Abstract
Cinnamyl alcohol dehydrogenase (CAD) is a key enzyme in lignin biosynthesis. However, little was known about CADs in melon. Five CAD-like genes were identified in the genome of melons, namely CmCAD1 to CmCAD5. The signal peptides analysis and CAD proteins prediction showed no typical signal peptides were found in all CmCADs and CmCAD proteins may locate in the cytoplasm. Multiple alignments implied that some motifs may be responsible for the high specificity of these CAD proteins, and may be one of the key residues in the catalytic mechanism. The phylogenetic tree revealed seven groups of CAD and melon CAD genes fell into four main groups. CmCAD1 and CmCAD2 belonged to the bona fide CAD group, in which these CAD genes, as representative from angiosperms, were involved in lignin synthesis. Other CmCADs were distributed in group II, V and VII, respectively. Semi-quantitative PCR and real time qPCR revealed differential expression of CmCADs, and CmCAD5 was expressed in different vegetative tissues except mature leaves, with the highest expression in flower, while CmCAD2 and CmCAD5 were strongly expressed in flesh during development. Promoter analysis revealed several motifs of CAD genes involved in the gene expression modulated by various hormones. Treatment of abscisic acid (ABA) elevated the expression of CmCADs in flesh, whereas the transcript levels of CmCAD1 and CmCAD5 were induced by auxin (IAA); Ethylene induced the expression of CmCADs, while 1-MCP repressed the effect, apart from CmCAD4. Taken together, these data suggested that CmCAD4 may be a pseudogene and that all other CmCADs may be involved in the lignin biosynthesis induced by both abiotic and biotic stresses and in tissue-specific developmental lignification through a CAD genes family network, and CmCAD2 may be the main CAD enzymes for lignification of melon flesh and CmCAD5 may also function in flower development.
Introduction
The lignification of tissues is thought to play a critical role in specialized conducting and supporting tissues of plants, facilitating water transport, providing mechanical strength, and defense against biotic and abiotic stresses [1]–[6]. Lignification is a complex process, which involves many intermediates and enzymes [7]. Cinnamyl alcohol dehydrogenase (CAD) catalyses the final step of the lignin biosynthesis, the conversion of cinnamyl aldehydes to alcohols, using NADPH as a cofactor [8]. Early reports on the identification of CAD enzymes have showed the CAD in gymnosperm is encoded by a single gene [9], [10]. However, CAD is encoded by a multigene family in angiosperm species. Complete sets of CAD genes and CAD-like genes have been identified in the genomes of model species and non-model plants [11]–[25].
Previous studies highlighted that CAD genes in angiosperm species were distributed in different classes according to phylogenetic tree [3], [23]–[27], which were part of a family of related proteins. Recent phylogenetic analysis suggested that the emergence of real lignin in the vascular plant lineage was associated with the origin of the bona fide CAD genes [28]. The primary genes involved in lignin biosynthesis,such as AtCAD4 and AtCAD5(Arabidopsis) [29], EgCAD2 (eucalyptus) [30] and OsCAD2 (rice) [23] have been characterized, which have a dominant role in normal tissue lignification. Furthermore, Other CADs could serve in other functions in different plant tissues. These function could range from a role in redundant (non-xylem) tissue lignification (example as a response to stress) [6], [20]–[22], [31], [32] and in biochemical processes unrelated to lignification [33]. Interestingly, there are differences in function between members of CAD genes family in angiosperm species; However, comprehensive analyses of lignified tissues of various Arabidopsis knockout mutants (for AtCAD5, 6, and 9) at different stages of growth/development indicated the presence of functionally redundant CAD metabolic networks [14], [15], [23], [29,]. The biochemical roles of CAD genes still need to be elucidated, particularly being differences in the lignin composition of tissues/plant species result from the different activities of several CAD isoenzymes, each with a different specificity. However, even though CADs have been investigated from a number of plant species, there are currently no reports on CAD in melons.
In this study we identified five CAD-like genes from the melons genome, and compared CAD sequences from a wide variety of plants, making full use of the available plant genome sequences (Arabidopsis, Oryza, Sorghum, Wheat, Populus, Medicago, et al.) as well as expressed sequence databases for species of basal angiosperms and gymnosperms. Alignment and phylogenetic analysis of the CAD gene family with related CAD proteins from other species indicated that five CmCADs were classified into four groups separately. We analyzed the structure and the promoter of melon CAD genes, and also investigated this CAD genes transcript in response to various fruit development stages and monitored tissue-specific expression. The effect of various plant hormones on CmCADs was also examined. We reported here the results of these analyses, suggesting that the CmCADs may be involved in melon fruit lignification induced by various hormones and during development and ripening and in tissue-specific developmental lignification.
Materials and Methods
Materials and treatments
All the experiments were carried out with ‘CaiHong7’, oriental sweet melons (Cucumis melo var. makuwa Makino). They were grown in pots (volume of 25 L, soil: peat: compost = 1∶1∶1) in a greenhouse under standard cultural practices for fertilization and pesticide treatments at Shenyang Agricultural University, Shenyang, China, from March to June in 2012. Freshly opened female flowers were sprayed with growth regulator ‘Fengchanji 2’ (a hormone complex, which mainly contains 4-chlorophenoxyacetic acid to increase the rate of fruit set; Shenyang Agricultural University) and tagged on the day of bloom to identify fruit of known age. Plants were trained as single stem, and two or three fruits per vine. Physiological maturity of this sweet melon is about thirty-six days after anthesis. Melons were harvested after 1, 5, 10, 15, 20, 25, 30, 33, 36, 39, 42, 45 and 48 days after anthesis. Mature leaf, developing leaf, pistillate flower, staminate flower, young stems and root tissues were collected from plants grown in a greenhouse for expression analysis.
Mature unripe oriental sweet melons were harvested at 30 days after anthesis (pre-climacteric stage) with the same node of the plant at a mature green stage, before the onset of ripening, and ripening was initiated by exposing the fruits to exogenous ethylene (100 µL/L) for 24 h in a closed 33 L chamber and then allowed to ripen for 12 days at 23°C in air only. Fruits that were allowed to undergo post-harvest ripening in air for 12 days without any exogenous ethylene treatment were treated as control. For 1-methylcyclopropene (1-MCP) (an ethylene perception inhibitor) treatment, mature unripe fruits were exposed to 100 µL/L 1-MCP for 12 h followed immediately by 100 µL/L ethylene treatment for 24 h and then kept at 23°C in air as in earlier cases. Three replicates were prepared for each treatment, and each replicate consisted of 15 fruit unless indicated otherwise. Fresh tissue was sampled every 48 h, frozen in liquid nitrogen and stored at −80°C until further use. For abscisic acid (ABA) and auxin (IAA) treatment, the fruit discs were dipped in a solution containing 100 µM ABA or IAA in 0.2% teepol (detergent) and vacuum infiltrated for 2 h. Infiltrated with 0.2% teepol were used as control fruits. All plant materials were frozen in liquid nitrogen and stored at −80°C. Three replications were carried out for abscisic acid (ABA) and auxin (IAA) treatment or control groups.
Identification of melon CAD genes
The keyword ‘cinnamyl alcohol dehydrogenase’ was used to search for the melon cinnamyl alcohol dehydrogenase sequences from the melon (Cucumis melon L.) genome (http://melonomics.net) [34]. In additional, to further confirm the accuracy of these genes, the predicted CAD-like gene sequences were compared to CAD proteins in other species by a BLASTp retrieve. Only those sequences with high score (>200) were selected.
Sequence analysis
The CAD protein prediction (amino acid number, calculated molecular weight and isoelectric points) was performed through SIB Bioinformatics Resource Portal (http://web.expasy.org/translate/, http://web.expasy.org/computepi/ and http://expasy.org/). The presence of functional domains were checked via NCBI's Conserved Domain Database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), PSIpred and PFP-FunDSeqE prediction server (www.expasy.ch/tools/#proteome; http://www.csbio.sjtu.edu.cn/bioinf/PFP-Fun DSeqE) (which allowed to identify a multi-domain structure in our CAD sequence). Multiple alignments were carried out with other known plant CAD proteins using Clustal W2 program and GENEDOC. Phylogenetic analyses of putative melon CAD proteins were carried out using MEGA5 (http://megasoftware.net) program based on the neighbor-joining method (minimum evolution criterion, bootstrap values performed on 1000 replicates). Promoter analysis was carried out by PLANT CARE program. The sequences were analysed for signal peptides using the SignalP4.1 server (http://www.cbs.dtu.dk/services/SignalP/) [24] and TargetP 1.1 Server. CmCAD subcellular localization prediction was performed by WoLF PSORT (http://wolfpsort.org/), CELLO v.2.5 (http://cello.life.nctu.edu.tw/), PSLpre (http://www.imtech.res.in/raghava/pslpred/index.html) and Loctree3 (https://rostlab.org/services/loctree3/). The disulfide bond was predicted in Scratch Protein Predictor server (http://scratch.proteomics.ics.uci.edu/).
RNA isolation and cDNA synthesis
Total RNA from fruit samples, leaves, stem, root, and flower material, were extracted using ultrapure RNA Kit following the manufacturer's recommendations (Kangwei Biotech, Beijing, China). RNA was suspended in RNase-free water (30 µL), treated with DNAse I (Promega, Madison, WI, USA) at 37°C for 50 min, re-precipitated and concentrated (40 µL). The RNA was measured by the NanoDrop Spectrophotometer ND-1000 and quality was checked by electrophoresis (28S rRNA/18S rRNA ratios). The cDNA was synthesized from total RNA (0.5–1 µg) by using M-MLV RTase cDNA Synthesis Kit following the manufacturer's instructions (Cat#D6130, TaKaRa, Tokyo, Japan).
Semi-quantitative PCR and real time qPCR
Transcript analysis was carried out by semi-quantitative PCR. For this, semi-quantitative PCR was carried out using cDNA prepared from 500 ng DNA free RNA from different fruit and vegetative tissues. PCR was performed using the gene-specific primers for each gene and 18SrRNA DNA fragment (148 bp) of melon as an internal control. Specific primers for each CAD gene were designed by Primer3 (http://frodo.wi.mit.edu/) and were listed in Table 1. All primers were designed to avoid detection of conserved regions. The PCR products were checked by agarose gel electrophoresis, and were also sequenced by the company (Sangon Biotech Co.Ltd., Shanghai, China) to confirm primer specificity. Twenty cycles were carried out for CmCADs.
Table 1. Semi-quantitative PCR and real time PCR primers.
| Gene | Primer | Sequence (5-3) |
| CmCAD1 | CmCAD1-F | GAGACGCAAGAAGTATTG |
| CmCAd1-R | ACTCAGGCATCTTACTAC | |
| CmCAD2 | CmCAD2-F | CTTACACTTACGAACTCAG |
| CmCAD2-R | CAACTTCCATCACTTCAC | |
| CmCAD3 | CmCAD3-F | CCACAACACATCAACCAT |
| CmCAD3-R | CATCCGCTAATCTTGCTTA | |
| CmCAD4 | CmCAD4-F | CATTGTTGTTCACGAGAG |
| CmCAD4-R | CCTATCACTCCAAGAGATT | |
| CmCAD5 | CmCAD5-F | GCTGTTAAGATTGCTAAGG |
| CmCAD5-R | GTAATCCATTGTCTCTGTTG | |
| 18srRNA | 18srRNA-F | AAACGGCTACCACATCCA |
| 18srRNA-R | CACCAGACTTGCCCTCCA |
Real time qRT-PCR was performed in a 20 µL reaction volume using SuperReal PreMix Plus (SYBR Green) (Cat.FP205, Tiangen Biotech, Beijing, China) on an ABI PRISM 7500 sequence-detection system according to manufacture's instructions. The gene-specific primers of real time qRT-PCR were the same as that of semi-quantitative PCR. Real-time qRT-PCR conditions were as follows: 50°C for 2 min, followed by 95°C for 10 min, then 45 cycles of 95°C for 15 s and 60°C for 1 min. All real-time qRT-PCR experiments were run in triplicate with different cDNAs synthesized from three biological replicates. Samples were run in triplicate on each 96-well plate. For each sample, a Ct (threshold sample) value was calculated from the amplification curves by selecting the optimal △Rn (emission of reporter dye over starting background fluorescence) in the exponential portion of the amplification plot. Relative fold differences were calculated based on the comparative Ct method using 18SrRNA DNA fragment (148 bp) of melon as an internal standard. To determine relative fold differences for each sample in each experiment, the Ct values for all CmCADs were normalized to the Ct value for 18SrRNA and was calculated using the formula 2−△△Ct. The means of CmCADs expression levels were calculated from three biological repeats, obtained from three independent experiments.
Statistical analysis
Data are expressed as mean values ± standard deviation of three independent experiments (n = 3). The data were analyzed by the analysis of variance (ANOVA) using the SPSS 13.0 statistics program, and significant differences were compared by a one-way ANOVA following Duncan's multiple range tests for each experiment at a P<0.05 level. The charts were generated by using Origin (version 8.0).
Results
Identification of melon CAD genes
All the predicted CAD genes in the melon genome were collected and compared with CAD genes in other species. In this case, the presence of functional domains was checked via NCBI's Conserved Domain Database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), and only those sequences having the same functional domains of CADs with other proved/putative species were selected as our target genes. Based on this information, 5 CAD genes, encoding full-or nearly full-length functional proteins, were identified in the melon genome. By ExPASy tools, we found that the longest protein of CADs consisted of 360 amino acid residues, and the shortest consisted of 355 amino acid residues. The ORF length ranged from 1068 to 1083 nucleotides. The predicted molecular weight and isoelectric points of all CADs proteins ranged from 38.42 kDa/5.68 to 39.62 kDa/6.63, respectively (Table 2). For unreported CAD genes in melon, we gave them a name by adding a number to their family name in the order in which they were searched.
Table 2. The information of CAD genes in melon.
| Gene | Gene accession No. | amino acid (aa) | ORF length | Melon predicted protein No. | Molecular weight (KD) | location | Isoelectric point |
| CmCAD1 | MELO3C019548 | 357 | 1074 | MELO3C019548 P1 | 39.20 | CM3.5_scaffold00038 | 5.96 |
| CmCAD2 | MELO3C018492 | 356 | 1071 | MELO3C018492 P1 | 39.40 | CM3.5_scaffold00034 | 5.68 |
| CmCAD3 | MELO3C003735 | 360 | 1083 | MELO3C003735 P1 | 39.62 | CM3.5_scaffold01596 | 6.63 |
| CmCAD4 | MELO3C005809 | 355 | 1068 | MELO3C005809 P1 | 38.42 | CM3.5_scaffold00005 | 5.99 |
| CmCAD5 | MELO3C023272 | 358 | 1077 | MELO3C023272 P1 | 38.87 | CM3.5_scaffold00059 | 6.14 |
Intron-exon structure of melon CAD genes
Structural analysis of the identified melon CAD genes revealed different intron-exon patterns both in relation to position and number of introns, which ranged from four to five per gene (Figure 1). Furthermore, substantial differences in the size between the exons were observed. Based on Figure 1, one individual intron may be missing in CmCAD1, CmCAD2 and CmCAD5, because introns located exactly at the same position have been present in the common ancestor [26]. Hence, the position and type of the introns should be strictly conserved between the different genes when present. Of the five identified introns, only CmCAD3 and 4 contain all five. Additionally, A 114-bp sequence was found in four sequences except CmCAD3 (Fig. 1). According to the model proposed by Youn et al. [35], this 114-bp sequence in CmCAD2 and CmCAD5 should encode the putative binding site-I for the monolignol substrate. The 114-bp sequence was identical to the second exon flanked by introns in CmCAD2 and CmCAD5. Relative to these, this sequence was identical to the fourth exon in CmCAD1 and CmCAD4, and it was unclear whether this sequence encoded the putative binding site.
Figure 1. Intron-exon structures of CAD genes from melon.
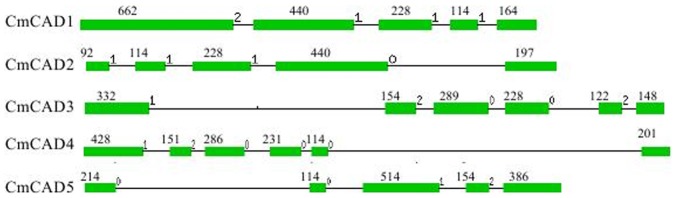
Exons and introns are indicated by open boxes and lines respectively. Numbers above boxes indicate the exon sizes. The intron sizes are not to scale. The names of CAD genes and intron-exon structure are indicated at the left and right sides respectively.
Phylogenetic analysis and characterization of the CmCAD genes family
To understand the possible functional divergence of the individual members of melon CAD genes family, phylogenetic analysis was performed using deduced amino acid sequences of melon CAD genes and CAD genes from other species. The phylogenetic tree revealed seven groups of CAD, and CmCADs were classified into four groups (Figure 2). CmCAD1 and CmCAD2 were positioned in the bona fide groupI, all of which have been characterized as bona fide CAD genes (groupI) involved in lignin biosynthesis [3]. CmCAD5 belonged to groupII; CmCAD4 clustered into groupV; CmCAD3 belonged to groupVII.
Figure 2. Phylogenetic relationship among CmCADs and CADs from other plant species.

The amino acid sequences were aligned by the Clustal W2 program, and the neighborjoining tree was drawn with TreeView. The corresponding GenBank and the melon genome (https://melonomics.net/) were noted in the phylogenetic tree and the accession number in the melon genome were CmCAD1 (MELO3C019548P1), CmCAD2 (MELO3C018492P1), CmACD3 (MELO3C003735P2), CmCAD4 (MELO3C005809P1) and CmCAD5 (MELO3C023272P1). The number for each interior branch was the percentage of bootstraps value (1000 replicates). Black circle denoted five CmCADs. CADs belong to the following plant species: Arabidopsis thaliana: AtCAD1 (AY288079), AtCAD2 (AY302077), AtCAD3 (AY302078), AtCAD4 (AY302081), AtCAD5 (AY302082), AtCAD6 (AY302075), AtCAD7 (AY302079), AtCAD8 (AY302080), and AtCAD9 (AY302076); Oryza sativa: OsCAD1 (AAN09864), OsCAD2 (DQ234272), OsCAD3 (AAP53892), OsCAD4 (BK003970), OsCAD5 (BK003971), OsCAD6 (CAD39907), OsCAD7 (CAE05206), OsCAD8A to D (BK003972), and OsCAD9 (AAN05338); Sorghum bicolor: SbCAD2 (Sb04g005950), SbCAD4-2 (Sb10g006300), SbCAD4-3 (Sb10g006290), SbCAD4-4 (Sb10g006280), SbCAD4-5 (Sb10g006270), SbCAD5 (Sb07g006090), SbCAD6 (Sb06g001430), SbCAD7 (Sb06g028240), SbCAD8-1 (Sb02g024220), SbCAD8-2 (Sb02g024210), and SbCAD8-4 (Sb02g024190); Triticum aestivum: TaCAD1 (GU563724), TaCAD2 (TC143210), TaCAD3 (TC143265), TaCAD4 (TC144004), TaCAD5 (TC149391), TaCAD6 (TC149393), TaCAD7 (TC170425), TaCAD8 (TC170426), TaCAD9 (TC170429), TaCAD10 (TC172690), and TaCAD11 (TC179401); Aralia cordata: AcCAD1 (D13991); Eucalyptus globulus: EgCAD1 (AF038561); Festuca arundinacea: FaCAD1a (AF188292); Lolium perenne: LpCAD1 (AF472591), LpCAD2 (AF472592), and LpCAD3 (AF010290); Medicago sativa: MsCAD1 (AF083333) and MsCAD2 (AF083332); Nicotiana tabacum: NtCAD1 (X62343) and NtCAD2 (X62344); Picea abies: PaCAD1 (X72675); Populus tremuloides: PtCAD1 (AF217957) and PtSAD (AF273256); Pinus taeda: PtaCAD1 (Z37992); Saccharum officinarum: SoCAD1 (AJ231135); Zea mays: ZmCAD1 (AJ005702) and ZmCAD2 (Y13733); Vitis vinifera: VvCAD (CBI34634.3), VvCAD6 (XP002269356.1), VvCAD9 (XP002279832.1). Cucumis sativus: CsCAD6 (XP004136373.1), CsCAD9 (XP004150677.1), CsCAD11 (XP004140716.1), CsCAD12 (XP004137094.1), CsCAD13 (XP004145884.1), CsCAD14 (XP004162965.1), Hordeum vulgare: HvCAD1A (BAJ84795.1), HvCAD1B (BAJ98188.1), HvCAD6 (BAK01962.1); Glycine max:GmCAD1 (XP003543132.1); Cicer arietinum: CaCAD1 (XP004485621.1); Gossypium hirsutum: GhCAD3 (ACQ59091.1); Ricinus communis: RcCAD (XP_002510582.1); Theobroma cacao: TcCAD9 (EOY15101.1); Fragaria vesca: FvCAD6 (XP004291336.1).
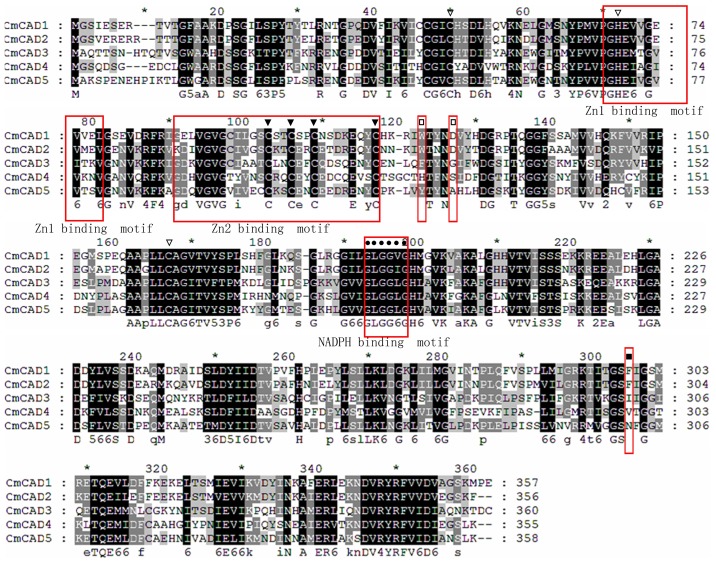
Multiple alignments revealed that the deduced amino acid sequence of CmCAD1 showed the highest homology with a CAD (GenBank no. ADO16245.1) in Ocimum tenuiflorum (99.17% identity); Other CmCADs showed the highest homology with corresponding CADs from Cucumis sativus (GenBank) (Figure S4–S7). The protein sequences of melon CAD genes were aligned against each other (Figure 3). However, only 77.07% identity was found between CmCAD1 and CmCAD2, and there were lower identity between CmCADs. All of the melon CADs had the highly conserved Zn1 catalytic center (C47, H69, and C163), the Zn-binding signature GHE(X)2G(X)5G(X)2V, the Zn2 structural motif (C100, C103, C106, and C114), and the NADPH-binding domain [GLGGV(L)G] motif (so-called Rossmann fold) (Figure 3), suggesting that these proteins appear to be zinc-dependent alcohol dehydrogenases and members of the plant CAD protein family and the medium-chain dehydrogenase/reductase (MDR) superfamily [36], [37].
Figure 3. Alignment of amino acid sequences of CmCADs.
Conserved important regions identified previously are marked as follows: white arrows denotes catalytic zinc ion coordinating residue, black arrows denotes structural Zn ion coordinating residue, the black circle denotes key residues for substrate specificity. The black square denotes key Phe299/Gly(300) residues for substrate specificity. The white square denotes key Trp199 and Asp123 residues for substrate binding. Locations of the Zn1, Zn2, and NADPH binding domains are shown in boxes. The alignment was performed with the ClustalW2 software program.
Out of all amino-acid residues in five CmCADs, 109 were invariant (30% identity). Phe299/Gly300 is a key determinant residue of substrate specificity [21], [38], and Trp119 and Asp123 are key residues in an optimal position for substrate binding [39], which were found in the sequence of CmCAD1 and CmCAD2. Additionally, previous researches also showed a few critical residues of identifying lignifying CADs, including His/Asp57, Leu58, Glu70, Cys/Ile/Val95, Ser120 and Ser212 [35], [40]. CmCAD1 and CmCAD2 had most of these critical residues, compared with other CmCADs (Figure 2). In Brachypodium, the amino-acid residues 58HL59 in the BdCAD5 sequence, believed to be part of the catalytic mechanism as a proton donor [24], were not found in CmCADs which contain either EW or DL/EL dipeptide, except for CmCAD4 which contains KL, indicating a change from E to K as a result of a G→A base transversion. This change in one of the proton-donating residues according to the model of Youn et al. [35] may lead to an inactive CmCAD4. Compared with the HL or DL motif present in monocot and eudicot CADs, increasing activity on a range of monolignals, an equivalent EW sequence motif at the H57/L58 positions of CmCAd3 and CmCAD5 may alter apparent substrate preferences [39].
No typical signal peptides were found in all CmCADs after analyzing their N-terminals using the SignalP software. SbCAD6 has an evolutionarily conserved SKL sequence at the C-terminus, which may serve as a signal peptide sequence to locate these enzymes in plant cell peroxisomes [17]. But no homology was found between the SKL sequence of CmCADs and those of other species through amino acids sequence comparison, suggesting that CmCADs may not be located in peroxisomes [17]. CmCADs subcellular localization prediction showed that these CAD genes may exist in the cytoplasm (Table S2). Moreover, the transmembrane topology predictions of five CmCADs using TMHMM 2.0 software and ABTMpro showed that there was no internal transmembrane segment in CmCADs. We also found there were 4 and 3 disulfide bonds in CmCAD1, CmCAD2, CmCAD3 and CmCAD4 proteins by Scratch protein Predictor, respectively, but there were 3 disulfide bonds in CmCAD5 protein.
Promoter sequence analysis
Analysis of promoter sequences of the melon CAD genes allowed us to identify several motifs that were known to be involved in the regulation of gene expression in various developmental and physiological processes. A promoter motif search showed that CmCAD promoter contained putative regulatory elements corresponding to known cis-elements of eukaryotic genes [19]. In melon CAD genes promoter, there were mainly two kinds of motifs, namely, cis-acting element involved in defense and stress responsiveness (such as hypoxia stress, heat stress) and cis-acting regulatory element involved in the response to various hormones, including some of these motifs involved in responses to biotic and abiotic stresses, such as auxin (IAA), ethylene, abscisic acid (ABA), salicylic acid (SA), and methyl jasmonate (MeJA) (Table S1) [19]. CmCAD3 and CmCAD4, which possess some motifs involved in stress responsiveness and in the response to various hormones, showed some differences in their sets of motifs. For instance, CmCAD3 also has motifs involved in response to wound and fungal elicitor responsive element, while CmCAD4 also possess MYB binding site involved in drought-inducibility. Other genes (CmCAD1, CmCAD2) possess elicitor responsive element and enhancer. These results indicated that transcriptional regulation of these CmCAD genes may be involved in fruit development and in the response to various stresses.
CmCADs expression in vegetative tissues
In order to investigate the transcript levels of these five CmCADs in different organs in melon plants, we collected samples of root, developing leaves, mature leaves, young stems, pistillate flower petals and staminate flower petals. Expression analysis using semi-quantitative PCR and real time qPCR showed that four of these CAD genes were expressed in these organs, but greatly varied in different tissues (Figure 4; Figure S1). Of the five CAD genes identified in melons, CmCAD5 expression was greatest in vegetative organs except mature leaves, in which CmCAD2, CmCAD3 and CmCAD5 were weakly expressed. CmCAD4 was either not expressed or expressed at very low levels in these tissues. Lignin biosynthesis genes are expected to be highly expressed in stems, where secondary cell walls are prevalent and lignification occurs, while remaining at relatively low levels in roots and especially leaves [41]. As might be expected, apart from CmCAD4, the other CmCAD were expressed at a slightly lower level in roots than in young stems, where lignin is also present, but these four CmCAD genes were highly expressed in developing leaves. Furthermore, CmCAD1, CmCAD2 and CmCAD3 showed significant expression differences between pistillate flower petals and staminate flower petals, with higher transcript levels in staminate flower petals.
Figure 4. Transcript levels of these five CmCAD in different melon organs.

The gene expressions of CmCAD in different organs in melon plants were determined by qRT-PCR in root, developing leaves, mature leaves, young stems, pistillate flower petals and staminate flower petals in melon plants. 18 s were used as internal control. The expression level of the genes in mature leaves was set as “1.0”. Data represent the means±SD (n = 3) of three biological samples. The experiments were repeated 3 times with similar results.
CmCADs expressions during melon fruit development
In the present study, five CmCAD expressions were analysed during melon fruit development. Real time qPCR and semi-quantitative PCR analysis indicated that these five CmCAD genes studied here were specifically expressed in fruit (Fig.5; Figure S1). The pattern of changes in transcript levels was similar for CmCAD1, CmCAD3 and CmCAD5 from 1 to 15days after anthesis with transient and sharp decrease at 15 days; while the expression of CmCAD2 was only expressed at 1 day. The expression of CmCAD1 and CmCAD3 showed an increase after 15 days, and gradually reduced after 30 days. In contrast, CmCAD2 and CmCAD5 consistently had higher expression in fruit after 30 days, with an increase in transcript abundance, which subsequently remained at a relatively constant level through to harvest. CmCAD4 was either not expressed or expressed at very low levels during fruit development.
Figure 5. CmCADs relative expression in developing stages of melon fruit after pollination were determined by qRT-PCR.
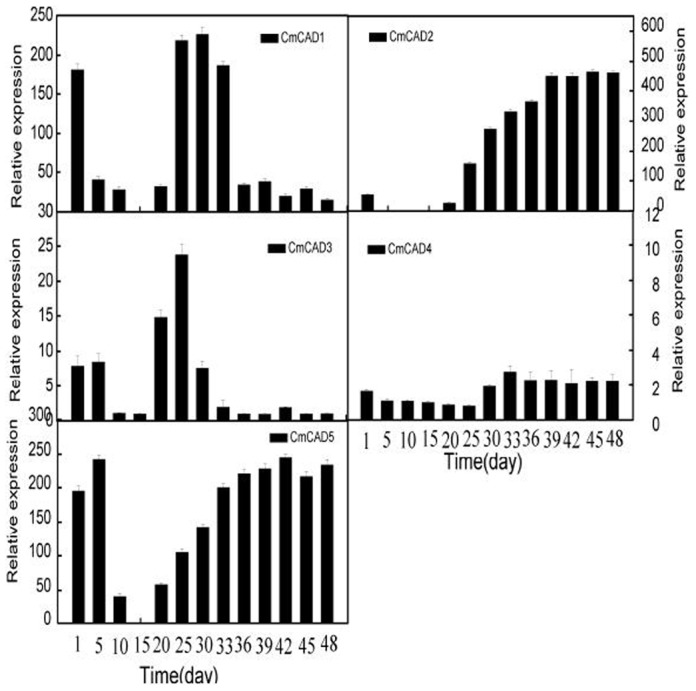
18 s were used as internal control. The expression level of CmCADs in melon fruit at 15days after pollination was set as “1.0”. Data represent the means±SD (n = 3) of three biological samples. The experiments were carried out in triplicate.
Effects of hormones on CmCAD genes expressions
Since ripening is also affected by other hormones like ABA and IAA, we studied the regulation of these genes in fruits by treatment with ABA and IAA. The real time qPCR and semi-quantitative PCR results revealed that CmCADs were induced by ABA (though expression was lower in case of ABA), except CmCAD4 (Figure 6; Figure S2). In contrast, IAA treatment strongly induced CmCAD1 and CmCAD5 expression. Other CmCADs were seemed to be insensitive to treatment with IAA and continued to be weakly expressed at a basal level.
Figure 6. The expression of CmCAD1, 2, 3, 4 and 5 in melon fruit after different hormonal treatments.
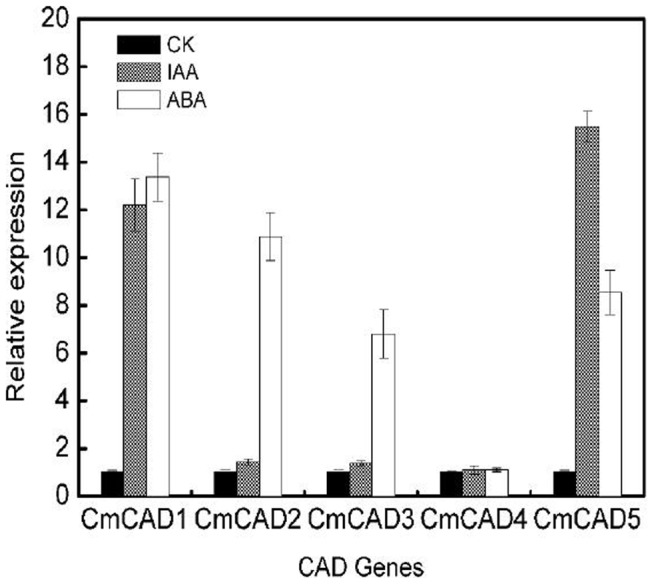
IAA and ABA (100 µM) treatments were given for 3 h as described in materials and methods section. Expression analysis was carried out by real time qPCR. For each gene, the relative abundance of mRNA was normalized against the 18S in the corresponding samples. The expression level of the genes in untreated melon fruit by IAA and ABA was set as “1.0”. Data represent the means±SD (n = 3) of three biological samples. The experiments were repeated 3 times with similar results.
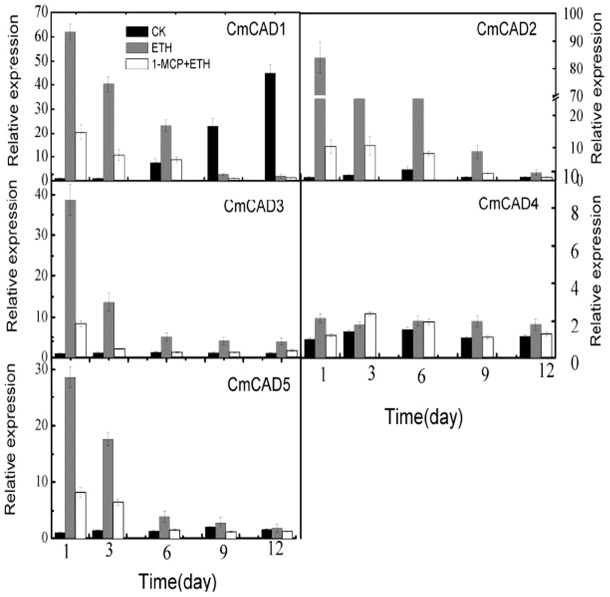
Effects of ethylene on CmCADs genes Expression
Since oriental sweet melons is a climacteric fruit which requires ethylene for initiation of ripening, we checked whether these five CAD genes were regulated by ethylene. Transcript accumulation of different CADs in melon was investigated during the course of ethylene induced ripening of harvested melon for 12 days. A basal level of expression of CmCADs transcript was detected in case of all the five CADs in ethylene untreated fruit on day 1. The transcript levels of CmCADs shot up on day 1 in ethylene treated fruit, apart from CmCAD4 (Figure 7; Figure S3). However, levels of CmCAD1, CmCAD2, and CmCAD5 transcript gradually decreased from day 1 levels in subsequent days, but levels of CmCAD3 rapidly reduced, and maintained throughout the progression of ripening thereafter. Of the five, CmCAD4 transcript showed the lowest steady state levels in all of treatments during the course of melon ripening. We checked the expression of CmCAD4 transcripts by semi-quantitative PCR at high annealing temperature using gene-specific primers to ensure that the transcript patterns were not due to cross hybridization. We found that CmCAD4 transcript levels were still lower and did not give clear results when 20 cycles of PCR were carried out as in case of other CAD genes. So for all semi-quantitative experiments related to CmCAD4, 35 cycles of PCR were carried out. Our results of semi-quantitative PCRs matched with real time qPCR analysis and confirmed that all the five CADs were expressed at different levels during melon ripening. The expressions of CmCADs were also studied in 1-MCP + ethylene treated fruits. All the five CmCADs showed transcript accumulation in 1-MCP + ethylene treated fruits, but the levels were far lower compared to the levels in ethylene treated fruits except CmCAD4, especially on day 1 post treatment (Figure 7; Figure S3). Since the patterns were same in real-time qRT-PCR and semi-quantitative PCR experiments, further studies needed to be carried out.
Figure 7. The expression levels of CmCADs after treatment with ethylene and 1-MCP.
The transcript levels of CsCADs were measured by real time qPCR in melon fruit treated, and 18S were used as internal control. The expression of the genes in untreated melon fruit after 1 day of storage was set to 1.0. Data represent the means±SD (n = 3) of three biological samples. The experiments were repeated 3 times with similar results.
Discussion
Cinnamyl alcohol dehydrogenase (CAD) is a major rate-limiting enzyme in lignin biosynthesis, and it catalyzes the conversion of coniferaldehyde, p-coumaraldehyde, and sinapaldehyde to guaiacyl, p-coumaryl, and syringyl monolignols, respectively. More importantly, the CAD gene family is related to stress responses during plant growth and is also closely associated with vegetative tissue, flower, pollen grains, fruit and seed maturation and aging [21], [42]. The CAD/CAD-like gene family has been investigated in a number of plant species such as wheat [3], tobacco [11], [13], Arabidopsis [14], [15], white spruce [16], sorghum [17], [18], populus [19], [20], sweet potato [21], brachypodium distachyon [24], tea [25], rice [26], [43], switch grass [44] and flax [45]. The aim of the present study was to identify and analyse genes coding for the CAD enzyme in melon.
Expression analysis in different tissue
In angiosperm, multiple homologous CAD genes, thought to have distinct roles, may participate in lignin biosynthesis in different tissues or during different growth and development of one type of tissue [24], [25]. In angiosperm and gymnosperm, expression analysis of CAD genes studied showed that they had different expression profiles. In Brachypodium distachyon, BdCAD5 was the only gene to be expressed in all tissues, with the highest expression in root and stem, and showed high sequence similarity to CAD genes biochemically characterized as bona fide CADs; Other BbCADs also were expressed in lignified and non-lignified tissues, but the relative expression varied across tissues [24]. CmCADs also showed different pattern in tissues and fruits during different developmental stages (Figure4 and 5; Figure S1). Except CmCAD4, the other CmCADs was highly expressed in young tissues (including developing leaves and young stems), with the same as results in ginkgo biloba [42], sweet potato [21] and tea [25]. Additionally, CmCAD5 had higher expression in flower, and we also found that CmCAD5 had an equivalent EW sequence motif, altering apparent substrate preferences, at the H57/L58 positions [39], but CmCAD5 lacked critical residues at the Trp119 and Asp123 positions, implying that CmCAD5 may participate in flower development or volatile synthesis. In Arabidopsis thaliana, AtCAD4, AtCAD5, AtCAD7 and AtCAD8 participated in lignin biosynthesis, but AtCAD4 was strongly expressed in flowers and roots; In contrast, the AtCAD5 gene was expressed in lignified roots and strongly expressed in pathogen-infected tissues [15], [19], [29]. OsCAD2 and SbCAD2 have been shown to be responsible for lignin biosynthesis, being related to hull and internode phenotypes in rice [46] and sorghum [18], respectively. These findings suggested that there seems to be the question of their functional redundancy in the CADs genes network. Therefore, we speculated that CmCADs might be involved in both lignin synthesis and pathogen defense. These results also suggested that plant CAD genes might also participate in other unknown functions or have evolved individual functions [42]. There is much to learn about CADs in dicotyledon.
Apart from lignin biosynthesis, biotic and abiotic stresses, plant CAD genes were also implicated in fruit tissue development and were closely related to tissue ageing. For example, loquat flesh tissue undergone lignification during ripening, which resulted to the increase in firmness, and CAD transcripts particularly accumulated during storage, modified by ethylene [47]. In the present study, the expression pattern of CmCADs differed greatly during fruit development, with CmCAD1, 2 and 3 weakly expressed or not expressed in early stages of development and CmCAD1 clearly expressed from 25 to 33 days after the anthesis, whereas CmCAD5 and CmCAD2 were strongly expressed during fruit development, apart from 15days after the anthesis (Figure 5; Figure S1). Hence, we speculated that both CmCAD2 and CmCAD5 could be involved in fruit development. While CmCAD2 only belonged to group I as bona fide CADs, and may be likely main candidate gene for lignin biosynthesis in melon. However, little information is available on the role of CAD in relation to the lignification of melon flesh tissue during fruit development and ripening. These findings implied that melon CAD genes might also be involved in the lignification of flesh tissue, and there were difference in function among family members.
There were significant expression differences between CmCAD4 and other CmCADs in different tissues and during development and ripening. These observed differences could partly be explained by the amino acids differences at position 58–59 (Figure 3). CmCAD4 had a KL motif, whereas other CmCADs had an either EW or DL/EL motif. Furthermore, based on our analysis of CmCADs, CmCAD4 had little key residues, and had a Ser123 instead of Asp123 which was suggested to be involved in determining the activity of all bona fide lignifying CADs; One mutation, D123S, resulted in a essentially inactive protein [39]. On the basis of the above results, it appears that CmCAD4 is a pseudogene. But we used the expressed sequence tag (EST) database in NCBI as a source of mRNA sequence bioinformatics for CmCAD4, and we found that CmCAD4 showed the highest homology with a Cucumis melo cDNA clone (GenBank no. JG465149.1) (92% identity), obtained from callus, from EST database of melon (CM-PEa library). Hence, It appears that CmCAD4 is specifically expressed in callus. Further researches are needed to confirm this speculation.
Induced expression analysis
We also studied the effect of ABA and IAA on CmCADs expression. ABA is extensively involved in the plant's response to abiotic stresses, such as drought, low temperature and osmotic stress, and also regulates a variety of growth and developmental processes, and can regulate the expression of relevant genes to increase plant adaptability [48]. ABA has been shown to induce CADs genes expression in sweet potato [21], ginkgo [42] and tea [25] after exposure to biotic or abiotic stresses. Real time qPCR and semi-quantitative PCR analysis of CmCADs in response of ABA suggested that ABA treatment increased the expressions of CmCAD1, 2, 3 and 5 (Figure 6; Figure S2). Purportedly, ABA-mediated plant responses to drought stress may be related to the regulation of relevant genes by the MYB transcription factor [44]. However, to the best of our information, there were no reports on effect of ABA or IAA on ripening specific CADs. Promoter analysis of the five melon CADs suggested the presence of ABA responsive ABRE motif [49] in the promoter of CmCAD1, 2, 3 and 5, and the promoter of CmCAD1 and CmCAD5 contains response elements (TGA) to IAA (Table S1). Therefore, the ABA-induced these CmCADs expression observed in present study may be related to the upstream MYB response elements. However this speculation has to be demonstrated by further cloning and function analyses of the promoter of CmCADs. Auxin (IAA) also plays a role in fruit development and ripening. Trainotti et al. [50] showed that there was an active crosstalk between IAA and ethylene that was important for the regulation of ripening. However, in case of melon, there were not reports about the inducing of ripening in mature fruit by IAA as done by ethylene. Of these, CmCAD1 and CmCAD5 expression were induced under IAA treatment (Figure 6; Figure S2), and other CAD genes were expressed at basal level but did not appear to be significantly regulated by IAA.
The promoter analysis of the five CAD genes revealed the presence ethylene responsive ERE motifs in CmCAD3 and CmCAD5 which have been shown to be responsive to ethylene treatment [51], [52] (Table S1). We found that the transcription level of CmCADs was obvious increase at 1 day after ethylene treatment and gradually decreased thereafter (Figure 7; Figure S3), apart from CmCAD4. However, CmCADs transcriptions were significantly suppressed by 1-MCP. Furthermore, ethylene was involved in lignification in Brassica chinensis and loquat flesh tissue by induced expression of BcCAD1-1 and BcCAD2 in loquat flesh and the expression of EjCAD1 and EjPOD genes, respectively [47], [53]; while 1-MCP down-regulated them. Induced GbCAD1 expression by ethylene may be related to enhancing PAL activity and subsequent product accumulation[42]. It is known to all that the biosynthesis of lignin in higher plants originates in the phenylalanine metabolic pathway. Therefore, the regulation of lignification and CmCADs expression of melon fruit tissue by ethylene during ripening may be related to the control in upstream of the phenylalanine metabolic pathway.
Additionally, we also identified several hormone-responsive cis-regulatory elements in the CmCADs promoter region, such as GARE, TATC-box, P-box (gibberellin), TCA-element (Salicylic acid), CCAAT-box, TGACG-motif and CGTCA-motif (MeJA)[19] (Table S1). A complex interplay of hormones is known to affect fruit development and ripening with auxin and GA being important during fruit expansion and ABA and ethylene for ripening [54]. In oriental sweet melon (Cucumis melo var. makuwa Makino), there are different ripening patterns within the fruit. The ripening of oriental sweet melon is initiated from the flesh and moves gradually towards the fruit cavity and the peel, and is earlier near the bottom and later at the carpopodium. The regulation pattern of ripening by hormones may selectively affect the expression of one or the other CADs. It also needs to be work out whether the regulation of hormones on different CAD gene members help in maintaining the net levels of CAD in fruit during ripening. These findings implied a complex hormonal regulation of the genes during fruit development and ripening and under stress conditions.
Conclusions
Taken together, we identified five CmCADs in melon, phylogenetic analysis indicated that they belonged to four different groups, and CmCAD genes may function in process of fruit tissue lignification and in lignin biosynthesis in xylem and under different stress conditions through a CAD genes network. On the transcript level, differential CmCADs expression suggested tight adaptation of the fruit to the developmental events and biotic and abiotic stresses as well as cell division. Promoter sequence analysis and subcellular localization prediction implied that CAD genes had different functions. The five isoforms respond differently to ABA and IAA, in addition to ripening related hormone ethylene, suggesting distinct metabolic roles for these genes. Further studies related to biochemical characterization of these CmCADs in order to confirm their putative function is needed.
Supporting Information
Transcript abundance of CmCAD1, 2, 3, 4 and 5 in vegetative tissues and developing stages of melon fruit (1–48). R, root; ML, mature leaf; DL, developing leaf; YS, young stem; PF, pistillate flower petal; SF, staminate flower petal. Stages and treatment were described in Section 2.5. RNA isolated from the samples. Expression analysis was carried out by semi-quantitative PCR using 18 S as an internal control.
(TIF)
mRNA abundance of CmCAD1, 2, 3, 4, and 5 in mature green fruit after treatment with different hormonal. Auxin and ABA (100 µM) treatments were given for 2 h as described in materials and methods section. Expression analysis was carried out by semi-quantitative PCR as described in Section 2.5 using actin as 18 S internal contrl.
(TIF)
CmCAD1, 2, 3, 4 and 5 accumulation during different stages of ethylene and 1-MCP treatment induced ripening of melon by semi-quantitative PCR. 1–12 indicate days after ethylene and 1-MCP treatment. The accumulation level of the genes in untreated melon fruit was control. 18 S was used as an internal control. All the treatments have been described in Section2.5.
(TIF)
Amino acid sequence alignment of melon CmCAD1 (MELO3C019548P1a) and CmCAD2 (MELO3C018492P1a) with closely related sequences of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD11 (XP_004140716.1b), CsCAD12 (XP_004137094.1b), Nicotiana tabacum NtCAD1 (X62343b), Populus tremuloides PtCAD1 (AF217957b), Arabidopsis thaliana AtCAD4 (AY302081b), AtCAD5 (AY302082b), Lolium perenne LpCAD3 (AF010290b), Festuca arundinacea FaCAD1a (AF188292b), Triticum aestivum TaCAD1 (ADI59734.1b), Oryza sativa OsCAD2 (NP_001046132.1), Zea mays ZmCAD2 (ACG45271.1b), Saccharum officinarum SoCAD1 (AJ231135b), Sorghum bicolor SbCAD2 (AEM63607.1b), Medicago sativa MsCAD2 (AF083332b), Eucalyptus globules EgCAD2 (CAA46585.1b) and Gossypium hirsutum GhCAD1 (ABZ01817.1b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: ( ) the black circle depicts the NADP binding site (aa 47–49, 52, 163, 167, 188–193, 211–212, 216, 232, 251–252, 254, 274–275, 298–300); (
) the black circle depicts the NADP binding site (aa 47–49, 52, 163, 167, 188–193, 211–212, 216, 232, 251–252, 254, 274–275, 298–300); ( )the grey circle depicts the substrate binding site (aa47, 49, 69, 95, 163, 300); (▽) white arrows depicts the catalytic Zn binding site (aa47, 69, 163); and (▾) black arrows depicts the structural Zn binding site (aa 100, 103, 106, 114). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/ and (b) GenBank.
)the grey circle depicts the substrate binding site (aa47, 49, 69, 95, 163, 300); (▽) white arrows depicts the catalytic Zn binding site (aa47, 69, 163); and (▾) black arrows depicts the structural Zn binding site (aa 100, 103, 106, 114). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/ and (b) GenBank.
(PPT)
Amino acid sequence alignment of melon CmACD3 (MELO3C003735P2a) with closely related sequences of of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD6 (XP_004136373.1b), Gossypium hirsutum GhCAD3 (ACQ59091.1b), Ricinus communis RcCAD (XP_002510582.1b), Theobroma cacao TcCAD9 (EOY15101.1b), Vitis vinifera VvCAD6 (XP_002269356.1b), Fragaria vesca FvCAD6 (XP_004291336.1b), Hordeum vulgare HvCAD6 (BAK01962.1b) and Sorghum bicolor SbCAD6 (XP_002446076.1b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: (•) the black circle depicts the NAD binding site (aa49–51, 54, 165, 169, 191–196, 214–215, 219, 235, 254–255, 257, 277–278, 301–303); (•)the grey circle depicts the substrate binding site (aa49, 51, 71, 97, 165, 303); (▽) white arrows depicts the catalytic Zn binding site (aa49, 71, 165); and (▾) black arrows depicts the structural Zn binding site (aa 102, 105, 108, 116). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/ and (b) GenBank.
(PPT)
Amino acid sequence alignment of melon CmCAD4 (MELO3C005809P1a) with closely related sequences of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD13 (XP004145884.1), CsCAD14 (XP004162965.1), Sorghum bicolor SbCAD4-2 (XP_002436635.1b), SbCAD4-3 (XP_002436634.1b), Oryza sativa OsCAD1 (AAN09864b), Zea mays ZmCAD4 (NP_001131273.1b), Hordeum vulgare HvCAD1A (BAJ84795.1b), HvCAD1B (BAJ98188.1b), Triticum aestivum TaCAD10 (TC172690c), Gossypium hirsutum GhCAD3 (ACQ59091.1b), Ricinus communis RcCAD (XP_002510582.1b), Theobroma cacao TcCAD9 (EOY15101.1b), Vitis vinifera VvCAD (CBI34634.3b), Theobroma cacao TcCAD4 (EOY23782.1b), Glycine max GmCAD1 (XP003543132.1b), Cicer arietinum CaCAD1 (XP004485621.1b) and Arabidopsis thaliana AtCAD1 (AY288079b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: ( ) the black circle depicts the NAD binding site (aa49–51, 54, 165, 169, 191–196, 214–215, 219, 235, 254–255, 257, 277–278, 301–303); (
) the black circle depicts the NAD binding site (aa49–51, 54, 165, 169, 191–196, 214–215, 219, 235, 254–255, 257, 277–278, 301–303); ( )the grey circle depicts the substrate binding site (aa49, 51, 71, 97, 165, 303); (▽) white arrows depicts the catalytic Zn binding site (aa49, 71, 165); and (▾) black arrows depicts the structural Zn binding site (aa 102, 105, 108, 116). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/, (b) GenBank and (c) http://compbio.dfci.harvard.edu/cgi-bin/tgi/Blast/index.cgi/1.
)the grey circle depicts the substrate binding site (aa49, 51, 71, 97, 165, 303); (▽) white arrows depicts the catalytic Zn binding site (aa49, 71, 165); and (▾) black arrows depicts the structural Zn binding site (aa 102, 105, 108, 116). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/, (b) GenBank and (c) http://compbio.dfci.harvard.edu/cgi-bin/tgi/Blast/index.cgi/1.
(PPT)
Amino acid sequence alignment of melon CmCAD5 (MELO3C023272P1a) with closely related sequences of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD9 (XP_004150677.1b), Populus trichocarpa PtCAD (XP_002300211.1b), Populus trichocarpa PtCAD6 (ABK94550.1b), Populus tomentosa PtCAD9 (AGU43751.1b), Vitis vinifera VvCAD9 (XP_002279832.1b), Arabidopsis thaliana AtCAD2 (AY302077b), AtCAD3 (AY302078b), AtCAD9 (AY302076b), Medicago sativa MsCAD1 (AF083333b) and PtSAD (AF273256b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: ( ) the black circle depicts the NAD binding site (aa50–52, 55, 166, 170, 191–196, 214–216, 219, 236, 254–255, 277–278, 301–303); (
) the black circle depicts the NAD binding site (aa50–52, 55, 166, 170, 191–196, 214–216, 219, 236, 254–255, 277–278, 301–303); ( )the grey circle depicts the substrate binding site (aa50, 52, 72, 98, 166, 303); (▽) white arrows depicts the catalytic Zn binding site (aa50, 72, 166); and (▾) black arrows depicts the structural Zn binding site (aa 103, 106, 109, 117). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/and (b) GenBank.
)the grey circle depicts the substrate binding site (aa50, 52, 72, 98, 166, 303); (▽) white arrows depicts the catalytic Zn binding site (aa50, 72, 166); and (▾) black arrows depicts the structural Zn binding site (aa 103, 106, 109, 117). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/and (b) GenBank.
(PPT)
List and description of nucleotide motifs discovered in promoter region of melon CAD genes.
(DOC)
CmCAD subcellular localization prediction.
(DOC)
Acknowledgments
We thank Qiang Chen, Deqing Lv, Yufan Tang and Tao Xu for excellent technical assistance.
Funding Statement
This study was financially supported by the National Natural Science Foundation of China (31272154). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boudet AM (2000) Lignins and lignification: selected issues. Plant Physiol Bioch 38: 81–96. [Google Scholar]
- 2. Frost CJ, Hunter MD (2008) Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol 178: 835–45. [DOI] [PubMed] [Google Scholar]
- 3. Ma QH (2010) Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J Exp Bot 10: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaur H, Shaker K, Heinzel N, Ralph J, Gális I, et al. (2012) Environmental Stresses of Field Growth Allow Cinnamyl Alcohol Dehydrogenase-Deficient Nicotiana attenuata Plants to Compensate for their Structural Deficiencies. Plant Physiol 159: 1545–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Domon JM, Baldwin L, Acket S, Caudeville E, Arnoult S, et al. (2013) Cell wall compositional modifications of Miscanthus ecotypes in response to cold acclimation. Phytochemistry 85: 51–61. [DOI] [PubMed] [Google Scholar]
- 6. Saidi MN, Bouaziz D, Hammami I, Namsi A, Drira N, et al. (2013) Alterations in lignin content and phenylpropanoids pathway in date palm (Phoenix dactylifera L.) tissues affected by brittle leaf disease. Plant Sci 211: 8–16. [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, et al. (2004) Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyl transferase affects phenylpropanoid biosynthesis. Plant Cell 16(6): 1446–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sattler SE, Funnell-Harris DL, Pedersen JF (2010) Brown midrib mutations and their importance to the utilization of maize, sorghum, and pearl millet lignocellulosic tissues. Plant Sci 178: 229–238. [Google Scholar]
- 9. O'Malley DM, Porter S, Sederoff RR (1992) Purification, characterization, and cloning of cinnamyl alcohol dehydrogenase in loblolly pine (Pinus taeda L.). Plant Physiol 98(4): 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galliano H, Cabane M, Eckerskorn C, Lottspeich F, Sandermann H, et al. (1993) Molecular cloning, sequence analysis and elicitor-/ozone-induced accumulation of cinnamyl alcohol dehydrogenase from Norway spruce (Picea abies). Plant Mol Biol 23: 145–156. [DOI] [PubMed] [Google Scholar]
- 11. Halpin C, Knight ME, Foxon GA, Campbell MM, Boudet AM, et al. (1994) Manipulation of lignin quality by downregulation of cinnamyl alcohol dehydrogenase. Plant J 6: 339–689. [Google Scholar]
- 12. MacKay JJ, Liu W, Whetten R, Sederoff RR, O'Malley DM (1995) Genetic analysis of cinnamyl alcohol dehydrogenase in loblolly pine: single gene inheritance, molecular characterization and evolution. Mol Gen Genet 247: 537–545. [DOI] [PubMed] [Google Scholar]
- 13. Damiani I, Morreel K, Danoun S, Goeminne G, Yahiaoui N, et al. (2005) Metabolite profiling reveals a role for atypical cinnamyl alcohol dehydrogenase CAD1 in the synthesis of coniferyl alcohol in tobacco xylem. Plant Mol Biolo 59: 753–769. [DOI] [PubMed] [Google Scholar]
- 14. Kim SJ, Kim MR, Bedgar DL, Moinuddin SGA, Cardenas CL, et al. (2004) Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis . P Nati Acad Sci USA 101: 1455–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim SJ, Kim KW, Cho MH, Franceschi VR, Davin LB, et al. (2007) Expression of cinnamyl alcohol dehydrogenases and their putative homologues during Arabidopsis thaliana growth and development: lessons for database annotations? Phytochemistry 68: 1957–1974. [DOI] [PubMed] [Google Scholar]
- 16. Bedon F, Levasseur C, Grima-Pettenati J, Seguin A, MacKay J (2009) Sequence analysis and functional characterization of the promoter of the Picea glauca Cinnamyl Alcohol Dehydrogenase gene in transgenic white spruce plants. Plant Cell Rep 28: 787–800. [DOI] [PubMed] [Google Scholar]
- 17. Saballos A, Ejeta G, Sanchez E, Kang C, Vermerris W (2009) A genomewide analysis of the cinnamyl alcohol dehydrogenase family in sorghum [Sorghum bicolor (L.) Moench] identifies SbCAD2 as the Brown midrib6 gene. Genetics 181(2): 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sattler SE, Saathoff AJ, Haas EJ, Palmer NA, Funnell-Harris DL, et al. (2009) A nonsense mutation in a cinnamyl alcohol dehydrogenase gene is responsible for the sorghum brown midrib6 phenotype. Plant Physiol 150: 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barakat A, Bagniewska-Zadworna A, Choi A, Plakkat U, DiLoreto D, et al. (2009) The cinnamyl alcohol dehydrogenase gene family in Populus: phylogeny, organization, and expression. BMC Plant Biol 9(1): 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barakat K, Bagniewska-Zadworna A, Frost AJ, Carlson JE (2010) Phylogeny and expression profiling of CAD and CAD-like genes in hybrid Populus (P. deltoides × P. nigra): evidence from herbivore damage for subfunctionalization and functional divergence. BMC Plant Biol 10: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim YH, Bae JM, Huh GH (2010) Transcriptional regulation of the cinnamyl alcohol dehydrogenase gene from sweetpotato in response to plant developmental stage and environmental stress. Plant Cell Rep 29: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saathoff AJ, Tobias CM, Sattler SE, Haas EJ, Twigg P, et al. (2011) Switchgrass Contains Two Cinnamyl Alcohol Dehydrogenases Involved in Lignin Formation. Bioenerg Res 4: 120–133. [Google Scholar]
- 23. Hirano K, Aya K, Kondo M, Okuno A, Morinaka Y, et al. (2012) OsCAD2 is the major CAD gene responsible for monolignol biosynthesis in rice culm. Plant Cell Rep 31: 91–101. [DOI] [PubMed] [Google Scholar]
- 24. Bukh C, Nord-Larsen PH, Rasmussen SK (2012) Phylogeny and structure of the cinnamyl alcohol dehydrogenase gene family in Brachypodium distachyon . J Exp Bot 17: 6223–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng WW, Zhang M, Wu JQ, Jiang ZZ, Tang L, et al. (2013) Molecular cloning, functional analysis of three cinnamyl alcohol dehydrogenase (CAD) genes in the leaves of tea plant, Camellia sinensis. J Plant Physiol 170(3): 272–282. [DOI] [PubMed] [Google Scholar]
- 26. Tobias CM, Chow EK (2005) Structure of the cinnamyl-alcohol dehydrogenase gene family in rice and promoter activity of a member associated with lignification. Planta 220: 678–688. [DOI] [PubMed] [Google Scholar]
- 27. Raes J, Rohde A, Christensen JH, Peer VY, Boerjan W (2003) Genome-wide characterization of the lignification toolbox in Arabidopsis . Plant Physiol 133(3): 1051–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo DM, Ran JH, Wang XQ (2010) Evolution of the cinnamyl/sinapyl alcohol dehydrogenase (CAD/SAD) gene family: the emergence of real lignin is associated with the origin of bona fide CAD. J Mol Evol 71: 202–218. [DOI] [PubMed] [Google Scholar]
- 29. Tronchet M, Balague C, Kroj T, Jouanin L, Roby D (2010) Cinnamyl alcohol dehydrogenases-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol Plant Pathol 11(1): 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goffner D, Joffroy I, Grima-Pettenati J, Halpin C, Knight ME, et al. (1992) Purification and characterization of isoforms of cinnamyl alcohol dehydrogenase from Eucalyptus xylem. Planta 188: 48–53. [DOI] [PubMed] [Google Scholar]
- 31. Bhuiyan NH, Selvara JG, Wei Y, King J (2009) Role of lignification in plant defense. Plant Signal Behav 4(2): 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Latreche K, Rahmania F (2011) High extracellular accumulation of p-hydroxybenzoic acid, p-hydroxycinnamic acid and p-hydroxybenzaldehyde in leaves of Phoenix dactylifera L. affected by the brittle leaf disease. Physiol Mol Plant p 76: 144–151. [Google Scholar]
- 33. Wang YZ, Dai MS, Zhang SJ, Shi ZB (2014) Exploring Candidate Genes for Pericarp Russet Pigmentation of Sand Pear (Pyrus pyrifolia) via RNA-Seq Data in Two Genotypes Contrasting for Pericarp Color. PLOS ONE 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordi GM, Andrej BJ, Walter S, Bourgeois M, Mir G, et al. (2012) The genome of melon (Cucumis melo L). PNAS 12054–15109.
- 35. Youn B, Camacho R, Moinuddin SGA, Lee C, Davin LB, et al. (2006) Crystal structures and catalytic mechanism of the Arabidopsis cinnamyl alcohol dehydrogenases AtCAD5 and AtCAD4. Org Biomol Chem 4: 1687–1697. [DOI] [PubMed] [Google Scholar]
- 36. Persson B, Krook M, Jornvall H (1991) Characteristics of short-chain alcohol dehydrogenases and related enzymes. Eur J Biochem 200: 537–543. [DOI] [PubMed] [Google Scholar]
- 37. McKie JH, Jaouhari R, Douglas KT, Goffner D, Feuillet C, et al. (1993) A molecular model for cinnamyl alcohol dehydrogenase, a plant aromatic alcohol dehydrogenase involved in lignification. BBA-Protein Struct M 1202: 61–69. [DOI] [PubMed] [Google Scholar]
- 38. McKie JH, Jaouhari R, Douglas KT, Goffner D, Feuillet C, et al. (1993) A molecular model for cinnamyl alcohol dehydrogenase, a plant aromatic alcohol dehydrogenase involved in lignification. BBA-Protein Struct M 1202, 61–69. [DOI] [PubMed] [Google Scholar]
- 39. Saathoff AJ, Hargrove MS, Haas EJ, Tobias CM, Twigg P, et al. (2012) Switchgrass PviCAD1: Understanding residues important for substrate preferences and activity. Appl Biochem Biotech 168: 1086–1100. [DOI] [PubMed] [Google Scholar]
- 40. Lauvergeat V, Kennedy K, Feuillet C, Mckie JH, Gorrichon L, et al. (1995) Site-directed mutagenesis of a serine residue in cinnamyl alcohol dehydrogenase, a plant NADPH-dependent dehydrogenase, affects the specificity for the coenzyme. Biochemistry 34: 12426–12434. [DOI] [PubMed] [Google Scholar]
- 41. Trabucco GM, Matos DA, Lee SJ, Saathoff AJ, Priest HD, et al. (2013) Functional characterization of cinnamyl alcohol dehydrogenase and caffeic acid O-methyltransferase in Brachypodium distachyon. BMC Biotechnol 13: 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng H, Li LL, Xu F, Cheng SY, Cao FL, et al. (2013) Expression patterns of a cinnamyl alcohol dehydrogenase gene involved in lignin biosynthesis and environmental stress in Ginkgo biloba. Mol Biol Rep 40: 707–721. [DOI] [PubMed] [Google Scholar]
- 43. Li X, Yang Y, Yao J, Chen G, Zhang Q, et al. (2009) Flexible Culm 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol Biol 69(6): 685–697. [DOI] [PubMed] [Google Scholar]
- 44. Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124(4): 1–17. [DOI] [PubMed] [Google Scholar]
- 45. Chantreau M, Grec S, Gutierrez L, Dalmais M, Pineau C, et al. (2013) PT-Flax (phenotyping and TILLinG of flax): development of a flax (Linum usitatissimum L.) mutant population and TILLinG platform for forward and reverse genetics. BMC Plant Biology 13: 159–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang KW, Qian Q, Huang ZJ, Wang YQ, Li M, et al. (2006) Gold Hull and internode2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiol 140: 972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shan LL, Li X, Wang P, Cai C, Zhang B, et al. (2008) Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta 227(6): 1243–1254. [DOI] [PubMed] [Google Scholar]
- 48. Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2(6): 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singh RK, Sane VA, Misra A, Ali SA, Nath P (2010) Differential expression of the mango alcohol dehydrogenase gene family during ripening. Phytochemistry 71: 1485–1494. [DOI] [PubMed] [Google Scholar]
- 50. Trainotti L, Tadiello A, Casadoro G (2007) The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 58 (12): 3299–3308. [DOI] [PubMed] [Google Scholar]
- 51. Tesniere C, Pradal M, El-Kereamy A, Torregrosa L, Chatelet P, et al. (2004) Involvement of ethylene signalling in a non-climacteric fruit: new elements regarding the regulation of ADH expression in grapevine. J Exp Bot 55: 2235–2240. [DOI] [PubMed] [Google Scholar]
- 52. Verries C, Pradal M, Chatelet P, Torregrosa L, Tesniere C (2004) Isolation and analysis of the promoter of VvAdh2, a grapevine (Vitis vinifera L.) ripeningrelated gene. Plant Sci 167: 1067–1074. [Google Scholar]
- 53. Zhang LB, Wang G, Chang JM, Liu JS, Cai JH, et al. (2010) Effects of 1-MCP and ethylene on expression of three CAD genes and lignification in stems of harvested Tsai Tai (Brassica chinensis). Food CHem 123(1): 32–40. [Google Scholar]
- 54. Srivastava A, Handa AK (2005) Hormonal regulation of tomato fruit development: a molecular perspective. J. Plant Growth Regul 24: 67–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transcript abundance of CmCAD1, 2, 3, 4 and 5 in vegetative tissues and developing stages of melon fruit (1–48). R, root; ML, mature leaf; DL, developing leaf; YS, young stem; PF, pistillate flower petal; SF, staminate flower petal. Stages and treatment were described in Section 2.5. RNA isolated from the samples. Expression analysis was carried out by semi-quantitative PCR using 18 S as an internal control.
(TIF)
mRNA abundance of CmCAD1, 2, 3, 4, and 5 in mature green fruit after treatment with different hormonal. Auxin and ABA (100 µM) treatments were given for 2 h as described in materials and methods section. Expression analysis was carried out by semi-quantitative PCR as described in Section 2.5 using actin as 18 S internal contrl.
(TIF)
CmCAD1, 2, 3, 4 and 5 accumulation during different stages of ethylene and 1-MCP treatment induced ripening of melon by semi-quantitative PCR. 1–12 indicate days after ethylene and 1-MCP treatment. The accumulation level of the genes in untreated melon fruit was control. 18 S was used as an internal control. All the treatments have been described in Section2.5.
(TIF)
Amino acid sequence alignment of melon CmCAD1 (MELO3C019548P1a) and CmCAD2 (MELO3C018492P1a) with closely related sequences of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD11 (XP_004140716.1b), CsCAD12 (XP_004137094.1b), Nicotiana tabacum NtCAD1 (X62343b), Populus tremuloides PtCAD1 (AF217957b), Arabidopsis thaliana AtCAD4 (AY302081b), AtCAD5 (AY302082b), Lolium perenne LpCAD3 (AF010290b), Festuca arundinacea FaCAD1a (AF188292b), Triticum aestivum TaCAD1 (ADI59734.1b), Oryza sativa OsCAD2 (NP_001046132.1), Zea mays ZmCAD2 (ACG45271.1b), Saccharum officinarum SoCAD1 (AJ231135b), Sorghum bicolor SbCAD2 (AEM63607.1b), Medicago sativa MsCAD2 (AF083332b), Eucalyptus globules EgCAD2 (CAA46585.1b) and Gossypium hirsutum GhCAD1 (ABZ01817.1b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: ( ) the black circle depicts the NADP binding site (aa 47–49, 52, 163, 167, 188–193, 211–212, 216, 232, 251–252, 254, 274–275, 298–300); (
) the black circle depicts the NADP binding site (aa 47–49, 52, 163, 167, 188–193, 211–212, 216, 232, 251–252, 254, 274–275, 298–300); ( )the grey circle depicts the substrate binding site (aa47, 49, 69, 95, 163, 300); (▽) white arrows depicts the catalytic Zn binding site (aa47, 69, 163); and (▾) black arrows depicts the structural Zn binding site (aa 100, 103, 106, 114). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/ and (b) GenBank.
)the grey circle depicts the substrate binding site (aa47, 49, 69, 95, 163, 300); (▽) white arrows depicts the catalytic Zn binding site (aa47, 69, 163); and (▾) black arrows depicts the structural Zn binding site (aa 100, 103, 106, 114). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/ and (b) GenBank.
(PPT)
Amino acid sequence alignment of melon CmACD3 (MELO3C003735P2a) with closely related sequences of of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD6 (XP_004136373.1b), Gossypium hirsutum GhCAD3 (ACQ59091.1b), Ricinus communis RcCAD (XP_002510582.1b), Theobroma cacao TcCAD9 (EOY15101.1b), Vitis vinifera VvCAD6 (XP_002269356.1b), Fragaria vesca FvCAD6 (XP_004291336.1b), Hordeum vulgare HvCAD6 (BAK01962.1b) and Sorghum bicolor SbCAD6 (XP_002446076.1b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: (•) the black circle depicts the NAD binding site (aa49–51, 54, 165, 169, 191–196, 214–215, 219, 235, 254–255, 257, 277–278, 301–303); (•)the grey circle depicts the substrate binding site (aa49, 51, 71, 97, 165, 303); (▽) white arrows depicts the catalytic Zn binding site (aa49, 71, 165); and (▾) black arrows depicts the structural Zn binding site (aa 102, 105, 108, 116). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/ and (b) GenBank.
(PPT)
Amino acid sequence alignment of melon CmCAD4 (MELO3C005809P1a) with closely related sequences of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD13 (XP004145884.1), CsCAD14 (XP004162965.1), Sorghum bicolor SbCAD4-2 (XP_002436635.1b), SbCAD4-3 (XP_002436634.1b), Oryza sativa OsCAD1 (AAN09864b), Zea mays ZmCAD4 (NP_001131273.1b), Hordeum vulgare HvCAD1A (BAJ84795.1b), HvCAD1B (BAJ98188.1b), Triticum aestivum TaCAD10 (TC172690c), Gossypium hirsutum GhCAD3 (ACQ59091.1b), Ricinus communis RcCAD (XP_002510582.1b), Theobroma cacao TcCAD9 (EOY15101.1b), Vitis vinifera VvCAD (CBI34634.3b), Theobroma cacao TcCAD4 (EOY23782.1b), Glycine max GmCAD1 (XP003543132.1b), Cicer arietinum CaCAD1 (XP004485621.1b) and Arabidopsis thaliana AtCAD1 (AY288079b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: ( ) the black circle depicts the NAD binding site (aa49–51, 54, 165, 169, 191–196, 214–215, 219, 235, 254–255, 257, 277–278, 301–303); (
) the black circle depicts the NAD binding site (aa49–51, 54, 165, 169, 191–196, 214–215, 219, 235, 254–255, 257, 277–278, 301–303); ( )the grey circle depicts the substrate binding site (aa49, 51, 71, 97, 165, 303); (▽) white arrows depicts the catalytic Zn binding site (aa49, 71, 165); and (▾) black arrows depicts the structural Zn binding site (aa 102, 105, 108, 116). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/, (b) GenBank and (c) http://compbio.dfci.harvard.edu/cgi-bin/tgi/Blast/index.cgi/1.
)the grey circle depicts the substrate binding site (aa49, 51, 71, 97, 165, 303); (▽) white arrows depicts the catalytic Zn binding site (aa49, 71, 165); and (▾) black arrows depicts the structural Zn binding site (aa 102, 105, 108, 116). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/, (b) GenBank and (c) http://compbio.dfci.harvard.edu/cgi-bin/tgi/Blast/index.cgi/1.
(PPT)
Amino acid sequence alignment of melon CmCAD5 (MELO3C023272P1a) with closely related sequences of other plants. GenBank accession numbers are as follows: Cucumis sativus CsCAD9 (XP_004150677.1b), Populus trichocarpa PtCAD (XP_002300211.1b), Populus trichocarpa PtCAD6 (ABK94550.1b), Populus tomentosa PtCAD9 (AGU43751.1b), Vitis vinifera VvCAD9 (XP_002279832.1b), Arabidopsis thaliana AtCAD2 (AY302077b), AtCAD3 (AY302078b), AtCAD9 (AY302076b), Medicago sativa MsCAD1 (AF083333b) and PtSAD (AF273256b). Conserved residues are shaded in black. The multi-domain architecture predicted by NCBI's CDD is marked: ( ) the black circle depicts the NAD binding site (aa50–52, 55, 166, 170, 191–196, 214–216, 219, 236, 254–255, 277–278, 301–303); (
) the black circle depicts the NAD binding site (aa50–52, 55, 166, 170, 191–196, 214–216, 219, 236, 254–255, 277–278, 301–303); ( )the grey circle depicts the substrate binding site (aa50, 52, 72, 98, 166, 303); (▽) white arrows depicts the catalytic Zn binding site (aa50, 72, 166); and (▾) black arrows depicts the structural Zn binding site (aa 103, 106, 109, 117). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/and (b) GenBank.
)the grey circle depicts the substrate binding site (aa50, 52, 72, 98, 166, 303); (▽) white arrows depicts the catalytic Zn binding site (aa50, 72, 166); and (▾) black arrows depicts the structural Zn binding site (aa 103, 106, 109, 117). Dark grey shading indicates similar residues in seven out of eight of the sequences and clear grey shading indicates similar residues in five out of eight of the sequences. The letters following the accession numbers in the legend of the figure indicate the source database: (a) https://melonomics.net/and (b) GenBank.
(PPT)
List and description of nucleotide motifs discovered in promoter region of melon CAD genes.
(DOC)
CmCAD subcellular localization prediction.
(DOC)