Abstract
Biliary atresia is the most common cholangiopathy of childhood. With complete obstruction of segments or the entire length of extrahepatic bile ducts, the timely pursuit of hepatoportoenterostomy is the best strategy to restore bile drainage. However, even with prompt surgical intervention, ongoing injury of intrahepatic bile ducts and progressive cholangiopathy lead to end-stage cirrhosis. The pace of disease progression is not uniform; it may relate to clinical forms of disease and/or staging of liver pathology at diagnosis. Although the etiology of disease is not yet defined, several biological processes have been linked to pathogenic mechanisms of bile duct injury. Among them, there is increasing evidence that the immune system targets the duct epithelium and disrupts bile flow. In this review, we discuss how careful clinical phenotyping, staging of disease, and pre-clinical research give insight into response to ongoing trials and rational design of new therapies to block progression of disease.
Keywords: Liver, Cholestasis, Cirrhosis, Children, Neonatal cholestasis, Transplantation
Introduction
Biliary atresia is commonly referred to as an obstructive cholangiopathy of infancy of unknown etiology and poorly defined pathogenesis. It is time to revise this statement. Yes, the etiology is unknown, but our understanding of pathogenesis is much greater. Recent patient-based studies and the use of in vitro systems coupled with an experimental model of disease have identified biological and cellular networks that regulate tissue pathology. Historically, several factors have been proposed to contribute to the pathogenesis of bile duct injury. Most of them are based on valuable clinical and tissue observations, but are limited in reproducibility and lack mechanistic data. Exceptions relate to genetic susceptibility of disease and activation of pro-inflammatory circuits as factors that interplay to produce the typical lesion of extrahepatic bile ducts, and perhaps to the intrahepatic injury that persists after surgical portoenterostomy. These biological processes have potential implications for ongoing and future clinical trials. What regulates susceptibility of disease? Is there enough information to justify immunosuppressive therapy? If so, what type(s) of therapy and which patients are likely to respond? To answer these questions, we present an overview of the disease, discuss phenotypic variations at diagnosis, and examine the rationale for therapeutic approaches to stop progression of the liver disease.
A progressive cholangiopathy of infancy
Biliary atresia results from an inflammatory and fibrosing obstruction of extrahepatic bile ducts. The incidence of disease has wide geographical distribution, affecting 1 in 5,000-18,000 live births (1). Studies have suggested a time-space clustering of cases and seasonal variation, associations with advanced maternal age and increased parity, and a tendency for early fetal losses. A slight female predominance (1.25:1) may be present, with rare familial recurrence and twin studies showing that most sets are discordant for the disease.
Biliary atresia is a disease of “mosts.” It is the most common cause of neonatal cholestasis and the most frequent indication for liver transplant in the pediatric population worldwide, accounting for 40 to 50% of all liver transplants in children (2). It is the most rapidly progressive cholangiopathy of infancy, completely obstructing extrahepatic bile ducts by the time the diagnosis is made. Tissue analysis points to a most intriguing pathology. In the pathology of extrahepatic bile ducts, there is loss of epithelium and extensive fibrosis (with occasional foci of inflammation). In contrast, intrahepatic bile ducts are typically hyperplastic, embedded in portal tracts with variable inflammation and fibrosis, and surrounded by lobules with features of cholestasis and giant cell transformation of hepatocytes. These pathological findings reflect the multifactorial nature of disease pathogenesis. Yet, the clinical hallmarks of the disease are simple: jaundice, acholic stools, and variable degrees of hepatosplenomegaly.
The clinical presentation shares features with other causes of neonatal cholestasis; therefore, the initial task for the clinician is to make a specific diagnosis in a timely fashion so that a surgical relief by hepatoportoenterostomy can be pursued promptly. Unfortunately, even after surgical intervention, biliary cirrhosis progresses in the vast majority of infants. These clinical features and the potential factors involved in pathogenesis of disease have been the subject of excellent reviews (1, 3, 4). Below, we review current concepts and emerging data suggesting that biliary atresia encompasses different phenotypes with potential relevant to clinical course and response to treatment.
Clinical forms or variants of disease
There is consistency in clinical features: affected infants present with jaundice (conjugated hyperbilirubinemia), acholic stools and dark-colored urine in the first few weeks of life. The infant may have normal growth at the time of diagnosis. The presence of hepatomegaly, splenomegaly, failure to thrive, pruritus, and coagulopathy depends on the stage of progression of disease. While these features are common to all infants, the time of onset of symptoms, the coexistence of non-hepatic congenital malformations, and anatomical variants of remnants of the extrahepatic bile ducts allow the subgrouping of patients into clinical forms (5).
Perinatal form of biliary atresia
Most infants fall into this clinical form, which typically develop symptoms after a jaundice-free interval after birth. The onset of cholestasis in a newborn who was previously well suggests that the disease results from a perinatal or early postnatal insult that targets the bile ducts, leading to an inflammatory and rapidly fibrosing obstruction of the duct lumen. Thus, this form is commonly referred to as “perinatal” or “acquired” biliary atresia.
Embryonic form of biliary atresia
Up to 15% of affected infants have an earlier onset of jaundice, often present at birth, and non-hepatic congenital malformations. This group of patients is referred to as having the “embryonic,” “congenital,” or “fetal” form of biliary atresia. Consistent with an earlier onset of bile duct injury, extrahepatic bile ducts may be absent (i.e., no sign of a fibrous cord at the time of exploratory laparotomy). Splenic abnormalities (asplenia, double spleen, and polysplenia) have been reported in 8-12% of the infants, occurring in isolation or in combination with one or more additional defects in a variant known as biliary atresia splenic malformation (BASM) syndrome (6, 7). As a group, these infants have higher association with maternal diabetes and may have worse outcome following portoenterostomy, with decreased transplant-free survival by 2 years of age.
Cystic variant of biliary atresia
The presence of a cystic malformation near the site of obstruction of the common bile duct constitutes an anatomic variant often referred to as “cystic biliary atresia;” it may be associated with improved bile drainage after portoenterostomy (8). Some of the infants with biliary cysts are detected prenatally during routine ultrasound examination of the fetus; jaundice and acholic stools may present soon after birth or after a variable period of time. A recent review of a large cohort with biliary atresia reported the presence of biliary cysts in ~8% of patients (9). In this series, infants with this cystic variant were younger at presentation, but a delay in performing a portoenterostomy beyond 70 days of age was associated with poor long-term survival with the native liver. The anatomical details of this variant and the differences in outcome raise the possibility that it differs in pathogenic mechanisms of disease.
The existence of clinical forms and variants forms the basis for the argument that biliary atresia encompasses a spectrum of disease phenotypes. The application of molecular techniques to study patients that are carefully catalogued into individual clinical forms may provide insight into the biological basis for clinical/anatomical variations, and perhaps may influence the response to different treatment protocols.
Stages of disease at diagnosis
In general, longer duration of disease implies progression of the tissue pathology. Consistent with this concept, diagnosis and surgical intervention in younger infants have been linked to improved bile flow after portoenterostomy and long-term outcome (4). However, age alone is not a uniform predictor of outcome (10), suggesting that other factors contribute to the clinical course. One potential factor is the type and extent of liver injury at diagnosis. This was suggested by an earlier report that infants with excessive hepatic inflammation at the time of diagnosis tended to have poor clinical outcome (11). However, other studies cast doubt about this association by reporting poor outcome in children with advanced hepatic fibrosis (12, 13) or no correlation between fibrosis and clinical outcome (14).
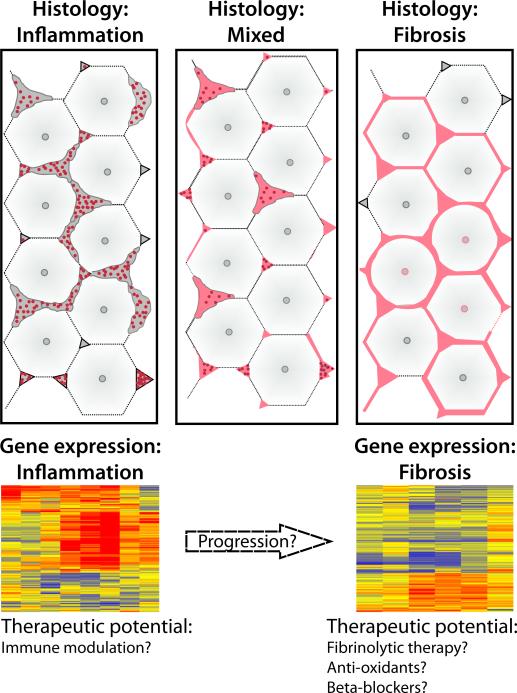
We recently re-examined the relationship between the degree of inflammation and fibrosis with the clinical course after portoenterostomy. Using histological grading of liver biopsies at diagnosis, we were able to assign 30% of the subjects into an inflammation group and 36% into fibrosis, with 34% remaining unclassified due to similar degrees of inflammation and fibrosis in the same biopsy (15). Neither histological group correlated with age at diagnosis, clinical parameters, or 2-year survival with the native liver. Reasoning that morphological quantification methods may be limited by sampling artifacts due to injury patterns that are not uniform among neighboring lobules, we generated gene expression signatures for biopsy fragments for the same patients. By differential profiling of gene expression and prediction analysis models, the majority of the subjects were grouped into inflammation or fibrosis. Although analysis of association using this molecular classification showed that infants with an inflammation signature were younger, age alone could not predict molecular grouping based on the observation that several infants younger than 8 weeks of age were classified into the fibrosis group. Interestingly, infants with the fibrosis signature had decreased transplant-free survival at 2 years of age (15).
The differences in molecular signature and the association of inflammation with younger age raise the possibility that the gene expression signatures reflect two distinct but inter-related stages of disease. The first stage, represented by younger patients with an inflammatory signature (most often but not exclusively at younger age), is placed biologically earlier in pathogenesis of disease, while the other patients may have transitioned to a more advanced stage of fibrosis (Figure 1). If this biological continuum exists, it is tempting to speculate that only those infants with molecular signature of inflammation might have a higher response rate to anti-inflammatory treatment, such as corticosteroids. Collaborative work through multi-center studies will make it possible to formally validate this approach and determine whether tailoring clinical trials to stages of disease that take into account the biological makeup of the patient will improve treatment response and long-term survival with the native liver.
FIGURE 1. Relationship between stages of liver disease and potential treatments.
(A) Diagram depicting the liver acinus with expanded portal spaces containing inflammatory cells (left panel) and advanced fibrosis (right panel); the center panel shows mixed histological features with both inflammation and fibrosis. (B) Example of gene expression cluster analyses with a distinct expression signature for inflammation or fibrosis. Levels of gene expression are shown as a color variation from red (high) to blue (low); yellow denotes baseline levels; columns represent patients and rows represent genes (based on reference 15).
Pathogenic mechanisms of disease
Observational studies and analyses of liver biopsies identiy five factors with potential role in pathogenesis of disease: 1) exposure to environmental toxins, 2) defect in fetal/prenatal circulation, 3) defect in morphogenesis of the biliary tract, 4) viral infection, and 5) inflammation (16, 17). Among these factors, there is limited evidence for a role of toxins and defects in prenatal circulation in pathogenesis of disease. Environmental toxins were implicated in the development of jaundice and bile duct obstruction in lambs and calves in New South Wales, Australia in 1964 and 1988 (18), but no causative agent was identified. Defects in prenatal circulation were implied from anatomical variants of hepatic artery described in some patients with biliary atresia, arterial hyperplasia/hypertrophy in liver specimens, and on the importance of arterial blood flow for integrity of bile ducts (19, 20). There are limited data supporting this mechanism. It is possible that arterial changes may represent a non-specific reaction of a diseased microenvironment that migh be rich in growth-promoting signals that favor angiogenesis. Thus, while it is attractive to think that toxins or microcirculatory changes trigger biological processes that lead to biliary atresia, the field awaits stronger linkage to mechanisms of disease.
Abnormal morphogenesis
The presence of ductal plate malformations in some livers at the time of diagnosis suggests that the pathogenesis of disease involves, at least in part, abnormalities of cell fate of the developing bile duct (21). Ductal plate is a bilayered tubular structure surrounded by thick mesenchyme that is formed near the portal vein between 11 and 13 weeks of gestation (22). The plate gradually disappears, except for a focal area where it forms a lumen and gives rise to intrahepatic bile ducts. Persistence of ductal plates postnatally in those infants with biliary atresia raises the possibility that abnormal mesenchymal support and improper remodeling of hilar ducts may be important pathogenic factors in early stages of disease. Although this anatomic detail has been linked to infants with the embryonic form of biliary atresia, a recent morphological analysis of several portal tracts from each of 8 infants with the perinatal form of biliary atresia and 6 with BASM identified ductal plate malformation in 10% of the portal tracts, with no predilection to either clinical forms of the disease (23).
In addition to these anatomic considerations, the presence of poly- or asplenia, cardiovascular defects, abdominal situs inversus, intestinal malrotation, and other anomalies point to potential defects in embryogenesis and asymmetric left-right determination of visceral organs (also known as laterality) (24). This concept obtained further support by the findings of abnormalities in organ symmetry and bile duct obstruction in the Inv transgenic mouse (25). Although mutational analyses in children with laterality defects and biliary atresia failed to identify mutations in the Inversin gene (26), the model is an example of how embryonic defects can cause obstruction of extrahepatic bile ducts. Consistent with this observation, the over-expression or inactivation of several other genes in mice has been shown to disrupt normal embryogenesis of the extrahepatic biliary system, producing loss of anatomical segments (example: gallbladder and cystic duct) without impairing growth into adulthood (Table 1).
Table 1.
Molecular circuits controlling morphogenesis of the biliary system: morphological consequences in mice with genetic mutations.
| Protein | IHBD | EHBD | Gallbladder |
|---|---|---|---|
| Jagged-Notch | Abnormal | Unaffected | Unaffected |
| Inv | Normal | Atresia | Normal |
| Hes1 | Unaffected | Hypoplasia | Agenesis |
| HNF6 | Ductal plate malformation and intrahepatic biliary cysts | Abnormal | Agenesis |
| HNF1β | Paucity of small IHBD and dysplasia of larger IHBD | Constrictions | Abnormal epithelium and dilated cystic duct |
| Foxf1 | Normal | Unaffected (?) | Small or absent; no epithelial cells |
| Foxm 1b | Agenesis | Normal | Undefined |
| Foxa1/Foxa2 | Hyperplasia of bile ducts | Undefined | Undefined |
| Sox17 | Normal | With ectopic pancreas | Absent |
| Lgr4 | Normal | Normal | Hypoplasia |
| Pdx1 | Normal or unchecked | Loss of Pegs | ?? |
IHBD: Intrahepatic bile ducts; EHBD: Extrahepatic bile ducts; PBG: Peribiliary glands
Although animal models provide proof-of-concept that defective embryogenesis can target the biliary system, there is paucity of similar data from patient-based studies. Promising observations have linked mutations in the CFC1 gene with patients with laterality defects (27, 28), and single nucleotide polymorphisms (SNPs) have been reported as potential susceptibility factors for biliary atresia (in general), such as CD14 and MIF (29, 30). A genome-wide association study of 200 Chinese children with biliary atresia identified high prevalence of SNP rs17095355 on 10q24 in children with biliary atresia, which was validated in an independent patient population of 124 infants (31). At this time, the biological relevance of this SNP is not known. The main limitation of these reports is the small patient population. Progress in the field will require comprehensive, whole-genome mutation surveys in a patient population that meets stringent criteria for adequate statistical analyses, followed by proof-of-principle experiments to explore the biological relevance of SNPs.
Viral infection
Viral infection is frequently cited as a likely etiological agent in the pathogenesis of biliary atresia. It was suggested by Landing several decades ago, who proposed that the biliary atresia phenotype results from exposure to virus(es) and resides along a continuum with neonatal intrahepatic cholestasis and choledocal cyst (32). Since then, several viruses have been implicated in biliary atresia, either from direct detection in injured livers and biliary remnants or the presence of serological markers of infection. The list includes cytomegalovirus, human papillomavirus, human herpes virus 6, Epstein-Barr virus, reovirus, and rotavirus in specific groups of patients, but the identification of individual viruses in tissues has been inconsistent in different populations (for detailed review see reference 33).
Further evidence for a viral etiology is the ability of viruses to induce cholangitis in young mice (example: reovirus) or obstruction of extrahepatic bile ducts in newborn mice (example: rotavirus). These animal models also provide insight as to why it may be difficult to consistently isolate viruses from affected tissues: the neonatal immune system efficiently clears the virus. For example, in the rotavirus-induced experimental biliary atresia, even highly sensitive amplification techniques fail to identify the virus after the bile duct becomes obstructed (34). Thus, future studies must use novel detection technologies or generate indirect evidence of viral infection, such as the report of a molecular footprint of an immune response to viral infection in children with biliary atresia (35).
There is increasing evidence from patient- and animal-based studies that inflammation plays a key role in pathogenesis of liver and bile duct injury in biliary atresia. It is less clear what triggers the inflammation and how it relates to other factors that have been proposed to participate in pathogenesis of the disease. Thus, a review of the evidence for these mechanisms is necessary to guide future research and to build a rationale for new trials based on the biology of disease.
Inflammation as effectors of biliary injury
Inflammation circuits are the most reproducible mechanisms implicated in disease pathogenesis from studies in humans and experimental models. Yet, subjecting patient cohorts to anti-inflammatory treatment does not uniformly improve clinical course. Among the potential reasons for this outcome is the lack of uniformity in stages of disease at the time of diagnosis (15).
Human studies
Several patient-based studies point to a role of the immune system in the pathogenesis of epithelial injury and duct obstruction. For example, cholangiocyte pyknosis and necrosis have been associated with infiltration of mononuclear cells in walls of interlobular bile ducts, duct walls at the portal hepatic, and remnants of extrahepatic bile ducts (36-38). Some of these cells are CD8+ T lymphocytes (39). CD4+ cells also populate affected livers and are associated with markers of pro-inflammatory activation, such as interferon-gamma (IFNγ), interleukin-2, the interleukin-2 receptor CD25, tumor necrosis factor-alpha (TNFα), and the transferrin receptor CD71 (40-43). More direct evidence for an effectors role of T lymphocytes emerged from a report that liver and bile duct remnants of patients with biliary atresia have oligoclonal expansion of CD4+ and CD8+ T cells (44). These are technically challenging experiments that add functional relevance to this group of antigen-specific T cells, and set the stage for future studies investigating their relationship to molecular epitopes in cholangiocytes.
Further evidence of a proinflammatory state derives from a large-scale gene expression analysis of liver biopsies from infants with biliary atresia. The approach identified a genetic footprint in which genes involved in Th1 response were activated at early stages of biliary atresia, with simultaneous but transient suppression of markers of humeral immunity (45). Other studies reported increased number and activation of Kupffer cells and the expression of the MHC class II antigen HLA-DR in the livers of infants with biliary atresia (41, 46, 47).
It is essential to recognize that patient-based studies often generate hypotheses that require direct testing using in vitro systems or animal models to strengthen the argument for causality. For example, the aberrant expression of HLA-DR by cholangiocytes led to the proposal that they may have antigen-presenting properties but testing this hypothesis in the laboratory found no evidence that virus-infected cholangiocytes induce lymphocyte proliferation (48). Therefore, observations in human tissues should be validated by mechanistic studies in the laboratory.
Animal studies
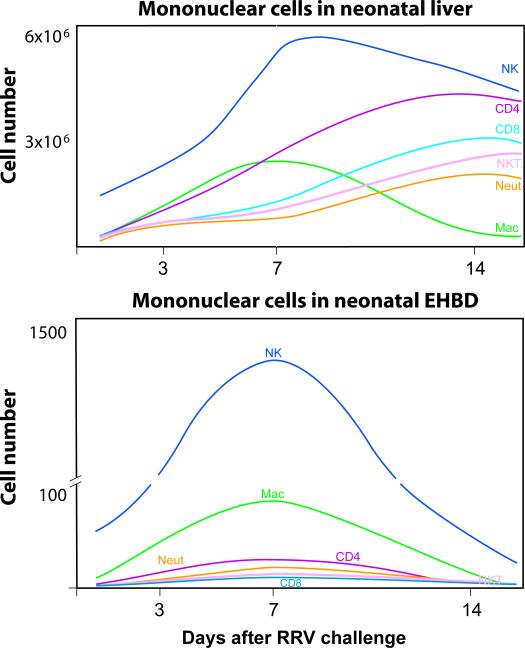
The description of biliary obstruction in newborn mice infected with Rhesus rotavirus type A (RRV) set the stage for the use of this model in mechanistic studies of biliary atresia (49, 50). Analyses of hepatic mononuclear cells and patterns of gene expression show that many clinical-pathological features are similar to the disease in humans (Table 2) (34, 51-55). The model provides opportunities for direct examination of extrahepatic bile ducts to investigate different stages of injury and progression to lumenal obstruction, tasks that cannot be performed in humans because of the advanced fibrosis in biliary remnants at diagnosis. Isolation of mononuclear cells from livers and extrahepatic bile ducts of naïve and RRV-infected newborn mice enabled for the first time the quantification of resident mononuclear cells and their rise after RRV infection (Figure1).
Table 2.
Comparison of clinical, histological, and immunological phenotypes between human biliary atresia and the rotavirus experimental mouse model
| Feature | Human disease | Mouse model |
|---|---|---|
| Onset of symptoms | Restricted to neonatal period | Restricted to neonatal period |
| Jaundice | Yes | Yes |
| Acholic stools | Yes | Yes |
| Virus etiology | Rotavirus, reovirus, HPV, CMV, others | Rotavirus |
| Bile duct proliferation | Yes | Yes* |
| Portal inflammation | Yes (variable intensity) | Yes |
| Fibrosis | Common at presentation | Not present until late in disease |
| Epithelial injury in EHBD | Yes | Yes |
| Obstruction of EHBD | Yes | Yes |
| Non-hepatic anomaly | Yes (in the embryonic form) | No |
| Hepatic CD4+ cells | Increased + Th1 program | Increased + Th1 program |
| Hepatic CD8+ T cells | Increased, activated | Increased, activated |
| Hepatic macrophages | Increased, activated | Increased, activated |
| Apoptosis | Biliary epithelium | Biliary epithelium |
| Molecular signature | Liver: Th1-like network EHBD: Not studied | Liver: Th1-like network EHBD: Th1-like network |
HPV: human papilloma virus; CMV: cytomegalovirus; EHBD: extra-hepatic bile ducts
Increased population of epithelial cells in portal tracts (without obvious proliferation of duct profiles)
Mechanisms of duct obstruction
The prevailing hypothesis that pro-inflammatory cytokines are important to the pathogenesis of biliary atresia has been tested in the RRV-mouse model. Based on the hepatic overexpression of IFNγ in livers of infants at the time of diagnosis, RRV was inoculated into newborn mice carrying an inactivating mutation in the Ifnγ gene. In these mice, cholestasis induced by RRV was transient, extrahepatic bile ducts were free of obstruction, and survival improved substantially (34). Improved cholestasis after RRV was also observed after the administration of antibodies to α2-integrin (56). In other studies, neither the constitutive loss of interleukin-12 nor the antibody depletion of TNFα after the onset of cholestasis prevented duct obstruction or the atresia phenotype (57, 58). These studies demonstrate that the influence on disease pathogenesis cannot be presumed to apply to all pro-inflammatory cytokines, even those with high levels of mRNA expression in affected livers.
Searching for the cellular basis of cytokine production and biliary obstruction, individual mononuclear cells have been depleted in newborn mice to examine their contribution to the atresia phenotype. In the first study, the loss o CD4+ cells decreased the expression of Th1 genes, but did not alter the duct injury and progression to obstruction (54). In contrast, the loss of CD8+ cells prevented duct obstruction after rotavirus infection while allowing the development of cholangitis (54). The similarities between the phenotypes produced by the loss of CD8+ cells or by Ifnγ gene mutation suggest that both factors work in parallel to promote duct obstruction (34, 54).
Mechanisms of epithelial injury
An even more dramatic effect on the atresia phenotype was produced by depletion of NK cells (55). In these experiments, investigators first found that incubation of hepatic NK cells isolated from RRV-infected mice lazed cholangiocytes in a contact-dependent fashion, with no lysis when the activating Nkg2d receptor was blocked on NK cells. Consistent with these in vitro studies, the depletion of NK cells or antibody blockade of Nkg2d immediately after birth prevented the development of jaundice in newborn mice infected with RRV (55). Detailed histological analysis of extrahepatic bile ducts revealed intact epithelium; without duct injury or obstruction, newborn mice grew into adulthood. Interestingly, this lack of duct injury or obstruction occurred despite the presence of the virus in the liver, which suggested that RRV alone is not sufficient to produce biliary obstruction, but requires NK cells to breach the continuity of the duct epithelium and initiate a cascade of events that produce the atresia phenotype.
In culture, cholangiocytes infected by RRV express variable levels of cytokines and chemokines (48, 59), but at levels that appear below the threshold to induce chemotaxis (60). Initiating signals appear to derive from two other cell types that are targeted by RRV: macrophages and dendritic cells. We detected RRV in hepatic macrophages, and found that Mip2/Cxcl2 secreted by the macrophage line Raw 264.7 induced neutrophil chemotaxis (60). In a study published as an abstract (61), we also found that dendritic cells harbor the virus and induce proliferation of T lymphocytes and activation of NK cells after RRV challenge. Collectively, these data suggest that the presence of RRV in macrophages and dendritic cells triggers an inflammatory response by effectors lymphocytes that, in the process of clearing the virus, induce bile duct injury by sensing infected cholangiocytes. The ability of NK and CD8+ T cells to also lye uninfected cholangiocytes raises the possibility of molecular mimicry and autoimmunity in pathogenesis of disease.
Postnatal development and disease susceptibility
One feature that deserves special note is the restriction of onset of disease to the first few months of life in infants and the first few days of life in mice, suggesting that the mechanisms of disease are substantially influenced by developmental factors. One likely factor is the influence of genes regulating embryogenesis, such as CFC1, SNP rs17095355, and others (see above); however, the lack of congenital malformations in the majority of patients opens the possibility for a greater influence of other biological processes. Among them is an inflammatory response triggered by the presence of rare maternal cells in the liver of affected infants (maternal chimerism) (62, 63). Evidence for this process is largely circumstantial at this time. Another relates to the paucity of regulatory T lymphocytes (Treg cells) in the liver and other peripheral tissues in the first 3 days of life in mice. Treg cells have important immunomodulatory function; their absence leads to an array of autoimmune phenotypes. Treg cells were reported to be nearly absent in the liver following RRV challenge in the first 3 days of life (64). In contrast, when RRV was injected at 7 days of age, a time when the liver was populated by Treg cells, mice were resistant to the biliary atresia phenotype. How these results apply to susceptibility of biliary injury in humans, however, is largely unknown at this time.
Working model of bile duct injury by the immune system
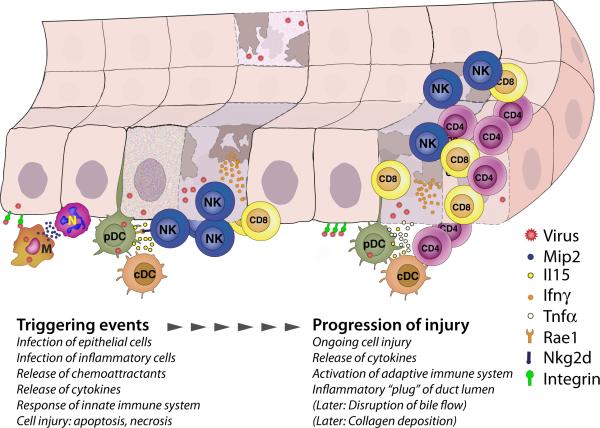
The combined genetic and cell depletion studies in mice uncover a continuum of biological events that produce obstruction of extrahepatic bile ducts in a fashion that recapitulates some features of the disease in humans. The events begin with a viral infection (example: rotavirus) that targets the bile duct epithelium and primes macrophages and dendritic cells (“initiating” phase). This is followed by activation of NK cells that injure cholangiocytes and disrupt mucosal continuity (phase of epithelial injury). An amplification of the adaptive immune response by CD4+ and CD8+ T cells and by the release of pro-inflammatory cytokines form a cellular plug at the site of epithelial injury (phase of obstruction), followed by collagen deposition to produce the atresia phenotype (Figure 3).
FIGURE 3. Mechanisms of epithelial injury in experimental biliary atresia.
Triggering events on the left show RRV infection of cholangiocytes and macrophages (M), and the release of Mip2 and other chemokines to attract neutrophils (N). Soon after infection, plasmacytoid and conventional dendritic cells (pDC and cDC, respectively) activate NK lymphocytes, which injure cholangiocytes by direct engagement and release of Ifnγ and other cytokines. Progression of injury is promoted by pDC and cDC-driven activation and expansion of CD4+ and CD8+ T cells and formation of an inflammatory “plug,” to be followed by disruption of bile flow and collagen deposition. Based on references 34, 54-56, 60, 61.
Considerations for designing clinical trials
Improved understanding of the variations in clinical phenotypes and pathogenesis of biliary atresia identifies at least three variables that should be taken into consideration when designing clinical trials: 1) clinical forms or variants of disease, 2) stages of liver pathology, and 3) types of intervention.
Clinical forms or variants of disease
At present, there are not enough data to justify a potential stratification of patients based on clinical phenotypes when designing clinical trials. However, based on reports of a decrease in long-term survival in children with BASM, it is important to examine the effect of this clinical form on trial end-points. The same applies to the group of infants with the cystic variant undergoing portoenterostomy beyond 70 days of age.
Stages of liver pathology
It is only logical to control for different stages of disease when designing clinical trials. Although staging has been done by histopathology of liver biopsies at diagnosis or time of enrollment, there is no standardized scoring system for biliary atresia and studies using histology measurements have not been reproduced successfully (11-14). A systematic evaluation of liver pathology, perhaps including biopsies from different lobes of the liver, might provide insight into the severity of liver disease and guide the type(s) of intervention with best chances to improve outcome. An emerging alternative is the use of molecular signatures obtained from liver biopsies, which has been shown preliminarily to group infants into inflammation or fibrosis (Figure 1) (15). Others include circulating levels of cytokines and chemokines at diagnosis (and during therapy to assess for response) (65), and hepatic elastography to measure stiffness as an indicator of fibrosis.
Types of intervention
The best therapeutic interventions are those that target key pathogenic mechanisms of biliary injury (such as the immune system) or progression of liver pathology (such as fibrosis). Corticosteroids are undergoing trials and have been justified based on its anti-inflammatory properties and the ability to stimulate bile flow. The use of corticosteroids after portoenterostomy assumes that the mechanisms of liver disease after portoenterostomy are similar to the processes that destroyed the extrahepatic bile duct. The only placebo-controlled trial published to date showed a trend of corticosteroid-treated infants to have improved bile drainage when the surgery was performed before 70 days of age (66). We now await the completion of an ongoing placebo-controlled multicenter trial in the U.S. designed to enroll a larger number of subjects (http://clinicaltrials.gov/ct2/show/NCT00061828). For this and future trials, it is important that the analysis includes innovative approaches to identify clinical, histological, molecular, and serum biomarkers that may influence response to therapy.
At least for selected group of patients, new treatment modalities should be considered. For example, would infants that show improved bile drainage after 1-2 months of corticosteroids benefit from additional low-dose immunosuppression? For those with clear evidence of prominent inflammation, either by validated histology scoring system or molecular signatures, would depletion of specific cell types or cytokines block progression of disease?
It is possible that future approaches to tame the disease will include targeting other biological processes, such as fibrosis, oxidative stress, and apoptosis. Bile acid enrichment with ursodeoxycholic acid is commonly used despite the lack of controlled trials. Regardless of the approach, the time is right to build on the current momentum for multi-center trials to carefully stage the disease, develop markers of response, and test innovative strategies to block progression of disease. Uncertainties are many; yet, the challenge to improve the health of children with biliary atresia is real and worthy.
Areas of future research in biliary atresia.
Molecular basis of clinical phenotypes
Application of molecular techniques to study patients that are carefully catalogued into individual clinical forms and variants of biliary atresia
Genome-side mutation survey to identify genetic susceptibility of disease
Stages of liver disease
Development and validation of histological scoring system(s) to stage the liver disease
Validation of molecular approaches to stage liver disease at diagnosis
Etiology of disease
Use of novel detection technologies to search for viral etiology
Search of molecular footprints of an immune response to viral infection
Pathogenesis of disease
Identification of antigen(s) that target(s) the immunologic injury to cholangiocytes
Cellular and molecular mechanisms of disease: Role of Treg cells, NKT cells, dendritic cells, microRNAs
Epigenetic mechanisms and the role of maternal chimerism in biliary atresia
Clinical trials
Design clinical trials tailored to stages of liver disease
Design clinical trials that take into account genetic predictors of treatment response
Two-step trial of immunosuppression: High-dose corticosteroid, followed by low-dose maintenance in subjects with initial bile drainage (early responders)
New clinical trials to target non-immune processes: anti-fibrotic agents, antioxidants, apoptosis inhibitors
FIGURE 2. Tissue population by mononuclear cells after RRV challenge.
Graphs depict the changes in the number of mononuclear cells of Balb/c mice at early phases (3 days) following RRV challenge in the first day of life, at the time of inflammatory obstruction of extrahepatic bile ducts (7 days), and at the time of atresia (14 days) (based on reference 55)
Acknowledgement
This work was supported by the NIH grants DK64008 and DK83781. Dr. Bezerra is the Cincinnati Principal Investigator of the NIDDK-funded Childhood Liver Disease, Research and Education Network (NIH grant DK62497).
Footnotes
Disclosure statement:
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Kazuhiko Bessho, Research Scholar, Pediatric Liver Care Center and Division of Pediatric Gastroenterology, Hepatology, and Nutrition of Cincinnati Children's Hospital Medical Center and the Department of Pediatrics, University of Cincinnati, Cincinnati, OH, USA
Jorge A. Bezerra, The William and Rebecca Balistreri Chair in Pediatric Hepatology, Professor of Pediatrics, and Director of the Digestive Health Center. Pediatric Liver Care Center and Division of Pediatric Gastroenterology, Hepatology of Nutrition of Cincinnati Children's Hospital Medical Center and the Department of Pediatrics, University of Cincinnati, Cincinnati, OH, USA
REFERENCES
- 1.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374:1704–13. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RA, Kleinman RE. Biliary atresia. J Pediatr Gastroenterol Nutr. 2002;35 doi: 10.1097/00005176-200207001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37:4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Sokol RJ, Shepherd RW, Superina R, et al. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46:566–81. doi: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohi R. Biliary atresia: A surgical perspective. Clin Liver Dis. 2000;4:779–804. doi: 10.1016/s1089-3261(05)70141-0. [DOI] [PubMed] [Google Scholar]
- 6.Davenport M, Savage M, Mowat AP, Howard ER. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery. 1993;113:662–8. [PubMed] [Google Scholar]
- 7.Shneider BL, Brown MB, Haber B, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148:467–74. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 8.Muise AM, Turner D, Wine E, et al. Biliary atresia with choledochal cyst: implications for classification. Clin Gastroenterol Hepatol. 2006;4:1411–4. doi: 10.1016/j.cgh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Davenport M, Caponcelli E, Livesey E, et al. Surgical Outcome in Biliary Atresia: Etiology Affects the Influence of Age at Surgery. Ann Surg. 2008;247:694–98. doi: 10.1097/SLA.0b013e3181638627. [DOI] [PubMed] [Google Scholar]
- 10.Volpert D, White F, Finegold MJ, et al. Outcome of early hepatic portoenterostomy for biliary atresia. J Pediatr Gastroenterol Nutr. 2001;32:265–9. doi: 10.1097/00005176-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Azarow KS, Phillips MJ, Sandler AD, et al. Biliary atresia: should all patients undergo a portoenterostomy? J Pediatr Surg. 1997;32:168–72. doi: 10.1016/s0022-3468(97)90173-1. discussion 72-4. [DOI] [PubMed] [Google Scholar]
- 12.Pape L, Olsson K, Petersen C, et al. Prognostic value of computerized quantification of liver fibrosis in children with biliary atresia. Liver Transpl. 2009;15:876–82. doi: 10.1002/lt.21711. [DOI] [PubMed] [Google Scholar]
- 13.Weerasooriya VS, White FV, Shepherd RW. Hepatic fibrosis and survival in biliary atresia. J Pediatr. 2004;144:123–5. doi: 10.1016/j.jpeds.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 14.Santos JL, Kieling CO, Meurer L, et al. The extent of biliary proliferation in liver biopsies from patients with biliary atresia at portoenterostomy is associated with the postoperative prognosis. J Pediatr Surg. 2009;44:695–701. doi: 10.1016/j.jpedsurg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Moyer K, Kaimal V, Pacheco C, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med. 2010;2:33. doi: 10.1186/gm154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balistreri WF, Grand R, Hoofnagle JH, et al. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–92. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- 17.Bezerra JA. The next challenge in pediatric cholestasis: deciphering the pathogenesis of biliary atresia. J Pediatr Gastroenterol Nutr 43 Suppl. 2006;1:S23–9. doi: 10.1097/01.mpg.0000228197.28056.2f. [DOI] [PubMed] [Google Scholar]
- 18.Harper P, Plant JW, Unger DB. Congenital biliary atresia and jaundice in lambs and calves. Aust Vet J. 1990;67:18–22. doi: 10.1111/j.1751-0813.1990.tb07385.x. [erratum appears in Aust Vet J 1990 May;67(5):167].
- 19.dos Santos JL, da Silveira TR, da Silva VD, et al. Medial thickening of hepatic artery branches in biliary atresia. A morphometric study. J Pediatr Surg. 2005;40:637–42. doi: 10.1016/j.jpedsurg.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Ho CW, Shioda K, Shirasaki K, et al. The pathogenesis of biliary atresia: a morphological study of the hepatobiliary system and the hepatic artery. J Pediatr Gastroenterol Nutr. 1993;16:53–60. doi: 10.1097/00005176-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Desmet VJ. Congenital diseases of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–83. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 22.Tan CE, Davenport M, Driver M, Howard ER. Does the morphology of the extrahepatic biliary remnants in biliary atresia influence survival? A review of 205 cases. J Pediatr Surgery. 1994;29:1459–64. doi: 10.1016/0022-3468(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 23.Pacheco MC, Campbell KM, Bove KE. Ductal plate malformation-like arrays in early explants after a Kasai procedure are independent of splenic malformation complex (heterotaxy). Pediatr Dev Pathol. 2009;12:355–60. doi: 10.2350/09-01-0598-OA.1. [DOI] [PubMed] [Google Scholar]
- 24.Carmi R, Magee CA, Neill CA, Karrer FM. Extrahepatic biliary atresia and associated anomalies: etiologic heterogeneity suggested by distinctive patterns of associations. Am J Med Gen. 1993;45:683–93. doi: 10.1002/ajmg.1320450606. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama T, Copeland NG, Jenkins NA, et al. Reversal of left-right asymmetry: a situs inversus mutation. Science. 1993;260:679–82. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- 26.Schon P, Tsuchiya K, Lenoir D, et al. Identification, genomic organization, chromosomal mapping and mutation analysis of the human INV gene, the ortholog of a murine gene implicated in left-right axis development and biliary atresia. Human Genetics. 2002;110:157–65. doi: 10.1007/s00439-001-0655-5. [DOI] [PubMed] [Google Scholar]
- 27.Davit-Spraul A, Baussan C, Hermeziu B, et al. CFC1 gene involvement in biliary atresia with polysplenia syndrome. J Pediatr Gastroenterol Nutr. 2008;46:111–2. doi: 10.1097/01.mpg.0000304465.60788.f4. [DOI] [PubMed] [Google Scholar]
- 28.Jacquemin E, Cresteil D, Raynaud N, Hadchouel M. CFC1 gene mutation and biliary atresia with polysplenia syndrome. J Pediatr Gastroenterol Hepatol Nutr. 2002;34:326–27. doi: 10.1097/00005176-200203000-00026. [DOI] [PubMed] [Google Scholar]
- 29.Arikan C, Berdeli A, Kilic M, et al. Polymorphisms of the ICAM-1 gene are associated with biliary atresia. Dig Dis Sci. 2008;53:2000–4. doi: 10.1007/s10620-007-9914-1. [DOI] [PubMed] [Google Scholar]
- 30.Shih HH, Lin TM, Chuang JH, et al. Promoter polymorphism of the CD14 endotoxin receptor gene is associated with biliary atresia and idiopathic neonatal cholestasis. Pediatrics. 2005;116:437–41. doi: 10.1542/peds.2004-1900. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Barcelo MM, Yeung MY, Miao XP, et al. Genome-wide association study identifies a susceptibility locus for biliary atresia on 10q24.2. Hum Mol Genet. 2010 doi: 10.1093/hmg/ddq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst--the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg. 1974;6:113–39. [PubMed] [Google Scholar]
- 33.Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis. 2007;27:233–42. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114:322–9. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Masri AN, Flemming P, Rodeck B, et al. Expression of the interferon-induced Mx proteins in biliary atresia. J Pediatr Surg. 2006;41:1139–43. doi: 10.1016/j.jpedsurg.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Bill AH, Haas JE, Foster GL. Biliary Atresia: histopathologic observations and reflections upon its natural history. J Pediatr Surg. 1977;12:977–82. doi: 10.1016/0022-3468(77)90609-1. [DOI] [PubMed] [Google Scholar]
- 37.Gosseye S, Otte JB, De Meyer R, Maldague P. A histological study of extrahepatic biliary atresia. Acta Paediatr Belg. 1977;30:85–90. [PubMed] [Google Scholar]
- 38.Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg. 1995;30:515–8. doi: 10.1016/0022-3468(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed AF, Ohtani H, Nio M, et al. CD8+ T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J Pathol. 2001;193:383–9. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path793>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 40.Broome U, Nemeth A, Hultcrantz R, Scheynius A. Different expression of HLA-DR and ICAM-1 in livers from patients with biliary atresia and Byler's disease. J Hepatol. 1997;26:857–62. doi: 10.1016/s0168-8278(97)80253-x. [DOI] [PubMed] [Google Scholar]
- 41.Davenport M, Gonde C, Redkar R, et al. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001;36:1017–25. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 42.Dillon PW, Belchis D, Minnick K, Tracy T. Differential expression of the major histocompatibility antigens and ICAM-1 on bile duct epithelial cells in biliary atresia. Tohoku J Exp Med. 1997;181:33–40. doi: 10.1620/tjem.181.33. [DOI] [PubMed] [Google Scholar]
- 43.Mack CL, Tucker RM, Sokol RJ, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res. 2004;56:79–87. doi: 10.1203/01.PDR.0000130480.51066.FB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mack CL, Falta MT, Sullivan AK, et al. Oligoclonal expansions of CD4+ and CD8+ T-cells in the target organ of patients with biliary atresia. Gastroenterology. 2007;133:278–87. doi: 10.1053/j.gastro.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bezerra JA, Tiao G, Ryckman FC, et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1563–659. doi: 10.1016/S0140-6736(02)11603-5. [DOI] [PubMed] [Google Scholar]
- 46.Tracy TF, Jr., Dillon P, Fox ES, et al. The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg. 1996;31:121–5. doi: 10.1016/s0022-3468(96)90333-4. discussion 25-6. [DOI] [PubMed] [Google Scholar]
- 47.Urushihara N, Iwagaki H, Yagi T, et al. Elevation of serum interleukin-18 levels and activation of Kupffer cells in biliary atresia. J Pediatr Surg. 2000;35:446–9. doi: 10.1016/s0022-3468(00)90211-2. [DOI] [PubMed] [Google Scholar]
- 48.Barnes BH, Tucker RM, Wehrmann F, et al. Cholangiocytes as immune modulators in rotavirus-induced murine biliary atresia. Liver Int. 2009;29:1253–61. doi: 10.1111/j.1478-3231.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen C, Biermanns D, Kuske M, et al. New aspects in a murine model for extrahepatic biliary atresia. J Pediatr Surg. 1997;32:1190–5. doi: 10.1016/s0022-3468(97)90680-1. [DOI] [PubMed] [Google Scholar]
- 50.Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res. 1993;33:394–9. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Carvalho E, Liu C, Shivakumar P, et al. Analysis of the Biliary Transcriptome in Experimental Biliary Atresia. Gastroenterology. 2005;129:713–17. doi: 10.1016/j.gastro.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 52.Leonhardt J, Stanulla M, von Wasielewski R, et al. Gene expression profile of the infective murine model for biliary atresia. Pediatr Surg Int. 2006;22:84–9. doi: 10.1007/s00383-005-1589-0. [DOI] [PubMed] [Google Scholar]
- 53.Mack CL, Tucker RM, Sokol RJ, Kotzin BL. Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol. 2005;115:200–9. doi: 10.1016/j.clim.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shivakumar P, Sabla G, Mohanty S, et al. Effector role of neonatal hepatic CD8+ lymphocytes in epithelial injury and autoimmunity in experimental biliary atresia. Gastroenterology. 2007;133:268–77. doi: 10.1053/j.gastro.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivakumar P, Sabla GE, Whitington P, et al. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest. 2009;119:2281–90. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jafri M, Donnelly B, Allen S, et al. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohanty SK, Shivakumar P, Sabla G, Bezerra JA. Loss of interleukin-12 modifies the pro-inflammatory response but does not prevent duct obstruction in experimental biliary atresia. BMC Gastroenterol. 2006;6:14. doi: 10.1186/1471-230X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tucker RM, Hendrickson RJ, Mukaida N, et al. Progressive biliary destruction is independent of a functional tumor necrosis factor-alpha pathway in a rhesus rotavirus-induced murine model of biliary atresia. Viral Immunol. 2007;20:34–43. doi: 10.1089/vim.2006.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jafri M, Donnelly B, Bondoc A, et al. Cholangiocyte secretion of chemokines in experimental biliary atresia. J Pediatr Surg. 2009;44:500–7. doi: 10.1016/j.jpedsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohanty SK, Ivantes CA, Mourya R, et al. Macrophages are targeted by rotavirus in experimental biliary atresia and induce neutrophil chemotaxis by mip2/cxcl2. Pediatr Res. 2010;67:345–51. doi: 10.1203/PDR.0b013e3181d22a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saxena V, Shivakumar P, Sabla GE, et al. Dendritic cells regulate injury of bile duct epithelium by activation of NK cells in experimental biliary atresia. Hepatology. 2009 [Google Scholar]
- 62.Hayashida M, Nishimoto Y, Matsuura T, et al. The evidence of maternal microchimerism in biliary atresia using fluorescent in situ hybridization. J Pediatr Surg. 2007;42:2097–101. doi: 10.1016/j.jpedsurg.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 63.Muraji T, Hosaka N, Irie N, et al. Maternal microchimerism in underlying pathogenesis of biliary atresia: quantification and phenotypes of maternal cells in the liver. Pediatrics. 2008;121:517–21. doi: 10.1542/peds.2007-0568. [DOI] [PubMed] [Google Scholar]
- 64.Miethke AG, Saxena V, Shivakumar P, et al. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol. 2010;52:718–26. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narayanaswamy B, Gonde C, Tredger JM, et al. Serial circulating markers of inflammation in biliary atresia--evolution of the post-operative inflammatory process. Hepatology. 2007;46:180–7. doi: 10.1002/hep.21701. [DOI] [PubMed] [Google Scholar]
- 66.Davenport M, Stringer MD, Tizzard SA, et al. Randomized, double-blind, placebo-controlled trial of corticosteroids after Kasai portoenterostomy for biliary atresia. Hepatology. 2007;46:1821–7. doi: 10.1002/hep.21873. [DOI] [PubMed] [Google Scholar]