Abstract
Weight suppression, the difference between highest past weight and current weight, is a robust predictor of clinical characteristics of bulimia nervosa; however, the influence of weight suppression in anorexia nervosa (AN) has been little studied, and no study to date has investigated the ways in which the relevance of weight suppression in AN may depend upon an individual’s current body mass index (BMI). The present study investigated weight suppression, BMI, and their interaction as cross-sectional and prospective predictors of psychological symptoms and weight in AN. Women with AN completed depression (Beck Depression Inventory-II) and eating disorder symptomatology measures (Eating Disorder Examination Questionnaire and Eating Disorders Inventory-3) at residential treatment admission (N = 350) and discharge (N = 238). Weight suppression and BMI were weakly correlated (r = −.22). At admission, BMI was positively correlated with all symptom measures except Restraint and depression scores. Weight suppression was also independently positively correlated with all measures except Weight Concern and Body Dissatisfaction subscale scores. In analyses examining discharge scores (including admission values as covariates), the admission weight suppression X BMI interaction consistently predicted post-treatment psychopathology. Controlling for weight gain in treatment and age, higher admission weight suppression predicted lower discharge scores (less symptom endorsement) among those with lower BMIs; among those with higher BMIs, higher weight suppression predicted higher discharge scores. These results are the first to demonstrate that absolute and relative weight status are joint indicators of AN severity and prognosis. These findings may have major implications for conceptualization and treatment of AN.
Keywords: Anorexia nervosa, weight suppression, treatment, outcome
Research suggests that weight suppression, defined as the difference between highest weight since reaching adult height and current body weight, has high cross-sectional and predictive relevance in bulimia nervosa (BN). The construct has been found to be positively correlated with frequency of objective bulimic episodes among individuals who meet criteria for a diagnosis of BN (Lowe, Thomas, Safer, & Butryn, 2007) and predicted, over a period of 8-year follow-up, longer time to first full remission from BN (Lowe et al., 2011). Findings among individuals with sub-threshold BN were similar: In a large sample of collegiate men and women, individuals with bulimic syndromes had higher weight suppression levels than those without bulimic syndromes, and greater weight suppression predicted increased likelihood of bulimic syndrome maintenance at 10-year follow-up (Keel & Heatherton, 2010). Butryn and colleagues (2006) found that greater weight suppression predicted worse outcomes in the cognitive-behavioral treatment of BN, though two studies did not replicate this finding (Carter, McIntosh, Joyce, & Bulik, 2008; Zunker et al., 2010).
In addition to robust prediction of bulimic symptomatology, weight suppression has also been found to predict weight gain. Among women with BN, results indicate that weight suppression, independent of body mass index (BMI), predicts weight gain both in the short term (Lowe, Davis, Lucks, Annunziato, & Butryn, 2006) and long term (over 5-year follow-up; D. B. Herzog et al., 2010). Of note, lower admission BMI was also found to predict short-term weight gain in treatment independently of weight suppression level (Lowe, Davis, et al., 2006).
Among individuals without eating disorders, evidence from experimenter-induced weight loss studies, which do not result in objectively low post-weight loss BMI levels, suggests that an individual’s highest past weight may serve as a biological marker to which the body may be metabolically driven to return (e.g., MacLean, Bergouignan, Cornier, & Jackman, 2011; Rosenbaum & Leibel, 2010). In more naturalistic studies of women without eating disorders, weight suppression has been found to predict weight gain (Lowe et al., 2006), even with the inclusion of resting metabolic rate and total energy expenditure as covariates (Stice, Durant, Burger, & Schoeller, 2011).
While much is known about the implications of weight suppression for BN and non-eating disordered populations, this construct is only beginning to be investigated among individuals with anorexia nervosa (AN). A large body of evidence supports the clinical importance of objectively low weight in AN, often defined as a BMI under 18.5 mg/kg2 (National Institutes of Health & National Heart, 1998), including a broad array of resulting physiological and metabolic abnormalities (Klein & Walsh, 2004; Leonard & Mehler, 2001; Monteleone, DiLieta, Castaldo, & Maj, 2004) and associated problematic psychological symptoms, including preoccupation with food and eating (e.g., Keys, Brozek, Henschel, Mickelsen, & Taylor, 1950). Patients with AN are all, by definition, low in objective weight; however, variability in highest past weights among individuals with AN (Coners, Remschmidt, & Hebebrand, 1999; Miyasaka et al., 2003) indicates a wide range of weight suppression levels that may also impact the biology and psychology of AN.
Despite a historical focus on the biological and psychological impact of objectively low BMI or percent of ideal body weight in AN, updates to diagnostic criteria reference consideration of both extent of weight loss and the current BMI of an individual with AN. Diagnostic criteria for AN, according to both Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association, 2000) and fifth edition (DSM-5) criteria (American Psychiatric Association, 2013), require an individual to restrict energy intake and reach a “significantly low body weight” below what is minimally “normal” for adults. In DSM-5, BMI also determines an individual’s current AN illness severity rating; however, the rationale for DSM-5 criteria development stated that the use of BMI alone to define a significantly low body weight “may not adequately reflect an individual’s…history,” and that the determination of whether weight is inappropriately low “is best made by the clinician in light of all relevant information” (American Psychiatric Association, 2012). Further, DSM-5 includes an “Other Specified Feeding or Eating Disorder” diagnosis of “atypical anorexia nervosa,” which includes individuals who have lost a significant amount of weight (i.e., who are high in weight suppression), but have not reached a weight that is below the objectively “normal range.” Thus, the field seems to be moving toward consideration of both objectively low weight and the degree of weight loss, including medical and psychological consequences of the degree of weight loss in AN (e.g., Watson & Andersen, 2003). Further research regarding the independent and joint influences of a patient’s weight loss and current weight status is needed to better determine how researchers and clinicians may best understand and incorporate this information in diagnosis, prevention, and treatment.
Preliminary evidence supports the biological and psychological significance of the construct of weight suppression for individuals with AN. For example, weight-restored (to at least 90% of ideal body weight, based on height) female patients with AN who were higher in weight suppression at the time of weight restoration had lower serum leptin levels and were less likely to have resumed menstruation before the time of hospital discharge (Klein et al., July 2011). Thus, weight suppression, beyond absolute weight status, may serve as an additional indicator of illness severity. Studies have also found that patients with the binge-eating/purging subtype of AN (AN-BP) have histories of higher maximum lifetime weight compared to patients with the restricting subtype (AN-R) and are more likely than patients with AN-R to report premorbid obesity (Garfinkel, Moldofsky, & Garner, 1980). Although individuals with AN-BP and those with AN-R are at a low body weight, the distance between highest lifetime weight and current weight (i.e., weight suppression) in individuals with AN-BP may be significantly greater. These findings suggest that weight suppression may be especially relevant to understanding bulimic behaviors in the context of AN. Further, as distance from highest adult weight has been found to predict weight gain in both non-eating disorder populations and in BN, it may also serve as a relevant predictor of symptomatology and weight gain in treatment in patients with AN. Most recently, in a large sample of patients with AN (N = 185), Wildes and Marcus (2012) found that while weight suppression was not related to achievement of a minimally adequate body weight, weight suppression, controlling for BMI, predicted total weight gain, a faster rate of weight gain, and likelihood of endorsing bulimic symptoms during intensive treatment.
Although research has begun to investigate the predictive power of weight suppression above and beyond BMI in AN, potential interactive effects of weight suppression and BMI have not been considered. Specifically, the extent to which the low BMI levels of patients with AN may moderate the relation between weight suppression and outcome has not yet been studied. Initial results in individuals with BN spectrum disorders support consideration of a joint influence of weight suppression and low BMI on eating disorder symptomatology in AN. BMI was found to moderate the relation between weight suppression and binge eating frequency in full and sub-threshold BN, with individuals highest in weight suppression and lowest in objective weight status reporting the highest binge eating frequencies (Butryn, Juarascio, & Lowe, 2011). Similarly, in addition to previously reported main effects of weight suppression in AN, the same degree of weight suppression may have a more dramatic impact on an individual depending on the severity of the resulting low BMI level, particularly because such weight loss may activate varying degrees of physiological starvation responses.
A conceptual representation of the relation between weight suppression and current and highest adult BMI is shown in Figure 1. For example, a 30 lb weight loss in an individual whose highest adult BMI was 24 kg/m2 (Figure 1, line C) may not have the same psychological and biological implications as a 30 lb weight loss in an individual whose highest adult BMI was 22 kg/m2 (Figure 1, line A). Further, weight loss to the same objectively low weight may have a more dramatic impact on individuals depending on degree of weight suppression. For example, weight loss to a BMI of 18 kg/m2 may have different implications for an individual whose highest adult BMI was 24 kg/m2 (Figure 1, line C) compared to an individual whose highest adult BMI was 19 kg/m2 (Figure 1, line D). As with BN, weight suppression among individuals with AN may have implications independent of or in interaction with current BMI for symptomatology, outcome, and diagnosis.
Figure 1.
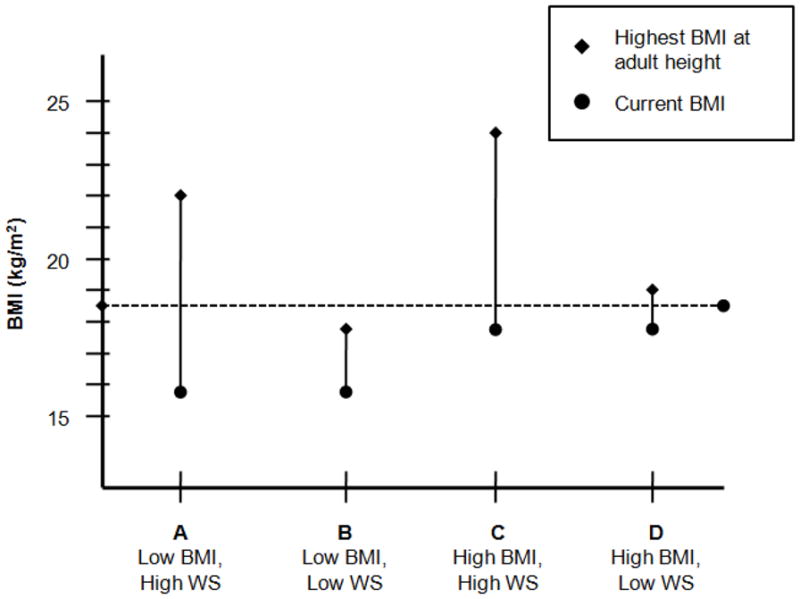
Conceptual representation of the relation between varying levels of weight suppression and current and highest adult body mass index (BMI; kg/m2) among patients with anorexia nervosa. The vertical bars represent weight suppression (WS), the difference between highest BMI at adult height (diamonds) and current BMI (circles).
Though the results reported by Wildes and Marcus (2012) mark an important first step in the investigation of weight suppression in AN, replication of these initial findings is needed. Further, weight suppression has not yet been investigated as a baseline correlate of psychological symptoms in AN or as a predictor of changes in psychological symptoms during treatment in this population. Previous research has examined weight suppression as a predictor of outcome above and beyond BMI, but the current study aims to examine weight suppression as a predictor of outcome in conjunction with BMI. Although understanding how these variables may relate to AN etiology is beyond the scope of our investigation, an improved understanding of illness course, potential maintenance factors, and predictors of treatment response seems a critical addition to the literature on this pernicious and treatment-resistant disorder.
Cross-sectional analyses examining weight suppression and BMI in relation to psychological symptoms at admission of course prevent determination of causality. Body dissatisfaction or eating concern, for example, may drive behaviors that result in weight suppression, and/or brain changes associated with high degrees of weight suppression to objectively low body weights may exacerbate preoccupation with weight and eating. Nevertheless, regardless of the direction of this relation, examination of cross-sectional associations may support consideration of both absolute and relative weight status in determining illness severity ratings.
The present study investigates, in a sample of women with AN admitted to residential treatment, whether level of weight suppression and BMI at admission, both independently and in interaction, 1) relate to admission symptomatology and 2) predict weight or symptom levels at discharge, controlling for baseline values. This examination includes individuals who were all receiving a fairly standardized residential inpatient treatment and were all required to gain weight. As a cross-sectional relation and a prognostic relation between variables may be different, the present study maintains consistency with previous literature examining the relevance of weight suppression in individuals with BN using a combination of cross-sectional and longitudinal analyses. Previous studies indicating that weight suppression is a correlate of eating disorder symptoms and predictor of treatment outcome in eating disorder populations have included BMI as a covariate; the current investigation represents an extension of these findings by examining the possibility that the cross-sectional and predictive effects of weight suppression may depend upon BMI level. We therefore conceptualize our analyses as tests of BMI as a moderator of the effects of weight suppression.
Methods
Participants
Study participants were patients admitted to one of two residential treatment facilities for eating disorders (one in Pennsylvania and one in Florida) between July 1, 2007 and December 31, 2008. Both treatment facilities are owned and operated by a large, national center for eating disorders. All individuals were eligible if they were admitted to one of these sites during the period of data collection and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994) criteria for AN, except for amenorrhea, as assessed by one of four psychiatrists. Individuals who met all criteria for standard AN except that, despite significant weight loss, the individual’s weight was within the normal range (above 18.5 kg/m2) were excluded from the present sample to maintain diagnostic consistency (n = 12, 3.3% of sample). Of the 337 women who responded, 77.2% denied taking oral contraceptives at the time of residential inpatient admission. Menstrual status data were available for 237 of the 260 women with AN who denied taking oral contraceptives, and 79.3% of these women had missed at least three periods in the four months before admission.
Because reliability of the treatment facilities’ psychiatrist diagnoses has not been established, self-report items from the Eating Disorder Examination Questionnaire (EDE-Q) served as a proxy measure of diagnostic reliability. Similar to procedures described in previous research (Wolk, Loeb, & Walsh, 2005), individuals were considered to meet AN diagnostic criterion B if they endorsed EDE-Q item 10 (fear of gaining weight) and were considered to meet criterion C if they endorsed item 11 (felt fat), item 22 (overvaluation of weight), or item 23 (overvaluation of shape) with a rating of 4 or higher (indicating clinically significant presence of that symptom on 16 days or more of the past 28 days). Information regarding menstrual status (criterion D) and birth control usage were also obtained from the EDE-Q, and amenorrhea was considered present if individuals endorsed missing three or more periods in the past four months. Approximately 40.3 % of the sample (n = 141) met all diagnostic criteria for AN, including amenorrhea, with an additional 21.7 % (n = 76) who otherwise met full criteria but were taking a form of birth control, preventing assessment of amenorrhea. Of those not taking birth control, 10 % met all diagnostic criteria except for amenorrhea (n = 36). Because research suggests that there is little difference between individuals who do and do not experience amenorrhea (e.g., Attia & Roberto, 2009; Dalle Grave, Calugi, & Marchesini, 2008; Roberto, Steinglass, Mayer, Attia, & Walsh, 2008), and this criterion was eliminated in DSM-5, individuals who met all criteria except amenorrhea were considered to have an EDE-Q-confirmed diagnosis of AN.
A total of 4.6 % did not meet criterion B (fear of fat, n = 16), and 22.9 % did not meet criterion C (disturbance in or undue influence of weight and shape, n = 80). Although these individuals did not endorse significant weight and shape concerns on the self-report questionnaire, because all patients included in analyses had been admitted to a residential treatment center for eating disorders and were admitted with a BMI under 18.5 kg/m2, it is likely that this 27.5% of the sample who did not meet these two criteria per EDE-Q responses were a clinically relevant group. Analyses were run both with and without the 27.5 % of the sample who did not meet either criterion B or C, and both results have been reported below (“all patients” vs. “confirmed-diagnosis patients”).
Approximately 35% of patients admitted to these facilities in Pennsylvania and Florida receive a diagnosis of AN. The 350 patients who completed the admission assessment represented approximately 80% of all patients with AN admitted during the study period. Study participants were all female, as only females are admitted to these treatment facilities. Approximately two-thirds of the sample (n = 238) also completed a discharge assessment. Mean length of stay in the program was 37.2 days (SD = 20.0).
Procedure
The study was approved by the research committee that oversees both treatment facilities, and access to and analysis of the data was approved by the Institutional Review Board at Drexel University. All participants provided informed consent prior to completing assessments. The measures administered were completed as part of the standard admission battery, and completion rates reflect the typical rates of completion at each of the two facilities. Admission questionnaires were administered via computer on the second day of treatment, and discharge questionnaires were completed in the same manner within the final two days of treatment.
The comprehensive treatment program at both facilities focused on normalization of eating patterns, weight gain or stabilization, and elimination of compensatory behaviors. Patients received an intensive program of individual, group, and family therapy, provided by a multi-disciplinary team consisting of psychiatrists, psychologists, nutritionists and nurses. Each patient received a nutritionist-developed, individualized meal plan designed to promote weight gain of two to three pounds per week. The theoretical orientation at both facilities is eclectic and is largely based on psychodynamic and feminist-relational theories.
Measures
Eating disorder symptomatology
The EDE-Q (Fairburn & Beglin, 2008) is a 36-item self-report questionnaire that focuses on eating disorder symptoms over the past 28 days. It was adapted from the Eating Disorder Examination (Fairburn & Cooper, 1993), an investigator-based, semi-structured interview. The EDE-Q contains Shape Concern, Weight Concern, Eating Concern, and Restraint subscales in addition to a Global scale score computed from the average of the four subscales. Number of missed menstrual periods in the past four months was also obtained from this questionnaire. Data from community and clinical populations indicate good concurrent validity of the EDE-Q for all features except binge eating (Fairburn & Beglin, 1994). Acceptable levels of internal consistency have been observed for the EDE-Q Global and subscale scores (Cronbach α coefficients above .70; Peterson et al., 2007), and the level of internal consistency was found to be acceptable in our sample (Cronbach’s α = .88 for Shape Concern, .82 for Weight Concern, .72 for Eating concern, .84 for Restraint, and .94 for Global subscale scores).
The Eating Disorders Inventory-3 (EDI-3; Garner, 2004) is a 96-item self-report inventory that measures eating disorder symptom severity and psychological dimensions associated with eating disorders. The EDI-3 is organized into 12 primary scales; however the current study included only the Drive for Thinness, Body Dissatisfaction, and Bulimia subscales. The inventory has adequate psychometric properties, and the test-retest reliability of these subscales among women diagnosed with eating disorders has been shown to be excellent (Clausen, Rosenvinge, Friborg, & Rokkedal, 2011). The level of internal consistency was found to be acceptable in our sample (Cronbach’s α = .88 for Drive for Thinness, .89 for Bulimia, and .91 for Body Dissatisfaction).
Depressive symptomatology
The Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996), a 21-item, multiple choice self-report measure, was used to assess depressive symptomatology. At both treatment facilities, the item assessing suicidality was excluded. The BDI-II has adequate test-retest reliability, internal consistency (Cronbach’s α = .93 in our sample), and convergent validity (Sprinkle et al., 2002; Steer, Ball, Ranieri, & Beck, 1997).
Weight status
Weights were measured using a digital scale and height was measured by stadiometer. BMI at admission and discharge was calculated as weight in kilograms divided by height in meters squared. Highest lifetime non-pregnancy weight at adult height was obtained via self-report from the cover page of the EDI-3. Weight suppression was calculated by subtracting weight at admission in kg from this reported highest weight in kg. The validity of recalled past weights has been supported by previous research (Tamakoshi et al., 2003), including a study of adolescents with AN and BN reporting a correlation of .92 between highest measured premorbid weight from school records and highest recalled premorbid weight (there were no differences between diagnostic groups; Swenne, Belfrage, Thurfjell, & Engström, 2005). Use of recalled highest past weight in the calculation of weight suppression is standard (Butryn, et al., 2006; Carter, et al., 2008; D. B. Herzog, et al., 2010; Keel & Heatherton, 2010; Lowe, et al., 2011; Lowe, Davis, et al., 2006; Lowe, et al., 2007; Zunker, et al., 2010). Rate of weight change from admission to discharge was calculated as (discharge weight in kg − admission weight in kg)/days in residential treatment.
Statistical Analysis
Hierarchical multiple regression analyses were used to test whether weight suppression, admission weight status (BMI), and their interaction would significantly predict symptomatology (BDI-II scores and EDE-Q and EDI-3 subscales) at admission and discharge. All variables included in computing interactions were centered. Cross-sectional analyses at baseline were examined by entering covariates in the first bock followed by weight suppression and BMI in the second block and the interaction term in the final block. Prospective analyses at discharge were examined by entering covariates and admission scores for the dependent variable in the first block, followed by weight suppression and BMI in the second block, and the interaction term in the final block. This approach adjusts all discharge outcome variables for their baseline values, permitting examination of post-treatment symptom levels regardless of admission illness severity. All variables within each block were entered simultaneously, and were not interpreted unless the block itself was significant (Cohen & Cohen, 1983).
Several variables (admission: weight suppression, EDE-Q Restraint, EDI-3 Drive for Thinness, EDI-3 Bulimia, age, duration of illness; discharge: EDI-3 Bulimia) did not satisfy criteria for a normal distribution (skewness and kurtosis not exceeding an absolute value of 1), therefore square root, log, and inverse transformations were computed for each variable and compared to identify which brought the distribution closest to normality. With the exception of age, for which a log transformation was used, all of these variables were transformed using a square root transformation. After transformation, all variables satisfied criteria for a normal distribution, and the transformed versions of these variables were used in all analyses reported below. All regression assumptions of linearity, normality, homoscedasticity of residuals, and non-multicollinearity were met. Outliers were identified using studentized residuals, leverage and Cook’s distances, and between zero and four cases were removed from each analysis in the results reported below. The pattern of results was similar when all cases were included in the analyses.
Cross-sectional analyses examining the prediction of menstrual status excluded participants who reported taking oral contraceptives, 22.9 % of the full sample. In addition, initial analyses indicated that BMI, weight suppression, and their interaction were predictive of weight change from admission to discharge; therefore, discharge analyses for other variables were conducted including weight change as a covariate. Additionally, preliminary analyses indicated that baseline weight suppression had a small to moderate correlation with age (r (360) = .33, p < .001) and duration of illness (r (357) = .25, p < .001). Therefore, these variables were considered for inclusion as covariates. Age and duration of illness were themselves highly correlated (r (347) = .81, p < .001) and therefore, to avoid violation of the multicollinearity assumption, could not be simultaneously entered into the regression. Results were rerun separately including each covariate. Results reported below include age as a covariate; however, results including the duration of illness variable were equivalent.
Results
Sample Characteristics
Demographic and clinical characteristics of the full sample and confirmed-diagnosis sample at admission and discharge are presented in Table 1. The majority of the full sample (91.4%) self-identified as Caucasian (3.1% Multi-racial or Other, 2.0% Hispanic, 1.7% African American, and 1.4% Asian). The majority of patients (85.7%, n = 300) received at least one co-morbid diagnosis from program psychiatrists, most commonly major depressive disorder (49.4%, n = 173), generalized anxiety disorder (15.7%, n = 55), depressive disorder not otherwise specified (14.9%, n = 52) and substance abuse disorders (13.4%, n = 47). Approximately half (55.7%) of study participants in the full sample reported at least one previous psychiatric hospitalization.
Table 1.
Means, Standard Deviations, and Sample Size at Admission and Discharge
| Variable | Admission – Full Sample | Admission – Confirmed-Diagnosis Sample | Discharge – Full Sample | Discharge – Confirmed- Diagnosis Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | N | M | SD | N | M | SD | |
| 1. Weight suppression (lbs) | 350 | 34.8 | 20.2 | 253 | 35.5 | 20.4 | ||||||
| 2. BMI (kg/m2) | 350 | 15.7 | 1.5 | 253 | 15.8 | 1.5 | 350 | 18.1 | 1.3 | 253 | 18.2 | 1.3 |
| 3. Highest adult BMI (kg/m2) | 350 | 21.5 | 3.4 | 253 | 21.7 | 3.4 | ||||||
| 4. Age (years) | 350 | 23.9 | 10.0 | 253 | 23.8 | 9.6 | ||||||
| 5. Duration of Illness (years) | 347 | 7.6 | 8.2 | 251 | 8.0 | 8.4 | ||||||
| EDE-Q Subscale Scores | ||||||||||||
| 6. Restraint Subscale | 349 | 4.3 | 1.6 | 253 | 4.9 | 1.1 | 238 | 1.4 | 1.2 | 171 | 1.6 | 1.3 |
| 7. Weight Concern | 349 | 4.0 | 1.6 | 253 | 4.0 | 1.1 | 238 | 3.0 | 1.6 | 171 | 3.5 | 1.5 |
| 8. Shape Concern | 349 | 4.5 | 1.5 | 253 | 5.2 | 0.8 | 238 | 3.8 | 1.6 | 171 | 4.3 | 1.4 |
| 9. Eating Concern | 349 | 3.5 | 1.4 | 253 | 4.8 | 1.0 | 238 | 1.9 | 1.2 | 171 | 2.2 | 1.1 |
| 10. Global | 349 | 4.1 | 1.3 | 253 | 4.7 | 0.7 | 238 | 2.5 | 1.3 | 171 | 2.9 | 1.2 |
| 11. Periods missed in the last 4 months | 285 | 3.0 | 1.5 | 136 | 3.2 | 1.3 | ||||||
| EDI-3 Subscale Scores | ||||||||||||
| 12. Drive for Thinness | 348 | 20.3 | 7.4 | 251 | 23.5 | 4.1 | 237 | 14.8 | 7.6 | 171 | 16.6 | 7.1 |
| 13. Bulimia | 348 | 6.6 | 7.2 | 251 | 7.4 | 7.3 | 237 | 2.5 | 3.4 | 171 | 2.7 | 3.3 |
| 14. Body Dissatisfaction | 348 | 26.3 | 10.4 | 251 | 30.3 | 8.4 | 236 | 23.5 | 9.9 | 170 | 26.2 | 9.0 |
| 15. Beck Depression Inventory-II Score | 343 | 31.9 | 13.3 | 247 | 35.7 | 11.6 | 237 | 16.2 | 12.0 | 171 | 18.2 | 12.3 |
Note. Means and sample sizes presented use untransformed version of variables.
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3.
Two-hundred and thirty eight participants (68.0 %) completed measures at discharge. Completers and non-completers were compared on all baseline demographic and outcome measures listed in Table 1. The only statistically significant difference between these groups was in length of stay, with completers staying an average of 40.7 days (SD = 21.4) compared to non-completers’ average stay of 29.6 days (SD = 14.1). Because of frequent, unanticipated cessation of insurance coverage and resulting early discharge from the treatment centers, the difference in length of stay was expected. As those who did not complete discharge assessments did not differ at admission in weight suppression, BMI, or other admission symptomatology levels from those who did, the completer group was considered to be representative of the full sample. Discharge analyses include completers only, with the exception of analyses of BMI and rate of weight gain, which was available for all participants (n = 350) because it was recorded in medical charts and thus did not depend upon patients completing discharge self-report measures.
Weight Suppression and BMI as Correlates of Symptomatology at Admission
Intercorrelations between all variables at admission are presented in Table 2. The correlation between baseline BMI and weight suppression was statistically significant but small (r (350) = −.22, p < .001), such that those who had lower BMIs were more highly weight suppressed. Statistical information for individual predictors presented in Tables 3 and 4 represent results from the final model after all predictors have been included. We compared these to coefficient results for predictor variables when including only steps 1 and 2 of the model (prior to the addition of the interaction term) and found that differences between the models in the size of standardized beta coefficients and semi-partial correlations were small in size, and that the statistical significance of individual predictors did not change between the two models for any outcome variable.
Table 2.
Intercorrelations at Admission
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Weight suppression (lbs) | - | .−22*** | .86*** | .31*** | .23*** | .09 | .03 | .06 | .09 | .07 | .19** | .03 | .15** | .04 | .12* |
| 2. BMI (kg/m2) | −.28*** | - | .25*** | −.03 | −.05 | .05 | .10 | .09 | .10 | .09 | −.22*** | .08 | .19*** | .12* | .07 |
| 3. Highest adult BMI (kg/m2) | .86*** | .19** | - | .29*** | .21*** | .11* | .07 | .08 | .12* | .10 | .07 | .06 | .22*** | .09 | .15** |
| 4. Age (years) | .37*** | −.001 | .37*** | - | .81*** | .08 | .08 | .06 | .06 | .08 | −.19*** | −.05 | .11* | .002 | .18*** |
| 5. Duration of Illness (years) | .29*** | −.01 | .30*** | .84*** | - | .19*** | .20*** | .19*** | .17*** | .21*** | −.19*** | .10 | .18*** | .14** | .26*** |
| EDE-Q Subscale Scores | |||||||||||||||
| 6. Restraint Subscale | .11 | −.05 | .07 | .12 | .14* | - | .67*** | .68*** | .61*** | .84*** | .01 | .66*** | .26*** | .56*** | .42*** |
| 7. Weight Concern | .02 | .12* | .08 | .15* | .16* | .37*** | - | .89*** | .67*** | .91*** | .06 | .76*** | .20*** | .78*** | .57*** |
| 8. Shape Concern | .06 | .15* | .11 | .07 | .11 | .35*** | .72*** | - | .70*** | .93*** | .12* | .77*** | .25*** | .82*** | .56*** |
| 9. Eating Concern | .12 | .08 | .15* | .05 | .08 | .36*** | .41*** | .43*** | - | .84*** | .13* | .61*** | .49*** | .57*** | .53*** |
| 10. Global | .10 | .09 | .12* | .12 | .15* | .70*** | .81*** | .79*** | .73*** | - | .09 | .80*** | .34*** | .77*** | .59*** |
| 11. Periods missed in the last 4 months | .21** | −.21** | .08 | −.21** | −.24*** | −.14* | −.05 | .02 | .05 | −.04 | - | .09 | −.01 | .10 | −.01 |
| EDI-3 Subscale Scores | |||||||||||||||
| 12. Drive for Thinness | −.002 | .02 | −.002 | −.13* | −.07 | .36*** | .54*** | .53*** | .33*** | .54*** | .05 | - | .26*** | .71*** | .52*** |
| 13. Bulimia | .11 | .17** | .18*** | .05 | .07 | .06 | .04 | .06 | .43*** | .20*** | −.04 | .09 | - | .12* | .18*** |
| 14. Body Dissatisfaction | .02 | .14* | .08 | .01 | .07 | .37*** | .64*** | .72*** | .35*** | .66*** | .02 | .52*** | −.04 | - | .53*** |
| 15. Beck Depression Inventory-II Score | .14* | .08 | .17** | .15** | .20** | .19** | .39*** | .33*** | .35*** | .41*** | −.04 | .26*** | .05 | .35*** | - |
Note. Correlations above the diagonal = results including all participants; Correlations below the diagonal = results including only participants with EDE-Q confirmed AN diagnosis.
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3.
p < .05
p < .01
p < .001
Table 3.
Relations among Admission Weight Suppression, Admission BMI, and Symptomatology at Admission – Results Including All Participants (N = 350)
| Outcome Variable | Block 1 | Block 2 | Block 3 | ||||
|---|---|---|---|---|---|---|---|
| ΔR2 | Age β b(SE) |
ΔR2 | WS β b(SE) |
BMI β b(SE) |
ΔR2 | WS × BMI β b(SE) |
|
| EDE-Q Subscale Scores | |||||||
| Restraint Subscale Score | .01* | .06 .08 (.07) |
.03* | .18** .07 (.02) |
.11 .03 (.02) |
.01 | −.10 −.03 (.02) |
| Weight Concern Score | .01* | .09 .38 (.25) |
.02* | .10 .14 (.08) |
.14* .14 (.06) |
.004 | −.06 −.06 (.06) |
| Shape Concern Score | .01 | .06 .23 (.22) |
.03* | .15* .19 (.08) |
.14** .14 (.05) |
.01 | −.08 −.07 (.05) |
| Eating Concern Score | .01 | .04 .14 (.22) |
.04** | .19*** .24 (.07) |
.15** .14 (.05) |
.01 | −.10 −.09 (.05) |
| Global Score | .01* | .07 .25 (.20) |
.03** | .17** .20 (.07) |
.15** .13 (.05) |
.01 | −.10 −.08 (.05) |
| EDI-3 Subscale Scores | |||||||
| Drive for Thinness Score | .001 | −.07 −.23 (.19) |
.02* | .14* .16 (.06) |
.12* .09 (.04) |
.01 | −.08 −.06 (.04) |
| Bulimia Score | .01* | .05 .20 (.22) |
.07*** | .21*** .29 (.08) |
.26*** .25 (.05) |
.01 | −.10 −.09 (.05) |
| Body Dissatisfaction Score | <.001 | −.01 −.18 (1.62) |
.03* | .10 .98 (.54) |
.17** 1.14 (.38) |
.01 | −.07 −.46 (.35) |
| BDI-II Score | .03*** | .13* 4.87 (2.04) |
.02* | .14* 1.68 (.69) |
.09 .82 (.49) |
.01 | −.07 −.58 (.45) |
| Number of Missed Menstrual Periods (N = 231) | .03** | −.26*** −.96 (.23) |
.16*** | .31*** .37 (.08) |
−.19** −.15 (.05) |
.001 | .03 .02 (.05) |
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3,
p < .05
p < .01
p < .001.
Table 4.
Relations among Admission Weight Suppression, Admission BMI, and Symptomatology at Admission – Results Including Only Participants with EDE-Q Confirmed Diagnosis of AN (N = 253)
| Outcome Variable | Block 1 | Block 2 | Block 3 | ||||
|---|---|---|---|---|---|---|---|
| ΔR2 | Age β b(SE) |
ΔR2 | WS β b(SE) |
BMI β b(SE) |
ΔR2 | WS × BMI β b(SE) |
|
| EDE-Q Subscale Scores | |||||||
| Restraint Subscale Score | .01 | .10 .10 (.07) |
.005 | .06 .02 (.02) |
−.03 −.01 (.02) |
<.001 | .01 .003 (.01) |
| Weight Concern Score | .02* | .16* .46 (.20) |
.02 | −.02 −.02 (.07) |
.10 .07 (.05) |
.01 | .08 .05 (.04) |
| Shape Concern Score | .01 | .05 .11 (.16) |
.03* | .07 .05 (.05) |
.15* .09 (.04) |
.01 | .08 .04 (.03) |
| Eating Concern Score | .003 | −.003 −.01 (.21) |
.02* | .15* .15 (.07) |
.12 .09 (.05) |
<.001 | .002 .001 (.04) |
| Global Score | .02 | .09 .20 (.15) |
.02 | .08 .06 (.05) |
.10 .05 (.03) |
.003 | .06 .03 (.03) |
| EDI-3 Subscale Scores | |||||||
| Drive for Thinness Score | .02* | −.14* −.35 (.17) |
.004 | .05 .04 (.05) |
.02 .01 (.04) |
.004 | .06 .03 (.03) |
| Bulimia Score | .002 | −.04 −.16 (.28) |
.07*** | .23** .29 (.09) |
.26*** .25 (.06) |
.01 | −.07 −.06 (.06) |
| Body Dissatisfaction Score | <.001 | −.02 −.37 (1.67) |
.02 | .06 (.46 (.54) |
.15* .83 (.38) |
.001 | .03 .16 (.33) |
| BDI-II Score | .02* | .11 3.49 (2.28) |
.02 | .12 1.27 (.75) |
.11 .86 (.54) |
.002 | .05 .33 (.45) |
| Number of Missed Menstrual Periods (N = 171) | .05** | −.34*** −1.26 (.28) |
.16*** | .36*** .41 (.09) |
−.12 −.10 (.06) |
<.001 | .01 .01 (.05) |
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3,
p < .05
p < .01
p < .001.
In analyses including the full sample, weight suppression and BMI were independently and positively associated with severity of symptomatology on all baseline measures (see Table 3). Controlling for age and weight suppression, BMI independently predicted all symptom measures except EDE-Q Restraint subscale and BDI-II scores. Controlling for age and BMI, weight suppression independently predicted all symptom measures except EDE-Q Weight Concern subscale and EDI-3 Body Dissatisfaction scores. After controlling for age and the independent effects of BMI and weight suppression, the weight suppression X BMI interaction was not a statistically significant predictor of any scores at admission.
When baseline results were re-run including only participants with an EDE-Q-confirmed AN diagnosis (N = 253), BMI was only found to be a statistically significant independent correlate of EDI-3 Bulimia subscale scores, and weight suppression was only found to be a significant correlate of EDI-3 Bulimia subscale scores and number of missed menstrual periods (see Table 4). Of note, as demonstrated in Table 1, removal of participants based on subclinical scores (below 4 out of 6) on certain EDE-Q items resulted in restricted ranges in the confirmed-diagnosis sample in EDE-Q subscale scores and scores on other outcome measures that were highly correlated with those subscales in the full sample (see Table 1 for differences in means and standard deviations in the two samples). This likely reduced correlations between those outcome variables and all other measures, including the proposed predictors (for example, bivariate correlations between outcome measures were reduced on average by r = .2 (range: .06–.33) in comparison to the full sample).
Weight Suppression and BMI as Predictors of Treatment Outcome
Means and standard deviations for outcome variables at discharge as well as intercorrelations between all predictor and discharge outcome measures are presented in Tables 1 and 5, respectively. Similar to admission results, statistical information for individual predictors presented in Tables 6 and 7 represent results from the final model after all predictors have been included. We again compared these to coefficient results for predictor variables when including only steps 1 and 2 of the model (prior to the addition of the interaction term) and found that differences between the models in the size of standardized beta coefficients and semi-partial correlations were small in size, and that the statistical significance of individual predictors did not change between the two models for any outcome variable, with the exception of two minor differences in predictors crossing the threshold for statistical significance in analyses restricted to the confirmed-diagnosis sample.1
Table 5.
Intercorrelations at Discharge
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Weight suppression (lbs) | - | .−22*** | .86*** | .31*** | .23*** | .05 | .06 | .02 | .03 | .04 | .03 | .09 | .05 | −.02 |
| 2. BMI (kg/m2)⟡ | −.28*** | - | .25*** | −.03 | −.05 | .02 | −.003 | −.01 | .05 | .02 | .16* | .12 | .11 | .16* |
| 3. Highest adult BMI (kg/m2) | .86*** | .19** | - | .29*** | .21*** | .07 | .06 | .003 | .05 | .05 | .11 | .13* | .09 | .07 |
| 4. Age (years) | .37*** | −.001 | .37*** | - | .81*** | −.17** | −.13 | −.16* | −.15* | −.17** | −.12 | −.04 | −.03 | −.02 |
| 5. Duration of Illness (years) | .29*** | −.01 | .30*** | .84*** | - | −.07 | −.08 | −.11 | −.08 | −.10 | −.05 | .004 | .03 | −.04 |
| EDE-Q Subscale Scores | ||||||||||||||
| 6. Restraint Subscale | .06 | .05 | .10 | −.22** | −.12 | - | .63*** | .59*** | .64*** | .78*** | .52*** | .18** | .50*** | .45*** |
| 7. Weight Concern | .08 | −.02 | .09 | −.12 | −.09 | .63*** | - | .77*** | .82*** | .89*** | .71*** | .32*** | .64*** | .55*** |
| 8. Shape Concern | .01 | −.01 | =.004 | −.19* | −.18* | .57*** | .75*** | - | .91*** | .93*** | .75*** | .23*** | .79*** | .53*** |
| 9. Eating Concern | .01 | .07 | .05 | −.17* | −.14 | .63*** | .81*** | .88*** | - | .95*** | .77*** | .25*** | .76*** | .61*** |
| 10. Global | .04 | .03 | .06 | −.20** | −.15 | .79*** | .89*** | .91*** | .94*** | - | .77*** | .27*** | .76*** | .60*** |
| EDI-3 Subscale Scores | ||||||||||||||
| 11. Drive for Thinness | .002 | .14 | .08 | −.14 | −.10 | .51*** | .69*** | .72*** | .76*** | .76*** | - | .35*** | .74*** | .59*** |
| 12. Bulimia | .03 | .12 | .08 | −.02 | .04 | .14 | .23** | .19* | .23** | .23** | .34*** | - | .19** | .30*** |
| 13. Body Dissatisfaction | .07 | .09 | .11 | −.01 | .01 | .51*** | .60*** | .74*** | .72*** | .73*** | .69*** | .16* | - | .53*** |
| 14. Beck Depression Inventory-II Score | −.05 | .18* | .05 | −.05 | −.05 | .47*** | .51*** | .50*** | .60*** | .59*** | .59*** | .26*** | .50*** | - |
Note. Correlations above the diagonal = results including all participants; Correlations below the diagonal = results including only patients with EDE-Q confirmed AN diagnosis.
= Correlations are with BMI at admission.
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3.
p < .05
p < .01
p < .001
Table 6.
Prediction of Symptomatology at Discharge – Results Including All Participants (N = 238)
| Outcome Variable | Block 1 | Block 2 | Block 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔR2 | Admission Score β b(SE) |
Age β b(SE) |
Weight Change β b(SE) |
ΔR2 | WS β b(SE) |
BMI β b(SE) |
ΔR2 | WS × BMI β b(SE) |
|
| BMI (N = 350) | .27*** | .58*** .51 (.04) |
−.09 −.33 (.17) |
.03*** | .20*** .23 (.06) |
.02** | −.15** −.12 (.04) |
||
| Rate of weight change (N = 350) | .001 | −.08 −.02 (.01) |
.12*** | .16** .01 (.004) |
−.28*** −.02 (.003) |
.003 | −.06 −.003 (.003) |
||
| EDE-Q Subscale Scores | |||||||||
| Restraint Subscale Score | .16*** | .31*** .778 (.16) |
−.24*** −.78 (.21) |
.20** .17 (.07) |
.01 | −.06 −.07 (.08) |
.09 .07 (.06) |
.03** | .18** .14 (.05) |
| Weight Concern Score | .35*** | .53*** .56 (.06) |
−.25*** −1.10 (.25) |
.29*** .33 (.08) |
.02* | .02 .03 (.09) |
.17** .19 (.08) |
.02** | .14** .13 (.05) |
| Shape Concern Score | .39*** | .68*** .68 (.06) |
−.24*** −1.06 (.24) |
.26*** .29 (.08) |
.003 | −.02 −.03 (.08) |
.07 .07 (.07) |
.01* | .12* .12 (.05) |
| Eating Concern Score | .25*** | .44*** .36 (.05) |
−.17** −.54 (.19) |
.27*** .22 (.06) |
.01 | −.02 −.02 (.07) |
.12 .09 (.06) |
.03** | .17** .12 (.04) |
| Global Score | .41*** | .58*** .56 (.05) |
−.25*** −.88 (.19) |
.28*** .24 (.06) |
.01 | −.02 −.02 (.07) |
.10 .09 (.06) |
.02** | .14** .11 (.04) |
| EDI-3 Subscale Scores | |||||||||
| Drive for Thinness Score | .28*** | .50*** 3.23 (.36) |
−.13* −2.78 (1.23) |
.07 .34 (.40) |
.01 | .01 .04 (.43) |
.13 .65 (.36) |
.03** | .17** .75 (.25) |
| Bulimia Score | .34*** | .59*** .44 (.04) |
−.10 −.31 (.17) |
.02 .01 (.05) |
.001 | −.03 −.03 (.06) |
.03 .02 (.05) |
.01 | .09 .06 (.04) |
| Body Dissatisfaction Score | .45*** | .66*** .65 (.05) |
−.03 −.70 (1.45) |
.17** 1.15 (.46) |
.01 | −.06 −.57 (.49) |
.09 .58 (.43) |
.02** | .13** .75 (.29) |
| BDI-II Score | .21*** | .45*** .40 (.05) |
−.11 −3.71 (2.06) |
.14 1.14 (.66) |
.02 | −.11 −1.23 (.72) |
.14 1.07 (.61) |
.01 | .11 .78 (.44) |
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3,
p < .05
p < .01
p < .001.
Table 7.
Prediction of Symptomatology at Discharge – Results Including Only Participants with EDE-Q Confirmed Diagnosis of AN (N = 171)
| Outcome Variable | Block 1 | Block 2 | Block 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔR2 | Admission Score β b(SE) |
Age β b(SE) |
Weight Change β b(SE) |
ΔR2 | WS β b(SE) |
BMI β b(SE) |
ΔR2 | WS × BMI β b(SE) |
|
| BMI (N = 253) | .27*** | .59*** .51 (.05) |
−.03 −.10 (.21) |
.01 | .15* .17 (.07) |
.02** | −.16** −.12 (.04) |
||
| Rate of weight Change (N = 253) | .001 | −.10 −.02 (.01) |
.12*** | .19** .01 (.005) |
−.25*** −.01 (.003) |
.01 | −.12 −.005 (.003) |
||
| EDE-Q Subscale Scores | |||||||||
| Restraint Subscale Score | .15*** | .23** .79 (.26) |
−.31*** −1.12 (.28) |
.27** .24 (.08) |
.03 | −.02 −.02 (.10) |
.20* .17 (.08) |
.04** | .20** .17 (.06) |
| Weight Concern Score | .15*** | .26*** .36 (.10) |
−.24** −1.01 (.33) |
.40*** .41 (.10) |
.04* | .02 .03 (.11) |
.24** .24 (.09) |
.04** | .23** .19 (.06) |
| Shape Concern Score | .18*** | .26*** .43 (.12) |
−.24** −.96 (.31) |
.38*** .37 (.09) |
.02 | −.01 −.01 (.10) |
.15 .14 (.09) |
.03** | .20** .16 (.06) |
| Eating Concern Score | .13*** | .22** .23 (.07) |
−.15 −.49 (.25) |
.39*** .31 (.08) |
.03 | −.01 −.01 (.09) |
.19* .14 (.07) |
.05** | .23** .16 (.05) |
| Global Score | .24*** | .34*** .50 (.10) |
−.28*** −.94 (.35) |
.39*** 4.32 (.08) |
.03 | .01 .01 (.08) |
.21* .16 (.07) |
.03** | .19** .13 (.05) |
| EDI-3 Subscale Scores | |||||||||
| Drive for Thinness Score | .10*** | .27*** 2.27 (.61) |
−.10 −2.10 (1.59) |
.16 .76 (.48) |
.03 | −.01 −.07 (.53) |
.18 .84 (.44) |
.06*** | .26*** 1.04 (.31) |
| Bulimia Score | .35*** | .61*** .45 (.05) |
−.06 −.16 (.20) |
.10 .07 (.06) |
.01 | −.08 −.07 (.07) |
.05 .04 (.06) |
.02* | .14* .09 (.04) |
| Body Dissatisfaction Score | .38*** | .56*** .59 (.07) |
.03 .69 (1.71) |
.26** 1.61 (.51) |
.01 | −.06 −.44 (.56) |
.13 .74 (.48) |
.03** | .20** 1.00 (.33) |
| BDI-II Score | .15*** | .36*** .37 (.08) |
−.09 −2.92 (2.69) |
.14 1.19 (.81) |
.02 | −.14 −1.53 (.92) |
.12 .96 (.79) |
.02* | .16* 1.16 (.55) |
Abbreviations. WS, weight suppression (kg); BMI, body mass index (kg/m2); EDE-Q, Eating Disorder Examination-Questionnaire; EDI-3, Eating Disorders Inventory-3,
p < .05
p < .01
p < .001.
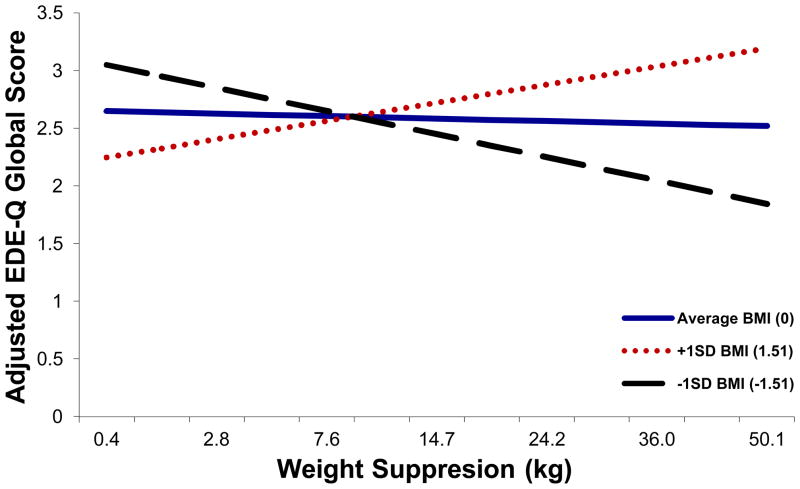
In prospective analyses, for almost all measures, the interaction between weight suppression and BMI at intake was the best predictor of discharge outcome, both in the full sample and in the confirmed-diagnosis sample (see Tables 6 and 7). Prediction of EDI-3 Bulimia subscale scores and BDI-II scores was only statistically significant in the confirmed-diagnosis sample (see Table 7). Examination of the interaction effects indicated that for most variables, with the exception of discharge weight, admission weight suppression was inversely associated with severity of symptoms at discharge among those with lower BMIs at admission (i.e., higher admission weight suppression was associated with better outcome among those who entered treatment at especially low BMIs). In contrast, among those with higher BMIs at admission, admission weight suppression was positively associated with severity of symptoms at discharge (i.e., higher weight suppression at admission was associated with poorer outcome among those who entered treatment at higher BMIs; see Figure 2).
Figure 2.
Relations among admission weight suppression, admission BMI, and EDE-Q Global scores at discharge. EDE-Q Global subscale score values graphed represent discharge values controlling for baseline values, age, and weight change over treatment, with all participants included. The relationship between weight suppression and EDE-Q Global score was significantly moderated by BMI (p = .007). The pattern of results was similar using only the confirmed-diagnosis sample, and for all statistically significant EDE-Q, EDI-3 and BDI-II outcome measures.
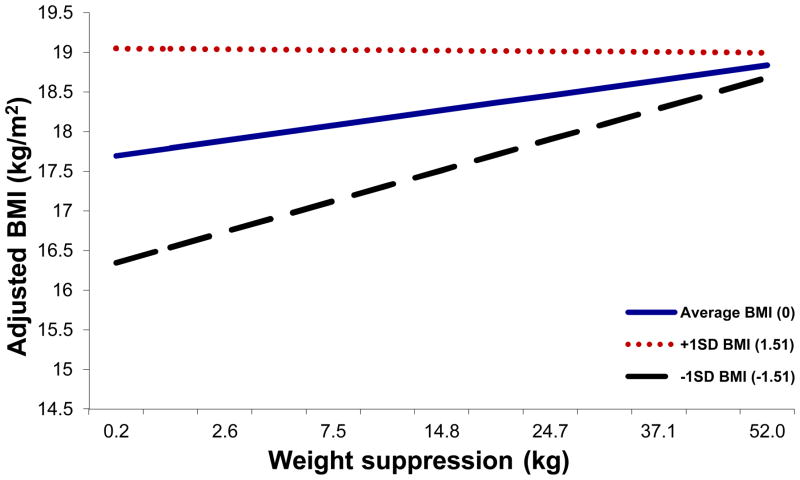
Results indicated that weight suppression consistently predicted higher BMIs at discharge, but this relation was strongest among those with lower BMIs at admission (see Figure 3). Rate of weight change was statistically significantly predicted independently by both BMI and weight suppression, but the weight suppression X BMI interaction was not a statistically significant predictor of rate of weight change (see Tables 6 and 7).
Figure 3.
Relations among admission weight suppression, admission BMI, and BMI at discharge. BMI values graphed represent discharge values controlling for baseline values and age, with all participants included. Weight suppression at admission was consistently positively associated with absolute weight status at discharge, but admission BMI moderated this relation (p =.006), which was strongest among those with lower BMIs at admission. The pattern of results was similar including only the confirmed-diagnosis sample.
To address the possibility that the observed relations between weight suppression, BMI and our outcome measures might be better accounted for by AN subtype, we conducted a series of exploratory analyses. Although individuals with higher weight suppression and individuals with higher BMIs at admission were more likely to be classified as AN-BP subtype, the addition of subtype as a covariate in analyses did not change the pattern of our results, nor did it alter the statistical significance of any of the previously reported prospective findings.2
Discussion
The body weights of individuals with AN are, by definition, objectively low, but previous research indicates varying weight histories among these individuals (Coners, et al., 1999; Miyasaka, et al., 2003). The field has historically focused on the significance of objectively low current weight status in AN, and the relevance of patients’ weight histories to AN symptomatology is only beginning to be considered. Only two previous studies (Klein, et al., July 2011; Wildes & Marcus, 2012) have examined weight suppression in AN. No study to date has investigated the possibility that, as has been found in BN, BMI may moderate the effects of degree of weight suppression on psychological and physiological symptoms. The low correlation between BMI and weight suppression in the present sample permitted extension of the initial findings of Wildes and Marcus (2012) and examination of the possibility that a patient’s level of weight suppression below her previous highest adult weight and her current, objectively low weight status may interact to predict weight gain achieved in treatment, the speed with which a patient gains weight in treatment, and the ways in which psychological symptoms change in response to treatment.
At admission to residential treatment, both BMI and weight suppression were independently and positively associated with several measures of psychopathology, including Shape Concern, Eating Concern, and Global EDE-Q scores as well as EDI-3 Bulimia and Drive for Thinness scores. In addition, higher weight suppression, controlling for BMI, was associated with higher EDE-Q Restraint and depression scores. Controlling for weight suppression, patients with higher BMIs had higher Weight Concern subscale scores and EDI-3 Body Dissatisfaction scores. Patients with lower BMIs, as would be expected, reported more missed menstrual periods. Interestingly, weight suppression was also associated with number of missed menstrual periods, even when controlling for BMI. These results suggest that higher weight suppression, above and beyond absolute weight status, and higher BMI, independently of weight suppression, are both associated with increased eating pathology, general distress, and physiological abnormalities. No statistically significant interaction between BMI and weight suppression was found for any admission variables.
While the positive relation between BMI and symptomatology is surprising in light of prior research on the psychological effects of starvation (Keys, et al., 1950), consistently higher levels of body dissatisfaction and concern with shape and weight at admission to residential treatment among patients with higher BMIs may be related to a desire to become objectively thinner, anticipated exposure to patients with lower BMIs in the treatment milieu, or anticipation of weight gain in treatment. The observed positive association between BMI and the EDI-3 Bulimia subscale at admission replicates prior findings that bulimic behaviors among patients with AN are associated with higher weight (e.g., Garner, Olmsted, Bohr, & Garfinkel, 1982; Eddy, Keel, Dorer, Delinksy, Franko, & Herzog, 2002). In addition, the observed association between weight suppression and higher EDI-3 Bulimia subscale scores, independently of BMI, replicates findings among patients with BN (Lowe, et al., 2007). Both findings are also consistent with the results of our exploratory examinations of AN subytpe, as individuals higher in weight suppression, independent of BMI, and individuals with higher BMI, independent of weight suppression, were more likely to be classified as AN-BP.
Higher weight suppression may be independently associated with greater levels of psychopathology for both psychological (e.g., increased food obsessions) and biological (e.g., abnormal serotonin levels; Bailer & Kaye, 2011) reasons; however, the cross-sectional nature of admission results precludes determination of the direction of causality. For instance, as previously noted, high body dissatisfaction might be associated with weight suppression because of greater motivation to lose weight, and the association between EDE-Q Restraint and weight suppression may reflect the fact that individuals high in weight suppression previously exerted greater restraint over eating in order to reach their more highly weight-suppressed state. Whether weight suppression exacerbates AN symptoms, or increased severity of eating disorder psychopathology motivates individuals to suppress their weight to a greater degree cannot be determined in the current investigation. Nevertheless, the observed associations between weight suppression and symptomatology, even when BMI was controlled, suggest that weight suppression is an indicator of illness severity above and beyond absolute weight status.
Of note, a number of the aforementioned associations at treatment admission were non-significant in analyses including only women whose AN diagnosis was confirmed by EDE-Q responses. In this confirmed-diagnosis sample, weight suppression was statistically significantly associated only with EDI-3 Bulimia and number of missed menstrual periods, while BMI was associated only with Shape Concern scores and EDI-3 Bulimia and Body Dissatisfaction. A likely explanation for the reduction in the number of significant associations in cross-sectional results between the full and confirmed-diagnosis samples is the previously-noted restricted ranges resulting from the use of cut-off scores on several EDE-Q items to define the confirmed-diagnosis sample. Reduced power in the confirmed-diagnosis sample also may have impacted our ability to detect effects. Given the limitations of determining diagnoses based on self-report items from the EDE-Q as well as the broadening of criteria for AN in DSM-5 (i.e., addition to criterion B of “persistent behavior that interferes with weight gain, even though at a significantly low weight” and addition to criterion C of “persistent lack of recognition of the seriousness of the current low body weight”), the results based on this restricted sample should be interpreted with caution. In addition, those who did not meet EDE-Q diagnostic criteria were at an objectively low weight, would likely meet criteria for a diagnosis of AN per DSM-5, and were admitted to residential treatment for an eating disorder; therefore, the full sample is likely clinically representative.
Unlike the cross-sectional findings, in which weight suppression and low BMI could represent mere consequences of eating disorder psychopathology, results of the longitudinal analyses indicate that baseline weight suppression interacts with BMI in the prediction of degree of symptom change over treatment, even when controlling for potential explanatory variables, including age and weight change during treatment. In prospective analyses including baseline values as covariates, the weight suppression X BMI interaction was the most consistent predictor of discharge symptomatology levels, including all of the EDE-Q subscales as well as EDI-3 Drive for Thinness and Body Dissatisfaction. The nature of the significant interaction was the same for all of the above variables: at lower admission BMIs, greater weight suppression predicted better scores at discharge, while among those with the highest BMIs at admission, greater weight suppression predicted higher scores at discharge. Notably, the prediction of psychological symptom outcome was independent of the amount of weight gained in treatment and patient age, and results of exploratory analyses indicate that these findings cannot be better accounted for by diagnostic subtype.
When analyses were restricted to the sample of women whose diagnosis was confirmed by EDE-Q, the weight suppression X BMI interaction predicted depression and EDI-3 Bulimia scores at discharge in addition to the variables mentioned above. Again, at lower admission BMIs, greater weight suppression predicted lower scores on these measures at discharge while among those with the highest BMIs at admission, greater weight suppression predicted higher discharge scores. Although results in the EDE-Q confirmed-diagnosis sample must be interpreted with caution given previously noted limitations, the interactive effect of weight suppression and BMI in predicting Bulimia subscale scores is particularly interesting in light of recent findings that patients with AN high in weight suppression at admission, regardless of admission BMI, are more likely to endorse bulimic behaviors while in treatment (Wildes & Marcus, 2012). The present results, while preliminary and only statistically significant in the EDE-Q-confirmed-diagnosis sample, suggest that high weight suppression in combination with higher BMI at admission may best predict increased bulimic symptoms over the course of treatment. As patients with AN with relatively higher BMIs and high levels of weight suppression at admission may be most comparable in absolute and relative weight status to previously-studied individuals with BN who are high in weight suppression, the current findings appear consistent with prior BN research (Butryn, et al., 2011).
Of note, effect sizes for the weight suppression X BMI interaction in both the full sample and confirmed-diagnosis sample are small and only explain an additional 2–6 % of the variance in outcome measures. Nonetheless, the pattern of results is markedly consistent across outcome measures, and effects were slightly larger in the confirmed-diagnosis sample.
One possible mechanism of the consistently observed weight suppression X BMI interaction may be differential psychological reactions to a given degree of weight gain as a function of individual weight history. Patients with AN with relatively higher BMIs and high weight suppression are more likely to have a history of being closer to overweight than those with higher BMIs and low weight suppression (e.g., see Figure 1, lines C and D). The prospect of weight regain may be more distressing for patients with a history of being near-overweight or overweight, as they may fear that weight regain will culminate in a return to their previous weight status. Thus, increased distress about gaining weight, even when amount of weight gained is held constant, may explain the higher discharge scores on measures of body dissatisfaction and concerns about weight and shape among individuals with high admission BMIs and high weight suppression. Further, those who are more distressed about weight gain may be more likely to resist increases in food intake in treatment, which could account for higher EDE-Q Restraint subscale scores at discharge in this group.
The inverse relation between weight suppression and discharge symptomatology found among patients with the lowest BMIs is somewhat surprising. Because amount of weight gained in treatment was included as a covariate, differential weight gain cannot explain this finding. The outcome may again be a result of differences in psychological reactions to weight gain. Patients with low BMIs and high weight suppression levels, while potentially less likely than patients with higher BMIs and high weight suppression levels to have a history of being overweight, have previously reached weights substantially higher than their low weights at admission to treatment (e.g., see Figure 1, line A). Conversely, patients with low BMIs and low weight suppression levels are more likely to have been chronically underweight and have never reached adult weights significantly higher than their admission weights (see Figure 1, line B). As a result, weight gain during treatment may be more distressing for these patients, who may reach or surpass their highest historical adult weights while in treatment. As previously noted, such differences in distress associated with weight gain, even when amount of weight gain is held constant, may account for differences in EDE-Q and EDI-3 scores at discharge.
Just as admission weight suppression’s prediction of discharge symptomatology depended upon admission BMI, so, too, did its prediction of weight gain over the course of treatment. As was found by Wildes and Marcus (2012), those higher in weight suppression gained more weight in treatment; however, in the present sample, weight suppression had the strongest relation with amount of weight gained in treatment among those with the lowest BMIs at admission, and at higher BMIs, the relation between weight suppression and weight gain was weak. Though the admission BMI of all patients was under 18.5 kg/m2, and all patients included in the sample were required to gain weight, the observed weaker relation between weight suppression and weight gain at higher admission BMIs may be related to these patients needing to gain comparatively less weight to reach their goal weight in treatment. The present study also replicated previous findings that those higher in weight suppression gain weight more quickly in treatment (Wildes & Marcus, 2012); however, unlike the results of Wildes and Marcus (2012), BMI was also an independent predictor of rate of weight gain such that those at lower BMIs gained weight more quickly.
The present results indicate that weight restoration may be most difficult for those who do not have a history of ever weighing significantly more than their current weight. Those who do have this history may find it easier to regain weight for both psychological and metabolic reasons. Prior results in BN as well as in non-clinical populations indicate that weight suppression is associated with proneness to later weight gain (e.g., D. B. Herzog, et al., 2010; Lowe, Davis, et al., 2006; Stice, et al., 2011), and evidence from studies of weight loss among both obese and normal-weight populations suggests that weight loss is associated with biological changes that promote weight regain (Leibel, Rosenbaum, & Hirsch, 1995; Rosenbaum & Leibel, 2010). Because the residential treatment program in the present study prescribed meals designed to promote a standardized rate of weight gain, the differences in amount and rate of weight gain as a function of admission weight suppression and BMI suggest differences in either compliance with treatment, biological mechanisms related to weight gain, such as level of energy expenditure, or both.
In terms of rate of weight gain, our findings indicate that high levels of weight suppression at admission, regardless of BMI, and lower BMIs at admission, regardless of weight suppression, are associated with the most rapid weight gain in treatment. Wildes and Marcus (2012) note that previous findings regarding the effects of rapid weight gain in treatment have been mixed: There is evidence to suggest that a faster rate of weight gain in treatment may be iatrogenic (T. Herzog, Zeeck, Hartmann, & Nickel, 2004; Lay, Jennen-Steinmetz, Reinhard, & Schmidt, 2002; Willer, Thuras, & Crow, 2005) and contrasting evidence that a faster rate of weight restoration predicts improved clinical outcome at one-year follow-up in patients with AN (Lund et al., 2009). In light of these previous, mixed findings, further research is needed to determine how our results concerning rate of weight gain may inform treatment recommendations.
In summary, results of the present study confirm recently reported findings that weight suppression predicts faster rate of weight gain during intensive treatment independently of admission BMI among patients with AN (Wildes & Marcus, 2012). As previously reported (Wildes & Marcus, 2012), higher weight suppression levels were associated with greater weight gain in treatment, but in the current sample the strength of this relation varied with BMI. The present study expands upon previous findings by examining the joint predictive effects of absolute and relative weight status on indices of psychological functioning. Results suggest that when amount of weight gained in treatment is held constant, an individual’s weight history and current weight status interact to predict her psychological reaction to the weight gain.
Taken together, the our results suggest that weight suppression is an important clinical indicator in AN and that it should be assessed and considered in conjunction with BMI or percent of ideal body weight (e.g., based on the Metropolitan Life Insurance Tables; Pai & Paloucek, 2000) in evaluating the illness severity and prognosis of AN patients. High weight suppression was associated with greater psychopathology at baseline, and, among patients with higher BMIs, predicted high levels of symptomatology at discharge. The combination of low weight suppression with low BMI may also represent a negative prognostic indicator.
These findings could carry important implications for the treatment of AN. Target weights in treatment are often set based on absolute weight status, but the statistically significant main effects of weight suppression and the weight suppression X BMI interactions found in the present study, if confirmed by further research, suggest that weight goals may need to be more idiographically determined. Standard guidelines of 90% of ideal body weight as a minimum “weight restoration” goal (American Psychiatric Association, 2006; Golden et al., 1997), for example, do not account for the influence of individual weight history found to be important in this sample and in others (Klein, et al., July 2011; Wildes & Marcus, 2012). Target weights based only on absolute weight status could contribute to higher rates of relapse and more difficult long-term weight stabilization and “normalization.”
Strengths of this study include the investigation of a novel construct in this population and the inclusion of a variety of psychological outcome measures. The large sample size is particularly important given the low prevalence of AN and the resulting difficulty of conducting large-scale research on the disorder. In addition, the study was conducted at large, community-based eating disorder treatment facilities, there were no exclusion criteria beyond an admission BMI threshold, and the length of stay was limited to approximately six weeks on average, supporting the “real-world” clinical significance of the results.
Limitations of this study must also be noted. First, our symptom measures were limited to self-report questionnaires which, particularly for the assessment of bulimic symptoms, may be prone to bias. This may be especially true among women with AN, to whom any eating in a treatment setting could be accompanied by a sense of “loss of control.” In addition, although there is evidence supporting the validity of self-reported highest past weights (Swenne, et al., 2005; Tamakoshi, et al., 2003), validity of self-reported weight history has not been studied among adults with AN. The lack of a rigorously standardized treatment approach, despite the advantage of ecological validity, is also a limitation. While weight gain during treatment was controlled in prospective analyses, we are unable to rule out the possibility that differences in the treatment approach across patients may have been a confounding variable.
Though the predominance of Caucasian women in the sample reflects the higher rates of Caucasians who seek treatment for eating disorders (American Psychiatric Association, 1993; Becker, Franko, Speck, & Herzog, 2003), the generalizability of these findings to other populations cannot be assumed. Further, menstrual status data provided one marker of physiological abnormality, but we were unable to directly assess physiological variables (e.g., hormones, electrolyte levels) in the study sample. An additional limitation is the high level of attrition in prospective analyses (32% of the initial sample did not complete discharge self-report measures). It is possible that those who did and did not complete discharge self-report measures differ in some meaningful way; however, analyses comparing these subsamples on all variables of interest yielded no statistically significant differences except in length of stay, which was expected.
In addition to replication of the current findings, future research should attempt to identify factors that may explain the relatively better symptom levels at discharge of women with higher levels of weight suppression and lower BMIs at admission. Further investigations should also extend the present work by examining weight suppression, BMI, and their interaction as predictors of the course of illness after discharge including time to remission, as has been investigated in BN (Lowe, et al., 2011). Both weight suppression and BMI were related to menstrual status at admission, and additional studies should investigate, over a longer time period, whether admission absolute and relative weight statuses predict resumption of menses. As the results of Klein and colleagues (July 2011) would suggest, individuals high in weight suppression may need to reach a higher BMI in order to resume regular menstruation and other physiological functions compared to patients with comparable admission BMIs and lower levels of weight suppression. The identification of biological markers associated with weight suppression in AN also represents an important direction for future research.
Acknowledgments
The authors thank Dr. Douglas Bunnell, Dr. Susan Ice, and Abby Costello at the Renfrew Center for Eating Disorders, as well as the patients who participated in this study. The authors also acknowledge Dr. J. Graham Thomas for his expert statistical guidance. This work was supported in part by grant F31MH097406 (LAB) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
In the confirmed-diagnosis sample, the statistical significance of BMI in predicting EDI Drive for Thinness (Full model: β = .18, Part = .13, t(162) = 1.89, p = .06; Partial model: β = .21, Part = .16, t(163) = 2.14, p = .03) and of weight suppression in predicting discharge BMI (Full model: β = .18, Part = .13, t(247) = 2.43, p = .02; Partial model: β = .12, Part = .10, t(248) = 1.89, p = .06) differ slightly between the two models.
A total of 46.3% of the sample were classified as AN-BP subtype. Results of hierarchical logistic regressions indicated that patients with higher weight suppression and with higher BMIs were more likely to be categorized as AN-BP (b = .31, SE = .11, Wald = 8.57, p = .003, Exp (B) = 1.36; and b = .30, SE = .08, Wald = 14.17, p <.001, Exp (B) = 1.34, respectively), but the weight suppression X BMI interaction was not related to subtype (p = .782). All cross-sectional and prospective analyses were repeated adding AN subtype to step 1 of hierarchical regressions. AN subtype was a statistically significant covariate in almost all cross-sectional models (with exception of BDI-II analysis, p = .07); however, subtype was not a statistically significant covariate in any models predicting discharge variables.
References
- American Psychiatric Association. Practice guideline for eating disorders. American Journal of Psychiatry. 1993;150:208–228. doi: 10.1176/ajp.150.2.212. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fouth Edition (DSM-IV) Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fouth Edition, Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Practice Guideline for the Treatment of Patients With Eating Disorders. 3. Washington, D.C: American Psychiatric Association (APA); 2006. [Google Scholar]
- American Psychiatric Association. DSM-5 Development. 2012;2012 from http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=26. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (DSM-5) Washington. D.C: American Psychiatric Association; 2013. [Google Scholar]
- Attia E, Roberto CA. Should amenorrhea be a diagnostic criterion for anorexia nervosa? International Journal of Eating Disorders. 2009;42(7):581–589. doi: 10.1002/eat.20720. [DOI] [PubMed] [Google Scholar]
- Bailer UF, Kaye WH. Serotonin: imaging findings in eating disorders. Current Topics in Behavioral Neurosciences. 2011;6:59–79. doi: 10.1007/7854_2010_78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Becker AE, Franko DL, Speck A, Herzog DB. Ethnicity and differential access to care for eating disorder symptoms. International Journal of Eating Disorders. 2003;33(2):205–212. doi: 10.1002/eat.10129. [DOI] [PubMed] [Google Scholar]
- Butryn ML, Juarascio A, Lowe MR. The relation of weight suppression and BMI to bulimic symptoms. International Journal of Eating Disorders. 2011;44(7):612–617. doi: 10.1002/eat.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. Journal of Abnormal Psychology. 2006;115:62–67. doi: 10.1037/0021-843X.115.1.62. [DOI] [PubMed] [Google Scholar]
- Carter FA, McIntosh VV, Joyce PR, Bulik CM. Weight suppression predicts weight gain over treatment but not treatment completion or outcome in bulimia nervosa. Journal of Abnormal Psychology. 2008;117:936–940. doi: 10.1037/a0013942. [DOI] [PubMed] [Google Scholar]
- Clausen L, Rosenvinge J, Friborg O, Rokkedal K. Validating the Eating Disorder Inventory-3 (EDI-3): A Comparison Between 561 Female Eating Disorders Patients and 878 Females from the General Population. Journal of Psychopathology and Behavioral Assessment. 2011;33(1):101–110. doi: 10.1007/s10862-010-9207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Coners H, Remschmidt H, Hebebrand J. The relationship between premorbid body weight, weight loss, and weight at referral in adolescent patients with anorexia nervosa. International Journal of Eating Disorders. 1999;26(2):171–178. doi: 10.1002/(sici)1098-108x(199909)26:2<171::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Dalle Grave R, Calugi S, Marchesini G. Is amenorrhea a clinically useful criterion for the diagnosis of anorexia nervosa? Behaviour Research and Therapy. 2008;46(12):1290–1294. doi: 10.1016/j.brat.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Eddy KT, Keel PK, Dorer DJ, Delinsky SS, Franko DL, H DB. Longitudinal comparison of anorexia nervosa subtypes. International Journal of Eating Disorders. 2002;31:191–201. doi: 10.1002/eat.10016. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin S. Eating Disorder Examination Questionnaire (EDE-Q 6.0) In: Fairburn CG, editor. Cognitive behavior therapy and eating disorders. New York, NY: Guilford Press; 2008. pp. 309–313. [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. International Journal of Eating Disorders. 1994;16(4):363–337. [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The eating disorders examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. 12. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- Garfinkel PE, Moldofsky H, Garner DM. The heterogeneity of anorexia nervosa: bulimia as a distinct subgroup. Archives of General Psychiatry. 1980;37(9):1036–1040. doi: 10.1001/archpsyc.1980.01780220074008. [DOI] [PubMed] [Google Scholar]
- Garner DM. Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2004. Eating Disorder Inventory-3. [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The Eating Attitudes Test: Psychometric features and clinical correlates. Psychological Medicine. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- Golden NH, Jacobson MS, Schebendach J, Solanto MV, Herz SM, Shenker IR. Resumption of menses in anorexia nervosa. Archives of Pediatrics and Adolescent Medicine. 1997;151(1):16–21. doi: 10.1001/archpedi.1997.02170380020003. [DOI] [PubMed] [Google Scholar]
- Herzog DB, Thomas JG, Kass AE, Eddy KT, Franko DL, Lowe MR. Weight suppression predicts weight change over 5 years in bulimia nervosa. Psychiatry Research. 2010;177(3):330–334. doi: 10.1016/j.psychres.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog T, Zeeck A, Hartmann A, Nickel T. Lower targets for weekly weight gain lead to better results in inpatient treatment of anorexia nervosa: a pilot study. European Eating Disorders Review. 2004;12(3):164–168. [Google Scholar]
- Keel PK, Heatherton TF. Weight suppression predicts maintenance and onset of bulimic syndromes at 10-year follow-up. Journal of Abnormal Psychology. 2010;119(2):268–275. doi: 10.1037/a0019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. Vol. 2. Minneapolis, MN: The University of Minnesota Press; 1950. [Google Scholar]
- Klein DA, Siegel M, Grunebaum Z, Wang Y, Chen H, Walsh BT. Leptin in Anorexia Nervosa: Relationship to Physical Activity and Weight Suppression. Paper presented at the The Annual Meeting of the Society for the Study of Ingestive Behavior; Clearwater, FL. Jul, 2011. [Google Scholar]
- Klein DA, Walsh BT. Eating disorders: clinical features and pathophysiology. Physiology & Behavior. 2004;81:359–374. doi: 10.1016/j.physbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Lay B, Jennen-Steinmetz C, Reinhard I, Schmidt MH. Characteristics of inpatient weight gain in adolescent anorexia nervosa: relation to speed of relapse and readmission. European Eating Disorders Review. 2002;10(1):22–40. [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Leonard D, Mehler PS. Medical issues in the patient with anorexia nervosa. Eating Behaviors. 2001;2(4):293–305. doi: 10.1016/s1471-0153(01)00058-7. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, et al. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite. 2006;47(1):83–90. doi: 10.1016/j.appet.2006.03.160. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Berner LA, Swanson SA, Clark VL, Eddy KT, Franko DL, et al. Weight suppression predicts time to remission from bulimia nervosa. Journal of Consulting & Clinical Psychology. 2011;79(6):772–776. doi: 10.1037/a0025714. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Davis W, Lucks D, Annunziato R, Butryn M. Weight suppression predicts weight gain during inpatient treatment of bulimia nervosa. Physiology & Behavior. 2006;87:487–492. doi: 10.1016/j.physbeh.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Thomas JG, Safer DL, Butryn ML. The relationship of weight suppression and dietary restraint to binge eating in bulimia nervosa. International Journal of Eating Disorders. 2007;40:640–644. doi: 10.1002/eat.20405. [DOI] [PubMed] [Google Scholar]
- Lund BC, Hernandez ER, Yates WR, Mitchell JR, McKee PA, Johnson CL. Rate of inpatient weight restoration predicts outcome in anorexia nervosa. International Journal of Eating Disorders. 2009;42(4):301–305. doi: 10.1002/eat.20634. [DOI] [PubMed] [Google Scholar]
- MacLean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301(3):R581–R600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka N, Yoshiuchi K, Yamanaka G, Sasaki T, Kumano H, Kuboki T. Relations among premorbid weight, referral weight, and psychological test scores for patients with anorexia nervosa. Psychological Reports. 2003;92(1):67–74. doi: 10.2466/pr0.2003.92.1.67. [DOI] [PubMed] [Google Scholar]
- Monteleone P, DiLieta A, Castaldo E, Maj M. Leptin functioning in eating disorders. CNS Spectrums. 2004;9(7):523–529. doi: 10.1017/s1092852900009615. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, & National Heart, Lung, & Blood Institute. Obesity. Washington, D. C: Department of Health and Human Services; 1998. [Google Scholar]
- Pai M, Paloucek F. The origin of the “ideal” body weight equations. The Annals of Pharmacotherapy. 2000;34(9):1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- Peterson CB, Crosby RD, Wonderlich SA, Joiner T, Crow SJ, Mitchell JE, et al. Psychometric properties of the eating disorder examination-questionnaire: Factor structure and internal consistency. International Journal of Eating Disorders. 2007;40(4):386–389. doi: 10.1002/eat.20373. [DOI] [PubMed] [Google Scholar]
- Roberto CA, Steinglass J, Mayer LES, Attia E, Walsh BT. The clinical significance of amenorrhea as a diagnostic criterion for anorexia nervosa. International Journal of Eating Disorders. 2008;41:559–563. doi: 10.1002/eat.20534. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. International Journal of Obesity. 2010;34(S1):S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, et al. Criterion validity, severity cut scores, and test-retest reliability of the Beck Depression Inventory-II in a university counseling center sample. Journal of Counseling Psychology. 2002;49(3):381–385. [Google Scholar]
- Steer R, Ball R, Ranieri WF, Beck AT. Further evidence for the construct validity of the Beck Depression Inventory-II with psychiatric outpatients. Psychological Reports. 1997;80(2):443–446. doi: 10.2466/pr0.1997.80.2.443. [DOI] [PubMed] [Google Scholar]
- Stice E, Durant S, Burger KS, Schoeller DA. Weight suppression and risk for future increases in body mass: effects of suppressed resting metabolic rate and energy expenditure. American Journal of Clinical Nutrition. 2011;94(1):7–11. doi: 10.3945/ajcn.110.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenne I, Belfrage E, Thurfjell B, Engström I. Accuracy of reported weight and menstrual status in teenage girls with eating disorders. International Journal of Eating Disorders. 2005;38(4):375–379. doi: 10.1002/eat.20199. [DOI] [PubMed] [Google Scholar]
- Tamakoshi K, Yatsuya H, Kondo T, Hirano T, Hori Y, Yoshida T, et al. The accuracy of long-term recall of past body weight in Japanese adult men. International Journal of Obesity and Related Metabolic Disorders. 2003;27:247–252. doi: 10.1038/sj.ijo.802195. [DOI] [PubMed] [Google Scholar]
- Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatrica Scandinavica. 2003;108(3):175–182. doi: 10.1034/j.1600-0447.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD. Weight suppression as a predictor of weight gain and response to intensive behavioral treatment in patients with anorexia nervosa. [doi: 10.1016/j.brat.2012.02.006] Behaviour Research and Therapy. 2012;50(4):266–274. doi: 10.1016/j.brat.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer MG, Thuras P, Crow SJ. Implications of the changing use of hospitalization to treat anorexia nervosa. The American Journal of Psychiatry. 2005;162(12):2374–2376. doi: 10.1176/appi.ajp.162.12.2374. [DOI] [PubMed] [Google Scholar]
- Wolk SL, Loeb KL, Walsh BT. Assessment of patients with anorexia nervosa: interview versus self-report. International Journal of Eating Disorders. 2005;37(2):92–99. doi: 10.1002/eat.20076. [DOI] [PubMed] [Google Scholar]
- Zunker C, Crosby RD, Mitchell JE, Wonderlich SA, Peterson CB, Crow SJ. Weight suppression as a predictor variable in treatment trials of bulimia nervosa and binge eating disorder. International Journal of Eating Disorders. 2010;44(8):727–730. doi: 10.1002/eat.20859. doi:10.1002/eat.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]