Abstract
Angiotensin II (Ang-II) stimulates vascular inflammation, oxidative stress, and formation and rupture of intracranial aneurysms in mice. Because angiotensin 1-7 (Ang-1-7) acts on Mas receptors and generally counteracts deleterious effects of Ang-II, we tested the hypothesis that Ang-1-7 attenuates formation and rupture of intracranial aneurysms. Intracranial aneurysms were induced in wild type and Mas receptor deficient mice using a combination of Ang-II-induced hypertension and intracranial injection of elastase in the basal cistern. Mice received elastase+Ang-II alone, or a combination of elastase+Ang-II+Ang-1-7. Aneurysm formation, prevalence of subarachnoid hemorrhage, mortality, and expression of molecules involved in vascular injury were assessed. Systolic blood pressure was similar in mice receiving elastase+Ang-II (148±5 mmHg, mean ±SE) or elastase+Ang-II+Ang-1-7 (144±5 mmHg). Aneurysm formation was also similar in mice receiving elastase+Ang-II (89%) or elastase+Ang-II+Ang-1-7 (84%). However, in mice that received elastase and Ang II, Ang-1-7 reduced mortality (from 64 to 36%, p<0.05) and prevalence of subarachnoid hemorrhage (from 75 to 48%, p<0.05). In cerebral arteries, expression of the inflammatory markers, Nox2 and catalase increased similarly in elastase+Ang-II or elastase+Ang-II+Ang-1-7 groups. Ang-1-7 increased expression of cyclooxygenase-2, and decreased expression of metalloproteinase 9 induced by elastase+Ang-II (p<0.05). In Mas receptor deficient mice, systolic blood pressure, mortality, and prevalence of subarachnoid hemorrhage were similar (p>0.05) in groups treated with elastase+Ang-II or elastase+Ang-II+Ang-1-7. Expression of Mas receptor was detected by immunohistochemistry in samples of human intracranial arteries and aneurysms. In conclusion, without attenuating Ang-II-induced hypertension, Ang-1-7 decreased mortality and rupture of intracranial aneurysms in mice, through a Mas receptor-dependent pathway.
Keywords: Angiotensin-1-7, intracranial aneurysm, subarachnoid hemorrhage, Mas receptor, hypertension
INTRODUCTION
With the exception of surgical interventions, treatment options for intracranial aneurysms are limited, thus greater insight into molecular mechanisms that control formation and rupture of intracranial aneurysms may lead to new treatment options. The wall of human intracranial aneurysms is rich in inflammatory cells and molecules1–3. Inflammation may contribute to formation of cerebral aneurysms, with disruption of the elastic membrane, which ultimately may contribute to aneurysm rupture. Angiotensin II (Ang-II) increases expression of pro-inflammatory cytokines and oxidative stress in blood vessels and stimulates remodeling of the extracellular matrix in blood vessels4. Although Ang-II plays a critical role in formation and rupture of abdominal aortic aneurysms (AAA)5, its role in formation and rupture of intracranial aneurysms is not clear.
Angiotensin 1-7 (Ang-1-7) acts as a functional antagonist of Ang-II6–9. Ang-1-7 is a product of the metabolism of Ang-II by the angiotensin converting enzyme type 2 (ACE2)7, 10, 11. When bound to the Mas receptor12, Ang-1-7 reduces inflammation and oxidative stress in peripheral vessels, articular and adipose tissue7, 13, 14. In the current study, we tested the hypothesis that Ang-1-7 decreases rupture of intracranial aneurysms.
METHODS
Experimental animals
Studies were performed in adult (11±1 Mo) wild type (WT) and Mas receptor deficient mice (Mas KO). The mice were bred on the C57BL6 background as described previously15. All experimental protocols and procedures conform to the National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Aneurysms were induced in mice according to published methods16, using the combination of stereotactic injection of elastase in the basal cistern and hypertension induced by systemic administration of Ang-II (or Ang-II+Ang-1-7)using osmoticminipumps. Systolic blood pressure was measured using the tail cuff method. Animals were monitored daily and sacrificed immediately if signs of neurological deficit were apparent, or after 3 weeks. Cerebral arteries isolated from mice with aneurysms, and shams were used for gene expression analysis by real time quantitative polymerase chain reaction.
Human Intracranial Aneurysms
Studies were approved by the University of Iowa Internal Review Board. Samples of intracranial aneurysms and arteries were collected from patients who underwent microsurgical clipping. Expression of Mas receptor was examined usingimmunostaining.
Drugs
Ang-1-7 and Ang-II were obtained from Bachem (Torrance, CA). All other reagents were obtained from Sigma (St Louis, MO).
Statistical analysis
Analysis was performed using Prism 6 (Graphpad, La Jolla, CA). Categorical data (incidence of aneurysms and subarachnoid hemorrhage) were compared between mice treated with Ang-II or Ang-II + Ang 1-7 using one-tailed Fisher’s exact test. Survival was analyzed with log rank (Mantel-Cox) test. Gene expression in cerebral arteries from sham, Ang II and AngII + Ang 1-7 WT mice was analyzed with one way Anova followed by Tukey’s post-hoc test. Gene expression in Mas receptor KO mice treated with Ang II or AngII Ang 1-7 was analyzed with unpaired t-test. A P value less than 0.05 was considered significant.
Additional information can be found in the Online Data Supplement at www.hyper.ahajournals.org
RESULTS
Effect of Angiotensin 1-7 in formation and rupture of intracranial aneurysms
Systolic pressure increased significantly after intracranial stereotactic injection of elastase and implantation of osmotic pumps containing Ang-II (148±5 mmHg, mean ±SE) or Ang-II + Ang-1-7 (144±5 mmHg) (P<0.05) (Figure 1A) vs baseline. Ang-II-induced hypertension was not attenuated by Ang-1-7 after 1, 2 or 3 weeks of treatment.
Figure 1.
Ang-1-7 does not attenuate Ang-II induced hypertension (A) in WT mice. (B) Ang-1-7 decreases mortality (*P<0.05) but does not attenuate aneurysm formation (C). Ang-1-7 decreased prevalence of subarachnoid hemorrhage (P<0.05). n= 28 WT mice treated with elastase+Ang-II and 25 WT mice treated with elastase+Ang-II+ Ang-1-7.
Compared to control mice (Figure 2A), more than 80% of hypertensive mice that received an intracranial injection of elastase displayed evidence of fusiform and/or saccular intracranial aneurysms during necropsy (Figure 2B). Most aneurysms were saccular or a mix of saccular and fusiform aneurysms; fewer than 20% were fusiform aneurysms. In some mice, ruptured aneurysms were identified near areas of subarachnoid hemorrhage (SAH) (Left panel in Figure 2B).
Figure 2.
(A) Cerebral blood vessels in a control mouse (scale bar=1mm) (left). Section of anterior communicating artery (right). (B) Cerebral arteries in situ (left) and after excision (center) from a mouse with several intracranial aneurysms and acute subarachnoid hemorrhage, and histological sections of intracranial aneurysms (right). Sections were stained with Masson’s trichrome (Art: artery; An: Aneurysms. Scale bar= 100µm).
Mortality was higher in mice treated with Ang-II [64% (18/28)] than in mice treated with Ang-II + Ang-1-7 [36% (9/25) p<0.05] (Figure 1B). Ang-1-7 did not attenuate formation of aneurysms (89% (25/28) Ang-II vs. 84% (21/25) Ang-II + Ang-1-7) (Figure 1C). Incidence of subarachnoid hemorrhage (SAH) was lower [48% (12/25)] in Ang-II + Ang-1-7 than in Ang-II treated mice [75% (21/28), p<0.05] (Figure 1D).
Formation and rupture of intracranial aneurysms in Mas KO mice
Similar studies were performed in Mas-KO mice. Increase in systolic pressure was similar in Mas-KO mice after intracranial stereotactic injection of elastase and infusion of Ang-II or Ang-II + Ang-1-7 (136±4 vs. 136±8 mmHg) respectively (Figure 3A). Mortality of Mas-KO mice treated with elastase and Ang-II was lower than in WT mice under the same treatment (P<0.05). In Mas KO mice Ang-1-7 did not reduce mortality (Figure 3B). Ang-1-7 did not attenuate formation of aneurysms in Mas-KO mice treated with Ang-II (84% 16/19 Ang-II vs. 100% (14/14) Ang-II + Ang-1-7 treated mice) (Figure 3C). Ang-1-7 did not reduce incidence of SAH: 53% (10/19) vs. 64% (9/14) in Mas-KO mice treated with Ang-II or Ang-II + Ang-1-7 respectively (p>0.05) (Figure 3D).
Figure 3.
Ang-1-7 does not attenuate Ang-II induced hypertension (A) in Mas-KO mice. Ang-1-7 does not decrease mortality (B) aneurysm formation (C) or prevalence of subarachnoid hemorrhage in Mas KO mice (P>0.05). n= to 19 Mas-KO mice treated with elastase+Ang-II and 15 Mas-KO mice treated with elastase+Ang-II+Ang-1-7.
Expression of genes involved in vascular injury
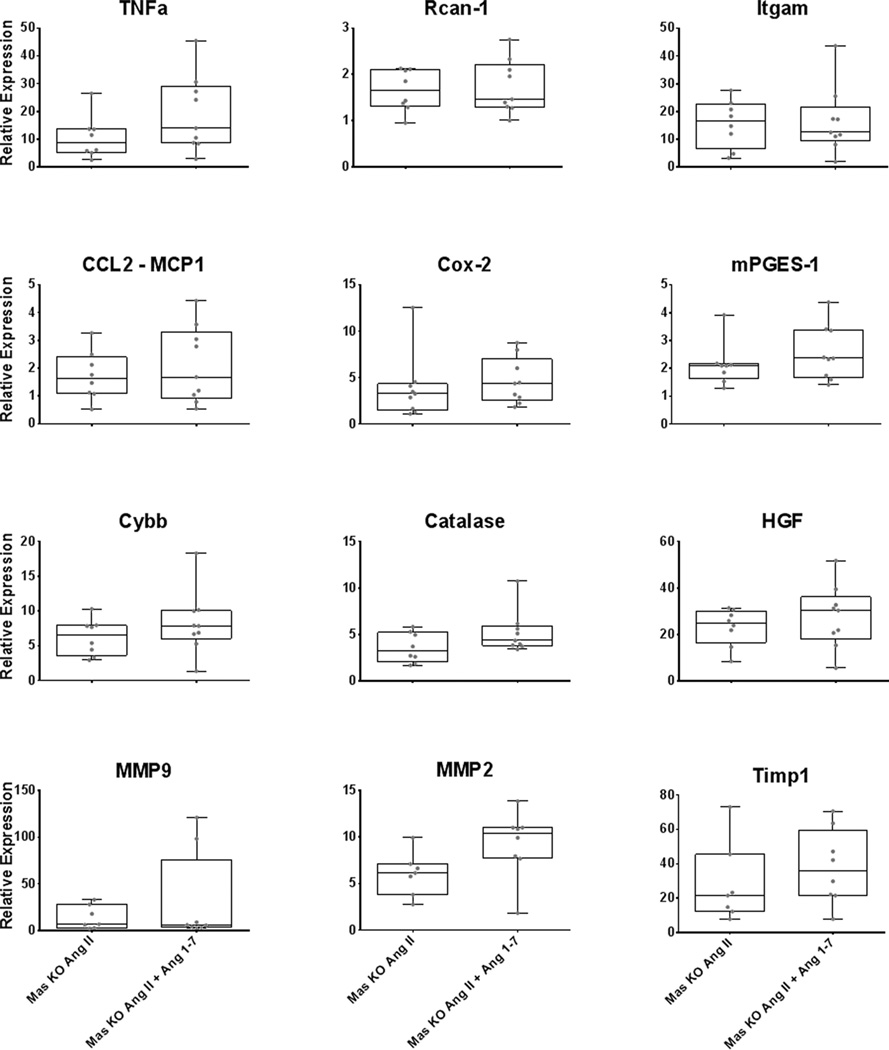
Expression of several genes involved in vascular inflammation, oxidative stress, and extracellular matrix remodeling were examined in cerebral arteries. In WT mice, intracranial injection of elastase and infusion of Ang-II increased expression of the proinflammatory cytokines TNFα, Itgam (a marker of macrophage infiltration), and the proinflammatory enzyme mPGES-1 (P<0.05) (Figure 4). Elastase+Ang-II also increased expression of Nox2, catalase, and the wound repair factor, HGF. Coinfusion of Ang-1-7 did not attenuate expression in cerebral arteries of inflammation mediators/markers or enzymes associated with oxidative stress, but notably increased expression of Cox2. Elastase+Ang-II increased expression of MMP-9, MMP-2, and TIMP-1. Coinfusion of Ang-1-7 markedly attenuated the increase in MMP-9 in cerebral arteries (Figure 4).
Figure 4.
Gene expression in cerebral arteries in WT control mice (sham), and mice in which intracranial aneurysms were induced (mice received elastase+Ang-II or elastase+Ang-II + Ang-1-7). *P<0.05 vs control, †=P<0.05 vs Ang-II. n=8 WT shams, n=8–15 WT mice with aneurysms treated with elastase+Ang-II and 8–12 WT mice with aneurysms treated with elastase+AngII+Ang-1-7.
In Mas receptor deficient mice, combination of intracranial injection of elastase and Ang-II also increased expression of molecules associated with inflammation, oxidative stress and vascular remodeling. In these mice, coinfusion of Ang-1-7 did not alter the changes in gene expression induced by elastase+Ang-II (Figure 5). In Mas-KO mice, coinfusion of Ang-1-7 did not attenuate the increased expression of MMP-9, nor increase expression of Cox2 induced by elastase+Ang-II.
Figure 5.
Gene expression in cerebral arteries from Mas receptor KO mice. Values are from mice treated withelastase+Ang-II or elastase+Ang-II+Ang-1-7, after induction of intracranial aneurysms (results were normalized to WT controls). No significant differences were found. n=7–8 Mas-KO mice with aneurysms treated with elastase+Ang-II and 8–9 Mas-KO mice with aneurysms treated with elastase+Ang-II+Ang-1-7.
Expression of Mas receptor in human aneurysms
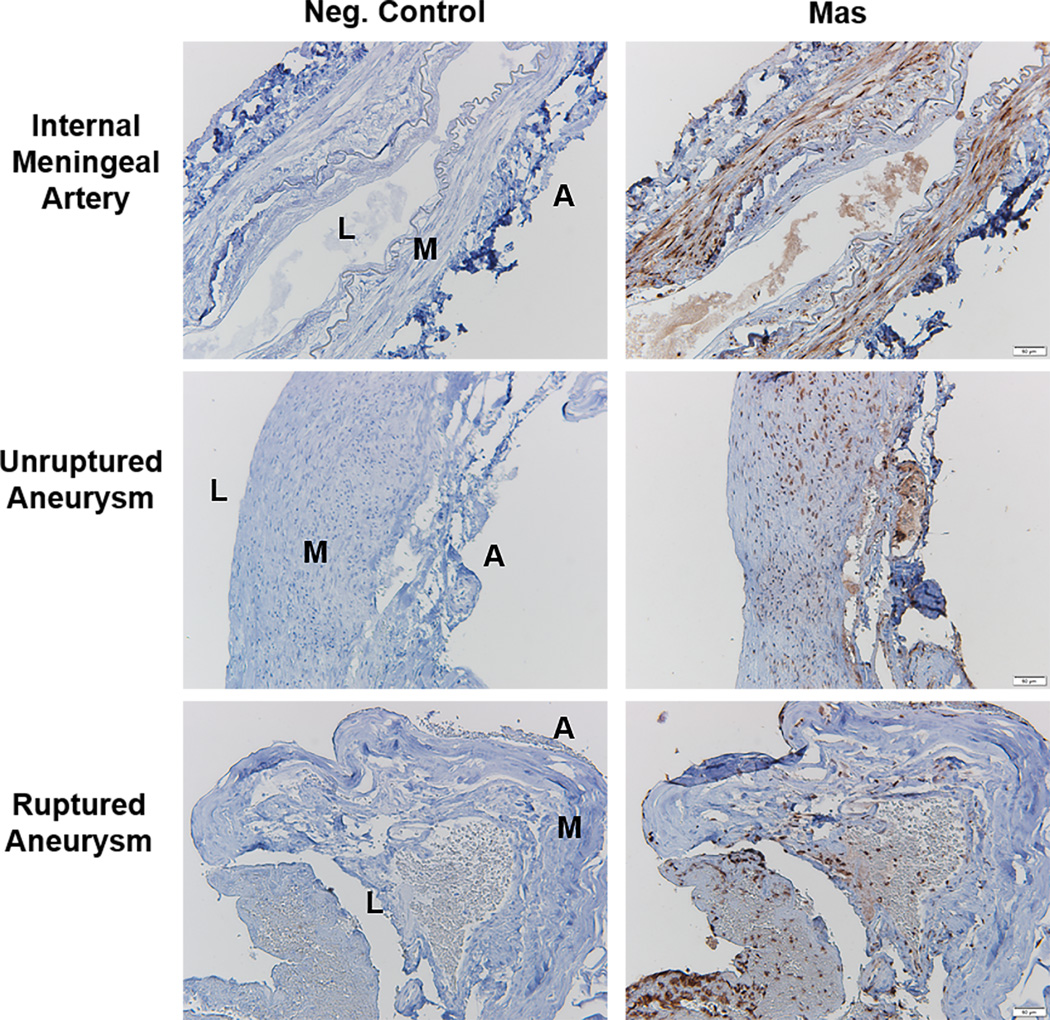
Mas receptor expression was demonstrated in media and intima of control human arteries (meningeal and superficial temporal arteries). Immunostaining for Mas was also positive in sections of unruptured and ruptured human intracranial aneurysms (Figure 6).
Figure 6.
Expression of Mas receptors in human intracranial aneurysms. Positive immunostaining for Mas was seen in samples from meningeal arteries, and in unruptured and ruptured intracranial aneurysms. Negative control immunostaining excluded the primary antibody for Mas. Images shown are representative of 5 meningeal or superficial temporal arteries and 5 unruptured and 3 ruptured aneurysms. L=Lumen; M=Media; A; Adventitia. Scale bar=50µm.
DISCUSSION
In the current study we replicated a model of intracranial aneurysms in mice16. As first demonstrated by Nuki et al16 cerebral aneurysms are produced in about 80 % of mice treated with Ang-II and intracranial injections of elastase. Using this model, we observed that Ang-1-7 decreased mortality and frequency of rupture of intracranial aneurysms in mice. Moreover protective effects of Ang-1-7 on aneurysm rupture were absent in Mas receptor deficient mice. Finally, Ang-1-7 decreased Ang-II-induced increases in expression of MMP-9 in cerebral arteries.
Angiotensin 1-7 has several protective effects in models of stroke17. Ang-1-7 decreased oxidative stress, apoptosis and autophagosome formation in spontaneously hypertensive rats (SHR)18, 19. Ang-1-7 decreased infarct size and neurological deficit after middle cerebral artery occlusion in rats20–22. Moreover, Ang-1-7 increased survival of stroke-prone spontaneously hypertensive rats23. In our study we focused on effects of Ang-1-7 in cerebral arteries in a model of intracranial aneurysms.
Because Ang-1-7 counteracts some of the deleterious effects of Ang-II6–9, we anticipated that Ang-1-7 might reduce susceptibility to cerebral aneurysms in this model. On the other hand, it was not clear whether Ang-1-7 would be sufficiently potent, especially against key mechanisms, to have a detectable effect on aneurysms. A broader implication of our findings is that hypertension, which is often associated with activation of the renin/angiotensin system, is a major risk factor for rupture of aneurysms, and Ang-1-7 may be effective in protection against rupture of aneurysms.
Ang-1-7 attenuated aneurysm rupture but did not reduce the hypertensive effect of Ang-II in our study. Antihypertensive effects of Ang-1-7 are not clear. Although Ang 1-7 decreased blood pressure in SHR24, 25, it failed to reduce blood pressure in other models of hypertension9, 26, 27. Our results agree with studies in which Ang-1-7 did not attenuate the increase in systolic blood pressure induced by Ang-II or DOCA salt9, 26, 27. Similarly, delivery of Ang-1-7 to the cerebral ventricles of SHR decreased brain damage but did not attenuate hypertension19, 23. Thus attenuation of aneurysm rupture by Ang-1-7 is not the result of an antihypertensive action of Ang-1-7.
Inflammation appears to play an important role in rupture of intracranial aneurysms. Proinflammatory enzymes such as Cox2 and mPGES1 are increased in the wall of ruptured cerebral aneurysms in humans1. Infiltration of leukocytes into the cerebral aneurysmal wall has also been found in humans2. Macrophage depletion, or decreased vascular macrophage infiltration in cerebral arteries from MCP-1 deficient mice, is associated with decreased aneurysm formation and rupture in mice3, 28. We found that Ang-II increased the expression of TNFα and mPGES1 in cerebral arteries and increased macrophage infiltration assessed by the specific macrophage marker Itgam.
Ang-1-7 has multiple beneficial actions in blood vessels7. Ang-1-7 dilates cerebral arteries29 and appears to attenuate neurological damage in stroke20–22. Ang-1-7 also increases survival and decreases the number of subcortical hemorrhages in stroke prone hypertensive rats23. Part of the protective effect of Ang-1-7 in stroke appears to be related to its modulatory effects on NFkB20 and inflammation22, 23. It was therefore of interest that Ang-1-7 decreased aneurysm rupture and mortality without decreasing Ang-II induced infiltration of macrophages or overexpression of TNFα and mPGES1 in cerebral arteries.
Ang-1-7 increased Cox-2 expression in cerebral arteries. Although Cox-2 is generally associated with inflammatory responses, it is also responsible for synthesis of prostacyclin, which is vasoprotective30. Protective effects of angiotensin 1-7 in the heart are attenuated by the cyclooxygenase inhibitor, indomethacin31. Thus, although Ang-1-7 did not appear to attenuate inflammation in our study, it is possible that some of the protective effects of Ang-1-7 may be mediated by increased synthesis of prostacyclin through the Cox-2 pathway.
Expression and activation of MMPs plays a critical role in aneurysm rupture. Increased expression of MMP-2 and MMP-9 is seen in ruptured cerebral aneurysms in patients32. Increased expression of MMP-2 and MMP-9 is associated with progression of cerebral aneurysms in rats33. Pharmacological inhibition of MMPs decreases aneurysm rupture in mice34. We found that Ang-1-7 attenuated Ang-II-induced increase in expression of MMP-9. Pathways by which Ang-1-7 or the Mas receptor regulate MMP expression are not known.
Ang-1-7 did not attenuate effects of intracranial injection of elastase and Ang-II in Mas deficient mice. Increased expression of Cox-2 by Ang-1-7 was not observed in Mas-KO mice. Moreover, in contrast to findings in WT mice, Ang-1-7 tended to increase the levels of MMP-2 and MMP-9 in cerebral arteries of Mas-KO mice with intracranial aneurysms. Because Ang1-7 is a weak agonist of Ang-II receptors35, we speculate that, in the absence of Mas receptors, Ang-1-7 may activate AT1 receptors for Ang-II and induce further vascular damage. Our studies in Mas-KO mice indicate that mice deficient in the receptor Mas had a lower mortality. This finding is puzzling, because most literature suggests that activation of Mas receptors generally plays a protective role in disease, thus its deletion would be expected to exacerbate vascular damage. There are, however, exceptions to this generalization, as Mas activation is associated with amelioration of renal36 and cardiac37 disease and liver steatosis38. In these experimental models, genetic deletion of Mas is associated with better outcomes36–38. Little is known about intracellular signaling pathways activated by Mas receptors, or regulation of other receptors such as AT1 by Mas. Thus, we speculate that when Ang-1-7 levels are low, Mas receptors may not signal, or may not regulate activation of pathways such as those activated by AT1 receptors. In contrast, in conditions in which Ang 1-7 levels increase, Mas is activated and can physiologically antagonize other pathways including the Ang-II pathway.
PERSPECTIVE
We demonstrated that the Mas receptor is expressed in the wall of human arteries and intracranial aneurysms. In addition, infusion of Ang-1-7 attenuated aneurysm rupture and mortality in a mouse model of intracranial aneurysms. Ang-1-7 did not decrease expression of markers of inflammation, but regulated the expression of MMP-9 and Cox-2. In conclusion, this study implies a potential novel therapeutic strategy for medical management of intracranial aneurysms. Future studies may explore pharmacological strategies to modulate Ang-1-7 signaling in human intracranial aneurysms via agonists of the Mas receptor.
Supplementary Material
Novelty and Significance.
What Is New?
In a mouse model of intracranial aneurysms, infusion of angiotensin 1-7 (Ang-1-7) reduced prevalence of subarachnoid hemorrhage and mortality.
Ang-1-7 did not attenuate markers of vascular inflammation, or oxidative stress, but reduced expression of MMP-9 and increased expression of Cox-2 in cerebral arteries of mice with intracranial aneurysms.
Protective effects of Ang-1-7 were not seen in mice deficient in Mas, the Ang-1-7 receptor.
What Is Relevant?
Hypertension and inflammation contribute to rupture of intracranial aneurysms.
The finding that Ang-1-7 reduces aneurysm rupture and mortality, without an effect on inflammation or blood pressure, may open a new therapeutic alternative for medical management of intracranial aneurysms.
Summary
Ang-1-7 protects against rupture of cerebral aneurysms, and decreases mortality, in a mouse model of intracranial aneurysms. Ang-1-7 did reduce blood pressure or cerebral vascular inflammation. Ang-1-7 reduced expression of MMP-9, a metalloprotease involved in the pathogenesis of aneurysm rupture. Ang-1-7 also increased Cox-2, an enzyme that synthesizes vasoprotective prostaglandins. Effects of angiotensin 1-7 are mediated by activation of the receptor Mas.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH grant HL-62984, and the Department of Veterans Affairs (BX001399). R.P was supported by a North Shore University-Brain Aneurysm Foundation grant and a Fulbright Scholarship.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Hasan D, Hashimoto T, Kung D, Macdonald RL, Winn HR, Heistad D. Upregulation of cyclooxygenase-2 (Cox-2) and microsomal prostaglandin E2 synthase-1 (mpges-1) in wall of ruptured human cerebral aneurysms: Preliminary results. Stroke. 2012;43:1964–1967. doi: 10.1161/STROKEAHA.112.655829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. J Neuroinflammation. 2012;9:222. doi: 10.1186/1742-2094-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1-7) inhibits angiotensin II-stimulated phosphorylation of map kinases in proximal tubular cells. Kidney Int. 2006;69:2212–2218. doi: 10.1038/sj.ki.5001509. [DOI] [PubMed] [Google Scholar]
- 9.McCollum LT, Gallagher PE, Ann Tallant E. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac remodeling associated with upregulation of dual-specificity phosphatase 1. Am J Physiol Heart Circ Physiol. 2012;302:H801–H810. doi: 10.1152/ajpheart.00908.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 11.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 12.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, Bretas TL, Bader M, de Sousa LP, da Silva TA, dos Santos RA, Simoes e Silva AC, Teixeira MM. Anti-inflammatory effects of the activation of the angiotensin-(1-7) receptor, mas, in experimental models of arthritis. J Immunol. 2010;185:5569–5576. doi: 10.4049/jimmunol.1000314. [DOI] [PubMed] [Google Scholar]
- 14.Santos SH, Fernandes LR, Pereira CS, Guimaraes AL, de Paula AM, Campagnole-Santos MJ, Alvarez-Leite JI, Bader M, Santos RA. Increased circulating angiotensin-(1-7) protects white adipose tissue against development of a proinflammatory state stimulated by a high-fat diet. Regul Pept. 2012;178:64–70. doi: 10.1016/j.regpep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Rabelo LA, Xu P, Todiras M, Sampaio WO, Buttgereit J, Bader M, Santos RA, Alenina N. Ablation of angiotensin (1-7) receptor mas in C57Bl/6 mice causes endothelial dysfunction. J Am Soc Hypertens. 2008;2:418–424. doi: 10.1016/j.jash.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pena Silva RA, Heistad DD. Promising neuroprotective effects of the angiotensin-(1-7)-angiotensin-converting enzyme 2-mas axis in stroke. Exp Physiol. 2014;99:342–343. doi: 10.1113/expphysiol.2013.076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang T, Gao L, Shi J, Lu J, Wang Y, Zhang Y. Angiotensin-(1-7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res. 2013;67:84–93. doi: 10.1016/j.phrs.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Jiang T, Gao L, Zhu XC, Yu JT, Shi JQ, Tan MS, Lu J, Tan L, Zhang YD. Angiotensin-(1-7) inhibits autophagy in the brain of spontaneously hypertensive rats. Pharmacol Res. 2013;71:61–68. doi: 10.1016/j.phrs.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Jiang T, Gao L, Guo J, Lu J, Wang Y, Zhang Y. Suppressing inflammation by inhibiting the nf-kappab pathway contributes to the neuroprotective effect of angiotensin-(1-7) in rats with permanent cerebral ischaemia. Br J Pharmacol. 2012;167:1520–1532. doi: 10.1111/j.1476-5381.2012.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mecca AP, Regenhardt RW, O'Connor TE, Joseph JP, Raizada MK, Katovich MJ, Sumners C. Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke. Exp Physiol. 2011;96:1084–1096. doi: 10.1113/expphysiol.2011.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C. Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology. 2013;71:154–163. doi: 10.1016/j.neuropharm.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, Banuelos C, Bizon JL, Reinhard MK, Sumners C. Centrally administered angiotensin-(1-7) increases the survival of stroke-prone spontaneously hypertensive rats. Exp Physiol. 2014;99:442–453. doi: 10.1113/expphysiol.2013.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benter IF, Ferrario CM, Morris M, Diz DI. Antihypertensive actions of angiotensin-(1-7) in spontaneously hypertensive rats. Am J Physiol. 1995;269:H313–H319. doi: 10.1152/ajpheart.1995.269.1.H313. [DOI] [PubMed] [Google Scholar]
- 25.Costa Ma, Lopez Verrilli Ma, Gomez Ka, Nakagawa P, Peña C, Arranz C, Gironacci MM. Angiotensin-(1-7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H1205–H1211. doi: 10.1152/ajpheart.00850.2009. [DOI] [PubMed] [Google Scholar]
- 26.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton Ta, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am J Physiol Heart Circ Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 27.Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1-7) prevents cardiac fibrosis in doca-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H2417–H2423. doi: 10.1152/ajpheart.01170.2005. [DOI] [PubMed] [Google Scholar]
- 28.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40:942–951. doi: 10.1161/STROKEAHA.108.532556. [DOI] [PubMed] [Google Scholar]
- 29.Feterik K, Smith L, Katusic ZS. Angiotensin-(1-7) causes endothelium-dependent relaxation in canine middle cerebral artery. Brain Res. 2000;873:75–82. doi: 10.1016/s0006-8993(00)02482-3. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Pure E, Funk CD, FitzGerald GA. Vascular cox-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003787. 132ra154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao X, Wang L, Yang C, He J, Wang X, Guo R, Lan A, Dong X, Yang Z, Wang H, Feng J, Ma H. Cyclooxygenase mediates cardioprotection of angiotensin-(1-7) against ischemia/reperfusion-induced injury through the inhibition of oxidative stress. Mol Med Rep. 2011;4:1145–1150. doi: 10.3892/mmr.2011.570. [DOI] [PubMed] [Google Scholar]
- 32.Marchese E, Vignati A, Albanese A, Nucci CG, Sabatino G, Tirpakova B, Lofrese G, Zelano G, Maira G. Comparative evaluation of genome-wide gene expression profiles in ruptured and unruptured human intracranial aneurysms. J Biol Regul Homeost Agents. 2010;24:185–195. [PubMed] [Google Scholar]
- 33.Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38:162–169. doi: 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
- 34.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T. Pharmacological stabilization of intracranial aneurysms in mice: A feasibility study. Stroke. 2012;43:2450–2456. doi: 10.1161/STROKEAHA.112.659821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handa RK. Metabolism alters the selectivity of angiotensin-(1-7) receptor ligands for angiotensin receptors. J Am Soc Nephrol. 2000;11:1377–1386. doi: 10.1681/ASN.V1181377. [DOI] [PubMed] [Google Scholar]
- 36.Esteban V, Heringer-Walther S, Sterner-Kock A, de Bruin R, van den Engel S, Wang Y, Mezzano S, Egido J, Schultheiss HP, Ruiz-Ortega M, Walther T. Angiotensin-(1-7) and the G protein-coupled receptor mas are key players in renal inflammation. PloS one. 2009;4:e5406. doi: 10.1371/journal.pone.0005406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T, Li Z, Dang H, Chen R, Liaw C, Tran TA, Boatman PD, Connolly DT, Adams JW. Inhibition of mas G-protein signaling improves coronary blood flow, reduces myocardial infarct size, and provides long-term cardioprotection. Am J Physiol Heart Circ Physiol. 2012;302:H299–H311. doi: 10.1152/ajpheart.00723.2011. [DOI] [PubMed] [Google Scholar]
- 38.Silva AR, Aguilar EC, Alvarez-Leite JI, da Silva RF, Arantes RM, Bader M, Alenina N, Pelli G, Lenglet S, Galan K, Montecucco F, Mach F, Santos SH, Santos RA. Mas receptor deficiency is associated with worsening of lipid profile and severe hepatic steatosis in ApoE-knockout mice. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1323–R1330. doi: 10.1152/ajpregu.00249.2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.