Abstract
beta zero-Thalassemia is an inherited disorder characterized by the absence of beta-globin polypeptides derived from the affected allele. The molecular basis for this deficiency is a mutation of the adult beta-globin structural gene or cis regulatory elements that control beta-globin gene expression. A mouse model of this disease would enable the testing of therapeutic regimens designed to correct the defect. Here we report a 16-kb deletion that includes both adult beta-like globin genes, beta maj and beta min, in mouse embryonic stem cells. Heterozygous animals derived from the targeted cells are severely anemic with dramatically reduced hemoglobin levels, abnormal red cell morphology, splenomegaly, and markedly increased reticulocyte counts. Homozygous animals die in utero; however, heterozygous mice are fertile and transmit the deleted allele to progeny. The anemic phenotype is completely rescued in progeny derived from mating beta zero-thalassemic animals with transgenic mice expressing high levels of human hemoglobin A. The beta zero-thalassemic mice can be used to test genetic therapies for beta zero-thalassemia and can be bred with transgenic mice expressing high levels of human hemoglobin HbS to produce an improved mouse model of sickle cell disease.
Full text
PDF




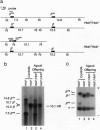
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Townes T. M. Synthesis of functional human hemoglobin in transgenic mice. Science. 1989 Sep 1;245(4921):971–973. doi: 10.1126/science.2772649. [DOI] [PubMed] [Google Scholar]
- Detloff P. J., Lewis J., John S. W., Shehee W. R., Langenbach R., Maeda N., Smithies O. Deletion and replacement of the mouse adult beta-globin genes by a "plug and socket" repeated targeting strategy. Mol Cell Biol. 1994 Oct;14(10):6936–6943. doi: 10.1128/mcb.14.10.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Transcriptional regulation of multigene loci: multilevel control. Trends Genet. 1993 Apr;9(4):134–137. doi: 10.1016/0168-9525(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Engel J. D. Developmental regulation of human beta-globin gene transcription: a switch of loyalties? Trends Genet. 1993 Sep;9(9):304–309. doi: 10.1016/0168-9525(93)90248-g. [DOI] [PubMed] [Google Scholar]
- Fabry M. E., Costantini F., Pachnis A., Suzuka S. M., Bank N., Aynedjian H. S., Factor S. M., Nagel R. L. High expression of human beta S- and alpha-globins in transgenic mice: erythrocyte abnormalities, organ damage, and the effect of hypoxia. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12155–12159. doi: 10.1073/pnas.89.24.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., Nagel R. L., Pachnis A., Suzuka S. M., Costantini F. High expression of human beta S- and alpha-globins in transgenic mice: hemoglobin composition and hematological consequences. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12150–12154. doi: 10.1073/pnas.89.24.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Fraser P., Vidal M. A., Hedges M. J., Ropers D., Luzzatto L., Grosveld F. A transgenic mouse model of sickle cell disorder. Nature. 1990 Jan 11;343(6254):183–185. doi: 10.1038/343183a0. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- McCune S. L., Reilly M. P., Chomo M. J., Asakura T., Townes T. M. Recombinant human hemoglobins designed for gene therapy of sickle cell disease. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9852–9856. doi: 10.1073/pnas.91.21.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992 May;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Witkowska H. E., Spangler E., Curtin P., Lubin B. H., Mohandas N., Clift S. M. Hypoxia-induced in vivo sickling of transgenic mouse red cells. J Clin Invest. 1991 Feb;87(2):639–647. doi: 10.1172/JCI115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Townes T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Behringer R. R. Human sickle hemoglobin in transgenic mice. Science. 1990 Feb 2;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- Shehee W. R., Loeb D. D., Adey N. B., Burton F. H., Casavant N. C., Cole P., Davies C. J., McGraw R. A., Schichman S. A., Severynse D. M. Nucleotide sequence of the BALB/c mouse beta-globin complex. J Mol Biol. 1989 Jan 5;205(1):41–62. doi: 10.1016/0022-2836(89)90363-x. [DOI] [PubMed] [Google Scholar]
- Shehee W. R., Oliver P., Smithies O. Lethal thalassemia after insertional disruption of the mouse major adult beta-globin gene. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3177–3181. doi: 10.1073/pnas.90.8.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Polsky F. I., Seidman J. G., Leder A., Edgell M. H., Leder P. A comparison of two cloned mouse beta-globin genes and their surrounding and intervening sequences. Cell. 1978 Jun;14(2):237–245. doi: 10.1016/0092-8674(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Behringer R. R. Human globin locus activation region (LAR): role in temporal control. Trends Genet. 1990 Jul;6(7):219–223. doi: 10.1016/0168-9525(90)90182-6. [DOI] [PubMed] [Google Scholar]
- Trudel M., De Paepe M. E., Chrétien N., Saadane N., Jacmain J., Sorette M., Hoang T., Beuzard Y. Sickle cell disease of transgenic SAD mice. Blood. 1994 Nov 1;84(9):3189–3197. [PubMed] [Google Scholar]
- Trudel M., Saadane N., Garel M. C., Bardakdjian-Michau J., Blouquit Y., Guerquin-Kern J. L., Rouyer-Fessard P., Vidaud D., Pachnis A., Roméo P. H. Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J. 1991 Nov;10(11):3157–3165. doi: 10.1002/j.1460-2075.1991.tb04877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S., Comer M. B., Jahn C. L., Hutchison C. A., 3rd, Edgell M. H. The adult beta-globin genes of the "single" type mouse C57BL. Cell. 1981 May;24(2):403–411. doi: 10.1016/0092-8674(81)90330-5. [DOI] [PubMed] [Google Scholar]