Abstract
The growth hormone/insulin-like growth factor-I (GH/IGF-I) axis is an important stimulator of collagen synthesis in connective tissue, but the effect of chronically altered GH/IGF-I levels on connective tissue of the muscle-tendon unit is not known. We studied three groups of mice; 1) giant transgenic mice that expressed bovine GH (bGH) and had high circulating levels of GH and IGF-I, 2) dwarf mice with a disrupted GH receptor gene (GHR−/−) leading to GH resistance and low circulating IGF-I, and 3) a wild-type control group (CTRL). We measured the ultra structure, collagen content and mRNA expression (targets: GAPDH, RPLP0, IGF-IEa, IGF-IR, COL1A1, COL3A1, TGF-β1, TGF-β2, TGF-β3, versican, scleraxis, tenascin C, fibronectin, fibromodulin, decorin) in the Achilles tendon, and the mRNA expression was also measured in calf muscle (same targets as tendon plus IGF-IEb, IGF-IEc). We found that GHR−/− mice had significantly lower collagen fibril volume fraction in Achilles tendon, as well as decreased mRNA expression of IGF-I isoforms and collagen types I and III in muscle compared to CTRL. In contrast, the mRNA expression of IGF-I isoforms and collagens in bGH mice was generally high in both tendon and muscle compared to CTRL. Mean collagen fibril diameter was significantly decreased with both high and low GH/IGF-I signaling, but the GHR−/− mouse tendons were most severely affected with a total loss of the normal bimodal diameter distribution. In conclusion, chronic manipulation of the GH/IGF-I axis influenced both morphology and mRNA levels of selected genes in the muscle-tendon unit of mice. Whereas only moderate structural changes were observed with up-regulation of GH/IGF-I axis, disruption of the GH receptor had pronounced effects upon tendon ultra structure.
Keywords: collagen turnover, IGF-I receptor, acromegaly, Laron syndrome, GH-deficiency
1. Introduction
Collagen is an essential and highly abundant protein in connective tissues, and the growth hormone (GH)/insulin-like growth factor-I (IGF-I) axis is believed to play a crucial role in regulating collagen and connective tissue structure during maturation. For example, GH stimulates the length growth of bones (Antoniazzi et al., 2010). In adulthood the impact of GH/IGF-I on connective tissue is more uncertain. Recently, hyper-physiologic levels of GH and IGF-I have been shown to stimulate collagen synthesis in healthy adult humans, both in tendon when GH or IGF-I is injected directly into patellar tendons, and in both tendon and muscle when GH is administrated systemically (Doessing et al., 2010a; Hansen et al., 2013; Vestergaard et al., 2012). In addition, GH and IGF-I are known to stimulate collagen synthesis in fibroblasts in vitro (Abrahamsson et al., 1991; Gillery et al., 1992; Ohlsson et al., 1992). Thus, a positive effect of GH/IGF-I on collagen synthesis is relatively well established. However, the effect of chronically altered GH/IGF-I levels on tendon and muscle connective tissue has only been studied to a very limited degree.
Existing data on chronic alterations in GH/IGF-I and the muscle-tendon unit comes from a study on patients with either chronic GH overexpression (acromegaly) or GH deficiency. In this study Doessing et al. (Doessing et al., 2010b) found a trend towards smaller mean collagen fibril diameter in the patellar tendon of acromegalic patients compared to the GH deficient patients. Furthermore, collagen mRNA expression was altered according to GH levels, but the collagen synthesis rates in tendon and muscle were not significantly different between the two patient groups. Although these results indicate a link between chronic changes in the GH/IGF-I axis and tendon ultra structure, these human patients are not optimal to study this system, since all patients are pharmaceutically treated and present almost normalized GH and IGF-I levels. Therefore, we set out to study tendon tissue in a situation with pronounced and chronic alterations in the GH/IGF-I axis: We used genetically altered mice for this purpose.
Two types of transgenic mice with markedly altered GH/IGF-I axis already exist; one expresses bovine GH (bGH) and the other has a disrupted GH receptor gene (GHR−/−). The giant bGH mice have approximately 2-times higher serum GH, as well as high hepatic IGF-I gene expression and serum IGF-I relative to littermate controls (Chen et al., 1997). In contrast, the dwarf GHR−/− mice have diminished GH signaling, leading to very low serum IGF-I level (Zhou et al., 1997). Both types of transgenic mice have been studied extensively with respect to metabolic disorders and lifespan changes (Coschigano et al., 2003; Berryman et al., 2004).
In this study, we used these transgenic mice and controls to investigate the effect of GH/IGF-I manipulation on Achilles tendon ultra structure, collagen concentration and mRNA levels of IGF-I, collagens and other matrix related components. Due to the technical challenges related to obtaining a reliable measure of gene-expression in the very small mouse tendons, we chose to supplement the tendon analysis with measures of mRNA expression in calf muscle tissue.
We hypothesized that chronic high systemic GH/IGF-I would stimulate IGF-I and collagen expression, and that increased collagen synthesis would lead to higher tendon collagen content and altered tendon ultra structure with higher collagen fibril volume fraction and number of fibrils per area compared to control. We expected the opposite effects in GHR−/− mice with chronic low GH/IGF-I. Furthermore, based on the observations by Doessing et al. on acromegalic and GH deficient patients (Doessing et al., 2010b), we hypothesized that mean collagen fibril diameters in tendons would be decreased with chronic high GH/IGF-I activity and increased with chronic low GH/IGF-I activity.
Results
2.1 Achilles tendon ultra structure analyzed by transmission electron microscopy (TEM)
TEM imaging showed ultra structural differences in the Achilles tendon with both chronic high and low GH/IGF-I activity, although the changes seemed more pronounced in the GHR−/− mice with low GH/IGF-I activity. The collagen volume fraction was significantly lower in the GHR−/− mice compared to control (CTRL) (61 ± 2 vs. 71 ± 1%, Figure 1A), while the bGH mice were similar to CTRL (bGH: 69 ± 1%, Figure 1A). In contrast, the number of collagen fibrils per area was significantly higher in the GHR−/− mice compared to CTRL mice (58.4 ± 1.6 vs. 48.5 ± 1.6 fibrils/μm2, Figure 1B). The mean collagen fibril diameter was lower in both the GHR−/− mice and bGH mice compared to CTRL mice (GHR−/−: 107.2 ± 2.7, bGH: 109.5 ± 2.3 and CTRL: 122.0 ± 2.1 nm, Figure 1C). However, when assessing the fibril diameter distributions (Figure 2 and 3), it is clear that the two types of transgenic mice present very different tendon ultra structures, and that the mean diameter does not provide a good description of the alterations induced by chronically high or low GH/IGF-I. As seen in Figure 2B and Figure 3B, the GHR−/− mice have lost the bimodal distribution of fibrils and show significantly more intermediate sized fibrils and significantly fewer large ones compared to the CTRL mice (Figure 2A and 3A). On the other hand, the bGH mice (Figure 2C and 3C) preserved the bimodal pattern, but had a significantly greater number of smaller fibrils and significantly fewer large ones compared to CTRL mice. Figure 3 shows representative TEM images of CTRL, GHR−/− and bGH mice.
Figure 1. Volume fraction, fibrils per area and mean diameter of Achilles tendon collagen fibrils.
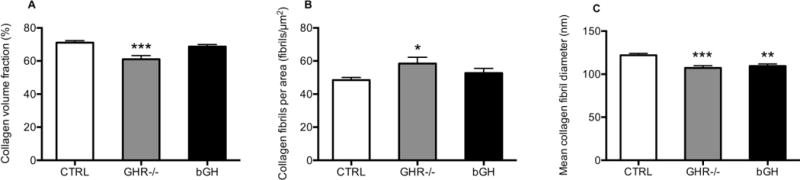
The proximal part of the Achilles tendon was analyzed by TEM imaging in control mice (CTRL, n=24) and transgenic mice with chronic low (GHR−/−, n=15) or high GH/IGF-I activity (bGH, n=15). The images were analyzed for collagen volume fraction (A), collagen fibrils per area (B) and mean collagen fibril diameter (C). Values are mean ± SEM (*p<0.05; ***p<0.001, only differences compared to control are shown).
Figure 2. Tendon collagen fibril diameter distributions.
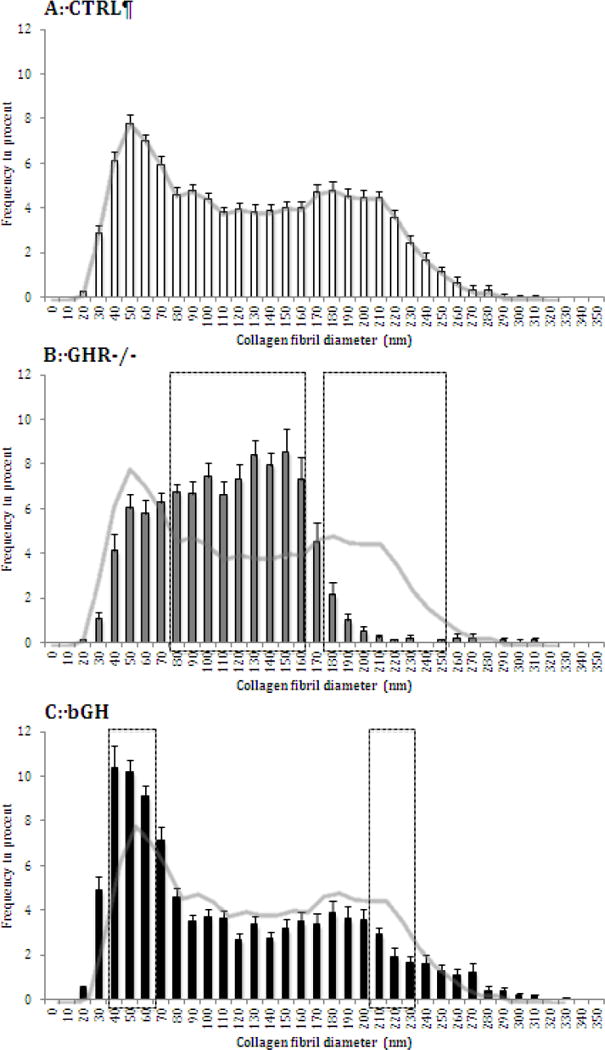
The proximal part of the Achilles tendon was analyzed by TEM imaging in control mice (CTRL, A, n=24) and transgenic mice with chronic low (GHR−/−, B, n=15) or high GH/IGF-I activity (bGH, C, n=15). For each mouse the collagen fibril diameters were measured and the distribution of these are shown for each group. The line-curve for CTRL is shown on A, B and C for easier comparison. The outlined areas in B and C represent diameters where the graphs differ significantly from CTRL (analyzed by functional data analysis, Supplementary data).
Figure 3. Representative TEM images of CTRL, GHR−/− and bGH mouse tendons.
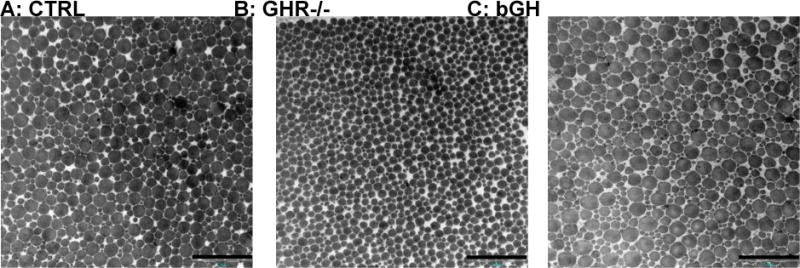
The proximal part of the Achilles tendon was analyzed by TEM imaging in control mice (CTRL) and transgenic mice with chronic low (GHR−/−) or high GH/IGF-I activity (bGH). The three images are representative for each group. Scale bars are 500 nm.
2.2 Hydroxyproline assay; Achilles tendon collagen content
The amount of hydroxyproline per total amount of amino acids in the Achilles tendon was equal in all 3 groups (Table 2), indicating that the amount of collagen in relation to total protein was constant despite the chronic GH/IGF-I alterations. Hydroxyproline and collagen content in relation to tendon sample dry-weights are also listed in Table 2. Hydroxyproline and collagen content per dry-weight was significantly lower in the bGH mice compared to CTRL mice. Collagen content per tendon dry-weight was calculated by multiplying the amount of hydroxyproline per dry-weight by 11.4 (the multiplying factor was calculated from the measured ratio between pure bovine tendon collagen mass and hydroxyproline mass).
Table 2.
Hydroxyproline and collagen content in Achilles tendon
| CTRL mice | GHR−/− mice | bGH mice | |
|---|---|---|---|
| Tendon sample dry weight (mg) | 0.65 ± 0.02 | 0.17 ± 0.01* | 0.81 ± 0.13 |
| HYP per total AA (molar ratio as percent) | 9.5 ± 0.1 | 9.4 ± 0.1 | 9.2 ± 0.1 |
| HYP per dry weight (μg/mg) | 78.6 ± 1.2 | 78.1 ± 1.8 | 70.8 ± 3.6* |
| Collagen per dry weight (μg/mg) | 896 ± 14 | 890 ± 21 | 807 ± 40* |
AA = amino acids, HYP = hydroxyproline.
Indicates a value significantly different from CTRL mice.
Data are presented as mean values ± SEM.
2.3 Tendon and muscle mRNA expression of IGF-I and collagen
We measured expression of all three isoforms of IGF-I in muscle, while only IGF-IEa was measured in tendon (as we did not expect to detect the other isoforms due to the limited amounts of tendon RNA). In muscle there was a clear connection between GH activity and IGF-I expression and we found that the expression of all three IGF-I isoforms was 3-fold lower in the GHR−/− mice compared to CTRL mice, while in the bGH mice the expression was 2-fold higher compared to CTRL (though not significant for IGF-IEb (p=0.12)) (Figure 4B). In tendon tissue a similar pattern for IGF-IEa was seen for the bGH mice though this did not reach significance (p=0.16) (Figure 4A). The expression of IGF-I receptor (IGF-IR) was below the detection level in tendon. In muscle it seemed to be inversely related to IGF-I expression, being 2-fold higher in the GHR−/− and 2-fold lower in the bGH mice compared to the CTRL mice (Figure 4B). This could indicate that the receptor is regulated by negative feedback in relation to IGF-I levels. The expression of collagen I and III (COL1A1 and COL3A1) was increased with high GH/IGF-I in the bGH mice in both tendon and muscle (Figure 5), and the increase was more pronounced in tendon (10 to 20-fold) than in muscle (2-fold, not significant for COL1A1, p=0.09). In muscle, the opposite effect was seen in the GHR−/− mice in muscle with a 2-fold lower collagen I and III expression compared to CTRL mice. In tendons from the GHR−/− mice we did not detect a decrease in collagen or in IGF-IEa expression (Figure 4A and 5A), but this could reflect the fact that our reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was expressed at a seemingly lower level in the tendons of GHR−/− mice, leading to a potentially 3-fold overestimation of the expression of mRNA targets in this group (see Supplementary Figure 9).
Figure 4. Tendon and muscle mRNA expression of IGF-I.
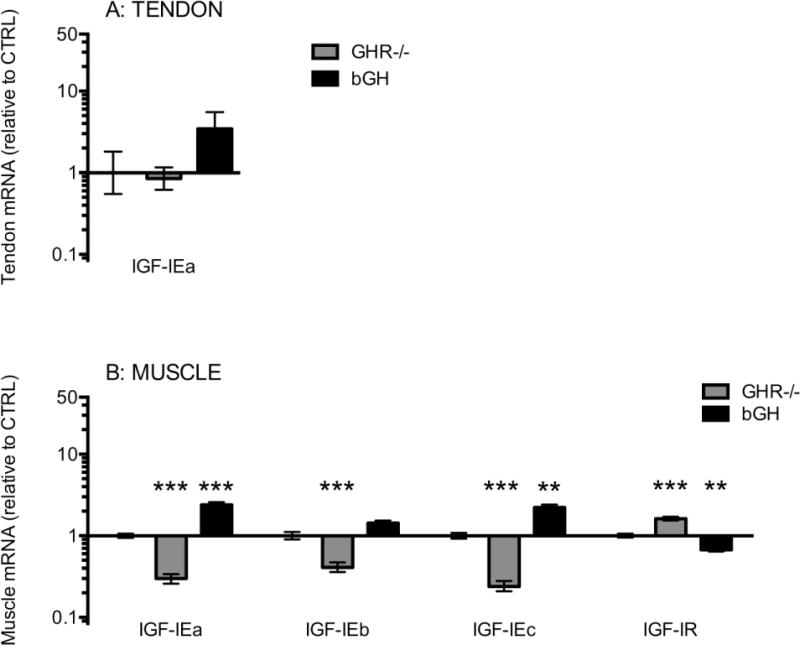
The figure shows IGF-IEa mRNA levels in Achilles tendon (A) and IGF-I isoforms (IEa, IEb and IEc) and IGF-IR mRNA levels in calf muscle (B). Mice with low GH/IGF-I activity (GHR−/−, grey bars, tendon: n=8, muscle: n=15) or high GH/IGF-I activity (bGH, black bars, tendon: n=7, muscle: n=14) are shown relative to control mice (CTRL, only error bars visible, tendon: n=12, muscle: n=24). All data are relative to GAPDH mRNA. IGF-IR = IGF-I receptor. Values are geometric mean ± back-transformed SEM (**p<0.01; ***p<0.001, only differences compared to control are shown).
Figure 5. Tendon and muscle mRNA expression of collagen I and III (COL1A1 and COL3A1).
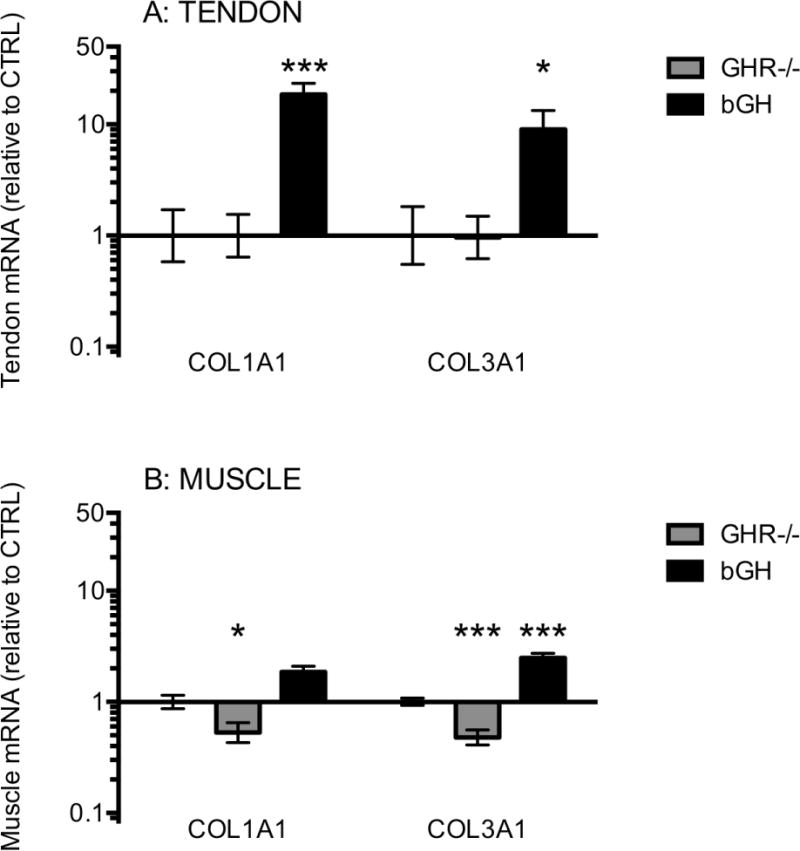
The figure shows collagen mRNA levels in Achilles tendon (A) and in calf muscle (B). Mice with low GH/IGF-I activity (GHR−/−, grey bars, tendon: n=8, muscle: n=15) or high GH/IGF-I activity (bGH, black bars, tendon: n=7, muscle: n=14) are shown relative to control mice (CTRL, only error bars visible, tendon: n=12, muscle: n=24). All data are relative to GAPDH mRNA. Values are geometric mean ± back-transformed SEM (*p<0.05; ***p<0.001, only differences compared to control are shown).
2.4 Tendon and muscle mRNA expression of matrix proteins and transforming growth factor-β (TGF-β)
We measured different matrix proteins in order to investigate any potential effects of GH/IGF-I manipulation on matrix regulation in tendon and muscle connective tissue (Figure 6). In tendon the expression of scleraxis, tenascin C and fibronectin was increased with high GH/IGF-I in the bGH mice compared to CTRL (Figure 6A). In muscle the expression of scleraxis, fibromodulin and decorin was decreased in the bGH mice compared to CTRL, while in the GHR−/− mice the expression of versican and fibronectin was decreased compared to CTRL (Figure 6B). We also measured TGF-β to investigate if there was any cross talk between IGF-I and this matrix growth factor. In tendon we found no significant differences in TGF-β1 or 2 (Figure 7A), and expression of TGF-β3 was below the detection level. In muscle with high GH/IGF-I in the bGH mice TGF-β2 was decreased and TGF-β3 was increased compared to CTRL (Figure 7B).
Figure 6. Tendon and muscle mRNA expression of matrix components.
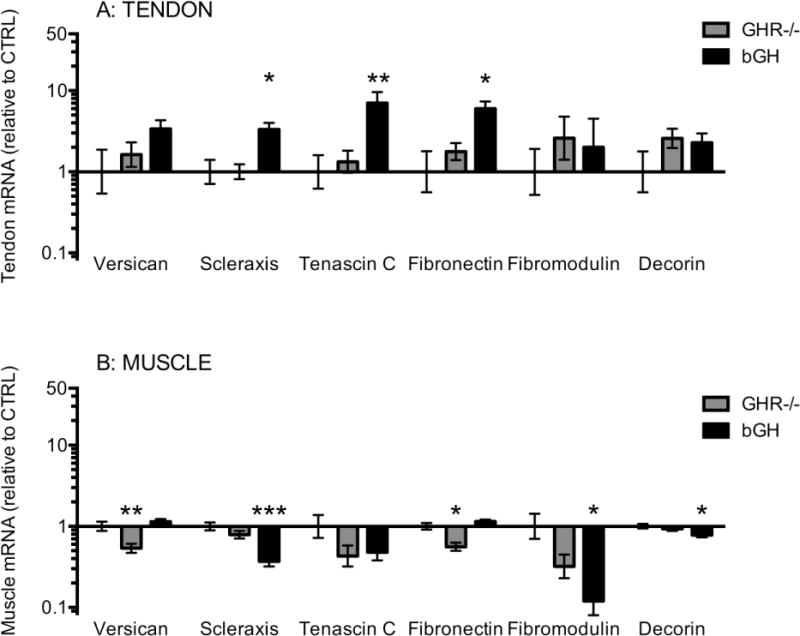
The figure shows matrix protein mRNA levels in Achilles tendon (A) and in calf muscle (B). Mice with low GH/IGF-I activity (GHR−/−, grey bars, tendon: n=8, muscle: n=15) or high GH/IGF-I activity (bGH, black bars, tendon: n=7, muscle: n=14) are shown relative to control mice (CTRL, only error bars visible, tendon: n=12, muscle: n=24). All data are relative to GAPDH mRNA. Values are geometric mean ± back-transformed SEM (*p<0.05; **p<0.01; ***p<0.001, only differences compared to control are shown).
Figure 7. Tendon and muscle mRNA expression of TGF-β.
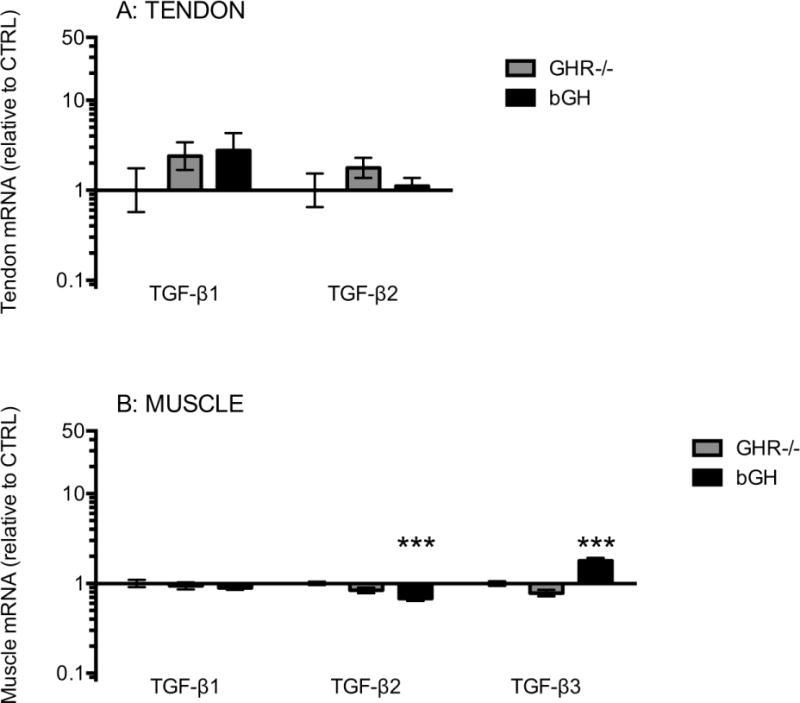
The figure shows TGF-β1 and 2 mRNA levels in Achilles tendon (A) and TGF-β1, 2 and 3 mRNA levels in calf muscle (B). Mice with low GH/IGF-I activity (GHR−/−, grey bars, tendon: n=8, muscle: n=15) or high GH/IGF-I activity (bGH, black bars, tendon: n=7, muscle: n=14) are shown relative to control mice (CTRL, only error bars visible, tendon: n=12, muscle: n=24). All data are relative to GAPDH mRNA. Values are geometric mean ± back-transformed SEM (***p<0.001, only differences compared to control are shown).
3. Discussion
The main finding in our study is that chronic alterations in the GH/IGF-I axis affect Achilles tendon ultra structure. The mice with low GH/IGF-I signaling, due to a defect GH receptor (GHR−/− mice), were found to have less tightly packed collagen fibrils (volume fraction), a smaller average collagen fibril diameter, and a loss of the bimodal distribution of fibril diameters that is normally seen in tendon tissue (Dyer and Enna, 1976). A doubling of GH and IGF-I levels (bGH mice) did not change the collagen fibril volume fraction, but it did affect the diameter distribution, presenting more small fibrils than in the control mice. Importantly, the bimodal distribution was maintained in the bGH mice. The concentration of collagen relative to total protein in the Achilles tendon tissue seemed unaffected by levels of GH and IGF-I. However, the chronic alterations of GH/IGF-I levels had a clear effect on tendon and skeletal muscle gene expression, with mRNA levels of IGF-I isoforms as well as collagens following the systemic levels of GH/IGF-I activity.
In adult tendon the distribution of collagen fibril diameters seems to follow a very preserved pattern. The bimodal distribution of fibril diameters have been found in various tendons in both rodents and humans (Dyer and Enna, 1976; Moore and Beaux, 1987; Ezura et al., 2000; Goh et al., 2012). The organization and growth of collagen fibrils depend on many factors, and knockout of different small leucine-rich proteoglycans (decorin, biglycan, fibromodulin and lumican) and regulatory fibril-forming collagen molecules (type V and XI) have individually been shown to change the diameter distribution of collagen fibrils in tendon (Danielson et al., 1997; Ezura et al., 2000; Wenstrup et al., 2011; Dunkman et al., 2013). However, the pattern we have seen in the mice lacking GH and IGF-I, with a total loss of the bimodal distribution, looks very severe compared to the other models. This emphasizes the necessity of these hormones in lateral growth and maturation of collagen fibrils.
We found that the collagen volume fraction was reduced to around 60% in the mice with low GH/IGF-I compared to around 70% in the control mice, but in contrast to our hypothesis, the mice with excessive GH/IGF-I did not have higher but similar collagen volume fraction compared to control mice. Most studies report tendon collagen volume fractions around 60–80% (Williams et al., 2008; Kongsgaard et al., 2010), and different interventions such as exercise and hormone therapy have failed to increase the collagen volume fraction (Hansen et al., 2009; Kongsgaard et al., 2010). This fits with our own finding in the bGH mice where collagen volume fraction remained unchanged despite a markedly increased collagen expression (10 to 20-fold) in tendon. Based on this it seems difficult to increase the normal volume fraction of collagen fibrils in tendon with hyper physiological stimuli. However, the low fibril volume fraction in the mice with disrupted GH/IGF-I signaling (GHR−/− mice), shows that physiological levels of GH and IGF-I are necessary for a normal packing of collagen fibrils.
It would have been interesting to relate our ultra structural, qualitative data with quantitative measures of tendon size. Unfortunately, it was not possible due to the practical difficulties in precisely dissecting and wet-weighing the very small mice tendons. Colao et al. found that acromegalic patients with active disease actually had thicker heel tendons measured with ultrasound (Colao et al., 1998), and it could therefore be speculated that excessive GH and IGF-I mainly affect the growth of the tendon and to a smaller degree affects tissue quality.
Since we were not able to measure the wet-weight of the Achilles tendons, we related the hydroxyproline content (and the thereby estimated collagen content) to both dry-weight and to total amino acids. These results showed that the ratio between hydroxyproline (or collagen) and the total protein content of the tendons was in general very constant and not affected by either high or low GH/IGF-I. The lower collagen fibril volume fraction found in the GHR−/− mice, must therefore have been due to increased water content between the fibrils rather than an increased amount of non-collagen protein. These results support the findings by Kyparos and co-workers, who found no changes in hydroxyproline per patellar tendon dry-weight after 14 days of GH treatment in GH deficient rats (Kyparos et al.,, 2002). However, to our surprise the bGH group had significantly lower hydroxyproline per dry-weight than the control group. We believe this has a practical explanation and does not reflect a true phenomenon. Since the bGH mice are 1.5 times larger than control mice and more than 4 times larger than the GHR−/− mice (Berryman et al., 2006), we were able to dissect the Achilles tendon closer to the calcaneus bone in these mice, and may have included fibrocartilage in some of these samples. This would explain the lower mean (and larger variation) of hydroxyproline per dry-weight content, due to a greater amount of sugar-containing components in fibrocartilage compared to tendon (Waggett et al., 1998).
Expression of mRNA was measured in both Achilles tendon and calf muscle tissue. Although muscle tissue was not the main focus of this investigation, we have previously observed that the gene-expression of collagen and IGF-I, in response to altered GH, is very similar in muscle and tendon (Doessing et al., 2010a). Therefore, due to the technical challenges related to obtaining a reliable measure of gene-expression in the very small mouse tendons (Table 2), we chose to include muscle tissue in the gene-expression analysis. As shown in the results the variation within groups is considerably smaller in the muscle samples and the expression of reference genes more stable, compared to tendon (Figures 4–7 and Supplementary Figure 9). Thus, our chances of reliably detecting effects of GH action were greater in the muscle samples, and in this way data from two tissue types supplement each other.
Expression of IGF-I mRNA in tendon and muscle overall seems to follow the systemic GH/IGF-I levels, and our findings on IGF-IEa and IGF-IEb muscle mRNA expression confirm an earlier study in the GHR−/− and bGH mice (Iida et al., 2004), and we add that muscle IGF-IEc mRNA expression follows the same pattern. Furthermore, the IGF-I receptor expression in muscle seems to be negatively correlated to the IGF-I level, at least at the mRNA level, showing low expression with high IGF-I expression (bGH mice) and the opposite in the GHR−/− mice. Despite these changes in the IGF-I receptor, collagen expression (I and III) still followed IGF-I expression in both types of transgenic mice in muscle and in the bGH mice in tendon (the absent effect on tendon collagen and IGF-I expression in the GHR−/− mice is potentially caused by changes in the reference gene in this group). The data for collagen expression indicate that fibroblasts in both tendon and muscle maintain an altered collagen production in response to long-term alterations in GH/IGF-I levels.
Although our results only reveal the mRNA expression and not the actual protein level, they fit with the study by Doessing et al. (Doessing et al., 2010a), where two weeks of GH administration to healthy males increased both mRNA expression and collagen synthesis in muscle and tendon. Furthermore, Delaughter et al. found that transgenic mice with sustained overexpression of IGF-I in the heart muscle had increased fibrosis, indicating increased collagen production (Delaughter et al., 1999), and Gosteli-Peter et al. found that muscle IGF-I protein levels were lower in male hypophysectomized rats compared to healthy controls (Gosteli-Peter et al., 1994). Somewhat in contrast, Kraemer et al. measured the protein levels of IGF-I in muscle from female mice, and found that it was increased in bGH mice but not decreased in GHR−/− mice (Kraemer et al., 2009). As proposed by Kraemer et al., the lack of decrease in muscle IGF-I protein could be a gender specific phenomenon in females, though further investigation is needed to verify this.
We measured other matrix related growth/transcription factors (TGF-β isoforms and scleraxis) and matrix proteins to investigate whether an alteration of GH/IGF-I signaling can lead to a general alteration of extracellular matrix homeostasis. Although, we did see changes in expression of some of these target genes, the data did not indicate a clear direction of the changes in relation to the level of GH/IGF-I activity, and several of the matrix components appeared to be regulated in the same direction both with high and low GH/IGF-I (Figure 6). An interesting observation however, is that in tendon tissue scleraxis was induced by high GH/IGF, while in muscle tissue it was clearly down regulated. Since scleraxis is involved in induction of collagen expression in fibroblasts (Léjard et al., 2007; Bagchi and Czubryt, 2012), it may be speculated that the regulation of collagen expression in response to high GH/IGF in tendon is related to scleraxis action while in muscle a different pathway could be active.
In conclusion, we found that chronic alterations in the GH/IGF-I axis affected Achilles tendon ultra structure and mRNA levels of IGF-I and collagen in tendon and in intramuscular connective tissue in our transgenic mouse model. These results emphasize that the stimulatory role of the GH/IGF-I axis on collagen synthesis in tendon tissue is necessary for normal collagen fibril organization, but that excessive GH/IGF-I levels with chronic up-regulation of collagen expression does not affect tendon structure in the exact opposite way, which could indicate a ceiling-effect of GH and IGF-I’s stimulating role on connective tissue.
4. Experimental procedures
4.1 Animals
The mice used in this study were produced and housed at Ohio University, US. Berryman and co-workers have published results and described these mice in 2006 (Berryman et al., 2006). In short, the bGH mice (n=15) were C57BL/6J male mice, and they were at the embryonic state injected with a metallothionein transcriptional regulatory element and a bGH cDNA containing the first intron of the gene leading to high serum GH and IGF-I levels. The GHR−/− male mice (n=15) were backcrossed for eight generations into the C57BL/6J strain and contained a homologous recombination that disrupted the fourth exon of the GHR binding protein gene disrupting the GH receptor and leading to low serum IGF-I. Because the bGH and GHR−/− mice were not exactly the same strain, they had two groups of age matched littermate male controls (NT (n=8) and WT (n=16), respectively). All four groups of mice were divided into sub-groups receiving either a normal or a high-fat diet, for the purpose of looking at metabolism in the previously published study (Berryman et al., 2006). Since no differences were found in any of the measured parameters between the two control groups (NT and WT) they were collapsed into one group (CTRL, n=24) in all analyses and graphs. All mice were killed at the age of 7 months, and the hind limbs quickly frozen at −80°C. These hind limbs were examined in the present study.
4.2 Preparation of muscle and tendon tissue
Muscle tissue (10–30 mg) was dissected from the posterior part of the lower limb for mRNA analysis while the tissue was still frozen. The dissected part was weighed and thereafter stored again at −80°C until analysis. After letting the remaining tissue thaw in room temperature the skin was removed, and the Achilles tendon (0.1–1 mg) was carefully harvested under a microscope. A smaller proximal portion of the Achilles tendon was saved for TEM imaging in 2% glutaraldehyde in 0.05 M sodium phosphate buffer (pH 7.2) at 4°C. The rest of the tendon was used for collagen content analysis and stored at −80°C degrees until analysis. Since we were not able to dissect the Achilles tendons in the frozen state but wanted to measure tendon mRNA expression, we used RNAlaterICE (Invitrogen, Life Technologies), which should inhibit RNAses and allow for dissection at room temperature without RNA damage. The contralateral hind limbs from the mice where two hind limbs were available (bGH: n=7, GHR−/−: n=8, NT: n=4, WT: n=8, all receiving the high-fat diet) were used for this purpose. While frozen, the hind limbs were trimmed to leave only the lower leg. This was immersed in a volume of RNAlaterICE approximately 10 times that of the tissue volume and left for 48 hours at −20°C. The Achilles tendons were then dissected at room temperature and stored at −80°C until further analyses. The dissection lasted no longer than 10 minutes for each sample. To verify that this procedure worked acceptably we also dissected a piece of muscle tissue from each of the frozen hind limbs, and ran these in parallel with the tendon samples. The data obtained from these muscle samples were compared with the data obtained from muscle samples extracted without use of RNAlaterICE (these results are only shown in Supplementary Figure 8).
4.3 TEM imaging of Achilles tendon cross-sections
The Achilles tendon samples for TEM were dehydrated in graded series of ethanol, transferred to propylene oxide and embedded in Epon (Hexion, Houston, US) according to standard procedures. Ultrathin sections were cut with a Reichert-Jung Ultracut E microtome and collected on Formvar membranes and stained with uranyl acetate and lead citrate. Digital images were obtained with a Philips CM100TEM (80 kV) equipped with a SIS MegaView2 camera. The examiner was blinded and saved ten randomly sampled electron micrographs of intercellular collagen from each mouse. Subsequently these micrographs were analyzed stereologically (C.A.S.T.-grid software, The International Stereology Center at Olympus, Denmark) to quantify the individual fibril diameters, the cross-sectional number of fibrils per area and the fraction of the cross-sectional area occupied by collagen fibrils (collagen fibril volume fraction).
2.4 Hydroxyproline assay for tendon collagen content
The samples were freeze-dried over 48 hours and the dry-weight was determined in a room with constant air humidity on a Metler Toledo Ultra-microbalance weight. Freeze-dried Achilles tendon samples were hydrolyzed in 500 μL 6 M HCl at 120 °C for 24 hours. Hereafter hydrolyzates were dried, rehydrated with distilled water and dried again (rehydration and drying repeated twice). The hydrolyzates were re-suspended and diluted appropriately in acetate-citrate buffer (0.6 % acetic acid, 130 mM citric acid, 440 mM sodium acetate, 425 mM sodium hydroxide). 150 μl diluted sample was mixed with 75 μl of chloramine-T solution (60 mM chloramine-T, 50% 1- propanol) and incubated at room temp for 20 min. 75 μl aldehyde perchloric acid solution (1 M 4-dimethylaminobenzaldehyde, 60% 1-propanol, 22% perchloric acid (70–72%)) was added to samples and they were incubated at 60°C for 25 min. Optical density was read at 570 nm and samples were compared to a standard curve made from known concentrations of hydroxyproline (Sigma, H1637) to determine hydroxyproline concentration. In addition to the unknown samples, we measured levels of hydroxyproline in purified bovine tendon collagen (Sigma, C9879) to determine the ratio between hydroxyproline and total amino acids in pure tendon collagen (explained further below). Hydroxyproline was related to both tendon sample dry-weight and to total amino acids.
2.5 Total amino acids
The total amino acid molar concentration in the tendon hydrolyzates was measured by adding 10 μl of hydrolyzate (appropriately diluted (20–400x, depending on tissue sample size)) and 190 μl of OPA-NAC buffer containing; 2.5 mM OPA (ortho-phthalaldehyde), 2.5 mM NAC (N-acetyl-L-cysteine), 40 mM borate, and 5 % ethanol (used to dissolve the OPA before mixing into final buffer), pH 9.5. Hydrolyzate and OPA-NAC buffer were mixed in a white microtiter plate (Costar # 3362) and fluorescence (ex340/em468) was read on a Perkin-Elmer Victor3 1420 multi-label counter. To estimate the amino acid concentration, the fluorescence of the samples was compared to the fluorescence of standard samples containing valine (Sigma, LAA21) in concentrations of 3000, 1000, 300, 100, 30, 10 and 3 μM.
4.6 RNA extraction
Tendon and muscle tissue was homogenized in 1 ml of TriReagent (Molecular Research Center, Inc., Cincinnati, OH) containing 5 stainless steel balls of 2.3 mm in diameter (BioSpec Products, Inc, Bartlesville, OK), and silicon-carbide sharp particles of 1 mm (BioSpec Products, Inc) (1 particle for muscle samples and 5 particles for tendon samples), by shaking in a FastPrep®-24 instrument (MP Biomedicals, Inc, France) at speed level 4 for 15 seconds. In order to obtain complete tissue homogenization of muscle samples the shaking process was repeated 3 times with ice cooling between each shaking step to avoid heating of the sample (tendon samples only shaken once). Following homogenization, bromo-chloropropane was added in order to separate the samples into an aqueous and an organic phase. Following isolation of the aqueous phase, RNA was precipitated using isopropanol (For tendon samples 4 μl of glycogen was added before the isopropanol to improve precipitation of RNA). The RNA pellet was then washed in ethanol and subsequently dissolved in RNAse-free water (20 ul for muscle and 10 ul for tendons). Muscle samples dissected in the frozen state were weighed prior to RNA extraction, while tissue submerged in RNAlateICE could not be reliably weighed. For all muscle samples total RNA concentrations were determined by spectroscopy at 260 nm and a good RNA integrity was ensured by gel electrophoresis. The tendon samples were very small and thus the amount of extracted RNA was too low to measure reliably, and similarly the quality could not be assessed. However, the tendon samples were purified in parallel with muscle samples, and these showed high RNA quality.
4.7 Real time RT PCR
For muscle samples 500 ng total RNA was converted into cDNA in 20 μl using the OmniScript reverse transcriptase (Qiagen, Valencia, CA) and 1 μM poly-dT (Invitrogen) according to the manufacture’s protocol (Qiagen). For tendon samples 4 μl of the total RNA suspension (10 ul) was converted to cDNA in 20 μl using the SensiScript reverse transcriptase (Qiagen, Valencia, CA) and 1 μM poly-dT (Invitrogen) according to the manufacture’s protocol (Qiagen). For each target mRNA, 0.25 μl of muscle cDNA (5 μl of a 20 fold dilution) or 0.5 μl of tendon cDNA (5 ul of a 10 fold dilution) was amplified in a 25 μl SYBR Green PCR reaction containing 1×Quantitect SYBR Green Master Mix (Qiagen) and 100 nM of each primer (Table 1). The amplification was monitored real-time using the MX3000P Real-time PCR machine (Stratagene, Santa Clara, CA). The Ct values were related to a standard curve made with known concentrations of DNA oligos (Ultramer™ oligos, Integrated DNA Technologies, Inc., Leuven, Belgium) with a DNA sequence corresponding to the sequence of the expected PCR product. Based on these Ct values, and accounting for the PCR efficiency, the relative difference between unknown samples was determined. The specificity of the PCR products was confirmed by comparing the melt curves for the unknown samples with melt curves of the DNA oligos. GAPDH was chosen as internal control, since GAPDH mRNA has been suggested to be constitutively expressed. To validate this assumption another unrelated “constitutive” RNA, RPLP0, was measured. In tendon samples a 3-fold increase in RPLP0 (or a decrease in GAPDH) was seen in the GHR−/− group (Figure 9A in Supplementary data).
Table 1.
Primers for real-time RT-PCR
| mRNA | Sense primer | Anti-sense primer | NM |
|---|---|---|---|
| GAPDH | CAGCAACTCCCACTCTTCCACCT | ACCACCCTGTTGCTGTAGCCGT | NM_008084.2 |
| RPLP0 | AGGCCCTGCACTCTCGCTTTC | GGGTACCCGATCTGCAGACACAC | NM_007475.5 |
| IGF-IEa | CTGACATGCCCAAGACTCAGAAGGA | AGGTCTTGTTTCCTGCACTTCCTCTAC | NM_001111275.1 |
| IGF-IEb | GCCACACTGACATGCCCAAGA | CCGTTACCTCCTCCTGTTCCCC | NM_184052.3 |
| IGF-IEc | AGTCCCCGTCCCTATCGACAAA | AGGTCTTGTTTCCTGCACTTCCTCTAC | NM_010512.4 |
| IGF-IR | GTGGCCAGAACCCGAGAACCC | GCCCCTCCGTACTTCCTGTACTCCT | NM_010513.2 |
| COL1A1 | CAGCACCCTTGTGGACGGCTG | GTCTTGCCCCAAGTTCCGGTGT | NM_007742.3 |
| COL3A1 | AGGCGAATTCAAGGCTGAAGGA | GTCTTGCTCCATTCCCCAGTGTG | NM_009930.2 |
| TGF-beta 1 | CAAGTGTGGAGCAACATGTGGAA | ATCAGTGGGGGTCAGCAGCC | NM_011577.1 |
| TGF-beta 2 | CGCGCTTTGGATGCTGCCTAC | TCCATTTCCATCCAAGATCCCTC | NM_009367.3 |
| TGF-beta 3 | TGCCAAAGAGATCCATAAATTCGACA | CCTTAGAGGTAATTCCTTTGGGGCA | NM_009368.3 |
| Versican | CCCCTGCAATTACCACCTCACCTA | TTCATCTTTCCAAAGGTCTTGGCATT | NM_001081249.1 |
| Scleraxis | TCTGCCTCAGCAACCAGAGAAAGT | TTGGCTGCTGTGGACCCTCCT | NM_198885.3 |
| Tenascin C | TGATGGGCAGATATGGGGACAA | TTTCGGAAGTTGCTGGGTCTCA | NM_011607.3 |
| Fibronectin | ACCACCGGCCACACCTACAACC | GCATGAAGCACTCAATGGGGC | NM_010233.1 |
| Fibromodulin | TCCTGTCAACACCAACCTGGAGA | CGTGCAGAAGCTGCTGATGGAG | NM_021355.3 |
| Decorin | GGTTGATGCACCCAGCCTGA | GATGCTGTTGAAGCTCAATCCCA | NM_001190451.1 |
Consequently the gene expression in the tendons of the GHR−/− group is potentially overestimated by 3-fold. In muscle the ratio between RPLP0 and GAPDH was also slightly different in the GHR−/−group (20 % increase in GAPDH relative to RPLP0) (Figure 9B in Supplementary data), however the differences in the target mRNAs in muscle were much higher and therefore not an artifact of changes in the reference mRNA.
4.8 Statistics
First we analyzed the two control groups (NT and WT) with two-way ANOVA (group*diet) and found no significant differences in any of the measured parameters, and the two control groups (NT and WT) were therefore collapsed into one control group in all further analyzes (CTRL, n=24). Differences between groups (bGH, GHR−/− and CTRL) were then evaluated with two-way ANOVA (group*diet) and Tukey Post hoc tests. Differences were only analyzed between the control group and each of the transgenic mouse groups, since it was not appropriate to compare the two groups of transgenic mice directly because the genetic backgrounds were not identical. No effect of diet was found, and high-fat and normal diet data were therefore pooled in all the graphical presentations for simpler interpretation (but in all statistical calculations the diet groups remained separate). All mRNA data were log-transformed for statistical analysis and shown as geometric means (back-transformed log-means) in graphs with error bars indicating relative SE (68% confidence interval) corresponding to SE for the log-transformed data. All two-way ANOVAs were performed in Sigma Plot 11 (Systat Software Inc., US) and graphs were drawn with Prism 5 (GraphPad Software Inc., US). For analysis of collagen fibril diameter distributions we used functional data analysis (Ramsey et al., 2009) to compare the groups (see Supplementary data, Figure 10). This was done using smoothing with a B-spline basis system. Estimation and permutation tests were carried out in R (R version 2.11.1) using the contributed R-package functional data analysis, which used in our context is illustrated in the book by Ramsay et al. (Ramsey et al., 2009). P-values below 0.05 were considered statistically significant, and data are presented as mean ± SEM unless otherwise stated.
Supplementary Material
Highlights.
We studied tendon and muscle in transgenic mice with GH/IGF-I alterations
Chronic high and low GH/IGF-I activity affects Achilles tendon ultra structure
Collagen and IGF-I mRNA expression in muscle followed systemic levels of GH/IGF-I
Collagen per total protein in tendon was not affected by GH/IGF-I alterations
Low GH/IGF-I more dramatically affected tendon than high GH/IGF-I
Acknowledgments
The authors would like to acknowledge Caroline Bøjstrup and Christina Hansen for their contribution with the TEM imaging and Anja Jokipii-Nielsen for her work with mRNA analyses. Financial support from the Weimann Foundation, Danish Medical Research Council, Lundbeck Foundation, Novo Nordisk Foundation, the State of Ohio’s Eminent Scholar Program, NIH grant (P01AG031736-01A1), AMVETS and Center for Healthy Aging is greatly appreciated. JJK is supported by a gift by Milton and Lawrence Goll.
Abbreviations
- bGH
Bovine growth hormone
- CTRL
control
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GH
growth hormone
- GHR
growth hormone receptor
- IGF-I
insulin-like growth factor-I
- IGF-IR
insulin-like growth factor-I receptor
- RPLP0
ribosomal protein large P0
- TGF-β
transforming growth factor-beta
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop R. 1991;9:495–502. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- Antoniazzi F, Monti E, Venturi G, Franceschi R, Doro F, Gatti D, Zamboni G, Tatò L. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol. 2010;163:479–487. doi: 10.1530/EJE-10-0208. [DOI] [PubMed] [Google Scholar]
- Bagchi R, Czubryt M. Synergistic roles of scleraxis and Smads in the regulation of collagen 1α2 gene expression. Biochim Biophys Acta. 2012;10:1936–1944. doi: 10.1016/j.bbamcr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of Growth Hormone on Susceptibility to Diet-Induced Obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- Chen NY, Chen WY, Striker LJ, Striker GE, Kopchick JJ. Co-expression of bovine growth hormone (GH) and human antagonist genes in transgenic mice. Endocrinology. 1997;138:851–854. doi: 10.1210/endo.138.2.5036. [DOI] [PubMed] [Google Scholar]
- Colao A, Marzullo P, Vallone G, Marino V, Annecchino M, Ferone D, De Brasi D, Scarpa R, Oriente P, Lombardi G. Reversibility of joint thickening in acromegalic patients: an ultrasonography study. J Clin Endocrinol Metab. 1998;83:2121–2125. doi: 10.1210/jcem.83.6.4865. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland A, Riders M, List E, Flyvberg A, Kopchick JJ. Deletion, But Not Antagonism, of the Mouse Growth Hormone Receptor Results in Severely Decreased Body Weights, Insulin, and Insulin-Like Growth Factor I Levels and Increased Life Span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Danielson K, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo R. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–9. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen MH, Flyvbjerg A, Kjaer M. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010a;588:341–51. doi: 10.1113/jphysiol.2009.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doessing S, Holm L, Heinemeier KM, Feldt-Rasmussen U, Schjerling P, Qvortrup K, Larsen JO, Nielsen RH, Flyvbjerg A, Kjaer M. GH and IGF1 levels are positively associated with musculotendinous collagen expression: experiments in acromegalic and GH deficiency patients. Eur J Endocrinol. 2010b;163:853–62. doi: 10.1530/EJE-10-0818. [DOI] [PubMed] [Google Scholar]
- Dunkman A, Buckley M, Mienaltowski M, Adams S, Thomas S, Satchell L, Kumar A, Pathmanathan L, Beason D, Iozzo R, Birk D, Soslowsky L. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer RF, Enna CD. Ultrastructural Features of Adult Human Tendon. Cell Tiss Res. 1976;259:247–259. doi: 10.1007/BF00215881. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE, Oldberg Å. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillery P, Leperre A, Maquart FX, Borel JP. Insulin-like growth factor-I (IGF-I) stimulates protein synthesis and collagen gene expression in monolayer and lattice cultures of fibroblasts. J Cell Physiol. 1992;152:389–396. doi: 10.1002/jcp.1041520221. [DOI] [PubMed] [Google Scholar]
- Goh KL, Holmes DF, Lu Y, Purslow PP, Kadler KE, Bechet D, Wess TJ. Bimodal collagen fibril diameter distributions direct age-related variations in tendon resilience and resistance to rupture. J Applied Physiol. 2012;113:878–888. doi: 10.1152/japplphysiol.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosteli-Peter MA, Winterhalter KH, Schmid C, Froesch ER, Zapf J. Expression and regulation of insulin-like growth factor-I (IGF-I) and IGF-binding protein messenger ribonucleic acid levels in tissues of hypophysectomized rats infused with IGF-I and growth hormone. Endocrinology. 1994;135:2558–2567. doi: 10.1210/endo.135.6.7527334. [DOI] [PubMed] [Google Scholar]
- Hansen M, Boesen A, Holm L, Flyvbjerg A, Langberg H, Kjaer M. Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scand J Med Sci Sports. 2013;23:614–619. doi: 10.1111/j.1600-0838.2011.01431.x. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kongsgaard M, Holm L, Skovgaard D, Magnusson SP, Qvortrup K, Larsen JO, Aagaard P, Dahl M, Serup A, Frystyk J, Flyvbjerg A, Langberg H, Kjaer M. Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol. 2009;106:1385–1393. doi: 10.1152/japplphysiol.90935.2008. [DOI] [PubMed] [Google Scholar]
- Iida K, Itoh E, Kim DS, del Rincon JP, Coschigano KT, Kopchick JJ, Thorner MO. Muscle mechano growth factor is preferentially induced by growth hormone in growth hormone-deficient lit/lit mice or bovine GH transgenic mice. J Physiol. 2004;560:341–349. doi: 10.1113/jphysiol.2004.069500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsgaard M, Qvortrup K, Larsen J, Aagaard P, Doessing S, Hansen P, Kjaer M, Magnusson SP. Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am J Sports Med. 2010;38:749–756. doi: 10.1177/0363546509350915. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Vingren JL, Schuenke MD, Kopchick JJ, Volek JS, Fragala MS, Häkkinen K, Jen-Ho, Thomas GA, Staron RS. Effect of circulating growth hormone on muscle IGF-I protein concentration in female mice with growth hormone receptor gene disruption. Growth Horm IGF Res. 2009;19:242–244. doi: 10.1016/j.ghir.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Kyparos A, Orth MW, Vailas AC, Martinez DA. Growth and maturational changes in dense fibrous connective tissue following 14 days of rhGH supplementation in the dwarf rat. Growth Horm IGF Res. 2002;12:367–373. doi: 10.1016/s1096-6374(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Léjard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl M, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the proalpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- Moore M, De Beaux A. A quantitative ultrastructural study of rat tendon from birth to maturity. J Anat. 1987;153:163–169. [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Nilsson A, Isaksson OG, Lindahl A. Effect of growth hormone and insulin-like growth factor-I on DNA synthesis and matrix production in rat epiphyseal chondrocytes in monolayer culture. J Endocrinol. 1992;133:291–300. doi: 10.1677/joe.0.1330291. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Hooker G, Graves S. Functional Data Analysis with R and MATLAB (Use R!) first. Spinger; New York: 2009. [Google Scholar]
- Vestergaard PF, Jorgensen JOL, Olesen JL, Bosnjak E, Holm L, Frystyk J, Langberg H, Kjaer M, Hansen M. Local Administration of Growth Hormone Stimulates Tendon Collagen Synthesis in Elderly Men. J Appl Physiol. 2012;113:1432–1438. doi: 10.1152/japplphysiol.00816.2012. [DOI] [PubMed] [Google Scholar]
- Waggett AD, Ralphs JR, Kwan AP, Woodnuttt D, Benjamin M. Characterization of Collagens and Proteoglycans at the Insertion of the Human Achilles Tendon. Matrix Biol. 1998;16:457–470. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI In developing tendon. J Biol Chem. 2011;10:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LN, Elder SH, Horstemeyer MF, Harbarger D. Variation of diameter distribution, number density, and area fraction of fibrils within five areas of the rabbit patellar tendon. Ann Anat. 2008;190:442–451. doi: 10.1016/j.aanat.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


