Abstract
Charles Darwin, while trying to devise a general theory of heredity from the observations of animal and plant breeders, discovered that domesticated mammals possess a distinctive and unusual suite of heritable traits not seen in their wild progenitors. Some of these traits also appear in domesticated birds and fish. The origin of Darwin’s “domestication syndrome” has remained a conundrum for more than 140 years. Most explanations focus on particular traits, while neglecting others, or on the possible selective factors involved in domestication rather than the underlying developmental and genetic causes of these traits. Here, we propose that the domestication syndrome results predominantly from mild neural crest cell deficits during embryonic development. Most of the modified traits, both morphological and physiological, can be readily explained as direct consequences of such deficiencies, while other traits are explicable as indirect consequences. We first show how the hypothesis can account for the multiple, apparently unrelated traits of the syndrome and then explore its genetic dimensions and predictions, reviewing the available genetic evidence. The article concludes with a brief discussion of some genetic and developmental questions raised by the idea, along with specific predictions and experimental tests.
A major gap in Charles Darwin’s theory of evolution, as presented in the first edition of The Origin of Species (Darwin 1859), was the absence of a theory of heredity. As Darwin knew, his theory of evolution required a distinct idea of how biological heredity worked, but in 1859 he was not prepared to offer one. His attempt to fill this gap came subsequently, in his massive, detailed study of inheritance, The Variation of Plants and Animals under Domestication (Darwin 1868). Written decades before there was a science of genetics, it relied primarily on the data produced by animal and plant breeders, hence on observations of domesticated animals and plants.
Darwin’s encyclopedic investigation of domesticated species revealed an intriguing phenomenon. From his survey of the animal breeding work, he found that domesticated mammals in general exhibit a suite of behavioral, physiological, and morphological traits not observed in their wild forebears. Today, the full set of these characteristics is known to include: increased docility and tameness, coat color changes, reductions in tooth size, changes in craniofacial morphology, alterations in ear and tail form (e.g., floppy ears), more frequent and nonseasonal estrus cycles, alterations in adrenocorticotropic hormone levels, changed concentrations of several neurotransmitters, prolongations in juvenile behavior, and reductions in both total brain size and of particular brain regions. The consistency of this extremely diverse set of phenotypic changes in domesticated mammals presents a major puzzle, as Darwin recognized. The suite seems to reflect something about the process of domestication per se, a conclusion strengthened by the finding that domesticated birds and even fish share some components of this spectrum of traits. Because Darwin published these findings just a few years after Mendel published his work, the hereditary basis of this phenomenon constitutes one of the oldest problems in genetics.
The general combination of traits in domesticated mammals is an ensemble that we will refer to as the “domestication syndrome” (DS) (adopting a term used for domesticated crop plants, e.g., Brown et al. 2008). We list its core components in Table 1. In this article, we will present a new hypothesis about the nature and origin of the DS, proposing that the unifying feature underlying its diverse traits is their shared developmental connection via neural crest cells, the multipotent stem cells that arise in vertebrate embryos from the dorsal part of the neural tube. After a brief review of previous thinking, we present our hypothesis and discuss how it can explain the main features of the syndrome. We then explore the genetic implications of the idea, including a brief look at some intriguing developmental, evolutionary, and genetic questions that the idea raises. We end with some predictions and a discussion of experiments and analyses to test the hypothesis.
Table 1. List of traits modified in the “domestication syndrome” in mammals*.
| Trait | Animal species | Location/source | References |
|---|---|---|---|
| Depigmentation (especially white patches, brown regions) | Mouse, rat, guinea pig, rabbit, dog, cat, fox, mink, ferret, pig, reindeer, sheep, goat, cattle, horse, camel, alpaca, and guanaco | Cranial and trunk | a |
| Floppy ears | Rabbit, dog, fox, pig, sheep, goat, cattle, and donkey | Cranial | b |
| Reduced ears | Rat, dog, cat, ferret, camel, alpaca, and guanaco | Cranial | c |
| Shorter muzzles | Mouse, dog, cat, fox, pig, sheep, goat, and cattle | Cranial | d |
| Smaller teeth | Mouse, dog, and pig | Cranial | e |
| Docility | All domesticated species | Cranial | f |
| Smaller brain or cranial capacity | Rat, guinea pig, gerbil, rabbit, pig, sheep, goat, cattle, yak, llama, camel, horse, donkey, ferret, cat, dog, and mink | Cranial | g |
| Reproductive cycles (more frequent estrous cycles) | Mouse, rat, gerbil, dog, cat, fox, goat, and guanaco | Cranial and trunk (HPG axis) | h |
| Neotenous (juvenile) behavior | Mouse, dog, fox, and bonobo | Cranial | i |
| Curly tails | Dog, fox, and pig | Trunk | j |
*Relative to those in the corresponding presumed wild ancestors.
Previous Thinking About the Genetic Basis of the Domestication Syndrome
There is no shortage of ideas about the nature of the domestication process and its effects on various domesticated animal species and breeds. Most of the explanations proposed, however, do not attempt to account for the full spectrum of the DS but instead concentrate on isolated elements, such as coat color changes or muzzle size, positing particular selective pressures imposed by humans that might have fostered those traits. If one further excludes specific traits deliberately selected in particular domesticated animals, such as increased meat or milk production in cattle, egg numbers in poultry, or wool quality in sheep, the number of genetic explanations for the traits of domesticated animals falls off even further. The few general ideas proposed in recent years have focused on possible general selective features that might pertain to living in captivity rather than attempting to provide genetic or mechanistic explanations for the syndrome (for example, Hemmer 1990; Leach 2003).
Darwin (1868) himself suggested two possible explanations. The first and most general was that the gentler “conditions of living” under domestication, in particular the improved diets provided to domesticated animals, induced these traits in some manner. He could not specify why precisely this should be the case for all of the traits seen nor why others were not produced. Furthermore, it was unclear how much of the DS was, in effect, induced environmentally in every generation, and how much had become hereditary. Nevertheless, it was a hypothesis and it made a prediction: that domesticated animals released into the wild would lose these traits over time. The evidence from escaped domesticated animals that he assessed was mixed and complicated by both the decreased survival rates of domesticates in the wild and, in some cases, by their interbreeding with wild relatives. Contemporary evidence clearly challenges the “conditions of living” hypothesis. For example, feral domesticates retain their small brains after as many as 40 generations back in the wild (Kruska 2005).
Darwin’s second explanation, which he offered more tentatively, was that the domestication-associated traits are a product of hybridization of different breeds or, even, related species. This is an interesting idea since it is true that hybridization can create novel properties, but it still does not explain why the particular traits of the DS and not others are seen in multiple species. Furthermore, the hybridization hypothesis has been experimentally tested and disproved as a general explanation in several species by rerunning the domestication process from essentially wild single stock progenitors, with no hybridization, and obtaining the DS nonetheless. This body of experimental domestication research has provided crucial information about the nature of the DS because it overcomes the difficulty of disentangling the initial genetic changes in the domestication of many breeds, which took place centuries or millennia ago, from the many later (and more specific) genetic changes resulting from subsequent bouts of selection (e.g., for meat, eggs, wool, etc.).
The first set of experimental domestication studies involved rats (King and Donaldson 1929; Castle 1947, as reviewed in Arbuckle 2005) but the most extensive body of work involves foxes (Vulpes vulpes), in a major research project initiated in 1959 by Dimitry K. Belyaev, in Novosibirsk, in the then Soviet Union. This experimental domestication procedure involved selective breeding, starting from effectively wild foxes, which had been obtained from fur farms where no attempt at domestication had been made. The protocol involved intensive selective breeding solely for increasing degrees of docility and tameness in successive generations, by selecting the tamest kits at each stage and then breeding only those. At each generation, the foxes were methodically examined for behavioral and/or morphological changes. After more than 50 years of selective breeding, this ongoing research program, now led by Lyudmilla Trut, has shown that the full suite of traits constituting the DS can arise relatively quickly under selection for tameness alone, in a species having no prior history of domestication. Analogous results have been found in rats and mink, under the same selective regime, by the same research group. For an early discussion, see Belyaev (1969), for recent reviews see Trut et al. (2004, 2009). This work established that the DS is truly a package of traits that develop semiconcurrently, not an assemblage of independently or sequentially acquired ones.
Following on from Darwin’s suggestion that the source of the changes in domesticated animals was ultimately in the gentler “conditions of living” provided by domestication, Belyaev (1979) argued that reduced stress levels in animals living in a protected anthropogenic environment caused multiple changes in hormonal responses and that these reset patterns of gene expression. In current terminology, he was proposing that the initial changes were epigenetic but that, with time, they became hereditable, fixed genetic changes. More recently, Trut and her colleagues have suggested that there is a single genetic regulatory network (GRN) underlying the traits of the DS and that the syndrome results from alterations in fairly “upstream” regulators, leading to dysregulation of downstream genetic modules involved in the development of the different tissue types affected in the DS (Trut et al. 2004). Those changes in upstream regulators could either be stable (germ-line transmissible) epimutations or true (genetic) mutations. In principle, this explanation can account for the diversity of traits exhibited in the DS.
There are two possible problems with this latter hypothesis, however. The first is the extended phenotypic domain of the proposed GRN, making it larger than any previously characterized. Currently known GRNs are dedicated to governing something simpler or smaller, such as demarcating specific regions of the early embryo (Davidson et al. 2002; Levine and Davidson 2005) or the development of particular structures such as insect wings (Abouheif and Wray 2002) or the mammalian pancreas (Poll et al. 2006). The second problem is that it posits upstream mutations (or epimutations) in the hypothesized network with dramatic, widespread effects, but which are not lethal. Although the higher levels of prenatal mortality seen among domesticated foxes relative to control, farm-bred animals indicates mild deleterious effects (L. Trut, personal communication), this is not to the extent that might be expected for disabling mutations in a large and early-acting GRN. Thus this explanation from the Novosibirsk group, postulating a single network that directly controls all the traits affected by the DS, is not without problems.
The Hypothesis: A Critical Role for the Neural Crest
Here, we present a new hypothesis that accounts in a straightforward and unified fashion for the disparate traits making up the domestication syndrome. Like previous researchers (e.g., Darwin 1875; Belyaev 1974; Trut 1999), we begin with the assumption that a primary selective pressure during the initial stages of domestication is on behavior, and in particular for tameness (lack of fearful or aggressive responses to human caretakers). Such a reduction in acute fear and long-term stress is a prerequisite to successful breeding in captivity (Darwin 1875; Belyaev 1974). Like the Novosibirsk group (Trut et al. 2009), we trace the mechanistic basis of tameness to reduced size and function of the adrenal glands, which play a central role in the physiology of both fear and stress responses. Adrenal hypofunction and reduced stress hormone levels are well documented in domesticated species and have been induced experimentally by selection for tameness during experimental domestication of foxes and rats (details below).
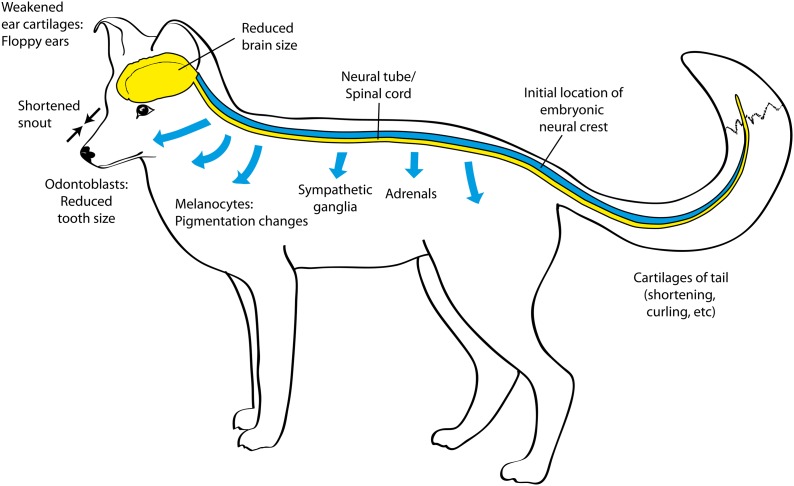
But the DS as a whole, with its diverse array of affected morphological traits, clearly cannot be caused simply by alterations of adrenal function. What, therefore, might be the common factor? What all of these diverse traits, including the adrenals, share is that their development is closely linked to neural crest cells (NCCs). NCCs are the vertebrate-specific class of stem cells that first appear during early embryogenesis at the dorsal edge (“crest”) of the neural tube and then migrate ventrally throughout the body in both the cranium and the trunk, giving rise to the cellular precursors of many cell and tissue types and indirectly promoting the development of others (Carlson 1999; Hall 1999; Gilbert 2003; Trainor 2014). The NCC-derived tissues include much of the skull, the sympathetic ganglia, the adrenal medulla, pigment-related melanoblasts in both head and trunk, and tooth precursors (odontoblasts). In the head specifically, cranial neural crest cells (CNCCs) are crucial precursors of bony, cartilaginous, and nervous components of the craniofacial region, including the jaws, hyoid, larynx, and external and middle ears. Although NCCs are not direct precursors of any part of the central nervous system (CNS) or of the adrenal cortex, they play important roles in the development of these tissues via postmigratory embryological interactions. In Figure 1, we indicate schematically the body sites affected in the DS and the routes of migration of NCCs.
Figure 1.
Developmental schematic of the “domestication syndrome” in relation to the neural crest. The blue tube indicates the approximate position of the neural crest in the early embryo, and the blue arrows indicate pathways of neural crest cell migration.
In the light of these well-established embryological observations, we posit that the multiple phenotypic changes characterizing the DS reflect a developmental reduction in neural crest cell input to all of these affected traits. In this view, the developmental source of the DS is singular, with the apparent diversity of phenotypic changes having a common foundation in the fact that all affected tissues are neural crest derivatives or influenced in their development by neural crest. In contrast to that unity of developmental cause, we also argue that the genetic changes underlying neural crest reduction are diverse and involve multiple genetic changes of moderate, quantitative effect. Our hypothesis is, to our knowledge, the only unified, mechanistically grounded explanation for all of the traits of the syndrome (though for a possible general role of the thyroid gland, see Crockford 2000).
In a nutshell, we suggest that initial selection for tameness leads to reduction of neural-crest-derived tissues of behavioral relevance, via multiple preexisting genetic variants that affect neural crest cell numbers at the final sites, and that this neural crest hypofunction produces, as an unselected byproduct, the morphological changes in pigmentation, jaws, teeth, ears, etc. exhibited in the DS. The hypothesized neural crest cell deficits in the DS could be produced via three routes: reduced numbers of original NCC formed, lesser migratory capabilities of NCC and consequently lower numbers at the final sites, or decreased proliferation of these cells at those sites. We suspect, however, that migration defects are particularly important. In this view, the characteristic DS phenotypes shown in parts of the body that are relatively distant from the sites of NCC origination, such as the face, limb extremities, tail, and belly midline, reflect lower probabilities of NCC reaching those sites in the requisite numbers. The stochastic, individual-to-individual variability in these pigmentation patterns is consistent with this idea.
A wide range of genes are known to play crucial roles in neural crest specification, migration, and postmigratory interactions. Given the biological importance of NCC-derived tissues, it is unsurprising that knockouts of these genes are frequently lethal in the homozygous organism, and often severely debilitating even in heterozygotes. Such conditions have long been known, and are given the generic designation “neurocristopathies” in medicine (Bolande 1974). In contrast to these pathologies, no genetic evidence indicates that the changes seen in domesticated animals are the result of mutations in any one specific “domestication” gene. Rather, the phenotypic changes seen in domesticates are quantitative, nonpathological, and of mainly moderate importance. Correspondingly, QTL analyses comparing experimentally domesticated rats and foxes with their respective wild-type progenitors typically implicate dozens of alleles of moderate effect (Trut et al. 2004; Albert et al. 2009, 2011; Kukekova et al. 2010) as do recent dog–wolf genetic comparisons (Vonholdt et al. 2010). Finally, experimental domestication studies show that responses of wild-type founder populations to selection for tameness are very rapid, even under outbreeding conditions, strongly suggesting that multiple existing alleles contribute to the response to selection, rather than new mutations or accumulation of recessive alleles to give homozygosity for those alleles (Trut et al. 2004, 2009). All of this is consistent, therefore, with the idea that the underlying genetic causation is polygenic and involves multiple alleles of relatively individually small effect, across many different genes. We expect that dozens of genes, all influencing neural crest development, migration, and interactions, provide the underlying genetic basis for the NCC hypofunction that, by our hypothesis, is at the root of the disparate set of features of the DS. Table 2 shows a sampling of these genes.
Table 2. Neural crest cell genes: Dosage effects and genetic interaction characteristics.
| Gene | Biochemical-molecular function | Organisms studied | Haploinsufficient effects | Genetic interactions w/ other NCC genes | Some references |
|---|---|---|---|---|---|
| Sox 10 | Transcription factor | Zebrafish, mouse, and human | + | + | Dutton et al. (2001); Stanchina et al. (2006) |
| Sox 9 | Transcription factor | Zebrafish, mouse, and human | + | + | Liu et al. (2013); Sahar et al. (2005) |
| Mitf | Transcription factor | Mouse, horse, and human | + | + | Hauswirth et al. (2012); Reissman and Ludwig (2013); Tachibana (2000) |
| Tcof1 | Nucleophosphoprotein | Human and mouse | + | + | Barlow et al. (2013); Shows and Shiang (2008) |
| FoxD3 | Transcription factor | Mouse and quail | ND | + | Thomas and Erickson (2009) |
| Pax3 | Transcription factor | Human, mouse, and horse | + | + | Epstein et al. (1993); Hauswirth et al. (2012); Moase and Trasler (1989) |
| Sox2 | HMG-transcription factor | Human and mouse | + | + | (Adameyko et al. 2012; Langer et al. 2013) |
| Chd7 | ATP-requiring chromatin remodeller protein | Human and mouse | + | + | Baipai et al. (2010) |
| Kit | Receptor protein tyrosine kinase | Human, mouse, horse, and dog | + | + | Fleischmann et al. (1991); Haase et al. (2009); Reissman and Ludwig (2013) |
| Magoh | Exon junction complex component | Human and mouse | + | + | Silver et al. (2013) |
| WSTF | Transcription factor | Human and mouse | + | ND | Barnett et al. (2012) |
| Fgf8 | Growth factor/signal transduction ligand | Human and mouse | + | ND | Frank et al. (2002) |
| piebald (s) | Endothelin-3 (ET-3) | Human and mouse | + | + | Robertson and Mason (1997); Stanchina et al. (2006) |
| piebald (l) | Endothelin-receptor B (ENDR-B) | Human and mouse | + | + | Pavan et al. (1995); Robertson and Mason (1997); Stanchina et al. (2006) |
| Ret | Receptor tyrosine kinase | Human, mouse, and rat | + | + | Borrego et al. (2013) |
| GDNF | Glial-derived neurotrophic factor | Zebrafish, mouse, and rat | + | ND | Flynn et al. (2007) |
NCC, neural crest cell; ND, not determined.
To this point, our argument has largely relied on correlations between aspects of the DS and neural crest cell behavior and genetics. Beyond such correlations, however, there is a considerable body of clinical and experimental work that provides direct empirical support for the hypothesis. We now briefly review this literature, starting with visible morphological changes and then turning to matters of behavior and neurobiology.
Morphological Components of the Domestication Syndrome
Pigmentation changes in domestication
Pigmentation changes from wild type are one of the most striking and consistent changes during domestication. All breeds of domesticated animals show areas of relative depigmentation in their fur coats, often as white spots or larger areas of the pelage, sometimes as brown patches (Darwin 1875; Belyaev 1974). Pigmentation changes represent one of the first traits to appear during the domestication of foxes, mink (Mustela vison) and rats (Rattus norvegicus) selected for tameness (Trut 1999; Trut et al. 2009). In these animals, the depigmented areas typically consist of irregular white patches found in preferred sites: just below the throat and above the eyes, the paws, and the tip of the tail. The neural crest cell connection here is expected, because such white areas usually lack melanocytes and melanocytes derive from neural crest cells. Because vertebrate pigmentation cells (chromatophores and melanocytes) derive from neural crest (Hall 1999; Gilbert 2003), these pigmentation changes are clearly generally consistent with our hypothesis. In general, areas of the body receiving delayed migration of NCCs are vulnerable to being depigmented (Jackson 1994; Yamaguchi et al. 2007; Mills and Patterson 2009).
Because pigmentation effects have been carefully studied and are relatively well understood, they provide a rich source of specific data, all of which (to our knowledge) is consistent with the neural crest hypothesis for DS. At least 125 genes are known to affect pigmentation in some way, at least 25 involving neural crest development and migration (Yamaguchi et al. 2007), many of which appear in Table 2, below. This extensive literature makes clear that the genetic basis for pigmentation changes is complex, with prominent pleiotropic and epistatic effects (cf. Reissman and Ludwig 2013). This provides one key basis for our argument that neural crest hypofunction in domestication does not result from one or a few genetic changes of large effect.
Our contention that the effects of any single gene mutation on neural crest derivatives depend on its interactions with a host of other genes is clearly supported by the variable clinical picture associated with Waardenburg syndrome. Waardenburg’s is a relatively common but highly variable genetic disease, the most common variants of which result from mutations in the Pax3 gene. Waardenburg syndrome is subdivided into four types, but the overall effects include the primary signs (deafness and pigmentation changes, particularly a white forelock) along with many other more variable symptoms, including white skin patches, cranial dysmorphology, very pale eyes, and absence of the enteric nervous system (Hirschsprung’s disease). All of these variable symptoms indicate disrupted NCC-related development. Neural-crest-derived melanocytes provide a required input to the inner ear, without which deafness results (cf. Jackson 1994), and the ganglia and neurons of the enteric nervous system, like the sympathetic nervous system, are derived from neural crest (Hall 1999; Gilbert 2003). The variable symptoms of Waardenburg syndrome are thus both consistent with our hypothesis and indicative of the complex and epistatic nature of genes affecting neural crest.
An example of multigene interactions in neural crest function is provided by the case of Splotch-delayed mutant mice studied as a possible murine model of Waardenburg syndrome (Asher et al. 1996). To better understand the genetic cause of the reduced penetrance and variable expressivity of mutant Pax3 alleles, Asher and colleagues started with the Pax3 mutation Splotch-delayed (Spd). This mutation originated in the highly inbred B6 mouse strain, where it leads to consistent pigmentation defects in otherwise normal heterozygotes (primarily a white belly “splotch”). But when this strain was crossed with Mus spretus and then those F1 backcrossed into the B6 mouse background, the Spd allele produced highly variable phenotypic effects, including both a range of pigmentation defects and, crucially for our hypothesis, cranial dysmorphologies such as reduced muzzle dimensions. The analysis showed that, regarding cranial changes, Pax3 interacts with at least two other genes, agouti and an unidentified sex-linked locus. The agouti locus is a key determinant of coat color in mammals, where the dominant allele leads to banding and striping (e.g., tabby cats) and the non-agouti locus to solid coat colors. This gene encodes a signaling peptide, ASIP, that influences melanocytes but also has multiple pleiotropic effects (Dreger and Schmutz 2011). Pigmentation penetrance was influenced by still another sex-linked gene, and Asher and colleagues hypothesized (Asher et al. 1996, p. 296) that all of these interactions are mediated via the multiple genes’ actions on neural crest cells.
Because so many genetic and developmental factors interact in pigmentation, it is not surprising that pigmentation defects show great interindividual and/or mutation-specific variability, even those derived from mutations in a single gene. For example, the well-studied W and Steel mutations in mice (which involve the Kit/Steel Factor signaling pathway) can produce overall albinism, white spotting, white feet, or a broad white “sash” depending on genetic background and the specific mutation (Jackson 1994). Because melanocytes derive from small discrete pools of neural crest cells that undergo circuitous migrations, depending on their final destination, the key expectation derived from this literature is that pigmentation should be depleted in domesticated species, but not that its pattern should take any specific form. Nonetheless, there is a clear tendency in both mouse mutants and domesticated species for pigmentation deficits to be more visible further from the site of origin of the neural crest, such as the paws or the midline of the belly (Jackson 1994; Yamaguchi et al. 2007; Mills and Patterson 2009).
In conclusion, the depigmentation changes seen in domesticated animals are clearly consistent with our hypothesis, and several of the more variable symptoms that go along with disorders primarily associated with pigmentation (e.g., reductions in the facial skeleton in Splotch mutant mice or loss of the enteric nervous system in some Waardenburg patients) support our suggestion that the core cause is a disruption in neural crest development.
Reduced facial skeleton (shorter snout and smaller jaws)
A second characteristic feature of domesticated mammals is a reduction in the upper and lower jaws and surrounding facial skeleton (“snout”) relative to wild-type ancestors (Darwin 1875; Belyaev 1974) (Although some particular dog breeds have been bred secondarily for shorter jaws, these do not bear directly on the DS, which concerns all breeds and species.) Furthermore, during the experimental domestication of foxes and mink by the Novosibirsk group, reduced muzzle and jaw size were early and general accompaniments of the selection for docility (Trut et al. 2009). The physical correlates of reduced muzzle size are the rounder and flatter faces seen in both species.
The upper and lower jaws and surrounding facial skeleton (snout) of mammals derive from two pairs of facial primordia, the maxillary (primary source of the upper jaws) and the mandibular (producing the lower jaws), along with the lateral and medial nasal processes. Both components of jaws develop following CNCC migration to the initial sites (Schilling and Le Pabic 2014). The size of the jaws is a function of the numbers of input NCCs, with smaller jaws reflecting smaller numbers since dramatic reductions in NCCs in the facial primordia greatly reduce development of the midface, including the jaws (Etchevers et al. 1999; Creuzet et al. 2004).
A well-understood human clinical syndrome directly relevant to jaw reduction is Treacher Collins syndrome, a primary symptom of which is reduced jaw size (micrognathia) and smaller zygomatic bones (facial hypoplasia). Other relevant aspects of this syndrome include tooth anomalies (tooth agenesis or deformities in 60% of cases) and malformed external ear cartilages (the ears can be absent, reduced, or malformed) along with conductive hearing loss caused by reductions or absence of the (neural crest derived) middle ear bones. Treacher Collins is typically (80–90% of the cases) caused by mutations in the TCOF1 gene, which encodes the Treacle protein involved in production of normal ribosomes. As typical for neurocristopathies, surviving Treacher Collins patients are heterozygotes, and the condition results from haploinsufficiency. Specifically, haploinsufficiency of TCOF1 leads to lowered levels of Treacle, which in turn leads to depletion of the NCC precursors of the first and second visceral arches (Dixon et al. 2006).
Another recently identified human neurocristopathy of interest is Mowat-Wilson syndrome, a rare syndrome featuring microcephaly, specific narrowing of the jaw, and a host of other neural-crest-related symptoms (e.g., changes in ear morphology, heart disease, and as for Waardenburg’s, variable presence of Hirschsprung’s disease) along with mental retardation and epilepsy. Interestingly, patients with this syndrome are reported to “have a happy demeanour with frequent smiling” (Mowat et al. 2003), consistent with the behavioral changes seen during animal domestication, considered below. The genetic basis of Mowat-Wilson is clear, mainly involving mutations of the ZEB2 gene, also known as SIP1 (Mowat et al. 2003). Again this syndrome results from haploinsufficiency, here caused by shortages of the ZEB2 protein during development. ZEB2 (Zinc finger E-box-binding homeobox 2) is a transcription factor involved in the Smad-mediated signaling cascade (hence the alternative name SIP1, for “Smad interacting protein 1”). The Xenopus homolog of this gene, XSIP1, has been well studied and is strongly expressed early in prospective neuroectoderm, then in the neural plate, and later in the neural tube and neural crest. This signal is a key developmental inducer of the epithelial-to-mesenchymal transition (EMT) of the neural crest. Without the signal, neural crest cells fail to delaminate from the neural tube.
Reduced tooth size
Another typical change associated with domestication in mammals is a reduction in the size of teeth relative to wild-type ancestors (Darwin 1875; Belyaev 1974). This is also consistent with our hypothesis because neural crest cells are directly involved in tooth development (Hall 1999; Le Douarin and Kalcheim 1999; Gilbert 2003). The largest part of each tooth develops from NCC-derived odontoblasts. The enamel-based crowns are produced from ameloblasts, which are not traditionally thought to be CNCC derived, but recent data suggest a neural crest contribution here as well (Wang et al. 2011). Reduced tooth size is thus predicted in theory if CNCC numbers are reduced. This prediction is borne out in practice by the reductions or absence of teeth common in Treacher Collins syndrome, the known neurocristopathy discussed above.
Floppy ears
Floppy ears are a component of the DS seen in one or more breeds of nearly every domesticated species while, in contrast, the only wild mammals with floppy ears are elephants (Darwin 1875). This trait can be understood in terms of the developmental sources of the outer ear: the mammalian pinna develops from a convergence of tissues from ectoderm, mesoderm, and endoderm as well as CNCCs derived from the hindbrain (Fekete 1999; Van De Water and Staecker 2005). Both cartilage and connective tissues of the pinna derive from NCC-derived cells of the first and second branchial arches, but the segments of NCC that are responsible for cartilage and connective tissues are not necessarily the same. The floppy-ear phenotype can thus be explained as the consequence of a mild deficiency of NCC-derived cartilage relative to the amount required for the erect ear phenotype, producing an inadequately stiffened pinna.
This theoretical explanation is borne out in practice by the ear reductions or absence observed in several neurocristopathies discussed above. (Both Treacher Collins and Mowat-Wilson sydromes are characterized by absent, reduced, or malformed ears). Of course, the small external ears of humans and rodents provide imperfect models for the elongated, erect ears of most carnivores and artiodactyls, and thus the biomechanical aspect of our explanation remains somewhat speculative. However, it is interesting to note that polychondritis, an autoimmune disorder specifically attacking collagens in the external ear, can lead to floppy “lop” ears even in humans (Rapini and Warner 2006), supporting a link between cartilage deficits and floppy ears.
Conclusions regarding morphological changes
As the brief review above makes clear, the main morphological components of the DS can all be explained by the derivation of the affected tissues from neural crest. Defects in neural crest production early in development are predicted to lead to reduced populations of melanocytes (leading to pigmentation changes), the chondrocytes of the facial skeleton (leading to reduced jaw and snout size), odontocytes (leading to reduced tooth size), and chondrocytes of the external ear (leading to reduced or floppy ears). All of these predictions are born out in practice both in clinical populations in the various neurocristopathies, as noted above, but in many cases can be directly tied to specific changes in neural crest proliferation or migration as verified in animal models.
As stressed initially when discussing pigmentation deficits, which are the best studied and understood of the neural-crest-related mutations, even changes in single genes can have diverse effects depending on the alleles of other interacting genes. Such epistatic effects, combined with the pleiotropy of most genes involved in neural crest, mean that the effects of neural crest reduction are quite variable both between and within species. We are aware of no single mutation or clinical syndrome that can reproduce all and only the phenotypic effects of the DS but this is as expected, given that we are not hypothesizing that the DS derives from changes in one or a few genes. Instead, the data above clearly support the co-occurrence of multiple components of the DS as combined effects of multiple NCC-affecting mutations and, when considered as a whole, all of the components are known to result from NCC-related genetic effects. Taken together with the experimental domestication data, these data strongly support a multigenic cause of the DS, centered upon the development of neural crest derivatives.
Behavioral and Neural Components of the Domestication Syndrome
We now turn to the behavioral components of the DS, focusing on the key phenotypic trait that is selected during domestication, tameness, and on its possible sources. We will also, however, briefly discuss other behavioral changes that take place in domestication and comment on the reductions in brain size frequently seen in domesticated species.
The mechanistic basis for tameness
A reduction in aggression and increase in docility (“tameness”), relative to their wild-type forebears, is the most prominent behavioral feature of all domesticated animals (Belyaev 1969). Experimental domestication studies in foxes, rats, and mink clearly demonstrate that selection on tameness alone, a behavioral trait, can lead to the multiple correlated morphological changes of the DS discussed above (Belyaev 1974; Trut 1999). As we have shown, most of these morphological traits can be causally linked to reductions in neural crest function. But how, precisely, does neural crest hypofunction lead to tameness? There are at least two possible routes, which are not mutually exclusive.
One well-studied component of tameness concerns the sympathetic nervous system, which governs “fight-or-flight” reactivity to novel or threatening stimuli. These behavioral responses rely crucially on the hypothalamic–pituitary–adrenal system (HPA axis), which acts to rapidly convert neurally derived perceptual information (“something strange or threatening is here!”) to hormonal signals, especially epinephrine (“adrenalin”), released from the NCC-derived adrenal medulla. This hormonal surge in turn prepares the body for fast powerful action (Sapolsky 1992). Acting in concert with this fast-acting response are slower stress responses, driven primarily by the adrenal cortex via corticosteroid hormones, that lead to longer-term elevated reactivity. Stress responses play a powerful role in the CNS and feed back to the brain to play a role in cognitive processing and learning about threatening or stressful situations (Sapolsky 1992).
The peripherally based fear/stress system appears to be down-regulated in domesticated mammals (Künzl and Sachser 1999) and provides a clear link between tameness and the neural crest, specifically via adrenal hypofunction and reduced adrenal size. In experimentally domesticated foxes and rats, the adrenal glands are smaller than in their unselected counterparts. In domesticated foxes the adrenals are already significantly reduced in utero (Osadschuk 1997) and multiple aspects of adrenal function are also down-regulated, including both baseline levels of adrenocorticoids and the response of adrenal cells to adrenocorticotropic hormone (ACTH), the upstream controller of stress-hormone release (Oskina 1997). As a result, after 45 generations of selection for tameness, domesticated foxes showed a substantial (three- to fivefold) reduction in both basal and stress-induced blood cortisol levels (Trut et al. 2009). Both adrenal gland size and blood corticosterone levels were also reduced in experimentally domesticated rats (Albert et al. 2008). Similar principles appear to apply in domesticated birds as well: domestic chickens are less fearful and reactive than wild junglefowl (Schütz et al. 2001), and basal cortisol levels are also reduced in domesticated Bengalese finches, relative to their (captive raised) wild-type ancestors (Suzuki et al. 2012).
Domestication thus consistently leads to a reduction in the peripheral physiological reaction to stressful stimuli or situations, which has a direct and immediate effect on tameness by reducing fearful reactivity. Belyaev, however, stressed a second and less direct, but equally important, developmental effect of sympathetic hypofunction in domesticated foxes (Belyaev 1984). Immediately after birth and for their first 1.5 months of life, the HPA axis of wild (unselected) fox kits is too immature to mount a full-blown stress and fear response, even though the kits move about and explore with their eyes open. At the end of this period the kits become highly reactive and fearful of strange animals including humans. Although domesticated foxes experience the same effect, the duration of immaturity for their HPA axis is much longer, i.e., 3–4 months. Domesticates therefore have a longer “socialization window.” Under normal captive rearing conditions domesticated kits are exposed to repeated interactions with human caretakers before the full physiological fear response is possible (Trut et al. 2004). Early human exposure means that caretakers are already perceptually recognized as low-threat stimuli by the time the HPA axis is mature, and thus humans do not initiate the neurally driven component of a fear response later in life (in this case, familiarity breeds indifference). Comparable differences are also seen between wolves (which have a 1.5-month socialization window) and dogs (where this window stretches from 4 to 10 months), as well as in laboratory mice selected for low aggression levels (Freedman et al. 1961; Gariépy et al. 2001). Crucially, even domesticated dogs become fearful and untameable for life if they are not socialized to humans within this extended window (Freedman et al. 1961; Scott 1962, 1964). Thus, a mild heterochronic delay in sympathetic reactivity, caused by delayed adrenal gland maturation, can have important life-long effects at higher cognitive levels, via a simple prolongation of a sensitive period for positive contact with humans.
Changes in the central nervous system
A third, poorly understood, neurobiological aspect of domestication involves changes in the central nervous system. Brain size is significantly reduced in most domesticated animals, relative to their wild-type ancestors, but to a variable degree (Kruska 1987, 1988a, 1996, 2005). This reduction, which is particularly prominent in the forebrain, is very significant in some species; for example, domestic pig brains are 35% smaller than expected for the same size wild boar, their presumed wild ancestor (Kruska 2005). Typical reductions are somewhat less extreme, roughly −20% in domesticated mink and −16% for horses (Kruska 2005). However, although this pattern is nearly universal, there are two exceptions: brain size reduction is very limited in domesticated foxes [only 2% relative to their known (untamed) predecessors; Trut et al. 1991] and no reduction in brain size has occurred during domestication in laboratory mice (Kruska 2005). Given that laboratory mice and domesticated foxes are nonetheless very tame, brain size reduction per se is clearly not a crucial determinant of tameness. Rather than overall brain size reduction, it seems more likely that reductions of particular components of the forebrain, such as the amygdala or other components of the limbic system, play the important CNS roles in tameness.
Another component of the DS potentially linked to reduced brain size is altered reproductive functions. The females of many domesticated species show more frequent estrus cycles and often lose strict seasonality in their breeding (Kruska 1988b; Hemmer 1990; Trut 1999). Presumably this involves the hypothalamic–pituitary–gonadal (HPG) axis, which governs reproductive cycles in mammals. Specifically, because the effects of the HPG axis on the female reproductive system are largely inhibitory (Hoehn and Marieb 2007), diminished functioning of the HPG axis would be expected to produce the DS-associated traits of accelerated reproductive maturation and reduced interbirth intervals. Another possibility involves the role of the pineal gland, a neural plate derivative that has not been thought to be NCC derived. The pineal plays a crucial role in setting occurrence of estrous cycles in relationship to day length. The pineal glands are smaller in domesticated than wild female foxes and produce lower melatonin levels (Kolesnikova 1991).
The developmental origins of brain size reduction, particularly forebrain regions, have traditionally been part of the mystery of the DS. However, recent data suggest an explanation that may again result from CNCC reductions. Although CNCCs are not direct progenitors of any part of the brain, including the limbic system, they play a crucial indirect role in forebrain development. In chick embryos, ablation of CNCCs from the neural tube posterior to the future telencephalon causes failure of development of major parts of the telencephalon, diencephalon, and mesencephalon (Etchevers et al. 1999; Creuzet et al. 2006). The reduction is due to ablation of an FGF8 signal emitted by the CNCC; in its absence, there is extensive apoptosis in the developing forebrain (Creuzet et al. 2004, 2006). If there is a quantitative relationship between CNCC numbers, FGF8 signal, and extent of forebrain cell precursor survival, then reduced CNCC numbers would affect development of components of the brain. While this suggestion remains hypothetical, it is testable, as discussed below.
Hereditary Foundations: Genetic vs. Epigenetic Changes
We now briefly reexamine the genetic foundations of the DS, in light of our hypothesis. To date, as we have stressed, no single gene mutants have been found that mimic the entire DS phenotype. Given the relative ease of selecting the condition, this is not surprising, since the spontaneous mutation rate of single genes, approximately 10−6/gene/generation, should be too low for efficient response to selection. Presumably, therefore, the DS is a polygenic condition. Furthermore, it probably does not require homozygosity of recessive alleles: the Novosibirsk group produced the DS in their selected animals by outbreeding, thus reducing homozygosity, using animals from different fox farm populations (Trut et al. 2004, 2009). The simplest inference, therefore, is that the DS arises from preexisting genetic variation consisting of mutations of small semidominant effects in several to many genes. Given that the DS has been produced in so many mammalian species, that preexisting variation might be common. A possible explanation for such ubiquity is that the alleles confer some sort of heterozygous advantage, as found in cystic fibrosis and sickle-cell anemia (Quintana-Murci and Barreiro 2010). Alternatively, the mutations may individually be of nearly neutral effect but when brought together, in various combinations, produce the dramatic phenotype of the DS. A possible relevant example of such polygenic synergism is the recent report that the “splashed white” phenotype in horses, identified by large white blazes, has such a multiple-gene basis (Hauswirth et al. 2012). As shown in that study also, not all the heritable variation required to produce the phenotype need be preexisting; in one of the horse lines, a new mutation contributed.
These general genetic characteristics of the DS accord with our hypothesis that it is neural crest genes specifically that are the ultimate source of the DS and, in particular, that it involves multiple mild loss-of-function mutations in several of these genes. Such genes should be individually dosage sensitive, a feature signaled by haploinsufficiency, and their mutations should exhibit interactions, additively or synergistically, to generate the effect. The available evidence on these genes fits these predictions and this material is summarized in Table 2. Correspondingly, mild loss-of-function mutations (hypmorphs) in such dosage-sensitive genes, which all affect the same cell type/developmental process, would be expected to give mutual enhancement of their effects. Finally, the large numbers of genes known to be required for NCC development or migration are consistent with the proposal of polygenic origins and, in principle, would constitute a large target size for the condition, facilitating its selection. (For information on NCC genetics, see reviews by Nikitina et al. 2009; Kulesa et al. 2010; Minoux and Rijli 2010).
Conventional point mutations, however, are not necessarily the sole genetic source of the condition. Recombination-generated alterations of particular repeat elements are correlated with morphological changes in carnivores (Fondon and Garner 2004) and might also be involved here (Trut et al. 2004). It is potentially relevant that some conserved noncoding elements (CNEs) are repeated elements (Kamal et al. 2006) and CNEs are associated with various neural crest genes associated with known neurocristopathies (Amiel et al. 2010). Recombination-generated changes among repeated elements can take place at much higher rates than point mutations.
It is also worth considering that some of the initial changes may not be DNA-sequence alterations but epigenetic changes. In particular, the Novosibirsk group has long argued that hormonal states in the mother, associated with the less stressful conditions of domesticity, are involved in generating the DS (Belyaev 1979; Trut et al. 2004, 2009). Findings consistent with this idea, though opposite in effect, involve maternal stresses in mice that create epigenetic chromatin state changes and behavioral phenotypes in offspring (Meany and Szyf 2005; Bagot and Meaney 2010). Whether epimutations have the requisite stability in trans-generational transmission to generate true heritable states is always a key question about their evolutionary potential (Slatkin 2009) but strong trans-generational transmissibility of epigenetic states has been shown for two genes affecting coat color patterns in the mouse, Agouti and AxinFu genes (Morgan et al. 1999; Rakyan et al. 2003). With respect to the DS, the most convincing evidence for an involvement of epigenetic effects concerns the “Star gene” in foxes, proposed by Belyaev to explain the initial appearance of a white forehead patch early in development of the DS. Star has a high rate of heritable change, in both directions, approximately 10−2 per generation, a rate far too high for conventional mutation (Belyaev et al. 1981; Trut et al. 2009). It is also possible that quasistable epimutations become converted to true genetic mutations (Karpinets and Foy 2005). We suggest that Belyaev’s hypothesis, positing inducible epimutations as initiating events in the DS, though unconventional, deserves reconsideration.
A final point: in different domesticated species, all three mechanisms—point mutations, recombination of repeat elements, and epimutations—might be involved but in different combinations and in different genes. Once the loci involved in the DS have been identified (see below), it should be possible to tell which explanations pertain to particular genes in specific breeds.
Predictions of the Neural Crest Hypothesis
A primary virtue of the hypothesis advanced above is its simplicity: it posits a unitary underlying cause for the seeming hodgepodge of traits characterizing domesticated species. However, a second important virtue is that it makes a number of predictions that can easily be tested using existing methods. Developmentally, our hypothesis predicts that NCC development will be altered in all domesticated species, relative to their wild-type ancestors. Genetically, we predict reduced expression of multiple genes affecting neural crest development, probably including some of those listed in Table 2.
In particular, we suggest that all domesticated birds and mammals will show reduction in initial NCC numbers (e.g., as caused by ZEB2 insufficiency in Mowat-Wilson syndrome) and/or delays or alteration of migratory behavior (as caused by diverse genes, including Pax3, MITF, Sox10, and EDNRB, and observed in different types of Waardenburg syndrome) leading to reduction in NCC numbers, or in their efficacy at the final site of action. This general prediction can be most easily tested in model organisms where the wild-type ancestor remains readily available (e.g., mice, rats, guinea pigs, and chickens) but should be possible for most domesticates though not cattle (whose wild-type ancestor, the aurochs, is extinct). For example, GFP-labeled neural crest cells from domestic chickens should be hypoactive when transplanted into wild junglefowl embryos, as should those from laboratory mice or rats in wild-type embryos. If such comparisons show fully normal NCC development and function in domesticated strains, our hypothesis would be falsified.
It should also be possible to create experimentally, genetically targeted mild reductions in NCC populations in nondomesticated mice and rats, whose consequences could then be examined. In particular, our predictions should be testable with engineered NCC genes that reduce the extent of CNCC migration. We predict quantitative relationships between reductions in NCC migration or proliferation and the various phenotypic traits associated with the DS, in viable animals produced by such experiments. Effects on forebrain development would be among those predicted. Again, a negative result would count heavily against the hypothesis.
Finally, and not least, if our idea is correct, some of the loci found to be associated with tameness in the recent rat and fox genetic analyses (cited below) will be found to be involved in NCC development, migration, or proliferation. Some of the genes listed in Table 2 might be in this set but there could be many others as well. If no NCC genes are found to be so implicated, our hypothesis can be rejected.
While the examples above do not exhaust the possibilities, and many more specific predictions about particular genes, signaling pathways, and NCC subtypes can be made, we hope that the short summary above shows that our hypothesis is not just compatible with a considerable body of existing research and data, but makes many testable predictions that can drive future research.
Conclusions
In this article, we have argued that all the facets of the domestication syndrome can be traced to mild neural crest cell deficits. In Figure 2, we summarize our proposal, depicting the routes by which those deficits can lead to the different traits of the syndrome.
Figure 2.
Diagrammatic representation of the neural crest hypothesis of the domestication syndrome, illustrating how selection for tameness, leading to decreased neural crest input into the sympathetic and adrenal systems, would cause the other observed components of the domestication syndrome as unselected by-products, resulting in a “mild neurocristopathy.” Arrows indicate predicted directions of influence on traits discussed in the text, as separated into direct and indirect developmental (mechanistic) effects.
The idea, of course, raises further questions concerning matters of developmental biology, neurobiology, and genetics. A particularly crucial developmental-neurobiological issue concerns the exact relationship between docility, the presumed initial target of human selection, and its connection to the hypothesized changes in neural development. Besides the factors discussed above, it is possible that under selection, docility results partly from a slowed pace of neural development, that would in turn cause a relatively immature emotional response to social threat, in particular a reduced fear–startle response, involving a chain of events which, in this case, was initiated by a mild neurocristopathy. While there must surely be other paths to docility, the relatively large genetic target for partial or mild neurocristopathic conditions, given the large number of genes involved and required for neural crest cell biology, could make this a favored route.
Many gaps and uncertainties remain in our proposal. For example, one trait, curly tails, though not a universal consequence of domestication, presently defies a direct explanation in our terms. Most importantly, however, the precise genetic—and epigenetic—bases of the domestication syndrome require further elucidation. The critical genetic questions concern the specific genes whose mutant forms are involved in creating the DS. Determining this will require detailed genetic analysis of the condition and preferably in several species, given our prediction that multiple and diverse neural crest cell genes might be involved. Recent progress in mapping the genes involved in domestication in the rat, fox, and dog lay the groundwork by providing a short list of candidate genes and as yet uncharacterized loci for testing our hypothesis (Albert et al. 2009; Kukekova et al. 2010; Vonholdt et al. 2010).
Supplementary Material
Acknowledgments
We thank Sophie Creuzet, Susan Dymecki, Daniel Lieberman, Maryellen Ruvolo, Kevin Shapiro, Lyudmilla Trut, and two anonymous reviewers for many helpful comments and criticisms. W.T.F. gratefully acknowledges support of ERC (European Research Council) Advanced Grant SOMACCA (The Syntax of the Mind – a Comparative Computational Approach) (no. 230604). In addition, we thank the Stellenbosch Institute of Advanced Study, where two of us (A.S.W. and R.W.W.) first started discussing these ideas, for support and for providing an excellent environment for doing creative work. R.W.W. also thanks Brian Hare and Tory Wobber for a decade of discussions about domestication.
Footnotes
Communicating editor: T. R. Magnuson
Literature Cited
- Abouheif E., Wray G. A., 2002. Evolution of the gene network underlying wing polyphenism in ants. Science 297: 249–252. [DOI] [PubMed] [Google Scholar]
- Adameyko I., Lallewend A., Furlan A., Zinin N., Aranda S., et al. , 2012. Sox 2 and MITF cross-regulatory interactions consolidate progenitor and melanocyte lineages in the cranial neural crest. Development 139: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert F. W., Shchepina O., Winter C., H. Römpler, D. Teupser et al, 2008. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm. Behav. 53: 413–421. [DOI] [PubMed] [Google Scholar]
- Albert F. W., Carlborg Ö., Plyusnina I., Besnier F., Hedwig D., et al. , 2009. Genetic architecture of tameness in a rat model of animal domestication. Genetics 182: 541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert F. W., Hodges E., Jensen J. D., Besnier F., Xuan Z., et al. , 2011. Targeted resequencing of a genomic region influencing tameness and aggression reveals multiple signals of positive selection. Heredity 2011: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J., Benko S., Gordon C. T., Lyonnet S., 2010. Disruption of long-distance highly conserved noncoding elements in neurocristopathies. Ann. N. Y. Acad. Sci. 1214: 34–46. [DOI] [PubMed] [Google Scholar]
- Arbuckle B. S., 2005. Experimental animal domestication and its application to the study of animal exploitation in Prehistory, First Steps of Animal Domestication, edited by Vigne J.-D., Peters J., Helmer D. Oxbow Books, Durham, UK. [Google Scholar]
- Asher J. H., Harrison R. W., Morell R., Carey M. L., Friedman T. B., 1996. Effects of Pax3 modifier genes on craniofacial morphology, pigmentation, and viability: a murine model of Waardenburg syndrome variation. Genomics 34: 285–298. [DOI] [PubMed] [Google Scholar]
- Bagot R. C., Meaney M. J., 2010. Epigenetics and the biological basis of gene x environment interactions. J. Am. Acad. Child Adolesc. Psychiatry 49: 752–772. [DOI] [PubMed] [Google Scholar]
- Baipai R., Chen D. A., Rada-Iglesias A., Zhang J., Xiong Y., et al. , 2010. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A., Dixon J., Dixon M., Trainor P. A., 2013. Tcof1 acts as a modifier of Pax3 during enteric nervous system development and in the pathogenesis of colonic aganglionosis. Hum. Mol. Genet. 22: 1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett C., Yazgan O., Kuo H.-C., Malakar T., Thomas T., et al. , 2012. Williams Syndrome Transcription Factor is critical for neural crest cell function in Xenopus laevis. Mech. Dev. 129: 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev D. K., 1969. Domestication of animals. Science J. 5: 47–52. [Google Scholar]
- Belyaev D. K., 1974. Domestication, plant and animal, pp. 936–942 in Encyclopaedia Britannica, Ed. 15, edited by Benton H. H. Encyclopedia Britannica–Helen Hemingway Benton Publishing, Chicago. [Google Scholar]
- Belyaev D. K., 1979. Destabilizing selection as a factor in domestication. J. Hered. 70: 301–308. [DOI] [PubMed] [Google Scholar]
- Belyaev D. K., 1984. Foxes, pp. 211–214 in Evolution of Domesticated Animals, edited by Mason I. L. Longman, New York. [Google Scholar]
- Belyaev D. K., Trut L. N., 1989. The convergent nature of incipient forms and the concept of destabilizing selection, pp. 155–169 in Vavilov’s Heritage in Modern Biology, edited by Ovchinnikov Y. A., Rapoport I. A. Nauka, Moscow. [Google Scholar]
- Belyaev D. K., Ruvinsky A. O., Trut L. N., 1981. Inherited activation-inactivation of the star gene in foxes: its bearing on the problem of domestication. J. Hered. 72: 267–274. [DOI] [PubMed] [Google Scholar]
- Bolande R. P., 1974. The neurocristopathies: a unifying concept of disease arising in neural crest maldevelopment. Hum. Pathol. 5: 409–429. [DOI] [PubMed] [Google Scholar]
- Borrego S., Ruiz-Ferrer M., Fernandez R. M., Antinolo G., 2013. Hirschsprung’s disease as a model of complex genetic etiology. Histol. Histopathol. 28: 1117–1136. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Jones M. K., Powell W., Allaby R. G., 2008. The complex origins of domesticated crops in the Fertile Crescent. Trends Ecol. Evol. 24: 103–109. [DOI] [PubMed] [Google Scholar]
- Carlson B. M., 1999. Human Embryology and Developmental Biology. C. V. Mosby, St. Louis. [Google Scholar]
- Castle W. E., 1947. The domestication of the rat. Proc. Natl. Acad. Sci. USA 33: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock J., 1999. A Natural History of Domesticated Mammals. Cambridge University Press, Cambridge. [Google Scholar]
- Creuzet S. E., Schuler B., Couly G., Le Douarin N. M., 2004. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc. Natl. Acad. Sci. USA 101: 4843–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzet S. E., Martinez S., Le Douarin N., 2006. The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc. Natl. Acad. Sci. USA 103: 14033–14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford, S. J., 2000 Dog evolution: a role for thyroid hormone physiology in domestication changes in Dogs Through Time: An Archaeological Perspective. Proceedings of the First ICAZ Symposium on the History of the Domestic Dog, edited by S. J. Crockford. Archaeological Reports International, Oxford. [Google Scholar]
- Crockford, S. J., 2004 Animal domestication and vertebrate speciation: a paradigm for the Origin of Species. Ph.D. Dissertation. University of Victoria, British Columbia. [Google Scholar]
- Crockford S. J., 2009. Evolutionary roots of iodine and thyroid hormones in cell-cell signaling. Integr. Comp. Biol. 49: 155–166. [DOI] [PubMed] [Google Scholar]
- Darwin C., 1859. On the Origin of Species. John Murray, London. [Google Scholar]
- Darwin C., 1868. The Variation in Animals and Plants under Domestication. John Murray, London. [Google Scholar]
- Darwin C., 1875. The Variation of Animals and Plants under Domestication. John Murray, London. [Google Scholar]
- Davidson E. H., Rast J. P., Oliveri P., Ransicka A., Calestania C., et al. , 2002. A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev. Biol. 246: 162–190. [DOI] [PubMed] [Google Scholar]
- Dixon J., Jones N. C., Sandell L. L., Jayasinghe S. M., Crane J., et al. , 2006. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl. Acad. Sci. USA 103: 13403–13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger D. L., Schmutz S. M., 2011. A SINE insertion causes the black- and-tan and saddle tan phenotypes in domestic dogs. J. Hered. 102: S11–S18. [DOI] [PubMed] [Google Scholar]
- Dutton K. A., Pauliny A., Lopes S. S., Elworthy S., Carney T. J., 2001. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest cell fates. Development 128: 4113–4125. [DOI] [PubMed] [Google Scholar]
- Epstein D. J., Vogan K. J., Trasler D. G., Gros P., 1993. A mutation within intron-3 of the Pax-3 gene produces aberrantly spliced mRNA transcripts in the splotch (Sp) mouse mutant. Proc. Natl. Acad. Sci. USA 93: 4213–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers H. C., Couly G. F., Vincent C., Le Douarin N. M., 1999. Anterior cephalic neural crest is required for forebrain viability. Development 126: 3533–3543. [DOI] [PubMed] [Google Scholar]
- Fekete D. M., 1999. Development of the vertebrate ear: insights from knockouts and mutants. Trends Neurosci. 22: 263–269. [DOI] [PubMed] [Google Scholar]
- Fleischmann R. A., Saltman V., Stastny V., Zneimer S., 1991. Deletion of the c-kit protooncogene in the human developmental defect piebald trait. Proc. Natl. Acad. Sci. USA 88: 10885–10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn B., Bergner A. J., Turner K. N., Young H. M., Anderson R. B., 2007. Effect of Gdnf haploinsufficiency on rate of migration and number of enteric neural crest-derived cells. Dev. Dyn. 236: 134–141. [DOI] [PubMed] [Google Scholar]
- Fondon J. W. I., Garner H. R., 2004. Molecular origins of rapid and continuous morphological evolution. Proc. Natl. Acad. Sci. USA 101: 18058–18063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. U., Fotheringham L. K., Brewer J. A., Muglia L. J., Tristani-Firouzi M., et al. , 2002. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 129: 4591–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D. G., King J. A., Elliot O., 1961. Critical period in the social development of dogs. Science 133: 1016–1017. [DOI] [PubMed] [Google Scholar]
- Gariépy, J.-L., D. J. Bauer, and R. B. Cairns, 2001. Selective breeding for differential aggression in mice provides evidence for heterochrony in social behaviours. Anim. Behav. 61: 933–947. [Google Scholar]
- Gilbert S. F., 2003. Developmental Biology. Sinauer, Sunderland, MA. [Google Scholar]
- Haase B., Brooks A. S., Tozaki T., Burger D., Poncet P.-A., et al. , 2009. Seven novel KIT mutations in horses with white coat colour phenotypes. Anim. Genet. 40: 623–629. [DOI] [PubMed] [Google Scholar]
- Hall B. K., 1999. The Neural Crest in Development and Evolution. Springer-Verlag, New York. [Google Scholar]
- Hare B., Wobber V., Wrangham R. W., 2012. The self-domestication hypothesis: bonobos evolved due to selection against male aggression. Anim. Behav. 83: 573–585. [Google Scholar]
- Hauswirth R., Haase B., Blatter M., Brooks A. S., Burger D., et al. , 2012. Mutations in MITF and Pax3 cause ‘’Splashed White” and other white spotting phenotypes in horses. PLoS Genet. 8: e1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer H., 1990. Domestication: The Decline of Environmental Appreciation. Cambridge University Press, Cambridge. [Google Scholar]
- Hoehn K., Marieb E. B., 2007. Human Anatomy and Physiology. Pearson Benjamin Cummings, San Francisco. [Google Scholar]
- Jackson I. J., 1994. Molecular and developmental genetics of mouse coat color. Annu. Rev. Genet. 28: 189–217. [DOI] [PubMed] [Google Scholar]
- Kamal M., Xie X., Lander E. S., 2006. A large family of ancient repeat elements in the human genome is under strong selection. Proc. Natl. Acad. Sci. USA 103: 2740–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinets T. V., Foy B. D., 2005. Tumorigenesis: the adaptation of mammalian cells to sustained stress environments by epigenetic alterations and succeeding matched mutations. Carcinogenesis 26: 1323–1334. [DOI] [PubMed] [Google Scholar]
- King, H. D., and H. H. Donaldson, 1929 Life processes and size of the body and organs in gray Norway rats during ten generations in captivity. American Anatomical Memoirs 14: 5–72.
- Kolesnikova, L. A., 1991 Peculiarities of the pineal structure and function in silver foxes: its changes under domestication, pp. 70–96 in Evolutionary-Genetic and Genetic-Physiological Aspects of Animal Domestication. ICG USSR, Novosibirsk (in Russian). [Google Scholar]
- Kruska D., 1987. How fast can total brain size change in mammals? J. Hirnforsch. 28: 59–70. [PubMed] [Google Scholar]
- Kruska D., 1988a Mammalian domestication and its effect on brain structure and behavior, Intelligence and Evolutionary Biology, edited by Jerison H. J., Jerison I. Academic Press, New York. [Google Scholar]
- Kruska D., 1988b Mammalian domestication and its effects on brain structure and behavior, pp. 211–250 in The Evolutionary Biology of Intelligence, edited by Jerison H. J., Jerison I. Springer-Verlag, Berlin. [Google Scholar]
- Kruska D., 1996. The effect of domestication on brain size and composition in the mink (Mustela vison). J. Zool. 239: 645–661. [Google Scholar]
- Kruska D. C., 2005. On the evolutionary significance of encephalization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain Behav. Evol. 65: 73–108. [DOI] [PubMed] [Google Scholar]
- Kukekova A. V., Trut L. N., Chase K., Kharlamova A. V., Johnson J. L., et al. , 2010. Mapping loci for fox domestication: deconstruction/reconstruction of a behavioral phenotype. Behav. Genet. 41: 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa P. M., Bailey J. C., Kasemeier-Kulesa J. C., Mclennan R., 2010. Cranial neural crest migration: new rules for an old road. Dev. Biol. 344: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzl, C., and N. Sachser, 1999. The behavioral endocrinology of domestication: a comparison between the domestic guinea pig (Cavia aperea f. porcellus) and its wild ancestor, the cavy (Cavia aperea). Horm. Behav. 35: 28–37. [DOI] [PubMed] [Google Scholar]
- Langer, L., K. Sulik, and L. Penny, 2013 Cleft palate in a mouse model of Sox2 haploinsufficiency. Cleft Palate Craniofac. J. 51: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Kalcheim C., 1999. The Neural Crest. Cambridge University Press, Cambridge. [Google Scholar]
- Leach H. M., 2003. Human domestication reconsidered. Curr. Anthropol. 44: 349–368. [Google Scholar]
- Levine M., Davidson E. H., 2005. Gene regulatory networks for development. Proc. Natl. Acad. Sci. USA 102: 4936–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. A., Wu M. H., Yan C. H., Chau B. K., So H., 2013. Phosphorylation of Sox9 is required for neural crest delamination and is regulated downstream of BMP and canonical Wnt signalling. Proc. Natl. Acad. Sci. USA 110: 2882–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meany M. J., Szyf M., 2005. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 7: 103–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills M. G., Patterson L. B., 2009. Not just black and white: pigment pattern development and evolution in vertebrates. Semin. Cell Dev. Biol. 20: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M., Rijli F. M., 2010. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137: 2605–2621. [DOI] [PubMed] [Google Scholar]
- Moase C. E., Trasler D. G., 1989. Spinal ganglia reduction in the splotch-delayed mouse neural tube defect mutant. Teratology 40: 67–75. [DOI] [PubMed] [Google Scholar]
- Morgan H. D., Sutherland H. G., Whitelaw E., 1999. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 23: 314–318. [DOI] [PubMed] [Google Scholar]
- Mowat D. R., Wilson M. J., Goossens M., 2003. Mowat-Wilson syndrome. J. Med. Genet. 40: 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina N., Sauka-Spengler T., Bronner-Fraser M., 2009. Gene regulatory networks in neural crest development and evolution. Curr. Top. Dev. Biol. 86: 1–14. [DOI] [PubMed] [Google Scholar]
- Osadschuk L. V., 1997. Effects of domestication on the adrenal cortisol production of silver foxes during embryonic development, Evolutionary-Genetic and Genetic-Physiological Aspects of Fur Animal Domestication, edited by Trut L. N., Osadschuk L. V. Scientifur, Oslo. [Google Scholar]
- Oskina I. N., 1997. Analysis of the functional state of the pituitary-adrenal axis during postnatal development of domesticated silver foxes (Vulpes vulpes), pp. 55–63 in Evolutionary-Genetic and Genetic-Physiological Aspects of Fur Animal Domestication, edited by Trut L. N., Osadschuk L. V. Scientifur, Oslo. [Google Scholar]
- Pavan W. J., Mac S., Cheng M., Tilghman S. M., 1995. Quantitative trait loci that modify the severity of spotting in piebald mice. Genome Res. 5: 29–41. [DOI] [PubMed] [Google Scholar]
- Poll A. V., Pierreux C. E., Lokmane L., Haumaitre C., Achouri Y., et al. , 2006. A vHNF/TCF2–HNF6 cascade regulates the transcription factor network that controls generation of pancreatic precursor cells. Diabetes 55: 61–69. [PubMed] [Google Scholar]
- Price E. O., 1999. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 65: 245–271. [Google Scholar]
- Quintana-Murci L., Barreiro L. B., 2010. The role played by natural selection on Mendelian traits in humans. Ann. N. Y. Acad. Sci. 1214: 1–17. [DOI] [PubMed] [Google Scholar]
- Rakyan V. K., Chong S., Champ M. E., Cuthbert P. C., Morgan H. D., et al. , 2003. Transgenerational inheritance of epigenetic states at the murine AxinFu allele occurs after maternal and paternal transmission. Proc. Natl. Acad. Sci. USA 100: 2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapini R. P., Warner N. B., 2006. Relapsing polychondritis. Clin. Dermatol. 24: 482–485. [DOI] [PubMed] [Google Scholar]
- Reissman M., Ludwig A., 2013. Pleiotropic effects of coat colour associated mutations in humans, mice and other mammals. Semin. Cell Dev. Biol. 24: 576–586. [DOI] [PubMed] [Google Scholar]
- Robertson K., Mason I., 1997. Hirschsprung’s disease: genetic mutations in mice and men. Gut 41: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar D. E., Longaker M. T., and N. Quarto, 2005. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev. Biol. 280: 344–361. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., 1992. Neuroendocrinology of the stress response, Behavioral Endocrinology, edited by Becker J. B., Breedlove S. M., Crews D. MIT Press, Cambridge, MA. [Google Scholar]
- Schilling T. F., Le Pabic P., 2014. Neural crest cells in craniofacial skeletal development, pp. 127–151 in Neural Crest Cells: Evolution, Development and Disease, edited by Trainor P. A. Academic Press, Amsterdam. [Google Scholar]
- Schütz, K. E., B. Forkman, and P. Jensen, 2001. Domestication effects on foraging strategy, social behaviour and different fear responses: a comparison between the red junglefowl (Gallus gallus) and a modern layer strain. Appl. Anim. Behav. Sci. 74: 1–14. [Google Scholar]
- Scott J. P., 1962. Critical periods in behavioral development. Science 138: 949–958. [DOI] [PubMed] [Google Scholar]
- Scott J. P., 1964. Genetics and the development of social behavior in dogs. Am. Zool. 4: 161–168. [DOI] [PubMed] [Google Scholar]
- Shows K. H., Shiang R., 2008. Regulation of the mouse Treacher Collins Syndrome homolog (Tcof1) promoter through differential repression of constitutive expression. DNA Cell Biol. 27: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D. L., Leeds H.-W. H., Miller E. E., Pavan W. J., 2013. The EJC component Magoh regulates proliferation and expansion of neural crest-derived melanocytes. Dev. Biol. 375: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatkin M. 2009. Epigenetic inheritance and the missing heritability problem. Genetics 182: 845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanchina L., Baral V., Robert F., Pingault V., Lemort N., et al. , 2006. Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev. Biol. 295: 232–249. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Yamada H., Kobayashi T., Okanoya K., 2012. Decreased fecal corticosterone levels due to domestication: a comparison between the white-backed munia (Lonchura striata) and its domesticated strain, the Bengalese finch (Lonchura striata var. domestica) with a suggestion for complex song evolution. J. Exp. Zool. 317: 561–570. [DOI] [PubMed] [Google Scholar]
- Tachibana M., 2000. MITF: a stream flowing for pigment cells. Pigment Cell Res. 13: 230–240. [DOI] [PubMed] [Google Scholar]
- Thomas A. J., Erickson C. A., 2009. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development 136: 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor P. A. (Editor), 2014. Neural Crest Cells: Evolution, Development and Disease. Academic Press, Amsterdam. [Google Scholar]
- Trut L., 1999. Early canid domestication: the farm-fox experiment. Am. Sci. 87: 160–168. [Google Scholar]
- Trut L., Oskina I., Kharlamova A., 2009. Animal evolution during domestication: the domesticated fox as a model. Bioessays 31: 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trut L. N., Dzerzhinsky F. J., Nikolsky V. S., 1991. Intracranial allometry and morphological changes in silver foxes (Vulpes vulpes) under domestication. Genetika 27: 1605–1611 (in Russian). [PubMed] [Google Scholar]
- Trut L. N., Plyusnina I., Oskina I. N., 2004. An experiment on fox domestication and debatable issues of evolution of the dog. Russ. J. Genet. 40: 644–655. [PubMed] [Google Scholar]
- Van De Water T. R., Staecker H., 2005. Otolaryngology: Basic Science and Clinical Review. Thieme, New York. [Google Scholar]
- Vonholdt B. M., Pollinger J. P., Lohmueller K. E., Han E., Parker H. G., et al. , 2010. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464: 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-K., Komatsu Y., Mishina Y., 2011. Potential contribution of neural crest cells to dental enamel formation. Biochem. Biophys. Res. Commun. 415: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Brenner M., Hearing V. J., 2007. The regulation of skin pigmentation. J. Biol. Chem. 282: 27557–27561. [DOI] [PubMed] [Google Scholar]
- Zeuner, F. E., 1963 A History of Domesticated Animals. Harper & Row, New York
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.