Abstract
Background
Coherent perception of the visual world requires orderly processing of spatially and temporally distributed visual information across the visual field. The organization of this visual information is impaired in schizophrenia. We previously found that visual temporal integration in patients is prolonged, using flashes presented to the central fovea. In this study, we investigated this temporal interaction in both the fovea and fairly far out in the peripheral visual field.
Methods
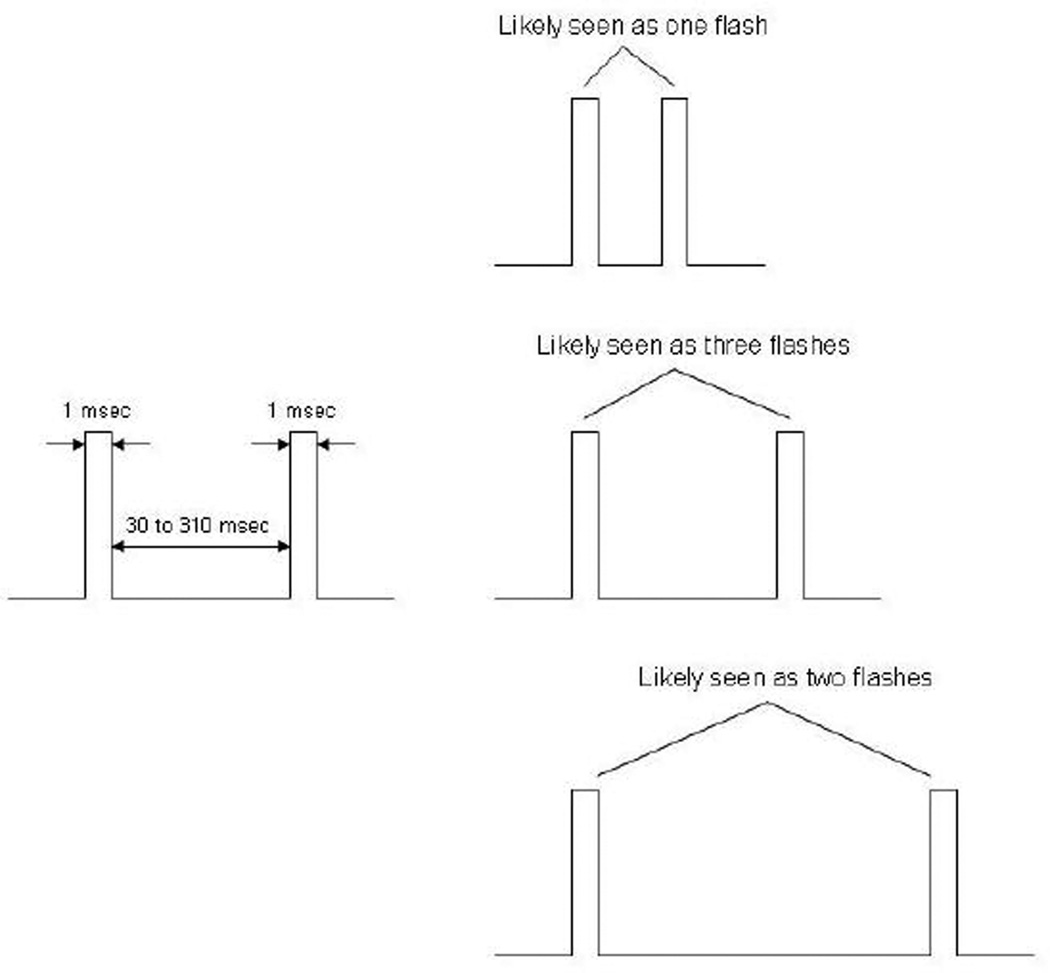
We used a ‘three-flash’ illusion paradigm in which two spatially-coincident light pulses (of 1 msec each) are perceived by healthy individuals as one, two or three flashes depending on the time interval between the pulses. In each trial, two light pulses were presented in the fovea or 34° out in the right visual field. The inter-stimulus pulse interval (ISI) ranged from 30 to 310 msec. The task for patients (n=28) and controls (n=26) was to indicate the number of flashes (one, two or three) perceived after each two-pulse presentation.
Results
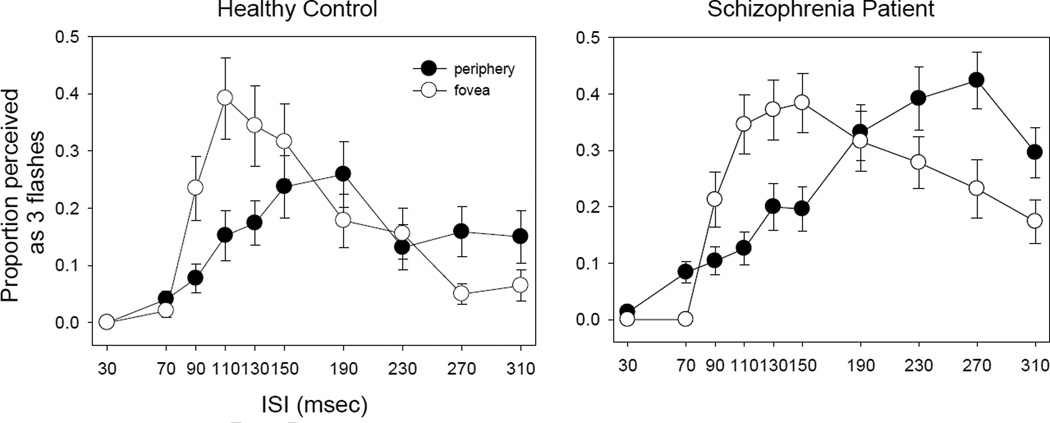
For the controls, the peak of three-flash illusion were shifted to longer ISIs (150 msec) in the periphery compared to the fovea (110 msec). For the patients, the three-flash illusion was greater and occurred at longer ISIs (270 msec in the periphery and 190 msec at the fovea).
Conclusion
Compared to central visual field, the range of temporal interactions in the periphery is prolonged to a greater extent in schizophrenia. This exacerbated temporal expansion in peripheral vision suggests a coarse temporal resolution for visual and cognitive organization in this mental disorder.
Keywords: schizophrenic, temporal processing, periphery, fovea, vision, perceptual organization
Introduction
The cardinal symptom of schizophrenia is disorganized thought and behavior, but we know much less about the basic perceptual and cognitive mechanisms that potentially contribute to this disorganization. Schizophrenia patients have deficient processing of visual signals (Butler et al. 2011; Green et al. 2009; Silverstein and Keane 2011). To provide sensory inputs for coherent thoughts and behaviors, visual processing must integrate spatially and temporally distributed information. This integration requires that visual information is gathered from large regions of the visual field, often well outside of the fovea.
Understanding this spatiotemporal integration in schizophrenia may shed light on where visual integration begins to fall apart. There are a number of studies done in the fovea on deficient processing of dynamic visual information in schizophrenia (Carroll et al. 2008; Chen 2011; Chen et al. 1999a; Chen et al. 2003; Chen et al. 1999b; Norton et al. 2008; O'Donnell et al. 1996; Parsons et al. 2013; Stuve et al. 1997). But surprisingly little is known about these processes in the peripheral field, where global and dynamic visual signals are preferentially processed.
Visual processes have different spatial and temporal characteristics in the central vs. peripheral fields. The periphery has a coarse resolution for processing spatial information. But for processing temporal information, the periphery appears to have a higher resolution than does the fovea, as shown in several ways. First, critical fusion frequency (CFF), or the highest flicker rate resolvable, increases towards the periphery (Hartmann et al. 1979; Nakayama and Tyler 1981). Second, temporal contrast modulation sensitivity extends to higher temporal frequency (Allen and Hess 1992; McKee and Taylor 1984). Third, temporal resolution as measured by the minimum perceivable temporal gap between two light pulses increases with eccentricity (providing the spatial size of the stimuli is scaled larger at greater eccentricity to compensate for cortical magnification) (Poggel et al. 2006). The periphery thus responds better than the fovea to larger spatial objects and faster temporal changes. These spatial and temporal properties allow visual processing in the periphery to serve as a rough spatial guide but a fine temporal meter to alert about major changes in the part of visual world that is not the current focus of attention.
Several studies have examined peripheral visual processing in schizophrenia, using either detection tasks or search tasks that engage multiple target positions. Two of the studies (Elahipanah et al. 2010; Granholm et al. 1996) showed that patients had degraded performance for detecting peripheral visual targets, but another study (Miller et al. 1990) showed relatively intact visual detection in periphery. Interestingly, under backward masking conditions, both patients (Butler et al. 1996; Green et al. 1994a) and their siblings (Green et al. 1997; Green et al. 2006; Sponheim et al. 2013) showed impairments in perception of the target location and target identification, with the targets presented at multiple possible peripheral locations. The impaired performances occurred primarily when temporal intervals between target and mask were brief (20 to 80 msec). These masking results are consistent with the notion that spatial and temporal processing in schizophrenia is altered within the peripheral field.
There are two critical issues regarding these studies on peripheral visual processing in schizophrenia. First, there was no direct comparison of temporal processing in the periphery versus fovea (Green et al. 1994b; Norton et al. 2008). Second, there was a possible confounding effect of spatial uncertainty, since the targets were presented into multiple possible spatial locations (Green et al. 1994b; Kraehenmann et al. 2012). The impaired performance might thus partly reflect difficulties of attending to many targets/locations at once. In the present study, we directly compare visual temporal processing in the fovea versus periphery, using a single stimulus that removes the problem of spatial uncertainty.
In an earlier study, we measured temporal interactions in the fovea in schizophrenia patients using the three-flash illusion paradigm (Norton et al. 2008). In this paradigm, two brief light pulses are sequentially presented in the same position, and the perception is that of either 1, 2 or 3 flashes. The three-flash illusion refers to the perception of three flashes while only two light pulses are presented (Bowen 1989). The number of flashes perceived is presumably determined by the interaction between the two separate temporal impulse response functions (IRF) generated by each of the two light pulses. The response to the light pulses provide information about the functional role of the IRF and the integration of the two IRFs produced by the two pulses (see Discussion), which include both linear and non-linear temporal processing of the visual system.
Our foveal study of three-flash illusion suggests that patients have prolonged temporal responses. In the present study, we compared the three-flash illusion in the fovea versus periphery, using both patients and healthy controls. Our working hypothesis is that the three-flash illusion 1) will occur at shorter ISIs for peripheral than for foveal targets (assuming that temporal processing is relatively fast in periphery), and 2) will occur at longer ISIs for patients than for controls (assuming that sluggish temporal processing is implicated in schizophrenia).
Methods and Materials
Subjects
Twenty-eight patients and 26 non-psychiatric controls participated in this study. The patients met criteria for schizophrenia or schizoaffective disorder based on an interview by independent clinicians using the SCID-IV (First, Spitzer, Gibbon, & Williams, 2002) and all available medical records. The clinicians were trained research assistants, psychologists, and psychiatrists. The patients were either inpatients from the Schizophrenia and Bipolar Disorders Program at McLean Hospital (n=6) or were outpatients who received treatment from McLean Hospital or local clinics (n=22). Fourteen of the patients had the diagnosis of schizophrenia and the other fourteen had the diagnosis of schizoaffective disorder (eight were bipolar-type and the other six were depressive-type). All patients were taking antipsychotic medication (mean chlorpromazine equivalent was 537.2 mg (SD: 358.1 mg) (Davis 1974; Woods 2003). Their average duration of illness was 18.0 years (10.4 years). The patients’ PANSS scores for the positive, negative and general scales ranged from 7 to 29 (mean: 15.8 (6.8)), 7 to 30 (mean: 13.6 (5.9)) and 16 to 48 (mean: 29.4 (10.6)), respectively.
Healthy controls were screened for Axis 1 disorders using an interview based on the SCID N/C (First et al. 2002). Patients and controls were excluded if they had recent (6 months) drug or alcohol abuse, or a history of brain injury or neurological disorders (such as seizure or stroke). The protocol was approved by Mclean Hospital Institution Review Board. All subjects signed informed consent in accordance with the IRB guidelines.
Demographic information is provided in Table 1. Patients and controls did not differ in age or sex. The groups were significantly different in terms of education (t = 2.4, p = 0.02) and IQ (t = 2.3, p = 0.02). Visual acuity of the participants was shown to be normal using Rosenbausm Pocket Eye Chart. The same groups of subjects had participated in our previous foveal study (Norton et al. 2008), but was tested for this study in a peripheral visual field as well as at the fovea with briefer light pulses (see below).
Table 1.
Demographic Information of the Sample
| Demographic variable Group |
Sex | Age | Verbal IQ* | Education* | Parental Education |
|---|---|---|---|---|---|
| Healthy Controls | F = 16 M = 10 |
43.0 (13.8) | 108.0 (9.2) | 15.9 (2.3) | 13.5 (2.6) |
| Schizophrenia Patients | F = 17 M = 11 |
40.3 (9.9) | 100.8 (12.3) | 14.1 (2.0) | 13.9 (2.1) |
Means are reported above standard deviations. Education and age are in years. Verbal IQ was measured using the WAIS – R (Wechsler 1981). Asterisk denotes significant group difference (p < 0.05).
Procedure
Light pulses
Light pulses were generated with a red light emitting diode (LED) which allows precise temporal control within the micro-second range. A Macintosh G3 system controlled the visual stimuli and recorded the subject’s responses. On each trial, light pulses of 1 msec were presented twice, with an inter-stimulus interval (ISI) of either 30, 70, 90, 110, 130, 150, 190, 230, 270 or 310 msec (Figure 1). There were 100 trials for the periphery and 100 trials for the fovea, with 10 trials devoted to each ISI in a quasi-random order. The whole procedure lasted 20 to 25 minutes.
Figure 1.
Schematic illustration of two light pulses (left) and their perception (right). The perception of one, two or three flashes, as depicted at top, bottom or middle panel, corresponds to short, long or medium inter-stimulus interval.
The LED was a nearly uniform disc (subtending 17 min arc) with a peak luminance 35 cd/m2. In separate runs, the LED target was presented at the fovea and the 34° right visual field (i.e. at 45 cm from fixation at a viewing distance of 66.7 cm).
Task
The LED target was viewed in a dimly-illuminated room. A fixation point was provided for the eccentric LED target. The fixation target was visible at all times and thus could be fixated well.) Subjects were instructed to press a button to initiate the presentation of the pulses, when they were fixating well. Under all conditions the pulse was clearly visible at a relatively high suprathreshold level As discussed earlier, all subjects were well practiced in the task.. The subject on each trial pressed buttons to indicate whether they saw 1, 2 or 3 flashes.
The dependent variable here was the perceived flash number in each trial.
Results
Three-flash illusion at the fovea and in periphery
The three-flash illusion differs significantly between patients and controls (Figure 2). A three way ANOVA (group × ISI × field) of 3 flash data showed significant effects on group (F=19.8, p<0.001) and ISI (F=14.5, p<0.001), but not on field (F=0.000, p=0.98). The interaction was significant between ISI and field (F=7.06, p<0.001), but not between group and ISI (F=2.37, p=0.12) or between group and field (F=0.48, p=0.49). The three way interaction was not significant (F=0.21, p=0.99).
Figure 2.
Comparison of three-flash illusion in the central and the peripheral fields. For both panels, x axis indicates interstimulus interval (ISI) whereas y axis indicates proportion of trial perceived as three flashes. The left panel is for controls and the right panel is for patients. The error bars represent ±1 standard error.
This analysis indicates that three-flash illusion differs between patients and controls but difference similarly occurs at the fovea and in the periphery (Figure 2). A combination of the absence of interactions between group and ISI or between group and field and the presence of group effect suggests similar alterations of temporal integration between the visual fields and across ISIs in patients. The significant interaction between field and ISI indicates that three-flash illusions at the fovea and in the periphery occur at different ISIs. The subsequent two sections will examine the responses for the fovea and the periphery respectively, comparing patients and control at each field position.
Perception of light pulse at the fovea
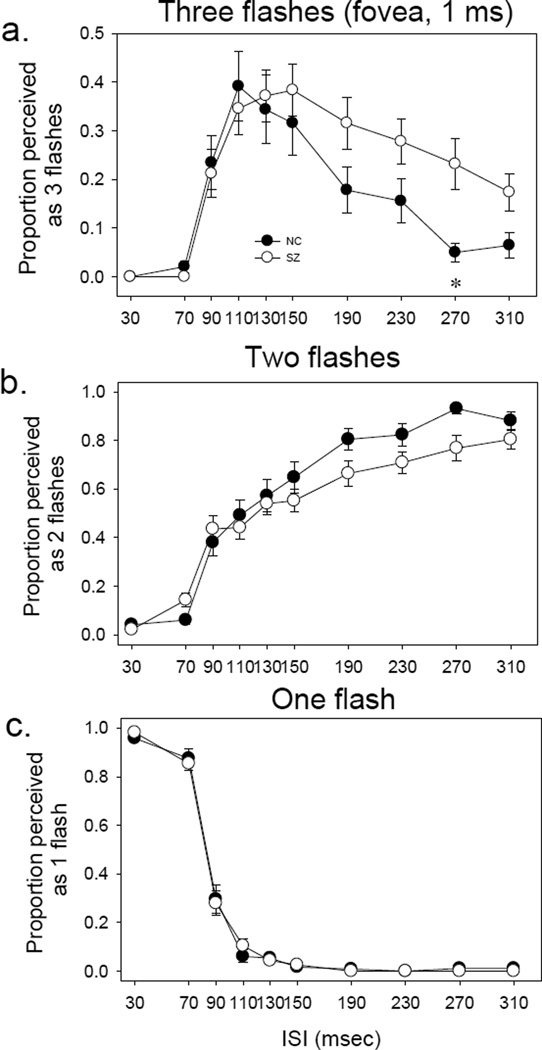
In Figure 3, each panel shows respectively the number of judgments of seeing 3 flashes, 2 flashes and 1 flash, as a function of ISI. For healthy controls, the three-flash illusion occurred frequently at intermediate ISIs (110 to 150 msec, the top panel). For patients, the three-flash illusion was more prominent, and occurred at somewhat longer ISIs (110 to 230 msec).
Figure 3.
Perception of one, two or three flashes in the central visual field. In all panels (a, b, and c), x axis indicates interstimulus interval (ISI) whereas y axis indicates the proportion of trial perceived as one (bottom), two (middle) or three (top) flashes. Note that the scale of y axis for panel a is amplified by a factor of two as compared to that for panels b and c, to better illustrate the three-flash illusion. The error bars represent ±1 standard error.
A three way ANOVA (group × ISI × flash number) showed a significant effect on flash # (F=307.7, p<0.001), but not on group (F=0.005, p=0.944) or ISI (F=0.183, p=0.996). The interaction was significant between group and flash # (F=4.32, p=0.013) and between ISI and flash # (F=113.2, p<0.001), but not between group and ISI (F=0.07, p=1.00). The three way interaction was not significant (F=1.34, p=0.15). This indicates that the perception of one (merging of two visual event), two (separate processing of two visual events) and three (interaction of two visual events) flashes differs from each other, and this difference is not the same between patients and controls.
A two way ANOVA (group × ISI) showed that for the ‘three flashes’, the main effects were significant for group (patient vs. control) (F=4.44, p=0.036) and for ISI (F=16.36, p<0.001), but the group-ISI interaction was not significant (F=1.06, p=0.38). Post hoc t tests yielded a significant group difference at 270 msec (t=2.52, p=0.016). The analyses indicated that patients had significant greater three-flash illusion at the fovea than did healthy controls.
The result of ‘two flashes’ is shown in the middle panel of Figure 3. ANOVA showed a significant main effect on ISI (F=57.25, p<0.001) (Figure 2b), but the group effect was not significant (F=2.62, p=0.106), nor was the group-ISI interaction (F=1.04, p=0.41).
The result of ‘one flash’ is shown in the bottom panel of Figure 3. ANOVA showed a significant main effect on ISI (F=238.79, p<0.001) (Figure 2c). The group effect was not significant (F=0.20, p=0.65), nor was the group-ISI interaction (F=0.30, p=0.97).
These results for the fovea replicate the general features of our previous study which was conducted solely at the fovea (Norton et al. 2008).
Perception of light pulse in periphery
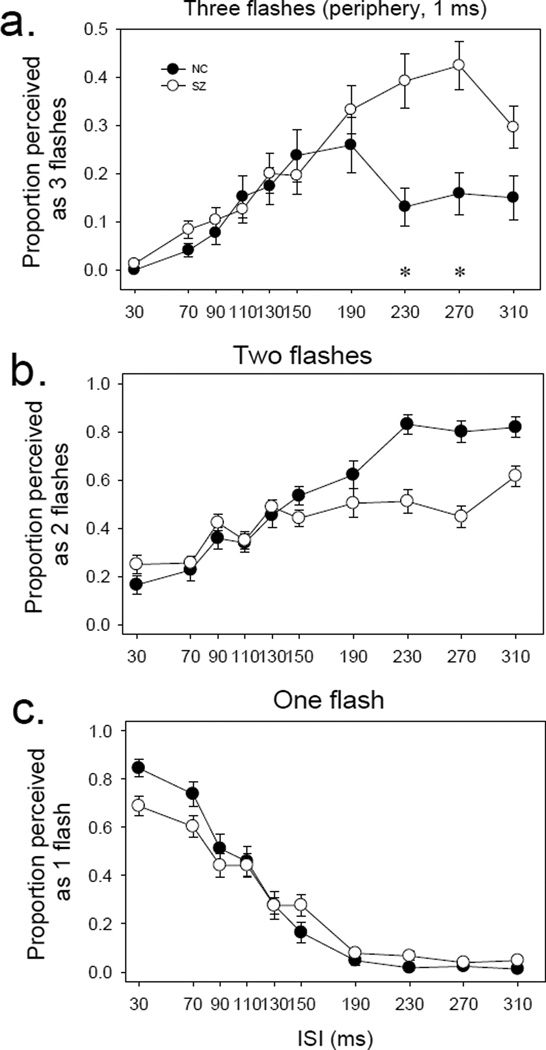
Figure 4, presented in a similar format, shows the results for the periphery. For healthy controls, the three-flash illusion occurred mostly at intermediate ISIs (150 to 190 msec) (Figure 3, top panel). The three-flash illusion was more prominent in patients, especially at long ISIs (230 to 310 msec).
Figure 4.
Perception of one, two or three flashes in the periphery. In all panels (a, b and c), x axis indicates inter-stimulus interval (ISI) whereas y axis indicates proportion of trial perceived as one (bottom), two (middle) or three (top) flashes. Note that the scale of y axis for panel a is amplified by a factor of two as compared to that for panels b and c, to better illustrate the three-flash illusion. The error bars represent ±1 standard error.
A three way ANOVA (group × ISI× flash #) showed significant effects on group (F=86.4, p<0.001), ISI (F=2.37, p=0.012) and flash # (F=38.7, p<0.001). The interaction was significant between group and ISI (F=2.43, p=0.01), between group and flash # (F=57.1, p<0.001) and between ISI and flash # (F=41.19, p<0.001). The three way interaction was not significant (F=0.81, p=0.69). This indicates that the perceptions of one (merging of two visual events), two (separate processing of two visual events) and three (temporal interaction of two visual events) flashes differs from each other, and this difference is not the same between patients and controls.
ANOVA on 3 flash data showed a significant effect on group (patient vs. control) (F=11.0, p=0.001) and on ISI (F=10.13, p<0.001). The group-ISI interaction was not significant (F=1.57, p=0.12). Post hoc t test indicated a significant group difference at the ISIs of 230 msec (t=2.16, p=0.037) and 270 msec (t=3.05, p=0.004). The analyses indicated that patients had significant greater three-flash illusion in the periphery than did healthy controls.
For the result of ‘two flashes’, ANOVA showed a significant main effect of group (F=181.99, p<0.001) and of ISI (F=8.21, p<0.001) (Figure 4, middle panel). But group-ISI interaction was not significant (F=1.11, p=0.36).
For the result of ‘one flash’, ANOVA showed a significant effect of ISI (F=68.52, p<0.001) (Figure 4, bottom panel). But the effect of group was not significant (F=0.95, p=0.33), nor was the group-ISI interaction (F=1.38, p=0.19).
Relationship between three-flash illusion and non-visual variables
For the patients, there was no significant correlation between either the PANSS subscales (positive, negative, general or conceptual disorganization) or CPZ and the peak prevalence of three-flash illusion for ISI at the fovea (150 msec) or in periphery (270 msec). The peak prevalence of the three-flash illusion was not correlated with the age or sex of the patients.
Discussion
We found that the temporal interaction of two peripheral visual pulses was shifted to a longer time scale in schizophrenia. Going between the fovea and the 34° right visual field, the peak of three-flash illusion changed from the ISIs of 110 to 150 msec in controls, and 190 to 270 msec in patients. The patients had more reports of the three-flash illusion at long ISIs (230 and 270 msec in periphery and 270 msec at the fovea). This temporal prolongation suggests that interactions of peripheral visual processes in schizophrenia have a coarse temporal resolution.
Visual processing deficits have been extensively demonstrated in schizophrenia (Butler and Javitt 2005; Butler et al. 2008; Chen et al. 2006), including those in the processing of temporal information (Carroll et al. 2008; Green et al. 1994c; Norton et al. 2008; Swerdlow et al. 2014; Wynn et al. 2013). However, there has been little focus on the processing outside the fovea, especially for dynamic temporal stimuli. This is surprising, for the processing of dynamic visual information plays a key role in peripheral vision.
Our initial expectation was that in the periphery, the three-flash illusion would peak at a shorter inter-stimulus interval (ISI), since the periphery typically has a faster temporal response than the fovea. But the present results were in the opposite direction: for healthy controls, the peak of three-flash illusion shifted from an ISI of 110 to 150 msec in going between the foveal location and the 34° periphery. This shift was greater for schizophrenia patients – from 190 msec at the fovea to 270 msec in the periphery.
There may be two interpretations for this unexpected result. First, the results showing a faster peripheral response are generally obtained with low contrast stimuli near threshold (Kelly 1961; Ninomiya et al. 1989) whereas the three-flash illusion here was only obtained with highly supra-threshold stimuli. Bowen, who discovered three-flash illusion, argued that the impulse response function (IRF) must be at least triphasic or of higher order (well above threshold) to account for the three-flash illusion (Bowen 1989). Note that going from a biphasic (near threshold) to a triphasic IRF represents a distinct nonlinearity of visual processing. For a linear system, increasing the contrast of the stimulus would simple scale the amplitude height of the IRF but would not change either the shape of the IRF (e. g. from biphasic to triphasic) or the duration of the IRF. The prolongation of the temporal window of the three flash illusions in our patients thus might reflect a nonlinearity problem - both a temporal dilation of the IRF and a change of shape (where the third and higher-order lobe of the IRF grows in relative size). Neurally, the IRF is determined by early processing in the visual system, i.e. in the retina (Buttner et al. 1975) and possibly the striate cortex (Chebkasov 1985). Thus, patients’ altered three-flash illusion shown here implicates an early visual processing problem,
Second, the three-flash illusion represents an integration of the two separate IRFs. But most of the faster peripheral responses measured in previous studies involved little temporal integration. So, the integration process itself might be prolonged in the periphery. Indeed, it was reported that two-pulse temporal integration functions were more prolonged in periphery compared to the fovea (MacAvoy et al. 1991). This is consistent with our results, except that the difference between the foveal and peripheral locations was further magnified in schizophrenia patients.
Peripheral visual processing is normally global and dynamic. The slowing down of peripheral visual processing in schizophrenia is consistent with the previous findings of selective impairment in the visual tasks involving global and dynamic stimuli (Chen et al. 2003; Chen et al. 1999b; O'Donnell et al. 1996). Global and dynamic visual signals are preferentially processed in the magno pathway, where neurons have large receptive fields and fast temporal response (Derrington and Lennie 1984; Livingstone and Hubel 1988a). It has been shown that magno neurons are increasingly represented with eccentricity (Azzopardi et al. 1999; Connolly and Van Essen 1984) (but also see (Livingstone and Hubel 1988b)). Magno pathway deficit has been hypothesized and shown as a major sensory mechanism for schizophrenia (Butler et al. 2001; Javitt 2009; Martinez et al. 2008). The present results showing a prolonged three-flash illusion in the periphery of our patients may thus implicate the magno pathways which are robustly represented in periphery.
The patients’ prolonged three-flash illusion in the periphery might affect comparison of dynamic stimuli across different positions of the visual field. It might blur the boundary between the visual events of which precise timing is critical. During motion-related behavioral activities such as driving and catching moving objects, critical visual inputs are received not only from central but also from peripheral visual fields. Sluggish temporal integration of visual inputs across fields would provide erroneous signals for subsequent perceptual and cognitive decision processes and lead to a delay in rendering judgments that are critically needed for the navigation of moving vehicles and targets. This requires further testing.
In summary, the present study showed a clear prolongation of the three-flash illusion in the periphery. This raises more questions. First, whether the temporal prolongation increases at yet further eccentricity needs to be evaluated. Second, larger size targets ought to be tested to compensate for changes in cortical size with eccentricity (Levi et al. 1985; Strasburger et al. 2011). Third, the correlation of the temporal prolongation and severity of psychotic symptoms ought to be further checked with larger sample size, since the previous study of the illusion in the fovea showed a positive correlation but the present study did not. Note that the clinical measures used here are largely static in nature whereas the visual temporal processing measure is dynamic in nature. It would be useful in future studies to assess patients’ ability to engage daily activities that continuingly change with time such as social interactions. Comparisons of visual temporal interactions and social functioning in patients may yield new insights. Fourth, the present patients were treated with antipsychotic medications. Although the medication doses (indexed by CPZ) were not significantly correlated with the size of the three-flash illusion, the exact role that the medications play in visual temporal processing remains unspecified.
Concluding remarks
This study found that temporal integration in periphery is substantially protracted in schizophrenia. This slowing-down process occurs in the purely temporal processing domain, and thus cannot be accounted by diverted attention of spatial context or attention to multiple target locations as in previous studies involving periphery visual field. The temporal delay in periphery may contribute to the disorganization of spatially and temporally distributed visual and cognitive information in patients.
Acknowledgment
This work was supported in part by NIH grant (R01 MH 096793). We thank Dr. Ken Nakayama for discussion in the early stage of the study and Dr. Dost Ongur for supervision of clinical evaluations. We also thank anonymous reviewers for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors declare no conflict of interest, financial or otherwise.
Contributions
YC designed the study, performed data analysis and wrote the first draft of the paper. DN tested subjects, performed data analysis and revised the paper. CS designed the study and revised the paper. All authors approved the final version of the paper.
References
- Allen D, Hess RF. Is the visual field temporally homogeneous? Vision Res. 1992;32(6):1075–1084. doi: 10.1016/0042-6989(92)90008-7. [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Jones KE, Cowey A. Uneven mapping of magnocellular and parvocellular projections from the lateral geniculate nucleus to the striate cortex in the macaque monkey. Vision Res. 1999;39(13):2179–2189. doi: 10.1016/s0042-6989(98)00319-8. [DOI] [PubMed] [Google Scholar]
- Bowen RW. Two pulses seen as three flashes: a superposition analysis. Vision Research. 1989;29(4):409–417. doi: 10.1016/0042-6989(89)90005-9. [DOI] [PubMed] [Google Scholar]
- Butler PD, Chen Y, Ford JM, Geyer MA, Silverstein SM, Green MF. Perceptual Measurement in Schizophrenia: Promising Electrophysiology and Neuroimaging Paradigms From CNTRICS. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40(4):295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18(2):151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158(7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64(1):40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner U, Grusser OJ, Schwanz E. The effect of area and intensity on the response of cat retinal ganglion cells to brief light flashes. Exp Brain Res. 1975;23(3):259–278. doi: 10.1007/BF00239739. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O'Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67(2):150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebkasov SA. Spatial distribution of activity of the neurons of the visual cortex during stimulation with a flash of diffused light. Neurosci Behav Physiol. 1985;15(4):296–303. doi: 10.1007/BF01185291. [DOI] [PubMed] [Google Scholar]
- Chen Y. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr Bull. 2011;37(4):709–715. doi: 10.1093/schbul/sbr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Norton D. Trait vs. state markers for schizophrenia: identification and characterization through visual processes. Current Psychiatry Reviews. 2006;2:431–438. doi: 10.2174/157340006778699729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci U S A. 1999a;96(8):4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophrenia Research. 2003;61(2–3):215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999b;56(2):149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- Connolly M, Van Essen D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J. Comp Neurol. 1984;226(4):544–564. doi: 10.1002/cne.902260408. [DOI] [PubMed] [Google Scholar]
- Davis JM. Dose equivalence of the antipsychotic drugs. Journal of Psychiatric Research. 1974;11:65–69. doi: 10.1016/0022-3956(74)90071-5. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, Reingold EM. Visual search performance among persons with schizophrenia as a function of target eccentricity. Neuropsychology. 2010;24(2):192–198. doi: 10.1037/a0017523. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JB. Structure Clinical Interview for DSM -IV-TR Axis I Disorders - Non-patient Edition (SCID-I/NP, 11/2002 revision) New York, NY: Biometric Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- Granholm E, Asarnow RF, Marder SR. Display visual angle and attentional scanpaths on the span of apprehension task in schizophrenia. J Abnorm Psychol. 1996;105(1):17–24. doi: 10.1037//0021-843x.105.1.17. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception Measurement in Clinical Trials of Schizophrenia: Promising Paradigms From CNTRICS. Schizophr Bull. 2009;35(1):163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54(5):465–472. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and backward visual masking in unaffected siblings of schizophrenic patients. Biol Psychiatry. 2006;59(5):446–451. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994a;51(12):939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Archives of General Psychiatry. 1994b;51(12):939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Archives of General Psychiatry. 1994c;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Lachenmayr B, Brettel H. The peripheral critical flicker frequency. Vision Res. 1979;19(9):1019–1023. doi: 10.1016/0042-6989(79)90227-x. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DH. Visual responses to time-dependent stimuli, I: amplitude sensitivity measurements. Journal of the Optical Society of America. 1961;51:422–429. doi: 10.1364/josa.51.000422. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, Vollenweider FX, Seifritz E, Kometer M. Crowding deficits in the visual periphery of schizophrenia patients. PLoS One. 2012;7(9):e45884. doi: 10.1371/journal.pone.0045884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Res. 1985;25(7):963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988a;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Do the relative mapping densities of the magno- and parvocellular systems vary with eccentricity? J Neurosci. 1988b;8(11):4334–4339. doi: 10.1523/JNEUROSCI.08-11-04334.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1(1):95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SP, Taylor DG. Discrimination of time: comparison of foveal and peripheral sensitivity. J Opt Soc Am A. 1984;1(6):620–627. doi: 10.1364/josaa.1.000620. [DOI] [PubMed] [Google Scholar]
- Miller MB, Chapman LJ, Chapman JP, Barnett EM. Schizophrenic deficit in span of apprehension. J Abnorm Psychol. 1990;99(3):313–316. doi: 10.1037//0021-843x.99.3.313. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Tyler CW. Psychophysical isolation of movement sensitivity by removal of familiar position cues. Vision Research. 1981;21(4):427–433. doi: 10.1016/0042-6989(81)90089-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya H, Arakawa O, Ikeda T. Visuo-motor reaction times of normal subjects and schizophrenics using inverting prisms. Jpn J Psychiatry Neurol. 1989;43(2):147–154. doi: 10.1111/j.1440-1819.1989.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Norton D, Ongur D, Stromeyer C, 3rd, Chen Y. Altered 'three-flash' illusion in response to two light pulses in schizophrenia. Schizophr Res. 2008;103(1–3):275–282. doi: 10.1016/j.schres.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153(5):687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- Parsons BD, Gandhi S, Aurbach EL, Williams N, Williams M, Wassef A, Eagleman DM. Lengthened temporal integration in schizophrenia. Neuropsychologia. 2013;51(2):372–376. doi: 10.1016/j.neuropsychologia.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Poggel DA, Treutwein B, Calmanti C, Strasburger H. Increasing the temporal g(r)ain: double-pulse resolution is affected by the size of the attention focus. Vision Res. 2006;46(18):2998–3008. doi: 10.1016/j.visres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Vision Science and Schizophrenia Research: Toward a Review of the Disorder Editors' Introduction to Special Section. Schizophr Bull. 2011;37(4):681–689. doi: 10.1093/schbul/sbr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, Sass SM, Noukki AL, Hegeman BM. Fragile early visual percepts mark genetic liability specific to schizophrenia. Schizophr Bull. 2013;39(4):839–847. doi: 10.1093/schbul/sbs041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger H, Rentschler I, Juttner M. Peripheral vision and pattern recognition: a review. J Vis. 2011;11(5):13. doi: 10.1167/11.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve T, Friedman L, Jesberger JA, Gilmore G, Strauss ME, Meltzer H. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychological Medicine. 1997;27(1):143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Sprock J, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, et al. Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophr Res. 2014;152(2–3):503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Mathis KI, Ford J, Breitmeyer BG, Green MF. Object substitution masking in schizophrenia: an event-related potential analysis. Front Psychol. 2013;4:30. doi: 10.3389/fpsyg.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]