Abstract
The disruption of neurotransmitter and neurotrophic factor signaling in the central nervous system (CNS) is implicated as the root cause of neuropsychiatric disorders, including schizophrenia, epilepsy, chronic pain, and depression. Therefore, identifying the underlying molecular mechanisms by which neurotransmitter and neurotrophic factor signaling regulates neuronal survival or growth may facilitate identification of more effective therapies for these disorders. Previously, our lab found that the heterotrimeric G protein, Gz, mediates crosstalk between G protein-coupled receptors and neurotrophin signaling in the neural cell line PC12. These data, combined with Gαz expression profiles - predominantly in neuronal cells with higher expression levels corresponding to developmental times of target tissue innervation - suggested that Gαz may play an important role in neurotrophin signaling and neuronal development. Here, we provide evidence in cortical neurons, both manipulated ex vivo and those cultured from Gz knockout mice, that Gαz is localized to axonal growth cones and plays a significant role in the development of axons of cortical neurons in the CNS. Our findings indicate that Gαz inhibits BDNF-stimulated axon growth in cortical neurons, establishing an endogenous role for Gαz in regulating neurotrophin signaling in the CNS.
Keywords: BDNF, GNAZ, G proteins, Neurotrophin
Introduction
Understanding the mechanisms by which neurons develop polarity and extend axons and dendrites is critical for understanding nervous system development and disorders related to this development. While a number of growth factors have been shown to impact neuron development, much is yet to be learned regarding the regulation of intracellular signaling networks that govern this process. Several lines of evidence indicate G protein coupled receptors (GPCRs) that play important roles in synaptic communication may also play a significant role in neuron development (McCobb et al., 1988; Ponimaskin et al., 2007; Prokosch et al., 2010; Reinoso et al., 1996).
Neurotransmitter monoamines, including norepinephrine, serotonin, and dopamine have been shown to augment (Lieske et al., 1999; Reinoso et al., 1996; Song et al., 2004) or inhibit (Haydon et al., 1984; Reinoso et al., 1996; Spencer et al., 1996) neurite growth in a highly context-specific manner. Additionally, several disorders that have been traditionally characterized by disregulation of monoamines have in recent years also been identified as having a developmental and/or neurotrophic basis, some examples include schizophrenia, chronic pain, epilepsy, and depression (Hendry et al., 2000; Hinton et al., 1990; Hisata et al., 2007; Ho and Wong, 2001; Hsu et al., 1979; Huang et al., 1999; Hughes et al., 2001). Together, these findings are suggestive of an important role for G proteins and GPCRs in the regulation of growth pathways during neuron development.
Gαz is a member of the Gαi subfamily of heterotrimeric G proteins, and couples to GPCRs accordingly. Gαz has been shown to preferentially couple to several types of GPCRs in cells and in vivo (Ho and Wong, 2001; Kimple et al., 2009), including the u-opioid (Hendry et al., 2000; Sanchez-Blazquez et al., 2009), α2-adrenergic (Kelleher et al., 2001; Meng and Casey, 2002; Yang et al., 2000), 5-HT1A serotonin (Oleskevich et al., 2005; Serres et al., 2000; van den Buuse et al., 2007), and D2 dopamine (Leck et al., 2006; van den Buuse et al., 2005; Yang et al., 2000) receptors. Coupling to these receptors has been primarily demonstrated through altered behavioral responses to receptor-specific agonists in wild-type and Gαz-null mice. In general, Gαz-null mice exhibit increased anxiety and depressive-like behaviors (Oleskevich et al., 2005; van den Buuse et al., 2007). Evidence for Gz coupling to 5-HT1A serotonin receptors comes from studies showing that Gαz-null mice are insensitive to induction of anxious behaviors by a 5-HT1A agonist (van den Buuse et al., 2007), and show significantly increased amplitudes of 5-HT-mediated potassium current and conductance in CA1 pyramidal neurons (Oleskevich et al., 2005). Evidence that Gz couples to the α2A-adrenergic receptor is supported by decreased platelet aggregation and impaired inhibition of cAMP formation in response to epinephrine in Gαz-null mice (Hsu et al., 1979; Kelleher et al., 2001; Yang et al., 2000, 2002). Gαz-null mice also exhibit a loss of the antidepressant effects of catecholamine reuptake inhibitors reboxitine and desipramine (Hendry et al., 2000; Yang et al., 2000). A role for Gz in dopaminergic signaling was first demonstrated with the finding that Gαz-null mice exhibited a highly exaggerated response to cocaine (Yang et al., 2000), and these mice are less sensitive to the impact a D2-specific receptor agonist in a number of behavioral and physiologic responses (Leck et al., 2006). Gαz-null mice also exhibited altered responses to amphetamine with regard to locomotor activity and prepulse inhibition response (Ralph et al., 1999; van den Buuse et al., 2005).
In almost every case of receptors that couple to Gz, there is evidence suggesting a developmental role for the pathways they control (Haydon et al., 1987; Koert et al., 2001; Prokosch et al., 2010; Reinoso et al., 1996). In this regard, we previously identified a regulatory role for Gαz in neurotrophin signaling and cellular differentiation. Stimulation of α2A-adrenergic receptors in PC12 cells expressing Gαz resulted in impairment of nerve growth factor (NGF)-mediated differentiation (Meng and Casey, 2002). This impact of Gαz on neurotrophin signaling was shown to be mediated through the monomeric GTPase Rap1; activated Gαz blunted both the activation of Rap1 and subsequent phosphorylation of Erk by NGF in PC12 cells (Meng and Casey, 2002).
Gαz tissue distribution is quite restricted, primarily to neuronal and neuroendocrine cells, and the protein has been shown to be highly expressed in cortex, hippocampus, cerebellum, and as well as in ganglion cells of the retina (Hinton et al., 1990; Kimple et al., 2009). In further support of a role for Gαz in neuronal development, Gαz mRNA is highest in sensory neurons near birth (E16 to P14), corresponding to the time of target tissue innervation (Kelleher et al., 1998). Together, these findings suggest an important role for Gz signaling in neuronal development. Here, we explored the impact of Gαz on cortical neuron development by examining morphological aspects of neurons at early developmental stages in wild-type and Gz-null neurons, as well as in several systems designed to modulate the activity of Gz. We found that Gαz plays a regulatory role in axon growth, particularly with regard to that stimulated by the neurotrophin BDNF.
Results
Cortical neurons from Gz-null mice exhibit enhanced axon growth
The localization Gαz specifically to neurons of the cortical, hippocampal and cerebellar regions of the CNS (Hinton et al., 1990), its developmental regulation (Kelleher et al., 2001; Schuller et al., 2001), and see Supplemental Fig. 1, combined with previous findings regarding its impact on cell growth and morphology (Meng and Casey, 2002), have indicated that Gαz expression increases at early postnatal stages, particularly with regard to cortical brain regions. Such expression suggests that Gαz might provide an important developmental context for regulation of neurotrophin-sensitive neuron development and led us to examine the impact of Gz on cortical neuron morphology and development.
In order to probe the functional impact of Gαz on neuron development, we first examined differences in morphology of cortical neurons cultured from wild-type and Gz-null mice. At early developmental stages (48 to 72 h in culture), cortical neurons cultured from Gαz-null mice demonstrated significantly enhanced axon growth compared to those cultured from wild-type mice (Fig. 1a,b,f,g). Neuron development has been well-characterized as occurring in a series of specific stages of development, with different modes of growth and polarization occurring at each stage (Craig and Banker, 1994; Polleux and Snider, 2010). In this model, initially described by Banker and colleagues, in developmental stage 1, a few hours after plating in culture, a neuron starts to sprout lamellipodia. By stage 2, a growth period of 12–24 h, neurites grow at equal rates and axons and dendrites are indistinguishable. By stage 3, one of the neurites has begun rapid growth and quickly becomes much longer than the other neurites, and cell polarity is apparent (Dotti et al., 1988; Goslin and Banker, 1989). At developmental stage 4, the slower-growing dendrites resume growth. Finally, by stage 5 neurons become mature and fully differentiated with dendritic spine and synapse formation. Thus, the differences in axon length between wild-type and Gz-null cortical neurons at early growth stages could be due to acceleration of the entire developmental process, or of growth during one particular developmental stage.
Fig. 1.
Axon growth is increased in cortical neurons lacking Gz. a) Axon length of wild-type and Gz-null mouse cortical neurons imaged live at 48 h in culture. Error bars indicate SEM; statistical significance as determined by t-test is denoted as p < 0.05 (*). Inset: Western blot from brain cortices of wild-type (WT) and Gz-null (Gz−/−) mice. b) Axon length of stage 3 cortical neurons from wild-type and Gz-null littermates imaged by fluorescence microscopy at 72 h in culture. At 72 h, neurons were fixed, stained with Tau-1, and imaged by fluorescence microscopy at 40× magnification. Error bars indicate SEM; statistical significance as determined by t-test is denoted as *** p < 0.0001. c) Length of longest dendrite from wild-type and Gz-null mouse cortical neurons imaged live at 48 h in culture (error bars indicate SEM, no significant difference by t-test). d) Length of longest dendrite stage 3 cortical neurons from wild-type and Gz-null littermates imaged by fluorescence microscopy at 72 h in culture (error bars indicate SEM, no statistical difference by t-test). e) Scholl analysis of wild-type and Gz-null cortical neurons at 48 h in culture (error bars SEM, no significant differences by t-test). f) Primary axon branches in stage 3 cortical neurons from wild-type and Gz-null mice. Error bars indicate SEM, statistical significance as determined by t-test is denoted by p < .05(*). g) Secondary axon branch points in stage 3 cortical neurons from wild-type and Gz-null mice. Error bars indicate SEM, statistical significance as determined by t-test is denoted by p < .05(*). h) Representative DIC images (20×) of wild-type and Gz−/− neurons 48 h in culture; size bar = 20 µm.
Initial characterization of the number of neurons populating each of the above-noted developmental stages did not reveal any significant differences between the two genotypes (Supplemental Table 1), suggesting that the difference observed in axon growth in the neurons from the Gαz-null mice was specifically due to an impact at the stage of axon development. Hence, we focused our attention on the axon growth of neurons specifically at stage 3 in their development; i.e. just as they begin to extend their specified axons. Fixed neurons were stained with Tau-1, an axonal marker, and neurons fitting into the stage 3 category, defined by the presence of Tau-1 staining and a clearly defined axon that was ≥2×the length of the longest dendrite, was measured. As shown in Fig. 1b, f and g, cortical neurons from Gz-null mice exhibited significantly enhanced axon growth and branching at stage 3 as compared to the wild-type counterparts. No significant differences in dendrite growth were observed between wild-type and Gz-null neurons at either 48 or 72 h in culture (Fig. 1c,d). These data indicate that loss of Gαz impacts the early stages of axon growth.
Localization of Gαz to axon growth cones
In order to better characterize the localization of Gz in cortical neurons, we constructed a YFP-Gαz fusion protein. Several groups have demonstrated that internal fluorescent protein fusions of Gα could be constructed without disrupting their functionality (Hughes et al., 2001; Hynes et al., 2004; Yu and Rasenick, 2002). For the expression of the FP-tagged Gαz, we used an internal fusion of Gαz modeled after a CFP-Gαs created by Berlot and colleagues by inserting YFP inside the constitutively-active variant of Gαz, GαzQ205L (Hynes et al., 2004). This YFP-Gαz internal fusion was characterized for its localization in HEK cells and it ability to inhibit cAMP (Supplemental Fig. 2). The YFP-Gαz Q205L displayed the same functionality as the untagged GzQ205L in each of these respects.
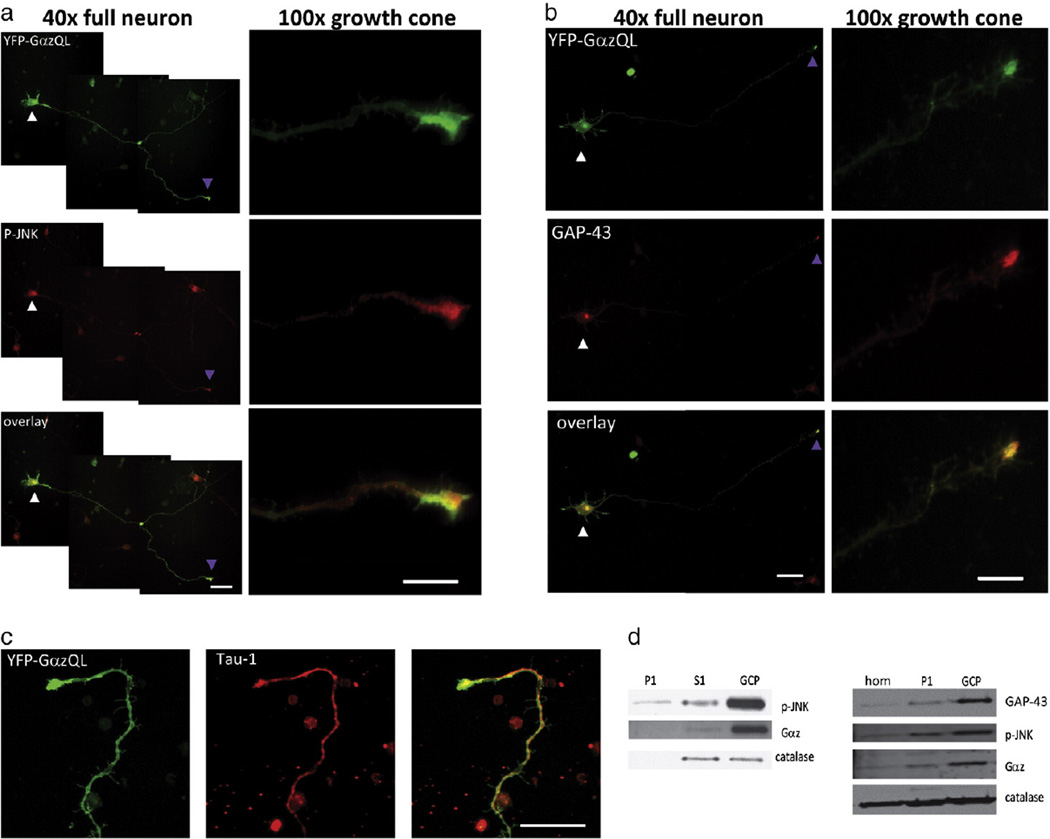
Expression of YFP-Gαz Q205L in cortical neurons revealed that the protein was localized at growth cones. YFP-GαzQL co-localized well with standard markers of axon growth cones, phospho-JNK (Fig. 2a) and GAP-43 (Fig. 2b), but also appears to a lesser extent to be present throughout the axon, including in filopodial protrusions (Fig. 2c). In further support of a specific targeting of Gαz to growth cone isolations, we performed differential centrifugation followed by a Ficoll gradient and found that endogenous Gαz was enriched with other growth cone proteins (Fig. 2d).
Fig. 2.
Gαz localizes to growth cones in rat cortical neurons. a, b) Rat cortical neurons were transfected with YFP-GαzQ205L, and stained with antibodies recognizing the axonal growth cone markers phospho-JNK (Panel A) or GAP-43 (Panel B). Neurons were imaged by fluorescence microscopy at 40× and 100× magnification. In each Panel, two imaging fields were required to capture the full neuron at 40× magnification, and the imaging fields are merged. Arrows indicate cell body (white) and axonal growth cone (purple). Size bars represent 30 um (40×) and 10 um (100×) according to magnification. c) Rat cortical neurons were transfected with YFP-GαzQ205L, and stained with the anti-Tau-1. Neurons were imaged by fluorescence microscopy at 40× magnification. An overexposed image of the axon terminus is displayed. d) Endogenous Gαz co-fractionates with growth cones (left, rat neurons 6 days in culture, right, P2 balbc/c mouse pups). Left: E18 rat cortical neurons were plated on 10 cm plates at 3 × 106 cells per plate. Cells were harvested 6 days post-plating and processed as described in Experimental Procedures into S1, P1 and growth cone pellet (GCP) components. Fractions were resolved by SDS-PAGE and immunoblot analysis performed using antibodies against phospho-JNK (Thr 183/Tyr 185, a marker of growth cones), Gαz, and catalase (a marker of mitochondria and peroxisomes). Results shown are from a single experiment that has been repeated three times with similar results. Right: Cortices from P2 mouse pups were extracted and fractionated to isolated growth cone pellet as described in experimental procedures. Whole homogenate, as well as P1 and growth cone pellet were resolved by SDS-PAGE and immunoblot analysis was performed for Gαz, phospho-JNK, GAP-43 and catalase. Results shown are from a single experiment that was repeated twice with similar results.
Activated Gαz inhibits BDNF-stimulated axon growth
The finding that cortical neurons lacking Gαz have enhanced axon growth raised the question as to the underlying cellular mechanism of this impact. We could envision three possible places where Gz could impact on axon development: i) on the inherent axon growth properties, ii) on the sensitivity of neurons to a growth factor in the media, or iii) on growth factor release, i.e. in an autocrine function. In order to further probe the underlying mechanism, we employed a single-cell based assay where axon growth of a few cells expressing a dominant-active variant of Gαz could be monitored amidst a neuronal culture predominantly expressing the endogenous levels of Gαz found in wild-type cortical neurons. To do this, we implemented a low-efficiency transfection method to express the fluorescent protein fusion of the dominant-active Gαz Q205L in ∼10% of the neuronal population.
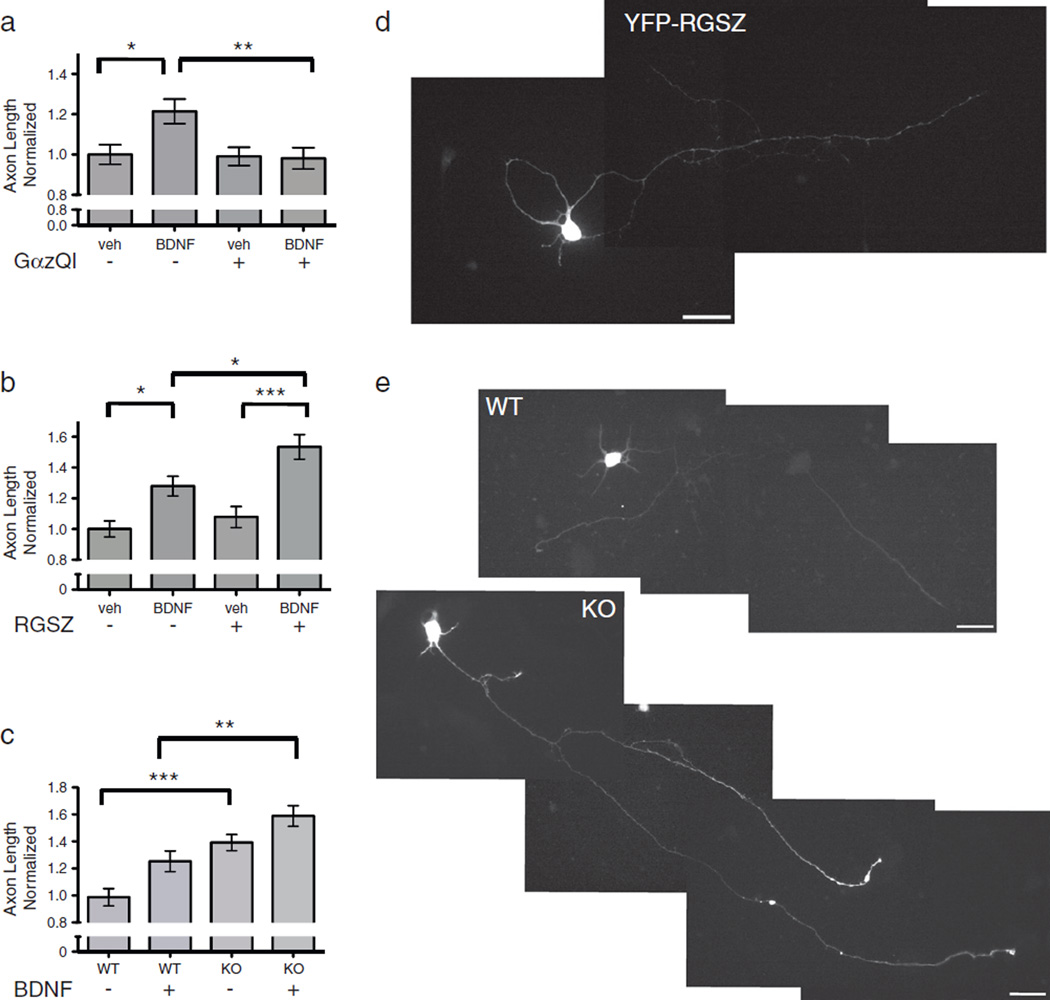
In cortical neurons isolated from wild-type mice cultured under basal conditions, no significant difference was observed in axon growth between those expressing the YFP control protein and those expressing YFP-GzQ205L (Fig. 3a). This finding suggested that Gz signaling does not impact the inherent growth properties of cortical neurons, but rather impacts either the response to, or release of, a growth factor that promotes axonal growth. In the YFP-GzQ205L-expressing neurons treated with BDNF, the activated Gαz completely inhibited BDNF-stimulated axon growth (Fig. 3a). In order to examine the role of endogenous Gαz on BDNF-stimulated axon growth, we then applied the assay in cells expressing a negative regulator of Gαz, RGSZ1. Using this approach of perturbing the function of the endogenous G protein, we observed a significant augmentation of BDNF-induced axon growth (Fig. 3b), but no differences were observed in basal levels of axon growth in the YFP-RGSZ expressing neurons.
Fig. 3.
Gz negatively regulates BDNF-stimulated axon growth. a) Impact of dominant-active Gαz on BDNF-stimulated axon growth. Rat cortical neurons were transfected with YFP or YFP-GαzQ205L, and allowed to recover. After 24 h, media was changed to low serum (2.5%). At 48 h post-transfection, neurons were treated with 50 ng/ml BDNF or vehicle in serum-free media for 24 h. Neurons were fixed and axons were stained for Tau-1. Developmental stage 3 neurons were imaged at 40× magnification, and the axon lengths were measured using Metamorph software. Statistical significance was calculated using a one-way ANOVA followed by a Tukey’s multiple comparison test, and is noted as follows: p < 0.05 (*), p < 0.01 (**). b) Impact of RGSZ, a negative regulator of Gz on BDNF-stimulated axon growth. Rat cortical neurons were transfected with YFP or a YFP-RGSZ construct, and allowed to recover. After 24 h, media was changed to low serum. At 48 h post-transfection, neurons were treated with 50 ng/ml BDNF or vehicle in serum-free media for 24 h. Neurons were fixed and axons were stained for Tau-1. Developmental stage3 neurons were imaged at 40×magnification, and the axon lengths were measured using Metamorph software. Statistical significance was calculated using a one-way ANOVA followed by a Tukey’s multiple comparison test, and is noted as follows: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***). c) Impact of BDNF on axon growth in the absence of Gz. Mouse cortical neurons (P2) were transfected with YFP, and allowed to recover. After 24 h, media was changed to low serum (2.5%). At 48 h post-transfection, neurons were treated with 50 ng/ml BDNF or vehicle in serum-free media for 24 h. Neurons were fixed and axons were stained for Tau-1. Developmental stage 3 neurons were imaged at 40×magnification, and the axon lengths were measured using Metamorph software. Statistical significance was calculated using a one-way ANOVA followed byaTukey’s multiple comparison test, and is noted as follows: p < 0.01 (**), p < .001. d) Representative image of YFP-RGSZ-expressing rat cortical neuron size bar indicates 30 µm E) Representative images of wild-type and Gz-null mouse cortical neurons, size bars indicate 30 µm.
We also examined the impact of BDNF on axon growth of cortical neurons from Gαz-null mice (Fig. 3c). However, while the neurons from Gαz-null mice exhibited enhanced axon growth as detailed above, it appears that this enhancement is close to the potential growth limit of these axons. Addition of BDNF to cortical cultures from Gαz-null mice enhanced the growth of these cells significantly beyond that seen with wild-type cells treated with BDNF, but the difference in growth induced by BDNF in the Gαz-null cortical neurons was not significantly greater than that induced by BDNF in the wild-type neurons, incontrast to what was observed when expressing the negative regulator, RGSZ (Fig. 3b). We postulate that the complete lack of Gz from the beginning of neuron development in the Gαz-null mice is likely responsible for these findings, and is suggestive of a mechanism whereby Gz regulates sensitivity to growth factor, rather than controlling an inherent growth property. Overall, however, these data provide compelling evidence for an endogenous role for Gz in regulating BDNF-stimulated axon growth.
Gαz impacts cell signaling pathways induced by BDNF
To begin to explore the biochemical mechanism by which Gz impacts BDNF-stimulated axon growth, we monitored several cell signaling responses downstream of BDNF in cortical cultures from wild-type and Gαz-null mice. Given previous data indicating that Gz acts through Rap1GAP to inhibit NGF-stimulated Rap1 activation and Erk phosphor-ylation in PC12 cells (Meng and Casey, 2002), we first looked at indicators down stream of Rap1incortical neurons. Several groups have noted that neurotrophin stimulation of neurite growth requires sustained activation of Rap1 and Erk pathways on timeframes of 30 to 60 min (Hisata et al., 2007). Hence, we monitored Erk and ribosomal protein S6 phosphorylation at 30 and 60 min following BDNF stimulation. Under basal conditions, no significant differences in either process were observed between the neurons cultured from wild-type and Gαz-null mice. Following treatment with BDNFfor30and60 min, how-ever, both pErk and pS6 levels were elevated in the cultures derived from the Gz-null mice than in those from the wild-type counterparts (Supplemental Fig. 4). These data suggest that Gz plays an endogenous role in regulating cell signaling responses commonly involved in the development and growth of cortical neurons in response to BDNF.
Discussion
The data presented here indicate that Gαz exerts an inhibitory effect on axon growth stimulated by the neurotrophic factor BDNF in cortical neurons. In addition to the enhanced axon growth in Gz-null cortical neurons observed at early developmental stages, both positive and negative regulation of Gz resulted in alterations in axon growth specifically in the presence of BDNF. The finding that axon growth is altered in the neurons lacking Gz under basal conditions, compared with no significant changes in axon growth in the absence of BDNF in the systems in which Gz signling is amplified or suppressed (i.e. GzQL and RGSZ overexpression), likely reflects differences in the nature of each of these manipulations. For one thing, the Gz-null neurons are missing Gz from the point of plating, when there is the most serum present in the media, while the YFP-GzQL and YFP-RGSZ would take ∼24 h to be expressed, when the serum concentrations are reduced. Furthermore, endogenous Gz and RGSZ are still present during the overexpression studies, thus it may take additional stimulation to bring forth the effects of the overexpressed proteins. Finally, we cannot rule out the possibility that depleting Gz from an entire population of cells, compared with reducing its activity in ∼10% of cells impacts the overall environment of the growth cultures. Nonetheless, the impact of modulation of Gz on BDNF-stimulated growth is clear and warrants further examination of downstream mechanisms.
Gz has several well-characterized effectors, adenylyl cyclase and Rap1GAP (Meng et al., 1999; Wong et al., 1992) that have also been shown to play a role in neurotrophin signaling and linked to neuron development (Goldberg et al., 2002; Hisata et al., 2007; Meyer-Franke et al., 1998). The finding here that Gαz is predominantly localized to axonal growth cones and regulates axon growth at early developmental stages is also suggestive of a critical role for Gαz in CNS biology.
The regulation of axon growth and guidance has important biological significance within the CNS, for example, functional target-tissue innervation and the possibility of axon regeneration. Axons, as the primary mode by which rapid communication occurs over long and highly specific distances in the CNS, provide a subject of study that may shed light on several neural disorders in several key ways. The growth rates of axons, and factors that influence this growth, are critical components for understanding and being able to manipulate nervous system development. Neurotrophins in particular have been characterized for their role in axon development. In this regard, it is interesting that Gαz expression appears to be higher at early postnatal stages, particularly in cortical areas where Gz expression has been shown to be especially high (Hinton et al., 1990). These data are consistent with a report indicating that Gαz mRNA is decreased beyond P21 in whole mouse brain (Kelleher et al., 2001). Together with our findings reported here regarding the impact of Gz on BDNF-stimulated growth pathways in developing cortical neurons, these data suggest a critical role for Gαz in neuronal development at time points in the brain where critical stages of cortical development, including axon extension and neuronal migration take place (Noctor et al., 2004).
Neurotrophin, and particularly BDNF, signaling has been shown to play a critical role in plasticity at early neonatal stages (Bonhoeffer, 1996; Huang et al., 1999). Furthermore, aberrant cortical development at these critical early stages has been linked to psychological disorders such as bipolar disorder and schizophrenia (Gogtay and Thompson, 2010; Johnston, 2009). In addition to its important developmental roles, BDNF is also involved in plasticity in the developed brain which, if dysregulated, can lead to affective disorders, neurodegeneration, chronic pain and epilepsy (Binder, 2004; Binder and Scharfman, 2004). In this context, our finding that Gαz can negatively regulate BDNF-induced signaling and axon growth of cortical neurons provides additional details for understanding the biological ramifications of perturbing such processes. Interestingly, studies of interacting partners of another Gαi subfamily member, Gαo2, has been implicated the Rap1GAP axis of Gαi signaling in axon growth as well (Baron et al., 2013; He et al., 2006). In a broader context, these findings suggest a mechanism linking heterotrimeric G protein signaling with that of neurotrophic growth pathways in the CNS, which may be particularly informative in the development of treatments for disorders where both of these signaling paradigms play crucial roles (Berger-Sweeney and Hohmann, 1997; Binder and Scharfman, 2004; Bloom, 1993; Duman and Monteggia, 2006).
Experimental methods
Materials
BDNF and natural mouse laminins were obtained from Invitrogen. Neurobasal media, B-27 supplement, and Glutamax were from GIBCO. Poly-D-Lysine was from Sigma. Fetal bovine serum was from Atlas. Antibodies to phospho-Erk (Thr202/Tyr204, #9101S), phospho-S6 ribosomal protein (Ser235/236, #2211S) and phospho-JNK (Thr183/Tyr185, #9251S) were obtained from Cell Signaling. The antisera to Gαz (2919B) was produced by a commercial supplier using a conjugated peptide antigen corresponding to Gαz residues 111–125: TGPAESKGEITPELL, and affinity-purified on a resin of the immobilized peptide. Anticatalase was obtained from Sigma, and anti-Tau-1 (mouse monoclonal, MAB3420) was from Millipore. GAP-43 was from Millipore and MAP2 was from Chemicon International.
Preparation of rat brain lysates
After decapitation, the hippocampus, cerebellum, cortex and olfactory lobe of rat brain were dissected washed three times in ice-cold phosphate buffered saline (PBS), and homogenized in ice-cold lysis buffer (50 mM HEPES pH 7.5, 1 mM EDTA, 3 mM dithiothreitol, 10 mM MgSO4, 1% polyoxyethylene-10-lauryl ether and a protease inhibitor mix). The homogenized lysates were centrifuged at 15,000 ×g for 30 min and the supernatants were collected. Protein concentration of the lysates were measured using Pierce BCA protein assay kit (Pierce Kit) and 30 µg of each sample was processed by SDS-PAGE and transferred to PVDF membrane; Western blots were probed using an anti-Gz 2919B antibody, a GAPDH (Abcam) and synapsin1 (Millipore) antibodies.
Mouse neuron cultures
Cortices from wild-type and Gz-null balb/c mice (P2) from the same litter were removed under sterile conditions and processed in parallel. Meninges were removed with forceps, and tissue was minced with spring scissors and placed in a dissociation solution containing 100 U Worthington papain suspension, 3.6 ml serum-free Neurobasal-A medium (GIBCO), 0.4 ml10 mMcysteine (Sigma). Tissues were then incubated while shaking at room temperature for 20 min. Each preparation was then centrifuged twice at 100 ×g for 3 min at 4 °C, replacing the papain solution with serum-free Neurobasal-A containing 10% fetal bovine serum, 0.4% B27 supplement and 1% Glutamax for each wash. The preparations then underwent further dissociation with a series of triturations with sequentially narrower fire-polished glass Pasteur pipettes. Cell numbers were determined using 0.4% Trypan blue stain and a hemacytometer.
Rat neuron cultures
Cortices from E18 (embryonic day 18) Sprague-Dawley rats were removed and placed in Hank’s balanced salt solution on ice. The tissue was minced and cells were dissociated by mechanical disruption using fire-polished glass Pasteur pipettes of sequentially narrower openings. Neurons were plated in plating media containing Neurobasal Medium containing 10% fetal bovine serum, 0.4% B27 supplement and 1% Glutamax. After 24 h, 1.5 ml (75%) of the media was changed to a serum-free version of the plating media.
Axon growth assay: live cell imaging
Cortical neurons from wild type and Gz-null mice were isolated from balb/c mouse littermates at age P2. Neurons were plated at a density of 250,000 per dish onto MatTek 35 mm gridded glass-bottom dishes coated for 24 h with each of 1 mg/ml poly-D-lysine (Sigma P0899) and 10 µg/m laminins (mouse natural, Invitrogen). After 24 h, media was replaced, lowering the serum concentration to 2.5%. At 48 h in culture, neurons were imaged by DIC at 20× magnification on a Zeiss Axio Observer at 37 °C and 5% CO2. Axon lengths were measured using Metamorph software. Scholl analysis was performed by counting the number of neurites crossing concentric circles separated by 20 µm. Only neurons with evident polarity (one neurite approximately twice the length of than others) were used for analysis; a t-test was used to determine statistical significance between wild-type and Gz-null conditions.
Axon growth assay: stage 3 fixed neurons
Cortical neurons from wild-type and Gz-null mice were prepared as described and plated on PDL- and laminin-coated cover-slips in 6-well dishes, at a density of 2 million cells per well, in Neurobasal-A media with B27 and Glutamax supplements and 10% FBS. Neurons (P1) from wild-type and Gz-null littermates were transfected with the YFP construct using the Amaxa Nucleofector® system. After 24 h, the media was replaced with media containing 2.5% FBS. After another 24 h media was replaced with serum-free media, and cells were treated with either 50 ng/ml BDNF or vehicle control (H20). At 72 h in culture (24 h after treatment), cells were rinsed twice in Dulbecco’s phosphate-buffered saline (+ glucose) and fixed in 4% paraformaldehyde. Developmental stage 3 neurons were imaged at 40× magnification, and the axon lengths were imaged using a Zeiss Axio Imager widefield fluorescence microscope and analyzed using Metamorph software. For each axon growth experiment approximately 30 neurons per conditions were measured in 3 independent experiments for a total of 90 neurons for each condition. Dendrite growth and axon branching were also analyzed using Metamorph software and were conducted for five cohorts of 12 neurons each for ∼60 neurons total. Axon branching is reported by the number of primary (branches directly off of the axon) or secondary (branches stemming off of other branches) branch points per neuron extending longer than a filopodial protrusion. In each case, a t-test was used to determine whether differences between wild-type and Gz-null neuron morphology were statistically significantly different.
Growth cone isolation
Growth cones were isolated from either P2 mouse cortical brain dissections or cultured rat cortical neurons as described previously (Gordon-Weeks and Lockerbie, 1984; Patterson and Skene, 1999). For cultured growth cones, E18 rat cortical neuron cultures were plated on 8 × 10 cm dishes at a density of 3 × 106 cells per plate and harvested after 6 days in culture. Plates or brain tissues were placed on ice and washed with PBS supplemented with 1 g/l D-glucose, and 36 mg/l sodium pyruvate, scraped in 4 ml of buffer (SHE) containing 320 mMsucrose,4 mMHepespH 7.4,2 mMEDTA,2 mMEGTA, mini EDTA-free protease inhibitor cocktail tablet (Roche), 50 mM sodium fluoride, 10 mM sodium pyrophosphate, and 1 mM sodium orthovanadate (activated). Cells were then lysed using 10 strokes of a motorized glass-teflon loose-fitting homogenizer at 900 rpm. The sample was centrifuged at 600 ×g, the supernatant (termed SIA) was saved, and the pellet was resuspended in 4 ml of the above SHE buffer. This suspension was centrifuged again at 600 ×g, and the resulting pellet (P1) was saved for SDS-PAGE while the supernatant (S1B) was combined with the previous supernatant (S1A), and a sample of this mixture (termed S1) was saved for SDS-PAGE. The remaining S1 was centrifuged for 15 min at 14,800 ×g, yielding a P2 pellet and an S3 fraction. The P2 pellet was resuspended in 1.5 mL SHE buffer, homogenized, divided in two, and loaded onto a Ficoll gradient (2 steps of 7% and 12%, 0.75 ml each, both made up in the SHE buffer). Ficoll gradients were centrifuged for 20 min at 214,000 ×g using a TLS-55 swinging bucket rotor. The interface between the Ficoll steps was collected (∼300 µL) and 300 µL SHE buffer was gradually added while mixing carefully. Finally, the sample was centrifuged for 15 min at 16,000 ×g at 4 °C. The resulting pellet was resuspended, assayed for protein content, and resolved by SDS-PAGE and immunoblot analysis. Blots were probed with anti-Gαz (2919B, see Materials), anti-pJNK Thr 183/Tyr 185 (Cell signaling), a well characterized marker of axonal growth cones (Oliva et al., 2006) and anti-catalase (Sigma). Additionally, the P2 mouse brain growth cone isolation was probed for GAP-43 (Millipore) to further confirm the distribution of growth cones to the GCP fraction.
Localization of YFP-Gαz in cortical neurons
Rat cortical neurons (E18) were prepared as described. Neurons were transfected by electroporation (Amaxa, Lonza) with YFP-Gαz Q205L; reactions contained 2.5 µg DNA and 3 million cells each. Following this protocol, cells were plated on coverslips coated with poly-D-lysine (Sigma) and natural mouse laminins (Invitrogen) at a density of 1 million cells per well of a 6-well dish in 2 ml rat neuron plating media (Neurobasal-GIBCO, supplemented with 0.4% B27 and 1% Glutamax). After 24 h the media was changed, reducing serum content to 2.5%. At 48 h post-plating, the media was changed, reducing serum content to 0.67%. At 72 h post-plating, cells were fixed in 4% PFA in 33% DPBS supplemented with 1 g/L D-Glucose, and 36 mg/L sodium pyruvate, and then 66% DBS overnight at 4 °C. Cells were permeabilized using 0.3% Triton in 5% goat serum in DPBS and incubated overnight at 4 °C, protected from light, in a 1:200 dilution of MAP2 (Chemicon International) as well as GAP-43 (Millipore), phospho-JNK (Cell Signaling), or Tau-1 (Millipore) in permeabilization buffer. Cells were then washed 6 × 20 min in permeabilization buffer, and TRITC-conjugated mouse secondary as well as Cy5-conjugated rabbit secondary antibodies were added for an overnight incubation at 4 °C. Finally, cells were washed twice for 20 min in permeabilization-buffer and rinsed once in DPBS containing glucose and pyruvate as above for 30 min. Each coverslip was mounted on a glass slide with 10 µl of mounting media (1% p-phenylenediamine in 90% glycerol, 10% PBB) with 4 µg/ml DAPI. Neurons were visualized by fluorescence microscopy using a Zeiss Axio Imager widefield fluorescence microscope using 40×/0.75 and 100×/1.4 objectives.
Axon growth assay: single cell assays
Rat cortical neurons (E18) were prepared as described. Neurons were transfected by electroporation with constructs containing YFP or the internally-tagged dominant active variant of Gαz, YFP-Gαz Q205L, or YFP-RGSZ1; reactions contained 2.5 µg DNA and 3 million cells each. Cells were then plated on coverslips coated with poly-D-lysine (Sigma) and natural mouse laminins (Invitrogen) at a density of 1 million cells per well of a 6-well dish in 2 ml rat neuron plating media (Neurobasal-GIBCO, supplemented with (0.4% B27 and 1% Glutamax). After 24 h the media was changed, reducing serum content to 2.5%. At 48 h post-plating, neurons were treated with 50 µg/ml BDNF or vehicle control in serum-free media. At 72 h post-plating, cells were fixed in 4% PFA in 33% DPBS supplemented with 1 g/L D-Glucose, and 36 mg/L sodium pyruvate, and then 66% DBS overnight at 4 °C. Cells were permeabilized in 5% goat serum using 0.3% Triton in DPBS and incubated overnight at 4 °C, protected from light, in a 1:200 dilution of Tau-1 (Millipore mouse monoclonal, MAB3420) in permeabilization buffer. Cells were then washed 6 × 20 min in permeabilization buffer, and TRITC-conjugated mouse secondary antibody added for an overnight incubation at 4 °C. Finally, cells were washed twice for 20 min in permeabilization-buffer and rinsed once in DPBS, containing glucose and pyruvate as above for 30 min. Each coverslip was mounted on a glass slide with 10 µl of mounting media (1% p-phenylenediamine in 90% glycerol, 10% PBB) with 4 µg/ml DAPI. Using a 40× objective, neurons were observed to identify those in particular in stage 3 of their development as described in the literature (Craig and Banker, 1994; Polleux and Snider, 2010). Briefly, the stages can be described as: (1) neuron starts to sprout lamellipodia, (2) axon and dendrites grow at equal rates, (3) axon has begun to grow more rapidly than dendrites, clear polarity is established (4) dendrite growth increases (5) full differentiation and synapse formation occur. All stage 3 neurons (i.e. those staining positive for Tau-1 and greater than twice the length of the next longest neurite) were imaged and analyzed for each condition. Approximately 30 neurons per condition were assessed for each group three times, for a total of 90 neurons. To determine whether differences were statistically significant, a one-way ANOVA was performed followed by a Tukey’s multiple comparison test.
Supplementary Material
Acknowledgments
We thank Missy Infante for the technical assistance. We thank James McNamara’s laboratory, and in particular Yanzhong Huang, for the generous gift of rat cortical neurons. We thank Michelle Kimple, Ian Cushman, A. Soren Leonard and J.H. Pate Skene for their helpful discussion and insight. This work was supported by the National Institutes of Health grant DK76488 and the Duke-NUS (National University of Singapore) Graduate Medical School Singapore.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.mcn.2013.12.004.
References
- Baron J, et al. The alpha-subunit of the trimeric GTPase Go2 regulates axonal growth. J. Neurochem. 2013;1246:782–794. doi: 10.1111/jnc.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav. Brain Res. 1997;862:121–142. doi: 10.1016/s0166-4328(96)02251-6. [DOI] [PubMed] [Google Scholar]
- Binder DK. The role of BDNF in epilepsy and other diseases of the mature nervous system. Adv. Exp. Med. Biol. 2004;548:34–56. doi: 10.1007/978-1-4757-6376-8_3. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;223:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE. Advancing a neurodevelopmental origin for schizophrenia. Arch. Gen. Psychiatry. 1993;503:224–227. doi: 10.1001/archpsyc.1993.01820150074008. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocor-tex. Curr. Opin. Neurobiol. 1996;61:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu. Rev. Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;84:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;5912:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;721:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, et al. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;335:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks PR, Lockerbie RO. Isolation and partial characterisation of neuro-nal growth cones from neonatal rat forebrain. Neuroscience. 1984;131:119–136. doi: 10.1016/0306-4522(84)90264-1. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 1989;1084:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, McCobb DP, Kater SB. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984;2264674:561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- Haydon PG, McCobb DP, Kater SB. The regulation of neurite outgrowth, growth cone motility, and electrical synaptogenesis by serotonin. J. Neurobiol. 1987;182:197–215. doi: 10.1002/neu.480180206. [DOI] [PubMed] [Google Scholar]
- He JC, Neves SR, Jordan JD, Iyengar R. Role of the Go/i signaling network in the regulation of neurite outgrowth. Can. J. Physiol. Pharmacol. 2006;847:687–694. doi: 10.1139/y06-025. [DOI] [PubMed] [Google Scholar]
- Hendry IA, et al. Hypertolerance to morphine in G(z alpha)-deficient mice. Brain Res. 2000;8701-2:10–19. doi: 10.1016/s0006-8993(00)02387-8. [DOI] [PubMed] [Google Scholar]
- Hinton DR, et al. Novel localization of a G protein, Gz-alpha, in neurons of brain and retina. J. Neurosci. Off. J. Soc. Neurosci. 1990;108:2763–2770. doi: 10.1523/JNEUROSCI.10-08-02763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisata S, et al. Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. J. Cell Biol. 2007;1785:843–860. doi: 10.1083/jcb.200610073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Wong YH. G(z) signaling: emerging divergence from G(i) signaling. On-cogene. 2001;2013:1615–1625. doi: 10.1038/sj.onc.1204190. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Knapp DR, Halushka PV. The effects of alpha adrenergic agents on human platelet aggregation. J. Pharmacol. Exp. Ther. 1979;2083:366–370. [PubMed] [Google Scholar]
- Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;986:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hughes TE, Zhang H, Logothetis DE, Berlot CH. Visualization of a functional Galpha q-green fluorescent protein fusion in living cells. Association with the plasma membrane is disrupted by mutational activation and by elimination of palmitoylation sites, but not be activation mediated by receptors or AlF4. J. Biol. Chem. 2001;2766:4227–4235. doi: 10.1074/jbc.M007608200. [DOI] [PubMed] [Google Scholar]
- Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J. Biol. Chem. 2004;27942:44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev. Disabil. Res. Rev. 2009;152:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- Kelleher KL, Matthaei KI, Leck KJ, Hendry IA. Developmental expression of messenger RNA levels of the alpha subunit of the GTP-binding protein, Gz, in the mouse nervous system. Brain Res. Dev. Brain Res. 1998;1072:247–253. doi: 10.1016/s0165-3806(98)00020-0. [DOI] [PubMed] [Google Scholar]
- Kelleher KL, Matthaei KI, Hendry IA. Targeted disruption of the mouse Gz-alpha gene: a role for Gz in platelet function? Thromb. Haemost. 2001;853:529–532. [PubMed] [Google Scholar]
- Kimple ME, Hultman R, Casey PJ. Signaling through Gz. In: Bradshaw RAD, Dennis E, editors. Handbook of Cell Signaling. 2nd edition. Academic Press; 2009. pp. 601–605. [Google Scholar]
- Koert CE, et al. Functional implications of neurotransmitter expression during ax-onal regeneration: serotonin, but not peptides, auto-regulate axon growth of an identified central neuron. J. Neurosci. 2001;2115:5597–5606. doi: 10.1523/JNEUROSCI.21-15-05597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leck KJ, et al. Gz proteins are functionally coupled to dopamine D2-like receptors in vivo. Neuropharmacology. 2006;513:597–605. doi: 10.1016/j.neuropharm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Lieske V, Bennett-Clarke CA, Rhoades RW. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience. 1999;903:967–974. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- McCobb DP, Haydon PG, Kater SB. Dopamine and serotonin inhibition of neurite elongation of different identified neurons. J. Neurosci. Res. 1988;191:19–26. doi: 10.1002/jnr.490190104. [DOI] [PubMed] [Google Scholar]
- Meng J, Casey PJ. Activation of Gz attenuates Rap1-mediated differentiation of PC12 cells. J. Biol. Chem. 2002;27745:43417–43424. doi: 10.1074/jbc.M204074200. [DOI] [PubMed] [Google Scholar]
- Meng J, Glick JL, Polakis P, Casey PJ. Functional interaction between Galpha(z) and Rap1GAP suggests a novel form of cellular cross-talk. J. Biol. Chem. 1999;27451:36663–36669. doi: 10.1074/jbc.274.51.36663. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;214:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;72:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Leck KJ, Matthaei K, Hendry IA. Enhanced serotonin response in the hippocampus of Galphaz protein knock-out mice. Neuroreport. 2005;169:921–925. doi: 10.1097/00001756-200506210-00009. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 2006;2637:9462–9470. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SI, Skene JH. A shift in protein S-palmitoylation, with persistence of growth-associated substrates, marks a critical period for synaptic plasticity in developing brain. J. Neurobiol. 1999;393:423–437. doi: 10.1002/(sici)1097-4695(19990605)39:3<423::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Polleux F, Snider W. Initiating and growing an axon. Cold Spring Harb. Perspect. Biol. 2010;24:a001925. doi: 10.1101/cshperspect.a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponimaskin E, Voyno-Yasenetskaya T, Richter DW, Schachner M, Dityatev A. Morphogenic signaling in neurons via neurotransmitter receptors and small GTPases. Mol. Neurobiol. 2007;353:278–287. doi: 10.1007/s12035-007-0023-0. [DOI] [PubMed] [Google Scholar]
- Prokosch V, Panagis L, Volk GF, Dermon C, Thanos S. Alpha2-adrenergic receptors and their core involvement in the process of axonal growth in retinal explants. Invest. Ophthalmol. Vis. Sci. 2010;5112:6688–6699. doi: 10.1167/iovs.09-4835. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J. Neurosci. 1999;1911:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso BS, Undie AS, Levitt P. Dopamine receptors mediate differential morphological effects on cerebral cortical neurons in vitro. J. Neurosci. Res. 1996;434:439–453. doi: 10.1002/(SICI)1097-4547(19960215)43:4<439::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Rodriguez-Munoz M, de la Torre-Madrid E, Garzon J. Brain-specific Galphaz interacts with Src tyrosine kinase to regulate Mu-opioid receptor-NMDAR signaling pathway. Cell. Signal. 2009;219:1444–1454. doi: 10.1016/j.cellsig.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Schuller U, Lamp EC, Schilling K. Developmental expression of heterotrimeric G-proteins in the murine cerebellar cortex. Histochem. Cell Biol. 2001;1162:149–159. doi: 10.1007/s004180100303. [DOI] [PubMed] [Google Scholar]
- Serres F, et al. Evidence that G(z)-proteins couple to hypothalamic 5-HT(1A) receptors in vivo. J. Neurosci. 2000;209:3095–3103. doi: 10.1523/JNEUROSCI.20-09-03095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZM, Abou-Zeid O, Fang YY. Alpha2a adrenoceptors regulate phosphorylation of microtubule-associated protein-2 in cultured cortical neurons. Neuroscience. 2004;1232:405–418. doi: 10.1016/j.neuroscience.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Spencer GE, Lukowiak K, Syed NI. Dopamine regulation of neurite outgrowth from identified Lymnaea neurons in culture. Cell. Mol. Neurobiol. 1996;165:577–589. doi: 10.1007/BF02152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Buuse M, et al. Enhanced effect of dopaminergic stimulation on prepulse inhibition in mice deficient in the alpha subunit of G(z) Psychopharmacology. 2005;1833:358–367. doi: 10.1007/s00213-005-0181-6. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Martin S, Holgate J, Matthaei K, Hendry I. Mice deficient in the alpha subunit of G(z) show changes in pre-pulse inhibition, anxiety and responses to 5-HT(1A) receptor stimulation, which are strongly dependent on the genetic background. Psychopharmacology (Berl) 2007;195:273–283. doi: 10.1007/s00213-007-0888-7. [DOI] [PubMed] [Google Scholar]
- Wong YH, Conklin BR, Bourne HR. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science. 1992;2555042:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc. Natl. Acad. Sci. U. S. A. 2000;9718:9984–9989. doi: 10.1073/pnas.180194597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, et al. Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J. Biol. Chem. 2002;27748:46035–46042. doi: 10.1074/jbc.M208519200. [DOI] [PubMed] [Google Scholar]
- Yu JZ, Rasenick MM. Real-time visualization of a fluorescent G(alpha)(s): dissociation of the activated G protein from plasma membrane. Mol. Pharmacol. 2002;612:352–359. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.