Abstract
Casino venues are often characterized by “warm” colors, reward-related sounds, and the presence of others. These factors have always been identified as a key factor in energizing gambling. However, few empirical studies have examined their impact on gambling behaviors. Here, we aimed to explore the impact of combined red light and casino-related sounds, with or without the presence of another participant, on gambling-related behaviors. Gambling behavior was estimated with the Iowa Gambling Task (IGT). Eighty non-gamblers participants took part in one of four experimental conditions (20 participants in each condition); (1) IGT without casino-related sound and under normal (white) light (control), (2) IGT with combined casino-related sound and red light (casino alone), (3) IGT with combined casino-related sound, red light and in front of another participant (casino competition—implicit), and (4) IGT with combined casino-related sound, red light and against another participant (casino competition—explicit). Results showed that, in contrast to the control condition, participants in the three “casino” conditions did not exhibit slower deck selection reaction time after losses than after rewards. Moreover, participants in the two “competition” conditions displayed lowered deck selection reaction time after losses and rewards, as compared with the control and the “casino alone” conditions. These findings suggest that casino environment may diminish the time used for reflecting and thinking before acting after losses. These findings are discussed along with the methodological limitations, potential directions for future studies, as well as implications to enhance prevention strategies of abnormal gambling.
Keywords: Decision-making, Gambling, Environment, Sounds, Lights, Competition
Introduction
Gambling is characterized by intermittent rewards and losses delivered on a variable ratio, which entails imperfect prediction of reward (Schultz 2002). As such, when we pull the lever and win some money during gambling, we experience a potent rush of pleasure, precisely because the reward was so uncertain or unexpected (Griffiths and Auer 2013; Redish et al. 2007). Another key factor involved in the attractiveness of gambling is that it often occurs in a typical environment, usually casino settings (Griffiths 1993; Hess and Diller 1969; Peller et al. 2008). Indeed, entering a casino is typically viewed as a pleasurable experience triggered by general noise, “warm” colors, and reward-related sounds (e.g., Finlay et al. 2006, 2007, 2010). In addition, playing the tables in a casino can be a disorienting experience, which can possibly impact at-risk gambling intention (Finlay et al. 2010; Marmurek et al. 2007). Specifically, due to a lack of clocks and natural daylight, casinos can simulate daylight during the dark hours to lure players into remaining at the tables and slot machines.
Thus, casino-related context constitutes a key factor in the repetition of gambling behaviors. Surprisingly, only a couple of empirical studies have investigated the impact of casino-related factors (e.g., fast sounds and “warm” lights) on gambling behaviors. Dixon et al. (2007) found that fast tempo music (e.g., >94 beats per minute) significantly heightened participant’s betting speed when gambling. In addition to fast sound, “warm” colors are often used in gambling environments (e.g., Griffiths and Swift 1992). For instance, red has been found to be stronger, more exciting, and more arousing than blue (e.g., Yoto et al. 2007). Stark et al. (1982) provide one of the only empirical contributions assessing the effects of colored light on gambling behavior. These authors found that gambling under red light (compared to blue light) led to more risk taking, higher stakes, and more frequent bets. More recently, Spenwyn et al. (2010) observed that the combined effects of both high tempo music and red light (but not red light or high tempo music conditions alone) result in faster bets in a computerized version of roulette. According to Spenwyn et al. (2010), their results are in accordance with Ward et al. (1992) notion that we process environments as a whole, so that the combined effects of situational characteristics will be most effective in influencing consumer’s behavior. More specifically, the fast music and red light may “fit” with participant’s expectations of a casino environment (Griffiths and Parke 2005), which is associated with both light and sounds. In other words, only the combination of light and music may induce faster play because it ‘mimics’ accurately gambling-related environment. As a result, participants may appraise the casino setting environment as appropriate and, therefore, may not be distracted by anything that could have been deemed inappropriate (Spenwyn et al. 2010).
Another main characteristic of the casino setting is the presence of others while gambling, which can have an energizing effect on gambling. For instance, in a recent study, Rockloff et al. (2011) have highlighted that gambling simultaneously with others participants increased the speed of betting on a simulated slot machine, as compared with the condition in which participants had to gamble alone. Moreover, despite the fact that gamblers are usually attempting to beat the odds against the machine, they are also in a sense in competition with others either implicitly or explicitly. More specifically, during explicit competition, individuals are clearly aware that their performance is being compared to at least one other performer (e.g., to compare gambling scores while gambling with friends). During implicit competition, however, individuals are involved in normative comparison (i.e., the competitive situation is not explicitly stated or agreed upon), they unofficially tend to compare their performance with that of another. Both implicit and explicit competitions can modify behavioral performance. For instance, Baumeister (1984) showed that performing a simple motor-skill task (e.g., golf putting) is altered when one has to perform the task simultaneously with another participant (i.e., implicit competition). With regard to explicit competition, several studies (e.g., Church 1962; Cross and Gill 1982; Seta et al. 1977) have shown that individuals performed motor-skill tasks faster during a one-to-one competition rather than alone.
In the present study, we aimed to examine the impact of light, sounds and pairs on decision-making during the Iowa Gambling Task (IGT; Bechara et al. 1994). The main reason for choosing the IGT is that, by contrast to a simulated slot machine paradigm, rewards and losses during this task are not randomly chosen. Indeed, the IGT involves probabilistic learning via monetary rewards and punishments specifically associated with four decks selection (A, B, C or D), where advantageous performance requires subjects to choose decks associated with low rewards but lower losses and to forego decks associated with large rewards but larger losses. In other words, advantageous decision-making during the IGT is in opposition with the profile of decision-making usually promoted within the casino setting (i.e., preference for choices featuring high short-term rewards). Hence, despite its lower ecological validity (as compared with a simulated slot machine), the use of the IGT allows to examine if casino-related environment could bias decision-making towards high short-term rewards rather than lower but long-term rewards.
In summary, the aim of this study was to explore the impact of casino-related context (i.e., sound, light and pairs) on (1) risky decision-making during the IGT, (2) response speed after rewards and losses, and (3) response shifting after rewards and net losses. Based on results from previous studies, we present two primary hypotheses: First, compared to a neutral situation context (i.e., participants performed the IGT alone with no sound and white light), the combination of casino-related sound and red light would modify participants’ IGT performances (i.e., more frequent selection of decks featuring high rewards but higher losses; lower reaction time after net losses; lower deck response shifting after net losses). Second, we hypothesized that performing the IGT face to face with another participants and under casino-related sounds and light would further modify participants’ IGT performances, as compared to the “neutral” and the “casino alone” conditions. Additionally, we also aimed to examine whether explicit competition context (i.e., participants performed the IGT face to face with another participant under casino-related light and sounds and are requested to perform better that his/her opponent) could further bias IGT performances, as compared to implicit competition context (i.e., participants performed the IGT face to face with another participant under casino-related light and sounds with no further instruction).
Methods
Participants
Eighty participants, including 56 male and 24 female subjects, aged 18–62 (M = 22.69, SD = 6.06), successfully completed the experiment from November 2012 to February 2013 following their recruitment from newspaper advertisements in Brussels, Belgium. To avoid biases, resulting from inside knowledge of how these tasks operate, Psychiatrists, Psychologists and other personnel having had psychological training were excluded from participation. None of the participants scored three or higher on the South Oaks Gambling Screen (SOGS, Lesieur and Blume 1987), which refer to low problem gambling. Moreover, on the SOGS, only twelve participants (15 %) reported playing the numbers or betting on lotteries occasionally (i.e., less than once a week). All remaining control participants reported not gambling at all.
Current Clinical Status
Current clinical status of depression and anxiety levels were rated with the Beck Depression Inventory (BDI; Beck et al. 1961), the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger 1983). Sensitivity to loss and reward was estimated with the BIS/BAS scale (Carver and White 1994). Impulsivity was examined with the UPPS scale (Whiteside and Lynam 2001). We also estimated the desire to win in interpersonal situations with the Revised Competitiveness Index (Houston et al. 1992, 2002).
The Iowa Gambling Task (IGT)
In this task, participants sat in front of four decks of cards that were identical in appearance, except for their labels A, B, C and D. They were told that the goal of the task was to earn as much money as possible. Participants were informed that each trial would consist of a deck selection and the turning over of one card from the selected deck to reveal the yield. Participants were informed that they were free to switch between decks at any time, and as often as desired. The net outcome of choosing from either deck A or deck B was a loss of five times the average per ten cards (referred to as disadvantageous decks), and the net outcome of choosing from either decks C or D was a gain of five times the average per ten cards (advantageous decks). The total number of trials was set at 100 card selections.
Design
The design comprised four between-subjects conditions: the “control” condition (alone, white light, no casino-related sounds), the “casino alone” condition (CA; alone, red light, casino-related sound), the “implicit competition casino” condition (CCI; IGT face to face with another participant, red light, casino-related sound) and the “explicit competition casino” condition (CCE; IGT face to face against another participant, red light, casino-related sound). The dependent measures were the participants’ the number of cards picked from the advantageous decks in each stage of 20 cards (five block of twenty trials), response speed after rewards and losses and response shifting after rewards and net losses.
Participants’ Subjective Appraisal of Experimental Manipulations on Their Affects
Directly after the experiment, we asked participants to fill-in a four-items form with a 7-point rating scale (from “extremely negatively” to “extremely positively”), which aimed at examining participants’ subjective appraisal of experimental manipulations on their affects: Item 1: “Did the experiment have influenced your mood during the task?”, Item 2: “Did the sounds have influenced your mood during the task?”, Item 3: “Did the light have influenced your mood during the task?”, Item 4: “Did the presence of the individual in front of you have influenced your mood during the task?”. Items 2, 3 were filled by the participants of the CA, CCE, CCI conditions only. Item 4 was filled by participants of the CCE and CCI conditions only.
Materials
The experiment took place in a room situated in The Laboratory of Medical Psychology and Addictology of the Brugmann University Hospital (Université Libre de Bruxelles, Belgium). The IGT (full screen) was run on 19 inches laptop computers. No sounds were induced by rewards and losses during the IGT. In the casino conditions, in order to fully expose participants to a red environment, the walls of the entire room were covered in dark (e.g., Spenwyn et al. 2010). In the control condition, walls of the room were white. The lighting in the room was manipulated using a 15 Watt white light or a 15 Watt red light, which were placed into the room’s main lighting. The casino-related sounds were chosen from a web database and referred to casino ambiance at slot machines. The casino-related sounds were opposed to a no sound condition rather than a slow music condition because casino sounds are not complementary to other kind of sounds or no vocal music (e.g., chill-out music, classical music). The tempo of the casino-related sounds was 121 beats per minute (bpm), which corresponds to fast tempo music (i.e., >94 bpm correspond to fast music;<72 bpm correspond to slow music; Milliman 1982). The music was uploaded onto an MP3 player and played through speakers that were positioned in the upper right corner of the room. The volume of the music remained the same for all conditions.
Procedure
Following ethical clearance participants were recruited by email and were asked to meet the experimenter outside the laboratory. Participants were randomly assigned to one of the four conditions before experiment day (with twenty participants in each condition). The day of the experiment, participants first filled a consent form, the Sate version of the Anxiety Inventory and then received an explanation of the IGT task. Participants were given the opportunity to ask any questions they had before entering the room. When they were satisfied that they understood the procedure that they will follow, the participants (one in the control and CA conditions; two in the CCI and CCE conditions) were then led to the room where the experiment took place. In the casino conditions (i.e., CA, CCI, CCE), background music was already playing and the red light was already on. In the CCI and the CCE conditions, participants performed the task in front of another participants. The two participants began the task simultaneously. No further instructions were given except in the CCE condition, in which participants were informed that they were competing with each other, and that they had to try to win more money than their opponent. Each participant started with $2,000 worth of virtual money. Participants were informed that they had to play until an “end” message was displayed on the screen. Directly after the IGT, participants were asked to quit the room and to fulfill the four items examining their appraisal of the experimental situation. They were then requested to complete the BDI, UPPS, STAI-S, STAI-T, BIS/BAS and the Competitiveness Index. Each participant received €10 for his or her participation. Participants were not remunerated as a function of their gambling performance.
Results
Demographics and Current Clinical Status
A description of demographic variables, scores on the BDI, the STAI, UPPS, BIS/BAS, Competitiveness Index and SOGS is presented in Table 1. The groups were similar in terms of age. There was an equal number of male and female within the four conditions. There was no significant between group difference on the BDI, STAI, UPPS, BIS/BAS, Competitiveness Index and SOGS. In addition, we observed no significant correlation (on the total number of participants and for each group separately) between measures of clinical status and the dependent measures (the number of cards picked from the advantageous decks in each stage of 20 cards, response speed after rewards and losses and response shifting after rewards and net losses).
Table 1.
Demographical data and standard deviations for the control and the casino conditions groups
| Control | CA | CCI | CCE | Test statistics | |
|---|---|---|---|---|---|
| n | 20 | 20 | 20 | 20 | |
| Age (SD) | 22.45 (5.27) | 21.85 (4.63) | 23.20 (8.19) | 23.25 (6.16) | F(3,76) = 0.58, NS |
| Male/Female | 14/6 | 14/6 | 14/6 | 14/6 | |
| BIS | 19.85 (3.78) | 20.15 (4.46) | 18.90 (2.93) | 20.45 (4.72) | F(3,76) = 0.52, NS |
| BAS drive | 11.05 (2.58) | 11.40 (1.88) | 11.70 (2.59) | 11.20 (2.07) | F(3,76) = 0.30, NS |
| BAS reward | 16.65 (2.11) | 16.70 (2.59) | 17.10 (1.61) | 17.20 (2.67) | F(3,76) = 0.29, NS |
| BAS fun-seeking | 11.50 (2.46) | 11.60 (2.83) | 12.45 (1.95) | 11.95 (2.32) | F(3,76) = 0.63, NS |
| UPPS urgency | 29.75 (4.91) | 31.05 (6.24) | 29.65 (4.83) | 29.20 (8.94) | F(3,76) = 0.30, NS |
| UPPS lack of premeditation | 30.20 (4.23) | 31.95 (4.19) | 31.80 (4.27) | 31.10 (4.38) | F(3,76) = 0.90, NS |
| UPPS lack of perseverance | 27.25 (4.66) | 28.40 (4.32) | 28.55 (5.42) | 28.10 (4.65) | F(3,76) = 0.29, NS |
| UPPS sensation-seeking | 28.10 (8.41) | 28.25 (7.43) | 28.85 (8.17) | 27.45 (7.11) | F(3,76) = 0.11, NS |
| BDI | 5.00 (3.34) | 3.75 (3.59) | 3.60 (3.93) | 5.25 (4.82) | F(3,76) = 0.91, NS |
| STAI-S before | 33.10 (8.64) | 32.75 (8.86) | 31.35 (6.10) | 31.70 (8.10) | F(3,76) = 0.89, NS |
| STAI-S after | 32.85 (7.61) | 31.80 (13.79) | 30.85 (8.26) | 30.45 (7.69) | F(3,76) = 0.24, NS |
| STAI-T | 40.10 (7.50) | 35.50 (15.86) | 38.75 (10.44) | 40.75 (13.27) | F(3,76) = 0.74, NS |
| Competitiveness Index | 39.35 (10.32) | 41.15 (11.56) | 36.10 (9.94) | 35.5 (8.31) | F(3,76) = 1.56, NS |
| SOGS | 0.65 (1.03) | 0.40 (0.88) | 0.20 (0.41) | 0.30 (0.65) | F(3,76) = 1.21, NS |
Values shown are the mean and standard deviations on each measure
BDI Beck Depression Inventory, STAI-E State version of the State-Trait Anxiety Inventory, STAI-T Trait version of the State-Trait Anxiety Inventory, SOGS South Oaks Gambling Screen
IGT Decision-Making Performance
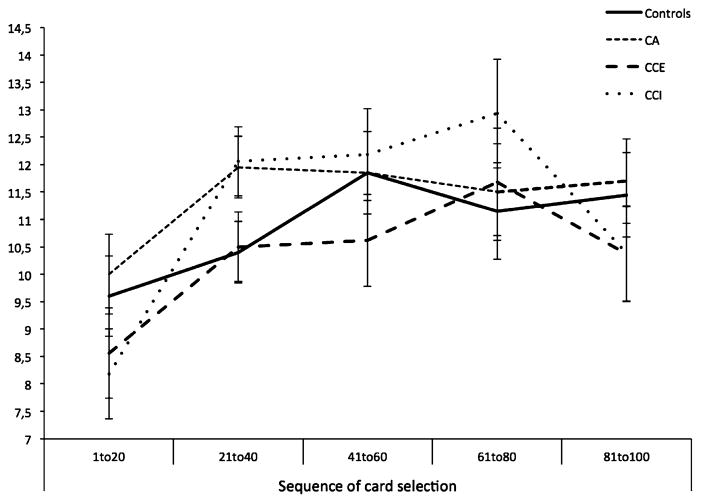
A repeated measures ANOVA was performed, with group as a between subjects factor, stage (5 blocks of 20 trials) as a within subjects factor, and the number of cards picked from the advantageous decks as the dependent measure. This analysis revealed an effect for stage, F(4,272) = 9.36, p < 0.001, ηg2 = 0.12, indicating that task performance increased during the consecutive stages of the task (see Fig. 1). However, there was no group effect, F(3,68) = 0.46, p = 0.46, ηg2 = 0.04, and no group by stage interaction, F(12,272) = 1.08, p = 0.43, ηg2 = 0.04. Additional one-sample t tests were undertaken in order to examine if advantageous deck selection on the last stage of the IGT differs from the chance level (test value = 10). These analyses that the mean of advantageous deck selection on the latter stage of the IGT significantly differ from the chance level in the control group, t(19) = 2.15, p < 0.05. We observed no significant difference in the three other groups, indicating the mean of advantageous deck selection on the latter stage of the IGT did not significantly differ from the chance level in CA, CCI, CCE groups.
Fig. 1.
Means of the total number of cards selected from the advantageous decks for each block of 20 card choices on the Iowa Gambling Task in the controls, CA, CCE and CCI groups. Error bars are the standard errors of the mean
IGT Response Speed After Net Rewards and Net Losses
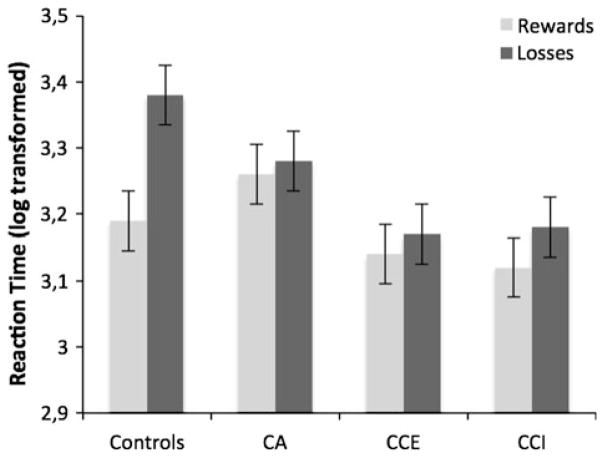
Median reaction times (RT) after net rewards and after net losses were calculated. Due to non-normality of these measures (median RT rewards; Kolmogrorov–Smirnov = 0.12, p < 0.05; median RT losses; Kolmogrorov–Smirnov = 0.17, p < 0.001), log-transformed data were entered into the model (median log(10) RT rewards; Kolmogrorov–Smir-nov = 0.08, p = 0.21; median log(10) RT losses; Kolmogrorov–Smirnov = 0.06, p = 0.89). A repeated measures ANOVA was performed with group as a between subjects factor, contingency (reward or loss) as a within subjects factor, and log(10) median reaction times as dependent measure. As depicted in Fig. 2, response speed after rewards was faster than after losses, F(1,76) = 11.34, p < 0.001, ηg2 = 0.13. The groups differed in reaction times, F(3,76) = 3.14, p < 0.05, ηg2 = 0.11. Pairwise comparisons indicated that the CCI and the CCE groups responded faster than the CA and the controls groups (p < 0.05). There was no difference between the CCI and the CCE groups. There was also no difference between the CA and the control groups. Importantly, an interaction effects of group by contingency were found, F(3,76) = 3.33, p < 0.05, ηg2 = 0.12, which indicated that controls were slower after losses than after rewards (see Fig. 2). This difference was not observed in the three other groups (see Fig. 2).
Fig. 2.
Mean response times (log 10 transformed) after receiving a net reward or a net loss on the Iowa Gambling Task by control, CA, CCE and CCI participants. Error bars are the standard errors of the mean
IGT Response Shifting After Net Rewards and Net Losses
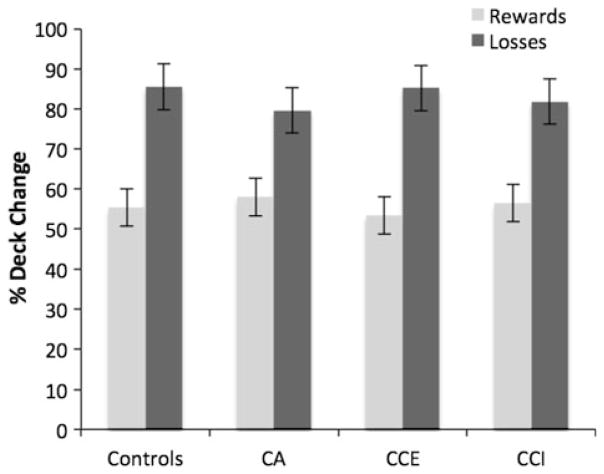
A repeated measures ANOVA with group as a between subjects factor and contingency (reward or loss) as a within subjects factor was performed to investigate whether net rewards or net losses resulted in change of deck choice on the consecutive trial. Percentage of change after rewards or after net losses was included as the dependent variable. An overall effect of contingency was present, F(1,76) = 107.38, p < 0.001, ηg2 = 0.59. As is depicted in Fig. 3, a higher percentage of change was present after losses than after rewards. No group effect was present, F(3,76) = 0.024, p = 0.99, ηg2 = 0.001, and no group by contingency interaction was found, F(3,76) = 0.76, p = 0.52, ηg2 = 0.03.
Fig. 3.
Percentage of change of deck on trials after a reward or a loss on the Iowa Gambling Task by control, CA, CCE and CCI participants. Error bars are the standard errors of the mean
Participants’ Subjective Appraisal of Experimental Manipulations on Their Affects
One-sample t tests (test value = 4) were undertaken in order to examine participants’ subjective appraisal of experimental manipulations. These analyses revealed that participants in the three casino-related conditions (CA: M = 4.70, SD = 1.21; CCI: M = 4.60, SD = 1.04, CCE: M = 4.71; SD = 1.34) but not in the control condition (M = 4.50; SD = 1.39), perceived that the experiment had positively affected their mood (Item 1; p < 0.05). Participants of the three casino conditions did not perceive that either the casino-related sound (Item 2; CA: M = 4.20, SD = 1.70; CCI: M = 3.80, SD = 1.61, CCE: M = 4.05; SD = 2.09) or the red light (Item 3; CA: M = 4.25, SD = 1.58; CCI: M = 3.80, SD = 1.28, CCE: M = 4.30; SD = 1.49) have influenced their mood (either positively or negatively) during the IGT. By contrast, in the competition conditions and according to participants, it appears that the presence of the other individual induced positive affect (Item 4), but only in the CCE group (CCI: M = 4.40, SD = 1.09, CCE: M = 4.85; SD = 1.38; p < 0.05).
A one-way ANOVA revealed that there was no significant between-group difference for Item 1, F(3,76) = 1.90, p = 0.14. There was no significant difference between the CA, CCI and CCE groups on for the perceived influence of casino-related sound and red light on affect during the experiment, F(2,57) = 0.25, p = 0.78, F(2,57) = 0.71, p = 0.49, respectively. There was no difference between the CCI and the CCE groups on the item examining the impact of the presence of the other participant while performing the IGT, t(40) = 1.13, p = 0.26.
Finally, correlation analyses (on the total number of participants and for each group separately) revealed that there was no significant association between participants’ subjective appraisal of experimental manipulations (Items 1, 2, 3 and 4) and IGT dependent measures deck selection, reaction speed and response shifting).
Discussion
The aim of the present study was to explore the impact of combined red light and casino-related sounds, with or without the presence of another participant, on decision-making behaviors, assessed with the Iowa Gambling Task (IGT). The main findings of the present research could be summarized as follows: In contrast to the control condition, participants in the casino conditions (casino alone, implicit and explicit competition conditions) did not exhibit slower deck selection reaction time after losses than after rewards. Moreover, participants in the competition conditions (both the implicit and the explicit) displayed lowered deck selection reaction time after losses and rewards, as compared to the control and the “casino alone” conditions. These results could not been explained by the intensity of anxiety, depression, impulsivity, competitiveness, sensitivity to loss and reward, as well gambling habits.
This study demonstrated that the combined effect of casino-related sound and red light modulate the reaction time associated with rewards and losses. In other words, we observed that participants in the control condition were slower after losses than after rewards whereas there was no difference in the three “casino-context” groups. This was the first time that the effect of the casino-related context on choice reaction time was estimated on the basis of previous choice-outcome. Indeed, previous studies (Stark et al. 1982; Dixon et al. 2007; Spenwyn et al. 2010) showed that fast sounds and/or red light increase participant’s betting speed when gambling, but independent of feedback contingency. In addition, in accordance with our hypotheses and previous research (Rockloff et al. 2011), we observed that performing the IGT face to face with another participant (with or without explicit instruction of competition) heightened the decision speed, independently of feedback contingency. Thus, our results suggest that gambling with others may be a key factor in increasing the betting speed while gambling within a casino-related context (induced here by the combination of casino-related sounds and red light). Nevertheless, because red light, casino-related sounds and the presence of pairs “mimics” accurately gambling-related environment, we cannot exclude that participants’ prior experience with casino settings (which might be low given the absence of gambling problem and the average young age of participants) played a role in significant effects observed in the present study. Similarly, participants’ attitudes and appraisals associated with casino settings are also likely to impact current findings. Further studies are needed to examine these issues.
The hypothesis that casino-related context would bias advantageous deck selection during the IGT was not supported, at least in this particular group of non-gamblers. Indeed, we observed no significant between-group difference on the profile of advantageous deck selection during the IGT (across the five stages of twenty trials). In addition, we observed no significant between-group difference with regard to deck response shifting after rewards and net losses. Interestingly, exploratory analyses showed that the mean of advantageous deck selection differ significantly from the chance level on the latter stage of the IGT only in the control group. Nevertheless, taken together, these results suggest that the impact of casino-related context may not impact deck selection directly. Besides, it also suggests that, even though a casino-related context may induce some behavioral changes in non-gamblers, these changes are not sufficient to over-ride the normal mechanisms of self-control, which consequently lead to disadvantageous behavioral decisions. Perhaps this explains why most casino visitors do not succumb to gambling addiction. In addition, it is noteworthy that the IGT may vary according to its level of uncertainty across trials (Brand et al. 2006). More specifically, selections during the second part of the IGT (trials 60–100) may be referred as decision-making under risk (i.e., situations of decision-making in which probabilities of reward and loss are known) because participants should have experienced the different win/loss contingencies enough to know which decks are risky and which are not (Brand et al. 2006). By contrast, the earlier blocks of the IGT refer to decision-making under ambiguity (i.e., situations of decision-making in which probabilities of reward and loss are unknown) because there has not been time for a participant to experience any of the win/loss contingencies during early deck choices (Brand et al. 2006). In this context, it is possible that gambling-related context has more impact on the latter stages of the IGT because a participant has to decide whether to take a risk or not, whereas, in the early stages of the IGT, deck selection is not yet associated with any explicit expected value. In this context, future studies should extend the experimental IGT situation (e.g., 120 trials instead of 100 trials) in order to examine if the effect of casino-related context enhance with the repetition of trials. Future studies should also include problem gamblers and determine whether these individuals have an increased vulnerability towards impairments in self-control and decision-making within a casino-related setting, as compared with non-gamblers.
In addition, based on several studies advancing that the casino atmosphere may impact emotions (Finlay et al. 2006, 2007, 2010), we also included an estimation of participants’ subjective appraisal of experimental manipulations on their affects. Interestingly, findings from this complementary examination suggest that the casino-related context increases positive affect. Nevertheless, this score was not associated with behavioral performance. Moreover, participants of the three casino conditions did not perceive that either the casino-related sound or the red light have influenced their affects (positively or negatively) during the IGT. However, these findings are to be taken with caution since the investigation of the psychological impact of casino-related design was only exploratory and complementary in the present study (i.e., estimated in the basis of four items). Therefore, additional research is needed in order to examine more thoroughly this question (e.g., using additional self-reports measures, such as The Environmental Pleasure Scale; Mehrabian and Wixen 1986, as in Finlay et al. 2010).
The fact that casino-related sounds were not separated from red light was based on a study by Spenwyn et al. (2010) in which the combined effects of both high tempo music and red light (but not red light or high tempo music conditions alone) result in faster bets in a computerized version of roulette. The fact that this was previously found helped to justify the exclusion of this condition in this experiment. However, we cannot rule out the possibility that the effects of sounds on decision-making reaction time were higher than those produced by red light and vice versa. In this context, additional studies are needed to examine the specific effect of sounds or light on decision-making during the IGT. Future studies should also examine the specific effect of the presence of pairs on IGT performance, that is, without casino-related sounds and red light. In the present study, participants were not remunerated as a function of their IGT performance. This lowers the ecological validity of the present design. Indeed, real monetary rewards and losses might have heightened the effect of sound, light and pairs on deck selection and choice reaction time during the IGT. In addition, participants recruited for this study were mainly young, which restricts the generalizability of current results. Nevertheless, the present finding also suggest that a casino–related context impacts decision-making behaviors in individuals (i.e., non-gamblers young adults) who are more inclined to develop their first life-experience with gambling (Wardle et al. 2007), and thus directly targeted by the gambling industry. Meanwhile, we also need to consider that not all young people are vulnerable and that most people have normal prefrontal mechanisms and are resilient to gambling addiction—perhaps only those with genetic or environmentally induced hypofrontality are more vulnerable and at a higher risk (e.g., Dackis and O’Brien 2005). In other words, we need to acknowledge both possibilities: that everyone who is exposed to casinos is vulnerable and at risk to gamble; or only a few who are predisposed and at higher risk to become gamblers, despite the fact that casino cues bring about behavioral changes even in those who are resistant to becoming gambling addicts. In this context, although present findings have some limitations, they also have potential implications for the prevention of the development and the maintenance of excessive gambling. For instance, one option would be to inform (beginners and frequent) gamblers on the specific impact of casino-related context on their gambling behaviors (Peller et al. 2008).
In conclusion, our findings suggest that sounds, lights and the presence of pairs while gambling may play a key role in energizing gambling behaviors. Therefore, the present findings can be used to enhance prevention strategies of abnormal gambling by targeting situational factors that could lead the individual to gamble beyond socially acceptable limits.
Contributor Information
Damien Brevers, Email: dbrevers@ulb.ac.be, Psychological Medicine Laboratory, Faculty of Medicine, Brugmann-campus, Université Libre de Bruxelles (ULB), 4 Place Van Gehuchten, 1020 Brussels, Belgium. Department of Psychology, Brain and Creativity Institute, University of Southern California, Los Angeles, CA, USA.
Xavier Noël, Psychological Medicine Laboratory, Faculty of Medicine, Brugmann-campus, Université Libre de Bruxelles (ULB), 4 Place Van Gehuchten, 1020 Brussels, Belgium.
Antoine Bechara, Department of Psychology, Brain and Creativity Institute, University of Southern California, Los Angeles, CA, USA.
Nora Vanavermaete, Psychological Medicine Laboratory, Faculty of Medicine, Brugmann-campus, Université Libre de Bruxelles (ULB), 4 Place Van Gehuchten, 1020 Brussels, Belgium.
Paul Verbanck, Psychological Medicine Laboratory, Faculty of Medicine, Brugmann-campus, Université Libre de Bruxelles (ULB), 4 Place Van Gehuchten, 1020 Brussels, Belgium.
Charles Kornreich, Psychological Medicine Laboratory, Faculty of Medicine, Brugmann-campus, Université Libre de Bruxelles (ULB), 4 Place Van Gehuchten, 1020 Brussels, Belgium.
References
- Baumeister RF. Choking under pressure: Self-consciousness and paradoxical effects of incentives on skillful performance. Journal of Personality and Social Psychology. 1984;46:610–620. doi: 10.1037//0022-3514.46.3.610. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Brand M, Labudda K, Markowitsch HJ. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Networks. 2006;19:1266–1276. doi: 10.1016/j.neunet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality Social Psychology. 1994;67:319–333. [Google Scholar]
- Church RM. The effects of competition on reaction time and palmar skin conductance. Journal of Abnormal and Social Psychology. 1962;65:32–40. doi: 10.1037/h0041901. [DOI] [PubMed] [Google Scholar]
- Cross JB, Gill DL. Competition and instructional set effects on the speed and accuracy of a throwing task. Research Quartely for Exercice and Sport. 1982;53:125–132. doi: 10.1080/02701367.1982.10605238. [DOI] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nature Neuroscience. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Dixon L, Trigg R, Griffiths M. An empirical investigation of music and gambling behaviour. International Gambling Studies. 2007;7:315–326. [Google Scholar]
- Finlay K, Kanetkar V, Londerville J, Marmurek HHC. The physical and psychological measurement of gambling environments. Environment and Behaviour. 2006;38:570–581. [Google Scholar]
- Finlay K, Marmurek HHC, Kanetkar V, Londerville J. Trait and state emotion congruence in simulate casinos: Effects on at-risk gambling intention and restoration. Journal of Environmental Psychology. 2007;27:166–175. [Google Scholar]
- Finlay K, Marmurek HHC, Kanetkar V, Londerville J. Casino Décor effects on gambling emotions and intentions. Environment and Behavior. 2010;42:524–545. [Google Scholar]
- Griffiths MD. Fruit machine gambling: The importance of structural characteristics. Journal of Gambling Studies. 1993;9:133–152. [Google Scholar]
- Griffiths MD, Auer M. The irrelevancy of game-type in the acquisition, development, and maintenance of problem gambling. Frontiers in Psychology. 2013 doi: 10.3389/fpsyg.2012.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths MD, Parke J. The psychology of music in gambling environments: An observational research note. Journal of Gambling Issues. 2005:13. http://www.camh.net/egambling/issue13_jgi_13_griffiths_2html.
- Griffiths MD, Swift G. The use of light and colour in gambling arcades: A pilot study. Society for the Study of Gambling Newsletter. 1992;21:16–22. [Google Scholar]
- Hess HF, Diller JV. Motivation for gambling as revealed in the marketing methods of the legitimate gaming industry. Psychological Reports. 1969;25:19–27. doi: 10.2466/pr0.1969.25.1.19. [DOI] [PubMed] [Google Scholar]
- Houston JM, Farese DM, LaDu T. Assessing competitiveness: A validation study of the competitiveness index. Personality and Individual Differences. 1992;13:1153–1156. [Google Scholar]
- Houston JM, Harris PB, McIntire S, Francis D. Revising the competitiveness index. Psychological Reports. 2002;90:31–34. doi: 10.2466/pr0.2002.90.1.31. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Marmurek HHC, Finlay K, Kanetkar V, Londerville J. The influence of music on estimates of at-risk gambling intentions: An analysis by casino design. International Gambling Studies. 2007;7:113–122. [Google Scholar]
- Mehrabian A, Wixen WJ. Preferences for individual video games as a function of their emotional effects on players. Journal of Applied Social Psychology. 1986;16:3–15. [Google Scholar]
- Milliman RE. Using background music to affect the behaviour of supermarket shoppers. Journal of Marketing. 1982;46:86–91. [Google Scholar]
- Peller AJ, LaPlante DA, Shaffer HJ. Parameters for safer gambling behavior: Examining the empirical research. Journal of Gambling Studies. 2008;24:519–534. doi: 10.1007/s10899-008-9097-5. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A, Kurth-Nelson Z. Reconciling reinforcement learning models with behavioral extinction and renewal: Implications for addiction, relapse, and problem gambling. Psychological Review. 2007;114:784–805. doi: 10.1037/0033-295X.114.3.784. [DOI] [PubMed] [Google Scholar]
- Rockloff MJ, Greer N, Fay C. The social contagion of gambling: How venue size contributes to player losses. Journal of Gambling Studies. 2011;27:487–497. doi: 10.1007/s10899-010-9220-2. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Seta JJ, Paulus PB, Risner HT. The effects of group composition and evaluation on task performance. Bulletin of the Psychonomic Society. 1977;9:115–117. [Google Scholar]
- Spenwyn J, Barrett DK, Griffiths MD. The role of light and music in gambling behaviour: An empirical pilot study. International Journal of Mental Health and Addiction. 2010;8:107–118. [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory: STAI (Eorm I) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Stark GM, Saunders DM, Wookey PE. Differential effects of red and blue coloured lighting on gambling behaviour. Current Psychological Research. 1982;2:95–99. [Google Scholar]
- Ward JC, Bitner MJ, Barnes J. Measuring the prototypicality and measuring of retail environments. Journal of Retailing. 1992;68:194–220. [Google Scholar]
- Wardle H, Sproston K, Orford J, Erens B, Griffiths M, Constantine R. British gambling prevalence survey. London: National Centre for Social Research; 2007. [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Yoto A, Katsuura T, Iwanaga K, Shimomura Y. Effects of object colour stimuli on human brain activities in perception and attention referred to EEG alpha band response. Journal of Physiological Anthropology. 2007;26:373–379. doi: 10.2114/jpa2.26.373. [DOI] [PubMed] [Google Scholar]