Preface
As we age, the innate immune system becomes dysregulated and is characterized by persistent inflammatory responses that involve multiple immune and non-immune cell types, and that vary depending on the cell activation state and tissue context. This ageing-associated basal inflammation, particularly in humans, is thought to be induced by factors including the reactivation of latent viral infections and the release of endogenous damage-associated ligands of pattern recognition receptors (PRRs). Innate immune cell functions, such as cell migration and PRR signalling, that are required to respond to pathogens or vaccines are also impaired in aged individuals. This immune dysregulation may affect conditions associated with chronic inflammation, such as atherosclerosis and Alzheimer’s disease.
The United Nations projects that the global human population over the age of 60 will increase by more than threefold (to nearly two billion individuals) during the first half of the 21st century, and by 2050 will exceed the size of the global population of young individuals (less than 15 years of age) 1. This unprecedented growth in the aged population is observed in both developed and developing nations. In the United States, individuals over the age of 65 currently comprise approximately 12% of the population, but account for over 35% of visits to general internists, 34% of prescription drug use, 50% of hospital stays, and 90% of nursing home residents 2 —reflecting in part increased morbidity and mortality from infectious diseases and poor responses to vaccination 3.
With regard to the adaptive immune system, there is evidence for broad-ranging, age-associated alterations in the development and function of B and T cells 4, 5, 6, 7, 8. The impact of ageing on the innate immune system had been less well studied, until recently. The diverse cell lineages that mediate innate immunity show heterogeneous ageing phenotypes that reflect their developmental, tissue and activation context. Overall, studies in aged mice (generally older than 20 months) and in humans over the age of 65 have shown that activation of the aged innate immune system results in dysregulated inflammation. This dysregulation involves both elevated basal inflammation (especially in humans) and an associated impairment in mounting efficient innate and adaptive immune responses to newly encountered pathogens or vaccine antigens. Indeed, a body of evidence, mainly from human studies, indicates that older adults have elevated levels of pro-inflammatory cytokines, clotting factors and acute phase reactants in the steady state 9-11, and the term ‘inflamm-ageing’ was coined to describe this phenomenon 12. Evaluation of several13, 14 (but not all 15) human cohorts have shown an association between elevated levels of cytokines, particularly of interleukin-6 (IL-6), and decline in innate immune function in older individuals.
The mechanisms that underlie this heightened ageing-associated basal inflammation remain incompletely understood, but seem to involve changes in the numbers and functions of innate immune cells, altered expression of pattern recognition receptors (PRRs), activation of PRRs by endogenous ligands associated with cellular damage, and aberrant signalling events downstream of PRR activation that lead to cytokine secretion. Here, we review evidence, predominantly from mouse and human studies, on ageing-associated alterations in innate immunity; although there are substantial similarities between aged humans and mice, there are also important differences (Table 1). These differences probably reflect various factors, including the characteristics of genetically homogeneous inbred mouse strains, as well as intrinsic species-specific differences between humans and mice16. We also discuss the implications of age-associated changes in innate immunity for improving responses to infection or vaccination and for age-associated chronic inflammatory diseases.
| Cell type | Decreased in ageing | Increased in ageing |
|---|---|---|
| Human | ||
| Haematopoietic stem cells |
|
|
| Neutrophils | ||
| Monocytes | ||
| Macrophages | ||
| Monocyte-derived DCs and myeloid DCs |
||
| Plasmacytoid DCs |
|
|
| Mouse | ||
| Haematopoietic stem cells |
||
| Neutrophils | ||
| Macrophages | ||
| Monocyte-derived DCs and myeloid DCs |
||
| Plasmacytoid DCs | ||
The ageing innate immune cell compartment
Studies in mice have shown that aged haematopoietic stem cells (HSCs) have reduced regenerative potential and fail to efficiently reconstitute myeloablated recipients following transplantation. Moreover, aged mouse HSCs are biased towards myeloid differentiation at the expense of lymphopoiesis 17-20, and there is also evidence for a similar skewing in human HSCs from older donors 21. The underlying mechanisms probably include both ageing-associated cell-intrinsic and microenvironmental changes22. For example, the presence of low levels of lipopolysaccharide (LPS) or chemokines, such as CC-chemokine ligand 5 (CCL5), may compromise HSC regenerative capacity or promote myeloid skewing23, 24. In addition, DNA damage in the form of double-stranded DNA breaks seems to be increased in HSCs from older humans 25. These alterations are associated with a decline in adaptive immunity and may contribute to an increased incidence of myeloid malignancies associated with ageing 26.
Most innate immune cell populations seem to either remain stable in size or decrease with age. For example, studies using the SENIEUR protocol (which selects for successfully aged adults without comorbid medical conditions) have reported no change or a mild decrease in the numbers of neutrophils 27, 28. In this regard, it is notable that neutrophilia was a risk factor for death in a multivariate analysis of one cohort of aged adults 29. Monocyte numbers are grossly unchanged in older adults 30, 31, although age-associated increases in CD14+CD16+ inflammatory monocytes have been reported 31-33. Finally, decreased percentages and numbers of plasmacytoid dendritic cells (pDCs) have been reported in older adults34, 35 (although not in all studies 36). Likewise, myeloid DCs (mDCs) also appear to be decreased or unchanged with age35, 37. The existing evidence indicates that basal inflammation in older individuals is not associated with increased numbers of myeloid cells, despite the skewing of aged HSCs to the myeloid cell lineage. This reflects the impaired bone marrow homing, proliferative responses and self-renewal capacity seen in aged HSCs with myeloid skewing 20.
Ageing and innate immune cell migration
Neutrophils are the first cells to migrate to pathogen-infected sites, and their migratory capacity has been extensively studied in the context of ageing. The speed of neutrophil movement, also known as chemokinesis, was found to be unperturbed in older individuals 38, but neutrophils from aged mice show impaired chemokinesis in response to IL-8 39. By contrast, chemotaxis (directional movement in response to a gradient of a stimulus) of neutrophils from older adults 38, 40 and mice 41-43 seems to be impaired. Moreover, impaired neutrophil chemotaxis was found to contribute to delayed wound healing in aged mice, a defect associated with lowered expression of intracellular adhesion molecule 1 (ICAM1)44 (Figure 1). However, diminished chemotaxis can result not only in reduced neutrophil migration to the sites of inflammation but also in defective neutrophil egress from inflamed tissues. In this context, local neutrophil-mediated inflammation was increased in aged, compared to young mice following burn-associated lung injury, and bacterial or viral infection 39, 45, 46. In the case of burn-associated injury, an age-associated decrease in the expression of CXC-chemokine receptor 2 (CXCR2) by neutrophils and an increase in the expression of ICAM1 by pulmonary endothelial cells contributed to increased neutrophil inflammation in the lungs39 (Figure 1). The findings that ICAM1 expression is increased in the burn model but is decreased in the wound healing model may reflect tissue context and differences between a systemic (burn) versus local response.
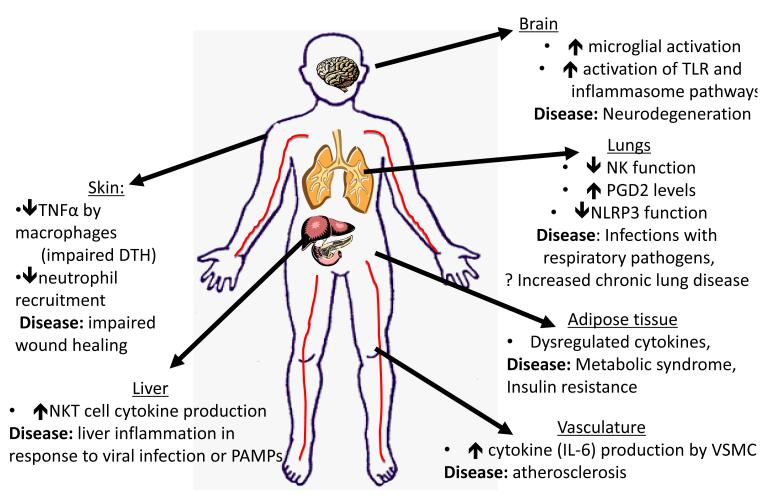
Figure 1. Organ-specific changes of the innate immune system associated with ageing and disease.
The effects of aging in various organ systems are depicted. Diminished innate immune responses are found in some cases—such as in skin, where a decrease in TNF production by macrophages results in decreased endothelial cell activation and diminished DTH responses. In addition, studies in mice have linked diminished neutrophil recruitment to impaired wound healing. Decreased NK cell function and induced NLRP3 function in response to influenza virus influence infection in the lung. However, there are also examples of dysregulated inflammatory responses found in aging. For example, in the lung following burn injury in a murine model, there is increased neutrophil inflammation. In the liver there is experimental data showing that NKT cells exhibit exaggerated inflammatory responses that contribute to immune pathology during viral infection or to TLR activators. In the brain, TLR and inflammasome signalling are elevated with aging, and are associated with microglial activation. Signalling via the NLRP3 inflammasome contributes to increased age-associated inflammation in adipose tissue as well. Finally, within the vasculature, increased basal IL-6 production by vascular smooth muscle cells has been found in aged rodents and non-human primates and may provide a potential explanation for the increased prevalence of atherosclerosis with aging.
Neutrophil migration in vitro can also be facilitated or inhibited depending on the presence of pro-inflammatory stimuli, including IL-8 and tumour necrosis factor (TNF)47, and such age-associated microenvironmental and tissue-related factors likely contribute to dysregulation of neutrophil activation and migration. Taken together, these results suggest that aged neutrophils have an impaired ability to traffic into and out of sites of infection. Notably, defects in the migration of mouse pulmonary DCs48 and human monocyte-derived DCs36 have also been reported, as further discussed below. Such alterations in cell trafficking with ageing could affect the initiation of innate immune responses at sites of infection.
Ageing and innate immune cell effector functions
Neutrophils
Neutrophils from older, compared to young, adults showed impaired phagocytosis of opsonized Escherichia coli or Streptococcus pneumoniae 49-51 and a diminished capacity to kill phagocytosed microorganisms 50, 52, 53. One potential mechanism contributing to an age-associated defect in intracellular bacteria killing in human neutrophils could involve the diminished expression of dihydroepiandrosterone sulfate (DHEAS), a circulating steroid that promotes superoxide generation in neutrophils 54.
Age-associated defects in neutrophil effector function have been associated with impaired signal transduction functions. Examples include impaired anti-apoptotic responses to granulocyte-macrophage colony-stimulating factor (GM-CSF) mediated through JAK (Janus kinase)–STAT (signal transducer and activator of transcription) tyrosine kinase 55 and phosphoinositide 3-kinase (PI3K)–AKT pathways 56. Neutrophils from older adults compared to young individuals show reduced basal expression of suppressor of cytokine signalling 1 (SOCS1) and SOCS3, which negatively regulate JAK–STAT signalling 56, and an impaired response to triggering receptor expressed on myeloid cells 1 (TREM1) 57, which is an immunoglobulin superfamily activating receptor that mediates the production of cytokines, chemokines and reactive oxygen species (ROS). This dysregulated signal transduction may reflect alterations in membrane lipid composition and lipid rafts that result in inappropriate localization or retention in membrane signalling domains. For example, the negative regulator SRC homology domain-containing protein tyrosine phosphatase 1 (SHP1) is excluded from lipid rafts following GM-CSF stimulation in neutrophils from young individuals, but is retained in rafts in neutrophils from older individuals 58. Taken together, these findings indicate that ageing impairs several signalling pathways in neutrophils, including the generation of the respiratory burst and apoptotic pathways, which may result in reduced protection to microbial infection.
Although in early studies neutrophil function was reported to be largely preserved in aged mice 59, 60, a recent study in aged mice indicated that neutrophils infected with methicillin-resistant Staphylococcus aureus (MRSA) exhibited decreased production of CXCL1 and CXCL253. Neutrophils also produce neutrophil extracellular traps (NETs), which are scaffolds of extruded chromatin that contain antimicrobial peptides and proteases such as elastase and myeloperoxidase that facilitate the capture and killing of pathogens 61. NET formation was diminished with age in a mouse model of MRSA infection 53, but it remains to be determined whether NETs are altered in aged humans.
NK and NKT cells
Natural killer (NK) cells show decreased cytotoxicity, lung infiltration and interferon-γ (IFNγ) production in a model of influenza virus infection in aged, compared to young, mice 22, 62 (Figure 1). In an ectromelia poxvirus model, NK cells from older mice (aged 14-18 months) showed a cell-intrinsic defect in migration to regional lymph nodes 63. Cytotoxicity of NK cells induced by type I IFNs appears decreased in aged mice 64, as does induced production of IFNγ and granzyme B for specific cytokine combinations 65.
In humans, NK cells can be broadly divided into a CD56low population that has cytotoxic activity and a CD56hi population that is responsible for cytokine production 66. CD56hi NK cells appear diminished in proportion and cytokine/chemokine secretion with ageing. In older adults, cytotoxicity is decreased on a per cell basis, with an expansion of the CD56low cytotoxic NK cell compartment 67-72. Notably, this diminished cytotoxicity is associated with an age-associated defect in the mobilization of perforin to the immunological synapse 73. Elucidating the mechanisms underlying age-associated changes in NK cell function may have particular clinical impact, as impaired NK cell function is associated with increased infection rates and mortality in older adult nursing home residents74.
Invariant NKT (iNKT) cells are characterized by CD1d-restricted recognition of endogenous and bacterial glycolipids 75. iNKT cell numbers are increased in aged versus young mice 76; by contrast, human iNKT cells in the blood decrease with age and show diminished proliferation in response to the CD1d ligand α-galactosylceramide 77, 78. In mice, NKT cells produce cytokines such as IL-17A, which contributes to immune pathology after viral infection 46. Consistent with this, studies in non-infectious murine models indicate that ageing enhances inflammatory responses by NKT cells, and that this leads to liver immunopathology79, 80 (Figure 1). Thus, NKT cells also contribute to innate immune dysregulation with ageing.
Macrophages and DCs
Various macrophage and DC effector functions in ageing individuals have been studied. Several reports have demonstrated an age-associated decline in nitric oxide production by mouse and rat macrophages 81-82. However, evaluation of the effects of aging on phagocytosis has produced mixed results. While several studies report preserved phagocytosis of bacterial targets by aged mouse macrophages 83, 84, studies of human monocytes and monocyte-derived DCs (MDDCs) suggest an age-associated impairment in phagocytosis 32, 36, and phagocytosis of apoptotic cells by both mouse macrophages and human MDDCs has been reported to be reduced36, 85. These findings raise the possibility of differential age-associated effects on specific phagocytic functions.
Studies of antigen-presenting cells (APCs) generally show impaired function with ageing 86, 87, 88 (with one exception89). For example, bone marrow-derived DCs (BMDCs) in aged compared to young mice were defective in controlling tumour growth in a B16 melanoma model 90. A recent study of infection with a modified version of Listeria monocytogenes demonstrated decreased expression of costimulatory proteins and impaired bacterial uptake by CD8α+ DCs in aged mice, particularly at early stages of infection (although LPS-induced costimulatory protein expression was comparable to young mice) 91. In oral infection of mice with the intracellular parasite Encephalitozoon cuniculi, CD11c+ DCs from lymph nodes in adult (not aged) mice had a decreased ability to prime T cells compared to DCs from young mice 92. This impaired function was associated with an age-associated decrease in IL-12 production by DCs that could be complemented by IL-15 treatment. Microenvironmental alterations, such as disruption of the architecture of the splenic marginal zone, may also contribute to impairments in antigen presentation93. Whether these processes are altered in human cells remains unclear; studies to date using relatively small numbers of subjects have shown preserved antigen presentation function in DCs and monocytes 94, 95.
Ageing and PRR signal transduction
Alterations in TLR expression
Ageing is associated with impaired PRR signalling which may, in part, be accounted for by the reported alterations in TLR protein expression in innate immune cells from older compared to young individuals. Decreased surface expression of TLR1 has been associated with diminished TLR1/TLR2-induced cytokine production in human monocytes 33, 96. Age-associated decreases in TLR1, TLR3 and TLR8 protein expression by mDCs 97 and in TLR7 and TLR9 expression by pDCs 37 have been reported as well. While substantial age-associated decreases in TLR gene expression have been reported in mice 98, this is less clear in humans. Concordant changes in both protein and gene expression have been reported, such as the age-associated increase in TLR5 mRNA and protein in adherent human monocytes 99, but in other cases changes in gene expression may not be sufficient to account for changes in TLR protein levels 97. This suggests that post-transcriptional mechanisms contribute to the decreased TLR protein levels; in human monocytes, total TLR1 protein expression was comparable in cells from young and older adults; thus, the reported decrease in cell surface TLR1 could result from mechanisms involving TLR1 transport to the plasma membrane 96.
TLR signalling and cytokine production in aged monocytes and macrophages
TLR signalling has a crucial role in linking innate and adaptive immune responses through the induction of expression of costimulatory molecules and pro-inflammatory cytokines. One of the first studies to evaluate TLR function in aged C57BL/6 mice revealed a generalized decrease in Tlr1-Tlr9 gene expression and TLR-induced TNF and IL-6 production by splenic and peritoneal macrophages, with decreased expression of TLR4 protein100. By contrast, in aged BALB/c mice, surface expression of TLR2 and TLR4 on peritoneal macrophages was unchanged. Nonetheless, TLR2- and TLR4-induced TNF and IL-6 production were reduced in macrophages from aged BALB/c mice, and associated with diminished MAPK p38 protein expression101, 102. An age-associated impairment in TLR2-dependent cytokine production and signalling was also reported in alveolar macrophages from BALB/c mice, in a model of S. pneumoniae infection 103 and in C57BL/6 macrophages responding to Porphyromonas gingivalis, with a decrease observed only for IL-6 production in aged BALB/c mice 84, 104. These findings provide evidence for age-associated decline in mouse TLR function, with variation in the underlying mechanisms that may in part reflect genetic background (Figure 2).
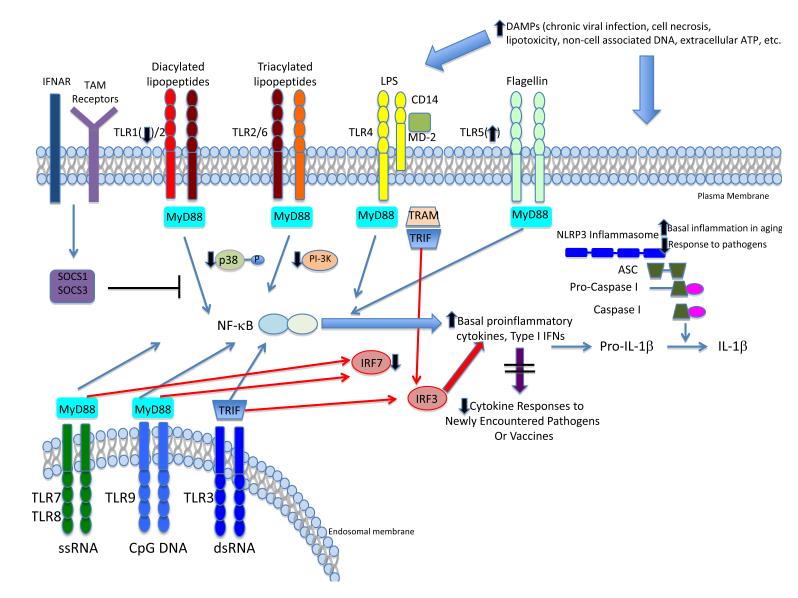
Figure 2. Effects of ageing on innate immune PRR signalling.
TLR and NLRP3 signalling pathways are depicted, with NF-κB-dependent pathways indicated in blue and type I interferon pathways indicated in red. With aging, elevated levels of PRR ligands (DAMPs) arising from cell damage, necrosis, lipotoxicity or other causes contribute to an elevated pro-inflammatory state, as manifested by increased basal levels of proinflammatory cytokines that may result in part from both TLR and NLRP3 signalling. Such basal activation may restrict responsiveness to new pathogens or vaccines, resulting in innate immune failure and impaired adaptive immune responses. Changes in protein expression for TLR1 and TLR5 in humans are indicated; results in mice suggest either a widespread decrease in TLR gene expression, or preserved expression with changes in intracellular signaling proteins (e.g. p38 MAP kinase). Abbreviations: TLR—Toll-like Receptor; IFNAR—type I Interferon receptor; MyD88—myeloid differentiation primary response 88; TRIF—Toll/IL1 receptor domain-containing adapter-inducing interferon-β; TRAM—TRIF-related adaptor molecule; IRF3—interferon regulatory factor 3; IRF7—interferon regulatory factor 7; NLRP3—NOD-like receptor family, pyrin domain containing 3; ASC—Apoptosis-associated speck-like protein containing a CARD.
In rhesus macaques, TLR2-TLR6-, TLR4- and TLR9-induced TNF and IL-6 production in monocytes were decreased in aged, compared to young, animals 105. In humans, monocytes from older adults produce less TNF and IL-6 in response to TLR1/TLR2 stimulation, with an associated decrease in MAP kinase signalling; decreased cell surface expression of TLR1, but not TLR2, was associated with this reduced cytokine production 96. In studies of cell subpopulations, TLR1/TLR2-induced cytokine production was decreased in all monocyte subsets, including classical CD14hiCD16−, inflammatory CD14hiCD16+ and non-classical CD14lowCD16hi monocytes, in older compared to young adults 33.
TLR-dependent expression of the costimulatory molecules CD80 and CD86 following TLR engagement in vitro was altered in monocytes from older adults, thereby potentially affecting the efficiency of antigen presentation. Notably, the extent of TLR-induced CD80 and CD86 expression strongly correlated with antibody response to the trivalent inactivated influenza vaccine 106. These studies of induced TLR function were notable for the use of multivariate statistical modeling to adjust for potential co-variates such as gender, race, comorbidities and medication use — such modeling is valuable in human studies given the considerable heterogeneity in human cohorts.
So, monocyte and macrophage function seems to be diminished overall in aged mice and humans compared to young individuals. However, these impairments occur in the presence of dysregulated or inappropriately persistent inflammatory responses. For example, basal and LPS-induced TNF production by CD14+CD16+ human monocytes are increased with age 32. In addition, human (adherent) monocytes exhibit an age-associated increase in TLR5-induced production of IL-8 and IL-6 and increases in TLR5-induced p38 and ERK phosphorylation 99. This increase in TLR5 signalling in monocytes from older adults was not appreciated in non-adherent cells 96, suggesting that adherence to plastic could result in partial activation that uncovered an age-associated pro-inflammatory bias. The maintenance, or even enhancement, of TLR5 activity with ageing might also be potentiated by an inflammatory environment in the setting of infection. Further studies are needed to clarify the underlying mechanisms in view of the potential use of TLR5 agonists as vaccine adjuvants 107 (Box 1), and association of TLR5 gene expression with human influenza virus vaccine antibody responses in young adults 108. Another example of dysregulated TLR responses came from studies of in vitro West Nile virus (WNV) infection: in WNV-infected macrophages from older adults, STAT1-dependent downregulation of TLR3 expression was impaired, which resulted in an inappropriate persistence of TLR3 activation that might contribute to the increased morbidity and mortality associated with WNV infection in older adults 109.
Box 1. TLR agonists as vaccine adjuvants for the elderly.
Recently, specific Toll-like receptor (TLR) activators, such as the lipopolysaccharide component lipid A, have been used as a strategy to boost immune protection following vaccination in aged individuals. This may be accomplished by the production of pro-inflammatory cytokines, which can enhance the responses of aged CD4+ T cells189. Administration of the TLR5 ligand flagellin has been associated with preserved innate immune responses in aged mice and elevated inflammatory responses by specific human cell types from older individuals99, 190. Indeed, a vaccine construct that comprised flagellin linked to peptides from the influenza virus haemagglutinin afforded protection against lung infection with influenza virus in aged mice 190. However, the protection induced in the aged mice was still inferior to that induced in young mice, which probably reflects defects in the adaptive immune response. A clinical study using a similar vaccine construct found that people over 65 years of age exhibited a robust humoral response without substantial side effects 191.
Several studies have used the TLR9 ligand CpG-containing oligonucleotides in combination with Fms-like tyrosine kinase 3 (FLT3) ligand as an adjuvanted intranasal vaccine against influenza virus and pneumococcus; both vaccines gave similar responses in young and aged mice 192,193. CpG oligonucleotides added to the pneumococcal conjugate vaccine also restored pneumococcal polysaccharide-specific antibody responses in aged mice 194. A recent human study found that a TLR4 agonist, glucopyranosyl lipid, enhances the ability of myeloid dendritic cells to respond to influenza virus infection in vitro195, and this was mediated by increased myeloid dendritic cell production of granzyme B195. Taken together, these studies indicate that TLR engagement has great potential to enhance immune protection in aged individuals, but targeted use of TLR agonists — alone or in combination — will be essential as different TLR activators may have distinct effects on overcoming defects in the adaptive immune system of older individuals.
Finally, the ageing-associated in vivo microenvironment is also likely to contribute to functional defects. For example, in a study of human delayed-type hypersensitivity responses to tuberculin purified protein derivative, impaired T cell migration in the skin of older adults was linked to impaired TNF production by skin macrophages and consequent impairment in endothelial cell activation (Figure 1). Notably, skin macrophages from older and young humans produced comparable levels of TNF ex vivo, which suggests that factors in the microenvironment are responsible for the impaired function in vivo 110.
TLR signalling and cytokine production in aged DCs
In mice, there is evidence for preserved DC function with ageing; mouse BMDCs111 and CD11c+ DCs that were isolated from the spleen and lymph nodes showed unperturbed TLR function 89, although lower levels of LPS-induced IL-6 and TNF production by DCs have been reported in a T cell receptor transgenic system 86. However, an age-associated decrease in type I IFN production by pDCs was found following in vitro or in vivo challenge with type 2 herpes simplex virus. This ageing-associated dysfunction was associated with decreased nuclear translocation of IFN regulatory factor 7 (IRF7). In addition, TLR9-dependent type I IFN responses in pDCs were augmented by treatment with anti-oxidants and in mice subjected to caloric restriction 112.
Another age-associated alteration of TLR function includes increased IL-23 expression by mouse BMDCs following combined stimulation with the TLR4 ligand LPS and the TLR7 agonist R848; this increase is associated with alterations in histone modification at the IL-23 p19 promoter 113 and similarly resulted in an age-associated increase in the production of prostaglandin E2 (PGE2) by BMDCs. Notably, PGE2 was found to substantially augment IL-23 production by DCs from aged but not young mice 114. As IL-23 promotes the differentiation of T helper 17 (TH17) cells, such increases in PGE2 and IL-23 production might underlie the potential increased predisposition to TH17 cell responses reported in aged individuals 115, 116. An age-associated increase in PGD2 production has been observed in lung tissue of mice infected with respiratory virus, and this increase was associated with impaired migration of pulmonary DCs to regional lymph nodes (Figure 1). Treatment of these mice with PGD2 antagonists resulted in improved DC migration and T cell responses 48.
Primary DCs from ageing humans have been reported to have impaired functions overall.. For example, the production of IL-12 downstream of TLR4 stimulation with LPS was reduced in myeloid DCs from older compared to young individuals 117. Another study revealed a generalized, age-associated decrease in TLR-induced cytokine production by mDCs and pDCs and this strongly correlated with reduced antibody responses to influenza virus immunization 97. Other studies of human pDCs have also shown age-associated changes in TLR7- and TLR9-induced cytokine production 37, influenza virus-induced type I and type III IFNs 118, 119, and WNV-induced type I IFN production 120. Taken together with other reports indicating that human pDC numbers in peripheral blood are decreased with age 34, 35, these findings suggest that the antiviral and antitumour functions of pDCs may be particularly affected in older adults. Strikingly, basal levels of intracellular cytokines in primary mDCs and pDCs have been found to be increased with ageing 97 — a finding that is consistent with the age-associated pro-inflammatory environment. Such a background of high cytokine production may reflect a degree of basal TLR activation that cannot be further augmented with additional TLR agonist treatment, and could contribute to failed innate immune responses to pathogens or vaccines (Figure 2).
Monocyte-derived DCs (MDDCs) from older compared to young adults showed increased TNF and IL-6 production following TLR4 or TLR8 stimulation; and TNF and IFNα production were also increased following exposure to self-DNA in vitro 36, 121. By contrast, in vitro assays of phagocytosis and a transwell assay of migration demonstrated reduced function by MDDCs from older adults. These phenotypes were associated with impaired PI3K signalling, which has been implicated as both a negative regulator of TLR signalling and a positive regulator of phagocytic function36. However, the possibility that the GM-CSF and IL-4 treatment used to obtain MDDCs could attenuate potential age-associated differences should also be considered.
However, ageing is not always associated with increased TLR signalling in human MDDCs. An age-associated decrease in CD80 and CD86 upregulation and in type I IFN production by MDDCs was observed after infection with WNV. This defect was associated with impaired induction of STAT1 and IRF7 expression120, similar to findings in primary human pDCs 119. Furthermore, WNV-infected MDDCs from older compared with young adults showed decreased nuclear translocation of the positive regulator IRF1 and increased expression of the TLR inhibitory protein AXL (also known as UFO) 120, which is a member of the TYRO3/AXL/MER (TAM) receptor family. Following activation of the type I IFN receptor (IFNAR)-STAT1 pathway, TAM receptors are expressed and hijack this pathway to produce the inhibitory proteins SOCS1 and SOCS3122; this mechanism provides a link to the STAT1-dependent decrease in type I IFN production that occurs following WNV infection. Taken together, these findings indicate that the effects of ageing on responses by DCs are complex, with evidence for both inappropriately impaired and augmented responses to PRR engagement that reflect cell type, activation state and tissue context (Table1 and Figure 2).
Signalling downstream of other PRRs
The effects of ageing on cytoplasmic PRRs, such as the NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs), remain to be completely elucidated. Notably, a recent study of aged Nlrp3–/– mice implicates the NLRP3 inflammasome in age-associated inflammation in adipose tissue and the brain, with aged knockout animals showing improvement in glucose tolerance and tests of memory and learning 123. By contrast, aged C57BL/6 mice showed reduced production of IL-1β and IL-18 following NLRP3 activation in the context of influenza virus infection 124. Taken together, these findings are again consistent with failure in innate immune activation in a setting of dysregulated inflammation (Figure 1).
PRR signalling is known to intersect with macroautophagy, which seems to be defective in various cell types from aged organisms 125; so it is probable that autophagic responses to intracellular pathogens are also impaired in older individuals. At the same time, defects in autophagy may influence PRR signalling via increased generation of ROS from dysfunctional mitochondria that ordinarily would be subject to macroautophagic degradation. As signalling downstream of both NLRP3 and RLR seem to be influenced by impaired autophagy 126-129, and as ROS production also generally increases with age, it is likely that PRR function in older individuals will be influenced by changes in autophagy 130.
Systemic factors contributing to innate immune ageing
Hormonal changes
Factors that are extrinsic to the immune system also contribute to the heightened pro-inflammatory environment associated with ageing. For example, both oestrogen and testosterone decrease IL-6 production in vitro and in vivo 131-134, and age-associated decreases in oestrogen or androgen production are associated with increases in basal pro-inflammatory cytokine levels 135, 136. Another non-immune contribution to the inflammatory environment is the neuroendocrine axis linking the central nervous system to the periphery via the hypothalamus. Knockdown of the histone deacetylase Sirtuin 1 in hypothalamic neurons induced a pro-inflammatory activated state in peripheral CD4+ T cells 137. Indeed, recent studies indicate that mammalian lifespan itself may be linked to an NF-κB-dependent inflammatory state in the hypothalamus, which occurs in aged mice and is linked to decreases in gonadotropin releasing hormone138. Further studies will be required to determine whether an analogous mechanism occurs in ageing humans.
Metabolic changes
Adipose tissue has immunological functions in addition to its well-known functions in energy storage and metabolism. Redistribution of adipose tissue and impairments in metabolic functions such as insulin sensitivity in older individuals is well described 139, and adipose tissue may be a source for age-associated increases in systemic levels of pro-inflammatory cytokines 140-143. Increased infiltration of macrophages in adipose tissue of aged mice is reminiscent of that seen in the setting of obesity, and supports the notion of obesity as a form of accelerated ageing in adipose tissue 144. Additional work on the biology of adipose tissue in the context of ageing will be required to obtain mechanistic insights. However, it is notable that saturated fatty acids and ceramides generated in the setting of lipotoxicity can activate the NLRP3 inflammasome 128, 145. In addition, a recent study identified a decrease in expression of Dicer, a protein that mediates microRNA processing, in adipose tissue from aged mice and human preadipocytes from older adults. Mice with an adipose tissue-specific knockout of Dicer had increased sensitivity to oxidative stress and expression of markers associated with senescence, which implicates miRNA regulation in adipose tissue ageing and its potential contribution to the age-associated pro-inflammatory environment146 (Figure 1).
DAMPs
Intriguing links between DNA damage and the activation of inflammatory responses comprise an additional potential contributing factor to the age-associated pro-inflammatory environment. For example, DNA damage from ionizing radiation has been associated with IL-6 production by epithelial cells147. Indeed, IL-1β, IL-6 and IL-8 are components of the senescence-associated secretory phenotype (SASP), which is the secretome of cytokines, growth factors, extracellular matrix proteins and proteases that are produced by senescent cells in response to DNA damage and that modify the local microenvironment 148. The SASP is also regulated by signalling via p38 MAPK 149 and by cell-associated IL-1α signalling 150. Notably, in a cohort of nonagenarians, levels of endogenous non-cell associated DNA were strongly associated with inflammatory markers such as C-reactive protein and were an independent risk factor for mortality 151. Such DNA, which could be released from senescent or necrotic cells, represents potential endogenous damage-associated molecular patterns (DAMPs) that activate innate immune responses. Several studies have shown that necrotic cells can activate the NLRP3 inflammasome, likely through the release of cellular ATP152-154, but it remains possible that additional PRRs, such as those that recognize nucleic acid motifs, could also participate in innate immune activation by endogenous ligands. In view of these findings, chronic infections, such as with cytomegalovirus (CMV) or other herpesviruses, ongoing endogenous DNA release by apoptotic cells or engagement of innate immune PRRs by other self ligands may all contribute to the increased inflammatory state associated with ageing. While chronic viral infections in humans depend on exposure, it remains unclear whether such pro-inflammatory DNA is intrinsic to ageing per se, or instead results from the consequences of comorbid disease or alterations in nutritional status in older adults.
Chronic and latent viral infections
Chronic viral infections, particularly with human CMV, have been linked to an age-associated pro-inflammatory environment155. CMV is known for cycles of asymptomatic reactivation throughout life, and such reactivation could contribute to an inflammatory micorenvironment156, 157. However, establishing causality, as opposed to associations, in humans has remained elusive. Although CMV reactivation has been shown to be associated with increased IL-6 and TNF levels and with impaired responses to influenza virus vaccine 158, 159, a recent longitudinal study that compared CMV seronegative older adults, those seroconverting to CMV and those who remained seropositive over a 10-year period failed to demonstrate an effect of CMV status on markers of inflammation 160. This study was the first to longitudinally evaluate individuals who seroconverted to CMV, and does not implicate CMV as the dominant driver of an age-associated pro-inflammatory environment. However, the number of new CMV seroconverters in this study was relatively small, and it is also conceivable that multiple rounds of CMV reactivation throughout life are needed to establish a systemic increase in inflammation. Further longitudinal studies over CMV are needed to address these issues.
Ageing and chronic inflammatory disease
The evidence discussed for age-associated innate immune dysregulation, in the setting of hormonal and metabolic aetiologies and endogenous ligands for PRRs, suggests that the resultant pro-inflammatory state will influence the pathogenesis of not only infectious diseases, but also conditions associated with chronic inflammation. Here, we illustrate the interface of innate immune activation with two examples of diseases that have a high prevalence in older adults — atherosclerosis and Alzheimer’s disease.
Atherosclerosis
Clinical and epidemiological studies have shown that ageing contributes to the development of atherosclerosis 161, which is a chronic inflammatory arterial disease characterized by the deposition of lipids within the arterial wall 162. Recently, TLR and NLRP3 signalling have been implicated in the development of this disease163, 164. It is not clear how ageing enhances the development of atherosclerosis, but elevated basal inflammatory responses may contribute to the chronic inflammation in the disease. It is also poorly understood how an atherosclerotic plaque becomes susceptible to rupture, and the exact nature of the complex thrombotic process after rupture, which include components of the immune system, the coagulation system and stromal cells. Vascular smooth muscle cells (VSMCs) that are harvested from the aortas of aged mice were found to have pro-atherogenic features, including increased production of pro-inflammatory cytokines, such as IL-6, and chemokines, such as CCL2 (Figure 1). TLR4 expression was also increased in aged VSMCs, and increased production of IL-6 was dependent on TLR4 and MyD88 165. VSMCs from ageing non-human primates also showed higher production of pro-inflammatory cytokines, such as IL-1β, TNF and CCL2166. In this study, the increased inflammatory response correlated with increased mitochondrial oxidative stress. Notably, treatment with resveratrol, which is a naturally occurring phenol that has been shown to extend life span in various experimental models, reversed the ageing-associated pro-inflammatory phenotype of VSMCs. However, it remains unclear how the inflammatory phenotype of aged VSMCs contributes to the pathogenesis of atherosclerosis.
Alzheimer’s disease
Dysregulated innate immune responses in the ageing brain may be relevant to various age-associated neurodegenerative conditions such as Alzheimer’s disease (Figure 1). PRRs are highly expressed in the brain, with expression of TLR1-TLR9 reported in both mouse and human microglia. Widespread TLR expression is also found in mouse astrocytes and cortical neurons, although in humans only TLR3 expression has been reported 167. Notably, TLR-induced cytokine production by mouse microglia is increased in aged compared to young mice. In humans, a gene expression microarray study of four regions of the brain in young (20-59 years old) and aged (60-99 years old) individuals, and in patients with Alzheimer’s disease (74-95 years old) revealed marked upregulation of TLR- and inflammasome-associated genes in older adults. In addition, a gene expression signature of microglial activation was also observed in older adults168.
The effect of TLR activation in the context of neurodegeneration has been investigated mainly in mouse models and seems to be complex. The β-amyloid peptide that is found in amyloid plaques that are deposited in the brains of individuals with Alzheimer’s disease induces a TLR-dependent innate immune response that may facilitate β-amyloid clearance 169-173. However, systemic inflammation, for example by administration of LPS, exacerbates β-amyloid plaque formation and cognitive impairment in a transgenic Alzheimer’s disease model 174, 175, and an association between systemic inflammation and Alzheimer’s disease has been reported in humans 176. Recent studies have also implicated the NLRP3 inflammasome in Alzheimer’s disease pathogenesis 177, 178. The beneficial and detrimental effects of innate immune engagement in older adults with Alzheimer’s disease may reflect the timing, location and extent of inflammation, and remains an area of active investigation.
Conclusions and future perspectives
Considerable progress has been made in elucidating age-associated alterations in innate immunity and their consequences. For some cell types, information on the effects of ageing is emerging; one example would be myeloid-derived suppressor cells (MDSCs) — a heterogeneous population of immature myeloid cells with potent T cell suppressive function 179. MDSCs are expanded in malignancies and other inflammatory states, and are also expanded in aged, compared to young mice 180 and humans 181. These findings are notable given the substantial age-associated increase in human cancers 182. A recent report in a mouse model of sterile inflammation described a neutrophil population that constitutively produced IFNγ and that conferred T cell resistance to MDSC suppression — this provides a potential mechanism for innate immune dysregulation that could potentiate chronic inflammation183. However, it remains to be seen whether this neutrophil population is present in aged humans or mice.
Understanding the biological basis for altered innate immunity and inflammation in ageing is a challenge with substantial clinical impact, as restoration or preservation of physiological function is likely to be of equal or greater importance than extension of lifespan. The study of well-characterized human cohorts with alterations in functional status, such as the geriatric syndrome of frailty184, offers the possibility of linking innate immune function and inflammation with biological mechanisms.
Integrative systems biology approaches are also beginning to yield insights into such mechanisms185. The use of computational approaches together with model systems will be crucial as our appreciation of the complexity of the immune system and its relationship to the environment increases. For example, the possibility that the gut microbiome may influence vaccine responsiveness 186, probably through engagement of the innate immune system, will require the synthesis of signatures of vaccine responses from gene expression, proteomic and immunological studies with known age-associated alterations in the composition of the human gut microbiome187, 188. These offer future prospects to the field of considerable challenge and opportunity.
Glossary terms
- Monocyte-derived DCs (MDDCs)
MDDCs can be generated in vitro from peripheral blood mononuclear cells in the presence of interleukin-4 and granulocyte-macrophage colony stimulating factor. MDDCs resemble myeloid DCs and may model the differentiation of DCs from monocytes that enter sites of inflammation.
- Macroautophagy
An evolutionarily conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation, through fusion to secondary lysosomes.
- Inflammasome
A multiprotein complex that consists of a NOD- and LRR-containing (NLR) protein, an adaptor protein and pro-caspase 1 and that upon assembly facilitates the caspase 1-mediated cleavage and production of mature cytokines such as interleukin-1β and interleukin-18.
- Conventional DCs (cDCs)
Specialized phagocytic antigen-presenting cells that have the classic stellate DC morphology. Mouse cDCs generally express CD11c, but are highly heterogeneous: CD8α+ and CD4+ cDC subsets in secondary lymphoid organs, and CD8α+CD103+ and CD4+CD11b+ cDC subsets in the periphery. Human cDCs are generally termed myeloid DCs and are lineage−MHC class II+CD11c+. Human equivalents to the mouse CD8α+ and CD4+ cDC subsets can be distinguished by expression of BDCA3 (CD141) and BDCA1 (CD1c), respectively.
- NOD-like receptors (NLRs)
A family of over 20 nucleotide-binding and oligomerization domain (NOD)-like cytoplasmic pattern recognition receptors that sense pathogens, toxins, endogenous danger signals (such as uric acid) and exogenous crystalline substances (such as alum, silica and asbestos) and induce inflammatory responses..
- Plasmacytoid DC (pDC)
An immature dendritic cell (DC) with a morphology that resembles that of a plasma cell.. Mouse pDCs express markers including B220, which is usually associated with the B cell lineage. In humans, pDCs express CD123 and BDCA2 (also known as CD303), and are CD11c-negative. These DCs are the main producers of type I interferons in response to viral infection.
- RIG-I-like receptors (RLRs)
A family of cytoplasmic pattern recognition receptors that are related to the RNA helicase retinoic acid inducible gene I (RIG-I) and that recognize viral single and double-stranded RNA and mediate antiviral responses such as type I interferon production.
References
- 1.Population Division. Department of Economic and Social Affairs. United Nations . World Population Ageing: 1950-2050. United Nations; New York: 2001. [Google Scholar]
- 2.Centers for Disease Control and Prevention . National Ambulatory Medical Survey. National Center for Health Statistics; 2005. [Google Scholar]
- 3.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis. 2000;30:931–933. doi: 10.1086/313792. [DOI] [PubMed] [Google Scholar]
- 4.Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age--age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- 5.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolich-Zugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24:356–364. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes L, Lefebvre JS. Age-related Deficiencies in Antigen-Specific CD4 T cell Responses: Lessons from Mouse Models. Aging Dis. 2011;2:374–381. [PMC free article] [PubMed] [Google Scholar]
- 8.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, et al. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 10.Fagiolo U, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 11.Mari D, et al. Hypercoagulability in centenarians: the paradox of successful aging. Blood. 1995;85:3144–3149. [PubMed] [Google Scholar]
- 12.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 15.Beharka AA, et al. Interleukin-6 production does not increase with age. J Gerontol A Biol Sci Med Sci. 2001;56:B81–88. doi: 10.1093/gerona/56.2.b81. [DOI] [PubMed] [Google Scholar]
- 16.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. Report that human hematopoietic stem cells from older adults show a bias toward myeloid, at the expense of lymphoid differentiation--mirroring findings in mouse HSCs.
- 22.Beli E, et al. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev. 2011;132:503–510. doi: 10.1016/j.mad.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ergen AV, Boles NC, Goodell MA. Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. 2012;119:2500–2509. doi: 10.1182/blood-2011-11-391730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esplin BL, et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rube CE, et al. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.eiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 27.Chatta GS, Price TH, Stratton JR, Dale DC. Aging and marrow neutrophil reserves. J Am Geriatr Soc. 1994;42:77–81. doi: 10.1111/j.1532-5415.1994.tb06077.x. [DOI] [PubMed] [Google Scholar]
- 28.De Martinis M, Modesti M, Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol. 2004;82:415–420. doi: 10.1111/j.0818-9641.2004.01242.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferrando-Martinez S, et al. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Dordr) 2013;35:251–259. doi: 10.1007/s11357-011-9341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratts RB, Weng NP. Homeostasis of lymphocytes and monocytes in frequent blood donors. Front Immunol. 2012;3:271. doi: 10.3389/fimmu.2012.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hearps AC, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 33.Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired Functions of Peripheral Blood Monocyte Subpopulations in Aged Humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garbe K, Bratke K, Wagner S, Virchow JC, Lommatzsch M. Plasmacytoid dendritic cells and their Toll-like receptor 9 expression selectively decrease with age. Hum Immunol. 2012;73:493–497. doi: 10.1016/j.humimm.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Orsini G, et al. Enumeration of human peripheral blood dendritic cells throughout the life. Int Immunol. 2012;24:347–356. doi: 10.1093/intimm/dxs006. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal A, et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. Reports an age-associated increase in TLR4 and TLR8-dependent cytokine production in human monocyte-derived DCs juxtaposed with impaired phagocytosis and chemotaxis and associated with decreased PI-3-kinase activation.
- 37.Jing Y, et al. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum Immunol. 2009;70:777–784. doi: 10.1016/j.humimm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]
- 39.Nomellini V, et al. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging Dis. 2012;3:234–247. [PMC free article] [PubMed] [Google Scholar]
- 40.Niwa Y, Kasama T, Miyachi Y, Kanoh T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44:1655–1664. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- 41.Murciano C, Yanez A, O’Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 42.Ren Z, et al. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J Med Microbiol. 2009;58:1559–1567. doi: 10.1099/jmm.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol. 2013;190:1746–1757. doi: 10.4049/jimmunol.1201213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez CR, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 46.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;6:446–456. doi: 10.1016/j.chom.2009.09.011. This paper employs a Herpes Simplex Virus infection model in mice to demonstrate that increased mortality of aged mice to HSV infection results from elevated IL-17 production in NKT cells, with enhanced neutrophil recruitment and activation in the liver and liver failure.
- 47.Luu NT, Rainger GE, Nash GB. Differential ability of exogenous chemotactic agents to disrupt transendothelial migration of flowing neutrophils. J Immunol. 2000;164:5961–5969. doi: 10.4049/jimmunol.164.11.5961. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. Uses murine infection models of several respiratory viruses (including influenza, SARS-CoV, RSV) to demonstrate an age-associated decrease in respiratory DC migration to draining lymph nodes that is associated with increased PGD2 levels in the lung and improved with PGD2 pharmacologic inhibition.
- 49.Butcher SK, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–886. [PubMed] [Google Scholar]
- 50.Fortin CF, McDonald PP, Lesur O, Fulop T., Jr. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11:873–882. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- 51.Simell B, et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011;29:1929–1934. doi: 10.1016/j.vaccine.2010.12.121. [DOI] [PubMed] [Google Scholar]
- 52.Fulop T, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 53.Tseng CW, et al. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin-resistant S. aureus. PLoS One. 2012;7:e41454. doi: 10.1371/journal.pone.0041454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radford DJ, et al. Dehdyroepiandrosterone sulfate directly activates protein kinase C-beta to increase human neutrophil superoxide generation. Mol Endocrinol. 2010;24:813–821. doi: 10.1210/me.2009-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortin CF, Larbi A, Dupuis G, Lesur O, Fulop T., Jr. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology. 2007;8:173–187. doi: 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- 56.Tortorella C, et al. Role of phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways in granulocyte macrophage-colony-stimulating factor failure to delay fas-induced neutrophil apoptosis in elderly humans. J Gerontol A Biol Sci Med Sci. 2006;61:1111–1118. doi: 10.1093/gerona/61.11.1111. [DOI] [PubMed] [Google Scholar]
- 57.Fortin CF, Lesur O, Fulop T., Jr. Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Fortin CF, Larbi A, Lesur O, Douziech N, Fulop T., Jr. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006;79:1061–1072. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- 59.Esposito AL, Poirier WJ, Clark CA. In vitro assessment of chemotaxis by peripheral blood neutrophils from adult and senescent C57BL/6 mice: correlation with in vivo responses to pulmonary infection with type 3 Streptococcus pneumoniae. Gerontology. 1990;36:2–11. doi: 10.1159/000213169. [DOI] [PubMed] [Google Scholar]
- 60.Lipschitz DA, Udupa KB. Influence of aging and protein deficiency on neutrophil function. J Gerontol. 1986;41:690–694. doi: 10.1093/geronj/41.6.690. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev. 2008;129:223–230. doi: 10.1016/j.mad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207:2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plett A, Murasko DM. Genetic differences in the age-associated decrease in inducibility of natural killer cells by interferon-alpha/beta. Mech Ageing Dev. 2000;112:197–215. doi: 10.1016/s0047-6374(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 65.Nogusa S, Murasko DM, Gardner EM. Differential effects of stimulatory factors on natural killer cell activities of young and aged mice. J Gerontol A Biol Sci Med Sci. 2012;67:947–954. doi: 10.1093/gerona/gls079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Almeida-Oliveira A, et al. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72:319–329. doi: 10.1016/j.humimm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Borrego F, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 69.Chidrawar SM, Khan N, Chan YL, Nayak L, Moss PA. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun Ageing. 2006;3:10. doi: 10.1186/1742-4933-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol. 2010;71:676–681. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Le Garff-Tavernier M, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 72.Solana R, et al. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Hazeldine J, Hampson P, Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell. 2012;11:751–759. doi: 10.1111/j.1474-9726.2012.00839.x. First study to implicate impaired perforin mobilization to the immunological synapse in age-associated impairment in NK cell cytotoxicity.
- 74.Ogata K, et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol. 2001;124:392–397. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peralbo E, Alonso C, Solana R. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42:703–708. doi: 10.1016/j.exger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 76.Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol. 2005;175:3102–3109. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- 77.DelaRosa O, et al. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213–217. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 78.Peralbo E, et al. Decreased frequency and proliferative response of invariant Valpha24Vbeta11 natural killer T (iNKT) cells in healthy elderly. Biogerontology. 2006;7:483–492. doi: 10.1007/s10522-006-9063-5. [DOI] [PubMed] [Google Scholar]
- 79.Kawabata T, et al. Functional alterations of liver innate immunity of mice with aging in response to CpG-oligodeoxynucleotide. Hepatology. 2008;48:1586–1597. doi: 10.1002/hep.22489. [DOI] [PubMed] [Google Scholar]
- 80.Inui T, et al. Age-associated augmentation of the synthetic ligand-mediated function of mouse NK1.1 ag(+) T cells: their cytokine production and hepatotoxicity in vivo and in vitro. J Immunol. 2002;169:6127–6132. doi: 10.4049/jimmunol.169.11.6127. [DOI] [PubMed] [Google Scholar]
- 81.Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175:1004–1007. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- 82.Tasat DR, Mancuso R, O’Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2:159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 83.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011;186:3441–3451. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang S, Domon H, Hosur KB, Wang M, Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mech Ageing Dev. 2009;130:538–546. doi: 10.1016/j.mad.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pereira LF, de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–194. doi: 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Plowden J, et al. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol. 2004;229:86–92. doi: 10.1016/j.cellimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Wong C, Goldstein DR. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 2013;25:535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan SY, et al. Phenotype and functions of conventional dendritic cells are not compromised in aged mice. Immunol Cell Biol. 2012;90:722–732. doi: 10.1038/icb.2011.104. [DOI] [PubMed] [Google Scholar]
- 90.Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li G, Smithey MJ, Rudd BD, Nikolich-Zugich J. Age-associated alterations in CD8alpha+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium. Aging Cell. 2012;11:968–977. doi: 10.1111/j.1474-9726.2012.00867.x. This study indicated that defects in murine CD8 alpha+ DCs with aging specifically impaired migration and the upregulation of costimulatory molecules, and may impair T cell responses in vivo to a model bacterial infection.
- 92.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–7984. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown KL, Gossner A, Mok S, Mabbott NA. The effects of host age on the transport of complement-bound complexes to the spleen and the pathogenesis of intravenous scrapie infection. J Virol. 2012;86:25–35. doi: 10.1128/JVI.05581-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark HL, et al. Characterization of MHC-II antigen presentation by B cells and monocytes from older individuals. Clin Immunol. 2012;144:172–177. doi: 10.1016/j.clim.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steger MM, Maczek C, Grubeck-Loebenstein B. Peripheral blood dendritic cells reinduce proliferation in in vitro aged T cell populations. Mech Ageing Dev. 1997;93:125–130. doi: 10.1016/s0047-6374(96)01835-0. [DOI] [PubMed] [Google Scholar]
- 96.van Duin D, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. This study employed multivariable mixed effects modeling to demonstrate an age-related decrease in TLR1/2 mediated cytokine production and TLR1 expression in monocytes from 159 human subjects.
- 97.Panda A, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. Demonstration of an extensive, age-associated decrease in TLR-dependent cytokine production in primary human mDCs and pDCs that reflects elevated basal levels of cytokine production in older adults, and is strongly associated with antibody response to influenza vaccination.
- 98.Renshaw M, et al. Cutting Edge: Impaired Toll-Like Receptor Expression and Function in Aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 99.Qian F, et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell. 2012;11:104–110. doi: 10.1111/j.1474-9726.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Renshaw M, et al. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 101.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 102.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 103.Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012;47:507–518. doi: 10.1016/j.exger.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaik-Dasthagirisaheb YB, Kantarci A, Gibson FC., 3rd Immune response of macrophages from young and aged mice to the oral pathogenic bacterium Porphyromonas gingivalis. Immun Ageing. 2010;7:15. doi: 10.1186/1742-4933-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Asquith M, et al. Age-dependent changes in innate immune phenotype and function in rhesus macaques (Macaca mulatta) Pathobiol Aging Age Relat Dis. 2012;2 doi: 10.3402/pba.v2i0.18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Duin D, et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 107.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakaya HI, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kong KF, et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. Demonstration of a DC-SIGN-dependent interaction with WNV resulting in a STAT1-dependent downregulation of TLR3 in human macrophages that is impaired in cells from older adults.
- 110.Agius E, et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. A study of age-associated impairment in human DTH responses that employed skin biopsy and suction blister samples to demonstrate diminished TNF-α production by dermal macrophages and an associated increase in Treg infiltration in the skin of older individuals.
- 111.Tesar BM, et al. Murine myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 112.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. Demonstration of a TLR9-dependent defect in pDC type I interferon production in aged mice associated with impaired IRF7 upregulation, with type I IFN production in aged mice increased with anti-oxidants or caloric restriction.
- 113.El Mezayen R, El Gazzar M, Myer R, High KP. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8:553–565. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Myer RG, El Mezayen R, High KP. Prostaglandin E2-dependent IL-23 production in aged murine dendritic cells. Exp Gerontol. 2010;45:834–841. doi: 10.1016/j.exger.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 116.Lee JS, et al. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Della Bella S, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 118.Canaday DH, et al. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J Clin Immunol. 2010;30:373–383. doi: 10.1007/s10875-010-9374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sridharan A, et al. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age (Dordr) 2011;33:363–376. doi: 10.1007/s11357-010-9191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qian F, et al. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 2011;203:1415–1424. doi: 10.1093/infdis/jir048. Demonstration that monocyte-derived DCs from older adults have diminished costimulatory protein expression and cytokine expression after WNV infection and TLR3 and TLR8 stimulation, with impaired STAT1 phosphorylation and upregulation of IRF7.
- 121.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182:1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Houm YH, et al. Canonical NLRP3 inflammasome links systemic low grade inflammation to functional decline in aging. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.09.010. in press. This manuscript demonstrates that age-associated inflammation in adipose tissue and brain is diminished in aged Nlrp3 knockout mice, with improvements in glucose tolerance and tests of learning and memory.
- 124.Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012;188:2815–2824. doi: 10.4049/jimmunol.1103051. First demonstration of an age-associated impairment in induced NLRP3 function in mice.
- 125.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tal MC, et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 130.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012;4:166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16:178–186. doi: 10.1006/cyto.2001.0962. [DOI] [PubMed] [Google Scholar]
- 132.Pottratz ST, Bellido T, Mocharla H, Crabb D, Manolagas SC. beta-Estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994;93:944–950. doi: 10.1172/JCI117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ray A, Prefontaine KE, Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269:12940–12946. [PubMed] [Google Scholar]
- 134.Yang BC, Liu CW, Chen YC, Yu CK. Exogenous dehydroepiandrosterone modified the expression of T helper-related cytokines in NZB/NZW F1 mice. Immunol Invest. 1998;27:291–302. doi: 10.3109/08820139809070902. [DOI] [PubMed] [Google Scholar]
- 135.Abu-Taha M, et al. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol. 2009;183:1393–1402. doi: 10.4049/jimmunol.0803157. [DOI] [PubMed] [Google Scholar]
- 136.Maggio M, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 137.Matarese G, et al. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proc Natl Acad Sci U S A. 2013;110:6193–6198. doi: 10.1073/pnas.1210644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tchkonia T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 141.Morin CL, Pagliassotti MJ, Windmiller D, Eckel RH. Adipose tissue-derived tumor necrosis factor-alpha activity is elevated in older rats. J Gerontol A Biol Sci Med Sci. 1997;52:B190–195. doi: 10.1093/gerona/52a.4.b190. [DOI] [PubMed] [Google Scholar]
- 142.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723–730. doi: 10.1093/gerona/glp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu D, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 144.Lumeng CN, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mori MA, et al. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. Demonstration that DNA damage associated with senescence induces the secretion of pro-inflammatory cytokines such as IL-6, suggesting that endogenous DNA could contribute to an age-associated increase in inflammation.
- 148.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jylhava J, Jylha M, Lehtimaki T, Hervonen A, Hurme M. Circulating cell-free DNA is associated with mortality and inflammatory markers in nonagenarians: the Vitality 90+ Study. Exp Gerontol. 2012;47:372–378. doi: 10.1016/j.exger.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 152.Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li H, Ambade A, Re F. Cutting edge: Necrosis activates the NLRP3 inflammasome. J Immunol. 2009;183:1528–1532. doi: 10.4049/jimmunol.0901080. [DOI] [PubMed] [Google Scholar]
- 155.Pawelec G, McElhaney JE, Aiello AE, Derhovanessian E. The impact of CMV infection on survival in older humans. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 156.Limaye AP, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stowe RP, et al. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trzonkowski P, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 160.Bartlett DB, et al. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell. 2012;11:912–915. doi: 10.1111/j.1474-9726.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- 161.Asia Pacific Cohort Studies Collaboration The impact of cardiovascular risk factors on the age-related excess risk of coronary heart disease. Int J Epidemiol. 2006;35:1025–1033. doi: 10.1093/ije/dyl058. [DOI] [PubMed] [Google Scholar]
- 162.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 163.Bjorkbacka H, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 164.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Song Y, et al. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2012;32:103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Csiszar A, et al. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011;81:825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 168.Cribbs DH, et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Michaud JP, Richard KL, Rivest S. Hematopoietic MyD88-adaptor protein acts as a natural defense mechanism for cognitive deficits in Alzheimer’s disease. Stem Cell Rev. 2012;8:898–904. doi: 10.1007/s12015-012-9356-9. [DOI] [PubMed] [Google Scholar]
- 172.Doi Y, et al. Microglia activated with the toll-like receptor 9 ligand CpG attenuate oligomeric amyloid {beta} neurotoxicity in in vitro and in vivo models of Alzheimer’s disease. Am J Pathol. 2009;175:2121–2132. doi: 10.2353/ajpath.2009.090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scholtzova H, et al. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer’s disease-related pathology. J Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee JW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sheng JG, et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 176.Holmes C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Heneka MT, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. J Immunol. 2011;186:697–707. doi: 10.4049/jimmunol.1002987. [DOI] [PubMed] [Google Scholar]
- 181.Verschoor CP, et al. Blood CD33(+)HLA-DR(−) myeloid-derived suppressor cells are increased with age and a history of cancer. J Leukoc Biol. 2013;93:633–637. doi: 10.1189/jlb.0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 183.Ryan SO, Johnson JL, Cobb BA. Neutrophils confer T cell resistance to myeloid-derived suppressor cell-mediated suppression to promote chronic inflammation. J Immunol. 2013;190:5037–5047. doi: 10.4049/jimmunol.1203404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li H, Manwani B, Leng SX. Frailty, inflammation, and immunity. Aging Dis. 2011;2:466–473. [PMC free article] [PubMed] [Google Scholar]
- 185.Furman D, et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol. 2013;9:659. doi: 10.1038/msb.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ferreira RB, Antunes LC, Finlay BB. Should the human microbiome be considered when developing vaccines? PLoS Pathog. 2010;6:e1001190. doi: 10.1371/journal.ppat.1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Biagi E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Claesson MJ, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 189.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leng J, et al. Efficacy of a vaccine that links viral epitopes to flagellin in protecting aged mice from influenza viral infection. Vaccine. 2011;29:8147–8155. doi: 10.1016/j.vaccine.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Taylor DN, et al. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 192.Asanuma H, et al. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine. 2012;30:803–812. doi: 10.1016/j.vaccine.2011.10.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morgan EL, Thoman ML, Sanderson SD, Phillips JA. A novel adjuvant for vaccine development in the aged. Vaccine. 2010;28:8275–8279. doi: 10.1016/j.vaccine.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G antipolysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect Immun. 2006;74:2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Behzad H, et al. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis. 2012;205:466–473. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]