Abstract
Aquatic habitats harbor a multitude of bacterial species. Many of these bacteria can act as pathogens to aquatic species and/or non-aquatic organisms, including humans, that come into contact with contaminated water sources or colonized aquatic organisms. In many instances, the bacteria are not pathogenic to the aquatic species they colonize and are only considered pathogens when they come into contact with humans. There is a general lack of knowledge about how the environmental lifestyle of these pathogens allows them to persist, replicate and produce the necessary pathogenic mechanisms to successfully transmit to the human host and cause disease. Recently, the zebrafish infectious disease model has emerged as an ideal system for examining aquatic pathogens, both in the aquatic environment and during infection of the human host. This review will focus on how the zebrafish has been used successfully to analyze the pathogenesis of aquatic bacterial pathogens.
Keywords: Zebrafish, aquatic pathogens, environmental reservoir, zoonotic, aquaculture, bacteria, infectious disease
1. Introduction
Aquatic pathogens are found in all water environments including marine, freshwater, brackish water, sewage, ground and even drinking water. Seawater alone harbors about 100 million bacteria per liter (Finkelstein and Oren, 2011). Humans can be exposed to all of these aquatic sources through various activities, ranging from consumption of contaminated seafood, ingestion of contaminated drinking water, recreational activities such as swimming, surfing, boating or fishing, exposure of open wounds to contaminated water sources or through handling of contaminated fish.
A significant source of human pathogens from aquatic habitats is the many fish species that are used for food consumption. Fish are an important part of the human diet worldwide and can therefore serve as vectors of human disease. Extensive growth in the aquaculture industry in the last 30 years has extended from an increased desire for lean protein and healthy fats, as well as depletion of fish species in natural waters from overfishing. In addition, restrictive quotas have been imposed for many wild caught fish in certain geographical areas, leading to further decline in fish availability in the food supply. To increase overall production to meet the rising demand, fish farms often have overcrowded ponds that can serve as hot transmission sites for fish disease and serve as bacterial reservoirs that can be passed on to humans during food preparation. In many parts of the world, fish that can harbor potential bacterial pathogens live in human water supplies, creating a reservoir that can be passed on to humans in drinking water. Incidences have been reported in which the source of a bacterial disease outbreak in wild fish came from a nearby contaminated aquaculture facility (Zlotkin et al., 1998). Therefore, one of our major sources of protein can be the origin for potential human pathogens and also be a major contributor to contamination of essential water supplies (Table 1).
Table 1.
Aquatic bacterial pathogens that cause disease in fish and humans.
| Organism | Symptoms in fish | Symptoms in humans | References |
|---|---|---|---|
| Aeromonas hydrophila | hemorrhagic septicemia, red sore disease and ulcerative infections | mild gastroenteritis, septicemia, necrotizing fasciitis and myonecrosis | (Janda and Abbott, 2010) |
| Edwardsiella tarda | septicemia, small cutaneous lesions, necrotic abscesses, petechial hemorrhaging of the fin and skin, rectal hernia, edema of the abdomen | Gastroenteritis, intestinal colitis, soft tissue infections, meningitis, peritonitis, septicemia, bacteremia, endocarditis and urosepsis | (Janda and Abbott, 2010; Mohanty and Sahoo, 2007; Schlenker and Surawicz, 2009; Tamada et al., 2009; Wang et al., 2005) |
| Mycobacterium marinum | skin erythema, raised scales, swollen abdomen, granulomatous lesions in the peritoneum, kidneys and ovaries | granulomatous skin and soft tissue infection | (Bonamonte et al., 2013; Gao et al., 2004; Huang et al., 2012; Jacobs et al., 2009; Wu et al., 2012) |
| Streptococcus agalactiae | sepsis and meningitis | early onset neonatal sepsis and meningitis | (Edmond et al., 2012; Evans et al., 2002; Skoff et al., 2009) |
| Streptococcus iniae | systemic infection with accompanying meningitis and panophthalmitis | bacteremic cellulitis with the rare complications of endocarditis, meningitis, arthritis, sepsis and toxic shock | (Agnew and Barnes, 2007; Bromage and Owens, 2002; Weinstein et al., 1996a; Weinstein et al., 1996b) |
| Vibrio alginolyticus | septicemia | gastrointestinal, wound and mucosal infections | (Blake et al., 1980; Dechet et al., 2008) |
| Vibrio cholerae | colonization of the intestinal track, mild edema, diarrhea | cholera disease causing dehydration and diarrhea | (Austin, 2010; Finkelstein and Oren, 2011; Runft et al., 2014; Sack et al., 2004) |
| Vibrio vulnificus | external haemorrhaging and ulcers, haemorrhaging of the gills, heart, liver and spleen | soft tissue infections, primary septicemia | (Blake et al., 1980; Dechet et al., 2008) |
For many years, zebrafish (Danio rerio) have served as an important tool of biomedical research, providing great advances in our understanding of vertebrate development and disease modeling. Importantly, in recent years, zebrafish have been used to effectively model human infectious disease (Allen and Neely, 2010; Davis et al., 2002; Meijer and Spaink, 2011; Neely et al., 2002; Phelps, 2005; Sullivan and Kim, 2008; van der Sar et al., 2004). Zebrafish pathogenesis closely reflects pathogenesis observed in diseased wild and farmed fish and clinical symptoms observed during human infections (Davis et al., 2002; Neely et al., 2002; Weinstein et al., 1997). Zebrafish can also become colonized by some aquatic pathogens without succumbing to disease, providing an excellent model for the role of fish as an “environmental reservoir” for aquatic pathogens (Runft et al., 2014). “Environmental reservoirs” describes locations that allow bacterial persistence and replication in the environment while favoring pathogen transmission to susceptible hosts (Vezzulli et al., 2010). This review will focus on recent research on zebrafish as experimental hosts of zoonotic aquatic bacterial pathogens. Using zebrafish as an aquatic colonization model of human pathogens can provide information on environmental reservoirs of pathogens and how they contribute to transmission of human disease. Of all the infectious diseases modeled using the zebrafish host, those diseases caused by aquatic organisms would appear to have the greatest potential to add to our knowledge of pathogenesis because of the environmental niche they share.
2. Utilizing zebrafish as an infectious disease model
Natural pathogens of fish, such as Mycobacterium marinum and Streptococcus iniae, have been utilized in zebrafish to provide excellent models for understanding the pathogenesis of human tuberculosis infections caused by Mycobacterium tuberculosis (see (Deng et al., 2011b; Krishnan et al., 2010; Lesley and Ramakrishnan, 2008; Meijer and Spaink, 2011; Pozos and Ramakrishnan, 2004; Ramakrishnan, 2013; Tobin and Ramakrishnan, 2008) for review and citations within) and human streptococcal systemic infections (Allen and Neely, 2011; Harvie et al., 2013; Locke et al., 2008; Lowe et al., 2007; Miller and Neely, 2005; Neely et al., 2002; Phelps, 2005). Using a model host allows comparison in side-by-side infections of bacterial mutants and wild type bacteria to examine the role of virulence genes in aquatic and human disease. Information gained from these analyses allows development of methods to better target infection either in the vector (fish) itself or in the human or animal host. One advantage of using the zebrafish as an infectious disease model is the ability to knockdown zebrafish genes during early life stages using morpholino technology. This method allows the researcher to suppress expression of a particular immune gene to determine its role in fighting disease. Furthermore, vaccine and treatment strategies studied in the zebrafish have the potential to be translated to aquaculture and to human clinics. Consequently, the use of the zebrafish for analysis of aquatic pathogens makes it an ideal infectious disease model.
Zebrafish disease models have utilized multiple life stages of the host, from embryos to adults (Meijer and Spaink, 2011). Because the innate immune system is active within 24 hours post fertilization (hpf) in zebrafish, but the adaptive immune system is not completely developed until 3-4 weeks later, important differences in required immune responses for fighting specific infections can be analyzed using the different stages of development (Clay et al., 2007; Clay et al., 2008; Davis et al., 2002; Lam et al., 2004; Meeker and Trede, 2008; Sullivan and Kim, 2008; Trede et al., 2004).
Multiple inoculation methods have been used to infect zebrafish and the success of a particular inoculation method depends on the ability of the pathogen to breach natural defense barriers. The outer mucus layer of the zebrafish provides a natural barrier to infection, much as the mucous membranes do in human tissues (Chang and Hwang, 2011). The epidermis and scales of the zebrafish also act as a protective layer, just as the epidermis and dead skin cell layer in humans act as a barrier to pathogens (Chang and Hwang, 2011). Natural routes of infection in the model system include simply adding bacteria to the tank water; bath immersion, in which the zebrafish are placed for a limited period of time in a high concentration of bacteria and then placed into fresh tank water; and dermal abrasion prior to bath immersion (Harriff et al., 2007; Neely et al., 2002; Phelan et al., 2005; Pressley et al., 2005; van Soest et al., 2011). Fish can also be infected or colonized by the oro-gastric route, by being exposed to inoculated tank water or allowed to feed on inoculated food sources (Kanther and Rawls, 2010; O’Toole and Kolter, 1998; Rawls et al., 2004; Runft et al., 2014; Szabady et al., 2009). All of these test the ability of the bacteria to breach natural barriers, or mimic inoculation conditions that would be encountered in the natural aquatic environment. However, they do not allow for an accurate measurement of the amount of bacteria delivered to the host.
Other methods of inoculation include oral gavage, in which bacteria are inserted into the esophagus through a small tube attached to a syringe (Cocchiaro and Rawls, 2013; Collymore et al., 2013). This allows inoculation into the gastrointestinal tract and is particularly important for analysis of pathogens that have a GI tropism. Although this method does provide more precision in determining the amount of bacteria inoculated than the natural routes discussed above, some of the inoculum may escape through the gills or be lost through the mouth. Therefore, subsequent bacterial counts taken shortly after inoculation can be inconsistent.
The most common route of inoculation is injection of bacteria directly into the host, which gives reliable reproducible infection results, but is not a natural route of infection. In adult zebrafish this includes intramuscular injection into the dorsal muscle or intraperitoneal injection (Neely et al., 2002; Phelps, 2005). Microinjection is also used for larval infections where bacteria are inoculated into the otic vesicle, the yolk sac, tail muscle, the tail vein, the caudal vein, or the Duct of Cuvier (Cosma et al., 2006; Li and Hu, 2012; Meijer and Spaink, 2011). All of these can be used to mimic a certain type of infection and to ask a specific question about the ability of the pathogen to survive in a particular tissue or to disseminate to other tissue environments.
3. Aquatic bacterial pathogens modeled in the Zebrafish
While mammalian models of human disease have proven invaluable in providing information on pathogenesis, they do not always accurately mimic disease symptoms that occur during human infections. Furthermore, some human pathogens do not readily infect mice, rats or rabbits. In the case of zoonotic infections, information on transmission from the vector to the human host is completely lacking. Many questions remain about the ability of the pathogen to persist during the environmental stage or in an alternate host, often in a non-pathogenic state, before being transferred to humans. Using the zebrafish to address these types of questions with aquatic pathogens provides an opportunity to acquire new knowledge that could be used to develop strategies to eliminate the pathogen prior to human exposure.
3.1 Aeromonas
Aeromonas species have had a devastating effect on the aquaculture industry with reports of major losses in fish populations worldwide resulting in great financial loss (Monette et al., 2006). Disease states in fish from Aeromonas hydrophila infections range from hemorrhagic septicemia, red sore disease and ulcerative infections in multiple species of fish (Janda and Abbott, 2010). Although the Aeromonads are mostly considered associated with aquatic environments i.e., found in fresh water, seawater, drinking water and groundwater, they are also ubiquitous in the environment, and are associated with a number of recreational habitats, animals and consumable products that can lead to human exposure and potential infection (Janda and Abbott, 2010). There are a plethora of clinical syndromes, both intestinal and extraintestinal, that Aeromonas can cause in humans ranging from relatively mild gastroenteritis to infections with high mortality rates such as septicemia, necrotizing fasciitis, and myonecrosis ((Janda and Abbott, 2010) and the references within). Serious wound infections can occur from simple abrasions if the affected area is exposed to contaminated water sources, such as streams, lakes and recreational waters. Therefore, aquatic habitats can serve as an environmental reservoir for human Aeromonas infection, which is now considered an emerging pathogen for humans (Igbinosa et al., 2012).
A. hydrophila is also a natural pathogen of zebrafish, making zebrafish an excellent model for the study of pathogenesis. A recent outbreak of hemorrhagic disease in laboratory zebrafish was isolated and identified to be A. hydrophila (Rodriguez et al., 2008). Naïve fish challenged with this strain by either IP injection or a wound-immersion route quickly developed the same symptoms as fish in the original outbreak. Importantly, injection with just the extracellular secreted products gave similar mortality, indicating that the major virulence factor(s) is secreted. Live bacteria and their extracellular secreted products were hemolytic against fish blood and cytotoxic to cultured zebrafish kidney cells. Pro-inflammatory cytokines (TNFα, IL-1β and IFNγ) and iNOS were strongly induced by infection with live bacteria as well as with extracellular secreted products alone. These results strongly indicate that A. hydrophila causes disease by triggering massive inflammation, mediated by the extracellular secreted products (Rodriguez et al., 2008). In support of these results, when Li et al. (2011) focused on three known virulence factors of A. hydrophila, the aerolysin, the cytotoxic enterotoxin and a serine protease, it was found that these three factors act synergistically in causing disease (Li et al., 2011). Isolates encoding none or only 1 or 2 of these virulence genes had similar LD50 when tested in the zebrafish model, whereas isolates with all three virulence genes had a reduced LD50. Isolates positive for all three virulence genes were also more hemolytic, more cytotoxic towards Vero cells, and had more proteolytic activity in their supernatants, suggesting that these virulence factors cause disease by tissue destruction (Li et al., 2011). However, there were strain specific differences, indicating that other virulence factors, beyond the three tested, also play a role in disease.
Much work has been done on finding ways to combat A. hydrophila infection in aquaculture to preserve fish populations as well as circumvent transmission to humans. Antibiotic treatment is one of the major options for treating an outbreak. However, use of inappropriate antibiotic treatment in the food supply has been highly controversial. One reason is that inappropriate antibiotic use can lead to development of antibiotic resistance in pathogens through selective mutations or transfer of antibiotic resistance genes between bacteria. Cantas et al. (2012) recently utilized the zebrafish infectious disease model to examine the role of antibiotic use in treating A. hydrophila infection (Cantas et al., 2012). Oral gavage was used to inoculate adult zebrafish with A. hydrophila resulting in systemic disease. Infected zebrafish were then treated with antibiotics that were either effective for the infection or at sub-inhibitory levels leading to ineffective treatment. Expression of innate immunity genes in the gut of the zebrafish was then measured under the different conditions. In addition, expression of bacterial antibiotic resistance plasmid transfer genes was examined to determine how the antibiotic treatments affected potential transfer of antibiotic resistance. Infection alone increased expression of IL-1β and IL-8, but not TNFα or complement factor C3. Infection plus effective antibiotic treatments increased all four examined immune genes, as did ineffective treatment with trimethoprim, sulphonamide or flumequine, but ineffective doses of tetracycline reduced expression of TNFα and C3 below levels measured in untreated infected fish. In addition, ineffective antibiotic treatment can also activate conjugative transfer genes of a bacterial antibiotic resistance plasmid, while transfer genes were repressed in bacteria during effective antibiotic treatment. Taken together, using zebrafish and A. hydrophila as a model, this study demonstrated the danger in ineffective antibiotic treatment leading to activation of resistance plasmid transfer genes, and in the case of tetracycline, repression of immune genes, whereas effective antibiotic concentrations not only kill the pathogen, but block transfer of resistance plasmids and can activate innate immunity genes to help the immune system clear the infection. This supports observations that single high dose antibiotic therapy can clear infection (Cantas et al., 2012), but highlights the importance of using antibiotics appropriately.
The zebrafish model has also been used to explore alternatives to antibiotic treatments to A. hydrophila infection. Many bacteria use “quorum sensing”, a signaling system based on population density, to communicate with each other. A. hydrophila use quorum sensing to regulate virulence gene expression using the signaling molecule acyl homoserine lactone (AHL) (Bi et al., 2007; Khajanchi et al., 2009; Vilches et al., 2009). One alternative to antibiotic use would be to block the quorum sensing signaling between bacteria using a treatment with an AHL degrading enzyme (lactonase), acting as a broad spectrum inhibitor of virulence. Zebrafish exposed to A. hydrophila infection by bath immersion that were subsequently fed with food supplemented with an AHL lactonase showed significantly reduced mortality compared to fish not treated with the enzyme providing a new potential therapy for infection (Cao et al., 2012).
Vaccination methods in fish populations are also being pursued as a mechanism for combating A. hydrophila infection. This reduces the dangers from broad scale antibiotic use in ponds, but is more labor-intensive as each fish needs to be treated. The zebrafish model has provided an excellent model for testing vaccination methods for many types of aquatic pathogens, including Aeromonas. One way in which to provide immunity to a population without having to vaccinate every fish is to use a vaccine that allows immunity to be transferred from mother to offspring. Wang et al. (2009) demonstrated that vaccination of breeding females with formalin-killed A. hydrophila provided significant protection to the embryos (Wang et al., 2009). This was not mediated by maternal transfer of antibody, but rather by maternal transfer of complement components C3 and Bf. Vaccination of breeding females caused a rise in C3 and Bf levels in both the whole body homogenate of the adult fish and in the extract of eggs produced by that fish. Treatment of the fish with an anti-C3 or anti-Bf antibody reversed these effects. Embryos from vaccinated and unvaccinated fish were challenged with live, fully virulent A. hydrophila. Embryos from vaccinated fish survived significantly more than those from unvaccinated fish. Embryos from vaccinated fish were able to kill the bacteria, providing evidence that the maternal derived complement components are able to lyse the pathogen (Wang et al., 2009).
In a follow-up study, vaccination of female zebrafish with the hapten-carrier trinitrophenylated bovine serum albumin TNP-BSA raised a specific antibody response in the fish (Wang et al., 2012a). These antibodies were transferred to the offspring of vaccinated females. Embryos from females vaccinated with TNP-BSA were less susceptible to A. hydrophila than embryos from unvaccinated females. This protection was IgM specific, as an anti-IgM treatment blocked protection. Embryos of vaccinated mothers were able to kill A. hydrophila suggesting that the protection is maternal antibody mediated bacterial lysis. These were not A. hydrophila specific antibodies, indicating that these maternal antibodies were able to act non-specifically in protecting the embryo from water-borne infection. This mechanism of specific maternal antibodies acting non-specifically against bacterial pathogens, in addition to maternally derived natural antibodies, may protect embryos from water-borne infection until development of the adaptive immune system (Wang et al., 2012a).
3.2 Edwardsiella
Edwardsiella tarda and Edwarsiella ictaluri can cause septicemia in fish populations from both freshwater and marine environments. While E. ictaluri only infects fish, E. tarda is a zoonotic pathogen that can infect both fish and humans (Janda and Abbott, 1993). Transmission to humans is through exposure to contaminated aquatic environments and infected fish or through ingestion of contaminated fish (Janda and Abbott, 1993). Gastroenteritis is the most common clinical syndrome observed in humans and can range from an asymptomatic carrier state to intestinal colitis (Schlenker and Surawicz, 2009). E. tarda can also cause extraintestinal infections including soft tissue infections, meningitis, peritonitis, septicemia, bacteremia, endocarditis and urosepsis (Wang et al., 2005). Although infections in humans are more rare than in fish, E. tarda septicemia infections occurring in individuals with impaired immune systems can be severe and result in high mortality rates up to 50% (Janda and Abbott, 1993; Tamada et al., 2009; Wang et al., 2005).
Along with the Aeromonads, the Edwardsiella bacterial species cause the most severe morbidity and mortality in aquaculture (Mohanty and Sahoo, 2007). Multiple species of fish are susceptible to edwardsiellosis including carp, tilapia, eel, catfish, mullet, salmon, trout and flounder. Conditions most favorable for E. tarda infection include high temperature, poor water quality and high organic content (Mohanty and Sahoo, 2007). These conditions can often occur in aquaculture farms without proper diligence to hygienic practices. The bacteria can survive in pond water and mud for long periods of time and can increase in concentration with increasing temperature and organic load (Mohanty and Sahoo, 2007). The clinical pathology of edwarsiellosis includes small cutaneous lesions that can develop into large necrotic abscesses, petechial hemorrhaging of the fin and skin, rectal hernia and swelling of the abdomen due to fluid accumulation (Park et al., 2012; Pressley et al., 2005). The ability of E. tarda to survive outside the host provides an environmental reservoir that can lead to outbreaks of infection in the fish population, and subsequently humans, when the conditions are favorable.
Experimental infection analyses have shown zebrafish to be an effective experimental host for E. tarda infection, exhibiting similar clinical pathology to that observed in naturally infected wild and farmed fish populations (Pressley et al., 2005). While larvae were susceptible to infection by bath immersion, adult zebrafish were only susceptible to infection if the dermis was abraded prior to bath immersion, or if the fish were injected with bacteria. Mortality was observed from all infection methods in both larvae and adult fish. Gross symptoms in adult fish included discoloration and petechial hemorrhages at the site of wounding, if infected via the abrasion-bath immersion method, and distended abdomen and characteristic peri-anal edema, if infected by IP injection (Pressley et al., 2005). The blood of infected fish contained inflammatory cells with intracellular bacteria in macrophages, similar to that shown in kidneys of experimentally infected rohu fish (Mohanty and Sahoo, 2007). Infected larvae showed systemic inflammation, leading to epithelial and muscle tissue destruction, and numerous inflammatory cells in the bloodstream, with intracellular bacteria in macrophages (Pressley et al., 2005).
To mimic the environment of a fish farm or site of high bacterial load in the natural environment, van Soest et al. (van Soest et al., 2011) used bath immersion as a route of infection. Their results indicated that infection levels, and especially immune responses, following immersion in E. tarda culture are highly variable from fish to fish. However, injection into the tail vein of the larva caused a more reproducible infectious dose and a more consistent induction of immune related genes. While bath immersion resulted in a range of 25-75% mortality, tail vein injection resulted in 100% mortality. Therefore, while the zebrafish can be used to mimic natural infection routes of E. tarda that may occur in the environment, using methods that produce 100% inoculation (such as injection) allows experimental analysis of virulence pathologies and host response by bypassing the first protective barriers to infection. Pro-inflammatory responses were measured at the single embryo level and showed a strong induction of the il-1b and mmp9 genes (van Soest et al., 2011). Pressley et al. (2005) also showed a strong upregulaton of IL-1β in both E. tarda infected larvae and adult zebrafish, as well as an increase in TNFα (Pressley et al., 2005). The results of a microarray performed on RNA isolated from infected zebrafish larvae revealed an increased expression of genes involved in defense and regulation of the immune response (van Soest et al., 2011). Results from these types of analyses with zebrafish provide new information about host response to E. tarda infection allowing development of more effective treatments.
Using a responsive experimental host also allows for analysis and identification of major virulence genes in the pathogen. Very little was known about the pathogenic mechanisms of E. tarda until recently. Using the zebrafish infectious disease model, several groups have been able to identify key factors in E. tarda pathogenesis. Xiao et al. (2012) showed E. tarda encodes two catalases, enzymes that allow the bacteria to break down hydrogen peroxide. Hydrogen peroxide is often the first line of defense used by the innate immune system against invading pathogens. Comparison of zebrafish infections with an E. tarda wild type strain and a strain that had a knockout of one of the catalase genes showed partial attenuation, with an approximate 10-fold increase in LD50. However, a strain with a deletion of both catalase genes showed a 100-fold increase in LD50 (Xiao et al., 2012). Similarly, Wang et al. (2012) showed that deletion of the rpoN gene, which encodes an alternative sigma factor implicated in multiple species as a transcriptional regulator of virulence genes, had an 8-fold increase in LD50 compared to infection with the wild type strain (Wang et al., 2012b). The ΔrpoN strain also had reduced ability to survive peroxide, acid, osmotic, and starvation stresses and produced less biofilm and chondroitinase, all of which could be implicated in virulence (Wang et al., 2012b). The invasin protein of E. tarda was examined for its role in virulence during zebrafish infection. Both deletion and overexpression of the invasion gene was analyzed. While deletion showed decreased virulence, most likely through reduced hemolytic activity and biofilm formation, overexpression of the invasin protein demonstrated higher adherence and internalization levels in cultured zebrafish epithelial cells (Dong et al., 2013).
Yu et al. (2012) took a different approach by analyzing the role in virulence of an antibiotic resistance plasmid (Yu et al., 2012). Virulence genes are often encoded on these plasmids, leading to transfer of virulence genes between bacterial populations and increased survival of bacteria in environments with high antimicrobial concentrations such as fish farms. An E. tarda strain cured of an antibiotic resistance plasmid showed attenuation in mortality in zebrafish as well as goldfish and Japanese flounder. Subsequent sequencing of the plasmid identified 84 open reading frames, revealing that multiple virulence genes other than the genes for antibiotic resistance are encoded on the plasmid harbored by E. tarda (Yu et al., 2012). All of these analyses provide new information on genes required for pathogenesis, identifying new targets for antimicrobial therapies.
A major reason for the success of the zebrafish infectious disease model is that the zebrafish and human immune systems are very similar, although there are some differences, including absence of lymph nodes and additional Toll-like receptors compared to their mammalian counterparts (Phelps and Neely, 2005; Trede et al., 2004) and the references within). This means that zebrafish can also be a good model for vaccine development and analysis of immunization against infections. In an effort to find a vaccine for E. tarda infections, Yang et al. (2013) examined the zebrafish immune response using a live attenuated E. tarda vaccine strain for immunization and a fully virulent E. tarda strain for the subsequent challenge. The vaccination led to 78% of fish surviving a challenge 4 weeks post-immunization whereas only 12% of naïve fish survived challenge. Immunity was mediated through TLR5 signaling and activated a CD8+ T-cell response through cross-presentation of extracellular antigen. CD8+ responses were induced to a higher level during the challenge phase than the immunization phase. Vaccination also induced specific antibody in the fish serum, indicating that vaccination activated both cell and humoral mediated immunity (Yang et al., 2013).
3.3 Mycobacteria
Mycobacterium marinum has been analyzed extensively in zebrafish as a model for human tuberculosis infections caused by M. tuberculosis (see (Deng et al., 2011b; Krishnan et al., 2010; Lesley and Ramakrishnan, 2008; Meijer and Spaink, 2011; Pozos and Ramakrishnan, 2004; Ramakrishnan, 2013; Tobin and Ramakrishnan, 2008) for review and citations within). However, M. marinum is also a human and fish pathogen in its own right. As a human pathogen, M. marinum most often causes a granulomatous skin and soft tissue infection in fish hobbyists named “fish tank granuloma”. This normally results from superficial wounding during routine fish tank maintenance. Additionally, infections have been linked to swimming in contaminated aquatic environments (Bonamonte et al., 2013; Wu et al., 2012). These infections can become severe, requiring prolonged multi-antibiotic therapy and/or surgical interventions (Bonamonte et al., 2013; Huang et al., 2012; Wu et al., 2012). In patients being treated with immunosuppressive drugs, these infections can be more prevalent and severe (Ferreira et al., 2012). Superficial infection can rarely disseminate to bones and joints or cause a pulmonary infection (Bonamonte et al., 2013; Wu et al., 2012). Mycobacteria are also natural pathogens of marine and freshwater fish (see (Gauthier and Rhodes, 2009; Jacobs et al., 2009) for review and citations within).
Fish kept in aquaculture farms and zebrafish research colonies are susceptible to natural infection by several species of Mycobacteria, including M. abscessus, M. chelonae, M. fortuitum, M. marinum, M. haemophilum and M. peregrinum. Most species are opportunistic pathogens but M. marinum and M. haemophilum are highly pathogenic. Bacteria are acquired from other infected fish or infected biofilm. (for more extensive discussion see (Whipps et al., 2012) and citations within).
To further examine the virulence of mycobacterial species isolated from research facilities, Watral and Kent (2007) experimentally infected zebrafish, by intraperitoneal injection, with several strains of M. marinum, M. abscessus, M. peregrinum, and M. chelonae (Watral and Kent, 2007). Only fish infected with M. marinum exhibited symptoms of disease and succumbed to infection. However, histopathologic examination of infected fish found granulomatous lesions in the peritoneum, kidneys and ovaries of fish infected with M. marinum and non-marinum species, indicating that these fish can be sub-clinically infected with Mycobacteria. Live bacteria were only consistently recovered from the fish infected with M. marinum, but some individual fish were asymptomatic carriers of other the other mycobacterial species. Symptoms of M. marinum disease in experimentally infected fish were skin erythema, raised scales, swollen abdomens and rarely, skin lesions (Watral and Kent, 2007).
To study possible natural transmission routes, Peterson et al. (2013) examined inoculation of M. marinum and M. chelonae by the oro-gastric route by using the single cell eukaryotic ciliate Paramecium caudatum as a vector (Peterson et al., 2013). Larval, juvenile and adult fish exhibited more extragastric (mainly kidney and spleen) granulomas containing Mycobacteria when fed paramecia infected with M. marinum or M. chelonae than when the bacteria were incorporated into a food gel matrix or free bacteria were added to the water. Gastrointestinal bacteria were observed for all infection routes, suggesting that bacteria are more virulent after passing through a ciliate host, and are better able to cause disseminated infection (Peterson et al., 2013).
Although the ESX-1 (RD1) transport system has been extensively studied for its role in virulence in mycobacteria (Abdallah et al., 2007; Champion and Cox, 2007), including in the zebrafish (Davis and Ramakrishnan, 2009; Volkman et al., 2010), the ESX-5 Type VII secretion system has more recently found to also play a role in virulence in many mycobacterial species. To examine the effect of the M. marinum type VII protein secretion system ESX-5 in establishment of granulomas in zebrafish, Weerdenburg et al. (2012) used the zebrafish model. Their analysis demonstrated that this protein secretion system serves a complex role in virulence (Weerdenburg et al., 2012). When 100 cfu of the ESX-5 mutant was microinjected into the caudal vein of zebrafish larvae 28 hours post fertilization, establishment of initial infection was mildly attenuated compared to injection with the wild type strain. Fewer bacteria were isolated from the infected larvae and granuloma formation was delayed in fish infected with the ESX-5 mutant than with wild type bacteria. However, adult fish infected with the ESX-5 mutant succumbed more rapidly to infection and had higher bacterial loads in the spleen and liver than fish infected with wild type bacteria. Since larvae do not yet have a functional adaptive immune system, the role of the adaptive immune system was examined to see if it was responsible for the discrepancy between attenuation in larvae and hypervirulence observed in adult fish. However, infection with the ESX-5 mutant into Rag1 deficient adult fish, which are deficient in an adaptive immune response, showed that the hypervirulent phenotype was not dependent on the adaptive immune system. Histopathologically, fish infected with the ESX-5 mutant bacteria developed granulomas sooner and in higher numbers than fish infected with wild type bacteria. Additionally, pro-inflammatory cytokines (IL-1β, TNF-α and IFN-γ) were more highly expressed in ESX-5 granulomas. Co-infection of wild type and ESX-5 bacteria, to determine if the secreted factors from the wild type could complement the hypervirulence of the ESX-5 mutant, showed that the ESX-5 mutant outcompeted the wild type strain. These results suggest that the ESX-5 protein secretion system functions to modulate M. marinum virulence and potentially contribute to persistence (Weerdenburg et al., 2012).
In addition to a type VII secretion system, the SecA2 alternative protein translocation system is another putative virulence factor of M. marinum. SecA2 mutants were attenuated in a larval zebrafish model of M. marinum infection (van der Woude et al., 2013). Fish were infected by microinjection into the caudal vein 24 hours post fertilization. Five days post-infection, bacterial loads were five times higher in fish infected with wild type than secA2 mutant bacteria. Mutant bacteria showed less aggregation in larvae and leukocytes formed smaller and less compact aggregates in fish infected with mutant bacteria. Complementation of the secA2 mutation restored wild type levels of bacterial load and aggregation with leukocytes (van der Woude et al., 2013).
One of the distinctive differences in Mycobacteria compared to other bacterial pathogens is the unique lipid components of the Mycobacterial cell wall. Recently, a study examining one of the proteins required for cell wall structure in M. marinum, MptC, encoding an α(1→2) mannosyltransferase, demonstrated that MptC also plays a role in virulence (Stoop et al., 2013). By examining both zebrafish adults and larvae, Stoop, et al (Stoop et al., 2013) took advantage of the ability to study the innate immune response to Mycobacterial infection in isolation from the adaptive immune response because of the later development of the adaptive immune system in zebrafish larvae. Examination of larval infection with an mptC mutant showed smaller bacterial aggregates, and mptC infected larvae had lower bacterial loads. Complementation with either the M. marinum or M. tuberculosis mptC gene restored wild type levels of bacterial growth and aggregation in vivo, indicating the M. marinum mptC gene serves the same function as in the human pathogen M. tuberculosis. However, virulence attenuation was greatly reduced when the same strains were used in an adult zebrafish intraperitoneal infection as both strains produced granulomas, although the mptC mutant produced fewer and showed less tissue destruction than fish infected with the wild type bacteria. Therefore, the larger magnitude of attenuation in larvae versus adult zebrafish indicates that MptC serves a role in virulence, but mainly in defense from innate immune mechanisms (Stoop et al., 2013).
In an effort to develop a vaccine to control M. marinum infection, Cui et al. (2012) examined the effectiveness of an attenuated strain of M. marinum with reduced replication in macrophages. In addition, heat killed M. marinum and extracellular secreted products were tested. Results showed that while all three vaccination methods led to the production of antibodies, only the live attenuated strain protected zebrafish from challenge with virulent M. marinum (Cui et al., 2010).
More recently, an important study examining the utility of zebrafish as a model for vaccination against mycobacterial disease was recently reported by Oksanen et al (Oksanen et al., 2013). They initially demonstrated that zebrafish could successfully be immunized against M. marinum by the BCG vaccine currently in clinical use to prevent human mycobacterial infection. Zebrafish were also shown to be able to stably express exogenous antigens following DNA vaccination, with expression lasting up to thirty days post vaccination. By vaccinating with three immunodominant antigens of M. marinum: Ag85B, CFP-10 and ESAT-6, individually and in combination, they were able to protect adult zebrafish from both slow and rapidly progressing M. marinum infection. Combination vaccination with all three antigens was able to induce a significantly higher IFN-γ response fourteen days post low dose M. marinum infection in vaccinated fish versus unvaccinated or fish vaccinated with a control plasmid. Following combination vaccination and a low dose challenge with M. marinum, vaccinated fish had fewer granulomas, and fewer affected organs. While combination vaccination and a high dose challenge of M. marinum, produced lower mortality and lower bacterial load in vaccinated fish. Therefore, this study demonstrates the effectiveness of pre-clinical vaccine research using the zebrafish infectious disease model (Oksanen et al., 2013).
3.4 Streptococcus
Streptococcosis in fish populations was first confirmed in Japan in the late 1950s (Hoshina et al., 1958). Since that time, multiple species of streptococci have been found to infect fish populations, but the major cause of economic losses from streptococcosis in aquaculture and wild fish populations are Streptococcus iniae and Streptococcus agalactiae. Both of these pathogens can also cause disease in humans and the aquatic habitat can act as a reservoir for human infection. Here we will discuss reports of the zebrafish infectious disease model to experimentally determine pathogenic mechanisms in both human and aquatic isolates of streptococci.
3.4.1 Streptococcus iniae
The first report of Streptococcus iniae infection was in 1976 in a captive Amazon freshwater river dolphin in which subcutaneous lesions called “golf ball disease” were described (Pier and Madin, 1976). However, in the last 35 years, S. iniae has emerged as a major finfish pathogen with a host range of over 27 species of fish from both freshwater and marine environments (Agnew and Barnes, 2007). While wild fish populations are just as susceptible to S. iniae infections, the most devastating effects of this pathogen have been observed in aquaculture with an estimated economic loss of over 100 million US dollars worldwide (Shoemaker et al., 2001). Although the type of disease presentation varies with fish species, most often a systemic infection occurs with accompanying meningitis and panophthalmitis (Bromage and Owens, 2002). In almost all cases, infection results in high morbidity and mortality.
S. iniae is also a zoonotic pathogen with the first reports of human infection occurring in 1995 after the handling of fresh fish from aquaculture (Weinstein et al., 1996a; Weinstein et al., 1996b). Infection in humans is usually a result of handling of contaminated fish and sustaining an injury, allowing access of the pathogen to the underlying dermis layer, most often of the hand. The resulting infection manifests as bacteremic cellulitis with the rare complications of endocarditis, meningitis, arthritis, sepsis and toxic shock in immunocompromised individuals (Hansen and Strassburger, 2000; Koh et al., 2009; Lau et al., 2003; Sun et al., 2007; Weinstein et al., 1996a; Weinstein et al., 1996b). Although there have only been about 30 reports of S. iniae infections in humans, it has been suggested that infections are most likely underreported because clinical identification relies on biochemical testing and S. iniae is currently not listed in commercial or clinical databases (Lau et al., 2003; Weinstein et al., 1997).
The first publication using zebrafish as an infectious disease model for streptococcosis demonstrated that the histopathology as well as the clinical symptoms of S. iniae disease mimicked that which was observed in farmed fish populations (Neely et al., 2002). Zebrafish succumbed to a systemic infection within 4 days post intramuscular injection with an LD50 of 5 × 103 cfu. S. iniae was isolated from the skin, heart, gall bladder and brain (Neely et al., 2002). Subsequently, a large-scale screen investigating virulence factors in S. iniae highlighted the advantage of using the zebrafish infectious disease model (Miller and Neely, 2005). A signature-tagged mutagenesis screen was performed to identify mutants that had not survived in the heart or the brain of the zebrafish at 24 hours post-infection. The screen identified 41 mutants that were attenuated in the zebrafish host, 50% of which had homology to virulence genes from other streptococcal pathogens. These results confirmed the effectiveness of the zebrafish disease model for identifying virulence genes required for infection in the host. Importantly, 10 of the transposon insertions were found to be in the capsule operon of S. iniae, confirming the importance of capsule for systemic infections (Miller and Neely, 2005). By comparing the sequence of the capsule operon of the 9117 virulent S. iniae strain to a commensal strain of S. iniae isolated from healthy fish, Lowe et al. (2007) were able to show that the commensal strain had a large deletion in the capsule operon encompassing several important capsule synthesis genes (Lowe et al., 2007). In addition, the ability of various capsule mutants to survive in the presence of the wild type strain (competitive indices) or the ability to disseminate and survive in different tissue environments was examined using the adult zebrafish, providing new information on regions of the capsule operon required for virulence (Lowe et al., 2007).
To better determine the role of the innate immune system in protection from S. iniae infection, a zebrafish larvae model was employed to visualize host-pathogen interactions in real time. An S. iniae wild type strain and a ΔcpsA capsule mutant strain were stained with the CellTracker red CMPTX dye prior to injection. Three zebrafish larvae strains were used as hosts: the Tg(mpeg1:dendra2) (Harvie et al., 2013) zebrafish line in which the macrophages are expressing EGFP; the Tg(mpx-dendra2) (Yoo and Huttenlocher, 2011) zebrafish line in which the neutrophils are expressing EGFP; and the Tg(mpx:mCherry-2A-rac2-d57n) zebrafish line in which neutrophils are expressing the inhibitory Rac2(D57N) mutation, making them unable to respond to inflammatory stimuli, including otic infection (Deng et al., 2011a). Injections were into the otic vesicle so as to observe recruitment of macrophages and neutrophils to the site of infection, as the otic cavity is usually free of leukocytes (Levraud et al., 2008). Injections of wild type S. iniae into larvae were lethal within 24 hours, while over 90% of larvae injected with ΔcpsA S. iniae survived. Both neutrophils and macrophages were visibly recruited to the site of infection and were observed to phagocytose bacteria. While depletion of both macrophages and neutrophils using Pu.1 morpholino injection led to an increased survival of the larvae, using a strain with impaired neutrophil function, but normal macrophage function, revealed that neutrophils are essential for control of S. iniae wild type infection (Harvie et al., 2013).
The cpsY gene, which is directly upstream of the capsule operon of S. iniae, was originally thought to be involved with capsule expression because of its homology to the LysR transcriptional regulator family (Koskiniemi et al., 1998). A cpsY deletion strain was also highly attenuated in the zebrafish infectious disease model and showed an inability to disseminate to the brain, a key factor in S. iniae pathogenesis in fish (Lowe et al., 2007). However, it was determined that CpsY was not regulating capsule in S. iniae (Lowe et al., 2007) or S. agalactiae (Shelver et al., 2003) and was instead involved in methionine biosynthesis and uptake (Bryan et al., 2008). To elucidate the role of CpsY in pathogenesis, Allen and Neely (2011) combined in vitro whole blood and human neutrophil assays along with in vivo zebrafish infections to determine that CpsY is required for survival within neutrophils (Allen and Neely, 2011). Subsequent research confirmed that in addition to methionine biosynthesis and uptake, CpsY stimulates cell wall stabilization through peptidoglycan O-acetylation and repression of autolysins (Allen and Neely, 2012). Therefore, as was shown by the larval infection model detailed above (Harvie et al., 2013), neutrophils play a key role in controlling infection of S. iniae; however, the pathogen has evolved specific mechanisms that allow survival in that environment.
In an effort to learn more about key virulence genes required for pathogenesis of S. iniae, Locke et al. (2008) performed pyrosequencing of an S. iniae strain isolated from the brain of a diseased hybrid striped bass (K288) (Locke et al., 2008). Their analysis revealed an M-like protein (called SiM), which had high homology to the well-studied surface-anchored M protein from Streptococcus pyogenes that is required for virulence (Fischetti, 1989; Phillips et al., 1981). In addition, they identified a gene that encodes a protein homologous to the C5a peptidases found in both S. pyogenes and S. agalactiae that cleaves the chemoattractant C5a of the complement system (Cleary et al., 1992). In vivo analyses in both zebrafish and hybrid striped bass of S. iniae mutants in the scpL and simA genes showed that the SiM protein plays a role in virulence, while the C5a peptidase did not. They further tested the SiM deletion strain as a vaccine candidate in the hybrid striped bass model and found it to be highly protective from a lethal dose of the wild type strain 90 days after vaccination (Locke et al., 2008).
López-Muñoz utilized the zebrafish-S. iniae disease model to determine the role of zebrafish IFNs during infections (López-Muñoz et al., 2009). Their results demonstrated that while both the group I and group II zfIFNs could protect against viral infection, only the group I zfIFN was able to protect zebrafish from S. iniae infection through the induction of proinflammatory genes. Interestingly, they also found that unlike with mammalian IFN-γ, zfIFN-γ was unable to produce the proinflammatory response to infections (López-Muñoz et al., 2009).
3.4.2 Streptococcus agalactiae
Streptococcus agalactiae, better known as Group B Streptococcus, (GBS) is a Gram-positive pathogen responsible for human and agricultural disease. Of all the streptococcal species, S. agalactiae appears to have the broadest host range for pathogenesis, having been isolated from both cold-blooded aquatic organisms and warm-blooded terrestrial animals. In humans, GBS is the leading cause of early onset neonatal sepsis and meningitis in the developed world (Edmond et al., 2012). GBS can also cause infections in non-pregnant adults and is considered as an emerging pathogen in immunocompromised and older individuals (Skoff et al., 2009). Outside of human infections, GBS is a major contributor to disease in agriculture, particularly bovine mastitis and fish sepsis.
Massive outbreaks of streptococcosis caused by GBS have been observed in both farmed and wild fish populations worldwide (Evans et al., 2002). There are also many reports examining the serotypes of strains isolated from different hosts to determine if certain serotypes are species specific (Evans et al., 2008; Pereira et al., 2010). There are only 9 serotypes of GBS and those that have been found to cause the most invasive disease in humans have also been isolated from aquatic mammals and fish (Delannoy et al., 2013). One study identified eating colonized fish as a risk factor for human colonization by GBS (Foxman et al., 2007).
Patterson et al. (2012) used the streptococcal-zebrafish model (Neely et al., 2002) to investigate disease characteristics of GBS (Patterson et al., 2012). Zebrafish infected with high doses of GBS by either the intramuscular or intraperitoneal route succumb to infection, whereas fish infected with a low dose can clear the infection. Bacteria are found within the bloodstream immediately after IP injection and dissemination to the brain was observed within 2 hours post injection. Inflammation within the brain was observed grossly as cerebral edema with higher expression of inflammatory cytokines IL-1β and IL-6 within the brain as compared to non-infected zebrafish (Patterson et al., 2012). This study accurately modeled the symptoms and response to meningitis observed in human GBS patients. Mutations in virulence genes of GBS led to attenuation in the zebrafish model, validating that factors important for human disease are also important for fish disease. Deletion of the transcriptional activator of capsule production cpsA leads to increased survival of fish infected by the IM route (Hanson et al., 2012) and cpsA mutants are attenuated in the ability to disseminate to the spleen, heart and brain (Fig 1, unpublished data from HMR). GBS strains carrying mutations in the hemolysin, cylE, a capsule transglycosylase, cpsD, or a two-component system responsible for virulence regulation, covRS, were attenuated in their ability to cause meningitis in the zebrafish. In contrast, the COH1 strain, which is hypervirulent in humans, caused increased concentration of GBS in the zebrafish blood and brain, resulting in increased lethality in zebrafish (Patterson et al., 2012).
Figure 1. Dissemination of S. agalactiae during zebrafish infection.
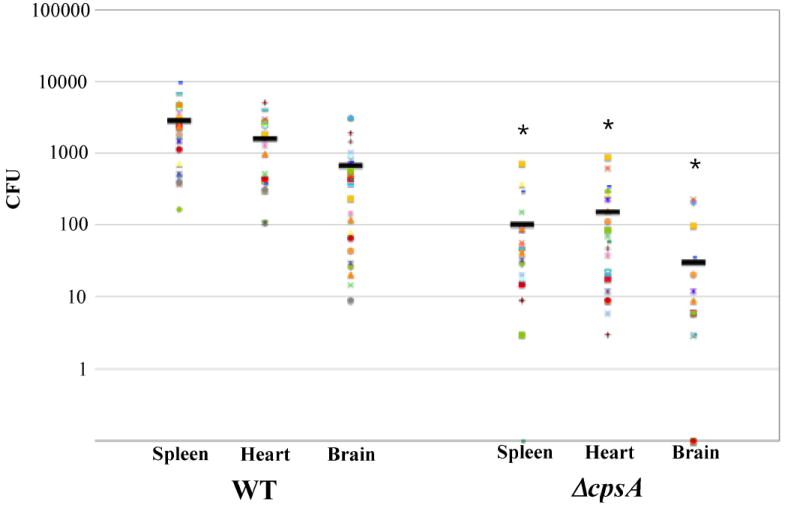
Adult zebrafish were injected intramuscularly with 106 CFU of S. agalactiae strain 515 or its isogenic ΔcpsA derivative. Four hours post injection, fish were euthanized and spleens, hearts and brains were isolated. Organs were homogenized and CFUs enumerated by viable plate count. Each dot represents 1 fish. (* p<0.01).
3.5 Vibrios
Of the human pathogens that can be found in seawater environments, Vibrios are the most predominant species (Auerbach et al., 1987). Infection in humans is most commonly acquired by wound contamination from exposure to contaminated water or aquatic organisms, the ingestion of contaminated water or consumption of raw or undercooked seafood (Finkelstein and Oren, 2011). The major clinical syndromes associated with Vibrio infection from aquatic environments are gastroenteritis, soft tissue infection and primary bacteremia (Austin, 2010; Blake et al., 1980). Soft tissue infections from wounds usually occur from recreational water activities such as swimming, surfing or boating, followed by fishing injuries and handling of seafood (Dechet et al., 2008). Although food borne infection is the most commonly recognized clinical syndrome from Vibrio aquatic exposure, soft tissue infections can result in high morbidity and mortality, leading the Centers of Disease Control and Prevention to recently add non-cholera vibriosis as a national notifiable disease (Dechet et al., 2008).
For non-food borne Vibrio infections, Vibrio vulnificus is the most common aquatic-associated infections in humans, followed by Vibrio alginolyticus and Vibrio parahemolyticus (Austin, 2010; Dechet et al., 2008). All three of these organisms have been modeled in the zebrafish (He et al., 2011; Pan et al., 2011; Paranjpye et al., 2013; Wang et al., 2008).
3.5.1. Vibrio alginlyticus
Vibrio alginolyticus causes septicemia in fish, as well as gastrointestinal, wound and mucosal infections in humans. To determine the role in pathogenesis in vivo of the twin-arginine translocation system (Tat) used by V. alginolyticus, He et al. (2011) utilized the zebrafish infectious disease model (He et al., 2011). The Tat system is used by the bacterium for translocating folded proteins and secretion of specific virulence factors as well as having roles in growth and motility. Construction and analysis of a strain deleted for the genes in the twin-arginine translocation system impacted biofilm formation and swarming motility and decreased secretion of extracellular proteases. Loss of these functions resulted in a reduction in virulence in the zebrafish compared to infection with the wild type strain (He et al., 2011). Similarly, Wang et al. (2008) used the zebrafish model to analyze mutants in iron uptake systems by demonstrating that the TonB1 and TonB2 systems are also required for systemic virulence of V. alginolyticus. Deletion of one locus resulted in an approximately one-log increase in LD50 and a double deletion resulted in over a 25-fold increase in LD50 (Wang et al., 2008).
3.5.2 Vibrio vulnificus
Vibrio vulnificus causes the greatest majority of soft tissue infections in humans from marine microorganisms (Finkelstein and Oren, 2011). However, the most lethal infection caused by V. vulnificus is primary septicemia, with a greater than 50% mortality rate (Blake et al., 1980).
V. vulnificus infection of zebrafish is lethal and shows a specific increase of innate immunity genes following V. vulnificus infection (Pan et al., 2011). Treatment of zebrafish with the antimicrobial peptide epinecidin-1 from Grouper (Epinephelus coioides) is able to protect zebrafish from V. vulnificus infection. Co-injection of epinecidin-1 conferred the best protection, but pre-treatment (one hour prior to injection of bacteria) or post-treatment (one hour after bacterial injection) also conferred protection. Fish surviving infection with epinecidin co-treatment were challenged again with bacteria 30 days post infection and had reduced mortality compared to naïve fish, indicating that epinecidin co-treatment acts similarly to a live-attenuated vaccine. Microarray analysis for inflammatory genes indicated that infection, epinecidin and co-treatment led to distinct transcriptional changes. Epinecidin acts as both an antibacterial and immunomodulatory molecule to confer protection to V. vulnificus infection (Pan et al., 2011).
3.5.3 Vibrio cholerae
Another Vibrio species that is responsible for a great deal of morbidity and mortality worldwide from consumption of contaminated seafood or water is Vibrio cholerae. Disease from V. cholerae manifests mainly as gastrointestinal symptoms, causing the disease of cholera, which leads to massive watery diarrhea resulting in dehydration and loss of electrolytes. Cholera afflicts an estimated 5 million people annually worldwide, causing over 100,000 deaths. Without rehydration therapy, cholera is lethal in 50-60% of patients; however quick treatment results in less than 1% mortality (Sack et al., 2004).
Like other Vibrio species, V. cholerae can be found associated with aquatic organisms (shellfish, insect egg masses, and plankton) as well as free swimming in water (Halpern et al., 2004; Hood et al., 1981; Rawlings et al., 2007; Senderovich et al., 2010). Much research has been done on the human pathogenesis of V. cholerae, and recently there has been increased interest on the environmental lifestyle and aquatic reservoirs of the organism, with hope of determining key factors required for transmission to humans. Current animal models for V. cholerae include the infant mouse model (Klose, 2000), the rabbit intestinal ileal loop model and the RITARD model (Formal et al., 1961; Spira and Sack, 1982; Spira et al., 1981). Unfortunately, neither the mouse nor the rabbit are natural hosts for V. cholerae and therefore, do not mimic human disease. Senderovich recently identified non-O1 V. cholerae in the intestinal tracts of multiple species of wild-caught fish (Senderovich et al., 2010), suggesting that V. cholerae may use vertebrate fish as an environmental reservoir, allowing for bacterial replication and transmission to water sources and other aquatic organisms.
Not much is known about the environmental lifestyle of V. cholerae or the key factors required for the pathogen to survive for long periods in an aquatic environment. Since previous mammalian models were not successful in reproducing human cholera symptoms, recent work investigated the efficacy of using the zebrafish host model as a model for cholera disease, and/or model as an environmental reservoir for V. cholerae (Runft et al., 2014). Runft et al. (2014) found that the zebrafish gut could be highly colonized over long periods of time by pandemic O1 serogroup V. cholerae strains through simple addition of the bacteria to the water. Furthermore, no prior manipulation of the natural intestinal flora is needed as is required with the other V. cholerae animal models, which suggests that colonization of the intestine in natural fish populations could easily occur with V. cholerae. Histology of infected zebrafish intestines illustrated that V. cholerae was closely attached to the intestinal epithelium and formed many micro-colonies in this environment (Runft et al., 2014). In an effort to mimic uptake of bacteria from both the water and the natural environment, providing a delivery vehicle of a contaminated food source was explored. Zebrafish rapidly eat freshly hatched brine shrimp as a natural food source. V. cholerae express chitin binding proteins and were found to bind readily to brine shrimp after a 15 minute incubation (Fig 2A & 2B). Addition of contaminated brine shrimp to an experimental tank of zebrafish resulted in an initial significant (p < 0.05) increase in colonization by 2 logs at 2 hours post inoculation compared to water inoculated fish (Fig 2C). However, by 24 hours post infection, levels of V. cholerae in the intestine were about the same as the water-inoculated fish (Runft et al., 2014).
Figure 2. Vibrio cholerae infection of zebrafish using brine shrimp as a vehicle.
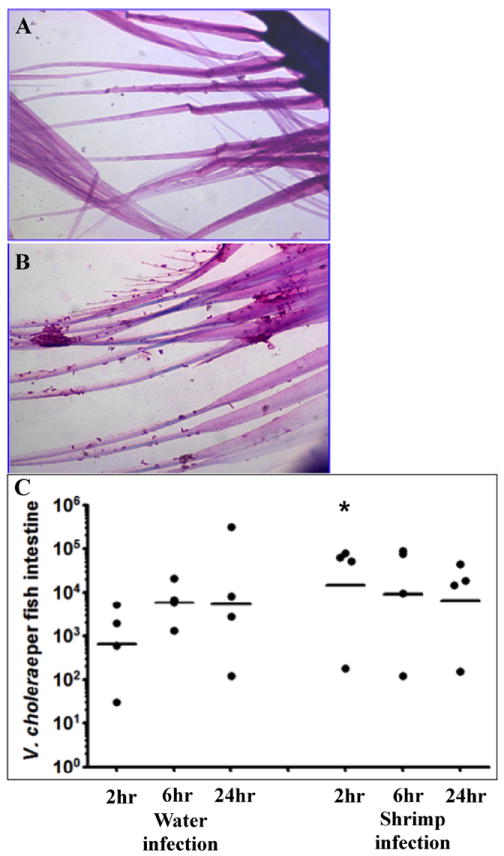
Brine shrimp cultures are produced using brine shrimp eggs (Artemia cysts) and a brine shrimp hatchery (Aquatic Eco-systems). Freshly hatched brine shrimp (< 24 hours post hatch) were removed from the hatchery and washed 3X in tap water to remove excess salt. One hundred microliters of washed brine shrimp were added to 1 ml of 108 cfu of V. cholerae and mixed gently on a rotator for 15 minutes at room temperature. A). Hematoxylin and eosin (H & E) stained brine shrimp without V. cholerae. B). H & E stained brine shrimp after incubation with V. cholerae. C). Time course of colonization for fish infected after water exposure or using brine shrimp as a vehicle. Each dot represents the data from one fish and the horizontal bar indicates the mean bacterial load per fish. Total colonization per intestine was calculated after plating serial dilutions of intestinal homogenate 24 hr post infection. * p < 0.05.
One of the most interesting findings from this study was the ability of infected fish to pass the infection to naïve fish. Zebrafish were placed in water contaminated with V. cholerae for 3 hours, followed by multiple washing steps. An exposed fish was then placed into an experimental tank with fresh water and multiple naïve fish. Importantly, previous work showed that V. cholerae cannot be isolated from the outer surface of the fish (Runft, D. L. nonpublished results). After 24 hours, the naïve fish population, exposed to the contaminated fish, was colonized with V. cholerae. This type of disease transmission cannot be analyzed using the mammalian host models. Moreover, this occurrence is very likely to happen in the natural environment and may be one way in which V. cholerae maintains its presence in an aquatic habitat, by transfer from host to host (Runft et al., 2014).
This new model for V. cholerae provides a more natural route of infection of any previous animal model. Furthermore, it goes beyond most animal models as it provides a method for exploring the environmental lifestyle of the organism and transmission characteristics of the pathogen from infected to non-infected host. Further study needs to be done to determine how colonization of the fish gut may prime the organism for infection of humans or allows the organism to become physiologically competent for transmission and subsequent infection of humans.
4. Summary
The zebrafish infectious disease model has emerged as a highly useful tool for analyzing pathogenesis caused by a number of organisms. More recently, analyses of aquatic pathogens in the zebrafish model have provided new information on how fish can act as environmental reservoirs for both human and aquatic infections. The zebrafish presents an ideal model in which to study these interactions because of its aquatic lifestyle as well as the ability accurately mimic disease characteristics observed during human infections. The ability of the aquatic pathogens to persist in the environment is a determining factor in the subsequent transmission to humans. Identifying the mechanisms required for successful pathogen colonization in the aquatic organisms most certainly will lead to future strategies to eliminate the pathogens from the aquatic environment in an effort to limit subsequent transmission to humans. In this way, instead of treating infections after they occur in the human host, we may be able to stop initial transmission. Here we focused on those pathogens that are found in the aquatic environment and serve as environmental reservoirs for infection of fish species and, importantly, as a mode of transmission to humans.
Highlights.
Review of analyses of aquatic bacterial pathogens in the zebrafish model
Fish can serve as environmental reservoirs for human disease
Zebrafish accurately mimic the clinical pathologies caused by aquatic bacterial pathogens
Acknowledgments
We are grateful to D. L. Runft for supplying data prior to publication and unpublished data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hannah M. Rowe, Email: hrowe@med.wayne.edu.
Jeffrey H. Withey, Email: jwithey@med.wayne.edu.
References
- Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- Agnew W, Barnes AC. Streptococcus iniae: an aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Veterinary microbiology. 2007;122:1–15. doi: 10.1016/j.vetmic.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Allen JP, Neely MN. Trolling for the ideal model host: zebrafish take the bait. Future Microbiol. 2010;5:563–569. doi: 10.2217/fmb.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Neely MN. The Streptococcus iniae transcriptional regulator CpsY is required for protection from neutrophil-mediated killing and proper growth in vitro. Infect Immun. 2011;79:4638–4648. doi: 10.1128/IAI.05567-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Neely MN. CpsY influences Streptococcus iniae cell wall adaptations important for neutrophil intracellular survival. Infect Immun. 2012;80:1707–1715. doi: 10.1128/IAI.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach PS, Yajko DM, Nassos PS, Kizer KW, McCosker JE, Geehr EC, Hadley WK. Bacteriology of the marine environment: implications for clinical therapy. Annals of emergency medicine. 1987;16:643–649. doi: 10.1016/s0196-0644(87)80061-6. [DOI] [PubMed] [Google Scholar]
- Austin B. Vibrios as causal agents of zoonoses. Veterinary microbiology. 2010;140:310–317. doi: 10.1016/j.vetmic.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Bi ZX, Liu YJ, Lu CP. Contribution of AhyR to virulence of Aeromonas hydrophila J-1. Research in veterinary science. 2007;83:150–156. doi: 10.1016/j.rvsc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Blake PA, Weaver RE, Hollis DG. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Bonamonte D, De Vito D, Vestita M, Delvecchio S, Ranieri LD, Santantonio M, Angelini G. Aquarium-borne Mycobacterium marinum skin infection. Report of 15 cases and review of the literature. European journal of dermatology : EJD. 2013;23:510–516. doi: 10.1684/ejd.2013.2103. [DOI] [PubMed] [Google Scholar]
- Bromage ES, Owens L. Infection of barramundi Lates calcarifer with Streptococcus iniae: effects of different routes of exposure. Dis Aquat Org. 2002;52:199–205. doi: 10.3354/dao052199. [DOI] [PubMed] [Google Scholar]
- Bryan JD, Liles R, Cvek U, Trutschl M, Shelver D. Global transcriptional profiling reveals Streptococcus agalactiae genes controlled by the MtaR transcription factor. BMC Genomics. 2008;9:607. doi: 10.1186/1471-2164-9-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantas L, Midtlyng PJ, Sorum H. Impact of antibiotic treatments on the expression of the R plasmid tra genes and on the host innate immune activity during pRAS1 bearing Aeromonas hydrophila infection in zebrafish (Danio rerio) BMC Microbiol. 2012;12:37. doi: 10.1186/1471-2180-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, He S, Zhou Z, Zhang M, Mao W, Zhang H, Yao B. Orally administered thermostable N-acyl homoserine lactonase from Bacillus sp. strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl Environ Microbiol. 2012;78:1899–1908. doi: 10.1128/AEM.06139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion PA, Cox JS. Protein secretion systems in Mycobacteria. Cell Microbiol. 2007;9:1376–1384. doi: 10.1111/j.1462-5822.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Hwang PP. Development of zebrafish epidermis. Birth defects research Part C, Embryo today : reviews. 2011;93:205–214. doi: 10.1002/bdrc.20215. [DOI] [PubMed] [Google Scholar]
- Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell host & microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary PP, Prahbu U, Dale JB, Wexler DE, Handley J. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect Immun. 1992;60:5219–5223. doi: 10.1128/iai.60.12.5219-5223.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchiaro JL, Rawls JF. Microgavage of zebrafish larvae. Journal of visualized experiments : JoVE. 2013:e4434. doi: 10.3791/4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collymore C, Rasmussen S, Tolwani RJ. Gavaging adult zebrafish. Journal of visualized experiments : JoVE. 2013 doi: 10.3791/50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma CL, Swaim LE, Volkman H, Ramakrishnan L, Davis JM. Zebrafish and frog models of Mycobacterium marinum infection. Curr Protoc Microbiol. 2006;Chapter 10(Unit 10B.12) doi: 10.1002/0471729256.mc10b02s3. [DOI] [PubMed] [Google Scholar]
- Cui Z, Samuel-Shaker D, Watral V, Kent ML. Attenuated Mycobacterium marinum protects zebrafish against mycobacteriosis. J Fish Dis. 2010;33:371–375. doi: 10.1111/j.1365-2761.2009.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechet AM, Yu PA, Koram N, Painter J. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997-2006. Clin Infect Dis. 2008;46:970–976. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- Delannoy CM, Crumlish M, Fontaine MC, Pollock J, Foster G, Dagleish MP, Turnbull JF, Zadoks RN. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013;13:41. doi: 10.1186/1471-2180-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Developmental cell. 2011a;21:735–745. doi: 10.1016/j.devcel.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Tang X, Hou M, Li C, Xie J. New insights into the pathogenesis of tuberculosis revealed by Mycobacterium marinum: the zebrafish model from the systems biology perspective. Critical reviews in eukaryotic gene expression. 2011b;21:337–345. doi: 10.1615/critreveukargeneexpr.v21.i4.40. [DOI] [PubMed] [Google Scholar]
- Dong X, Fan X, Wang B, Shi X, Zhang XH. Invasin of Edwardsiella tarda is essential for its haemolytic activity, biofilm formation and virulence towards fish. J Appl Microbiol. 2013;115:12–19. doi: 10.1111/jam.12198. [DOI] [PubMed] [Google Scholar]
- Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379:547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Bohnsack JF, Klesius PH, Whiting AA, Garcia JC, Shoemaker CA, Takahashi S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: a dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. J Med Microbiol. 2008;57:1369–1376. doi: 10.1099/jmm.0.47815-0. [DOI] [PubMed] [Google Scholar]
- Evans JJ, Klesius PH, Gilbert PM, Shoemaker CA, Al Sarawi MA, Landsberg J, Duremdez R, Al Marzouk A, Al Zenki S. Characterization of beta-haemolytic Group B Streptococcus agalactiae in cultured seabream, Sparus auratus L., and wild mullet, Liza klunzingeri (Day), in Kuwait. Journal of FIsh Diseases. 2002;25:505–513. [Google Scholar]
- Ferreira J, Grochowsky J, Krakower D, Zuromskis P, Baden R, Cheifetz AS. Mycobacterium marinum: an increasingly common opportunistic infection in patients on infliximab. The American journal of gastroenterology. 2012;107:1268–1269. doi: 10.1038/ajg.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R, Oren I. Soft tissue infections caused by marine bacterial pathogens: epidemiology, diagnosis, and management. Current infectious disease reports. 2011;13:470–477. doi: 10.1007/s11908-011-0199-3. [DOI] [PubMed] [Google Scholar]
- Fischetti VA. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal SB, Kundel D, Schneider H, Kunevn, Sprinz H. Studies with Vibrio cholerae in the ligated loop of the rabbit intestine. British journal of experimental pathology. 1961;42:504–510. [PMC free article] [PubMed] [Google Scholar]
- Foxman B, Gillespie BW, Manning SD, Marrs CF. Risk factors for group B streptococcal colonization: potential for different transmission systems by capsular type. Annals of epidemiology. 2007;17:854–862. doi: 10.1016/j.annepidem.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- Gauthier DT, Rhodes MW. Mycobacteriosis in fishes: a review. Veterinary journal (London, England : 1997) 2009;180:33–47. doi: 10.1016/j.tvjl.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Halpern M, Broza YB, Mittler S, Arakawa E, Broza M. Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microbial ecology. 2004;47:341–349. doi: 10.1007/s00248-003-2007-6. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Strassburger P. Description of an ectothermic TCR coreceptor, CD8 alpha, in rainbow trout. J Immunol. 2000;164:3132–3139. doi: 10.4049/jimmunol.164.6.3132. [DOI] [PubMed] [Google Scholar]
- Hanson BR, Runft DL, Streeter C, Kumar A, Carion TW, Neely MN. Functional analysis of the CpsA protein of Streptococcus agalactiae. J Bacteriol. 2012;194:1668–1678. doi: 10.1128/JB.06373-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriff MJ, Bermudez LE, Kent ML. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. J Fish Dis. 2007;30:587–600. doi: 10.1111/j.1365-2761.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Harvie EA, Green JM, Neely MN, Huttenlocher A. Innate Immune Response to Streptococcus iniae Infection in Zebrafish Larvae. Infect Immun. 2013;81:110–121. doi: 10.1128/IAI.00642-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Wang Q, Sheng L, Liu Q, Zhang Y. Functional characterization of Vibrio alginolyticus twin-arginine translocation system: its roles in biofilm formation, extracellular protease activity, and virulence towards fish. Current microbiology. 2011;62:1193–1199. doi: 10.1007/s00284-010-9844-6. [DOI] [PubMed] [Google Scholar]
- Hood MA, Ness GE, Rodrick GE. Isolation of Vibrio cholerae serotype O1 from the eastern oyster, Crassostrea virginica. Appl Environ Microbiol. 1981;41:559–560. doi: 10.1128/aem.41.2.559-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina T, Sano T, Morimoto Y. A streptococcus pathogenic to fish. J Tokyo Univ Fish. 1958;44:57–68. [Google Scholar]
- Huang Y, Xu X, Liu Y, Wu K, Zhang W, Liu P, Zeng X, Sun J, Jiang Y, Wang H. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clinical interventions in aging. 2012;7:533–538. doi: 10.2147/CIA.S36371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. TheScientificWorldJournal. 2012;2012:625023. doi: 10.1100/2012/625023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Stine CB, Baya AM, Kent ML. A review of mycobacteriosis in marine fish. J Fish Dis. 2009;32:119–130. doi: 10.1111/j.1365-2761.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Janda JM, Abbott SL. Infections associated with the genus Edwardsiella: the role of Edwardsiella tarda in human disease. Clin Infect Dis. 1993;17:742–748. doi: 10.1093/clinids/17.4.742. [DOI] [PubMed] [Google Scholar]
- Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanther M, Rawls JF. Host-microbe interactions in the developing zebrafish. Curr Opin Immunol. 2010;22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajanchi BK, Sha J, Kozlova EV, Erova TE, Suarez G, Sierra JC, Popov VL, Horneman AJ, Chopra AK. N-acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology. 2009;155:3518–3531. doi: 10.1099/mic.0.031575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose KE. The suckling mouse model of cholera. Trends Microbiol. 2000;8:189–191. doi: 10.1016/s0966-842x(00)01721-2. [DOI] [PubMed] [Google Scholar]
- Koh TH, Sng LH, Yuen SM, Thomas CK, Tan PL, Tan SH, Wong NS. Streptococcal cellulitis following preparation of fresh raw seafood. Zoonoses and public health. 2009;56:206–208. doi: 10.1111/j.1863-2378.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- Koskiniemi S, Sellin M, Norgren M. Identification of two genes, cpsX and cpsY, with putative regulatory function on capsule expression in group B streptococci. FEMS Immunol Med Microbiol. 1998;21:159–168. doi: 10.1111/j.1574-695X.1998.tb01162.x. [DOI] [PubMed] [Google Scholar]
- Krishnan N, Robertson BD, Thwaites G. The mechanisms and consequences of the extra-pulmonary dissemination of Mycobacterium tuberculosis. Tuberculosis (Edinburgh, Scotland) 2010;90:361–366. doi: 10.1016/j.tube.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Lau SK, Woo PC, Tse H, Leung KW, Wong SS, Yuen KY. Invasive Streptococcus iniae infections outside North America. J Clin Microbiol. 2003;41:1004–1009. doi: 10.1128/JCM.41.3.1004-1009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley R, Ramakrishnan L. Insights into early mycobacterial pathogenesis from the zebrafish. Curr Opin Microbiol. 2008;11:277–283. doi: 10.1016/j.mib.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levraud JP, Colucci-Guyon E, Redd MJ, Lutfalla G, Herbomel P. In vivo analysis of zebrafish innate immunity. Methods in molecular biology (Clifton, N J) 2008;415:337–363. doi: 10.1007/978-1-59745-570-1_20. [DOI] [PubMed] [Google Scholar]
- Li J, Ni XD, Liu YJ, Lu CP. Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J Appl Microbiol. 2011;110:823–830. doi: 10.1111/j.1365-2672.2011.04944.x. [DOI] [PubMed] [Google Scholar]
- Li YJ, Hu B. Establishment of multi-site infection model in zebrafish larvae for studying Staphylococcus aureus infectious disease. Journal of genetics and genomics = Yi chuan xue bao. 2012;39:521–534. doi: 10.1016/j.jgg.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Locke JB, Aziz RK, Vicknair MR, Nizet V, Buchanan JT. Streptococcus iniae M-like protein contributes to virulence in fish and is a target for live attenuated vaccine development. PLoS ONE. 2008;3:e2824. doi: 10.1371/journal.pone.0002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Muñoz A, Roca FJ, Meseguer J, Mulero V. New insights into the evolution of IFNs: zebrafish group II IFNs induce a rapid and transient expression of IFN-dependent genes and display powerful antiviral activities. J Immunol. 2009;182:3440–3449. doi: 10.4049/jimmunol.0802528. [DOI] [PubMed] [Google Scholar]
- Lowe BA, Miller JD, Neely MN. Analysis of the polysaccharide capsule of the systemic pathogen Streptococcus iniae and its implications in virulence. Infect Immun. 2007;75:1255–1264. doi: 10.1128/IAI.01484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker ND, Trede NS. Immunology and zebrafish: spawning new models of human disease. Dev Comp Immunol. 2008;32:745–757. doi: 10.1016/j.dci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Meijer AH, Spaink HP. Host-pathogen interactions made transparent with the zebrafish model. Curr Drug Targets. 2011;12:1000–1017. doi: 10.2174/138945011795677809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Neely MN. Large-scale screen highlights the importance of capsule for virulence in the zoonotic pathogen Streptococcus iniae. Infect Immun. 2005;73:921–934. doi: 10.1128/IAI.73.2.921-934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BR, Sahoo PK. Edwardsiellosis in fish: a brief review. Journal of biosciences. 2007;32:1331–1344. doi: 10.1007/s12038-007-0143-8. [DOI] [PubMed] [Google Scholar]
- Monette S, Dallaire AD, Mingelbier M, Groman D, Uhland C, Richard JP, Paillard G, Johannson LM, Chivers DP, Ferguson HW, Leighton FA, Simko E. Massive mortality of common carp (Cyprinus carpio carpio) in the St. Lawrence River in 2001: diagnostic investigation and experimental induction of lymphocytic encephalitis. Veterinary pathology. 2006;43:302–310. doi: 10.1354/vp.43-3-302. [DOI] [PubMed] [Google Scholar]
- Neely M, Pfeifer J, Caparon MG. Streptococcus-zebrafish model of bacterial pathogenesis. Infect Immun. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Oksanen KE, Halfpenny NJ, Sherwood E, Harjula SK, Hammaren MM, Ahava MJ, Pajula ET, Lahtinen MJ, Parikka M, Ramet M. An adult zebrafish model for preclinical tuberculosis vaccine development. Vaccine. 2013;31:5202–5209. doi: 10.1016/j.vaccine.2013.08.093. [DOI] [PubMed] [Google Scholar]
- Pan CY, Wu JL, Hui CF, Lin CH, Chen JY. Insights into the antibacterial and immunomodulatory functions of the antimicrobial peptide, epinecidin-1, against Vibrio vulnificus infection in zebrafish. Fish Shellfish Immunol. 2011;31:1019–1025. doi: 10.1016/j.fsi.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Paranjpye RN, Myers MS, Yount EC, Thompson JL. Zebrafish as a model for Vibrio parahaemolyticus virulence. Microbiology. 2013;159:2605–2615. doi: 10.1099/mic.0.067637-0. [DOI] [PubMed] [Google Scholar]
- Park SB, Aoki T, Jung TS. Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish. Veterinary research. 2012;43:67. doi: 10.1186/1297-9716-43-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H, Saralahti A, Parikka M, Dramsi S, Trieu-Cuot P, Poyart C, Rounioja S, Ramet M. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev Comp Immunol. 2012;38:447–455. doi: 10.1016/j.dci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Pereira UP, Mian GF, Oliveira IC, Benchetrit LC, Costa GM, Figueiredo HC. Genotyping of Streptococcus agalactiae strains isolated from fish, human and cattle and their virulence potential in Nile tilapia. Veterinary microbiology. 2010;140:186–192. doi: 10.1016/j.vetmic.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Peterson TS, Ferguson JA, Watral VG, Mutoji KN, Ennis DG, Kent ML. Paramecium caudatum enhances transmission and infectivity of Mycobacterium marinum and M. chelonae in zebrafish Danio rerio. Diseases of aquatic organisms. 2013;106:229–239. doi: 10.3354/dao02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan PE, Mellon MT, Kim CH. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio) Molecular immunology. 2005;42:1057–1071. doi: 10.1016/j.molimm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Phelps HA, Neely MN. Evolution of the zebrafish model: from development to immunity and infectious disease. Zebrafish. 2005;2:87–103. doi: 10.1089/zeb.2005.2.87. [DOI] [PubMed] [Google Scholar]
- Phelps HA, Neely MN. Evolution of the Zebrafish Model: From Development to Immunity and Infectious Disease. Zebrafish. 2005;2:87–103. doi: 10.1089/zeb.2005.2.87. [DOI] [PubMed] [Google Scholar]
- Phillips GN, Jr, Flicker PF, Cohen C, Manjula BN, Fischetti VA. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981;78:4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G, Madin S. Streptococcous iniae sp.nov., a beta-hemolytic streptococcus isolated from an Amazon fresh-water dolphin, Inia geoffrensis. Int J Syst Bacteriol. 1976;26:545–553. [Google Scholar]
- Pozos TC, Ramakrishnan L. New models for the study of Mycobacterium-host interactions. Curr Opin Immunol. 2004;16:499–505. doi: 10.1016/j.coi.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Pressley ME, Phelan PE, 3rd, Witten PE, Mellon MT, Kim CH. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev Comp Immunol. 2005;29:501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L. Looking within the zebrafish to understand the tuberculous granuloma. Advances in experimental medicine and biology. 2013;783:251–266. doi: 10.1007/978-1-4614-6111-1_13. [DOI] [PubMed] [Google Scholar]
- Rawlings TK, Ruiz GM, Colwell RR. Association of Vibrio cholerae O1 El Tor and O139 Bengal with the Copepods Acartia tonsa and Eurytemora affinis. Appl Environ Microbiol. 2007;73:7926–7933. doi: 10.1128/AEM.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez I, Novoa B, Figueras A. Immune response of zebrafish (Danio rerio) against a newly isolated bacterial pathogen Aeromonas hydrophila. Fish Shellfish Immunol. 2008;25:239–249. doi: 10.1016/j.fsi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol. 2014;80:1710–1717. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- Schlenker C, Surawicz CM. Emerging infections of the gastrointestinal tract. Best practice & research Clinical gastroenterology. 2009;23:89–99. doi: 10.1016/j.bpg.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Senderovich Y, Izhaki I, Halpern M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One. 2010;5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelver D, Rajagopal L, Harris TO, Rubens CE. MtaR, a regulator of methionine transport, is critical for survival of group B streptococcus in vivo. J Bacteriol. 2003;185:6592–6599. doi: 10.1128/JB.185.22.6592-6599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker CA, Klesius PH, Evans JJ. Prevalence of Streptococcus iniae in tilapia, hybrid striped bass, and channel catfish on commercial fish farms in the United States. American journal of veterinary research. 2001;62:174–177. doi: 10.2460/ajvr.2001.62.174. [DOI] [PubMed] [Google Scholar]
- Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T, Schrag SJ. Increasing Burden of Invasive Group B Streptococcal Disease in Nonpregnant Adults, 1990–2007. Clinical Infectious Diseases. 2009;49:85. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- Spira WM, Sack RB. Kinetics of early cholera infection in the removable intestinal tie-adult rabbit diarrhea model. Infect Immun. 1982;35:952–957. doi: 10.1128/iai.35.3.952-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira WM, Sack RB, Froehlich JL. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981;32:739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop EJ, Mishra AK, Driessen NN, van Stempvoort G, Bouchier P, Verboom T, van Leeuwen LM, Sparrius M, Raadsen SA, van Zon M, van der Wel NN, Besra GS, Geurtsen J, Bitter W, Appelmelk BJ, van der Sar AM. Mannan core branching of lipo(arabino)mannan is required for mycobacterial virulence in the context of innate immunity. Cell Microbiol. 2013;15:2093–2108. doi: 10.1111/cmi.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C, Kim CH. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immunol. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Sun JR, Yan JC, Yeh CY, Lee SY, Lu JJ. Invasive infection with Streptococcus iniae in Taiwan. J Med Microbiol. 2007;56:1246–1249. doi: 10.1099/jmm.0.47180-0. [DOI] [PubMed] [Google Scholar]
- Szabady RL, Lokuta MA, Walters KB, Huttenlocher A, Welch RA. Modulation of neutrophil function by a secreted mucinase of Escherichia coli O157:H7. PLoS Pathog. 2009;5:e1000320. doi: 10.1371/journal.ppat.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada T, Koganemaru H, Matsumoto K, Hitomi S. Urosepsis caused by Edwardsiella tarda. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2009;15:191–194. doi: 10.1007/s10156-009-0678-8. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Ramakrishnan L. Comparative pathogenesis of Mycobacterium marinu and Mycobacterium tuberculosis. Cell Microbiol. 2008;10:1027–1039. doi: 10.1111/j.1462-5822.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- van der Sar AM, Appelmelk BJ, Vandenbroucke-Grauls CM, Bitter W. A star with stripes: zebrafish as an infection model. Trends Microbiol. 2004;12:451–457. doi: 10.1016/j.tim.2004.08.001. [DOI] [PubMed] [Google Scholar]
- van der Woude AD, Stoop EJ, Stiess M, Wang S, Ummels R, van Stempvoort G, Piersma SR, Cascioferro A, Jimenez CR, Houben EN, Luirink J, Pieters J, van der Sar AM, Bitter W. Analysis of SecA2-dependent substrates in Mycobacterium marinum identifies protein kinase G (PknG) as a virulence effector. Cell Microbiol. 2013 doi: 10.1111/cmi.12221. [DOI] [PubMed] [Google Scholar]
- van Soest JJ, Stockhammer OW, Ordas A, Bloemberg GV, Spaink HP, Meijer AH. Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda. BMC immunology. 2011;12:58. doi: 10.1186/1471-2172-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli L, Pruzzo C, Huq A, Colwell RR. Environmental reservoirs of Vibrio cholerae and their role in cholera. Environmental microbiology reports. 2010;2:27–33. doi: 10.1111/j.1758-2229.2009.00128.x. [DOI] [PubMed] [Google Scholar]
- Vilches S, Jimenez N, Tomas JM, Merino S. Aeromonas hydrophila AH-3 type III secretion system expression and regulatory network. Appl Environ Microbiol. 2009;75:6382–6392. doi: 10.1128/AEM.00222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ji D, Shao J, Zhang S. Maternal transfer and protective role of antibodies in zebrafish Danio rerio. Molecular immunology. 2012a;51:332–336. doi: 10.1016/j.molimm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Wang IK, Kuo HL, Chen YM, Lin CL, Chang HY, Chuang FR, Lee MH. Extraintestinal manifestations of Edwardsiella tarda infection. International journal of clinical practice. 2005;59:917–921. doi: 10.1111/j.1742-1241.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Wang K, Liu E, Song S, Wang X, Zhu Y, Ye J, Zhang H. Characterization of Edwardsiella tarda rpoN: roles in sigma(70) family regulation, growth, stress adaption and virulence toward fish. Archives of microbiology. 2012b;194:493–504. doi: 10.1007/s00203-011-0786-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu Q, Cao X, Yang M, Zhang Y. Characterization of two TonB systems in marine fish pathogen Vibrio alginolyticus: their roles in iron utilization and virulence. Archives of microbiology. 2008;190:595–603. doi: 10.1007/s00203-008-0407-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang S, Tong Z, Li L, Wang G. Maternal transfer and protective role of the alternative complement components in zebrafish Danio rerio. PLoS One. 2009;4:e4498. doi: 10.1371/journal.pone.0004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watral V, Kent ML. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2007;145:55–60. doi: 10.1016/j.cbpc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Weerdenburg EM, Abdallah AM, Mitra S, de Punder K, van der Wel NN, Bird S, Appelmelk BJ, Bitter W, van der Sar AM. ESX-5-deficient Mycobacterium marinum is hypervirulent in adult zebrafish. Cell Microbiol. 2012;14:728–739. doi: 10.1111/j.1462-5822.2012.01755.x. [DOI] [PubMed] [Google Scholar]
- Weinstein MR, Litt M, Kertesz DA, Wyper P, Rose D, Coulter M, McGeer A, Facklam R, Ostach C, Willey B, Borczyk A, Low DE. Invasive infections due to a fish pathogen, Streptococcus iniae. New Engl J Med. 1997;337:589–594. doi: 10.1056/NEJM199708283370902. [DOI] [PubMed] [Google Scholar]
- Weinstein MR, Low DE, McGeer A, Willey B, Rose D, Coulter M, Wyper P, Borczyk A, Lovgren M. Invasive infection due to Streptococcus iniae: a new or previously unrecognized disease. CCDR. 1996a;22:129–132. [PubMed] [Google Scholar]
- Weinstein MR, Low DE, McGeer A, Willey B, Rose D, Coulter M, Wyper P, Borczyk A, Lovgren M. Invasive infection with Streptococcus iniae-Ontario, 1995-1996. MMWR Weekly. 1996b [PubMed] [Google Scholar]
- Whipps CM, Lieggi C, Wagner R. Mycobacteriosis in zebrafish colonies. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2012;53:95–105. doi: 10.1093/ilar.53.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TS, Chiu CH, Yang CH, Leu HS, Huang CT, Chen YC, Wu TL, Chang PY, Su LH, Kuo AJ, Chia JH, Lu CC, Lai HC. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296. doi: 10.1371/journal.pone.0041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Chen T, Wang Q, Zhang Y. Comparative analysis of the roles of catalases KatB and KatG in the physiological fitness and pathogenesis of fish pathogen Edwardsiella tarda. Letters in applied microbiology. 2012;54:425–432. doi: 10.1111/j.1472-765X.2012.03225.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Liu Q, Ni C, Li S, Wu H, Wang Q, Xiao J, Zhang Y. Gene expression profiling in live attenuated Edwardsiella tarda vaccine immunized and challenged zebrafish: insights into the basic mechanisms of protection seen in immunized fish. Dev Comp Immunol. 2013;40:132–141. doi: 10.1016/j.dci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Huttenlocher A. Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. Journal of leukocyte biology. 2011;89:661–667. doi: 10.1189/jlb.1010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JE, Cho MY, Kim JW, Kang HY. Large antibiotic-resistance plasmid of Edwardsiella tarda contributes to virulence in fish. Microb Pathog. 2012;52:259–266. doi: 10.1016/j.micpath.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Zlotkin A, Hershko H, Eldar A. Possible transmission of Streptococcus iniae from wild fish to cultured marine fish. Appl Environ Microbiol. 1998;64:4065–4067. doi: 10.1128/aem.64.10.4065-4067.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


