± 0.2 | - | - | 15.0 ± 0.5 | 3.8 ± 0.1 | |
| 2 | None | 657.5 ± 21.5 | 82.5 ± 18.5 | 12.4 ± 2.4 | - | - | 24 ± 7.0 | 3.6 ± 0.9 |
| PDGF | 1762.5 ± 120.5 | 159 ± 28 | 8.9 ± 0.9 | - | - | 175 ± 8.0 | 10 ± 1.1 | |
| FGF | 2599 ± 136.0 | 305 ± 31 | 11.8 ± 1.8 | - | - | 179 ± 12.0 | 6.9 ± 0.1 | |
| 3 | None | 649 ± 17 | - | 31 ± 1 | 5.1 ± 0.3 | 20.5 ± 5.5 | 3.1 ± 0.7 | |
| PDGF | 1452.5 ± 84.5 | - | - | 67.5 ± 8.5 | 3.8 ± 0.2 | 163 ± 2.6 | 9.2 ± 1.0 | |
| FGF | 2349.5 ± 27.5 | - | - | 156.5 ± 11.5 | 6.9 ± 0.2 | 169.5 ± 29.5 | 7.2 ± 1.3 | |
| b Dual Expression of
MyoD/Cyclin A or Myogenin/Cyclin A in C2 Cultures | ||||||
|---|---|---|---|---|---|---|
| Number of Positive Cells Per
Actual Number of Fields Anayzeda |
||||||
| Days in 2% FCS |
Growth Factor Addeda |
Actual Number of Fields Analyzed per Plate |
MyoD/Cyclin A |
Myogenin/Cyclin A |
||
| All MyoD+ | MyoD+ which are also Cyclin A+ |
All Myogenin+ | Myogenin+ which are also Cyclin A+ |
|||
| 1 | None | 23 | 4 | 0 | - | - |
| 24 | 15 | 4 | - | - | ||
| PDGF | 20 | 11 | 6 | - | - | |
| 20 | 11 | 2 | - | - | ||
| FGF | 20 | 17 | 0 | - | - | |
| 20 | 11 | 0 | ||||
| 3 | None | 10 | 101 | 7 | 35 | 3 |
| 10 | 64 | 1 | 32 | 2 | ||
| PDGF | 10 | 187 | 9 | 60 | 1 | |
| 10 | 131 | 8 | 76 | 0 | ||
| FGF | 10 | 274 | 13 | 155 | 10 | |
| 10 | 336 | 17 | 170 | 2 | ||
Cultures were initiated at 4.8 × 104 cells per 35 mm plate using proliferation medium. Following 24 hours cultures were switched to the 2% FCS medium ± growth factors. Numbers reflect mononucleated cells. Fusion into myotubes was not observed at any of the time points. Data for day 3 are based on assaying 10 microscopic fields per plate and represent the average of duplicate plates. Data for day 1 were obtained by analyzing 20–24 microscopic fields per plate using duplicate plates; numbers were then averaged per ten fields per plate.
For all details refer to Table IVa
Myogenic Characteristics of the ‘Pre-MyoD’ Cells
Data shown in the previous sections have indicated that the majority, if not all, of the cells responding mitogenically to PDGF-BB or FGF-2 have not yet entered the MyoD — myogenin expression program. In the study described in this section, we attempted to determine what myogenic traits these growth factor-responsive, ‘pre-MyoD’ cells might express.
First we analyzed the immunoreactivity of the cells with an anti-desmin antibody (panel D in Figure 4). Desmin was examined as proliferating myoblasts from rodents and chickens can express desmin (Kaufman and Foster, 1988; Allen et al., 1991; Maley et al., 1994; Dlugosz et al., 1983; Yablonka-Reuveni and Nameroff, 1990). Following an exhaustive analysis of desmin expression by C2 cultures maintained in the 2% FCS (±PDGF or ±FGF) we concluded that during the first several days in culture only 5–15% of the cells were desmin+ regardless of the absence or presence of the growth factors (data not shown, experiments were repeated with cultures initiated al different cell densities). Desmin+ cells were present in culture even before the appearance of myogenin+ cells, and initially their number was higher than the number of MyoD+ cells. These suggest that in C2 cells desmin is expressed before the transition into the MyoD — myogenin program. However, MyoD+ cells were not always positive for desmin. Furthermore, while some desmin+ cells were positive for cyclin A, many cyclin A+ cells in the C2 cultures did not express desmin. Thus, the expression of desmin has not proved to be a general characteristic of proliferating or MyoD+ myoblasts in C2 cultures. Micrographs showing C2 cultures immunostained with the monoclonal antibody against desmin and a polyclonal antibody against MyoD are shown in Figure 4. It is note worthy the in agreement with our study at the protein level, the study of Li et al. (1994) have observed a very low level of mRNA transcripts for desmin in proliferating C2C12 myoblasts. This desmin transcript level gradually increased following transition into low serum medium and differentiation.
Second, we examined the immunoreactivity of the cells with an antibody against Myf5 (panel E in Figure 4). Myf5 was examined as its mRNA was detected in proliferating C2 cultures (Montarras et al., 1996). Furthermore, Myf5 can be expressed prior to MyoD during early development (Cossu el al., 1996a; 1996b). The immunostainability of C2 cells maintained in 2% FCS (±PDGF or ±FGF) varied for cells within individual plates; the majority of the cells demonstrated negative nuclei, some cells demonstrated very bright nuclei (Figure 4) and a third group of cells demonstrated nuclei with above background staining. Additionally the cytoplasm of some cells was positive as well (Figure 4). This range of nuclear and cytoplasmic staining was identified throughout the culture days examined, making exact quantification of the Myf5+ cells difficult. Double immunostaining assays with the polyclonal antibody against Myf5 and the monoclonal antibodies against MyoD or myogenin have led us to conclude that many of the brightly stained Myf5 cells were negative for MyoD and most, if not all, were negative for myogenin. Altogether, the data suggest that although at least some of the ‘pre-MyoD’ cells were positive for Myf5, the expression of Myf5 was not a uniform characteristic of the ‘pre-MyoD’ cells.
DISCUSSION
The present study was undertaken to further analyze the role of PDGF-BB during myogenesis of the mouse-derived C2 myoblasts. The analysis of cell growth by monitoring the actual number of cells, combined with immunocytochemistry to detect proliferating cells and to trace the temporal expression of different myogenic-specific proteins, has provided a more direct means to analyze possible influences of growth factors as the cells progress through proliferation and the subsequent steps of differentiation. In addition to focusing on the mitogenic influences of PDGF-BB during myogenesis of C2 cells (and comparing them to the influences of FGF-2), we were able to dissect the differentiation process of the C2 cells into multiple steps and further inquire if those steps are affected by the growth factors.
Influence of PDGF-BB on Cell Proliferation and the Appearance of Myosin+ Cells
In the first part of the investigation we attempted to gain further understanding concerning the proliferation of C2 cells. The degree of the effect of PDGF on cell proliferation seems to be cell density related. The effect of PDGF is immediately apparent in cultures initiated at the higher density. However, the mitogenic influence of PDGF in the lower density cultures is not striking at first, but develops gradually with time in culture. As the ‘low density’ cells are further maintained in culture, proliferation is enhanced by PDGF, suggesting that levels of PDGF in the serum (or potentially other mitogens which PDGF can substitue for) became rate limiting when cell density increased. The frequency of the BrdU+ cells suggests that cells in the lower density cultures (but not in the high density ones) are capable of proliferation during the first day in culture without PDGF at rates which are comparable to cells receiving PDGF (46% versus 55% BrdU+ cells in the absence or presence of PDGF, respectively). This represents a minimal value, capturing only those cells that were in the DNA synthesizing phase during the 2-hour exposure to BrdU. Hence, the high number of BrdU+ observed in the day 1 low density cultures suggests that the cells are highly synchronized at this stage. Cultures receiving PDGF demonstrate a decline in the frequency of BrdU+ cells in progressive times in culture despite the increase in total cell numbers. This was observed in both low density and high density cultures (panels A, B, E and F in Figure 1). This somewhat paradoxical observation is likely to reflect a reduction in the synchronicity of the proliferating cells with time in culture; upon losing cell synchronicity with progressive time in culture the 2-hour pulse with BudR is likely capturing less S-phase cells. A more prolonged exposure to BudR can capture more S-phase cells in C2 cultures (data not shown). However, as discussed in the Results section, we avoided in the present study such a prolonged exposure to the drug because it can interfere with the progression through myogenesis. The study of the myosin+ cells has shown that differentiation takes place in both the higher and lower density cultures. However, the data indicate that myosin+ cells accumulate at a slower rate when PDGF is present. To further resolve the effect of PDGF-BB during myogenesis of C2 cells we analyzed the influence of PDGF on the phenotypic transitions of the cells before becoming myosin+.
Influences of PDGF-BB on the Appearance of MyoD+ and/or Myogenin+ Cells
Employing double immunoflourescence, we analyzed the temporal appearance of cells positive for MyoD and/or myogenin in the absence or presence of PDGF (Table I and Figure 2). In contrast to immunostaining with individual antibodies against either MyoD or myogenin (e.g., Yoshida et al., 1996), the dual immunostaining approach allowed us to differentiate between MyoD+ cells which did or did not express myogenin, and vise versa. This approach has provided a more accurate measure for the state of the cells transiting through the differentiation program in the absence or presence of the growth factor. In agreement with the previous set of experiments, the data showed that PDGF supports an increase in total cell numbers and further indicated that the majority, if not all, of these cells which responded mitogenically to PDGF were negative for MyoD or myogenin. This suggests that the PDGF-responding cells are in a compartment which precedes the expression of detectable levels of MyoD protein (i.e., ‘pre-MyoD’ state). The possibility that the cells responding to PDGF represent a fraction of the cells that can never enter the MyoD — myogenin program (i.e., ‘differentiation-defective’ cells) is not supported by our studies. The mononucleated cells present in well-fused cultures yield myogenic cultures upon passaging the original culture, indicating that the cells which ‘fail’ to differentiate in the initial passage are myogenic (data not shown). Additionally, the clonal studies have shown that the majority, if not all, of C2 cells can give rise to clones undergoing MyoD — myogenin expression program. There are negative cells even within these myogenic clones; these negative cells are positioned more peripherial in the clone and differentiate later.
While PDGF enhances the number of cells which have not yet entered the MyoD — myogenin program, the analysis of cells with specific myogenic phenotypes further suggests that the progression through the myogenic program occurs at different rates in the absence or presence of PDGF. Data in Table I and Figure 2 indicate that the frequency of ‘myogenic phenotype’ cells increased between days 1 and 4 by about 4 fold for cultures lacking PDGF while in cultures which received PDGF the frequency of ‘myogenic phenotype’ cells increased only by 2 fold. At day 1 the frequency of such myogenic phenotype cells was similar (about 10%) regardless of the absence or presence of PDGF. The cell phenotype whose frequency is most affected by PDGF is the MyoD+/myogenin+ phenotype; in the absence of PDGF the frequency of mononucleated cells positive for both MyoD and myogenin is far higher in control compared to PDGF-treated cultures in both day 2 and day 3 time points. Eventually, by culture day 4, there is a decline in the frequency of MyoD+/myogenin+ cells in control cultures correlated with a rise in the frequency of cells fused into myotubes (panels D and F in Figure 2). The tight correlation between the fall in the frequency of the MyoD+/myogenin+ cells and the increase in the frequency of cells fused into myotubes indicates that many of the fusing cells have originated from the MyoD+/myogenin+ cell pool.
The timing of appearance of cells positive for MyoD and/or myogenin in the regular and clonal cultures suggests that the MyoD+ cells transit into the MyoD+/myogenin+ compartment, and that in some instances the latter cells further transit into the MyoD−/myogenin+ phenotype and/or fuse into myotubes. However, results in Figure 2 indicate that the frequency of the MyoD+/myogenin+ differs appreciably in the absence or presence of PDGF while the frequency of the MyoD+/myogenin− cells does not (panels E and F in Figure 2). This raises a question regarding the source of the MyoD+/myogenin+ cells in cultures lacking PDGF. We favor the possibility that many of these cells become positive for myogenin at the same time, or just shortly after, they become positive for MyoD. Our investigations with the cultures initiated at lower density also suggest that the frequency of myogenin+ cells is reduced in cultures receiving PDGF (Table III). We have not yet resolved the means by which PDGF might delay the appearance of the myogenin+ cells. Nevertheless, we propose that PDGF affect differentiation independently of its mitogenic role and we favor the possibility that PDGF delays the appearance of myogenin+ cells by influencing another agent(s) that is involved in inducing differentiation. One potential candidate is insulin-like growth factor-II (IGF-II). Several studies have demonstrated that C2 cells produce endogenous IGF-II which can act in an autocrine manner to enhance myoblast differentiation (Florini et al., 1991; Montarras et al., 1996). Additionally, the level of IGF-II produced by C2 cells progressively increases with time in culture following the transition of the cells to a medium containing 2% serum (Tollefsen et al., 1989). A robust increase in the levels of IGF-II transcripts was shown to occur not only when C2 cultures were differentiating in low-serum medium, but also when the cultures become crowded in serum-rich medium; this increase correlated with an increase in the level of myogenin transcripts (Montarras et al., 1996). It is possible then, that PDGF-BB may suppress the emergence of myogenin+ cells by influencing the levels of IGF-II or its receptors. This possibility has not yet been tested by us, but a precedence for the influence of PDGF-BB on reducing levels of IGF-II or on enhancing levels of IGF binding proteins has been documented in other systems (Giannella-Neto et al, 1992; Gabbitas et al., 1994; Canalis and Gabbitas, 1995).
In the doubly immunostained clones we generally observed a gradient, from the center of the clone to the periphery, of cells undergoing MyoD — myogenin expression program. Most regions initially contained MyoD+/myogenin− cells (or showed many more MyoD+/myogenin− cells than myogenin+ cells); subsequently the number of myogenin+ cells increased, also following this “rippling” gradient. Myotubes (which only began appearing in the clonal cultures by the third day in the 2% FCS medium) were also formed first in the center of the clone. Cells in the central parts of the clones were probably born first and reached a state of higher cell density before cells in the periphery of the clone. Hence, it is possible that the differentiation gradient reflects different microenvironments within the clone. With the view that IGF-II is produced by C2 cells and can enhance differentiation, it is possible that such a differentiation promoting agent is more concentrated in the center of the clone, contributing to the differentiation gradient observed. It is interesting to note that the initiation of differentiation at the central region of myogenic clones was also observed by Hauschka (1974) in his studies of human myoblasts; in the latter study differentiation was determined by the apperance of myotubes. The present clonal analysis (Table II) further suggests that the progression into myogenin expressing is somewhat retarded in clones treated with PDGF compared to control. Nevertheless, the influences of PDGF on delaying differentiation might no longer be effective in the central, more crowded region of the clone. Indeed, PDGF does not block (but only delays) the differentiation process in our routine cultures (i.e., low and high density), and as cultures become more crowded they eventually demonstrate high levels of differentiated cells regardless of the presence of the factor. Correlative with the inability of PDGF to influence differentiation of crowded cultures, Montarras et al. (1996) showed that transcript levels for both IGF-II and myogenin are dramatically enhanced in confluent C2 cultures even when maintained in serum-rich medium. Likewise, we have seen that when C2 cultures are maintained in serum-rich medium, the transition of the cells into the MyoD+ state and subsequently into the myogenin+ state, although initially delayed by one to two days, is eventually as robust as in the serum-poor medium.
Influences of FGF-2 on Proliferation and Transitions into the MyoD — Myogenin Expression Program
While the initial goal of the study was the analysis of PDGF influences, we have analyzed in many experiments the influences of both FGF-2 and PDGF-BB. In general, we have seen a somewhat similar pattern of influences by the two growth factors. However, although the presence of each of the factors lead to an increase in the number of the ‘pre-MyoD’ cells, we consistently observed that FGF promoted a higher number of cells than PDGF. Analyzing a wide range of concentrations of PDGF, we concluded that this difference can not be attributed to suboptimal levels of PDGF. We also noted that despite the higher number of cells in the FGF-treated cultures, the frequency of cyclin A+ cells was consistently lower during the initial days in cultures receiving FGF compared to cultures receiving PDGF (Table IVa). It is possible that this apparently paradoxical correlation may be a reflection of an abbreviated cell cycle time when FGF is added to the cultures. Since cyclin A is required for DNA replication and for the M/G2 transition, the protein is likely to be expressed during at least part of the G2 phase; hence, if the G2 phase is accelerated in the presence of FGF-2, than perhaps fewer cyclin A+ cells will be present.
The influences of FGF-2 on the progression through the MyoD — myogenin program were also similar to what we observed with PDGF. During the initial days in the lower density FGF generated an increase in total cell number as well as in the number of MyoD+ cells. In later days in the lower density culture (or earlier on in the higher density culture) the presence of FGF eventually lead to a decrease in the frequency of myogenin+ cells (for example see Table III). A recent study in which C2C12 cells were maintained in serum-free medium, suggested that FGF-2 promotes proliferation while suppressing the frequency of MyoD+ cells and myogenin+ cells (Yoshida et al., 1996). The latter study did not provide a time course of the emergence of the MyoD+ or myogenin+ cells, nor did it distinguish between MyoD+ cells which were potentially co-expressing myogenin. It is therefore possible that the cells suppressed by FGF-2 in the latter study are the MyoD+/myogenin+ cells; in such a case, the findings of Yoshida and colleagues will be in agreement with our observations regarding the reduction in the frequency of MyoD+/myogenin+ cells. We speculate, as for PDGF, that FGF-2 may retard the emergence of the myogenin+ cells by influencing IGF-II levels. Indeed, it has been indicated that the addition of FGF to C2 cultures led to a dramatic reduction in the steady state, low levels of IGF-II in proliferating myoblasts (Montarras et al., 1996).
Recently, a subclone of C2 cells which was stably transfected with antisense IGF-II cDNA has been shown to undergo apoptosis when maintained in low serum but cell survival and cell cycle progression could be regained with the addition of exogenous IGFs, PDGF-BB or FGF-2 (Stewart and Rotwein, 1996). In our studies, which employ ‘standard’ C2 cells, we found very few apoptotic cells when cultures are maintained in either serum-rich or serum-poor medium as shown by fragmented and condensed nuclei. Thus, the increase in cell numbers supported by PDGF-BB and FGF-2 in our studies is due to mitogenic influences of the agents.
Transitions of the Myogenin+ Cells into the MEF2A+ State
Recent studies have indicated that members of the MEF2 family of transcription factors act cooperatively with the MRF family during myogenesis (Molkentin el al., 1995). Additional studies by Molkentin et al. (1996) have recognized the presence of the four members of the MEF2 family in extracts of differentiating C2 cultures. We were able to further dissect the temporal expression of MEF2A by differentiating myoblasts via double immunostaining. We concluded that cells become positive for MEF2A only after they became positive for myogenin, and furthermore, cells transit to becoming myosin+ only after they became positive for MEF2A. We further analyzed the possibility that the transition of the myogenin+ cells into the MEF2A+ state might be influenced by PDGF-BB or FGF-2 and concluded that this transition is not influenced by the mitogens. We analyzed this possibility especially in view of the recent report on C2C12 cells which suggested that myogenin+ cells acquire the post-mitotic, myosin+ state only after becoming positive for the cell cycle inhibitor p21 (Andrés and Walsh, 1996). Hence, it was possible that the transitions of the myogenin+ cells might potentially be influenced by growth factors. Although not studied at length, we have used the same antibody against p21 described in the study of Andrés and Walsh (1996) to examine the time that myogenin+ cells become positive for the cell cycle inhibitor compared to the transition into the MEF2A+ state. Our limited study suggested that the time course for the transitions of the myogenin+ cells into the p21+ or MEF2A+ state was similar. However, whereas the immunostaining of MEF2A seems to be nearly always within myogenin+ cells, the strong immunostaining with the anti-p2l antibody was also exhibited by many cells which were negative for myogenin.
In summary, in the present study we extended our investigation on the mitogenic influences of PDGF-BB during myogenesis of C2 myoblasts. While PDGF is a mitogen for the cells, it also suppresses (but does not abolish) the transition of the C2 cells into the MyoD+/myogenin+ state. It is attractive to suggest, although still not fully examined, that the enhancement of proliferation and the delay in the progression into the MyoD/myogenin state represents two independent influences of PDGF. The present investigation suggests that FGF-2 is somehow a more ‘efficient’ mitogen than PDGF-BB, generating more ‘pre-MyoD’ cells. However, other aspects of myogenesis examined by us were similarly influenced by PDGF-BB and FGF-2. The transition of the cells into the MyoD+ state is initially not suppressed by the mitogens, but eventually, the frequency of the cells undergoing differentiation is reduced in the presence of PDGF-BB or FGF-2. We have begun researching the ways by which PDGF-BB may influence proliferation of C2 myoblasts. Our studies suggest that PDGF-BB (as well as FGF-2) transduce their mitogenic signals through the mitogen-activated protein kinase cascade (Z. Yablonka-Reuveni, A. Rivera, and J. Campbell, work in progress). We are also devising approaches for analyzing the possible basis for the influences of PDGF-BB and FGF-2 on the delay in the emergence of myogenin+ cells. The collective influences of the two mitogens during myogenesis of C2 myoblasts in culture may represent some of the means acting in vivo to regulate the number of proliferating and differentiating myoblasts available for muscle growth and repair.
Acknowledgements
We are grateful to Dr. David Han for suggesting the use of the anti-MEF2A antibody and for helpful discussions while preparing the manuscript. We are also grateful to Dr. David Graves for thoughtful comments on the manuscript. We thank many colleagues for providing us with valuable reagents: Drs. C. Hart and R. Seifert (PDGF-BB), Dr. S. Hauschka (FGF-2), Dr. S. Alemá (anti-MyoD, polyclonal), Drs. P. Houghton and P. Dias (anti-MyoD, monoclonal), Dr. W. Wright (anti-myogenin), Dr. S. Konieczny (anti-Myf5), Drs. J. Roberts and E. Firpo (anti-cyclin A and anti-cyclin E). The monoclonal antibodies against myosin (MF20) and against desmin (D3) were originally developed in Dr. D.A. Fischman’s laboratory and were obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, and the Department of Biology, University of Iowa, Iowa City, IA, under contract NO1-HD-6-2915 from the NICHD. This work was supported in parts by grants to Z.Y.-R. from the Muscular Dystrophy Association, the Cooperative State Research Service - U.S. Department of Agriculture (Agreements No. 93-37206-9301 and 95-37206-2356), and the National Institutes of Health (AR39677 and AG 13798).
References
- Allen RD, Rankin LL, Greene EA, Boxhorn LK, Johnson SE, Taylor RG, Pierce PR. Desmin is present in proliferating rat muscle satellite cells but not in bovine muscle satellite cells. J. Cell. Physiol. 1991;149:525–535. doi: 10.1002/jcp.1041490323. [DOI] [PubMed] [Google Scholar]
- Andrés V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Biochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R, Holtzer H. Inhibition of myoblast fusion after one round of DNA synthesis in 5-bromodeoxyuridine. J. Cell Biol. 1970;44:134–150. doi: 10.1083/jcb.44.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E, Gabbitas B. Skeletal growth factors regulate the synthesis of insulin-like growth factor binding protein-5 in bone cell culture. J. Biol. Chem. 1995;270:10771–10776. doi: 10.1074/jbc.270.18.10771. [DOI] [PubMed] [Google Scholar]
- Cardoso MC, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: Cyclin A and cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L. Platelet-derived growth factor receptor signals. J. Biol. Chem. 1994;269:32023–32026. [PubMed] [Google Scholar]
- Clegg CH, Linkhart TA, Olwin BB, Hauschka SD. Growth factor control of skeletal muscle differentiation: Commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J. Cell Biol. 1987;105:949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DR, Doumit ME, Merkel RA. Transforming growth factor-beta, basic fibroblast growth factor, and platelet-derived growth factor-BB interact to affect proliferation of clonaly derived porcine satellite cells. J Cell Physiol. 1993;157:307–312. doi: 10.1002/jcp.1041570213. [DOI] [PubMed] [Google Scholar]
- Cook DR, Doumit ME, Merkel RA. Ractopamine, cAMP and forskolin enhance the mitogenic activity of platelet-derived growth factor-BB on cultured porcine satellite cells. BAM (Basic and Applied Myology) 1995;5:57–64. [Google Scholar]
- Cossu G, Tajbakhsh S, Buckingham M. How is myogenesis initiated in the embryo? Trends in Genetics. 1996a;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- Cossu G, Kelly R, Tajbakhsh S, Di Donna S, Vivarelli E, Buckingham M. Activation of different myogenic pathways: myf-5 is induced by the neural tube and MyoD by the dorsal ectoderm in mouse paraxial mesoderm. Development. 1996b;122:429–437. doi: 10.1242/dev.122.2.429. [DOI] [PubMed] [Google Scholar]
- Danto SI, Fischman DA. Immunohistochemical analysis of intermediate filaments in embryonic heart cells with monoclonal antibodies to desmin. J. Cell Biol. 1984;98:2179–2191. doi: 10.1083/jcb.98.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias P, Parham DM, Shapiro DN, Tapscott SJ, Houghton PJ. Monoclonal antibodies to the myogenic regulatory protein MyoD1: Epitope mapping and diagnostic utility. Cancer Res. 1992;52:6431–6439. [PubMed] [Google Scholar]
- Dlugosz AA, Tapscott SJ, Holtzer H. Effects of phorbol 12-myristate 13-acetate on the differentiation program of embryonic chick skeletal muscle. Cancer Res. 1983;43:2780–2789. [PubMed] [Google Scholar]
- Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J. Biol. Chem. 1991;266:15917–15923. [PubMed] [Google Scholar]
- Gabbitas B, Pash J, Canalis E. Regulation of insulin-like growth factor-II synthesis in bone cell cultures by skeletal growth factors. Endocrinology. 1994;135:284–289. doi: 10.1210/endo.135.1.8013362. [DOI] [PubMed] [Google Scholar]
- Giannella-Neto D, Kamyar A, Sharifi B, Pirola CJ, Kupfer J, Rosenfeld RG, Forrester JS, Fagin JA. Platelet-derived growth factor isoforms decreases insulin-like growth factor I gene expression in rat vascular smooth muscle cells and selectively stimulates the biosynthesis of insulin-like growth factor binding protein 4. Circ. Res. 1992;71:646–656. doi: 10.1161/01.res.71.3.646. [DOI] [PubMed] [Google Scholar]
- Girard F, Strausfeld U, Fernandez A, Lamb NJC. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Weseman J, Moran JS, Lindstrom J. Effect of fibroblast growth factor on the division and fusion of bovine myoblasts. J. Cell Biol. 1976;70:395–405. doi: 10.1083/jcb.70.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z. Molecular and Cell Biology of Muscle Regeneration. In: Partridge T, editor. Molecular and Cell Biology of Muscular Dystrophy. London: Chapman & Hall; 1993. pp. 210–256. [DOI] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Hannon K, Kudla AJ, McAvoy MJ, Clase KL, Olwin BB. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 1996;132:1151–1159. doi: 10.1083/jcb.132.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS, Bandman E, Yahlonka-Reuveni Z. Myoblasts from embryonic and adult skeletal muscle regulate myosin expression differently. Dev. Biol. 1991;148:249–260. doi: 10.1016/0012-1606(91)90334-y. [DOI] [PubMed] [Google Scholar]
- Hauschka SD. Clonal analysts of vertebrate myogenesis. II. Environmental influences upon human muscle differentiation. Dev. Biol. 1974;37:329–344. doi: 10.1016/0012-1606(74)90153-5. [DOI] [PubMed] [Google Scholar]
- Heldin C-H. Structural and functional studies on platelet-derived growth factor. EMBO J. 1992;11:4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF. Expression of the muscle regulatory MRF4 during somite and skeletal myofiber development. Dev. Biol. 1991;147:144–156. doi: 10.1016/s0012-1606(05)80014-4. [DOI] [PubMed] [Google Scholar]
- Jin P, Farmer K, Ringertz NR, Sejersen T. Proliferation and differentiation of human fetal myoblasts is regulated by PDGF-BB. Differentiation. 1993;54:47–54. doi: 10.1111/j.1432-0436.1993.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Jin P, Sejersen T, Ringertz NR. Recombinant platelet-derived growth factor-BB stimulates growth and inhibits differentiation of rat L6 myoblasts. J Biol. Chem. 1991;266:1245–1249. [PubMed] [Google Scholar]
- Kaufman SJ, Foster RF. Replicating myoblasts express a muscle-specific phenotype. Proc. Natl. Acad. Sci. USA. 1988;185:9606–9610. doi: 10.1073/pnas.85.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts JM. Human cyclin E, a new cyclin that interacts with two members of the cdc2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J. Cell Biol. 1994;124:827–841. doi: 10.1083/jcb.124.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RW, Hauschka SD. EGF responsiveness and receptor regulation in normal and differentiation-defective mouse myoblasts. Dev. Biol. 1994;105:48–58. doi: 10.1016/0012-1606(84)90260-4. [DOI] [PubMed] [Google Scholar]
- Maley MAL, Fan Y, Beilharz MW, Grounds MD. Intrinsic differences in MyoD and myogenin expression between primary cultures of SLJ/J and BALB/C skeletal muscle. Exp. Cell Res. 1994;211:99–107. doi: 10.1006/excr.1994.1064. [DOI] [PubMed] [Google Scholar]
- McFarland DC, Pesall JE, Gilkerson KK. The influence of growth factors on turkey embryonic myoblasts and satellite cells in vitro . General Comp. Endocrinol. 1993;89:415–424. doi: 10.1006/gcen.1993.1049. [DOI] [PubMed] [Google Scholar]
- Megency LA, Rudnicki MA. Determination versus differentiation and the MyoD family of transcription factors. Biochem. Ceil Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- Minshull J, Golsteyn R, Hill C, Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Black B, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Firulli AB, Black BL, Martin JF, Hustad CM, Copeland N, Jenkins N, Lyons G, Olson EN. MEF2B is a potent transactivator expressed in early myogenic lineages. Mol. Cell. Biol. 1996;16:3814–3824. doi: 10.1128/mcb.16.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras D, Aurade F, Johnson T, Ilan J, Gros F, Pinset C. Autonomous differentiation in the mouse myogenic cell line, C2, involves a mutual positive control between insulin-like growth factor II and MyoD, operating as early as at the myoblast stage. J Cell Sci. 1996;109:551–560. doi: 10.1242/jcs.109.3.551. [DOI] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev. Biol. 1992;154:216–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Molec. Endocrinol. 1993;7:369–1378. doi: 10.1210/mend.7.11.8114752. [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini P, Flejou J-F, De Mitri MS, Pisi E, Franco D, Bréchot C. Structure and expression of cyclin A gene in human primary liver cancer. Correlation with flow cytometric parameters. J. Hepatol. 1995;23:47–52. doi: 10.1016/0168-8278(95)80310-6. [DOI] [PubMed] [Google Scholar]
- Salminen A, Braun T, Buchberger A, Simone J, Winter B, Arnold A-H. Transcription of the muscle regulatory gene MYF4 is regulated by serum components, peptide growth factors and signaling pathways involving G proteins. J. Cell Biol. 1991;115:905–917. doi: 10.1083/jcb.115.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapek SX, Rhee J, Spicer DB, Lassar AB. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, Bowen-Pope DF. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J. Biol. Chem. 1989;264:8771–8778. [PubMed] [Google Scholar]
- Smith TH, Block NE, Rhodes SJ, Konieczny SF, Miller JB. A unique pattern of expression of the four muscle regulatory factor proteins distinguishes somitic from embryonic, fetal and newborn mouse myogenic cells. Development. 1993;117:1125–1133. doi: 10.1242/dev.117.3.1125. [DOI] [PubMed] [Google Scholar]
- Smith CK, II, Janncy MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, and differentiation of rat skeletal muscle satellite cells. J. Cell. Physiol. 1994;159:379–385. doi: 10.1002/jcp.1041590222. [DOI] [PubMed] [Google Scholar]
- Sorrentino V, Pepperkok R, Davis RL, Ansorge W, Philipson L. Cell proliferation inhibited by MyoD1 independently of myogenic differentiation. Nature. 1990;345:813–815. doi: 10.1038/345813a0. [DOI] [PubMed] [Google Scholar]
- Stewart CEH, Rotwein P. Insulin-like growth factor-II is an autocrine survival factor for differentiating myoblasts. J. Biol. Chem. 1996;271:11330–11338. doi: 10.1074/jbc.271.19.11330. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Davis RL, Thayer MJ, Cheng P-F, Weintraub H, Lassar AB. MyoD1: A nuclear phosphoprotein requiring a myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Lassar AB, Davis RL, Weintraub H. 5-bromo-2’ deoxyuridine blocks myogenesis by extinguishing expression of MyoD1. Science. 1989;245:532–536. doi: 10.1126/science.2547249. [DOI] [PubMed] [Google Scholar]
- Thorburn AM, Walton PA, Feramisco JR. MyoD induced cell cycle arrest is associated with increased nuclear affinity of the Rb protein. Molec. Biol. Cell. 1993;4:705–713. doi: 10.1091/mbc.4.7.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen S, Sadow JL, Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc. Natl. Acad. Sci. USA. 1989;86:1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya TB, Rhodes SJ, Taparowsky EJ, Konieczny SF. Fibroblast growth factor and transforming growth factor βrepress transcription of the myogenic regulatory gene MyoD1. Molec. Cell Biol. 1989;9:3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, Maller J. Role of cyclin A in the dependence of mitosis on completion of DNA replication. Nature. 1991;354:314–317. doi: 10.1038/354314a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: Redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to myoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Development and postnatal regulation of adult myoblasts. Microscopy Research and Technique. 1995a;30:366–380. doi: 10.1002/jemt.1070300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. Myogenesis in the chicken: The onset of differentiation of adult myoblasts is influenced by tissue factors. BAM (Basic and Applied Myology) 1995b;5:33–42. [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Balestreri TM, Bowen-Pope DF. Regulation of proliferation and differentiation of myoblasts derived from adult mouse skeletal muscle by specific isoforms of PDGF. J. Cell Biol. 1990;111:1623–1629. doi: 10.1083/jcb.111.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Nameroff M. Temporal differences in desmin expression between myoblasts from embryonic and adult chicken skeletal muscle. Differentiation. 1990;45:21–28. doi: 10.1111/j.1432-0436.1990.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seifert RA. Proliferation of chicken myoblasts is regulated by specific isoforms of platelet-derived growth factor: Evidence for differences between myoblasts from mid and late stages of embryogenesis. Dev. Biol. 1993;156:307–318. doi: 10.1006/dbio.1993.1079. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Fujiswa-Sehara A, Taki T, Arai K, Nabeshima Y. Lysophoshatidic acid and bFGF control different modes in proliferating myoblasts. J. Cell Biol. 1996;132:181–193. doi: 10.1083/jcb.132.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]