Summary
The type VI secretion system (T6SS) is a widespread molecular weapon deployed by many Proteobacteria to target effectors/toxins into both eukaryotic and prokaryotic cells. We report that Agrobacterium tumefaciens, a soil bacterium that triggers tumorigenesis in plants, produces a family of type VI DNase effectors (Tde) that are distinct from previously known polymorphic toxins and nucleases. Tde exhibits an antibacterial DNase activity that relies on a conserved HxxD motif and can be counteracted by a cognate immunity protein, Tdi. In vitro, A. tumefaciens T6SS could kill Escherichia coli but triggered a lethal counterattack by Pseudomonas aeruginosa upon injection of the Tde toxins. However, in an in planta coinfection assay, A. tumefaciens used Tde effectors to attack both siblings cells and P. aeruginosa to ultimately gain a competitive advantage. Such acquired T6SS-dependent fitness in vivo and conservation of Tde-Tdi couples in bacteria highlights a widespread antibacterial weapon beneficial for niche colonization.
Graphical Abstract
Highlights
-
•
A type VI secretion DNase effector (Tde) family identified in Agrobacterium tumefaciens
-
•
Tde exhibits antibacterial DNase activity and is counteracted by immunity protein Tdi
-
•
Tde confers a competitive advantage to A. tumefaciens during host colonization
-
•
Tde and Tdi superfamilies are broadly conserved in bacteria
Type VI secretion systems (T6SSs) are weapons involved in interbacterial competition. Ma et al. report the discovery of a class of T6SS-dependent DNases that support the survival and fitness of the plant pathogen Agrobacterium tumefaciens against competitors such as Pseudomonas aeruginosa during plant colonization.
Introduction
Bacteria produce diverse toxic compounds, including diffusible small molecules such as antibiotics, that allow them to thrive in a competitive environment. They can also produce and secrete enzymatic toxins targeting nucleic acids, membrane lipids, or the peptidoglycan of competing bacterial cells (Benz and Meinhart, 2014; Braun and Patzer, 2013). The type VI secretion system (T6SS) is a molecular machine found in most Proteobacteria (Cascales, 2008; Filloux et al., 2008) and can deliver effectors to both eukaryotic (Pukatzki et al., 2007) and prokaryotic cells, which appear to be the major targets (Dong et al., 2013; English et al., 2012; Hood et al., 2010; Russell et al., 2011, 2012, 2013).
Functional and structural studies have shown that the T6SS nanomachine shares remarkable similarities with the bacteriophage tail structure (Basler et al., 2012; Brunet et al., 2014; Kapitein et al., 2013; Leiman et al., 2009). The system contains a TssB-TssC contractile sheath, which is proposed to accommodate the Hcp-VgrG tail tube/puncturing device. The contraction of the sheath leads to the propelling of Hcp, VgrG, and T6SS effectors across bacterial membranes (Basler et al., 2012; Bönemann et al., 2010; Kapitein et al., 2013; Leiman et al., 2009). Time-lapse fluorescent experiments highlighted the dynamics of this mechanism by revealing “T6SS dueling” between interacting cells (Basler et al., 2013; Basler and Mekalanos, 2012; Ho et al., 2014; LeRoux et al., 2012).
To date, only a few toxins have been biochemically characterized and shown to contribute to the bactericidal activity mediated by the T6SS (Russell et al., 2014). The most remarkable examples are the cell-wall-degrading effectors that include the type VI secretion amidase effector (Tae) and type VI secretion glycoside hydrolase effector (Tge) superfamilies (Russell et al., 2011, 2012; Whitney et al., 2013). The Tae family includes Tse1 from Pseudomonas aeruginosa (Russell et al., 2011) and Ssp1 or Ssp2 from Serratia marcescens (English et al., 2012). The Tge family includes the Tse3 muramidase from P. aeruginosa (Russell et al., 2011) and Tge2 and Tge3 from Pseudomonas protegens (Whitney et al., 2013). VgrG3 from Vibrio cholerae (Brooks et al., 2013; Dong et al., 2013) represents another effector family with a distinct muramidase fold unrelated to the Tge family (Russell et al., 2014). These enzymes are injected into the periplasm of target cells, where they hydrolyze the peptidoglycan, thereby inducing cell lysis (Brooks et al., 2013; English et al., 2012; Russell et al., 2011; Whitney et al., 2013). The phospholipase Tle superfamilies represent an additional set of T6SS toxins. By degrading phosphotidylethanolamine, a major constituent of bacterial membranes, these effectors challenge the membrane integrity of target cells (Russell et al., 2013).
A recent study reported the nuclease activity of two proteins, RhsA and RhsB from Dickeya dadantii, containing NS_2 and HNH endonuclease domains, respectively, which cause the degradation of cellular DNA and confer an intraspecies competitive advantage (Koskiniemi et al., 2013). However, whether the D. dadantii antibacterial activity mostly relies on the DNase activity, and whether Rhs proteins are delivered by a dedicated T6SS machine remains to be determined (Russell et al., 2014).
Agrobacterium tumefaciens is a soil bacterium that triggers tumorigenesis in plants by delivering T-DNA from bacterial cells into host plant cells through a type IV secretion system (T4SS) (Alvarez-Martinez and Christie, 2009; Gelvin, 2010). Although not essential for tumorigenesis (Wu et al., 2008), the A. tumefaciens T6SS is activated at both transcriptional (Wu et al., 2012) and posttranslational levels (Lin et al., 2014) when sensing acidity, a signal enriched in the plant wound site and apoplast. Here, using A. tumefaciens as a model organism, we report the discovery of a type VI DNase effector (Tde) family that exhibits potent antibacterial activity. The toxic activity of the Tde DNase is counteracted by a cognate immunity protein, here called Tdi. The T6SS increases the fitness of A. tumefaciens during in planta colonization, and the bacterium uses Tde to attack both intraspecies and interspecies bacterial competitors. The widespread conservation of the Tde toxin and Tdi immunity across bacterial genomes suggests that an appropriate combination of a functional T6SS and a broad toxin repertoire is key to niche colonization within a polymicrobial environment.
Results
Atu4350 Is an A. tumefaciens T6SS-Dependent Effector
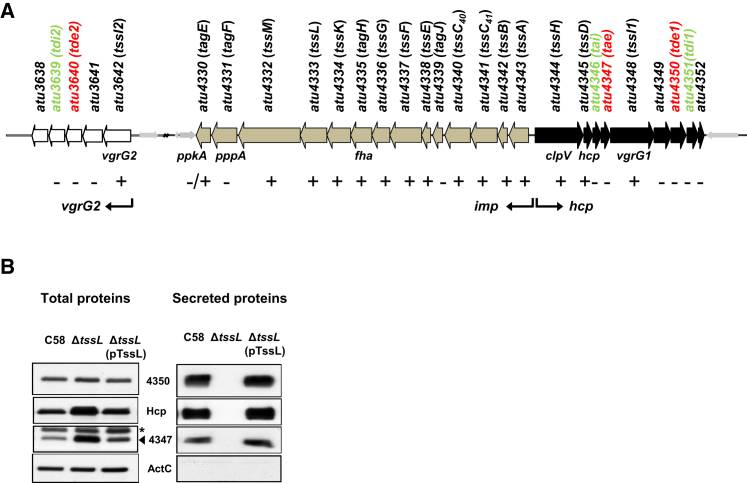
A. tumefaciens strain C58 contains a T6SS gene cluster in which 14 of 23 genes are essential for the assembly of a functional type VI secretion machinery (Lin et al., 2013). The other genes are dispensable because the secretion of Hcp, a hallmark for T6SS activity, is not significantly affected in corresponding mutants (Figure 1A) (Lin et al., 2013). The gene atu4347, which is located in the so-called hcp operon (Figure 1A), encodes a T6SS-secreted protein predicted to act as a peptidoglycan amidase (Lin et al., 2013). The gene atu4347 and its neighboring gene atu4346 encode proteins orthologous to the S. marcescens T6SS antibacterial toxin secreted small protein (Ssp), belonging to the amidase family 4, and a cognate immunity, classified as resistance-associated protein (Rap), respectively (English et al., 2012; Russell et al., 2012). Because several genes encoded in the hcp operon (Figure 1A) are dispensable for type VI secretion, additional T6SS toxin-immunity gene pairs may exist within this operon.
Figure 1.
Atu4350 Is an A. tumefaciens T6SS-Dependent Effector
(A) A. tumefaciens T6SS consists of the major T6SS gene cluster containing two operons, imp (in gray; atu4343 to atu4330) and hcp (in black; atu4344 to atu4352), and another divergent operon named vgrG2 (in white; atu3642 to atu3638) (Lin et al., 2013). The genes are indicated with locus/common names and/or designated as tss (type VI secretion) or tag (type VI secretion-associated gene) based on the proposed nomenclature (Shalom et al., 2007). The three toxins and their cognate immunity proteins identified in this study are indicated in red and green, respectively. The genes, which are essential, nonessential, or partially required for Hcp secretion, are flagged as (+), (−) or (−/+), respectively.
(B) Secretion of Atu4350 is T6SS dependent. Total and secreted proteins were isolated from wild-type C58, ΔtssL mutant, and the complemented strain ΔtssL(pTssL) grown on AB-MES minimal agar (pH 5.5) for 24 hr at 25°C for western blot analysis of nonsecreted protein ActC (Liu et al., 2008), Hcp, and Atu4347, known T6SS-dependent secreted proteins. Asterisk ∗ indicates the cross-reacting band of the antibody against Atu4347.
Attempts to delete the atu4351 gene were unsuccesful (Lin et al., 2013), which suggests that it may encode for a potential immunity protein protecting against the activity of a cognate toxin. This toxin is probably encoded by the adjacent gene, atu4350, and the secretion of Atu4350 is indeed readily detectable with growth of A. tumefaciens on acidic AB-MES minimal medium (pH 5.5), as was shown for the secretion of Hcp or Atu4347 (Figure 1B) (Lin et al., 2013, 2014; Ma et al., 2009, 2012; Wu et al., 2012; Wu et al., 2008). The secretion of Atu4350 is T6SS dependent, since it was abolished in a T6SS mutant, ΔtssL (Figure 1B).
A Superfamily of Type VI DNase Effectors
Atu4350 is annotated as a hypothetical protein, and no functional domains were identified by a BLASTP search of the NCBI database. A screening of the Pfam database linked the Atu4350 protein to a recently identified superfamily containing the putative domain toxin_43 (PF15604) (Zhang et al., 2012). This superfamily displays a conserved putative catalytic motif HxxD and exhibits an all-alpha helical fold feature (Figures 2A; Figure S1 available online). Furthermore, the members of this family are distinct from known polymorphic toxins and have been tentatively assigned a putative RNase activity (Zhang et al., 2012).
Figure 2.
A Superfamily of Type VI DNase Effectors
(A) Partial sequence alignment of the representative Tde superfamily proteins that contain the toxin_43 domain showing the conserved HxxD catalytic motif. The locus tag and organism name are on the left, and the amino acid position of residues in the alignment is indicated on each side of the sequences. The conserved amino acid residues are shaded in black for identity and in gray for similarity. Asterisks (∗) indicate amino acids in the HxxD catalytic motif, which were targeted for mutagenesis.
(B) In vitro DNase activity assay. The integrity of plasmid DNA (pTrc200) coincubated with purified proteins of the wild-type 4350 (WT) or the H190A D193A catalytic site mutant in the presence (+) or absence (−) of Mg2+ at 37°C for 1 hr was visualized with 1% agarose gel. Plasmid DNA with buffer (−) was a control.
(C) Detection of DNA fragmentation by TUNEL assay and analysis by cell sorting. E. coli cells containing pJN105 (vector) or derivatives expressing the wild-type Atu4350 or H190A D193A catalytic site mutant were induced by L-arabinose. Cells were fixed and stained with FITC-dUTP to detect the fragmented DNA by monitoring fluorescence intensity (indicated on the x axis) by cell sorting. FITC-labeled cells are indicated as positive, and cells with background FITC signal are indicated as negative. The counts for cell sorting are indicated on the y axis. Similar results were obtained from at least two independent experiments. See also Figures S1 and S2.
To investigate whether Atu4350 harbors a nuclease activity, we overexpressed a C-terminal His6-tagged fusion of the protein in Escherichia coli. Atu4350 was then purified in the presence of Atu4349, which resulted in increased Atu4350 yield and stability (Figures S2A and S2B). Atu4350 did not display a detectable RNase activity in vitro (Figure S2C). Instead, it showed a Mg2+-dependent DNase activity, as seen by the rapid degradation of supercoiled plasmidic DNA (pTrc200) (Figure 2B). The conserved HxxD motif is required for this DNase activity, since an Atu4350 derivative bearing amino acid substitutions within this motif (H190A D193A) lost its ability to degrade the pTrc200 plasmid (Figure 2B). To assess the DNase activity in vivo, the atu4350 gene and its derivatives were cloned under the control of an arabinose-inducible pBAD promoter in the plasmid pJN105. Induction of atu4350 expression resulted in rapid degradation of the pTrc200 and pJN105 (or derivatives) plasmids (Figure S2D). Cells producing the Atu4350 variant with substitutions in the HxxD motif showed no DNase activity (Figure S2D). The Atu4350-dependent DNA fragmentation was also characterized by using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) with 3′-OH termini of DNA breaks labeled with FITC-dUTP. TUNEL-positive cells (FITC labeled) were observed in E. coli cells producing only wild-type Atu4350 but not the Atu4350 variant (H190A D193A) (Figure 2C). More precisely, ∼50% of cells expressing Atu4350 but only ∼8% of cells producing the Atu4350 variant (H190A D193A) showed FITC labeling. Our results establish that Atu4350 is a bona fide DNase.
Three Toxin-Immunity Pairs in A. tumefaciens
The A. tumefaciens T6SS activity also relies on the expression of an operon encoding vgrG2, which is functionally redundant with vgrG1 for Hcp secretion (Lin et al., 2013) (Figure 1A). Standard bioinformatic tools showed that Atu3640 and Atu3639, encoded within the so-called vgrG2 operon (Figure 1A), are homologuous to Atu4350 and Atu4351, respectively (Figures 2A, S1, and S3). As observed with Atu4350, Atu3640 also possesses a C-terminal toxin_43 domain, and production of Atu3640 in E. coli cells caused rapid degradation of plasmidic DNA (Figure S2E).
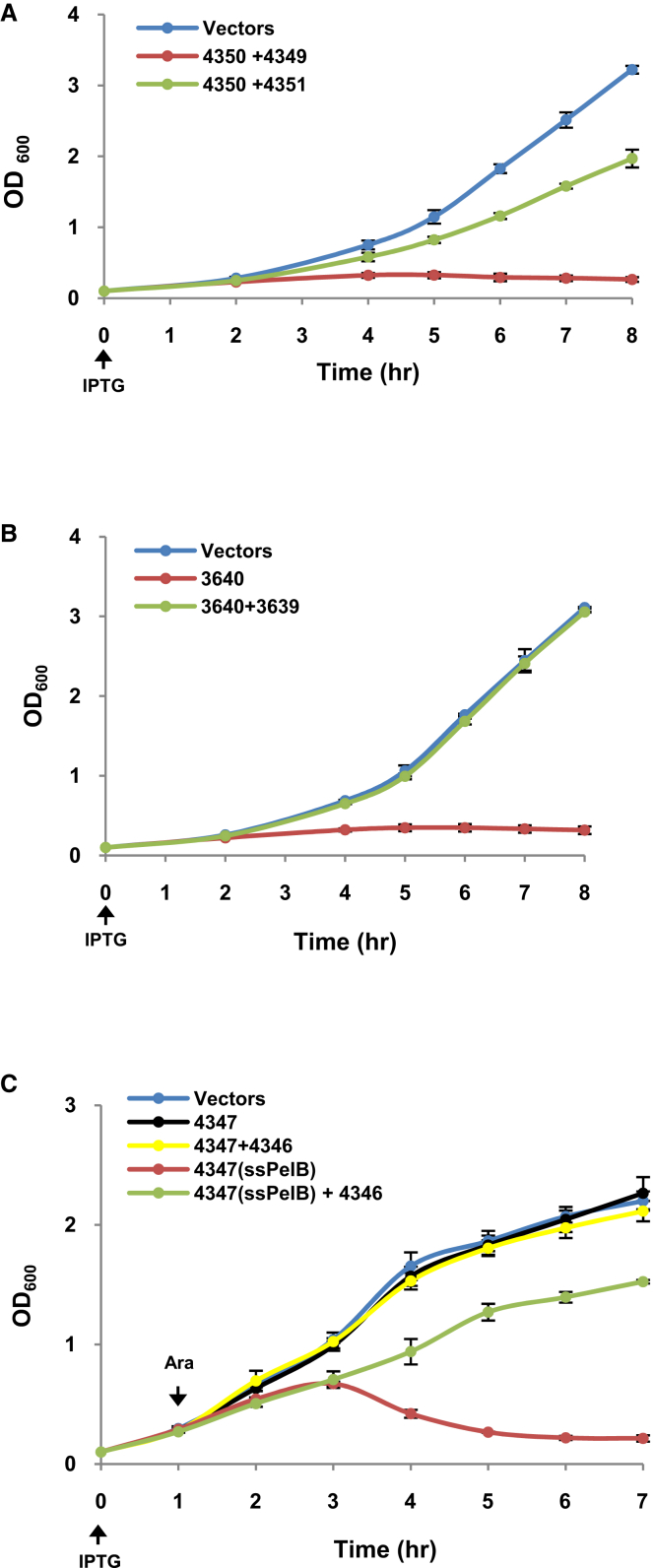
Collectively, our results suggest that Atu4350-Atu4351 and Atu3640-Atu3639, together with the Atu4347-Atu4346 proteins, are potential T6SS toxin-immunity pairs in A. tumefaciens. Atu4350 and Atu3640 have DNase activity, whereas Atu4347 is a putative peptidoglycan amidase (English et al., 2012). We used a strategy based on the coproduction of a given toxin-immunity pair to investigate the role of the putative immunity in protecting against the adverse effects of the toxin. The toxin gene was cloned under the control of an inducible promoter, whereas the putative cognate immunity gene was expressed from a compatible plasmid. The growth of A. tumefaciens cells harboring the vector controls increased steadily over time, with no growth upon induction of atu4350 and atu3640 expression (Figures 3A and 3B). The growth inhibition exerted by Atu4350 and Atu3640 was readily alleviated by the coexpression of the cognate immunity genes atu4351 and atu3639, respectively (Figures 3A and 3B). Atu4350 and Atu3640 exert a toxic effect via their DNase activity when produced within the cytoplasm, whereas the putative peptidoglycan amidase activity of Atu4347 is likely to occur within the periplasm. Indeed, the fusion of Atu4347 to a cleavable N-terminal Sec-dependent signal peptide (ssPelB) led to a significant growth inhibition (Figure 3C). The growth inhibitory effect of Atu4347 was neutralized by the coexpression of the cognate immunity gene atu4346, predicted to encode a protein bearing a typical N-terminal signal peptide (data not shown).
Figure 3.
Three Toxin-Immunity Pair Analysis
(A and B) Cultures of A. tumefaciens wild-type C58 harboring the vectors (pTrc200 and pRL662) or derivatives were supplemented with 1 mM IPTG (at time 0 hr) for growth curve analysis. Atu4350 was produced from plasmid pTrc200, and the putative immunity protein Atu4351 or Atu4349 was constitutively expressed from plasmid pRL662 (A). Atu3640 was produced from plasmid pTrc200, and the putative immunity protein Atu3639 was constitutively expressed from plasmid pRL662 (B).
(C) E. coli DH10B cultures were induced at 0 hr with 1 mM IPTG for 1 hr to produce the putative immunity protein Atu4346 from plasmid pTrc200, then L-arabinose (Ara) induction of Atu4347 with or without signal peptide (ssPelB) from plasmid pJN105. Cell growth was monitored by measuring OD600 at 1 hr intervals. The growth of control cells carrying empty vectors was monitored in parallel. Data are mean ±SE of three (A) or two ([B] and [C]) independent experiments.
In conclusion, we identified three toxin-immunity pairs. The Atu4347-Atu4346 pair belongs to the family type VI secretion amidase effector and immunity (Tae-Tai), and the toxin likely targets the peptidoglycan. Atu4350 and Atu3640 represent a family of T6SS toxins and are named Tde1 and Tde2, respectively, for Tde. Their cognate immunity proteins Atu4351 and Atu3639 are named Tdi1 and Tdi2, respectively.
The A. tumefaciens T6SS Has a Role in Bacterial Competition
The role of the three A. tumefaciens T6SS toxins Tae, Tde1, and Tde2 was assessed in bacterial competition, with T6SS-negative E. coli K12 cells used as prey cells (Dong et al., 2013; English et al., 2012; Hachani et al., 2013; Hood et al., 2010; Russell et al., 2011, 2012, 2013). A. tumefaciens and E. coli strains carrying gentamicin resistance were cocultured on LB (pH 7.0) or acidic AB-MES (pH 5.5) agar, and E. coli survival was monitored by counting gentamicin-resistant colony-forming units. E. coli survival was greatly reduced when cocultured with wild-type A. tumefaciens strain C58, as compared to E. coli alone or the A. tumefaciens T6SS mutant, ΔtssL (Figures S4A and S4B). Importantly, a strain presenting a functional T6SS, as shown by the high levels of Hcp secretion (Figure S5A), but lacking all toxin-immunity pairs (Δ3TIs) was unable to kill E. coli. These results demonstrate the antibacterial activity of the A. tumefaciens T6SS, which is relying on at least one of the three identified toxins, Tae, Tde1, or Tde2.
Tde Toxins Equip A. tumefaciens with a Plant Colonization Advantage
Despite its usefulness in identifying T6SS antibacterial activity, the E. coli K12 model does not provide information on whether a specific set of toxins can be advantageous for A. tumefaciens. Thus, we investigated the function of the T6SS antibacterial activity during interbacterial competition between A. tumefaciens strains. The A. tumefaciens attacker strain was mixed with target strains carrying gentamicin resistance to allow the quantification of surviving cells. Although Tde1 and Tae were readily secreted when bacteria were grown on acidic AB-MES agar plate (Figure 1B), the A. tumefaciens wild-type C58 strain had no significant growth advantage when cocultured with the strain Δ3TIs (Figure S4C).
However, the above described phenotypes may result from the limitations of an in vitro setup, which prompted us to assess the T6SS antibacterial activity in an environment closer to the in vivo situation. We thus assessed whether a functional T6SS and the associated toxins may give A. tumefaciens an advantage for survival inside the host plant. We used a combination of A. tumefaciens strains, which contain attacker and target cells, in coinfection assays. These strains carried the plasmid pRL662 encoding gentamicin resistance or pTrc200 conferring spectinomycin resistance, which allowed for selecting surviving cells within what we define here as the target cell population. The assay involved coinfiltration of A. tumefaciens attacker and target strains into Nicotiana benthamiana leaves (Anand et al., 2007). Coinfection with the A. tumefaciens wild-type C58 attacker strain caused a ∼5-fold decrease in surviving cell numbers of the Δ3TIs target strain in comparison to the C58 target strain (Figure 4A). In contrast, coincubation of the Δ3TIs target strain with an attacker strain lacking a functional T6SS, ΔtssL, or the three T6SS toxins, Δ3TIs, resulted in wild-type levels of fitness. These results strongly suggest that the A. tumefaciens T6SS and its associated toxins provide a competitive advantage to this bacterium during plant colonization.
Figure 4.
A. tumefaciens Intraspecies Competition In Planta
The A. tumefaciens attacker strain was mixed with the target strain harboring plasmids pRL662 or pTrc200 at 10:1 (attacker: target) ratio and infiltrated into N. benthamiana leaves. The survival of target cells was quantified by counting CFUs on antibiotics-containing LB agar.
(A) Attackers are wild-type C58, ΔtssL, or Δ3TIs (Δtae-tai, Δtde1-tdi1, Δtde2-tdi2) coinfected with target strains C58 or Δ3TIs.
(B) Attacker wild-type C58 was tested against target mutants lacking single (Δtae-tai, Δ4349-tde1-tdi1, or Δtde2-tdi2) or triple toxin-immunity pairs (Δ3TIs).
(C) The attacker strain C58 was coinfected with the target strains (Δ4349-tde1-tdi1 or Δtde2-tdi2) harboring plasmid pTrc200 (Vector) or derivatives expressing the cognate immunity gene.
(D) Attacker strains containing vector pTrc200 (Vec) or derivatives expressing wild-type (WT) or catalytic site mutants of Tde1 (H190A D193A, H190A, or D193A) were tested against the target mutant strain Δ4349-tde1-tdi1 harboring pRL662 plasmid. Data are mean ±SE ([B]: n = 3; [A], [C], and [D]: n = 4). Significant difference compared with C58 or Vec was denoted as ∗∗∗ = p < 0.0005, ∗∗ = p < 0.005, and ∗ = p < 0.05. See also Figures S4 and S5.
We monitored the contribution of each individual toxin-immunity pair in this experimental model. Target strains lacking Tde1-Tdi1 or Tde2-Tdi2 toxin-immunity pairs lost their competitive advantage against the wild-type C58 attacker (Figure 4B). Furthermore, the expression of a tdi immunity gene in the absence of the corresponding tde toxin gene was sufficient to protect the target strain against killing by the C58 attacker (Figure 4C). In contrast, the Δtae-tai mutant showed wild-type levels of fitness, which suggests that both Tde1 and Tde2, but not Tae, are crucial for A. tumefaciens competition during colonization in planta (Figure 4B). These observations are further supported by evidence showing that the presence of either of the tde-tdi toxin-immunity pairs is sufficient to attack the Δ3TIs target strain, but this ability is lost if the attacker is a double tde-tdi deletion mutant (Δtde1-tdi1Δtde2-tdi2) (Figure S4D). Importantly, attacking strains producing any variants of the Tde1 proteins (H190A, D193A, or H190A D193A substitutions) were unable to inhibit the growth of target cells (Figure 4D), which suggests that the Tde DNase activity was essential for providing the competitive advantage. Of note, mutations in the HxxD motif did not affect the secretion of Tde1, Hcp, or Tae (Figure S5B). These observations highlight the decisive role played by the Tde DNase toxins and their cognate immunity proteins in the fitness of A. tumefaciens during the colonization of the plant host.
A. tumefaciens T6SS Toxins Trigger a P. aeruginosa Counterattack In Vitro
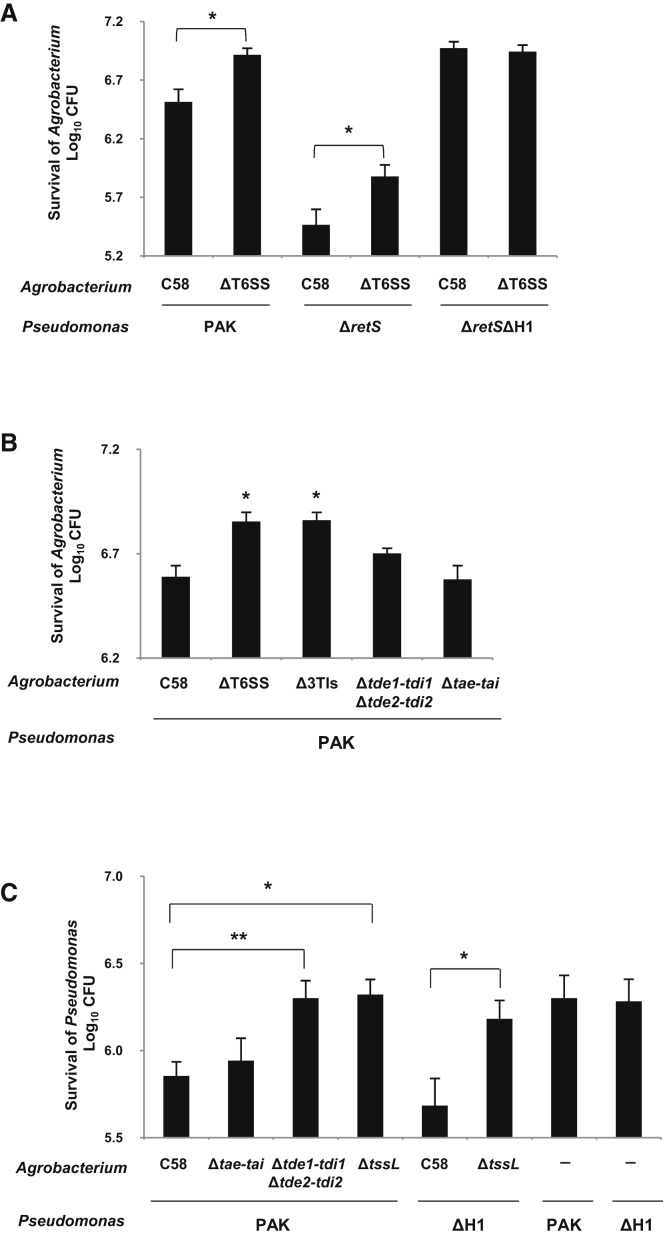
Because multiple microbial taxa coexist as communities to compete for resources, we further investigated the impact of the Agrobacterium T6SS activity in the frame of an interspecies context. P. aeruginosa is an opportunistic pathogen for humans and plants (Rahme et al., 1995), but it also coexists with A. tumefaciens as common residents in freshwater, bulk soil, and the rhizosphere (Hu et al., 2003; Schmeisser et al., 2003; Troxler et al., 1997). We examined A. tumefaciens-P. aeruginosa competition in both in vitro and in vivo assays. For competition assay in vitro, we designed coculture conditions on LB agar (pH 7.0) for which type VI secretion is observed in both strains (Hachani et al., 2011) (Figure S5C) and measured the competition outcomes. Even though A. tumefaciens and P. aeruginosa cells were cocultured in equal amounts, P. aeruginosa outcompeted A. tumefaciens by at least 100-fold after 16 hr of coincubation (Figure S4E). H1-T6SS is constitutively active in the P. aeruginosa strain PAKΔretS (Hachani et al., 2011), and this strain exerted a stronger inhibition on A. tumefaciens growth than the wild-type PAK strain (Figure 5A). Strikingly, upon contact with P. aeruginosa, the number of viable A. tumefaciens wild-type C58 cells was ∼5-fold lower than the isogenic ΔT6SS strain, suggesting that A. tumefaciens T6SS activity can trigger a P. aeruginosa counterattack. P. aeruginosa H1-T6SS is required for this counterattack as a mutant lacking this cluster (ΔretSΔH1) was unresponsive to A. tumefaciens (Figure 5A). An A. tumefaciens mutant lacking all three pairs of toxin-immunity genes (Δ3TIs) displayed a higher survival rate when cocultured with the P. aeruginosa wild-type strain (Figure 5B). Because the A. tumefaciens strain Δ3TIs was still T6SS active (as shown by Hcp secretion) (Figure S5A), the presence of a functional T6SS may not be sufficient for A. tumefaciens to trigger a P. aeruginosa counterattack. Of note, the A. tumefaciens wild-type C58, as well as the isogenic variants Δtde1-tdi1Δtde2-tdi2 and Δtae-tae mutants, could still deliver at least one T6SS toxin and were all killed by P. aeruginosa (Figure 5B). These data suggest that the injection of A. tumefaciens T6SS toxins was required to trigger a P. aeruginosa counterattack.
Figure 5.
A. tumefaciens-P. aeruginosa Competition Assays
(A and B) P. aeruginosa and A. tumefaciens cells were mixed equally and cocultured on LB agar ([A] and [B]) or coinfected in planta (C).
(A) P. aeruginosa wild-type PAK, PAKΔretS (ΔretS), or PAKΔretSΔH1 (ΔretSΔH1) was cocultured with A. tumefaciens wild-type C58 or T6SS mutant (ΔT6SS).
(B) P. aeruginosa PAK was mixed with one of the A. tumefaciens strains C58, ΔT6SS, Δ3TIs, Δtde1-tdi1Δtde2-tdi2, or Δtae-tai mutant.
(C) Cells of P. aeruginosa and A. tumefaciens harboring pRL662 derivative were mixed equally and infiltrated into N. benthamiana leaves. P. aeruginosa cell number was scored after 16 hr incubation at 37°C on LB agar without any antibiotics. Data are mean ±SE ([A]: n = 4–6; [B] and [C]: n = 3–4). Significant difference compared with C58 was denoted as ∗∗ = p < 0.005 and ∗ = p < 0.05. See also Figures S4 and S5.
A. tumefaciens Uses Tde as a Weapon against P. aeruginosa In Planta
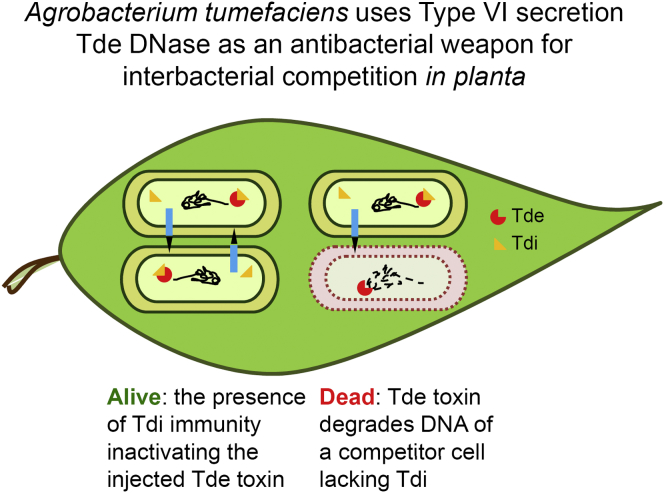
The advantage provided by the Tde toxins to A. tumefaciens when grown in planta (Figure 4) but not in vitro (Figure S4C) underlines the importance of a physiologically relevant environment for studying bacterial fitness. Thus, we investigated whether the relationship between A. tumefaciens and P. aeruginosa could differ in planta. Remarkably, the survival of P. aeruginosa wild-type PAK and its isogenic H1-T6SS mutant (ΔH1) was reduced by ∼5-fold following 24 hr coinfection with A. tumefaceins wild-type C58 in leaves of N. benthamiana (Figure 5C). In contrast, we detected no significant growth difference for A. tumefaciens strains grown alone or coinfected with P. aeruginosa inside the host plant (Figure S4F). The P. aeruginosa attack against A. tumefaciens observed in vitro may be totally inefficient or prevented in planta. Furthermore, the Δtae-tai strain retained the ability to attack P. aeruginosa, but ΔtssL or a strain lacking both tde-tdi (Δtde1-tdi1Δtde2-tdi2) were unable to kill P. aeruginosa (Figure 5C). During plant colonization, A. tumefaciens is able to attack P. aeruginosa by using a functional T6SS and the Tde toxins, whereas the Tae toxin does not seem to act as a potent effector in this context. All together, the Tde DNase toxins may be pivotal antibacterial toxins that A. tumefaciens uses against competitors during in planta colonization, as shown by the different competition scenarios illustrated in Figure 6.
Figure 6.

Illustration of A. tumefaciens Interbacterial Competition during In Planta Colonization
A. tumefaciens wild-type C58 (WT, green) injects Tde toxin (red or green circle) via the T6SS puncturing device drawn between the cells.
(A) None of the A. tumefaciens siblings is killed because of the presence of the Tdi immunity protein (orange or light green triangle) inactivating the injected Tde toxin from the WT.
(B) With Δtae-tai lacking an amidase toxin-immunity pair (light blue), no killing occurs because Tae toxin is not the major antibacterial weapon during in planta colonization.
(C) Injection of Tde toxin from WT A. tumefaciens to its sibling Δtde-tdi mutant (light blue) lacking the cognate immunity protein results in cell death caused by degradation of cellular DNA.
(D) Injection of Tde toxin from WT A. tumefaciens to P. aeruginosa (pink) results in cell death caused by degradation of cellular DNA.
The Tde-Tdi Couple Is Conserved among Bacterial Species
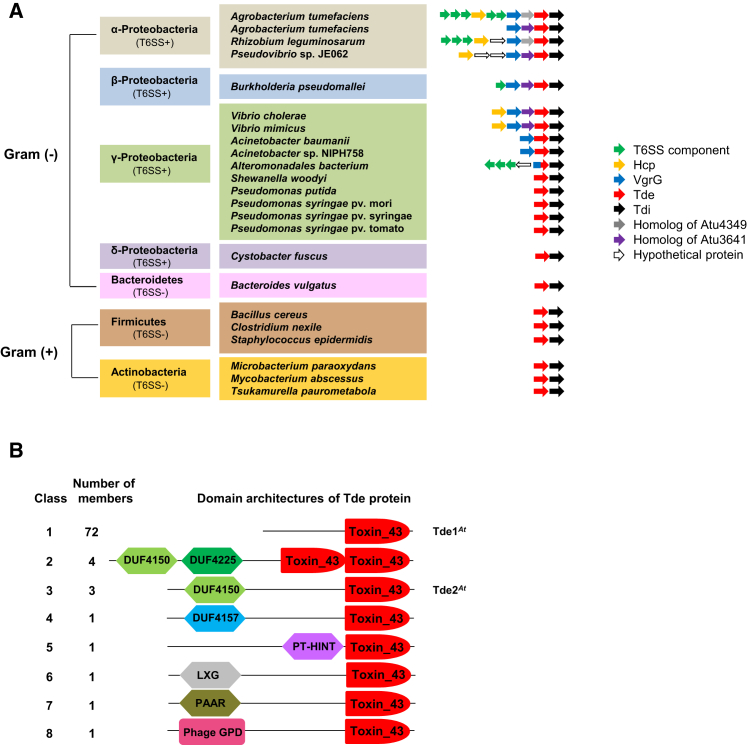
The identification of Tde toxins and the characterization of their role in plant colonization by A. tumefaciens prompted us to explore whether the Tde family is prevalent in plant-associated bacteria. The results obtained by BLASTP sequence homology search and the information extracted from the Pfam database highlighted the conservation of Tde-like proteins harboring the putative toxin_43 domain across several bacterial phyla (Figure 7A). The Tde-like superfamily can be divided into eight classes depending on the domain organization of the protein, ranging from a single (class 1) or tandem toxin_43 domains (class 2) to fusion with other domains with known or yet-to-be-identified functions (classes 3 to 8) (Figure 7B). Tde1 belongs to class 1, the most frequent, and contains only an identifiable C-terminal toxin_43 domain. Tde2 falls in class 3 and displays a domain of unknown function, DUF4150, within its N-terminal region. According to the Pfam database, this domain shows similarity to the recently characterized proline-alanine-alanine-arginine (PAAR) domain (Shneider et al., 2013), which can also be found in class 7. A direct sequence alignment between DUF4150 and PAAR motif-containing proteins revealed significant conservation between the two domains and suggests that DUF4150 could act as a PAAR-like protein (Figure S6).
Figure 7.
Conservation of Tde-Tdi Families in Bacteria
(A) Representatives of the Tde family (shown in Figure 2A) from Gram (−) Proteobacteria and Bacteroidetes and Gram (+) Firmicutes and Actinobacteria phyla. The genetic organization is deducted from the genome context survey by BLASTP analysis and homologous genes are color-coded according to their known or predicted functions. The presence (indicated as T6SS+) or absence of T6SS (indicated as T6SS−) is based on the BLASTP analysis of the conserved T6SS components TssM, TssB, VgrG, and Hcp.
(B) Eight classes of toxin_43 superfamily (PF15604). Proteins containing the toxin_43 domains are classified into eight classes/architectures according to the Pfam database. The graphical domain composition shows distinct domain organizations from a single to tandem toxin_43 domain fused to domains with known or unknown functions. The number of protein members found in each class is shown and classification of Tde1At (A. tumefaciens Tde1) as class 1 and Tde2At (A. tumefaciens Tde2) as class 3 is indicated. Detailed information for all class members and domain descriptions can be found in the Pfam PF15604 database (http://pfam.xfam.org/family/toxin_43). See also Figures S1, S3, S6, and S7.
The immunity proteins Tdi1 and Tdi2 contain an uncharacterized GAD-like and DUF1851 domains, which are well-conserved features in other putative Tdi homologs (Figure S3). Notably, the tde-tdi gene pair is conserved in Gram-negative Proteobacteria harboring T6SS features and highly prevalent in a wide range of plant pathogens (e.g., Pseudomonas syringae pv. syringae, Pseudomonas syringae pv. tomato), symbionts (e.g., Rhizobium leguminosarum), and plant growth-promoting bacteria (e.g., Pseudomonas putida), which further suggests their potential role for colonization in planta. The tde-tdi gene pair is also found in T6SS-negative organisms including Gram-positive Firmicutes (e.g., Bacillus cereus, Staphylococcus epidermidis) and Actinobacteria (e.g., Mycobacterium abscessus) as well as Gram-negative Bacteroidetes (e.g., Bacteroides vulgatus) (Figure 7A). This observation would imply the presence of alternative secretion mechanisms for Tde transport or other functions yet to be identified in this subset of microorganisms.
Discussion
In a form of bacterial warfare involving the T6SS nanomachine, peptidoglycan (English et al., 2012; Russell et al., 2011, 2012) and membrane lipids (Russell et al., 2013) were shown to be the main targets for T6SS toxins. Our discovery of a superfamily of DNases (Tde), together with the recently identified VgrG-dependent Rhs DNases (Koskiniemi et al., 2013) and predicted polymorphic nuclease toxins (Zhang et al., 2012), expands the repertoire of characterized T6SS-dependent antibacterial toxins. The Tde DNase toxins identified in this present study do not share homology with Rhs or any other characterized bacterial DNases (Figure S7), which suggests a unique biochemical activity for the Tde toxins.
The widespread presence of tde-tdi couples in divergent bacterial phyla reveals the conservation of this family of toxin-immunity pairs. The presence of a genetic linkage between vgrG and tde-tdi genes in most analyzed Proteobacteria agrees with previous observations that vgrG genes are often linked to genes encoding toxins (Koskiniemi et al., 2013; Russell et al., 2013). Two recent reports further demonstrated the requirement of the cognate VgrG for specific toxin-mediated antibacterial activity (Hachani et al., 2014; Whitney et al., 2014). Considering the genetic linkage between vgrG1 and tde1-tdi1 or vgrG2 and tde2-tdi2 in A. tumefaciens, VgrG1 and VgrG2 may bind specifically to Tde1 and Tde2, respectively, either directly or indirectly, to facilitate their secretion and delivery in the target cells.
Interestingly, the domain modularity observable in the Tde superfamily further supports the use of distinct transport mechanisms for each Tde class, as was generally suggested for the T6SS (Shneider et al., 2013). For example, Tde1 contains only a recognizable C-terminal toxin_43 domain, whereas Tde2 contains an additional N-terminal DUF4150 domain that shares sequence similarity with PAAR motif-containing proteins. This PAAR superfamily of proteins was recently described to sharpen the VgrG spike and to act as an adaptor to facilitate T6SS-mediated secretion of a broad range of toxins (Filloux, 2013; Shneider et al., 2013). Thus, the DUF4150 motif within the Tde2 toxin may be required to adapt or connect the protein at the tip of a VgrG spike to allow for delivery. The DUF4150 domain is also found in class 2 Tde toxins and can have a similar function for this subclass of proteins. Additional adaptor domains including known PAAR domain and other uncharacterized domains located at the N-terminal sequence of different Tde subclasses may be candidates for this function. In contrast, independent adaptor domains could be involved, as it would be the case for Tde1, which does not display any recognizable domain at its N terminus.
Of note, the importance of the T6SS and its associated toxins varies substantially depending on which set of bacteria are placed in competition and whether this occurs during in vitro or in vivo situations. Our findings that A. tumefaciens was outcompeted by P. aeruginosa in vitro is consistent with previous observation for significant competitive advantage of P. aeruginosa over A. tumefaciens in both planktonic and biofilm growth (An et al., 2006). The mechanisms for the domination of P. aeruginosa involve a faster growth rate, motility, and an unknown compound(s) capable of dispersal and inhibition of A. tumefaciens biofilm (An et al., 2006; Hibbing and Fuqua, 2012). Interestingly, in addition to its obvious growth advantage over A. tumefaciens under laboratory growth conditions, P. aeruginosa further triggers a lethal counterattack against T6SS-active A. tumefaciens. This phenomenon is clearly reminiscent of the recently described T6SS-dueling behavior (Basler and Mekalanos, 2012), with P. aeruginosa using a “tit-for-tat” strategy to counterattack threatening cells such as Vibrio cholerae or Acinetobacter baylyi (Basler et al., 2013). In regards to the A. tumefaciens-P. aeruginosa competition in vitro, the danger signal sensed by P. aeruginosa may be represented by the injected toxin and not the T6SS machinery itself. P. aeruginosa was recently found to induce a lethal T6SS counterattack in response to the T4SS mating system (Ho et al., 2013). In our study, the “T6SS counter-attack” trigger was not restricted to the Tde injection but was also effective with the injection of Tae, which alters the integrity of the bacterial cell envelope. Thus, the P. aeruginosa T6SS response may result from sensing a wide variety of cellular perturbation, including DNA damage or membrane/cell wall damage.
The competition outcomes and the relationship between A. tumefaciens and P. aeruginosa appear to vary greatly when switching from an in vitro to an in vivo environmental context. Inside the host plant, A. tumefaciens exhibits the T6SS- and Tde-dependent competitive advantage over P. aeruginosa, which suggests that the plant environment is a crucial determinant for the selection of the fittest A. tumefaciens strains. It is also striking that this competitive advantage for A. tumefaciens during intraspecies interaction is only observed in planta but not during in vitro growth, even though both antibacterial activity and type VI secretion were readily detected in vitro. While the molecular mechanisms and biological significance underlying this observation await future investigation, we speculated that A. tumefaciens may be able to recognize Agrobacterium or Rhizobiaceae-specific components that are absent in other distantly related bacteria such as E. coli and P. aeruginosa and choose not to attack its own siblings during free-living environment. Once A. tumefaciens successfully infects the host plant, A. tumefaciens may adjust its antibacterial stragtegy to attack all other nonisogenic bacteria at both intraspecies and interspecies levels, aiming to secure the nutrient for its own replication in the apoplast. It is worth mentioning that Agrobacterium T6SS may be also regulated by nutrients as type VI secretion is active in neutral rich medium 523 (Wu et al., 2008) or LB (Figure S5C) but not in minimal AB-MES medium (pH 7.0) (Wu et al., 2012). Thus, A. tumefaciens seems to regulate T6SS activity at multiple levels with complex mechanisms in response to different environmental cues. Therefore, beyond acidity (Wu et al., 2012), additional plant signal(s) may be required to trigger the ability of A. tumefaciens in differentiating self from nonself in order to attack coexisting competitors in the same ecological niche. Recent findings for a role of T6SS in export of self-identity proteins to provide a competitive advantage and territoriality in the social bacterium Proteus mirabilis indeed support the importance of self-recognition in interbacterial interactions (Alteri et al., 2013; Wenren et al., 2013).
The use of Tde as an antibacterial toxin to increase the fitness of A. tumefaciens during plant colonization lends support to their key role in a physiological and ecological context. This finding presents an unprecedented role of T6SS effector activity for bacterial competitive advantage at both intraspecies and interspecies levels inside a plant host. The distribution of tandem tde-tdi genes in the genomes of plant-associated bacteria suggests the conservation of this mechanism among other phytobacteria. Similar benefits were observed in the human pathogen V. cholerae during colonization of the infant rabbit intestine (Fu et al., 2013). Whereas A. tumefaciens uses the Tde DNases as major weapons to attack both its own siblings and P. aeruginosa during in planta colonization, V. cholerae delivers VgrG3 to target peptidoglycan of competing siblings for survival inside the animal host. In both cases, the cognate immunity is essential for this in vivo competitive advantage and sufficient to protect the toxin-producing bacterium from killing. In conclusion, the in vivo fitness advantage conferred by the T6SS for both plant and animal pathogens offers a unique perspective in the evaluation of the T6SS in the host, particularly within a polymicrobial environment.
Experimental Procedures
Bacterial Strains and Plasmids
Strains, plasmids, and primer sequences used in this study are in Tables S1 and S2. E. coli and P. aeruginosa strains were grown in LB, whereas 523 medium (Kado and Heskett, 1970) was routinely used for A. tumefaciens strains unless indicated. Growth conditions and mutant construction are as previously described (Lossi et al., 2013; Ma et al., 2009).
Bioinformatics Analysis
All sequences identified in this study were obtained from the NCBI database (http://www.ncbi.nlm.nih.gov/). Tde family proteins were identified by a BLASTP search with the amino acid sequence of the toxin_43 domain (defined by Pfam database, http://pfam.xfam.org/) for Tde1 (Atu4350) and Tde2 (Atu3640) against the non-redundant protein database to identify the Tde homologs with E value < 10−4 and extracted from the Pfam toxin_43 (PF15604) database. The Tde family was aligned by use of ClustalW on EMBL-EBI website (http://www.ebi.ac.uk/), and the secondary structure for the Tde1 toxin_43 domain was predicted by using the PSIPRED server (http://bioinf.cs.ucl.ac.uk/psipred/). Sequence logos were generated manually by examining the genome context of the neighbor genes. The presence of a signal peptide was predicted by using SignalP (http://www.cbs.dtu.dk/services/SignalP/).
In Vitro DNase Activity Assay
Plasmid DNA of pTrc200 (1 μg) was incubated with purified C-terminal His-tagged Tde1 or Tde1 derivative (H190A D193A) (0.5 μg) in 15 μl of 10 mM Tris/HCl (pH 7.5) for 1 hr at 37°C in the presence or absence of 2 mM Mg2+. Plasmid DNA with sample buffer served as a control. The integrity of DNA was visualized on 1% agarose gel. Tde proteins were overexpressed and purified from E. coli by nickel chromatography with details described in Supplemental Experimental Procedures.
Plasmid DNA Degradation Analysis in E. coli Cells
Overnight cultures of E. coli DH10B strain harboring the empty vectors or derivatives expressing Tde toxins were harvested and adjusted to an OD600 0.3 containing 0.2% L-arabinose for a further 2 hr to produce Tde toxins. Equal cell mass was collected, and plasmid DNA was extracted within an equal volume for DNA gel analysis.
Secretion Assay
Secretion assay from liquid culture was performed in LB or AB-MES for 4–6 hr at 25°C, as previously described (Ma et al., 2009). For detecting secretion on agar plate, A. tumefaciens cells were grown in liquid 523 for 16 hr at 28°C. The harvested cells were adjusted to OD600 1 with AB-MES (pH 5.5) (Lai and Kado, 1998), and 100 μl of cell suspension was spread and incubated on an AB-MES (pH 5.5) agar plate for 24 hr at 25°C. Cells were collected in 5 ml AB-MES (pH 5.5) and secreted protein was analyzed as described (Ma et al., 2009).
Growth Inhibition Assay
Overnight cultures of E. coli DH10B strain harboring vectors or their derivatives were adjusted to OD600 0.1. Expression of the tested immunity protein was induced by 1 mM IPTG for 1 hr before L-arabinose (0.2% final concentration) was added to induce expression of the toxin. For growth inhibition assay with A. tumefaciens, overnight cultures of A. tumefaciens C58 strain harboring empty vectors or their derivatives were adjusted to OD600 0.1. The tested immunity protein was constitutively expressed, and the toxin protein was induced with 1 mM IPTG. The growth was monitored by measuring OD600 at 1 hr intervals.
Interbacterial Competition Assay
The in planta competition assay was carried out by infiltration of bacterial cells into leaves of Nicotiana benthamiana, and the bacterial cell number was counted after 24 hr incubation at room temperature. Interbacterial competition assay on agar plate was performed by coculture on LB (pH 7.0) or AB-MES (pH 5.5) agar at 25°C for 16 hr. The competition outcome was quantified by counting colony forming units (CFU) on selective LB agar. All assays were performed with at least three independent experiments or a minimum of three biological replicates from two independent experiments. Data represent mean ±SE of all biological replicates. Statistics were calculated by Student’s t test, and the p value was denoted as ∗∗∗ = p < 0.0005, ∗∗ = p < 0.005, and ∗ = p < 0.05. Detailed methods and associated references are described in Supplemental Experimental Procedures.
TUNEL and Fluorescence-Activated Cell Sorting Analysis
Overnight culture of E. coli DH10B strains harboring the pJN105 vector or derivatives expressing Tde toxins were harvested, fixed, and stained by Apo-Direct Kit (BD Bioscience), and the intensity of fluorescence was determined by MoFlo XDP Cell Sorter (Beckman Coulter) and Summit V 5.2 software. Detailed methods and associated references are described in Supplemental Experimental Procedures.
Acknowledgments
The authors acknowledge Fred Ausubel, Jen Sheen, Chih-Horng Kuo, and Hanna Yuan for critical reading of this manuscript and the members of the Lai and Filloux laboratories for discussion. We also thank the technical assistance of Wen-Ching Lin with the secretion assay and the Flow Cytometry Core Facility and DNA Sequencing Laboratories at the Institute of Plant and Microbial Biology, Academia Sinica, for fluorescence-activated cell sorting analysis and DNA sequencing, respectively. This work was supported by the 2011 Taiwan Initiative Research Cooperation among Top Universities between UK and Taiwan from the National Science Council (NSC 100-2911-I-001-038) to E.M.L. and A.F., research grants from the National Science Council (NSC 98-2311-B-001 -002 -MY3 and NSC 101-2321-B-001 -033 -) to E.M.L., and the Medical Research Council grant MR/K001930/1 and the Wellcome Trust grant WT091939 to A.F. L.S.M. received postdoctoral fellowships from the National Science Council (NSC 100-2911-I-001-038) and Academia Sinica. J.S.L. received a postdoctoral fellowship from the National Science Council (101-2321-B-001 -033 -).
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Contributor Information
Alain Filloux, Email: a.filloux@imperial.ac.uk.
Erh-Min Lai, Email: emlai@gate.sinica.edu.tw.
Supplemental Information
References
- Alteri C.J., Himpsl S.D., Pickens S.R., Lindner J.R., Zora J.S., Miller J.E., Arno P.D., Straight S.W., Mobley H.L. Multicellular bacteria deploy the type VI secretion system to preemptively strike neighboring cells. PLoS Pathog. 2013;9:e1003608. doi: 10.1371/journal.ppat.1003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Martinez C.E., Christie P.J. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An D., Danhorn T., Fuqua C., Parsek M.R. Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proc. Natl. Acad. Sci. USA. 2006;103:3828–3833. doi: 10.1073/pnas.0511323103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Vaghchhipawala Z., Ryu C.M., Kang L., Wang K., del-Pozo O., Martin G.B., Mysore K.S. Identification and characterization of plant genes involved in Agrobacterium-mediated plant transformation by virus-induced gene silencing. Mol. Plant Microbe Interact. 2007;20:41–52. doi: 10.1094/MPMI-20-0041. [DOI] [PubMed] [Google Scholar]
- Basler M., Mekalanos J.J. Type 6 secretion dynamics within and between bacterial cells. Science. 2012;337:815. doi: 10.1126/science.1222901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M., Pilhofer M., Henderson G.P., Jensen G.J., Mekalanos J.J. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012;483:182–186. doi: 10.1038/nature10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M., Ho B.T., Mekalanos J.J. Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell. 2013;152:884–894. doi: 10.1016/j.cell.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz J., Meinhart A. Antibacterial effector/immunity systems: it’s just the tip of the iceberg. Curr. Opin. Microbiol. 2014;17:1–10. doi: 10.1016/j.mib.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Bönemann G., Pietrosiuk A., Mogk A. Tubules and donuts: a type VI secretion story. Mol. Microbiol. 2010;76:815–821. doi: 10.1111/j.1365-2958.2010.07171.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Patzer S.I. Intercellular communication by related bacterial protein toxins: colicins, contact-dependent inhibitors, and proteins exported by the type VI secretion system. FEMS Microbiol. Lett. 2013;345:13–21. doi: 10.1111/1574-6968.12180. [DOI] [PubMed] [Google Scholar]
- Brooks T.M., Unterweger D., Bachmann V., Kostiuk B., Pukatzki S. Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J. Biol. Chem. 2013;288:7618–7625. doi: 10.1074/jbc.M112.436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet Y.R., Hénin J., Celia H., Cascales E. Type VI secretion and bacteriophage tail tubes share a common assembly pathway. EMBO Rep. 2014;15:315–321. doi: 10.1002/embr.201337936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E. The type VI secretion toolkit. EMBO Rep. 2008;9:735–741. doi: 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T.G., Ho B.T., Yoder-Himes D.R., Mekalanos J.J. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2013;110:2623–2628. doi: 10.1073/pnas.1222783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English G., Trunk K., Rao V.A., Srikannathasan V., Hunter W.N., Coulthurst S.J. New secreted toxins and immunity proteins encoded within the Type VI secretion system gene cluster of Serratia marcescens. Mol. Microbiol. 2012;86:921–936. doi: 10.1111/mmi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A. Microbiology: a weapon for bacterial warfare. Nature. 2013;500:284–285. doi: 10.1038/nature12545. [DOI] [PubMed] [Google Scholar]
- Filloux A., Hachani A., Bleves S. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology. 2008;154:1570–1583. doi: 10.1099/mic.0.2008/016840-0. [DOI] [PubMed] [Google Scholar]
- Fu Y., Waldor M.K., Mekalanos J.J. Tn-Seq analysis of vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe. 2013;14:652–663. doi: 10.1016/j.chom.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin S.B. Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu. Rev. Phytopathol. 2010;48:45–68. doi: 10.1146/annurev-phyto-080508-081852. [DOI] [PubMed] [Google Scholar]
- Hachani A., Lossi N.S., Hamilton A., Jones C., Bleves S., Albesa-Jové D., Filloux A. Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. J. Biol. Chem. 2011;286:12317–12327. doi: 10.1074/jbc.M110.193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachani A., Lossi N.S., Filloux A. A visual assay to monitor T6SS-mediated bacterial competition. J. Vis. Exp. 2013;73:e50103. doi: 10.3791/50103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachani A., Allsopp L.P., Oduko Y., Filloux A. The VgrG proteins are “A la carte” delivery systems for bacterial type VI effectors. J. Biol. Chem. 2014 doi: 10.1074/jbc.M114.563429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbing M.E., Fuqua C. Inhibition and dispersal of Agrobacterium tumefaciens biofilms by a small diffusible Pseudomonas aeruginosa exoproduct(s) Arch. Microbiol. 2012;194:391–403. doi: 10.1007/s00203-011-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B.T., Basler M., Mekalanos J.J. Type 6 secretion system-mediated immunity to type 4 secretion system-mediated gene transfer. Science. 2013;342:250–253. doi: 10.1126/science.1243745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B.T., Dong T.G., Mekalanos J.J. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe. 2014;15:9–21. doi: 10.1016/j.chom.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood R.D., Singh P., Hsu F., Güvener T., Carl M.A., Trinidad R.R., Silverman J.M., Ohlson B.B., Hicks K.G., Plemel R.L. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.Y., Fan Y., Lin Y.H., Zhang H.B., Ong S.L., Dong N., Xu J.L., Ng W.J., Zhang L.H. Microbial diversity and prevalence of virulent pathogens in biofilms developed in a water reclamation system. Res. Microbiol. 2003;154:623–629. doi: 10.1016/j.resmic.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kado C.I., Heskett M.G. Selective media for isolation of Agrobacterium, Carynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathology. 1970;60:969–976. doi: 10.1094/phyto-60-969. [DOI] [PubMed] [Google Scholar]
- Kapitein N., Bönemann G., Pietrosiuk A., Seyffer F., Hausser I., Locker J.K., Mogk A. ClpV recycles VipA/VipB tubules and prevents non-productive tubule formation to ensure efficient type VI protein secretion. Mol. Microbiol. 2013;87:1013–1028. doi: 10.1111/mmi.12147. [DOI] [PubMed] [Google Scholar]
- Koskiniemi S., Lamoureux J.G., Nikolakakis K.C., t’Kint de Roodenbeke C., Kaplan M.D., Low D.A., Hayes C.S. Rhs proteins from diverse bacteria mediate intercellular competition. Proc. Natl. Acad. Sci. USA. 2013;110:7032–7037. doi: 10.1073/pnas.1300627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E.M., Kado C.I. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 1998;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman P.G., Basler M., Ramagopal U.A., Bonanno J.B., Sauder J.M., Pukatzki S., Burley S.K., Almo S.C., Mekalanos J.J. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. USA. 2009;106:4154–4159. doi: 10.1073/pnas.0813360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoux M., De Leon J.A., Kuwada N.J., Russell A.B., Pinto-Santini D., Hood R.D., Agnello D.M., Robertson S.M., Wiggins P.A., Mougous J.D. Quantitative single-cell characterization of bacterial interactions reveals type VI secretion is a double-edged sword. Proc. Natl. Acad. Sci. USA. 2012;109:19804–19809. doi: 10.1073/pnas.1213963109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.S., Ma L.S., Lai E.M. Systematic dissection of the agrobacterium type VI secretion system reveals machinery and secreted components for subcomplex formation. PLoS ONE. 2013;8:e67647. doi: 10.1371/journal.pone.0067647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.S., Wu H.H., Hsu P.H., Ma L.S., Pang Y.Y., Tsai M.D., Lai E.M. Fha interaction with phosphothreonine of TssL activates type VI secretion in Agrobacterium tumefaciens. PLoS Pathog. 2014;10:e1003991. doi: 10.1371/journal.ppat.1003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A.C., Shih H.W., Hsu T., Lai E.M. A citrate-inducible gene, encoding a putative tricarboxylate transporter, is downregulated by the organic solvent DMSO in Agrobacterium tumefaciens. J. Appl. Microbiol. 2008;105:1372–1383. doi: 10.1111/j.1365-2672.2008.03874.x. [DOI] [PubMed] [Google Scholar]
- Lossi N.S., Manoli E., Förster A., Dajani R., Pape T., Freemont P., Filloux A. The HsiB1C1 (TssB-TssC) complex of the Pseudomonas aeruginosa type VI secretion system forms a bacteriophage tail sheathlike structure. J. Biol. Chem. 2013;288:7536–7548. doi: 10.1074/jbc.M112.439273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.S., Lin J.S., Lai E.M. An IcmF family protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its walker a motif is required for type VI secretion system-mediated Hcp secretion in Agrobacterium tumefaciens. J. Bacteriol. 2009;191:4316–4329. doi: 10.1128/JB.00029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.S., Narberhaus F., Lai E.M. IcmF family protein TssM exhibits ATPase activity and energizes type VI secretion. J. Biol. Chem. 2012;287:15610–15621. doi: 10.1074/jbc.M111.301630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S., Ma A.T., Revel A.T., Sturtevant D., Mekalanos J.J. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. USA. 2007;104:15508–15513. doi: 10.1073/pnas.0706532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahme L.G., Stevens E.J., Wolfort S.F., Shao J., Tompkins R.G., Ausubel F.M. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- Russell A.B., Hood R.D., Bui N.K., LeRoux M., Vollmer W., Mougous J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–347. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.B., Singh P., Brittnacher M., Bui N.K., Hood R.D., Carl M.A., Agnello D.M., Schwarz S., Goodlett D.R., Vollmer W., Mougous J.D. A widespread bacterial type VI secretion effector superfamily identified using a heuristic approach. Cell Host Microbe. 2012;11:538–549. doi: 10.1016/j.chom.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.B., LeRoux M., Hathazi K., Agnello D.M., Ishikawa T., Wiggins P.A., Wai S.N., Mougous J.D. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature. 2013;496:508–512. doi: 10.1038/nature12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.B., Peterson S.B., Mougous J.D. Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 2014;12:137–148. doi: 10.1038/nrmicro3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser C., Stöckigt C., Raasch C., Wingender J., Timmis K.N., Wenderoth D.F., Flemming H.C., Liesegang H., Schmitz R.A., Jaeger K.E., Streit W.R. Metagenome survey of biofilms in drinking-water networks. Appl. Environ. Microbiol. 2003;69:7298–7309. doi: 10.1128/AEM.69.12.7298-7309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom G., Shaw J.G., Thomas M.S. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153:2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- Shneider M.M., Buth S.A., Ho B.T., Basler M., Mekalanos J.J., Leiman P.G. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature. 2013;500:350–353. doi: 10.1038/nature12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler J., Azelvandre P., Zala M., Defago G., Haas D. Conjugative transfer of chromosomal genes between fluorescent Pseudomonads in the rhizosphere of wheat. Appl. Environ. Microbiol. 1997;63:213–219. doi: 10.1128/aem.63.1.213-219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenren L.M., Sullivan N.L., Cardarelli L., Septer A.N., Gibbs K.A. Two independent pathways for self-recognition in Proteus mirabilis are linked by type VI-dependent export. MBio. 2013;4 doi: 10.1128/mBio.00374-13. e00374–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J.C., Chou S., Russell A.B., Biboy J., Gardiner T.E., Ferrin M.A., Brittnacher M., Vollmer W., Mougous J.D. Identification, structure, and function of a novel type VI secretion peptidoglycan glycoside hydrolase effector-immunity pair. J. Biol. Chem. 2013;288:26616–26624. doi: 10.1074/jbc.M113.488320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J.C., Beck C.M., Goo Y.A., Russell A.B., Harding B.N., De Leon J.A., Cunningham D.A., Tran B.Q., Low D.A., Goodlett D.R. Genetically distinct pathways guide effector export through the type VI secretion system. Mol. Microbiol. 2014;92:529–542. doi: 10.1111/mmi.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.Y., Chung P.C., Shih H.W., Wen S.R., Lai E.M. Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens. J. Bacteriol. 2008;190:2841–2850. doi: 10.1128/JB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.F., Lin J.S., Shaw G.C., Lai E.M. Acid-induced type VI secretion system is regulated by ExoR-ChvG/ChvI signaling cascade in Agrobacterium tumefaciens. PLoS Pathog. 2012;8:e1002938. doi: 10.1371/journal.ppat.1002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., de Souza R.F., Anantharaman V., Iyer L.M., Aravind L. Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol. Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.