Abstract
Soluble circulating low density lipoprotein receptor-related protein-1 (sLRP) provides key plasma binding activity for Alzheimer’s disease (AD) amyloid β-peptide (Aβ). sLRP normally binds 70–90% of plasma Aβ preventing free Aβ access to the brain. In AD, Aβ binding to sLRP is compromised by increased levels of oxidized sLRP which does not bind Aβ. Here, we determined plasma oxidized sLRP and Aβ40/42 sLRP-bound, other proteins-bound and free plasma fractions, cerebrospinal fluid (CSF) tau/Aβ42 ratios and mini-mental state examination (MMSE) scores in patients with mild cognitive impairment (MCI) who progressed to AD (MCI-AD, n=14), AD (n=14) and neurologically healthy controls (n=14) recruited from the Göteborg MCI study. In MCI-AD patients prior to conversion to AD and AD patients, the respective increases in oxidized sLRP and free plasma Aβ40 and Aβ42 levels were 4.9 and 3.7-fold, 1.8 and 1.7-fold and 4.3 and 3.3-fold (P < 0.05, ANOVA with Tuckey post-hoc test). In MCI-AD and AD patients increases in oxidized sLRP and free plasma Aβ40 and Aβ42 correlated with increases in CSF tau/Aβ42 ratios and reductions in MMSE scores (P < 0.05, Pearson analysis). A heterogenous group of ‘stable’ MCI patients that was followed over 2–4 years (n=24) had normal CSF tau/Aβ42 ratios but increased oxidized sLRP levels (P < 0.05, Student’s t test). Data suggests that a deficient sLRP-Aβ binding might precede and correlate later in disease with an increase in the tau/Aβ42 CSF ratio and global cognitive decline in MCI individuals converting into AD, and therefore is an early biomarker for AD-type dementia.
Keywords: Mild cognitive impairment, Alzheimer’s disease, Aging, Biomarker, sLRP
INTRODUCTION
Low-density lipoprotein receptor-related protein-1 (LRP) is a multifunctional scavenger receptor of the LDL receptor family [1]. LRP plays a major role in cellular transport of cholesterol associated with apolipoprotein E (apoE) containing lipoproteins. LRP has a 515 kDa extracellular α chain that contains four ligand binding domains that bind a diverse array of ∼ 40 structurally and functionally unrelated ligands [1] including Alzheimer’s disease neurotoxin, amyloid-β-peptide (Aβ) [2]. The 85 kDa transmembrane cytoplasmic β chain of LRP is responsible for cell signaling regulating endocytosis and interactions with several intracellular adaptor proteins [1]. Although LRP has been regarded mainly as an endocytotic cargo transporter, recent studies have shown that LRP transports several ligands transcellularly across the blood-brain barrier (BBB) including Aβ [2,3], tissue plasminogen activator [4], apoE2 and apoE3 [5] and a family of Kunitz domain-derived peptides [6]. It has been also shown that the cell surface LRP in brain vascular cells provides a major clearance route for Aβ by promoting its efflux from brain to blood [2,3,7–9], whereas LRP in the liver mediates systemic Aβ clearance [10].
β-secretase cleaves the N-terminal extracellular domain of LRP releasing soluble LRP (sLRP) [11] which circulates in plasma [12,13]. Recently, we have demonstrated that circulating plasma sLRP provides a key endogenous peripheral plasma ‘sink’ activity for Aβ by promoting a continuous removal of Aβ from brain [13]. In neurologically healthy humans and mice, sLRP normally binds 70–90% of circulating Aβ preventing free Aβ access to the brain [13]. In AD patients and AD transgenic mice, however, Aβ binding to sLRP is compromised by increased levels of oxidized sLRP, a form of sLRP which does not bind Aβ [13]. An increase in oxidized sLRP levels results in elevated levels of free Aβ40 and Aβ42 that can re-enter the brain as shown in different animal models [13–16]. Recombinant LRP fragments can effectively replace oxidized sLRP and sequester free Aβ in plasma in AD patients and AD transgenic mice which reduces Aβ-related pathology [13].
A long asymptomatic period precedes cognitive decline in AD [17–19]. Therefore, defining early events and biomarkers of dementia is critical for therapeutic interventions. Here, we studied the relationship between sLRP-mediated peripheral plasma binding activity of Aβ and the cerebrospinal fluid (CSF) tau/Aβ42 ratio as a predictor of neuronal injury and cognitive decline [17–19] and global cognitive outcome in patients with mild cognitive impairment (MCI) and AD.
MATERIALS AND METHODS
Patients
We studied a total of 66 patients and controls recruited from the Göteborg MCI study [20,21] including patients with MCI that progressed to AD (MCI-AD group, n = 14), AD patients (n = 14) and neurologically healthy age-matched controls (n = 14). In addition, we studied a heterogenous group of ‘stable’ MCI patients (n = 24) who did not develop dementia during a follow-up period of 2–4 years (mean 3.5 years; MCI group). The MCI-AD patients had a diagnosis of MCI at baseline and converted later into AD during a follow-up period of more than 2 years (mean 2.3 years). Details about inclusion and exclusion criteria for the Göteborg MCI study have been reported in previous publications [20,21]. Subjects with major depressive and other severe psychiatric disorders were excluded, whereas subjects with minor depressive symptoms and mild anxiety were not [21].
A summary of demographic data for patients included in the present study is given in Table 1. Briefly, controls, MCI-AD, AD and MCI patients were in average 68, 65, 69 and 64 year old. As expected, both MCI-AD and AD groups had a high frequency of apolipoprotein E4 (APOE4) allele (i.e., 64%) compared to a heterogenous MCI group (21%) and controls (29%).
Table 1.
Demographic data
| Control | MCI-AD | AD | MCI | |
|---|---|---|---|---|
| Age (± s.d.) | 67.64 ± 5.41 | 65 ± 7.66 | 69.14 ± 7.09 | 63.79 ± 7.0 |
| Gender (male/female) | 6/8 (42/58%) | 4/10 (28/72%) | 6/8 (42/58%) | 12/12 (50/50%) |
| APOE4 carriers (%) | 29% | 64% | 64% | 21% |
At baseline and at biannual follow-ups, patients and controls underwent neurological and psychiatric examinations, cognitive assessment and neuropsychological tests, and sampling of blood and cerebrospinal fluid (CSF), as described in detail in previous publications [20–22]. The diagnoses of MCI and AD type dementia were based on criteria as described below and were made by a clinician blinded to the results of the patients exams, as we described previously [20–22]. The inclusion criterion of a progressive cognitive impairment for more than six months had to be anamnestically verified.
The criteria of MCI were those described by Petersen [23] which consist of: (i) memory complaint, preferably corroborated by an informant, (ii) objective memory impairment adjusted for age and education, (iii) preservation of general cognitive functioning, (iv) no or minimal impairment of daily life activities, and (v) not fulfilling the DSM-IIIR (Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised) criteria of dementia. The patients who received a diagnosis of AD were required to meet the DSM-IIIR criteria of dementia and the criteria of probable AD defined by National Institute of Neurological and Communicative Disorders-Stroke/Alzheimer’s disease and Related Disorders Association (NINCDS-ADRDA) [24].
Healthy controls were recruited mainly from senior citizens organizations, while a few were spouses of the study patients. Controls were not included if they had subjective or objective signs of cognitive disorder as assessed from the above procedure. All controls were followed clinically for 2 years. Patients and controls gave their informed consent to participate in the study, which was conducted according to the provisions of the Helsinki Declaration and approved by the ethics committees of Göteborg University, Sweden.
Non-fasting plasma samples were collected in the morning by venipuncture in tubes containing ethylenediaminetetracetic acid (EDTA) as anticoagulant. CSF was obtained at baseline by lumbar puncture through the L3/L4 or L4/L5 interspace. The lumbar punctures were performed in the morning to avoid any influence on the result from diurnal fluctuations in biomarker levels. The CSF, collected in polypropylene tubes, was submitted to centrifugation at 2,000 x g at 4°C for 10 min. The ensuing supernatant was aliquoted in screw-cap polypropylene tubes and stored at −80°C to await biochemical analyses.
Procedures
sLRP purification from human plasma
sLRP was isolated from human plasma as described [12] and used to generate the standard curve in human sLRP specific enzyme-linked immunosorbent (ELISA) assay. Briefly, protease inhibitors (1 mM phenylmethylsulfonyl fluoride, Cat. No. P7626, 2 mM benzamidine, Cat. No. B6506, 2 mM bacitracin, Cat. No. 11702, 1 µM leupeptin, Cat. No. L2884, Sigma, St. Louis, MO) were added to plasma, the pH adjusted by adding 1:10 volume of 0.1 M 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, N-(2-Hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid (HEPES, Cat. No. H4034, Sigma), pH 7.4, 10 IU/ml heparin (Cat. No. H3149, Sigma), 5 mM Ca2+, followed by centrifugation (4°C, 20,000g, 30 min) and filtration through a 0.2 µm filter. Plasma was next passed through a α2-macroglobulin (Cat. No. M6159, Sigma) affinity column, washed with HBSC (HEPES, 20 mM, calcium, 2mM, and 0.1% Tween 20) pH 7.4, and eluted with 25 mM EDTA, 20 mM 2-(N-Morpholino) ethanesulfonic acid (MES, Cat. No. M3671, Sigma), pH 6.0. The eluted fraction was dialyzed against PBS overnight at 4 °C and concentrated using a 30 kDa molecular cut-off Microcon® centrifugal filter unit (Cat. No. 42409, Millipore Corp., Billerica, MA). The protein concentration was determined using the BCA protein assay kit (Cat. No. 23225, Thermo Fisher Scientific, Rockford, IL) and the purity by SilverStain (Cat. No. 24597, Thermo Fisher Scientific).
Human sLRP specific ELISA
Plasma sLRP levels were determined by humanspecific sLRP ELISA, as described [12]. Briefly, 96 well LockWell™, MaxiSorp plates (Cat. No. 446469, Nunc, Thermo Fisher Scientific) (were coated overnight with receptor associated protein (RAP) (Cat. No. RA02, Oxford Biomedical Research, Oxford, MI) (1 µg/well) in bicarbonate buffer and blocked with HBSC buffer (HEPES, 20 mM, calcium, 2 mM, 1% BSA and 0.1% Tween 20), for 30 min at 37°C. Plasma samples and/or purified human plasma-derived sLRP standards, diluted 100-fold in blocking buffer, were added to each well, incubated for 1 h at room temperature, washed extensively with HBSC, and then incubated with the N-terminus human specific LRP 8G1 antibody (Cat. No. 438190, 1:500, EMD Biosciences), followed by horseradish peroxidase (HRP) conjugated goat anti-mouse secondary antibody (Cat. No. A4789, Sigma) (1:5000) for 1 h at room temperature. We added 3,3',5,5'-tetramethylbenzidine substrate (Cat. No. 53-00–01, KPL, Haithersburg, MD) and stopped the reaction with 1 N HCl. Absorbance was determined at 450 nm, and plasma sLRP concentrations were calculated from the standard curve.
Oxidized sLRP
We determined oxidized plasma sLRP levels from the sLRP carbonyl content by first forming hydrazone derivatives with 2, 4-dinitrophenylhydrazide (DNPH), as described [13]. Plasma sLRP (2 µg) was immunoprecipitated with 8G1 human specific LRP antibody (EMD Biosciences) using a protein G immnoprecipitation kit (Cat. No. 11719386001, Roche Diagnostics GmbH, Mannheim, Germany). sLRP was retrieved by boiling for 10 min in 18 µL 10% SDS followed by centrifugation at 6,000 g for 1 min. The supernatant containing sLRP was incubated at a room temperature for 15 min with DNPH (Cat. No. S7150; OxyBlot™ Protein Oxidation Detection Kit, Millipore). The supernatant was removed, and the derivatized sLRP precipitated in 10% trichloroacetic acid (TCA) (Cat. No. T6399, Sigma), and separated by centrifugation at 10,000 g for 2 min. The pellet was washed 5 times with a cold ethanol/acetone mixture (1:1), dried at 37°C for 15 min and resuspended in 6 M guanidine hydrochloride (Cat. No. 24110, Thermo Fisher Scientific). The guanidine-protein blank was prepared by following the above procedure in the absence of DNPH-protein derivatization. The absorption maximum of the derivatized protein was about 375 nm. We obtained the difference in absorption between the DNPH-derivatized sLRP and the blank, and determined the concentration of sLRP hydrazone derivatives using the reference absorbtivity of 21 mM−1cm−1, as described [13].
Human Aβ40 and Aβ42 ELISAs
The levels of human endogenous Aβ40 and Aβ42 in different plasma fractions were determined with human specific ELISA kits (Cat. No. KHB3481 and KHB3544, Invitrogen), respectively, according to the manufacturer’s instructions, as we reported [13].
sLRP-bound human Aβ in plasma
Plasma was IgG-depleted by incubation with with protein G beads (Cat. No. 11719408001, Roche) and incubated with human specific LRP antibody (8G1, EMD Biosciences) overnight at 4°C in the presence of protein G agarose (Protein G immnoprecipitation kit, Roche Diagnostics). The agarose was recovered by centrifugation and washed according to instructions in the protein G immunoprecipitation kit. The washed agarose beads containing the sLRP associated Aβ were resuspended in 10 µl of 5 M guanidine hydrochloride/50 mM Tris.Cl, pH 8.0 and mixed for 4 h at room temperature. sLRP-bound Aβ40 and Aβ42 levels were determined with human specific ELISAs (see above) using 25-fold diluted samples.
Free unbound Aβ in plasma
sLRP-depleted plasma supernatants were subjected to ultrafiltration with Microcon® 30 kDa molecular weight cutoff (Cat. No. 42409, Millipore Corp., Bedford, MA). The filtrate was further purified and concentrated using PepClean™ C-18 spin columns (Cat. No. 89870, Thermo Scientific) according to manufacturer’s instructions. The proteins were eluted from the column with 60 µl of 70% acetonitrile (Cat. No. 27,071-7, Aldrich, Milwaukee, WI), vacuum dried and reconstituted in 5 µl of 5 M guanidine hydrochloride/50 mM Tris.Cl, pH 8.0. Human Aβ40 and Aβ42 levels in the filtrate fractions were determined by ELISAs, as described earlier [13].
Aβ bound to other proteins in plasma
After ultrafiltration with Microcon® 30 kDa molecular weight cutoff, human Aβ40 and Aβ42 levels in the retentate fractions were determined by ELISAs, as described earlier [13].
Intrassay %CV for Aβ40, Aβ42 and sLRP ELISA’s were 2.5, 8.5 and 5.5, respectively.
Aβ42 and tau in CSF
CSF total tau (T-tau) concentration was determined using a sandwich ELISA (Innotest hTAU-Ag, Innogenetics, Gent, Belgium) specifically constructed to measure all tau isoforms irrespectively of phosphorylation status, as previously described [25]. Aβ1–42 levels were determined using a sandwich ELISA (INNOTEST® β- AMYLOID(1–42), Innogenetics, Gent, Belgium), specifically constructed to measure Aβ containing both the first and 42nd amino acid, as previously described [26]. Please note that we did not have CSF samples for two MCI-AD and four AD patients.
Statistical analysis
To test the differences between controls and MCI-AD and AD we used one-way analysis of variance (one way ANOVA) followed by Tuckey’s post hoc test. The differences were considered to be significant at P < 0.05. All values were mean ± SEM. To test the difference between controls and MCI, we used Student’s t test. Pearson correlation coefficient (r) was used for determining correlations. Graphpad Prism 5.0 statistical program was used for analysis.
RESULTS
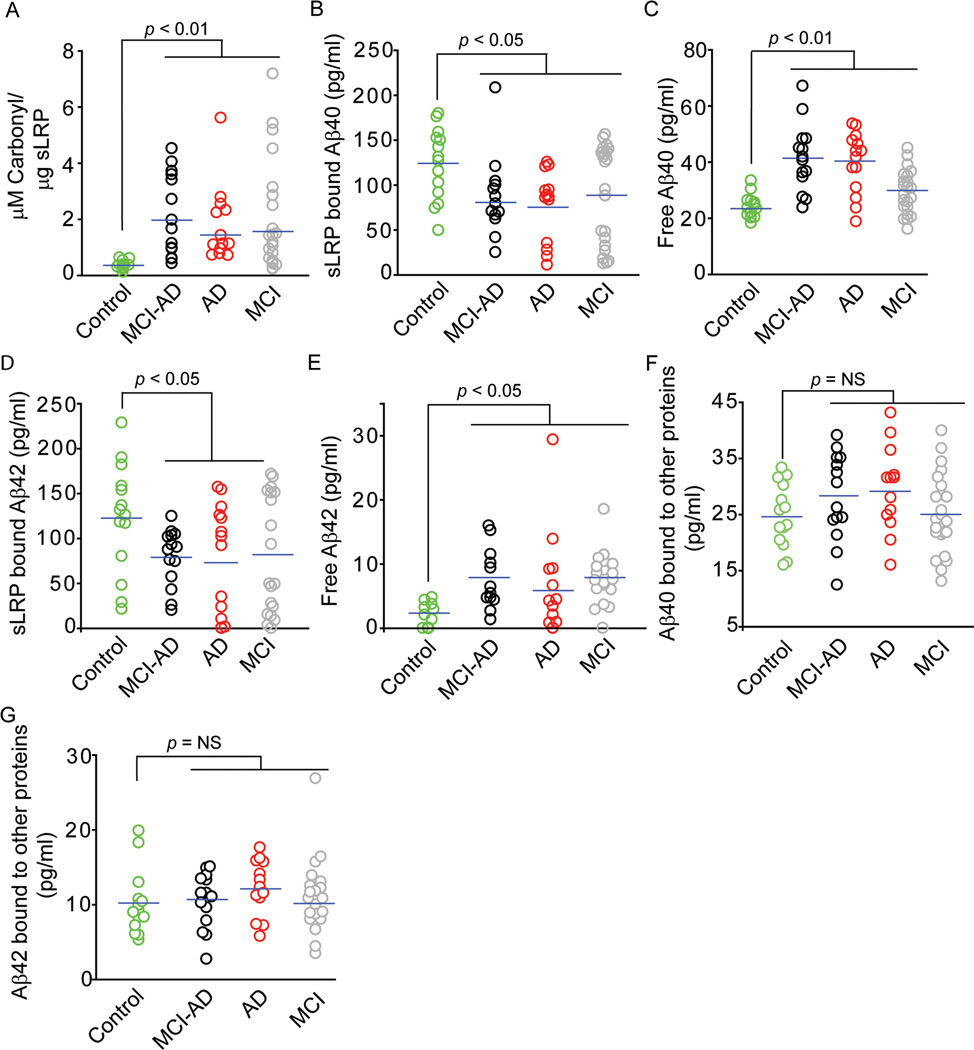
Individuals with MCI who progressed to AD (MCI-AD) and AD patients compared to controls had 4.9 and 3.7-fold increase in oxidized sLRP levels (p < 0.01; Fig. 1A), a form of sLRP which does not bind Aβ [13], respectively. Consistent with these findings, MCI-AD and AD patients had 28–34% and 35–38% significant reductions in sLRP-bound plasma Aβ40 and Aβ42 (p < 0.05) (Fig. 1B and 1D), and 80% and 72% and 4.3 and 3.3-fold increases in free plasma Aβ40 and Aβ42 levels (Fig. 1C and 1E), respectively. Fractions of Aβ40 and Aβ42 bound to other proteins did not change significantly in MCI-AD and AD groups compared to controls (Fig. 1F–G).
Fig. 1. Changes in oxidized plasma sLRP levels and plasma sLRP bound, free and other proteins bound Aβ fractions.
Oxidized plasma sLRP (carbonyl content) (A), sLRP-bound plasma Aβ40 levels (B), free plasma Aβ40 levels (C), sLRP-bound plasma Aβ42 levels (D), free plasma Aβ42 levels (E), other proteins bound Aβ40 levels (F) and other proteins bound Aβ42 levels (G) in neurologically normal healthy controls (green circles), MCI-AD (black circles), AD (red circles) and stable MCI (gray circles) patients. Points represent individual values from n = 14–24 individuals per group.
As reported [13], in terms of total Aβ levels data in Fig. 1 indicates that in neurologically healthy controls the majority of Aβ40 (i.e., 71%) and Aβ42 (i.e., 91%) binds to sLRP, and a minor portion, i.e., 14% of Aβ40 and 8% of Aβ42, binds to other proteins; the remaining fractions represent free Aβ40 ( i.e., 15% of total Aβ40) and free Aβ42 (i.e., 1% of total Aβ42). In MCI-AD and AD groups, there was a significant reduction in sLRP-bound Aβ40 and Aβ42 fractions to 50–55% and 80% of total Aβ40 and Aβ42, respectively, compared to 71% for sLRP-bound Aβ40 and 91% for sLRP-bound Aβ42 in controls. The changes in Aβ redistribution in MCI-AD and AD patients were associated with the corresponding increases in free Aβ40 and Aβ42 fractions to 28% and 8% of their respective total Aβ levels.
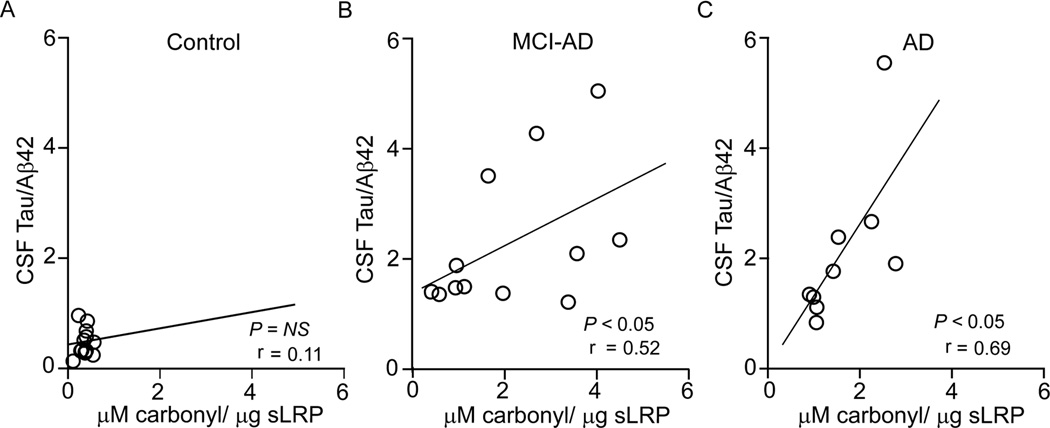
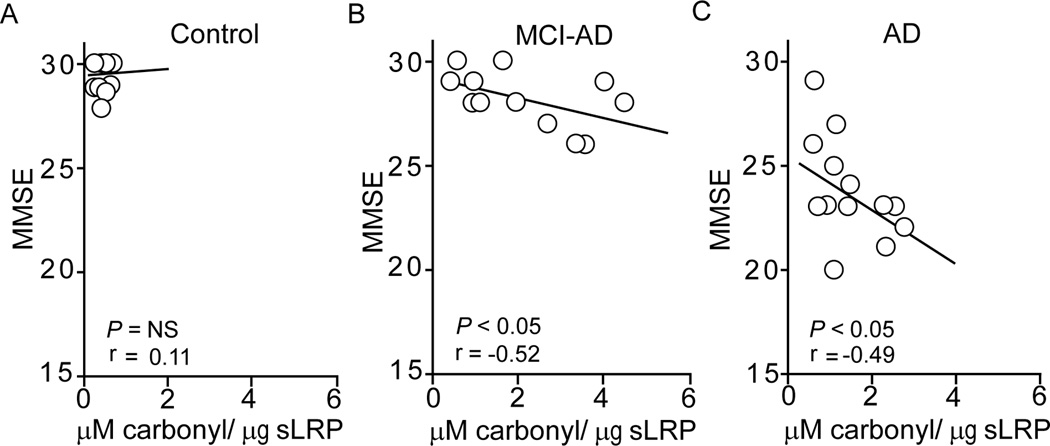
Compared to controls who did not show a correlation between the CSF tau/Aβ42 ratios and oxidized sLRP plasma levels (Fig. 2A), both MCI-AD and AD patients exhibited a significant positive correlation between the CSF tau/Aβ42 ratios and oxidized sLRP levels (r = 0.52; p < 0.05; r = 0.69; p < 0.05) (Fig. 2B–C). The analysis of MMSE scores and oxidized sLRP levels indicated no correlation in controls (Fig. 3A). In contrast, there was a significant negative correlation in MCI-AD patients and AD patients (Fig. 3B–C; r = −0.52; p < 0.05; r = −0.49; p < 0.05).
Fig. 2. The relationship between the CSF tau/Aβ42 ratio and oxidized plasma sLRP.
The CSF tau/Aβ42 ratio plotted against oxidized plasma sLRP (carbonyl content) levels in control subjects (A) and MCI-AD (B) and AD (C) patients. Pearson correlation coefficient (r) was used to determine the correlation between the two studied parameters. Points represent individual values from n = 10–14 individuals per group.
Fig. 3. The relationship between MMSE scores and oxidized plasma sLRP levels.
The relationship between MMSE scores and oxidized plasma sLRP (carbonyl content) levels in controls (A) and MCI-AD (B) and AD (C) patients. Pearson correlation coefficient (r) was used to determine the correlation between the two studied parameters. Points represent individual values from n = 14 individuals per group.
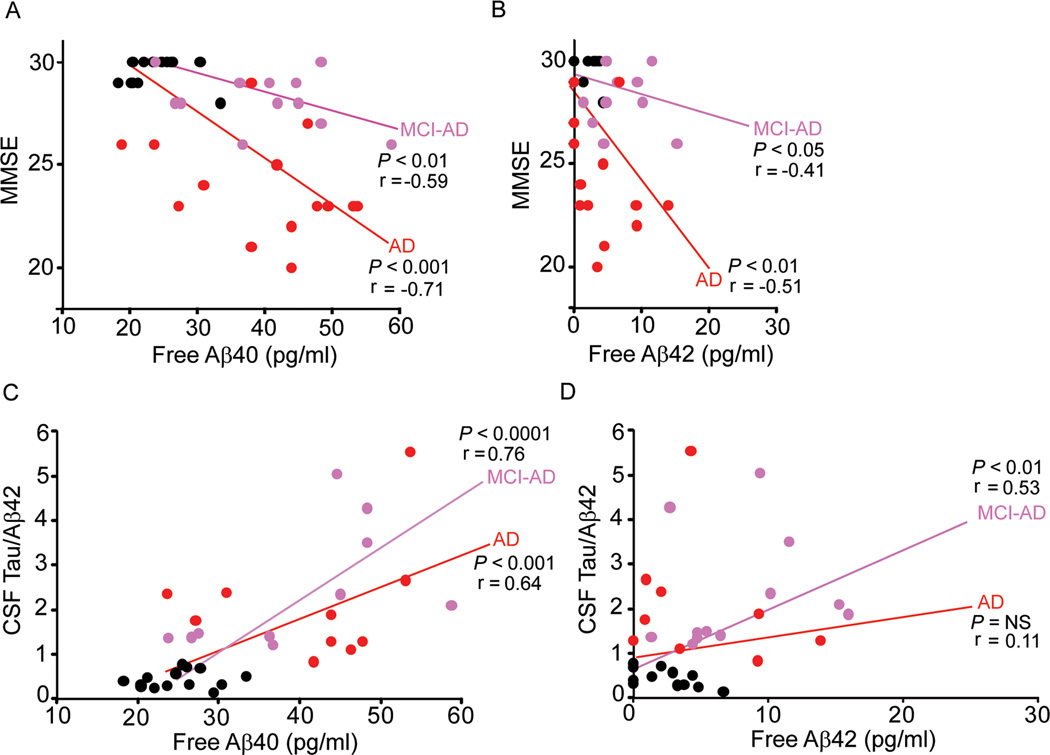
The analysis of the MMSE scores and free plasma Aβ40 levels (Fig. 4A; r = −0.59; p < 0.01; r = −0.71; p < 0.001 ) and Aβ42 levels (Fig. 4B; r = −0.41; p < 0.05; r = −0.51; p < 0.01) indicated a significant negative correlation in MCI-AD and AD groups compared to controls. In contrast, there was no correlation between free plasma Aβ levels and MMSE scores in controls.
Fig. 4. The relationship between the MMSE scores, the CSF tau/Aβ42 ratio and plasma free Aβ40 and 42 levels.
The relationship between the MMSE scores and plasma free Aβ40 (A) and free Aβ42 (B) levels in controls and MCI-AD ( ) and AD (
) and AD ( ) patients. The number of individuals in all groups was n = 14. The relationship between the CSF tau/Aβ42 ratio and plasma free Aβ40 (C) and free Aβ42 (D) levels in controls and MCI-AD (
) patients. The number of individuals in all groups was n = 14. The relationship between the CSF tau/Aβ42 ratio and plasma free Aβ40 (C) and free Aβ42 (D) levels in controls and MCI-AD ( ) and AD (
) and AD ( ) patients. The number of individuals per group ranged from 10 to 14. Pearson correlation coefficient (r) was used to determine the correlation between the two studied parameters.
) patients. The number of individuals per group ranged from 10 to 14. Pearson correlation coefficient (r) was used to determine the correlation between the two studied parameters.
The analysis of CSF tau/Aβ42 ratios and free plasma Aβ40 levels (Fig. 4C; r = 0.76; p < 0.0001; r = 0.64; p < 0.001) showed a significant positive correlation in the MCI-AD and AD groups, and no correlation in controls. A similar analysis of CSF tau/Aβ42 ratios and free plasma Aβ42 levels showed a significant positive correlation only in MCI-AD group (Fig. 4D; r = 0.53; p < 0.01); there was no correlation in AD group or controls.
We also studied a heterogenous group of so-called ‘stable’ MCI individuals who did not develop any type of dementia within the 3.5 years of follow-up and did not show changes in the CSF tau/Aβ42 ratios. These MCI patients exhibited a large variation in oxidized sLRP levels. One half of these MCI patients (12/24) had oxidized sLRP levels within a range found in normal healthy controls, whereas another half (12/24) had significantly higher levels which contributed to a significant 4.2-fold increase compared to neurologically healthy controls (p < 0.01; Fig. 1A). Therefore, our finding of an increase in oxidized sLRP in ‘stable’ MCI group should be interpreted cautiously as it likely reflects sLRP oxidation in a subgroup of patients.
Consistent with an increase in oxidized sLRP, MCI patients had reductions in sLRP-bound Aβ40 and Aβ42 plasma fractions by 28% and 32%, respectively (Fig. 1B and 1D). A more detailed analysis indicated that these differences could be attributed to a subgroup of 12/24 patients who had pronounced reductions in sLRP-bound Aβ, while 50% of the MCI patients (12/24) had normal values of sLRP-bound Aβ. Finally, corresponding to these findings, the MCI group showed a modest 27% increase in free plasma Aβ40 levels and somewhat more robust increase in free Aβ42 plasma levels compared to controls (Fig. 1C and 1E). Compared to controls, the MCI group did not show changes in Aβ40 and Aβ42 fractions bound to other proteins (Fig. 1F and 1G).
DISCUSSION
This study shows that disruption in sLRP-mediated peripheral ‘sink’ activity for Aβ is already present in patients diagnosed with MCI at baseline who progressed later to AD, i.e., in the so-called MCI-AD group. In both MCI-AD and AD patients there was a significant and comparable increase in oxidized plasma sLRP as indicated by increased sLRP carbonylation. Protein carbonylation is an established marker of oxidative stress [27] and oxidative damage to proteins is one of the earliest events in AD [19,28,29]. Importantly, oxidation of plasma sLRP results in a form of sLRP that does not bind plasma Aβ [13]. Consistent with these findings both MCI-AD and AD patients had elevated levels of free plasma Aβ40 and Aβ42. In MCI-AD patients, sLRP oxidation and the resulting increases in free plasma Aβ40 and Aβ42 levels that were found at an early MCI stage correlated at the time of AD conversion with increases in the CSF tau/Aβ42 ratios and reductions in the MMSE scores comparable to those seen in AD patients. This data suggests that an impaired sLRP-mediated peripheral binding of plasma Aβ during an early MCI stage is highly predictive of progression into AD dementia.
A heterogeneous group of so-called ‘stable’ MCI patients did not have changes in the CSF tau/Aβ42 ratios and MMSE scores and did not develop dementia during the studied 3.5 years of follow-up. However, as emphasized in the Results, about 50% of ‘stable’ MCI patients had elevated oxidized sLRP levels, whereas the remaining 50% had normal oxidized sLRP levels. At this point we do not have longitudinal data to determine whether changes in a subgroup of 50% MCI patients with elevated oxidized sLRP are predictive of conversion into AD, as shown for the MCI-AD group. Given that conversion from MCI to dementia occurs at a rate of 10–15% per year with ∼ 80% conversion by the sixth year of follow-up [30], it is tempting to speculate that about 50% of currently ‘stable’ MCI patients with elevated oxidized sLRP levels at baseline will eventually convert into AD over the next 3 years. The future longitudinal studies in MCI patients should address whether a disrupted sLRP-Aβ binding activity is specific for MCI conversion into AD, or it can be also found in other types of dementia, as for example in individuals with vascular dementia. These future studies should also address for how long a disrupted sLRP peripheral Aβ binding can co-exist with a diagnosis of ‘stable’ MCI.
The capillary beds of peripheral organs allow free exchanges of solutes between blood and the interstitial fluid [31]. In contrast, the BBB is sealed by the tight junctions precluding free and/or rapid entry of polar molecules into the brain such as small peptides [32,33] unless there is a carrier-mediated and/or receptor-mediated transport mechanism for these molecules at the BBB, as for example for rapid transport of amino acids [34] or slow transport of neuroactive peptides [35]. In rodent and primate models of AD, free circulating Aβ40 and Aβ42 can cross the BBB via a concentration-dependent specialized mechanism [36–42] and/or across a disrupted BBB [15]. In primates and rodents, free plasma Aβ entering the brain typically deposits onto the pre-existing amyloid on blood vessels [38,41] and brain parenchyma [42] and can bind to neurons inducing an oxidant injury [14,15]. The receptor for advanced glycation end products (RAGE) mediates Aβ transport from blood to brain, oxidant stress and neuroinflammatory response at the BBB [14,28]. Brain vasculature in AD expresses increased levels of RAGE [14,43,44] and reduced LRP levels [2,16] associated with Aβ cerebrovascular and/or brain accumulation. Some forms of Aβ are not cleared efficiently from brain as for example the Dutch isoform [45]. The rising plasma free Aβ concentrations that we show in the present study and the progressively increasing BBB RAGE expression (to transport the Aβ substrate from plasma into brain) throughout various neuropathologic stages from MCI to AD [46] can combine to exacerbate the AD evolution in the CNS.
Earlier studies in AD showed unchanged levels of plasma Aβ (as reviewed by Blennow et al. [19]). However, elevated levels of plasma Aβ42 and/or Aβ40 have been also reported in AD [47,48] raising a possibility that plasma Aβ may contribute to brain Aβ accumulation [28]. Studies in preclinical AD have been inconclusive, some suggesting plasma Aβ42 does not predict a subsequent development of AD (see in Blennow et al. [19]), others showing elevated plasma Aβ42 precedes AD [47,49]. The population-based Rotterdam study revealed that increased plasma Aβ40, but not Aβ42, was associated with a risk of developing AD and/or vascular dementia [50].
Reproducible and accurate measurements of Aβ in plasma are challenging because of the hydrophobic nature of the full length peptide and the heterogeneity of truncated Aβ fragments [22], and because most of plasma Aβ,i.e., 70–90%, is normally bound to sLRP [13]. Earlier studies did not distinguish between sLRP-bound Aβ that does not cross the BBB [13] and free circulating Aβ that can re-enter the brain [13–15,36–42]. Based on studies with free Aβ in rodents and primates one would expect that humans with chronically elevated levels of free circulating Aβ, as for example patients with MCI who converted into AD and AD patients, will have a continuous influx of free plasma Aβ into the brain contributing to the formation of amyloid plaques [28,38,41,42] and oxidant stress [14,15].
Most studies agree that an elevated CSF tau/Aβ42 ratio is predictive of AD [17–19,21,51,52], and that an increase in tau/Aβ42 CSF ratio reflects tau release from injured or dead neurons and/or reduced Aβ42 CSF levels due to enhanced Aβ aggregation in the brain. A positive correlation between the CSF tau/Aβ42 ratio and oxidized sLRP in MCI-AD and AD individuals, and a negative correlation between MMSE scores and oxidized sLRP indicates that faulty sLRP ‘sink’ activity precedes a typical AD CSF tau/Aβ42 profile and global cognitive decline.
In summary, our data suggests that impairments in sLRP-mediated Aβ peripheral binding also known as peripheral plasma ‘sink’ activity for Aβ is a useful early biomarker in MCI patients progressing to AD, and is an early event in the disease process preceding AD. The future longitudinal studies on a larger cohort of MCI patients and healthy individuals should determine the sensitivity and specificity of oxidized sLRP plasma test for AD, i.e., the percent of MCI patients who develop AD but do not have increased levels of oxidized sLRP, and the percent of MCI patients who have elevated levels of oxidized sLRP but do not develop AD and subsequently convert into other types of dementia (e.g., vascular) or remain diagnosed as ‘stable’ MCI.
ACKNOWLEDGEMENTS
This study was supported by US National Institutes of Health (NIH) grants R37NS34467 and R37AG023084 to BVZ.
Footnotes
Conflicts of interest
BVZ is the scientific founder of Socratech L.L.C., a startup biotechnology company with a mission to develop new therapeutic approaches for stroke and Alzheimer’s disease. BVZ is co-inventor on patents pertaining use of sLRP fragments as a potential therapy for Alzheimer’s disease.
References
Because of the vastness of this research area and a limited number of references, we had in some instances to cite only reviews. We apologize to our colleagues whose important original contributions were not acknowledged.
- 1.Herz J, Chen Y, Masiulis I, Zhou L. Expanding functions of lipoprotein receptors. J Lipid Res. 2009;50:S287–S292. doi: 10.1194/jlr.R800077-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deane R, Wu Z, Sagare A, Davis J, Yan SD, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid β-peptide interaction mediates differential brain efflux of Aβ isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Shibata M, Yamada S, Ram Kumar S, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benchenane K, Berezowski V, Ali C, Fernández-Monreal M, López-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. doi: 10.1161/01.CIR.0000163542.48611.A2. [DOI] [PubMed] [Google Scholar]
- 5.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeule M, Currie J-C, Bertrand Y, Ché C, Nguyen T, Régina A, Gabathuler R, Castaigne J-P, Béliveau R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B. Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med. 2000;6:718–719. doi: 10.1038/77397. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, Gau H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 9.Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, Streb JW, Guo H, Rubio A, Van Nostrand W, Miano JM, Zlokovic BV. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamaki C, Ohtsuki S, Iwatsubo T, Hashimoto T, Yamada K, Yabuki C, Terasaki T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 11.Von Arnim CAF, Kinoshita A, Peltan ID, Tangredi MM, Herl L, Lee BM, Spoelgen R, Hshieh TT, Ranganathan S, Battey FD, Liu C-X, Bacskai BJ, Sever S, Irizarry MC, Strickland DK, Hyman BT. The low density lipoprotein receptor-related protein (LRP) is a novel β-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 12.Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- 13.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-β by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane R, Yan SD, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 15.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer’s disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 16.Donahue JE, Flaherty SL, Johanson CE, Duncan IIIJA, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 17.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller G. Alzheimer’s biomarker initiative hits its stride. Science. 2009;326:386–389. doi: 10.1126/science.326_386. [DOI] [PubMed] [Google Scholar]
- 19.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer’s disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 20.Nordlund A, Rolstad S, Hellstrom P, Sjogren M, Hansen S, Wallin A. The Goteberg MCI study: mild cognitive impairment is a heterogenous condition. J Neurol Neurosug Psychiatry. 2005;76:1485–1490. doi: 10.1136/jnnp.2004.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordlund A, Rolstad S, Klang O, Edman Å, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Göteborg MCI study. J Neurol Neurosurg Psychiatry. 2010;81:541–546. doi: 10.1136/jnnp.2008.171066. [DOI] [PubMed] [Google Scholar]
- 22.Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, Wallin A, Minthon L, Blennow K. Evaluation of plasma Aβ40 and Aβ42 as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Int Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, VanMechelen E. Tau protein in cerebrospinal fluid: a biochemical diagnostic marker for axonal degeneration in Alzheimer’s disease? Mol Chem Neuropath. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 26.Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid β-amyloid(1–42) in Alzheimer’s disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki YJ, Carini M, Butterfield DA. Protein carbonylation. Antioxi Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Baldeiras I, Santana I, Proença MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J Alz Dis. 2008;15:117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- 30.Lovell MA. Similarities and differences between mild cognitive impairment and Alzheimer’s disease. J Alz Dis. 2010;19:219. doi: 10.3233/JAD-2010-1263. [DOI] [PubMed] [Google Scholar]
- 31.Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–248. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- 32.Zloković BV, Segal MB, Begley DJ, Davson H, Rakić L. Permeability of the blood-cerebrospinal fluid and blood-brain barriers to thyrotropin-releasing hormone. Brain Res. 1985;358:191–199. doi: 10.1016/0006-8993(85)90963-1. [DOI] [PubMed] [Google Scholar]
- 33.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 34.Segal MB, Preston JE, Collis CS, Zlokovic BV. Kinetics and Na independence of amino acid uptake by blood side of perfused sheep choroid plexus. Am J Physiol. 1990;258:F1288–F1294. doi: 10.1152/ajprenal.1990.258.5.F1288. [DOI] [PubMed] [Google Scholar]
- 35.Zlokovic BV, Mackic JB, Djuricic B, Davson H. Kinetic analysis of leucine-enkephalin cellular uptake at the luminal side of the blood-brain barrier of an in situ perfused guinea-pig brain. J Neurochem. 1989;53:1333–1340. doi: 10.1111/j.1471-4159.1989.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 36.Martel CL, Mackic JB, McComb JG, Ghiso J, Zlokovic BV. Blood-brain barrier uptake of the 40 and 42 amino acid sequences of circulating Alzheimer’s amyloid β in guinea pigs. Neurosci Lett. 1996;206:157–160. doi: 10.1016/s0304-3940(96)12462-9. [DOI] [PubMed] [Google Scholar]
- 37.Ghersi-Egea JF, Gorevic PD, Ghiso J, Frangione B, Patlak CS, Fenstermacher JD. Fate of cerebrospinal fluid-borne amyloid β-peptide: rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem. 1996;67:880–883. doi: 10.1046/j.1471-4159.1996.67020880.x. [DOI] [PubMed] [Google Scholar]
- 38.Ghilardi JR, Catton M, Stimson ER, Rogers S, Walker LC, Maggio JE, Mantyh PW. Intra-arterial infusion of [125I]Aβ1–40 labels amyloid deposits in the aged primate brain in vivo. Neuroreport. 1996;7:2607–2611. doi: 10.1097/00001756-199611040-00040. [DOI] [PubMed] [Google Scholar]
- 39.Poduslo JF, Curran GL, Haggard JJ, Biere AL, Selkoe DJ. Permeability and residual plasma volume of human, Dutch variant, and rat amyloid β protein 1–40 at the blood-brain barrier. Neurobiol Dis. 1997;4:27–34. doi: 10.1006/nbdi.1997.0132. [DOI] [PubMed] [Google Scholar]
- 40.Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, Zlokovic BV. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mackic JB, Weiss MH, Miao W, Kirkman E, Ghiso J, Calero M, Bading J, Frangione B, Zlokovic BV. Cerebrovascular accumulation and increased blood-brain barrier permeability to circulating Alzheimer’s amyloid beta peptide in aged squirrel monkey with cerebral amyloid angiopathy. J Neurochem. 1998;70:210–215. doi: 10.1046/j.1471-4159.1998.70010210.x. [DOI] [PubMed] [Google Scholar]
- 42.Mackic JB, Bading J, Ghiso J, Walker L, Wisniewski T, Frangione B, Zlokovic BV. Circulating amyloid-β peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol. 2002;38:303–313. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- 43.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 44.Silverberg GD, Miller MC, Messier AA, Majmudar S, Machan JT, Donahue JE, Stopa EG, Johanson CE. Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J Neuropath Exp Neurol. 2010;69:98–108. doi: 10.1097/NEN.0b013e3181c8ad2f. [DOI] [PubMed] [Google Scholar]
- 45.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substituition at codon 22 reduces clearance of Alzheimer’s amyloid-β peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 46.Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayeux R, Honig LS, Tang M-X, Manly J, Stern Y, Schupf N, Mehta PD. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortalilty, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 48.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer’s disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 49.Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 50.Van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta (1–40) and Abeta (1–42) and risk of dementia : a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 51.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 52.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka S-K, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, se Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jönhagen ME, Minthon L, Winblad B, Blennow K. CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]