Abstract
Corticostriatal projections are essential components of forebrain circuits widely involved in motivated behavior. These axonal projections are formed by two distinct classes of cortical neurons, intratelencephalic (IT) and pyramidal tract (PT) type neurons. Convergent evidence points to IT/PT differentiation of the corticostriatal system at all levels of functional organization, from cellular signaling mechanisms to circuit topology. There is also growing evidence for IT/PT imbalance as an etiological factor in neurodevelopmental, neuropsychiatric, and movement disorders – autism, amyotrophic lateral sclerosis, obsessive-compulsive disorder, schizophrenia, Huntington’s and Parkinson’s diseases, and major depression are highlighted here.
Cortex, basal ganglia, and thalamus are interconnected in large-scale loops intimately involved in forebrain function 1. Connectivity between cortex and striatum is directional: cortex communicates monosynaptically to striatum, but striatum communicates only indirectly to cortex via polysynaptic downstream circuits. This arrangement appears to be crucial for the corticostriatal (CStr) system’s roles in cognitive and motor functions, which include action selection, motor control, sequence learning, habit formation, and more 2. Recent work is revealing CStr involvement in a spectrum of neurological and neuropsychiatric disorders. This review aims to consolidate observations at the “upstream” (intracortical) end of CStr connectivity, spotlighting the salient functional properties of the two major classes of CStr neurons in the intact brain. It then addresses recent progress towards identifying synaptic and cellular changes affecting CStr connectivity in neuropsychiatric and movement/motor disorders, with an emphasis on diseases for which the evidence indicates differential involvement of the two types of CStr neurons.
Anatomy and classification of CStr neurons
CStr projection neurons are widely distributed across cortex. Two of the three major classes of cortical excitatory neurons, as defined by axonal anatomy, constitute distinct types of CStr neurons 3-5 (reviewed in 6-9) (Fig. 1A). Intratelencephalic (IT) neurons project ipsi- or bilaterally (via the external capsule and corpus callosum) within the telencephalon (cortex and striatum), and not to extratelencephalic regions such as thalamus or brainstem. These projections have complex topography, with certain parts of cortex projecting to certain parts of striatum 1, 10. IT neurons occur in layers 2-6. Those in layer 2/3 typically have only corticocortical (CCor) projections, whereas those in layers 5A, 5B, and 6 have both CCor and CCstr projections (collectively, at least; quantitative information is still lacking about the extent to which single deeper-layer neurons have these dual projections). For convenience, these will be referred to as IT-CCor and IT-CStr neurons, respectively, but the dual CStr/CCor arbors of the latter should be kept in mind. Here, the attempt is made to refer to IT neurons as IT-CStr and IT-CCor where data support this distinction, but in many cases this is not yet possible. (Layer 4 stellate cells can be considered an IT subclass having only local axonal projections.) Pyramidal tract (PT) neurons, restricted to layer 5B, project to brainstem and in some cases also spinal cord (via the internal capsule, cerebral peduncle, and pyramidal tract), with branches to ipsilateral cortex and numerous subcortical regions; however, their intracerebral axons do not cross the corpus callosum or other commissures to the contralateral hemisphere. CStr neurons are either IT or PT, not both (Fig. 1B). Layer 6 corticothalamic (CT) neurons form a third class of cortical projection neurons, innervating thalamus, including the reticular nucleus, but not striatum.
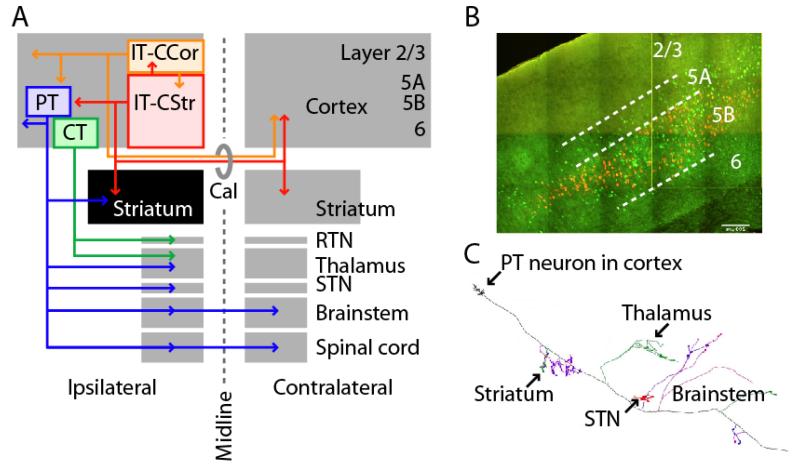
Figure 1. Long-range axonal projections define two classes of CStr neurons.
(A) Pyramidal tract (PT) neurons (blue) project to ipsilateral striatum, thalamus, subthalamic nucleus (STN), and many brainstem and spinal cord regions. Intratelencephalic (IT) neurons project ipsi- or bilaterally (via corpus callosum, Cal) within the cerebral hemispheres to cortex (IT-CCol, orange), and many of these also to striatum (IT-CStr, red). The ipsilateral striatum (black) is unique in receiving CStr input from both IT and PT neurons. Layer 6 corticothalamic neurons (CT, green) project only to thalamus and its reticular nucleus (RTN). (B) Retrogradely labeled corticospinal (PT-CSpi) and callosally projecting IT-CStr neurons in mouse motor cortex (T. Kiritani). IT (green) and PT (orange) neurons are intermingled in layer 5B, but not double-labeled. (C) A single PT neuron’s axon is multiprojectional, sending branches to many subcortical areas. Modified from 11.
A remarkable feature of PT neurons is that they innervate multiple targets including striatum, thalamus, subthalamic nucleus, midbrain, pontine nuclei, and other brainstem areas en route to their caudal-most destinations in the brainstem and spinal cord 5, 11 (Fig. 1C). Although particular sub-types of PT neurons are referred to based on the axonal branch of interest – e.g. corticospinal (PT-CSpi) or corticopontine (PT-CPn) neurons – it should be remembered that they are highly multiprojectional. A clear but often overlooked implication of this is that the behavioral functions of PT neurons presumably derive from their output projections in toto, not just a subset of branches. Further research is needed to clarify the details and significance of IT and PT branching patterns.
It is striking that the far-ranging axons of IT and PT neurons appear to overlap only in two places: ipsilateral cortex and, of particular relevance here, ispilateral striatum. In other words, striatum is unique among all subcortical areas in receiving both IT (bilaterally) and PT (ipsilaterally) inputs.
Knowledge is sparse about how these two classes may be differentially involved in behavior, but the available evidence suggests that IT and PT neurons play distinct roles in cognition and movement control 6, 7, 12-16.
Intracortical circuits
The computations carried out in any cortical area involve not only long-range inputs and outputs but also local intracortical circuits. The accumulating evidence, mostly from rodent studies, indicates that CStr neurons are integrated into these local circuits in stereotypic ways that may carry significant implications for how information is processed directly upstream of striatum.
Layer 2/3 excitatory connectivity to CStr neurons
Layer 2/3→5 connections form a prominent interlaminar pathway within local excitatory networks in the cortex 17-22. In mouse motor cortex, the connectivity from layer 2/3 pyramidal neurons onto projection neurons in layers 5A and 5B depends on both the precise sub-layer location of the postsynaptic neurons and their IT/PT identity 23 (Fig. 2A). Sub-layers of layer 2/3 also appear to target IT versus PT neurons differentially in layers 5A/B (layer 2→IT, layer 3→PT) suggesting an intracortical parallel pathway organization feeding into the IT and PT output channels. This circuit topology implies that IT and PT neurons collect information from separate upstream cortical networks to relay to their downstream striatal targets23, 24. Identifying those upstream networks remains an important goal for understanding how corticocortical pathways selectively engage the IT and PT output channels of the cortex.
Figure 2. Intracortical circuits are IT/PT-specific and hierarchical.
(A) Excitatory connections across layers differentially target IT and PT neurons. Layer 2 neurons (orange triangles, left) tend to target PT neurons (blue) in upper 5B, and IT neurons (red) in layer 5A but not 5B, whereas layer 3 neurons (orange triangles, right) tend to target PT neurons (blue) in upper 5B, and not IT neurons 23. This arrangement implies that distinct upstream networks carrying different functional information feed into IT and PT neurons. (B) Excitatory connectivity within layer 5B differs for IT and PT neurons 27-29. IT neurons (red) excite each other and PT neurons; PT neurons (blue) excite each other but not IT neurons. This hierarchical arrangement means that PT neurons are hodologically compartmentalized downstream from IT neurons within the local excitatory network. (C) Microcircuits involving inhibitory interneurons converge on both and IT and PT neurons 37. Fast-spiking (FS) interneurons are preferentially excited by both IT and PT neurons in layer 5A/B. In contrast, low-threshold-spiking (LTS) interneurons are excited by layer 2/3 neurons (orange). Both FS and LTS interneurons inhibit both IT and PT neurons. An implication is that IT and PT neurons share common inhibitory microcircuit mechanisms, which can, however, be differentially accessed by upstream circuits targeting upper versus deeper cortical layers.
Hierarchical connectivity
Neural systems are hierarchically organized. For example, spinal motor neurons are controlled by neurons in central pattern generator (CPG) networks, in turn influenced by CPG controller neurons 25. In cortex, inter-areal corticocortical pathways are hierarchically arranged 26, but at the level of local circuits this has been challenging to assess, in part because recurrent connectivity is abundant (see next section). However, recent studies of CStr neurons have shown hierarchical connectivity in local circuits as well.
A fundamental question is whether and how IT and PT neurons are interconnected: is there intracortical cross-talk between these two major CStr output channels? Paired recordings from corticopontine (PT-CPn) and callosally projecting IT-CStr neurons in the medial prefrontal cortex (mPFC) of the rat show unidirectional IT→PT connectivity 27. Similarly, paired recordings from layer 5 IT-CCor and PT-corticotectal neurons in mouse visual cortex show asymmetric connectivity, biased in the IT→PT direction 28. Paired recordings from corticospinal (PT-CSpi) and IT-CStr neurons in layer 5B of mouse motor cortex show essentially unidirectional IT→PT connectivity 29. This appears to generalize, as IT neurons in layers 2/3 and 5A also connect unidirectionally with PT neurons 23, 29. Collectively these studies suggest a hierarchical IT→PT principle in which PT neurons receive, but do not send, monosynaptic signals in the local excitatory network (Fig. 2B). Although this places them downstream within the cortical circuit, they can still play active computational roles, communicating with each other directly and providing feedback inhibition to the IT network (discussed below).
Recurrent connectivity
This aspect of connectivity, a basic and prominent feature of cortical circuits, has been proposed to amplify, integrate, distribute, and temporarily store information within subsets of neurons 30-32. In the case of CStr neurons, the convergent output of such. Recurrent connectivity is high in rat and ferret PFC, and higher between PT-CPn than between IT-CStr neurons 27, 34. In contrast, in mouse motor cortex, recurrent excitatory connectivity is higher for IT-CStr than for PT-CSpi neurons 29. Similarly, higher IT-IT and lower PT-PT connectivity is seen in mouse barrel and visual cortex, respectively 28. While the basic circuit organization appears conserved across areas and species, with recurrent connections within projection classes being present (Fig. 2B), the quantitative differences in connection rates may reflect system-specific (limbic versus sensorimotor) and area-specific functional specializations, species differences, or a combination of factors. Interestingly, both the intracortical 29 and intraspinal 35 outputs of corticospinal neurons are relatively facilitating, raising the possibility that similar synaptic dynamics pertain to the striatal and other outputs of PT neurons as well.
Connectivity between CStr and GABAergic neurons
Although excitatory cortical circuits are sharply IT/PT-differentiated, the integration of GABAergic interneurons into local circuits appears to follow other rules. For example, several types of interneurons, and especially fast spiking (FS) and low threshold spiking (LTS) interneurons, converge on both IT-CStr and PT-CSpi neurons 36, 37. FS interneurons in layers 5A/B in turn receive primarily intralaminar excitation from both IT and PT neurons, whereas LTS interneurons in these layers receive primarily interlaminar excitation, from layer 2/3 37. Consequently, the integration of interneurons into microcircuits with CStr neurons appears to be interneuron- and layer-specific, but relatively IT/PT-nonspecific (Fig. 2C). Much remains to be done to characterize the inhibitory mechanisms regulating IT/PT signaling.
Intrinsic properties
A cortical neuron’s function is set not only by its connectivity – a question of “who talks to whom” – but, equally importantly, by its electrophysiology – a question of “how” it responds to inputs to generate a particular pattern of outputs. Recent work is revealing markedly differentiated intrinsic properties of IT and PT neurons.
Hyperpolarization-activated current (Ih)
Ih is a mixed cation current encoded by HCN subunits that is dually regulated by voltage and cyclic nucleotides and produces “sag” potentials in response to current injection 38. Ih is selectively expressed in subsets of neurons throughout the nervous system, and is of special interest because it filters both excitatory and inhibitory synaptic events, generates electrical resonance, is enriched in apical dendrites of cortical neurons, and is under neuromodulatory control 38, 39. Since the initial observations of Ih in cat Betz cells 40, numerous studies establish that Ih is greater in PT compared to IT neurons (Fig. 3A); for example, PT-CPn or PT-corticothalamic versus IT-CCor neurons in rat mPFC 41, 42, and PT-CSpi versus IT-CStr neurons in mouse motor cortex 43. Congruous with the electrophysiology, HCN1 mRNA levels are higher in PT than in IT neurons 43. This projection-specific HCN expression pattern is one of many molecular distinctions between IT and PT neurons that can now be drawn (Fig. 3C). A speculated significance of PT-specific Ih expression is that its neuromodulation could regulate the transfer of cortical activity to downstream circuits; for example, locus ceruleus activity could thereby selectively enhance PT output 43 (Fig. 3B).
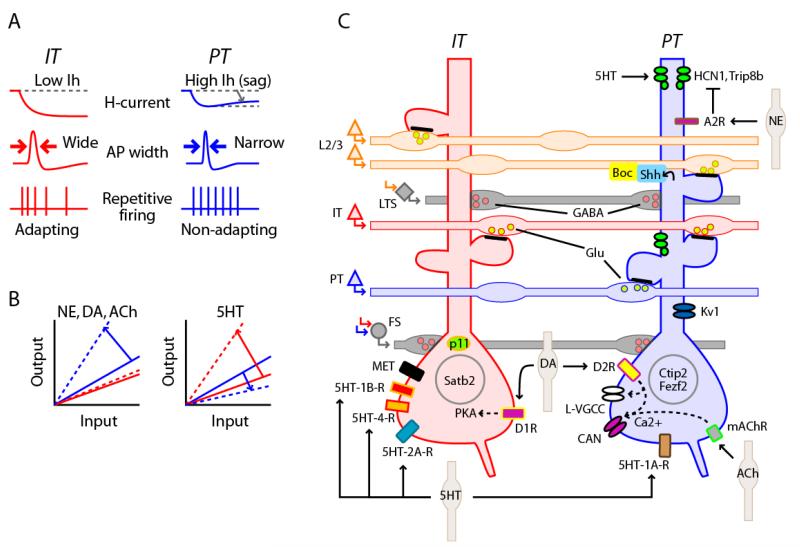
Figure 3. IT and PT neurons have extensively differentiated intrinsic electrophysiology, neuromodulatory properties, and molecular profiles.
(A) Intrinsic properties. Schematics depicting electrophysiological differences. PT neurons have more hyperpolarization-activated current (Ih), appeared as “sag” of the membrane potential during steps of injected current (top row, right). Action potentials are relatively wide (long duration) in IT neurons (middle row, left, arrows), and narrow (fast, short duration) in PT neurons (middle row, right, arrows). Repetitive firing in IT neurons typically follows an adapting (phasic) pattern (bottom row, left), but in PT neurons often follows a more non-adapting (tonic, sustained) pattern 41, 43, 45. (B) Neuromodulation. Left, Schematic depicting PT-specific increase in the transfer function relating synaptic input to spiking output, observed or inferred for NE, DA, and ACh (dashed lines) 41-43. Right, serotonin (5HT) has an opposite effect on PT neurons, increasing the excitability of IT neurons 69. These schematics, although greatly oversimplified, are intended to convey a sense of the diverse and either opposing or augmenting actions of neuromodulators on IT and PT neurons. (C) Molecular expression. IT neurons (left) express the transcription factor Satb2, the calcium-binding protein p11, the receptor tyrosine kinase Met, protein kinase A (PKA), types 1B-, 2A-, and 4-5HT receptor, and D1 dopamine receptors. PT neurons (right) express the transcription factors Ctip2 and Fezf2, muscarinic mAChR receptors, 5HT-1A receptors, D2 dopamine receptors, L-type voltage gated calcium channels (L-VGCC), calcium-activated non-selective cation channels (CAN), Kv1 potassium channels, HCN1 and Trip8b channel subunits, type A2 adrenoreceptors, and sonic hedgehog (shh), which interacts with Boc in presynaptic axons from layer 2/3 neurons. Whereas most of the molecules shown here are expressed differentially in IT and PT neurons, others, particularly ion channels such as L-VGCC, may be expressed in both IT and PT neurons, but are likely regulated by distinct signaling mechanisms. This schematic represents a simplified view of current information, with varying degrees of experimental support for the various features, and in some cases not fully capturing the complexity of the available data; for example, Kv1 channel expression is high in PT neurons in motor but not somatosensory cortex 50. This graphical summary is intended as a starting pointing for further refinement as new information about the types and subcellular distributions of molecules and signaling pathways is gained.
Spike-related properties
PT neurons have relatively fast (short-duration) action potentials, as observed in cat 44, mouse 45, and primate 46, 47 motor cortex. Repetitive firing patterns also differ: PT neurons tend to fire in a tonic/non-adapting or accelerating pattern, and IT neurons in a more phasic/adapting pattern 27, 41, 45, 48-50 (Fig. 3A). This difference in repetitive firing behavior arises from PT-specific expression of D-type current (Kv1 channels) in the case of mouse motor cortex neurons, which, however, is not observed in somatosensory cortex, indicating areal specificity in this particular firing property 50. The relationship between current stimulation intensity and evoked spike rate is also more linear for PT neurons 45. These properties generally suggest that PT neurons are designed for more reliable firing, especially at higher frequencies. Interestingly, there is evidence that back-propagated action potentials, implicated in dendritic integration and plasticity, are more attenuated and frequency-dependent in IT than in PT neurons 51. Exactly how these differences in intrinsic properties translate into behavioral functions has yet to be determined, but the findings reviewed above constitute a substrate for IT/PT-differentiated electrical signaling in vivo 14.
Neuromodulation
Neuromodulation can exert powerful effects on neural circuits. The same circuit can be reconfigured by neuromodulators to perform different computations 52-54. In the cortex, four neuromodulatory systems are of particular interest: noradrenergic input from the locus ceruleus, dopaminergic input from the ventral tegmental area, serotonergic input from the dorsal raphe, and cholinergic input from the nucleus basalis. We are a long way from comprehending how these neuromodulatory systems operate together in a dynamic and coordinated manner during behavior, but, as reviewed here, for each system, current research is beginning to show evidence of dichotomous influences of these neuromodulatory systems on IT and PT neurons. These findings, based largely on ex vivo experiments, lay the groundwork for future studies to address how neuromodulation shapes CStr function during behavior. Dysfunction in neuromodulatory systems is important in disease states as well, as discussed later.
Norepinephrine (NE)
A role for NE in working memory has been proposed based on its modulation of recurrent connectivity in mPFC networks, through cAMP-induced closure of HCN channels 55. Similarly, Ih in PT neurons (see above) endows them with selective sensitivity to noradrenergic agents, in this case implying that NE may regulate CStr output selectively via the PT channel (Fig. 3B). For example, NE could thereby alter the efficacy by which thalamic input to apical dendrites influences cortical output in a PT-selective manner. Details of the intracellular signaling mechanisms still need to be acquired, and these ideas have yet to be substantiated in vivo. Indeed, in an earlier study, NE applied to motor cortex in awake primates often reduced firing rates (for ~70% of cells, including some PT neurons, with no effect on the others), but more so prior to than during movements, producing a net sharpening of responses 56. New strategies, such as optogenetic activation of locus ceruleus axons 57, will undoubtedly facilitate efforts to clarify how NE afferents shape IT and PT signaling in vivo.
Dopamine (DA)
Here, too, IT/PT-specificity is evident. An earlier study in rat cortex reported expression of D1 and D2 receptors in IT but not in PT neurons 58. Supporting this, a recent study 59 in mouse mPFC reported restriction of D1 receptor expression to a subset of IT-type layer 5 neurons, with DA agonists promoting firing in a PKA (but not PKC) dependent manner. However, in contrast with the earlier report, another recent study of mouse PFC 42 found evidence for D2 receptor expression in PT but not IT neurons; only PT neurons were modulated (enhanced afterdepolarization, promoting increased firing) by a D2 agonist, an effect that was NMDA receptor and Ca2+ dependent, with evidence implicating synaptic NMDA receptors, L-type voltage-gated calcium channels, and a calcium-activated non-selective cation current (ICAN) in generating the increased excitability. Similarly, PT-specific D2 receptor expression was shown in ferret mPFC slices, where DA induced burst firing in PT-type layer 5 neurons during layer 2/3 stimulation, an effect that was sensitive to D2-selective drugs and also to the widely used antipsychotic drug haloperidol 60. Given the importance of DA in CStr function 61-64, including the influence of cortical DA on corticostriatal connectivity as it relates to human behavior such as decision-making 65, the dopaminergic modulation of IT/PT neurons merits extensive further investigation.
Serotonin (5HT)
5HT has long been known to have heterogeneous effects on cortical neurons 66, 67. Application of 5HT to large Betz cells (i.e., PT neurons) increases Ih, an observation that, in light of the decrease in Ih induced by NE (see above), illustrates how two neuromodulators can coordinately regulate excitability through opposing actions on the same ionic conductance. Recent evidence indicates that neuromodulation by 5HT is also IT/PT-specific. Expression of 5HT-2A receptors is highest in layer 5A 68, which is enriched in IT-CStr neurons. In mouse mPFC, 5HT excites (or biphasically inhibits-excites) IT neurons, but inhibits PT neurons 69 (see also 70); the inhibition occurs via 5HT-1A receptors and the excitation via 5HT-2A receptors. This pattern appears complementary to NE modulation (see above, and Fig. 3B). IT-CStr neurons also appear to have higher expression than PT neurons of 5HT-1B and 5HT-4 receptors 71. As discussed later, differential IT/PT serotonin signaling has significant disease implications.
Acetylcholine (ACh)
The projection-specificity of ACh effects on layer 5 neurons is incompletely understood; although various cholinergic effects have been reported for experiments with unlabeled pyramidal neurons, information is limited about the IT/PT-specificity. For example, a recent study reports fast cholinergic modulation of excitatory inputs to layer 5 pyramidal neurons through nicotinic ACh receptors containing α7 subunits72. However, in another recent study 41, persistent firing was induced by cholinergic agonists specifically in PT-CPn and not IT neurons; this effect was ascribed to an increased afterdepolarization mediated by muscarinic activation of ICAN, similar to the ICAN-mediated DA effect mentioned above (see also 73). This provides another example of the convergence of neuromodulatory signaling pathways on the same ionic conductance, but in this case with similar actions.
One challenge will be to dissect the cholinergic mechanisms mediated by long-range axons from the basal forebrain versus the local axons of cholinergic cortical neurons. Optogenetic approaches have potential for this by rapidly and selectively evoking ACh release from basal forebrain inputs 74, 75. For all the neuromodulatory systems, the release of modulators by axons in the cortex is likely to affect not only IT and PT neurons but to have additional and complex effects, such as modulating interneurons 74.
Downstream organization: IT/PT projections to striatal neurons and pathways
Although this review chiefly addresses the upstream (intracortical) organization of CStr circuits, an intimately related issue is the downstream organization within the striatum and other areas of the basal ganglia (Fig. 4A). How does IT/PT map onto the major dimensions of basal ganglia organization?
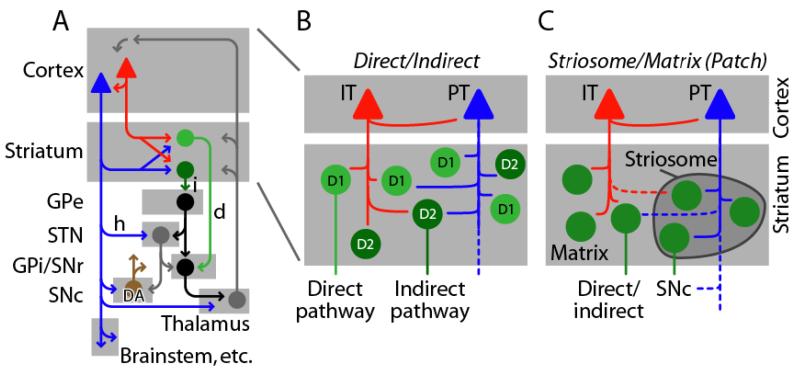
Figure 4. IT/PT innervation of striatum may be differentiated along multiple dimensions of striatal circuit organization.
(A) Simplified schematic illustrating that the CStr projection is an integral component in cortico-basal ganglia-thalamocortical loops. IT neurons (red) project only to striatum, but PT neurons (blue) project to striatum and additional parts of the basal ganglia, particularly to excitatory neurons (gray) in the subthalamic nucleus (STN) via the hyperdirect pathway (h), and to dopaminergic (DA) neurons (brown) in the substantia nigra pars compacta (SNc). GABAergic medium spiny neurons (MSNs) in the striatum project either via the direct pathway (d, light green) or indirect pathway (i, dark green) to GABAergic projection neurons (black) further downstream in the basal ganglia, including the external segment of globus pallidus (GPe), and the internal GP and SN pars reticulata (GPi/SNr). The STN also projects back to cortex (not shown) 196. (B) Direct-pathway MSNs (light green) express D1 DA recptors and indirect-pathway MSNs (dark green) express D2 DA receptors. Both cell types receive both IT and PT inputs, with mixed evidence for cell-type specific biases in the targeting. (C) Striatum is also parceled into matrix (light gray area) and striosome (dark gray zone) compartments. CStr innervation of MSNs in these compartments is biased towards PT→striosome connectivity (right), for CStr projections originating from limbic cortex 87.
IT→striatum, PT→multiple basal ganglia
As noted above, striatum is the sole subcortical area where IT and PT inputs converge. However, a conspicuous but sometimes overseen feature is that PT axons additionally innervate other parts of the basal ganglia (Fig. 4A). In particular, the PT projection to STN 11 (the hyperdirect pathway) is of interest for its role in movement disorders (see below). PT neurons also innervate dopaminergic neurons in the substantia nigra pars compacta (SNc) 11, 76, 77, which is of interest because the SNc is the source of dopaminergic input to the basal ganglia.
Direct and indirect pathways
Striatum contains two major classes of medium spiny neurons (MSNs) with characteristic axonal projections (Fig. 4A). Direct-pathway MSNs (dMSNs) project to the internal segment of globus pallidus (GPi) and substantia nigra pars reticulata (SNr), and express D1-type DA receptors. Indirect-pathway MSN (iMSNs) project polysynaptically to those nuclei via the external segment of the GP (GPe) and the subthalamic nucleus (STN), and express D2-type DA receptors (reviewed in 61).
Anatomical data indicate a pattern of CStr innervation biased towards IT→direct and PT→indirect connectivity within striatum 78 (reviewed in 6, 7) (Fig. 4B). However, a study using in vivo electrophysiology challenged this based on evidence that dMSNs and iMSNs receive similar amounts of IT and PT synaptic input 79. There is also evidence for greater activation of iMSNs following cortical stimulation 80. Further work is needed to resolve this crucial aspect of CStr organization 61.
Striatum is involved in many aspects of behavior including reward-based learning, habits, and action selection, and there is evidence that both the direct and indirect pathways contribute actively during behavior (e.g. 81, 82). Regarding the motor control functions of striatal circuits, the direct pathway is considered to promote intended actions and the indirect pathway to suppress unwanted movements 16, 61, 83-85 (but see 82). Building on these concepts, a recently proposed hypothesis for CStr function 86 extends the direct/indirect organization upstream to IT/PT neurons in the cortex. This model posits that IT neurons drive the direct pathway with signals representing the action to be executed in the immediate future (i.e., planned actions). These signals, through a combination of the large-scale loops through the basal ganglia and thalamus 1 (Fig. 4A) and the unidirectional IT→PT intracortical connectivity, end up being transferred to the PT-CPn neurons, which then drive the indirect pathway with signals representing the action currently being executed. Meanwhile the IT neurons are being updated with the next action-to-be-executed, and the process repeats itself. This model offers a compelling and well-defined conceptual framework to guide further experiments. In general, both the accuracy and complexity of basal ganglia circuit models will increase as experimental observations – e.g. PT innervation of STN and SNc – are incorporated.
Patch (striosome) and matrix compartments
There is also evidence for selective IT/PT innervation of these two intermixed striatal compartments (reviewed in 87) (Fig. 4C). In particular, PT neurons in prelimbic cortex appear to target striosomes preferentially, which is of interest because striosomes in turn project to SNc. Anatomical studies also indicate direct monosynaptic corticonigral innervation (by PT neurons) in parallel with this polysynaptic corticonigral pathway 11, 76, 77. More work on these circuits is needed to understand in detail the intersection of IT/PT and patch/matrix organization, especially as the latter exhibits regional gradients and other complexities.
Corticostriatal connectivity in disease – evidence of IT/PT imbalance
We now move from the IT/PT organization of the intact CStr system to consider how these cells and circuits are affected in disease states. It should be kept in mind that these disorders are complex and involve multiple systems; the focus on CStr connectivity neither excludes nor detracts from the involvement and importance of other brain circuits.
Autism spectrum disorder (ASD)
The most common form of ASD, non-syndromic autism, is arguably a predominantly IT-related disorder. Its diagnosis rests on a clinical picture of primarily cerebral dysfunction, with relative sparing of subcerebral systems, which are more often severely affected in syndromic neurodevelopmental disorders such as Rett syndrome88. In non-syndromic ASD, motor abnormalities include repetitive behaviors such as stereotypies, with relative sparing of long-tract (i.e., corticospinal) functions associated with PT projections (e.g. strength, dexterity) 89. In light of the anatomy of the CStr system (see above), observations of corpus callosum thinning 90-92, mild cortical thickening 93, and abnormal interhemispheric synchronization 94 all demonstrate IT involvement. Functional imaging studies in humans have emphasized long-range corticocortical (IT-CCor) connections, but IT-CStr projections are likely involved, given the dual cortical and striatal projections of many IT neurons (see above). Indeed, a recent study showed abnormally increased resting-state functional connectivity between widespread regions of striatum and multiple cortical areas 95. Interestingly, pontine involvement was noted as well, a reminder that subcortical systems are also undoubtedly affected in ASD 88.
Numerous studies of ASD-associated genes demonstrate CStr involvement. Mutations in neuroligins (NL) are associated with autism 96, and NL-1 knockout mice exhibit ASD-like repetitive behaviors and abnormal CStr synapses (reduced NMDA/AMPA) 97. Mutations in Shank3, a postsynaptic scaffolding protein expressed in MSNs, cause the ASD-related 22q13-deletion (Phelan–McDermid) syndrome 98. Shank3 knockout mice exhibit self-injurious repetitive grooming behavior and abnormal social interactions, and changes at CStr synapses 99. These animal studies collectively support the hypothesis of CStr dysfunction in ASDs, although the IT/PT specificity has yet to be worked out.
Human genetic studies identify the receptor tyrosine kinase MET as an autism risk allele 100, 101. Carriers show atypical activation/deactivation responses to social stimuli, and reduced corticocortical connectivity 102. In human, monkey, and mouse brains, MET is expressed in IT but not PT neurons 103. In Met conditional knockout mice, intracortical excitatory circuits show abnormally increased strength (hyperconnectivity), specifically for IT-CStr and not PT-CPn neurons 104, and altered dendritic morphology of striatal spiny neurons 105. These findings lend support to the popular concept that cortical circuits in non-syndromic ASD are altered towards local-circuit hyperconnectivity and long-range under-connectivity 90, 106-108. It remains to be evaluated whether local-circuit hyperconnectivity occurs in human ASD, and what the behavioral correlates of these changes are.
Collectively, these multiple lines of evidence from both human studies and animal models suggest a major role of IT neurons in ASD pathogenesis (Fig. 5).
Figure 5. CStr-related changes in autism spectrum disorder (ASD), amyotrophic lateral sclerosis (ALS), and major depressive disorder (MDD).
(A) Many changes in ASD are IT-related. Changes (depicted by yellow circles) observed in human ASD include: (1) mildly increased cortical thickness, (2) abnormal interhemispheric and long-range corticocortical synchrony, resulting in a relative disconnection of IT neurons in the contralateral cortex (dashed lines), and (3) thinning of corpus callosum (gray ellipse). Changes observed in mouse models include: (4) increased excitatory synaptic connectivity (small black arrows) from layer 2/3 neurons (orange) to layer 5 IT neurons (red) but not PT neurons (blue), in Met knockout mice; (5) changes at CStr synapses in Shank3 knockout mouse, and changes in striatal neuron dendrites in Met knockout mice. There is (6) relative sparing of pyramidal system functions. (B) Many changes in ALS are PT-related. Changes (depicted by yellow circles) observed include: (1) early cortical hyperexcitability, and hyperactivity during movement; (2) degenerative changes in layer 2/3 neurons; (3) selective degeneration of corticospinal (PT) neurons, with no known pathology of IT-CStr neurons; (4) reduction in PV immunoreactivity in human ALS cortex, but an increase in PV-positive cells in the SOD1 mouse model of ALS; (5) abnormal long-term plasticity at CStr synapses. (C) IT-CStr-related changes in MDD. Changes affecting the CStr projection in MDD include abnormal activity in (1) cortex and (2) striatum in human imaging studies, and (3) IT-CStr-specific upregulation of the calcium-binding protein p11 and 5HT-4 serotonin receptors induced by chronic treatment with the antidepressant fluoxetine (FLX) 181.
Amyotrophic lateral sclerosis (ALS) and related disorders
In contrast to diseases such as autism in which IT neurons are implicated, ALS, primary lateral sclerosis (PLS), and hereditary spastic paraplegia (HSP) are conditions that predominantly affect PT neurons, particularly corticospinal neurons (upper motor neurons) (Fig. 5B). Spastic paralysis can signify PT-specific cortical pathology. However, ALS is a multisystem disease, and neurodegeneration can extend to substantia nigra, globus pallidus, and thalamus, commensurate with the extrapyramidal signs sometimes present clinically 109-111. It remains unclear which, if any, of these are specifically CStr changes or due to other mechanisms, an issue challenging to resolve due to the multisystem nature of the disease. An exception is the case of HSP variants associated with a thin corpus callosum 112, as this indicates pathology of IT axons.
Cortical changes extend beyond corticospinal neurons. Early in the disease the motor cortex is hyperexcitable and hyperactive during movements, possibly due to decreased inhibition, increased excitation, or both 113-119. Reduced staining for parvalbumin (PV, a marker of GABAergic neurons) has been noted in post-mortem ALS cortex, independent of Betz cell depletion 120. With disease progression the cortex becomes hypoexcitable and corticospinal neurons degenerate, paralleling clinical progression. Degenerative changes observed in layer 2/3 120, 121 are intriguing as this layer excites corticospinal neurons (see above).
SOD1 and subsequently many other genes have been tied to familial ALS 122, 123, leading to mouse models, particularly those carrying SOD1 mutations, used extensively to investigate disease mechanisms 124. In motor cortex of SOD1-G93A mice, PV-positive interneurons are abnormally abundant 125. In light of the reduced PV immunoreactivity reported in human ALS tissue (see above), additional studies are needed to resolve this apparent discordance. Abnormalities in long-term synaptic plasticity at CStr synapses have also been found in SOD1-mutant mice, but attributed to pathology involving SNc rather than PT inputs 126, again reflecting some of the complexities and challenges in studying CStr function in multisystem diseases. An additional consideration is that long-term plasticity of CStr synapses depends on brain state 127. Much remains to be learned about IT/PT and CStr signaling abnormalities in ALS and related PT-predominant disorders.
Obsessive-compulsive disorder (OCD)
CStr dysfunction is considered a major factor in OCD pathogenesis, as formulated in the “corticostriatal loop hypothesis” 128 and consistent with the CStr system’s role in repetitive behaviors in general 129. Human imaging studies have shown increased functional connectivity in a subset of CStr circuits, referred to as the “OCD circuit”, encompassing orbitofrontal (OFC), prefrontal (PFC), and anterior cingulate areas of the cortex, the rostral caudate and ventral striatum, and thalamus (reviewed in 128, 130-132) (Fig. 6). Functional imaging has shown increased or otherwise abnormal functional connectivity in these CStr pathways 133-135. An increase, rather than decrease, broadly agrees with the clinical phenomenology of positive, rather than negative, signs. Furthermore, the increased functional connectivity in a subset of cortico-basal ganglia-thalamocortical loops accords with the proposal of OCD as a thalamocortical dysrhythmia 136, 137. Indeed, thalamocortical dysrhythmia, essentially a calcium channelopathy in thalamic relay neurons, has been proposed as an etiological mechanism in diverse neuropsychiatric and movement disorders, including many discussed here (ASD, PD, SZ, MDD).
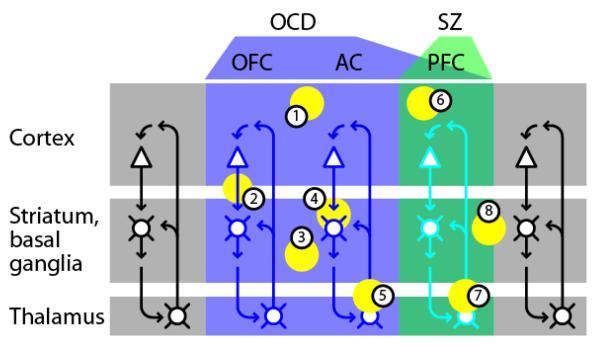
Figure 6. CStr-related changes in obsessive-compulsive disorder (OCD) and schizophrenia (SZ) involve subsets of CStr circuits, with some overlap.
In OCD (blue shading), the circuits primarily affected are CStr circuits involving orbitofrontal (OFC), anterior cingulate (AC), and prefrontal (PFC) cortex. SZ primarily involves dorsolateral PFC circuits. Both disorders involve hyperactivity in these circuits. CStr circuits in other regions appear to be spared. Neuron coloring portrays the differential involvement of the same basic circuits in these disorders. Abnormalities in OCD include: (1) cortical changes, with abnormal frontal activity seen by functional imaging; (2) CStr changes, including increased fronto-striatal connectivity; (3) structural and functional striatal changes, particularly in ventral striatum; (4) changes at CStr synapses, demonstrated in mouse models of OCD; (5) thalamic abnormalities, including increased activity in imaging studies, and thalamocortical dysrhythmia. Abnormalities in SZ include: (6) cortical changes, primarily in dorsolateral PFC, including reduced thickness and functional connectivity with cortex and striatum, and abnormal rhythms; (7) thalamocortical dysrhythmia; (8) striatal changes, including reduced volume associated with SZ risk alleles.
Evidence from mouse models supports this idea of CStr dysfunction in OCD. Sapap3-mutant mice show remarkable OCD-like behaviors and abnormal CStr connectivity 138. Slitrk5-knockout mice also exhibit OCD-like symptoms, and show increased neural activity in CStr areas 139. These mice do not show gross motor abnormalities or problems with motor learning, perhaps reflecting preponderantly IT-related pathology and sparing of PT functions. Indeed, the clinical features of OCD would seem to point in the direction of IT-predominant pathology. However, the part of the basal ganglia most affected in OCD, the ventral striatum, is enriched in striosomes, which (as discussed above, and reviewed in 87) receive PT innervation. The relative involvement of IT and PT projections remains to be elucidated in this disorder. The possibility of IT/PT dysregulation in Tourette’s syndrome, a tic disorder closely related to OCD 128, 130, also remains to be tested.
Schizophrenia (SZ)
CStr involvement in SZ is a longstanding idea 140, 141. Only certain sub-networks within the large scale loops involving cortex, basal ganglia, and thalamus seem to be affected (Fig. 6), a concept similar to that discussed above for OCD and one that pertains to other complex neuropsychiatric and movement disorders as well 141, 142. In alignment with this idea, a recent study 143 found evidence for impairments in cognitive skill learning, with preservation of motor skill learning ability. This is of interest in light of evidence for a prominent role of the CStr system in this latter type of learning (e.g. 144; see Box 2). Human imaging studies show decreased long-range functional connectivity in certain corticocortical and CStr (i.e., IT-predominant) projections 145 – similar in concept to the pattern of circuit changes thought to occur in ASD (see above), but involving other subsets of forebrain loops. Recent studies have shown that the SZ risk genes PPP1R1B, AKT1, and PRODH are associated with reductions in striatal size and increased functional fronto-striatal connectivity 146-148.
Box 2. Corticostriatal plasticity: implications for brain-machine interfaces (BMI).
The dynamic nature of cortical activity has long been appreciated. Earlier studies showed that monkeys could be trained to modulate the activities of motor cortex neurons, independent of motor activity 194. This is essentially the fundamental principle of BMI; i.e., training a subject to control a machine by learning to control the activity of a subset of its cortical neurons. The possibility that this type of cortical plasticity could reside specifically in CStr circuits was recently investigated 144. Rats implanted with tetrodes in motor cortex were successfully trained to modulate activity of cortical neurons to change the frequency of auditory tones, without generating movements. This demonstrates a vital role for CStr plasticity not only in motor-based learning 195 but also in the volitional and goal-directed learning of abstract skills. An implication for BMI is that CStr plasticity may be particularly promising as a source for driving BMI command signals.
The cortical area most implicated in SZ etiology is the dorsolateral PFC, and current ideas about the cellular basis for SZ etiology center on the roles of interneurons, gamma rhythms, NMDA receptors, DA, and 5HT 149-151. A possible IT/PT angle on SZ has recently emerged with the findings of IT/PT-specific neuromodulatory mechanisms, particularly DA and 5HT (see above). Abnormal cortical 5HT signaling appears to be a major factor in the complex etiology of SZ, an idea originally inspired by the similarities between psychotic states and the hallucinogenic effects of the 5HT-2A agonists LSD and psilocybin, and supported by various lines of evidence in human studies and animal models, including the high expression of 5HT-2A receptors in PFC and ventral pallidum 152. Abnormal cortical DA signaling is also implicated 153. Abnormalities in these neuromodulatory systems are therefore of great interest in the context of IT/PT organization, as recent evidence indicates that CStr neurons respond divergently to 5HT and DA agonists, through differential expression of 5HT and DA receptor subclasses in IT and PT neurons (discussed above).
Huntington’s disease (HD)
Despite the widespread brain expression of the huntingtin gene product, htt, the neurodegenerative process in HD mainly (but not exclusively) affects the cortex (especially frontal association and motor areas) and striatum (especially the anterior caudate), and thus the CStr projection 154-156 (Fig. 7). In striatum, iMSNs are preferentially affected 157, 158. In cortex, htt is broadly expressed in pyramidal neurons and inhibitory interneurons in all layers. However, in HD, although cell loss is extensive, it is restricted to pyramidal neurons in middle and deeper layers 159-161. The multilaminar involvement, plus corpus callosum thinning 162, 163, clearly implicates IT neurons, but the loss of SMI32-immunopositive neurons also implicates PT neurons. However, PT involvement appears to be mainly intracortical, as TMS studies have shown normal motor responses (indicating normal corticospinal conductance) but with higher cortical thresholds and reduced amplitudes (reviewed in 164).
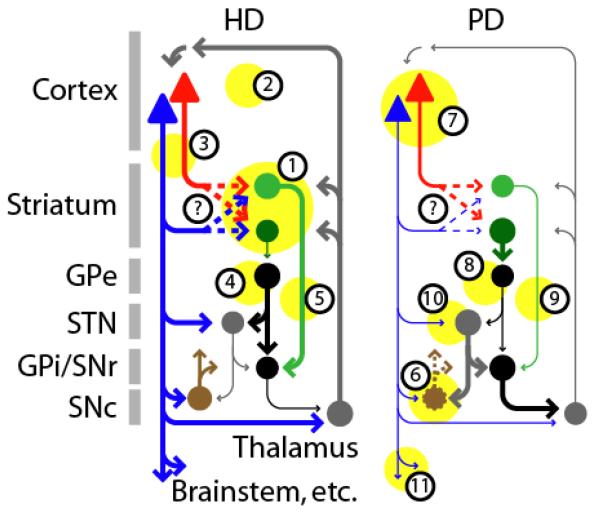
Figure 7. CStr changes are prominent in HD and PD but the differential involvement of IT and PT neurons is not fully understood.
Changes (depicted by yellow circles and line thicknesses) in HD, a hyperkinetic movement disorder, include: (1) neurodegenerative atrophy of striatum, particularly the caudate, preferentially affecting indirect-pathway MSNs (dark green), and (2) of cortex, across several layers, likely involving IT (red) and possibly PT (blue) neurons; (3) dysfunction in CStr projections. Network effects include (4) decrease of indirect pathway (dark green) and (5) increase of direct pathway (light green) activity. The net effect of increasing cortical output via PT neurons is thought to correlate with the hyperkinetic movements typical of HD. Changes in PD, a hypokinetic movement disorder, include: (6) neurodegeneration of SNc neurons (brown; dashed lines); (7) decreased activity in PT (blue) but not IT (red) neurons in the cortex. Network effects include (8) decrease of direct (light green) and (9) increase of indirect (dark green) pathway activity. The net effect of decreasing cortical output via PT neurons is thought to correlate with the hypokinetic movements typical of PD. (10) Abnormalities in the hyperdirect pathway from PT neurons to excitatory neurons (gray) in the STN have been implicated in abnormal rhythms in PD, and deep-brain stimulation in the STN can be an effective therapy. (11) Reduced drive in corticospinal projections is proposed as a downstream consequence of dysfunction in basal ganglia circuits. Modified from 171, 172. In both HD and PD, the possibility of abnormal IT/PT connectivity to direct/indirect MSNs has not yet been tested (question marks and dashed arrows).
In multiple animal models, cortical pyramidal neurons are electrophysiologically abnormal even before onset of motor deficits, including changes in excitatory and inhibitory synaptic inputs resulting in hyperexcitability 165. Cortical changes predict the onset and severity of symptoms 166. Dysfunction in CStr projections is well established (Fig. 7), and results from mouse models have shown a progressive disconnection of cortex and striatum with disease progression 167 (reviewed in 155). A recent study using multiple HD mouse models demonstrated consistent involvement of the CStr system, albeit with a range of severity 168. The possibility of IT/PT specificity of these changes remains to be tested. Investigations of dMSNs and iMSNs in mouse models of HD have shown complex effects evolving during disease progression 169, implying similar complexity for the cortical changes. Adding to the complexity is evidence that cortical interneurons, which also express htt but are not lost in the human disease 159, 160, contribute to the disease process through cell-cell interactions 170.
Parkinson’s disease (PD)
PD research has naturally focused on basal ganglia due to the locus of primary pathology in SNc. However, there is increasing interest in the cortical and CStr contributions to the disease process, and the available evidence suggests IT/PT-specific changes (Fig. 7). Models of the circuit changes in PD 171, 172 posit a reduced cortical drive to PT neurons. A study directly testing the cell-type specificity in motor cortex in parkinsonian primates found reduced firing rates mostly for PT and not IT neurons 14. An important area for future studies is to assess the changes in corticostriatal connectivity from IT/PT onto the key striatal cell types and pathways in PD models. This would enable abnormalities in the CStr system to be tied to those in intrastriatal circuits, such as the observed reduction of excitatory input to iMSNs 173.
The idea of abnormal PT activity in PD is also of interest because these neurons (particularly corticospinal neurons) convey cortical commands to the subcerebral motor system, and because they monosynaptically innervate glutamatergic neurons in STN and dopaminergic neurons in SNc (see above). For example, in DA-depleted states the STN is hyperactive, broadly consistent with the predictions of the direct/indirect model (Fig. 7). However, there is also reduced activity in STN-projecting PT neurons 174, and hypersynchronous oscillatory activity, which may reflect STN hypersensitivity to PT inputs, amplified by dysfunction elsewhere in the circuit 175. Deep-brain stimulation (DBS) of the STN is used to alleviate PD symptoms 176. New evidence suggests an intracortical basis for its efficacy, through antidromic activation (via the hyperdirect pathway) of PT neurons in the cortex 177. Indeed, human fMRI data show motor cortex activation during STN-DBS 178. In a mouse PD model, motor abnormalities are mitigated by selective optogenetic stimulation of PT neurons, mimicking STN-DBS 179. STN-DBS appears to be a case of a successful PT-selective neurointervention.
Major depressive disorder (MDD)
In MDD, cortex and striatum are prominent among the many brain regions showing abnormal activity in human imaging studies 180. A recent study in mice underscores the relevance of IT/PT-specific neuromodulation to disease states, showing that chronic treatment with a common antidepressant, fluoxetine (FLX), acts selectively on IT-CStr neurons in layer 5A to increase expression of p11 (a calcium binding protein) and 5HT-4 receptors, an effect not observed for PT neurons 181 (Fig. 5C). The efficacy of FLX as a first-line treatment for OCD 130 suggests it may have similar IT-specificity in that disorder. This is an excellent example of how the kinds of manipulations that are possible in mouse models can reveal cell-type specific molecular mechanisms directly relevant to human disease.
Conclusions and Perspective
Taking an IT/PT view of CStr connectivity brings into focus a striking bipartite organization, with distinct and contrasting properties evident at essentially every level of neural organization, including long-range projections (the initial basis for the IT/PT classification), cortical microcircuits, intrinsic properties, molecular specification, and neuromodulation. Indeed, IT and PT neurons differ in almost every regard except for one cardinal property: they both innervate striatum. The evolutionary conservation of IT/PT organization across species and cortical areas is consistent with the fundamental nature of its wide-ranging roles in motivated behavior.
The concept of excitation/inhibition (E/I) ratio, or balance, has been useful for understanding circuit-level changes in a wide variety of disease states 182. Similarly, the evidence reviewed here suggests that “IT/PT balance” may constitute a useful concept for approaching a range of neuropsychiatric, neurodevelopmental, and neurological disorders. Indeed, just as the “I” side of E/I balance is determined by distinct classes of interneurons, the “E” side is likely to depend particularly on IT and PT neurons.
Given the overarching nature of the IT/PT in CStr organization in the intact brain, it would be surprising if IT/PT imbalance was not important in disease states, and as reviewed here there is converging evidence that this is the case in many disorders. Currently, the strength of the available data for IT/PT involvement is variable, and in some cases tenuous. But the collective evidence across the spectrum of disorders reviewed here suggests that it may be useful to distinguish disorders that predominantly involve IT neurons (e.g. SZ, ASDs, and OCD) and those that predominantly involve PT (e.g. ALS, HSP, and probably PD). Indeed, even in other disorders in which the pathological process affects IT and PT neurons non-selectively (e.g. cerebrovascular disease, multiple sclerosis), the balance of IT/PT output to striatum may be perturbed, with concomitant clinical manifestations. In principle, it should be possible to develop therapeutic interventions with IT/PT selectivity, for example by exploiting their differential molecular and neuromodulatory mechanisms, or, as exemplified by STN-DBS in PD, their long-range axonal projections.
Box 1. Molecular-developmental specification of CStr neurons and their intracortical circuits.
Molecular mechanisms involved in cell-fate specification of IT and PT neurons have been worked out in considerable detail, particularly the roles of Ctip2 and Fezf2 in the case of PT neurons, and Satb2 for IT neurons 8, 183-186. IT-CStr neurons closely resemble layer 2/3 IT-CCor neurons in their molecular expression profiles, but differ in sharing with PT neurons – but in a developmentally transient manner – a subset of cell-fate related molecules, potentially explaining their striatal innervation 187. Recent work has pinpointed molecular mechanisms that establish layer 2/3→PT connections, showing that the synaptic connectivity depends on expression of the Shh-receptor Boc (Brother of CDO) in presynaptic layer 2/3 pyramidal neurons and sonic hedgehog (Shh) in postsynaptic PT neurons 188, 189. How cell-type specification across layers relates to regional specification of cortical areas 190-192 remains unclear, but further efforts in this area may illuminate the basis both for inter-areal functional differences within classes of CStr projection neurons (e.g. 50, 193) and for the striking sub-regional specificity of many of the disorders involving CStr neurons discussed below.
Acknowlegements
I am grateful to M. D. Bevan, D. J. Surmeier, and K. Svoboda for comments and suggestions, and to T. Kiritani for the image in Fig. 1. Support: NIH/NINDS grant NS061963.
Glossary
- Hierarchical connectivity
The concept that one class of neurons is functionally downstream from another. The two classes may be in separate areas, or locally intermingled. This feedforward arrangement implies control of the downstream population by the upstream population.
- Recurrent connectivity
The concept that neurons within a class connect with one another, implying feedback communication within the network.
REFERENCES
- 1.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 2.Pennartz CM, et al. Corticostriatal interactions during learning, memory processing, and decision making. J Neurosci. 2009;29:12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferino F, Thierry AM, Saffroy M, Glowinski J. Interhemispheric and subcortical collaterals of medial prefrontal cortical neurons in the rat. Brain Res. 1987;417:257–266. doi: 10.1016/0006-8993(87)90450-1. [DOI] [PubMed] [Google Scholar]
- 4.Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- 5.Levesque M, Charara A, Gagnon S, Parent A, Deschenes M. Corticostriatal projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1996;709:311–315. doi: 10.1016/0006-8993(95)01333-4. [DOI] [PubMed] [Google Scholar]
- 6.Reiner A. Organization of Corticostriatal Projection Neuron Types. In: Steiner H, Tseng KY, editors. of Basal Ganglia Structure and Function: A Decade of Progress. 2010. [Google Scholar]
- 7.Reiner A, Hart NM, Lei W, Deng Y. Corticostriatal projection neurons - dichotomous types and dichotomous functions. Front Neuroanat. 2010;4:142. doi: 10.3389/fnana.2010.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fame RM, Macdonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Verstynen TD, Badre D, Jarbo K, Schneider W. Microstructural organizational patterns in the human corticostriatal system. J Neurophysiol. 2012;107:2984–2995. doi: 10.1152/jn.00995.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci. 2012;32:5990–5999. doi: 10.1523/JNEUROSCI.5717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner RS, DeLong MR. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb Cortex. 2011;21:1362–1378. doi: 10.1093/cercor/bhq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauswein E, Fromm C, Preuss A. Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res. 1989;493:198–203. doi: 10.1016/0006-8993(89)91018-4. [DOI] [PubMed] [Google Scholar]
- 16.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Weiler N, Wood L, Yu J, Solla SA, Shepherd GMG. Top-down laminar organization of the excitatory network in motor cortex. Nat Neurosci. 2008;11:360–366. doi: 10.1038/nn2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J, et al. Local-circuit phenotypes of layer 5 neurons in motor-frontal cortex of YFP-H mice. Front Neural Circuits. 2008;2:1–8. doi: 10.3389/neuro.04.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooks BM, et al. Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol. 2011;9:e1000572. doi: 10.1371/journal.pbio.1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- 22.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CT, Sheets PL, Kiritani T, Shepherd GMG. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci. 2010;13:739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko T, Cho R, Li Y, Nomura S, Mizuno N. Predominant information transfer from layer III pyramidal neurons to corticospinal neurons. J Comp Neurol. 2000;423:52–65. doi: 10.1002/1096-9861(20000717)423:1<52::aid-cne5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 26.Markov NT, Kennedy H. The importance of being hierarchical. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiritani T, Wickersham IR, Seung HS, Shepherd GM. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J Neurosci. 2012;32:4992–5001. doi: 10.1523/JNEUROSCI.4759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas RJ, Koch C, Mahowald M, Martin KA, Suarez HH. Recurrent excitation in neocortical circuits. Science. 1995;269:981–985. doi: 10.1126/science.7638624. [DOI] [PubMed] [Google Scholar]
- 31.Lubke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci. 2000;20:5300–5311. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- 35.Phillips CG, Porter R. The Pyramidal Projection to Motoneurones of Some Muscle Groups of the Baboon’s Forelimb. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka YH, et al. Local connections of layer 5 GABAergic interneurons to corticospinal neurons. Frontiers in Neural Circuits. 2011;5:1–14. doi: 10.3389/fncir.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apicella A, Wickersham IR, Seung HS, Shepherd GMG. Laminarly orthogonal excitation of fast spiking and low threshold spiking interneurons in mouse motor cortex. Journal of Neuroscience. 2012;32:7021–7033. doi: 10.1523/JNEUROSCI.0011-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 39.Berger T, Larkum ME, Luscher HR. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- 40.Spain WJ, Schwindt PC, Crill WE. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987;57:1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- 41.Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gee S, et al. Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. J Neurosci. 2012;32:4959–4971. doi: 10.1523/JNEUROSCI.5835-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheets PL, et al. Corticospinal-specific HCN expression in mouse motor cortex: Ih-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. Journal of Neurophysiology. 2011;106:2216–2231. doi: 10.1152/jn.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Zhang JJ, Hu GY, Wu CP. Electrophysiological and morphological properties of pyramidal and nonpyramidal neurons in the cat motor cortex in vitro. Neuroscience. 1996;73:39–55. doi: 10.1016/0306-4522(96)00009-7. [DOI] [PubMed] [Google Scholar]
- 45.Suter BA, Migliore M, Shepherd GMG. Intrinsic electrophysiology of mouse corticospinal neurons: a class-specific triad of spike-related properties. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs184. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D, Fetz EE. Characteristic membrane potential trajectories in primate sensorimotor cortex neurons recorded in vivo. J Neurophysiol. 2005;94:2713–2725. doi: 10.1152/jn.00024.2005. [DOI] [PubMed] [Google Scholar]
- 47.Vigneswaran G, Kraskov A, Lemon RN. Large identified pyramidal cells in macaque motor and premotor cortex exhibit “thin spikes”: implications for cell type classification. J Neurosci. 2011;31:14235–14242. doi: 10.1523/JNEUROSCI.3142-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason A, Larkman A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex: II. Electrophysiology. Journal of Neuroscience. 1990;10:1415–1428. doi: 10.1523/JNEUROSCI.10-05-01415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- 50.Miller MN, Okaty BW, Nelson SB. Region-specific spike-frequency acceleration in layer 5 pyramidal neurons mediated by Kv1 subunits. J Neurosci. 2008;28:13716–13726. doi: 10.1523/JNEUROSCI.2940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grewe BF, Bonnan A, Frick A. Back-Propagation of Physiological Action Potential Output in Dendrites of Slender-Tufted L5A Pyramidal Neurons. Front Cell Neurosci. 2010;4:13. doi: 10.3389/fncel.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 53.Blitz DM, Nusbaum MP. Neural circuit flexibility in a small sensorimotor system. Curr Opin Neurobiol. 2011;21:544–552. doi: 10.1016/j.conb.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 56.Matsumura M, Sawaguchi T, Kubota K. Modulation of neuronal activities by iontophoretically applied catecholamines and acetylcholine in the primate motor cortex during a visual reaction-time task. Neurosci Res. 1990;8:138–145. doi: 10.1016/0168-0102(90)90066-n. [DOI] [PubMed] [Google Scholar]
- 57.Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 59.Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Goldman-Rakic PS. D2 receptor regulation of synaptic burst firing in prefrontal cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2004;101:5093–5098. doi: 10.1073/pnas.0400954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa RM. Plastic corticostriatal circuits for action learning: what’s dopamine got to do with it? Ann N Y Acad Sci. 2007;1104:172–191. doi: 10.1196/annals.1390.015. [DOI] [PubMed] [Google Scholar]
- 63.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012;32:9402–9409. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies MF, Deisz RA, Prince DA, Peroutka SJ. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987;423:347–352. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- 67.Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- 68.Weber ET, Andrade R. Htr2a Gene and 5-HT(2A) Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avesar D, Gulledge AT. Selective serotonergic excitation of callosal projection neurons. Frontiers in Neural Circuits. 2012;6:1–11. doi: 10.3389/fncir.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Co-expression of serotonin 5-HT(1B) and 5-HT(4) receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology. 2011;61:442–450. doi: 10.1016/j.neuropharm.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 72.Poorthuis RB, et al. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23:148–161. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-alpha7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 2012;32:3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kalmbach A, Hedrick T, Waters J. Selective optogenetic stimulation of cholinergic axons in neocortex. J Neurophysiol. 2012;107:2008–2019. doi: 10.1152/jn.00870.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakai ST. Corticonigral projections from area 6 in the raccoon. Exp Brain Res. 1988;73:498–504. doi: 10.1007/BF00406607. [DOI] [PubMed] [Google Scholar]
- 77.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 78.Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- 80.Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costa RM, et al. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52:359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 82.Cui G, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013 doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 84.Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morita K, Morishima M, Sakai K, Kawaguchi Y. Reinforcement learning: computing the temporal difference of values via distinct corticostriatal pathways. Trends Neurosci. 2012;35:457–467. doi: 10.1016/j.tins.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Crittenden JR, Graybiel AM. Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat. 2011;5:59. doi: 10.3389/fnana.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shepherd GMG, Katz DM. Synaptic microcircuit dysfunction in genetic models of neurodevelopmental disorders: focus on Mecp2 and Met. Curr Opin Neurobiol. 2011;21:827–833. doi: 10.1016/j.conb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gowen E, Hamilton A. Motor Abilities in Autism: A Review Using a Computational Context. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1574-0. [DOI] [PubMed] [Google Scholar]
- 90.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keary CJ, et al. Corpus callosum volume and neurocognition in autism. J Autism Dev Disord. 2009;39:834–841. doi: 10.1007/s10803-009-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A two-year longitudinal MRI study of the corpus callosum in autism. J Autism Dev Disord. 2012;42:2312–2322. doi: 10.1007/s10803-012-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schumann CM, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dinstein I, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–1225. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Martino A, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blundell J, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phelan K, McDermid HE. The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome) Mol Syndromol. 2012;2:186–201. doi: 10.1159/000334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell DB, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Judson MC, Eagleson KL, Levitt P. A new synaptic player leading to autism risk: Met receptor tyrosine kinase. J Neurodev Disord. 2011;3:282–292. doi: 10.1007/s11689-011-9081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rudie JD, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Judson MC, Bergman MY, Campbell DB, Eagleson KL, Levitt P. Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain. J Comp Neurol. 2009;513:511–531. doi: 10.1002/cne.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qiu S, Anderson CT, Levitt P, Shepherd GM. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated met receptor tyrosine kinase. J Neurosci. 2011;31:5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Judson MC, Eagleson KL, Wang L, Levitt P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J Comp Neurol. 2010;518:4463–4478. doi: 10.1002/cne.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Frith C. Is autism a disconnection disorder? Lancet Neurol. 2004;3:577. doi: 10.1016/S1474-4422(04)00875-0. [DOI] [PubMed] [Google Scholar]
- 108.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 109.Borasio GD, et al. Dopaminergic deficit in amyotrophic lateral sclerosis assessed with [I-123] IPT single photon emission computed tomography. J Neurol Neurosurg Psychiatry. 1998;65:263–265. doi: 10.1136/jnnp.65.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desai J, Swash M. Extrapyramidal involvement in amyotrophic lateral sclerosis: backward falls and retropulsion. J Neurol Neurosurg Psychiatry. 1999;67:214–216. doi: 10.1136/jnnp.67.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma KR, Sheriff S, Maudsley A, Govind V. Diffusion Tensor Imaging of Basal Ganglia and Thalamus in Amyotrophic Lateral Sclerosis. J Neuroimaging. 2012 doi: 10.1111/j.1552-6569.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boukhris A, et al. A new locus (SPG46) maps to 9p21.2-q21.12 in a Tunisian family with a complicated autosomal recessive hereditary spastic paraplegia with mental impairment and thin corpus callosum. Neurogenetics. 2010;11:441–448. doi: 10.1007/s10048-010-0249-2. [DOI] [PubMed] [Google Scholar]
- 113.Eisen A, Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:564–573. doi: 10.1002/mus.1042. [DOI] [PubMed] [Google Scholar]
- 114.Zanette G, et al. Changes in motor cortex inhibition over time in patients with amyotrophic lateral sclerosis. J Neurol. 2002;249:1723–1728. doi: 10.1007/s00415-002-0926-7. [DOI] [PubMed] [Google Scholar]
- 115.Vucic S, Cheah BC, Kiernan MC. Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Exp Neurol. 2009;220:177–182. doi: 10.1016/j.expneurol.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 116.Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- 117.Attarian S, Pouget J, Schmied A. Changes in cortically induced inhibition in amyotrophic lateral sclerosis with time. Muscle Nerve. 2009;39:310–317. doi: 10.1002/mus.21137. [DOI] [PubMed] [Google Scholar]
- 118.Turner MR, Leigh PN. Positron emission tomography (PET)--its potential to provide surrogate markers in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 2):S17–22. doi: 10.1080/14660820052415781. [DOI] [PubMed] [Google Scholar]
- 119.Brooks BR, et al. Functional magnetic resonance imaging (fMRI) clinical studies in ALS--paradigms, problems and promises. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 2):S23–32. doi: 10.1080/14660820052415790. [DOI] [PubMed] [Google Scholar]
- 120.Nihei K, McKee AC, Kowall NW. Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol. 1993;86:55–64. doi: 10.1007/BF00454899. [DOI] [PubMed] [Google Scholar]
- 121.Brion S, Plas J. Lesions of the motor cortex in amyotrophic lateral sclerosis. Encephale. 1986;12:81–87. [PubMed] [Google Scholar]
- 122.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 123.Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009;10:769–782. doi: 10.1038/nrg2680. [DOI] [PubMed] [Google Scholar]
- 124.Tovar YRLB, Santa-Cruz LD, Tapia R. Experimental models for the study of neurodegeneration in amyotrophic lateral sclerosis. Mol Neurodegener. 2009;4:31. doi: 10.1186/1750-1326-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Minciacchi D, Kassa RM, Del Tongo C, Mariotti R, Bentivoglio M. Voronoi-based spatial analysis reveals selective interneuron changes in the cortex of FALS mice. Exp Neurol. 2009;215:77–86. doi: 10.1016/j.expneurol.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Geracitano R, et al. Altered long-term corticostriatal synaptic plasticity in transgenic mice overexpressing human CU/ZN superoxide dismutase (GLY(93)-->ALA) mutation. Neuroscience. 2003;118:399–408. doi: 10.1016/s0306-4522(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 127.Stoetzner CR, Pettibone JR, Berke JD. State-dependent plasticity of the corticostriatal pathway. Neuroscience. 2010;165:1013–1018. doi: 10.1016/j.neuroscience.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 129.Langen M, Kas MJ, Staal WG, van Engeland H, Durston S. The neurobiology of repetitive behavior: of mice. Neurosci Biobehav Rev. 2011;35:345–355. doi: 10.1016/j.neubiorev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Aouizerate B, et al. Pathophysiology of obsessive-compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 131.Menzies L, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harrison BJ, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 134.Sakai Y, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry. 2011;26:463–469. doi: 10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 135.Beucke JC, et al. Altered cingulostriatal coupling in obsessive-compulsive disorder. Brain Connect. 2012;2:191–202. doi: 10.1089/brain.2012.0078. [DOI] [PubMed] [Google Scholar]
- 136.Schulman JJ, et al. Imaging of thalamocortical dysrhythmia in neuropsychiatry. Front Hum Neurosci. 2011;5:69. doi: 10.3389/fnhum.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shmelkov SV, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kleist K. Schizophrenic symptoms and cerebral pathology. J Ment Sci. 1960;106:246–255. doi: 10.1192/bjp.106.442.246. [DOI] [PubMed] [Google Scholar]
- 141.Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16:391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- 142.Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- 143.Foerde K, et al. Selective corticostriatal dysfunction in schizophrenia: examination of motor and cognitive skill learning. Neuropsychology. 2008;22:100–109. doi: 10.1037/0894-4105.22.1.100. [DOI] [PubMed] [Google Scholar]
- 144.Koralek AC, Jin X, Long Ii JD, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012;483:331–335. doi: 10.1038/nature10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 146.Kempf L, et al. Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function. PLoS Genet. 2008;4:e1000252. doi: 10.1371/journal.pgen.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meyer-Lindenberg A, et al. Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J Clin Invest. 2007;117:672–682. doi: 10.1172/JCI30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tan HY, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 150.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22:537–544. doi: 10.1016/j.conb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 153.Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- 154.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 155.Cepeda C, Wu N, Andre VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Prog Neurobiol. 2007;81:253–271. doi: 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eidelberg D, Surmeier DJ. Brain networks in Huntington disease. J Clin Invest. 2011;121:484–492. doi: 10.1172/JCI45646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deng YP, et al. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 158.Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Exp Neurol. 2008;211:227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cudkowicz M, Kowall NW. Degeneration of pyramidal projection neurons in Huntington’s disease cortex. Ann Neurol. 1990;27:200–204. doi: 10.1002/ana.410270217. [DOI] [PubMed] [Google Scholar]
- 160.Macdonald V, Halliday G. Pyramidal cell loss in motor cortices in Huntington’s disease. Neurobiol Dis. 2002;10:378–386. doi: 10.1006/nbdi.2002.0528. [DOI] [PubMed] [Google Scholar]
- 161.Hedreen JC, Peyser CE, Folstein SE, Ross CA. Neuronal loss in layers V and VI of cerebral cortex in Huntington’s disease. Neurosci Lett. 1991;133:257–261. doi: 10.1016/0304-3940(91)90583-f. [DOI] [PubMed] [Google Scholar]
- 162.Rosas HD, et al. Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical “disconnection”. Neuroimage. 2010;49:2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Di Paola M, et al. Multimodal MRI analysis of the corpus callosum reveals white matter differences in presymptomatic and early Huntington’s disease. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhr360. in press. [DOI] [PubMed] [Google Scholar]
- 164.Berardelli A, et al. Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord. 1999;14:398–403. doi: 10.1002/1531-8257(199905)14:3<398::aid-mds1003>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 165.Cummings DM, et al. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington’s disease. J Neurosci. 2009;29:10371–10386. doi: 10.1523/JNEUROSCI.1592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Laforet GA, et al. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington’s disease. J Neurosci. 2001;21:9112–9123. doi: 10.1523/JNEUROSCI.21-23-09112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hong SL, et al. Dysfunctional Behavioral Modulation of Corticostriatal Communication in the R6/2 Mouse Model of Huntington’s Disease. PLoS One. 2012;7:e47026. doi: 10.1371/journal.pone.0047026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miller BR, Walker AG, Barton SJ, Rebec GV. Dysregulated Neuronal Activity Patterns Implicate Corticostriatal Circuit Dysfunction in Multiple Rodent Models of Huntington’s Disease. Front Syst Neurosci. 2011;5:26. doi: 10.3389/fnsys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Andre VM, Fisher YE, Levine MS. Altered Balance of Activity in the Striatal Direct and Indirect Pathways in Mouse Models of Huntington’s Disease. Front Syst Neurosci. 2011;5:46. doi: 10.3389/fnsys.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gu X, et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 171.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 172.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 173.Day M, et al. Selectiveelimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 174.Orieux G, Francois C, Feger J, Hirsch EC. Consequences of dopaminergic denervation on the metabolic activity of the cortical neurons projecting to the subthalamic nucleus in the rat. J Neurosci. 2002;22:8762–8770. doi: 10.1523/JNEUROSCI.22-19-08762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wilson CJ, Bevan MD. Intrinsic dynamics and synaptic inputs control the activity patterns of subthalamic nucleus neurons in health and in Parkinson’s disease. Neuroscience. 2011;198:54–68. doi: 10.1016/j.neuroscience.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol. 2012;11:429–442. doi: 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- 177.Li Q, et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron. 2012;76:1030–1041. doi: 10.1016/j.neuron.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 178.Mueller K, Jech R, Schroeter ML. New England Journal of Medicine. 2013. Deep brain stimulation for Parkinson’s disease: letter to the editor; pp. 482–483. [DOI] [PubMed] [Google Scholar]
- 179.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 181.Schmidt EF, et al. Identification of the cortical neurons that mediate antidepressant responses. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 183.Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 184.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Woodworth MB, Custo Greig L, Kriegstein AR, Macklis JD. SnapShot: Cortical Development. Cell. 2012;151:918–918 e911. doi: 10.1016/j.cell.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shim S, Kwan KY, Li M, Lefebvre V, Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sohur US, Padmanabhan HK, Kotchetkov IS, Menezes JR, Macklis JD. Anatomic and Molecular Development of Corticostriatal Projection Neurons in Mice. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harwell CC, et al. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron. 2012;73:1116–1126. doi: 10.1016/j.neuron.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Courchet J, Polleux F. Sonic hedgehog, BOC, and synaptic development: new players for an old game. Neuron. 2012;73:1055–1058. doi: 10.1016/j.neuron.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 190.Tomassy GS, et al. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci U S A. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16:25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 192.Alfano C, Studer M. Neocortical arealization: Evolution, mechanisms and open questions. Developmental Neurobiology. 2012 doi: 10.1002/dneu.22067. [DOI] [PubMed] [Google Scholar]
- 193.Groh A, et al. Cell-Type Specific Properties of Pyramidal Neurons in Neocortex Underlying a Layout that Is Modifiable Depending on the Cortical Area. Cereb Cortex. 2010;20:826–836. doi: 10.1093/cercor/bhp152. [DOI] [PubMed] [Google Scholar]
- 194.Fetz EE. Operant conditioning of cortical unit activity. Science. 1969;163:955–958. doi: 10.1126/science.163.3870.955. [DOI] [PubMed] [Google Scholar]
- 195.Yin HH, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Degos B, Deniau JM, Le Cam J, Mailly P, Maurice N. Evidence for a direct subthalamo-cortical loop circuit in the rat. Eur J Neurosci. 2008;27:2599–2610. doi: 10.1111/j.1460-9568.2008.06229.x. [DOI] [PubMed] [Google Scholar]