Abstract
Purpose
The purpose of this pilot study is to investigate the utility of, and areas of refinement for, digital photography as an educational tool for food logging in obese patients with type 2 diabetes (T2DM).
Methods
Thirty-three patients aged 18-70 with T2DM, BMI at least 30 kg/m2, and A1C 7.5-9% were recruited from an endocrinology clinic and randomized to a week of food logging using a digital camera (DC) or paper diary (PD), crossing over for week two. Patients then viewed a presentation about dietary effects on blood glucose, using patient DC and blood glucose entries. Outcomes of adherence (based on number of weekly entries), changes in mean blood glucose and frequency of blood glucose checks, and patient satisfaction were compared between methods. Patient feedback on the DC intervention and presentation was also analyzed.
Results
Thirty patients completed the study. Adherence was identical across methods. The mean difference in number of entries was not significant between methods. This difference increased and neared statistical significance (favoring DC) among patients who were adherent for at least one week (21 entries, with 2 entries per day for 5 of 7 days, n=25). Mean blood glucose did not significantly decrease in either method. Patient satisfaction was similar between interventions. Feedback indicated concerns over photograph accuracy, forgetting to use the cameras, and embarrassment using them in public.
Conclusion
Though comparable to PD in adherence, blood glucose changes, and patient satisfaction in this pilot trial, patient feedback suggested specific areas of refinement to maximize utility of DC-based food logging as an educational tool in T2DM.
Introduction
Educational interventions focusing on dietary modification in type 2 diabetes (T2DM) have been associated with improvements in nutritional knowledge,1, 2 quality of life,3 cholesterol profiles,4 glycemic control,2, 5, 6 and weight loss.2, 3 Dietary self-monitoring is often a component of these successful interventions, as it provides an opportunity for self-evaluation and motivation for changing behavior.7, 8 Historically, paper diaries (PD) have been the most common means of dietary self-monitoring.9 Strict adherence with paper diaries has only ranged from approximately 45 to 55 percent by 8 to 24 weeks,10–12 likely due to the fact that patients often find this method tedious and difficult to accurately maintain over time.12, 13 Recent studies have employed technology-based replacements for paper diaries, such as personal digital assistants (PDA), internet-based tools, and cellular phone applications.11, 14–17 These interventions have often shown improvements over paper diaries in adherence,11 patient satisfaction,15 glycemic control,15 and/or accuracy.16 However, they may still have some limitations. First, they are often costly to design or use, and may not be easily adapted for certain patient populations (including the elderly and poorly educated, who may have difficulty reading, using, and accessing the technology).18, 19 Second, where these methods often utilize written or verbal feedback to augment understanding and self-evaluation, visual feedback may be preferable. Visual feedback may improve many patients’ abilities to recall prior lessons for use in future situations.20 In addition, self-regulatory and social cognitive theories suggest that for an educational tool to influence sustained behavioral change, patients need to a) see pathways for improvement through self-evaluation within concrete settings, and b) see a potential relationship of the observed behavior with downstream consequences.21, 22 Digital camera (DC) photographs for food logging, when linked to post-meal blood glucose readings, may provide powerful, concrete opportunities for self-evaluation of dietary effects on blood glucose for patients with diabetes. Results from previous studies show that combining images with written or verbal information (such as DC-based food logging and corresponding blood glucose values) results in increased retention,23, 24 understanding,25, 26 and future problem solving23, 24, 26 compared to written or verbal information alone. Despite a theoretical basis and promising results from these studies, digital photography for food logging has not been thoroughly studied in the obese patients with T2DM. A short 10-patient pilot study in patients with T2DM suggested that patient satisfaction and comfort with photography-based food logging is high.27 Small studies in general adult and pediatric populations similarly lend support that photography is preferable,28, 29 of comparable accuracy,28, 30–32 reliable,33 and easier to use27, 28, 34 compared to traditional food logging methods such as paper diaries.
The present pilot study aimed to assess the utility of digital camera-based food logging as a patient educational tool by first examining a) weekly adherence, b) associations with changes in mean weekly blood glucose and frequency of glucose checks, and c) patient satisfaction, using paper diaries for comparison.. Second, the authors aimed to understand possible areas of refinement for the DC intervention by analyzing participant feedback on a) their experiences with the digital cameras, and b) a presentation on the effects of diet on blood glucose values using patients’ digital photographs and pre- and post-meal blood glucose values.
Methods
Research Design
This was a 2-week, single-center, randomized crossover pilot study. Eligible patients who completed enrollment procedures were randomly assigned to a week-long DC or PD intervention first, crossing over to the other intervention for the second week. The main advantage of the crossover design is that each subject serves as his or her own control, obviating the need for a separate control group. This eliminates potentially problematic variability between groups and decreases the necessary sample size.35 The primary disadvantage is a potential “carry-over,” or treatment sequence effect, which can be addressed via a wash out period or by analytic methods (employed in this study).36
Recruitment Procedures
Patients were recruited from one university-based endocrinology clinic in Ann Arbor, Michigan, which is staffed by physicians, podiatrists, nutritionists, and diabetes educators. The patient population served by the clinic is racially, ethnically, and socioeconomically diverse. The research protocols were approved by the University of Michigan Institutional Review Board, and all participants provided written informed consent. From January through March 2013, adult patients aged 18-70 with T2DM and an upcoming routine clinic visit were initially identified through electronic medical records. Patients were approached if they met the following additional inclusion criteria: (1) body mass index of at least 30 kg/m2, and (2) an A1C between 7.5 and 9% at the current clinic visit or within the past 6 weeks if not measured. Exclusion criteria were (1) pregnancy, (2) no or limited internet access (to complete surveys on each intervention), and (3) severe physical or psychiatric conditions precluding adherence to the study protocol.
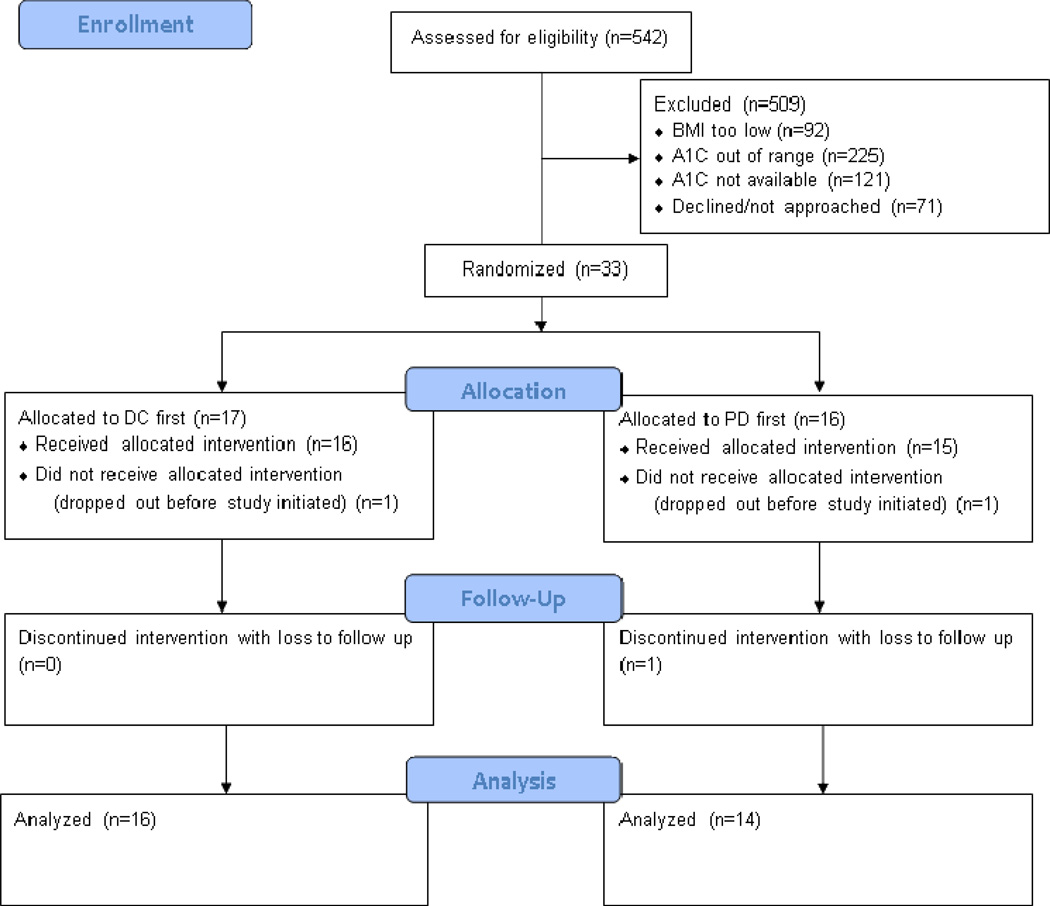
As shown in figure 1, 542 patients were identified by electronic medical records as potentially eligible. Of these, 438 patients did not meet the additional inclusion criteria, either due to out of range or unavailable BMI (n=92) or A1C (n=346). An additional 71 patients met all inclusion criteria but declined or were unable to be approached. In all, 33 eligible patients completed enrollment procedures and were randomly assigned to the DC (n=17) or PD (n=16) intervention first.
Figure 1.
Digital Camera Intervention
Patients were provided with a ViviCam 46™ (Vivitar, Edison, NJ) digital camera for one week of the study. This camera was selected because of its affordability (Manufacturer’s Suggested Retail Price approximately $25) and simple interface. During the appropriate week, patients were instructed to take pictures from approximately two feet away of all meals and snacks before consumption, and to verify that each picture turned out clearly before consuming the meal. Patients tested the camera before beginning the study and were able to contact the authors for troubleshooting. Memory cards and batteries were also provided, allowing patients to use the camera as much as was necessary to log all meals and snacks for the week. Time stamps were automatically recorded for each picture.
Paper Diary Intervention
Patients were also provided with several paper diary forms for the other study week. Patients were instructed to record all meals and snacks within circles representing “plates.” They also filled out the day of entry on each page and the time of consumption under each plate. The authors provided patients with detailed examples before study initiation and ensured that all of their questions were answered.
Across both study weeks, patients were told to continue checking their blood glucose in their usual fashion. Fifty glucose testing strips compatible with their meter were provided to each patient to ensure adequate supply for the study. They were told that the values and timing of their blood glucose readings may be used with their food entries in an anonymous educational opportunity at the end-of-study presentation (see below).
Digital Camera Presentation
After completion of the two study weeks, patients returned to the endocrinology clinic to view a one hour presentation primarily focused on the effect of diet on blood glucose, given by an endocrinologist. The first component of the presentation included an overview of diabetes and diabetes care, including the role of dietary self-monitoring. The second component utilized patients’ anonymous meal and snack photographs, accompanied by pre- and post-meal blood glucose values. Representative photographs were considered for the presentation if they were accompanied by pre-meal and post-meal blood glucose readings within approximately two hours of meal consumption. A schematic of the slides used (with a photograph that was not taken by a patient) is shown in figure 2. The presentation also included well-balanced examples of meals and snacks with associated pre- and post-meal blood glucose values, as photographed by the patients.
Figure 2.
Measurement Strategy
Demographic and clinical characteristics were assessed at baseline from review of electronic medical record data and confirmed with each patient. Adherence rates for each DC and PD week were categorized as strong adherence (SA) if there were at least 21 entries (e.g, photographs or diary entries, depending on the week), with at least 2 entries per day for all 7 days. Mild adherence (MA) was defined as at least 21 entries, with at least 2 entries per day for 5 of 7 days. Overall adherence for each intervention was defined as the sum of those attaining SA or MA for the intervention. A minimal participation (MP) subgroup excluded patients who were not at last mildly adherent for one study week. The number of weekly entries across each week was collected and the following entries were excluded: blurry photographs, paper diary entries for meals that were 3 words or less, and entries that were only beverages (as patients were not explicitly instructed to do this). Short-term glycemic control was assessed by change in mean blood glucose values and frequency of blood glucose checks across three weeks: week 0 (the week before study initiation), study week 1, and study week 2.
After each of the two study weeks, patients completed an identical anonymous internet-based survey of 4 questions on a 9-point Likert scale, assessing patients’ satisfaction, enjoyment, perceived ease of use, and enthusiasm for continuing each intervention. Each survey also included a write-in section allowing patients to submit feedback on the intervention they had just completed. After the digital camera presentation, patients completed a third anonymous survey of 2 questions assessing understanding of presentation content and their perceived future utility of DC-based food logging, plus a write-in section.
Analytic Strategy
Randomization was carried out to achieve balance across the pre-treatment variables age, A1C, BMI, gender, and insulin use.37 Quantitative comparisons of interest between DC and PD interventions included adherence rates; mean weekly food entries, blood glucose and frequency of glucose checks; and Likert scale responses on satisfaction surveys. Adherence rates and mean differences in weekly entries between methods were compared using McNemar’s test for categorical outcomes and paired t-tests for continuous outcomes. To determine independent associations between each logging method and the outcomes of a) mean weekly blood glucose values (based on all readings recorded by patients) and b) frequency of blood glucose checks, multivariate analyses were conducted using generalized estimating equations38 to account for the repeated measures within subjects. These models also controlled for treatment sequence, and for the day within the week using a linear trend. The distributions of Likert scale survey responses for a given intervention were similar across the two study weeks, so summary statistics were generated by combining weekly responses for each question by intervention. To determine recurring themes and areas of refinement for the DC intervention, data from the write-in portion of the DC survey is presented as a qualitative analysis derived from coding of the feedback. Data from the post-presentation survey is presented as median and interquartile range of Likert score responses for the two questions plus illustrative comments submitted in the write-in portion. Statistical comparisons, when made, were conducted with ActivePython 2.7 (ActiveState Software, Vancouver, BC)39 and SAS version 9.3 (SAS Institute, Cary, NC)40 with two-sided tests at a 0.05 significance level.
Results
Characteristics of the study participants
Participants’ ages ranged from 31 to 70 years with a mean age of 55.8 years (SD=9.0 years). Fifteen (45.4%) of the 33 participants were women. Characteristics of participants in the DC-first group did not differ significantly from participants in the PD-first group (table 1). One patient each in the DC-first and PD-first group dropped out of the study before its initiation. One additional patient in the PD-first group dropped out at the beginning of the second week and was lost to follow-up, yielding a total of 16 patients in the DC-first group and 14 patients in the PD-first group whose data was available for paired analysis, as shown in the CONSORT41 diagram (figure 1).
Table 1.
Baseline characteristics of participants by treatment group
| Characteristica | Digital Camera Intervention (DC) first (n=17) |
Paper Diary Intervention (PD) first (n=16) |
|---|---|---|
| Age (mean ± SD) | 55.6 ± 8.1 | 55.9 ± 10.2 |
| Sex (% male ± SD) | 52.9 ± 0.5 | 56.3 ± 0.5 |
| A1C (% ± SD) | 8.2 ± 0.5 | 8.2 ± 0.4 |
| BMI (kg/m2 ± SD) | 36.3 ± 5.3 | 36.3 ± 5.9 |
| Taking insulin (% ± SD) | 82.4 ± 0.4 | 81.3 ± 0.4 |
Food Logging Adherence
As shown in table 2, overall adherence (SA+MA) was 60.6% for both methods. On average, participants recorded more weekly DC entries than PD entries, but the difference was not significant (24.0 DC vs. 21.5 PD, P=0.20). In subgroup analyses, this difference increased and approached statistical significance when only patients with SA for both weeks (n=8) and only patients with MP (n=25) were analyzed, respectively (31.0 vs. 25.3, P=0.07; 27.2 vs. 23.0, P=0.05).
Table 2.
Key pilot trial outcomes, by intervention
| Outcome | Digital Camera Intervention (DC) (n=30) |
Paper Diary Intervention (PD) (n=30) |
P valuea |
|---|---|---|---|
| Overall adherence (SA+MA, %) | 60.6 | 60.6 | 1.0 |
| Mean number of weekly entries (95% CI) | |||
| All participants | 24.0 (19.8, 28.2) | 21.5 (18.8, 24.2) | 0.20 |
| SA both weeks only (n=8) | 31.0 (22.1, 39.9) | 25.3 (21.2, 29.3) | 0.07 |
| Minimal participation (n=25) | 27.2 (23.3, 31.0) | 23.0 (20.2, 25.8) | 0.05 |
| Change in mean blood glucose from baseline (%, 95% CI) | −5.8 (−12.4, 1.5) | −5.0 (−11.3, 1.8) | 0.11b, 0.14b |
| Change in mean weekly frequency of blood glucose checks from baseline (%, 95% CI) |
53.0 (25.7, 86.4) | 51.1 (30.2, 75.4) | <0.0001b, <0.0001b |
Blood Glucose and Frequency of Blood Glucose Checks
Compared to baseline, mean weekly blood glucose values decreased under both methods, but the change was not statistically significant from baseline or between interventions (adjusted change for DC -5.8%, P=0.11; PD -5.0%, P=0.14). Both methods significantly increased frequency of blood glucose checks from baseline (adjusted change for DC 53.0%; PD 51.1%, P<0.0001 for both), but the extent of the change was similar between methods (table 2). Changes in mean weekly blood glucose and frequency of blood glucose checks were not significantly different between methods on subgroup analyses of SA and MP patients only (not shown).
Patient Satisfaction Scores
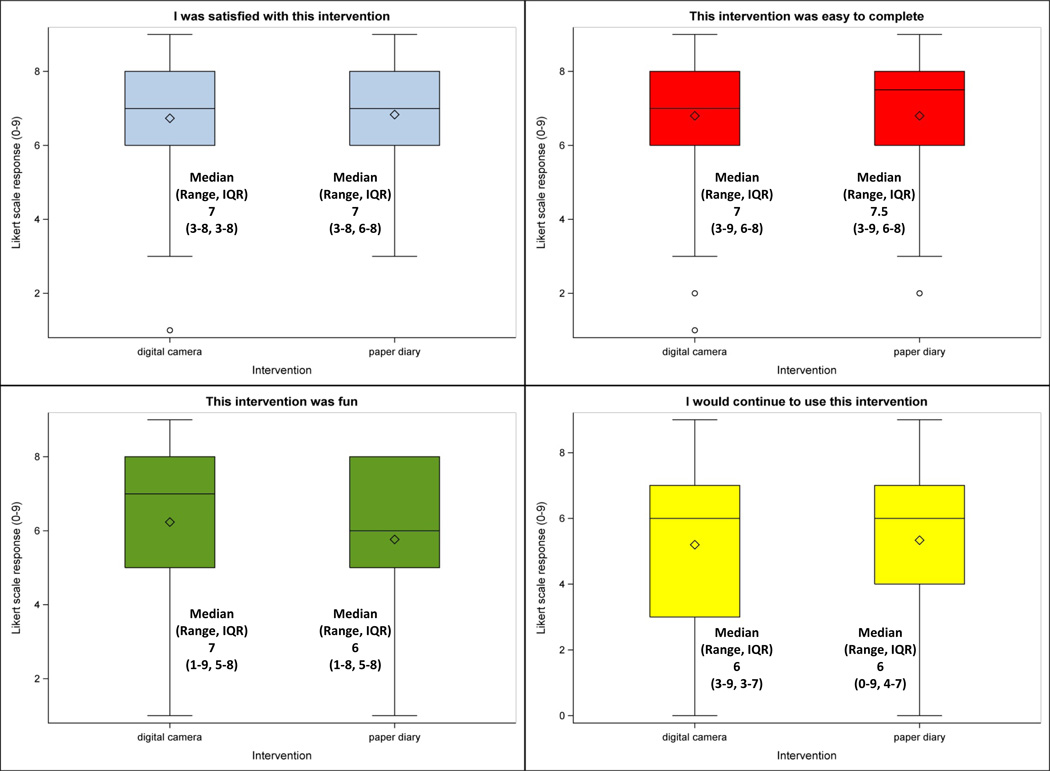
Quantitative results of the post-intervention patient satisfaction surveys are shown in figure 3. The median responses were similar for each question and across each intervention, and approximately corresponded to “somewhat agree” to “agree” (7 DC vs. 7 PD, 7 vs. 7.5, 7 vs. 6, and 6 vs. 6). Patients slightly favored the paper diaries for their ease of use (7 vs. 7.5) but slightly favored the digital cameras for which intervention was most fun (7 vs. 6). For most of the questions across each intervention, patients’ ranges of responses were consistent, with the exceptions of “I was satisfied with this intervention” (interquartile range 5 DC vs. 2 PD) and “I would continue to use this intervention” (range 6 vs. 9).
Figure 3.
Participant feedback for the DC Intervention
Table 3 shows themes recurring at least twice through the coded write-in comments on the DC surveys. Of the 20 comments submitted, the most common theme (n=7) was concern about accuracy of the photographs. Since patients were expected to take pictures before consuming the meal, many were concerned that this does not accurately represent the quantity actually eaten. There was also concern that, without ability to annotate the photograph, it is hard to depict the specific characteristics of the meal or snack (e.g., they may not receive credit for eating “low fat” or “light” foods). Forgetting to take photographs and forgetting the camera were also common experiences. The former seemed to be a particular problem with snacks, and the latter when dining out. Some participants also experienced embarrassment when using the digital cameras at a restaurant and difficulty using the camera.
Table 3.
Participant feedback for the digital camera intervention
| Theme | na | Examples |
|---|---|---|
| Concerned about accuracy (quantity actually eaten and/or content) | 7 | "One thing that appears to be missing, is a way to track what you actually eating. For example, one day for lunch I ordered a Turkey Rueben [sic] and fries. In the end, I only ate 1/2 of the sandwich and 1/2 of the fries." |
| "I did not know what to do if I photographed a meal and then did not eat it all." | ||
| "Pictures do not tell the whole story such as portion size, hidden condiments, healthiness of the food, or be unrecognizable as too [sic] what the food actually is." | ||
| "I do not feel that the pictures give an accurate portrayal of what a person is eating because you can't tell from a picture if someone used light food or fat free food or what." | ||
| Had camera but forgot to take pictures | 4 | "I found it difficult to take picture of absolutely everything that went into my mouth all the time." |
| "…[I]t was hard to take a picture of snacks. Sometime[s] I would grab a handful of something not even thinking about it." | ||
| Did not bring camera with them | 6 | "It is difficult to remember to take camera with me and to remember to take picture before eating." |
| "A couple times I…left the camara [sic] at home." | ||
| "… [It] is dificult [sic] to have the camera handy at all times due to variable schedule and location[s]." | ||
| "This might work if you used a camera such as one that you carried with you normally but it doesn't with one that is carried around as a special effort. Too hard to have it with you for every snack and when you eat out." | ||
| Embarrassment taking pictures while out | 2 | "I would never use the camara [sic] method. People are looking at you when you are out to eat." |
| "I felt really weird taking pichures [sic] of food in [r]estaurants." | ||
| Difficulty operating camera | 2 | "I found that sometimes, I took pictures of the surroundings when I was trying to review what the camera had taken." |
| "I had some issues with the supplied camera that caused me to miss one entry." |
Participant feedback for the DC Presentation
The median Likert scale responses to the two survey questions shown in table 4 corresponded to “agree.” Overall enthusiasm for the presentation was high. Comments demonstrated that many found the presentation to be helpful and motivating. Many comments during the presentation itself and on the survey called particular attention to the use of the photographs to demonstrate the effect of diet on blood glucose (figure 2).
Table 4.
Results and feedback for the presentation utilizing participants' digital photographs
| Question | Result(s) (n=29) |
|---|---|
| “I have a better understanding of healthy and unhealthy food choices after this presentation.” | 7 (6-8)a |
| "I think this presentation will help me make smarter food choices in the future." | 7 (6-8)a |
| Comments on the presentationb | "Presentation was very positive and informative." |
| "Great ideas in this presentation." | |
| "A good recap and hopefully it will recharge my desire to eat better." | |
| "I think there could have been more examples of good balanced snacks or meals." |
Conclusion
This preliminary pilot trial provides several important lessons on the potential utility of digital cameras as an educational tool for dietary self-monitoring in T2DM. The DC adherence rate, while identical to the PD intervention and consistent with other methods in the literature,10–12 was lower than expected for a short study. This may be explained, in part, by the participants’ feedback on the DC intervention. Patients, especially those who are overweight, are often concerned that their healthcare provider attributes excess weight or other health problems to personal flaws such as laziness and ignorance.42 Patients may feel that meal photographs, without an accompanying opportunity for further explanation of the food, may exacerbate this type of perceived judgment. Patients should therefore be given an opportunity to annotate their entries in a way that is sustainable for the long term. This may begin with encouraging elaboration of each entry during visits with their nutritionist, diabetes educator, or clinician, but using a voice-recorder may also be worthwhile. Such technology has already been developed and has shown encouraging results, based on the results of a small pilot study in Australia.27 Patients were not instructed to take post-consumption photographs for this study, as the focus was not to document or calculate intake. However, adding such photographs may allay patient concerns over pre-meal photographs being used in isolation, as well as provide additional educational opportunities on portion sizes of meals.
Some patients also felt they were being judged by the public when using the cameras at a restaurant. In addition, many patients forgot to bring their camera to restaurants in the first place. These findings suggest that a more subtle, easier to remember device should be considered. A cellular phone may be a viable option, as patients carry it with them regularly and most have photographing capabilities. This technology is also readily available and has been studied,17, 33 but not systemically in patients with T2DM. This adjustment, in addition to allowing patients to annotate their entries, may significantly increase their DC use. Based on the subgroup analyses presented, this may more conclusively demonstrate an increase in entries compared to paper diaries, and consequently maximize the potential benefits.
The changes in mean weekly blood glucose and frequency of blood glucose checks were comparable between DC and PD interventions. However, the unique ability of the digital cameras to provide visual and concrete feedback may at least partly explain the favorable response to the presentation. Feedback reinforced the potential power in using the patients’ digital photographs as a teaching strategy about the effect of food choices on post-meal blood glucose values. Such exercises should be an integral part of digital camera-based food logging in the future.
There did not appear to be a meaningful difference in patient satisfaction between interventions. This is in conflict with prior studies assessing patient satisfaction of camera-based versus paper diary-based food logging. 27–29, 34 This may be at least partly explained by the concerns voiced in patient feedback of the DC intervention. In addition, some prior pilot studies were shorter than the present study,27 which may not adequately allow patients to develop a complete opinion of the intervention.
The present study has important limitations. The sample size was small and the follow-up period was short. This study therefore may not allow generalizability for the entire population of patients with T2DM or for longer interventions. However, randomization was successful across various characteristics and there was no evidence of nonrandom drop-out across the methods, suggesting that the results are unlikely to be significantly biased. Second, the measurement of adherence in this study utilized criteria based on number of entries rather than weighted caloric intake. Previous studies have also used number of entries or “days used”-based criteria for adherence,10, 12 and accurately measuring caloric intake was not possible given limited resources. Given the heterogeneity in adherence definitions in the literature, caution should be taken when directly comparing adherence in this study to that in previous work. In addition, interpretation of the clinical endpoints in this trial, including adherence and effect on glycemic control, should be interpreted in the context of a preliminary, “proof of concept” pilot trial. The authors plan to conduct longer studies to re-assess logging behavior in a time frame consistent with previously published reports, as well as assess effect on glycemic control by more stable markers such as A1C. Finally, the quantitative components of the patient satisfaction surveys (e.g., Likert scale responses) were not compared using statistical testing because the surveys were erroneously anonymized before responses could be paired across weeks. In addition, there was one extra survey submitted after week one in the DC-first group, and it was unclear when the extra survey was filled out. The data presented in this report dropped the survey whose responses were closest to the mean for each question. However, alternatives such as dropping the last survey submitted and the most DC-favorable survey (to bias toward the null) yielded negligibly different results that would not have changed inferences.
Implications for Practice
Among obese patients with T2DM, DC-based food logging was comparable to PD-based food logging in terms of adherence, change in blood glucose and frequency of blood glucose checks, and patient satisfaction in this randomized, crossover pilot trial. However, patient subgroups that a) included only those who were strongly adherent for both study weeks, or b) excluded those who were not adherent for at least one week of the study—trended toward using DC for food logging more often. Patient feedback of the DC intervention and DC presentation provided several important areas of refinement in order to maximize the educational value and patient use of this novel intervention. Integration of methods to annotate photos and perhaps finding a more subtle and easy-to-remember medium for taking food photographs will likely decrease perceptions of judgment and embarrassment, as well as decrease the frequency of patients’ forgetting to record entries. To further prove and refine the concept of DC-based food logging in this population, larger trials with longer duration of follow up—including assessment of glycemic control markers before and after the presentation(s)—should be performed. It would also likely be worthwhile to gain more patient feedback through focus groups and interviews. In addition, future studies could address the usefulness of this approach as a component of diabetes self-management education or diabetes self-management support interventions to help patients understand the impact of food on blood glucose using concrete visual examples.
Acknowledgments
Financial Support: This work was funded by a Pilot Seed Grant awarded to BJE and LD from the Michigan Institute for Clinical and Health Research (MICHR), which is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2UL1TR000433. It also utilized the Michigan Center for Diabetes Translational Research funded by NIH P30DK092926 from the National Institute of Diabetes and Digestive and Kidney Diseases.
This work was funded by a Pilot Seed Grant awarded to BJE and LD from the Michigan Institute for Clinical and Health Research (MICHR), which is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2UL1TR000433. It also utilized the Behavioral, Clinical and Health Systems Research Core of the Michigan Center for Diabetes Translational Research funded by NIH P30DK092926 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The authors wish to thank Sonja Hughbanks for her administrative support.
Footnotes
Conflicts of Interest: None to disclose.
This work has been presented, in part, at the American Association of Clinical Endocrinologists Annual Meeting in Phoenix, AZ, May 1-5, 2013.
References
- 1.Kendall PA, Jansen GR. Educating patients with diabetes: comparison of nutrient-based and exchange group methods. J Am Diet Assoc. 1990 Feb;90(2):238–243. [PubMed] [Google Scholar]
- 2.Turnin MC, Beddok RH, Clottes JP, et al. Telematic expert system Diabeto. New tool for diet self-monitoring for diabetic patients. Diabetes Care. 1992 Feb;15(2):204–212. doi: 10.2337/diacare.15.2.204. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non-insulin-dependent diabetes mellitus. J Gen Intern Med. 1987 Jul-Aug;2(4):220–228. doi: 10.1007/BF02596443. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RM, Wilson DK, Hartwell SL, Merino KL, Wallace JP. Prospective evaluation of HDL cholesterol changes after diet and physical conditioning programs for patients with type II diabetes mellitus. Diabetes Care. 1985 Jul-Aug;8(4):343–348. doi: 10.2337/diacare.8.4.343. [DOI] [PubMed] [Google Scholar]
- 5.Franz MJ, Monk A, Barry B, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non-insulin-dependent diabetes mellitus: a randomized, controlled clinical trial. J Am Diet Assoc. 1995 Sep;95(9):1009–1017. doi: 10.1016/S0002-8223(95)00276-6. [DOI] [PubMed] [Google Scholar]
- 6.Hahn JM, Gordon DH. "Learn, taste, and share": a diabetes nutrition education program developed, marketed, and presented by the community. Diabetes Educ. 1998 Mar-Apr;24(2):153–154. doi: 10.1177/014572179802400204. 161. [DOI] [PubMed] [Google Scholar]
- 7.Burke LE, Warziski M, Starrett T, et al. Self-monitoring dietary intake: current and future practices. J Ren Nutr. 2005 Jul;15(3):281–290. doi: 10.1016/j.jrn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Kanfer FH. Self-monitoring: Methodological limitations and clinical applications. Journal of Consulting and Clinical Psychology. 1970;35(2):148–152. [Google Scholar]
- 9.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011 Jan;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obes Res. 1998 May;6(3):219–224. doi: 10.1002/j.1550-8528.1998.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 11.Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011 Feb;19(2):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eat Behav. 2009 Dec;10(4):220–227. doi: 10.1016/j.eatbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone A. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemp Clin Trials. 2008 Mar;29(2):182–193. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yon BA, Johnson RK, Harvey-Berino J, Gold BC, Howard AB. Personal digital assistants are comparable to traditional diaries for dietary self-monitoring during a weight loss program. J Behav Med. 2007 Apr;30(2):165–175. doi: 10.1007/s10865-006-9092-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsang MW, Mok M, Kam G, et al. Improvement in diabetes control with a monitoring system based on a hand-held, touch-screen electronic diary. J Telemed Telecare. 2001;7(1):47–50. doi: 10.1258/1357633011936138. [DOI] [PubMed] [Google Scholar]
- 16.Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr. 2008 Apr;27(2):280–286. doi: 10.1080/07315724.2008.10719701. [DOI] [PubMed] [Google Scholar]
- 17.Rusin M, Arsand E, Hartvigsen G. Functionalities and input methods for recording food intake: A systematic review. Int J Med Inform. 2013 Feb 13; doi: 10.1016/j.ijmedinf.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Demiris G, Finkelstein SM, Speedie SM. Considerations for the Design of a Web-based Clinical Monitoring and Educational System for Elderly Patients. Journal of the American Medical Informatics Association. 2001 Sep 1;8(5):468–472. doi: 10.1136/jamia.2001.0080468. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Or CKL, Karsh B-T. A Systematic Review of Patient Acceptance of Consumer Health Information Technology. Journal of the American Medical Informatics Association. 2009 Jul 1;16(4):550–560. doi: 10.1197/jamia.M2888. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MA, Horowitz TS, Wolfe JM. Auditory recognition memory is inferior to visual recognition memory. Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):6008–6010. doi: 10.1073/pnas.0811884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandura A. Health promotion from the perspective of social cognitive theory. Psychology & Health. 1998;13(4):623–649. 1998/07/01. [Google Scholar]
- 22.Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: A perceptual-cognitive approach. Psychology & Health. 1998;13(4):717–733. 1998/07/01. [Google Scholar]
- 23.Mayer R, Gallini J. When is an illustration worth ten thousand words? Journal of Educational Psychology. 1990;82(4):715–726. [Google Scholar]
- 24.Mayer R, Anderson R. The instructive animation: Helping students build connections between words and pictures in multimedia learning. Journal of Educational Psychology. 1992;84(4):444–452. [Google Scholar]
- 25.Mayer RE. Systematic thinking fostered by illustrations in scientific text. Journal of Educational Psychology. 1989;81(2):240–246. [Google Scholar]
- 26.Mayer R, Anderson R. Animations Need Narrations: An Experimental Test of a Dual-Coding Hypothesis. Journal of Educational Psychology. 1991;83(4):484–490. [Google Scholar]
- 27.Rollo ME, Ash S, Lyons-Wall P, Russell A. Trial of a mobile phone method for recording dietary intake in adults with type 2 diabetes: evaluation and implications for future applications. J Telemed Telecare. 2011;17(6):318–323. doi: 10.1258/jtt.2011.100906. [DOI] [PubMed] [Google Scholar]
- 28.Bird G, Elwood PC. The dietary intakes of subjects estimated from photographs compared with a weighed record. Hum Nutr Appl Nutr. 1983 Dec;37(6):470–473. [PubMed] [Google Scholar]
- 29.Higgins JA, LaSalle AL, Zhaoxing P, et al. Validation of photographic food records in children: are pictures really worth a thousand words? Eur J Clin Nutr. 2009 Aug;63(8):1025–1033. doi: 10.1038/ejcn.2009.12. [DOI] [PubMed] [Google Scholar]
- 30.Williamson DA, Allen HR, Martin PD, Alfonso AJ, Gerald B, Hunt A. Comparison of digital photography to weighed and visual estimation of portion sizes. J Am Diet Assoc. 2003 Sep;103(9):1139–1145. doi: 10.1016/s0002-8223(03)00974-x. [DOI] [PubMed] [Google Scholar]
- 31.Williamson DA, Allen HR, Martin PD, Alfonso A, Gerald B, Hunt A. Digital photography: a new method for estimating food intake in cafeteria settings. Eat Weight Disord. 2004 Mar;9(1):24–28. doi: 10.1007/BF03325041. [DOI] [PubMed] [Google Scholar]
- 32.Wang DH, Kogashiwa M, Ohta S, Kira S. Validity and reliability of a dietary assessment method: the application of a digital camera with a mobile phone card attachment. J Nutr Sci Vitaminol (Tokyo) 2002 Dec;48(6):498–504. doi: 10.3177/jnsv.48.498. [DOI] [PubMed] [Google Scholar]
- 33.Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, Anton SD. A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. Br J Nutr. 2009 Feb;101(3):446–456. doi: 10.1017/S0007114508027438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang DH, Kogashiwa M, Kira S. Development of a new instrument for evaluating individuals' dietary intakes. J Am Diet Assoc. 2006 Oct;106(10):1588–1593. doi: 10.1016/j.jada.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Melot C. New designs for clinical trials. Crit Care Med. 2009 Jan;37(1 Suppl):S59–S64. doi: 10.1097/CCM.0b013e318192111a. [DOI] [PubMed] [Google Scholar]
- 36.Senn S. Cross-over trials in Statistics in Medicine: the first '25' years. Stat Med. 2006 Oct 30;25(20):3430–3442. doi: 10.1002/sim.2706. [DOI] [PubMed] [Google Scholar]
- 37.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975 Mar;31(1):103–115. [PubMed] [Google Scholar]
- 38.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986 Apr 1;73(1):13–22. 1986. [Google Scholar]
- 39.ActivePython [computer program] Version 2.7. Vancouver: ActiveState Software Inc. 2011 [Google Scholar]
- 40.SAS [computer program] Version 9.3. Cary: SAS Institute Inc; 2011. [Google Scholar]
- 41.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001 Apr 17;134(8):657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MB, Chambliss HO, Brownell KD, Blair SN, Billington C. Weight bias among health professionals specializing in obesity. Obes Res. 2003 Sep;11(9):1033–1039. doi: 10.1038/oby.2003.142. [DOI] [PubMed] [Google Scholar]