Abstract
Behavioral inhibition, a temperament identifiable in infancy, is associated with heightened withdrawal from social encounters. Prior studies raise particular interest in the striatum, which responds uniquely to monetary gains in behaviorally inhibited children followed into adolescence. Although behavioral manifestations of inhibition are expressed primarily in the social domain, it remains unclear whether observed striatal alterations to monetary incentives also extend to social contexts. In the current study, imaging data were acquired from 39 participants (17 males, 22 females; ages 16–18 years) characterized since infancy on measures of behavioral inhibition. A social evaluation task was used to assess neural response to anticipation and receipt of positive and negative feedback from novel peers, classified by participants as being of high or low interest. As with monetary rewards, striatal response patterns differed during both anticipation and receipt of social reward between behaviorally inhibited and noninhibited adolescents. The current results, when combined with prior findings, suggest that early-life temperament predicts altered striatal response in both social and nonsocial contexts and provide support for continuity between temperament measured in early childhood and neural response to social signals measured in late adolescence and early adulthood.
Heightened interest in peer affiliation represents a major aspect of adolescent development, driven in part by an escalating desire to be liked by and gain acceptance from one’s peers (Brown, 2004; Rubin, Bukowski, & Parker, 2006). The high motivation for peer acceptance orients adolescents’ thoughts and behavior (e.g., dressing like members of a certain clique) toward obtaining peer approval and avoiding peer criticism (Allen, Porter, McFarland, Marsh, & McElhaney, 2005). Given the salience of peers’ opinions in adolescence, it is not surprising that being accepted or rejected by peers impacts an individual’s social experiences and emotional adjustment (La Greca & Lopez, 1998; Muris, Meesters, Merckelbach, Sermon, & Zwakhalen, 1998; Silverman, La Greca, & Wasserstein, 1995).
For example, adolescents who report high levels of acceptance by their peers also demonstrate social competence, have more intimate friendships, are generally popular, and have high self-esteem (Rubin et al., 2006). Adolescents who are rejected by their peers engage in social avoidance and experience higher levels of anxiety, depression, suicidality, excessive risk-taking, and substance use (Rubin et al., 2006). Moreover, these relationships are bidirectional; socially reticent adolescents often alter their social behavior by withdrawing from peers, which both limits socialization opportunities and increases vulnerability to peer rejection. Although peer affiliation is a universal issue in adolescence, the affect associated with peer acceptance and rejection may be uniquely modulated by distinct types of temperament.
Temperament is a biologically based, early-emerging tendency to react in specific behavioral, emotional, and physiological ways to one’s surroundings. These tendencies are identifiable in infancy and continue to predict behavior later in life (Kagan, 1997). This is not to say that temperament is destiny, as environmental conditions interact with temperament in shaping behavior (Degnan & Fox, 2007; Kagan & Fox, 2007). Temperament is thought to provide a biased weighting to the relative functional role of neural circuits within different periods of development. Because behavior often reflects the output of interactions among many such circuits, temperamental biases may remain latent with regard to behavioral dispositions. Early-life temperament has been associated with unique patterns of brain activation during adolescence, even in the absence of a link between temperament and outward indicators of psychopathology or behavioral abnormalities (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011; Jarcho et al., 2012). The enduring scaffold of temperament as a biologically based predisposition may be most readily observed in physiological measures, particularly measures that directly reflect aspects of brain function. Assessments of brain function may help identify profiles of temperament-linked patterns of physiological responding that indicate potential vulnerability for psychopathology. These patterns may be particularly salient in certain contexts or key developmental periods.
Behavioral inhibition, one of the most widely studied forms of temperament, is characterized by hyperreactivity to novelty in infancy and extreme social reticence in early to middle childhood (Coplan, Rubin, Fox, Calkins, & Stewart, 1994; Fox, Henderson, Marshall, Nichols, & Ghera, 2005). This social reticence occurs despite a strong motivation for interactions with peers (Fox et al., 2005; Rubin, Coplan, & Bowker, 2009). Although behavioral inhibition represents a normative, nonpathological trait, it does share behavioral and neurobiological features with some pathological states, particularly social anxiety disorder (SAD; Chronis-Tuscano et al., 2009; Degnan & Fox, 2007; Perez-Edgar & Fox, 2005; Pine, 1999). Nevertheless, fewer than half of all children with behavioral inhibition manifest signs of SAD, which suggests that the association between behavioral inhibition and SAD can remain latent, perhaps only manifesting transiently when an individual is placed in challenging social circumstances (Degnan & Fox, 2007).
The fear associated with peer rejection can constrain social engagement among children with behavioral inhibition when they are faced with novel social encounters (Rubin et al., 2009). In these contexts, youth with behavioral inhibition tend to avoid initiating interactions with unfamiliar peers (Asendorpf & Meier, 1993; Rubin et al., 2009) and, as a result, they infrequently experience positive peer feedback (Chen, DeSouza, Chen, & Wang, 2006; Rimm-Kaufman & Kagan, 2005). Furthermore, when children with behavioral inhibition do interact with unfamiliar peers, they often fault themselves for both their own avoidance behaviors and negative encounters (Burgess, Wojslawowicz, Rubin, Rose-Krasnor, & Booth-LaForce, 2006). Over time, this pattern of reticent social engagement and avoidance may produce a dynamic and recurrent cycle; the behavioral avoidance and self-criticism associated with inhibited temperament may reinforce each other and decrease both engagement in and motivation for future peer interactions.
This cyclical process of social disengagement may be moderated by experience, however. In other words, early tendencies associated with behavioral inhibition may lead to the avoidance of social situations in some children, culminating in the overt expression of SAD. In other children with behavioral inhibition, however, distinct social experiences may allow the child to mature in a fashion that supports positive social behavior and minimizes expression of impairing social avoidance. For example, young children with the temperament of behavioral inhibition who experience nonmaternal childcare are less likely to exhibit anxious behaviors later in childhood (Almas et al., 2011). Even in behaviorally inhibited (BI) children without SAD, however, underlying biased tendencies could persist, leaving the child with a latent, unexpressed risk profile. Thus, an early tendency to avoid social contact may result in psychopathology for some but not other children with early-life behavioral inhibition. In BI children with or without overt SAD, however, the lasting physiological imprint may manifest in measures of brain function (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011; Jarcho et al., 2012; Perez-Edgar et al., 2013).
One major question that arises in light of prior neuroimaging data on behavioral inhibition concerns the contexts in which a latent imprint might be expressed. Prior neuroimaging studies used procedures that are relatively removed from the contexts in which behavioral aspects of inhibition typically are expressed (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011). These prior studies have probed behavior and brain function with neurocognitive tasks, as opposed to real-world social encounters which typically trigger the defining features of behavioral inhibition. It is reasonable to expect that, in an ecologically valid social context involving potential peer interaction and evaluation, psychiatrically healthy adolescents characterized by high levels of childhood behavioral inhibition have an underlying biology that differs from children who entered life without behavioral inhibition. Such possibilities can be tested by applying emerging understandings in neuroscience to the study of a longitudinally followed cohort of infants characterized by their temperament.
Unique neural responses in BI and behaviorally noninhibited (BN) youth have been documented in the context of prior work on motivation for nonsocial incentives. These differences are found in the striatum, a multicomponent region (e.g., nucleus accumbens, caudate nucleus, putamen), which is central to motivated behavior. Specifically, BI versus BN adolescents show heightened striatal response when anticipating nonsocial rewards (Guyer et al., 2006), particularly those contingent on performance (Bar-Haim et al., 2009). In contrast, BI versus BN adolescents show blunted striatal response when receiving performance-contingent positive feedback in a nonsocial task (Helfinstein et al., 2011). Thus, early-childhood behavioral inhibition is associated with altered striatal responses in both anticipatory and consummatory phases of nonsocial reward processing in adolescence.
The striatal findings associated with behavioral inhibition show unique subregional patterns of activation as a function of the type of task and reward process under study. Such disparity in subregional striatal recruitment is expected based on the functional specialization of the striatal components, which is well described in seminal works on the basal ganglia physiology (Haber & Knutson, 2010). However, in humans, the functional discrimination across the various striatal modules is less clear, which is attributable to substantial information integration across these regions and the difficulty in assessing their role in isolation. According to a classic dorsal–ventral distinction, ventral structures (e.g., nucleus accumbens) preferentially code for the processing of emotional values and their translation into motivation, while dorsal structures (e.g., caudate, putamen) are more closely linked to cognitive and motor responses (Di Martino et al., 2008). Other striatal organizational models have also been described (Atallah, Lopez-Paniagua, Rudy, & O’Reilly, 2007;Mattfeld, Gluck, & Stark, 2011; Voorn, Vanderschuren, Groenewegen, Robbins, & Pennartz, 2004), all outlining both distinct and overlapping functions of striatal subregions. A full understanding of the specific contribution of each striatal subregion to behavioral inhibition is still nascent, however.
To date, neuroimaging research on reward processing and temperament has focused on only monetary incentives. However, virtually all motivated behaviors, including those associated with social reward, engage the striatum (Izuma, Saito, & Sadato, 2008; Jones et al., 2011; Kampe, Frith, Dolan, & Frith, 2001). For the BI child, social scenarios emerge as among the most salient circumstances that the child faces and are often associated with negative rather than positive affect (Boivin, Hymel, & Burkowski, 1995; Hanish & Guerra, 2000). As a result, one might expect the previous findings of altered striatal response to monetary reward to extend to a social context, and the current study considers this possibility. It is important that, in addition to moderating affective motivation, the striatum also contributes to learning contingencies associated with appetitive stimuli (Schultz, 2006). Thus, dysfunctional striatal responses in social contexts may be a key component in the cyclical nature of social disengagement associated with the BI temperament.
One major question arising from prior neuroimaging work concerns the degree to which temperament in early childhood continues to predict striatal response to socially salient events, even after the child has endured social experiences that could alter their underlying neural response patterns (Cicchetti & Tucker, 1994). To date, no neuroimaging study has addressed this question, although available neuroimaging research in adolescents and adults, considered as a group, has shown that peer acceptance versus rejection induces activation in the striatum and positive affect in typically developing adolescents (Gunther Moor, van Leijenhorst, Rombouts, Crone, & Van der Molen, 2010; Guyer, Choate, Pine, & Nelson, 2012). Other studies link the striatum to social reinforcement learning (Jones et al., 2011). These studies report multiple striatal subregions, including the caudate, putamen, and nucleus accumbens, operating as part of a unit with distinct subspecializations. Because response patterns in particular subregions may provide insights for future work, we retained this division here. The striatum also acts as part of a distributed neural circuit, encompassing multiple other cortical and subcortical structures (Hazy, Frank, & O’Reilly, 2010). For example, signal change in the fusiform gyrus and superior temporal gyrus (STG) is expected given the role of these regions in social cognition and social affect (Blakemore, 2008; Gunther Moor et al., 2010; Guyer, Choate, Pine, et al., 2012; Nelson, Leibenluft, McClure, & Pine, 2005; Pfeifer, Lieberman, & Dapretto, 2007; Saxe, 2006). In the context of the current social evaluation task, relevant circuitry would include the fusiform gyrus, amygdala, and thalamus, as well as the distributed association cortex of the temporal and frontal regions. While we examined striatal response in primary analyses, we also considered in secondary analyses associations with these other brain regions implicated in social cognition and social threat.
The present study examined striatal function in a social context that adolescents encounter daily (Guyer, Choate, Pine, et al., 2012). This allowed us to create a highly salient social milieu, even in the limited environment of a brain scanner. We used this context to assess adolescent variations in striatal response to social reward as a function of early-childhood behavioral inhibition. We hypothesized that early-childhood temperament continues to predict late adolescent response to social feedback. Moreover, we expected patterns of striatal responding in this social context to resemble patterns observed previously for monetary incentives. We expected BI adolescents, relative to BN adolescents, to show striatal hyperactivity while anticipating peer feedback, consistent with prior data on striatal response to anticipated monetary reward (Bar-Haim et al., 2009; Guyer et al., 2006). In contrast, we expected BI adolescents, relative to BN adolescents, to show striatal hypoactivity upon receiving acceptance feedback, again, consistent with prior data on striatal response to receipt of monetary reward. We also conducted exploratory whole-brain analyses to examine group differences among more broadly distributed regions previously implicated in social behavior. As in our past work documenting striatal functional differences between BI and BN adolescents, we did not expect to find group differences in task performance or psychopathology.
Method
Participants
A total of 433 4-month-olds were initially screened on reactivity to novel sensory stimuli; 153 were selected for a longitudinal study based on their extreme reactivity scores (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Individual differences in behavioral inhibition were assessed at four time points. At ages 14 and 24 months, children were presented with novel and unfamiliar objects and people during laboratory assessments (Fox et al., 2001). At ages 4 and 7 years, children’s reticent behavior with unfamiliar peers was measured using Rubin’s Play Observation Scale (Rubin, 1989). Maternal ratings of their child’s social fear were collected at 14 and 24 months with the Toddler Behavior Assessment Questionnaire (Goldsmith, 1996) and of their child’s shyness at ages 4 and 7 years with the Colorado Child Temperament Inventory (Rowe & Plomin, 1977). To create temperament scores, observed behavior and maternal-report scores across the four time points were taken from the entire cohort and standardized. These z scores were averaged to create a composite temperament score (M = −0.008, SD = 0.64, range = −1.33 to 2.82). A median split of the composite score was used to create BI and BN groups within the full cohort.
Participants were recruited for the current study in late adolescence based on their early temperament grouping. A psychiatric interview was then conducted to assess the presence of clinical diagnoses. This assessment determined the range of diagnoses among these participants as well as whether participants were taking psychotropic medication. Because medications have been shown to influence neural activation, participants currently receiving psychotropic medications were not eligible for study participation. For ethical reasons, participants presenting with serious, untreated psychopathology were also excluded. These two exclusion criteria limited the number of acutely symptomatic participants. Participants who met study eligibility criteria were representative of the larger cohort from which they were drawn with respect to temperament scores (0.09 for participants vs. −0.03 for the remaining cohort, p = 0.44).
Forty-nine of 58 participants eligible for the neuroimaging study completed scanning (9 met neuroimaging exclusion criteria, such as dental braces). Data from 10 participants were not usable because of motion .3 mm or lack of task deception (n = 3) resulting in a final sample of 39 participants (17 males). Thus, 80% of eligible participants provided usable data. This resulted in two groups who reflected extreme high (BI: n = 18, 9 males) and low (BN: n = 21, 8 males) temperament scores. The difference in temperament scores between groups arose by design, in that groups were formed in such a way that they differed in their temperament, with a large effect size (Cohen d = 3.42; Table 1).
Table 1.
Sample characteristics
| BI (n = 18) |
BN (n = 21) |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Statistic | p |
| Age (years) | 17.85 | 1.38 | 17.93 | 1.80 | t (37) = 0.17 | .87 |
| WASI FS IQ score | 114.28 | 10.74 | 113.52 | 12.25 | t (37) = 0.20 | .84 |
| BIC score | 0.89 | 0.64 | −0.63 | 0.18 | t (37) = 10.39 | <.001 |
| SCARED scorea | 10.55 | 7.18 | 8.16 | 6.94 | t (32) = 0.99 | .33 |
| Social Anxiety Scale | 37.47 | 11.30 | 42.56 | 11.52 | t (29) = 1.24 | .22 |
| n | % | n | % | |||
| Male | 9 | 50 | 8 | 38 | χ2 (1, 39) = 0.56 | .53 |
| Parent education ≥ college levelb | 15 | 88.2 | 16 | 84.2 | χ2 (1, 36) = 0.12 | .73 |
Note: BI, behaviorally inhibited; BN, behaviorally noninhibited; WASI FS IQ, Wechsler Abbreviated Scale of Intelligence full-scale IQ; BIC, behavioral inhibition composite; SCARED, Screen for Child Anxiety Related Emotional Disorders.
Scores ≥25 indicate the presence of an anxiety disorder.
Data are missing for three participants.
The groups did not differ on demographic characteristics or anxiety measures (Table 1). Participants were all Caucasian and resided in middle-class families, reflective of the demographics of the full cohort from which they were recruited. Overall anxiety was assessed with the Screen for Child Anxiety Related Emotional Disorders (Birmaher et al., 1997) averaged across adolescent and parent reports. Feelings of social anxiety in the context of peer relations were assessed with the self-reported Social Anxiety Scale—Adolescents (La Greca & Lopez, 1998). As indicated above, each participant and his or her parent was interviewed separately by an experienced clinician with the Schedule for Affective Disorders and Schizophrenia for School Aged Children—Present and Lifetime Version (Kaufman et al., 1997) to ascertain diagnoses of current psychiatric illness. As noted above, participants on medication or in need of acute treatment were not eligible. However, clinical psychopathology status of some participants did not meet exclusionary criteria. One BI case had comorbid social anxiety and depression, one BI case had attention-deficit/hyperactivity disorder, and two BN cases had generalized anxiety disorder. Findings were not affected when these participants were excluded. To maximize representativeness, these participants were retained in the analysis.
Procedures were approved by the Institutional Review Board and both participants and their parents provided written informed assent/consent to participate in the study.
Neuroimaging task
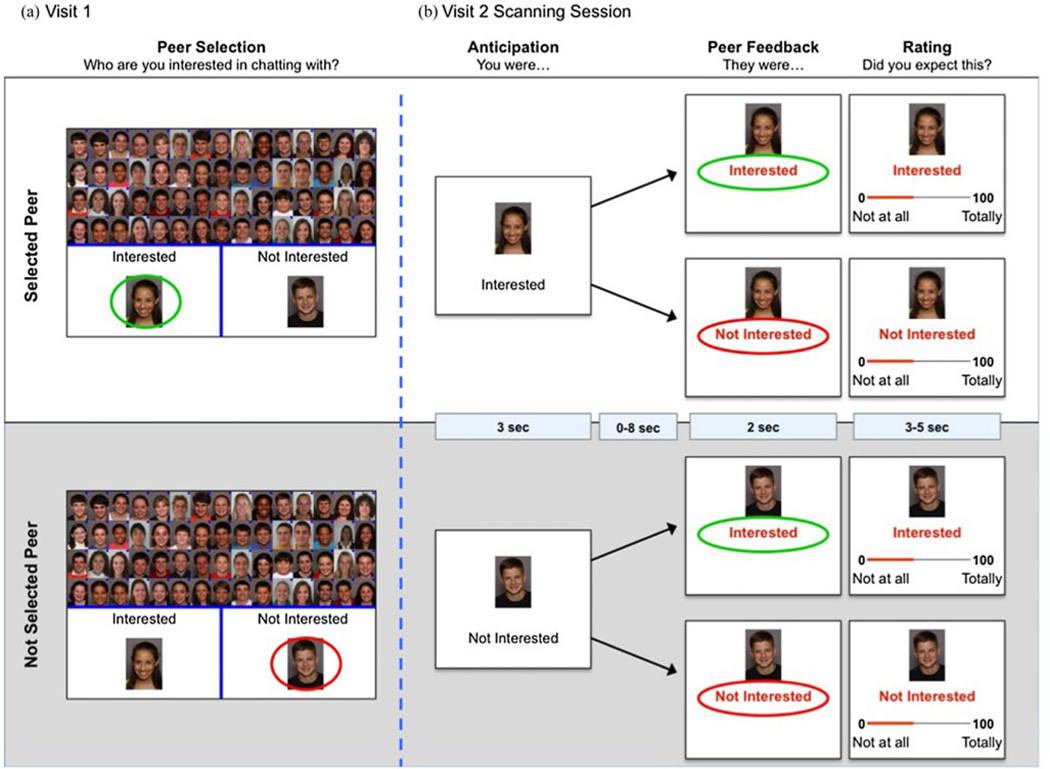
Participants completed a variant of the chatroom task (Figure 1), which simulates several aspects of social evaluation and feedback (Guyer, Choate, Pine, et al., 2012; Guyer et al., 2008; Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Lau et al., 2011). The task was administered during two laboratory visits. On Visit 1, participants were told that the purpose of the study was to learn more about internet-based social interaction and that during the subsequent visit they would chat online with another participant deemed a good match for them. Participants were photographed and asked to provide biographical information, which they were told would be shared with other study participants. Participants were then asked to sort photographs of 60 peers into a group of 30 with whom they would and 30 with whom they would not like to chat (Figure 1a). Participants were told that, although personal information was collected from other participants, technical factors prevented us from sharing this information, forcing participants to sort peers “based upon looks alone.” While participants only saw peers’ photographs, participants were led to believe that the peers would see all of the information that the study participant had provided during Visit 1. This strategy increased the salience of peer feedback and minimized potential distracting information and between-subject heterogeneity in psychological processes engaged during picture viewing. After the sort, photographs were shown again with the words “You were Interested/Not Interested” to remind participants of their choices. Participants rated each of six factors that may have influenced their choices: age, gender, friendliness, attractiveness, likeability, and “fun-ness,” rated as either relevant or not. Stimuli were 60 digital headshots of 11- to 20-year-old actors (30 males) posing happy expressions with direct gaze (Egger et al., 2011). E-prime 1.1 software was used to administer the task (Psychological Software Tools, Pittsburgh, PA).
Figure 1.
(Color online) An overview of the chatroom task paradigm. Participants completed the paradigm across two visits. (a) During their first visit, which occurred approximately 2 weeks prior to scanning, participants selected 30 peers they were interested in chatting with and 30 peers they were not interested in chatting with. (b) On their second visit, participants completed the next task phases while undergoing a functional magnetic resonance imaging scan. A typical trial time course is depicted.
The participants returned to the lab 2 weeks later for a functional neuroimaging scan. All 60 photographs were viewed in random order during each of two tasks. During Task 1, participants rated how much they thought each person in the photograph was interested in them. During Task 2, participants received feedback from each peer indicating acceptance or rejection. Only results from this second task are considered here, given our focus on neural response to social feedback. After completing the scanning session, all participants underwent a standardized debriefing procedure that has been used in prior studies providing deceptive social feedback (Guyer, Choate, Pine, et al., 2012; Guyer et al., 2009; Lau et al., 2011). The debriefing procedure involved use of a structured questionnaire to record participants’ experience with the task (e.g., “How happy were you when someone expressed interest in chatting with you?”), followed bya structured one-on-one debriefing interview concerning the actual study procedures and their rationale. During this debriefing interview participants were told about the deceptive nature of feedback. Of the 49 participants who were scanned, 46 indicated that they believed they would interact with another putative participant and answered the series of debriefing questions in away consistent with this report and thus were considered “deceived.” No adverse effects associated with deception occurred after participants were debriefed postscan.
Each trial started with the anticipation event (3 s), when the photographwas displayed and the participant was reminded of their prior selection. The feedback event (2 s) was followed with “They were Interested/Not interested” displayed under the picture (Figure 1b). Because striatal response varies during reward anticipation and receipt (Ernst & Fudge, 2009; van Leijenhorst, Crone, & Bunge, 2006), task parameters dissociated these processes with variable-duration jitter (presentation of a fixation cross 0–8000 ms) between response anticipation and feedback, and between trial end and subsequent anticipation event. In addition, because striatal response was expected to vary by participants’ selections of peers, trials were binned on this variable (Figure 1a). Prior neuroimaging studies suggest that striatal response is modulated by expectation (Gunther Moor et al., 2010). Such an effect in neuroimaging data is consistent with considerable basic science work in nonhuman samples showing that the striatum responds to violations of expected contingencies (Hazy et al., 2010). Focusing participants’ attention on rating how much they expected the received feedback was designed to increase the salience of expectancy violations and confirmations. After feedback was received, participants rated their expectation of the feedback on a 100-point scale (3–5 s; Figure 1b). Although participants were told the feedback was genuine, pictures were randomly assigned by computer algorithm to deliver acceptance/rejection feedback, with equal numbers for each feedback type.
Functional magnetic resonance imaging acquisition
Scanning occurred in a General Electric (Waukesha, WI) Signa 3-T magnet. Stimuli were projected onto a screen at the foot of the scanner bed and viewed with mirrors mounted on the head coil. Foam padding was used to constrain head motion. A hand-held two-button response device was used to record participants’ ratings (Research Services Branch, NIMH, Bethesda, MD). Each brain volume (367 total) consisted of 36 2.6-mm interleaved slices, acquired axially using a T2*-weighted gradient echo pulse sequence with 2300 ms repetition time, 25 ms echo time, 908 flip angle, 2.5 × 2.5 × 2.6 mm voxels, 96 × 96 matrix, and 24 cm field of view. Four dummy acquisitions were obtained before task onset for signal stabilization. A high-resolution structural image was acquired per subject using a T1-weighted standardized magnetization prepared spoiled gradient recalled echo sequence: 124 1.2 mm thick axial slices, 7.816 repetition time, 3.024 echo time, 6° flip angle, 256 × 192 matrix, and 22 cm field of view.
Data analysis
Functional magnetic resonance imaging data were preprocessed and analyzed using Analysis of Functional and Neural Images (AFNI) software (Cox, 1996). Preprocessing included corrections for slice timing and motion, reslicing to 2-mm isotropic voxels, warping into standard space, spatial smoothing to a 6-mm full-width half-maximum Gaussian kernel, and normalizing blood oxygen level-dependent signal intensity to percentage signal change. Movement artifact was mitigated by including six motion correction parameters in the statistical model as nuisance covariates, along with a covariate for mean intensity and linear drift.
The statistical model was a gamma variate basis function convolved with the hemodynamic response function provided in AFNI. Modeled events of interest were set to the onset of each picture for anticipation events and the onset of feedback for acceptance/rejection events. A general linear model determined the beta value and t statistic of all events at the single-subject level. This was followed by group-level, random-effects analyses of variance (ANOVA). Response to anticipation events was modeled as a 2 × 2 repeated measures ANOVA to examine group (BI, BN) and participant peer selection (selected, not selected) effects. Response to feedback events was modeled as a 2×2×2 repeated measures ANOVA to examine group (BI, BN), participant peer selection (selected, not selected), and stimulus feedback (acceptance, rejection) effects. Of note, with this design, effects of both acceptance and rejection are modeled. While we expected group differences in response to peer acceptance, we also examined group differences in response to rejection.
Our main analyses used a region of interest (ROI) approach focused on the striatum following past work (Bar-Haim et al., 2009; Guyer et al., 2006). Masks were created separately for the caudate, nucleus accumbens, and putamen regions using the AFNI Talairach Daemon data set and a threshold of p < .005 with a spatial extent of 10 contiguous 2-mm voxels (80 mm3). This threshold was determined by Monte Carlo simulations for voxels of the masks to determine the spatial extent of a cluster required for a p < .05 ROI correction.
Secondary whole-brain analyses were also conducted for which the initial statistical threshold was set at p < .005. To reduce Type I errors, this initial threshold was made more stringent by requiring a spatial extent of 50 contiguous 2-mm voxels (400 mm3). Whole-brain analyses were conducted to evaluate recruitment of regions beyond the striatum, given their role in social cognition (Blakemore, 2008; Haxby, Hoffman, & Gobbini, 2000; Nelson et al., 2005; Saxe, 2006). This included the amygdala, fusiform gyrus, anterior cingulate cortex, and other cortical regions in the STG and ventral prefrontal cortices.
Post hoc analyses on extracted signal change values were conducted using SPSS (Chicago) to clarify main and interaction effects that emerged in significant clusters. Behavioral data were analyzed in SPSS. Groups were compared using t tests on the percent of peers participants selected (N = 30) due to being the same age, being the same gender, looking friendly, looking attractive, the peer would like them, and the peer seems like fun and did not select (N = 30) due to not being the same age, not being the same gender, looking unfriendly, looking unattractive, the peer would not like them, and the peer does not seems like fun. A 2 (group) × 2 (peer selection) × 2 (feedback) repeated measures ANOVA was used to compare the groups on expectation ratings for each feedback type from each peer. Effect sizes were calculated as Cohen d.
Results
Behavioral data
Reasons for peer selections
Group differences were found in participants’ reasons for not selecting peers. Looking “unfriendly” was used more frequently to justify not selecting a peer by the BI group (M = 0.21, SD = 0.18) than the BN group (M = 0.09, SD = 0.12), t (37) = 2.48, p = .02, d = 0.80. Similarly, the BI group used “did not seem like fun” to justify not selecting a peer more often (M = 0.37, SD = 0.26) than did the BN group (M = 0.20, SD = 0.26), t (37) = 2.01, p = .05, d = 0.65. The BI and BN groups did not differ on any other measure that quantified the reasons for choices to select or not select peers.
Expectation of feedback
Expectancy ratings were examined in a Group × Peer Selection × Feedback repeated measures ANOVA. No significant main or interaction effects with group were found. However, there was a significant Selection × Feedback interaction, F (1, 37)=45.10, p < .001, d=2.21. All participants endorsed higher expectation of receiving acceptance feedback from selected peers (M = 62.55, SE = 2.05) than from not-selected peers (M = 47.57, SE = 2.18; p < .001). One-sample t tests showed that participants rated expectations above the scale midpoint for selected peers only, t (38) = 31.06, p < .001. In contrast, for expectancy of negative feedback, participants endorsed higher expectation from peers they had not selected (M = 59.90, SE = 2.16) versus peers they had selected (M = 41.93, SE = 2.23; p < .001), with ratings of unselected peers significantly above the scale midpoint, t (38) = 28.17, p < .001.
Neuroimaging data
Striatal ROI analysis
Anticipation of feedback
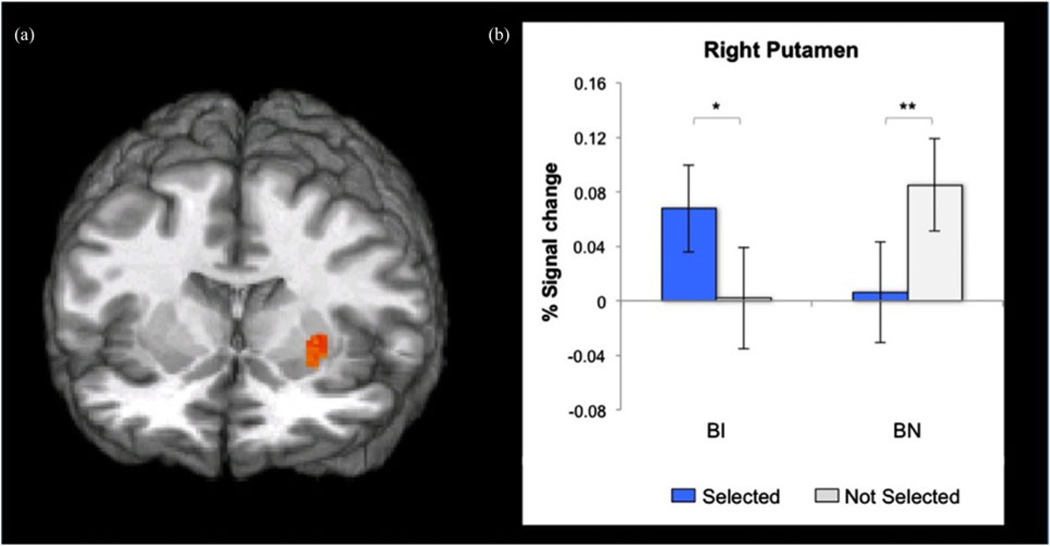
Anticipation analyses focused on whether the groups differed in activation in the three a priori striatal ROIs during anticipation of feedback from selected versus not-selected peers. A significant Group × Peer Selection interaction was found with a large effect in the right putamen, F (1, 37) = 15.85, p < .001, d = 1.31 (Table 2, Figure 2a). Extracted signal change estimates from this cluster revealed distinct patterns of striatal hyperactivation within each group (Figure 2b). The BI group exhibited increased putamen activation when anticipating feedback from selected peers versus not-selected peers ( p = .02, d = 1.63). In contrast, the BN group had greater putamen activation when anticipating feedback from not-selected versus selected peers (p = .003, d = 2.09). Thus, anticipation of feedback engaged the striatum in both BI and BN adolescents with a large effect size in each group. However, the relative pattern of responding markedly differed in the two groups.
Table 2.
Significant clusters based on region of interest analyses of main and interaction effects of group, selection, and feedback on striatal activation during anticipation of and response to feedback
| Comparison | Region | Peak Voxel Coordinates | No. of Voxels | F | p |
|---|---|---|---|---|---|
| Anticipation phase | |||||
| Group × Selection | R. putamen | 27, −1, 4 | 80 | 15.85 | <.001 |
| Feedback phase | |||||
| Group × Selection × Feedback | R. caudate | 15, 21, 2 | 13 | 12.10 | .001 |
Note: The statistical threshold for all effects was set at p < .005 with a spatial extent correction of 80 mm3 (10 contiguous 2-mm voxels). R., right; N=39, df =1, 37.
Figure 2.
(Color online) Anticipation of feedback in the region of interest analysis. (a) Statistical maps (y = −6, z = 0) showing a Group × Peer Selection interaction effect centered on the right putamen (peak coordinates: 27, −1, 4; p < .001). (b) Behaviorally inhibited (BI) versus behaviorally noninhibited (BN) adolescents showed greater putamen activation when anticipating feedback from selected peers. In contrast, BN versus BI adolescents show greater putamen activation when anticipating feedback from not-selected peers. *p < .05. **p < .01.
Receipt of feedback
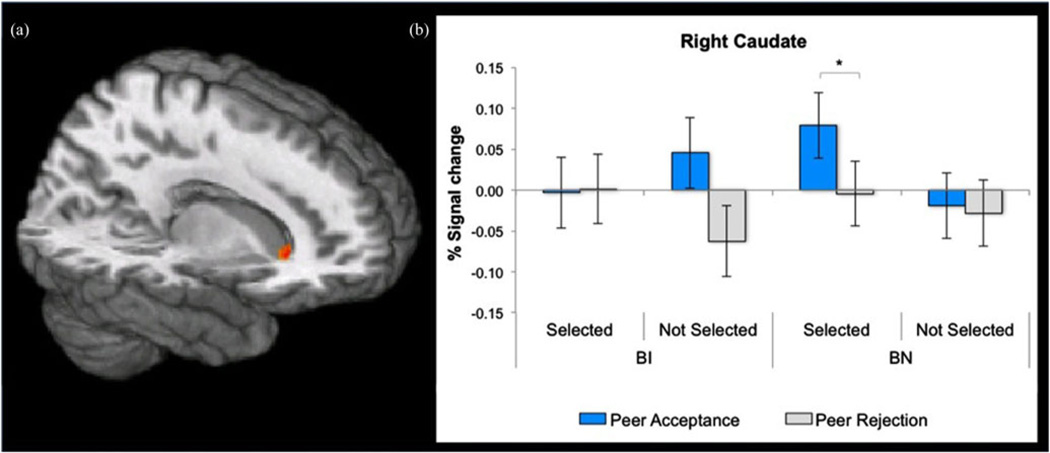
Feedback analyses focused on group differences in striatal responses when receiving acceptance and rejection from selected and not-selected peers. Again, large effect size group differences emerged. Overall, these differences manifested in a significant three-way Group × Peer-Selection × Feedback interaction in the caudate, F (1, 37) = 12.10, p , .001, d = 1.14 (Table 2, Figure 3a). Extracted signal change estimates were examined to decompose this three-way interaction (Figure 3b). Post hoc analyses indicated heightened striatal engagement selectively in the BN group. Specifically, differences within the BN group manifested as greater caudate activation to acceptance versus rejection from selected peers ( p < .05, d = 1.35; Figure 3b). In contrast, within the BI group, differences were not found in response to either feedback type from selected peers; at a trend level of significance, the BI group showed a greater response to acceptance versus rejection from not-selected peers (p = .07, d = 1.23). No main effects of group emerged, nor did any clusters exceed threshold for two-way Group × Peer Selection or Group × Feedback interactions.
Figure 3.
(Color online) Receipt of feedback in the region of interest analysis. (a) Statistical maps (x = 13, z = −1) showing a Group × Peer Selection × Feedback interaction effect centered on the caudate (peak coordinates: 15, 21, 2). (b) Among behaviorally noninhibited (BN) adolescents, greater caudate activation occurred in response to acceptance versus rejection feedback from selected peers. This pattern was not seen in behaviorally inhibited (BI) adolescents. *p < .05.
Whole brain analysis
Anticipation of feedback
A Group × Peer Selection interaction effect was found within the right precentral gyrus, F (1, 37) = 12.53, p < .001, d = 1.16 (Table 3). Because of our focus on regions within the social-information processing network (Nelson et al., 2005), this effect was not decomposed further. No main effects of group or peer selection were found.
Table 3.
Significant clusters based on a whole-brain analysis of main and interaction effects of group, selection, and feedback on neural activation during anticipation of and response to feedback
| Comparison | Region | BA | Peak Voxel Coordinates | No. of Voxels | F | p |
|---|---|---|---|---|---|---|
| Anticipation phase | ||||||
| Group × Selection | R. precentral gyrus | 3 | 52, −15, 37 | 61 | 12.53 | <.001 |
| Feedback phase | ||||||
| Group | L. fusiform | 37 | −45, −49, −18 | 54 | 15.65 | <.001 |
| Group × Selection | L. precuneus | 7 | −5, −57, 42 | 76 | 11.53 | <.005 |
| Group × Feedback | R. thalamus | — | 1, −9, 14 | 56 | 18.10 | <.001 |
| Group × Selection × Feedback | L. posterior cingulate | 23 | −3, −33, 16 | 93 | 14.01 | <.001 |
| L. occipital gyrus | 18 | −43, −87, 0 | 79 | 17.08 | <.001 | |
| L. superior temporal gyrus | 22 | −47, −23, −4 | 65 | 15.54 | <.001 | |
| R. occipital gyrus | 19 | 49, −81, 4 | 52 | 17.18 | <.001 | |
| R. middle temporal gyrus | 22 | 55, −45, 2 | 51 | 14.12 | <.001 | |
| L. occipital gyrus | 37 | −59, −71, 4 | 51 | 14.57 | <.001 |
Note: The statistical threshold for all effects was set at p < .005 with a spatial extent correction of 400 mm3 (50 contiguous 2-mm voxels). R., right; L., left; BA, Brodmann area; (—) BA does not apply; N = 39, df = 1, 37.
Receipt of feedback
As expected, group differences in several regions involved in social cognition (e.g., fusiform, STG) emerged (Table 3). No group differences were found in the amygdala, however. Amain effect of group on response to feedback was found on the left fusiform gyrus, F (1, 37) = 15.65, p < .001, d=1.30 (Table 3, Figure 4a). Signal change estimates extracted from this cluster (peak coordinates: −45, −49, −18) indicated heightened fusiform activation in the BI versus BN group, regardless of peer selection or feedback type (Figure 4b).
Figure 4.
(Color online) Receipt of feedback in the whole brain analysis. (a) Statistical maps (z = −18) showing a group main effect centered on the left fusiform gyrus (peak coordinates: −45, −49, −18). (b) Behaviorally inhibited (BI) versus behaviorally noninhibited (BN) adolescents showed greater fusiform activation across all feedback events. *p < .05.
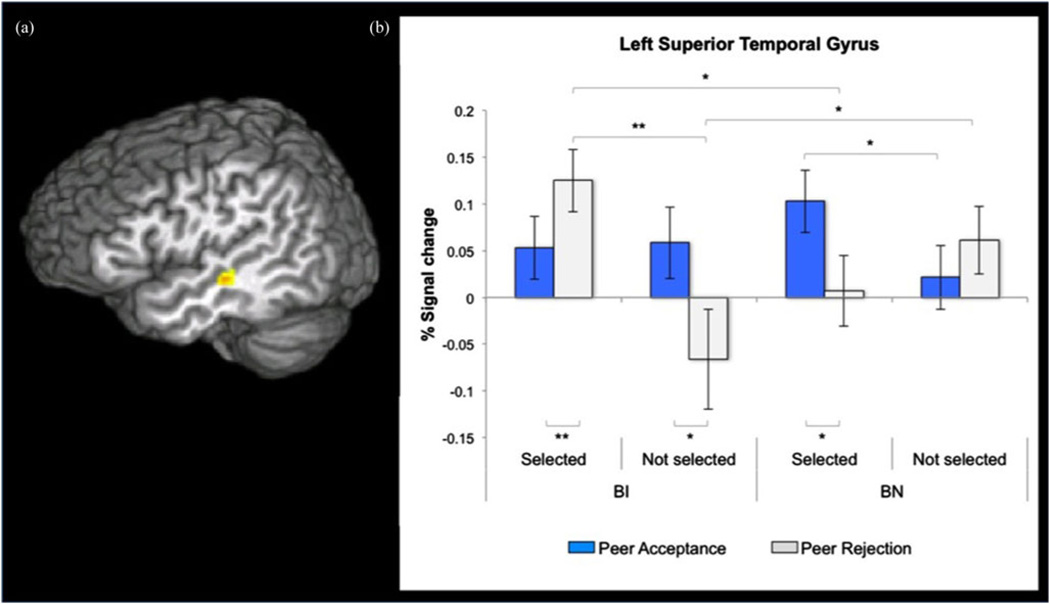
A Group × Peer Selection × Feedback interaction effect was found for a cluster in the left STG, F (1, 37) = 15.54, p < .001, d = 1.29 (Table 3, Figure 5a). Post hoc analyses of extracted signal change estimates from the STG cluster showed several significant between- and within-group differences in STG response to feedback type from selected and not-selected peers (Figure 5b). The BI versus BN group comparison showed significantly greater STG activation to rejection from selected peers (p = .03, d = 1.52), whereas the BN versus BI group comparison showed greater STG activation to rejection from not-selected peers (p = .05, d = 1.33). Within the BN group, greater STG response emerged to acceptance feedback from selected versus not-selected peers (p < .05, d = 1.35) and to acceptance versus rejection from selected peers ( p = .005, d = 1.95). Within the BI group, greater STG response was seen to rejection from selected versus not-selected peers (p = .001, d = 2.50), rejection versus acceptance from selected peers (p < .05, d = 1.35), and acceptance versus rejection from not-selected peers (p = .03, d = 1.54).
Figure 5.
(Color online) Receipt of feedback in the whole brain analysis. (a) Statistical maps (x = −47) showing a Group × Peer Selection × Feedback interaction effect centered on left superior temporal gyrus (STG) activation (peak coordinates: −47, −23, −5). (b) Behaviorally noninhibited (BN) adolescents showed greater STG activation to acceptance versus rejection feedback from selected peers. Behaviorally inhibited (BI) adolescents displayed heightened STG activation to rejection versus acceptance from selected peers and greater deactivation to rejection versus acceptance from nonselected peers. *p < .05. **p < .01.
Discussion
In this study we used an ecologically valid social evaluation paradigm that mimics a form of common peer-interaction contexts saturating the lives of today’s adolescents. We used this paradigm to compare adolescents characterized by BI versus BN temperament, as prospectively documented at multiple time points from infancy through early childhood. These two groups of adolescents showed distinct striatal responses when anticipating and receiving social feedback, which represent situations that can trigger withdrawal or anxiety in BI individuals. Consistent with prior work on striatal response to monetary rewards (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011), striatal sensitivity varied as a function of temperament, the peer delivering the feedback, and feedback valence.
When awaiting feedback from selected versus not-selected peers, putamen activity was heightened in BI adolescents. In contrast, when anticipating feedback from not-selected versus selected peers, BN adolescents showed heightened putamen activity. These patterns resemble the heightened striatal response, particularly in the putamen, observed in BI adolescents when anticipating monetary rewards (Guyer et al., 2006). It is interesting that, across these studies, such neural correlates consistently manifest in groups of adolescents who show no readily apparent differences in behavior, based on multiple rating scales or task performance at the time of scanning.
Studies of rodents and nonhuman primates indicate that within a reward context the putamen supports the selection of actions and the formation of habits (Hazy et al., 2010;Muranishi et al., 2011). This role contrasts with functions attributed to other striatal subregions, including regions that support aspects of reward-value processing (Haruno & Kawato, 2006; Muranishi et al., 2011; Peters & Buchel, 2010) or that are more flexibly engaged as a function of changes in goals (Hazy et al., 2010). Although previous work has demonstrated more extensive striatal responding among BI individuals to anticipated monetary rewards (Bar-Haim et al., 2009; Guyer et al., 2006), in the present study, heightened anticipatory activity was constrained to the putamen. It is also of note that prior findings of between-group differences in striatal function depended on task parameters. In some contexts, BI individuals show increased striatal activation; whereas in others, it is BN individuals who show such increased activation (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011; Jarcho et al., 2012). Nonetheless, because this is the first report of striatal response to social stimuli of differential salience, it is difficult to compare directly the current and prior findings. In the present study, the BI group demonstrated potentiated putamen activity when anticipating salient feedback from peers selected for an interaction. This may reflect BI individuals’ emotional sensitivity to social evaluation that may occur because of their investment in the peers they selected. The BN group had potentiated putamen activity when anticipating feedback from nonsalient (not-selected) peers. This latter finding may reflect that the BN group is more flexibly engaged than the BI group and prepared to update their goals given that feedback from unselected peers would be less expected than from selected peers. The BN group may also be more sensitive than the BI group to the potential consequence of their own rejection of others.
In contrast to anticipation, receipt of feedback revealed a markedly different pattern of temperament-related striatal responses. Caudate response was amplified during social reward delivery for the BN group. This pattern is consistent with other data on social reward processing (Gunther Moor et al., 2010; Guyer, Choate, Pine, et al., 2012; Guyer et al., 2009; Izuma et al., 2008; Jones et al., 2011; Kampe et al., 2001; Spreckelmeyer et al., 2009). It is interesting that caudate activation in BN adolescents occurred selectively to social reward delivery from selected peers but not to positive feedback from not-selected peers or to negative feedback from either set of peers. For the BN group, reward-value processing appears to be selectively engaged by positive feedback from desired peers.
Relative to BN adolescents, the caudate of BI adolescents was relatively unresponsive to social feedback, particularly positive feedback from selected peers. This pattern in behavioral inhibition appears atypical, relative to findings observed in other research linking the caudate to reward delivery (Helfinstein et al., 2011). As with group differences during reward anticipation, the blunted striatal response to reward receipt among BI adolescents may reflect a lasting imprint of early-childhood behavioral inhibition on neural processing of social reward (Guyer et al., 2006). Thus, blunted caudate responding may manifest specifically in contexts where strong social motivation exists amidst concerns over negative consequences of social interactions (Fox et al., 2005; Rubin et al., 2009). Although behavioral reports indicated that all adolescents expected positive feedback from selected peers and negative feedback from not-selected peers, the current data suggest that, at the neurocircuitry level, the BI group’s neural response to feedback may be viewed as atypical. Specifically, the striatum may be highly responsive when anticipating stimuli selected by the individual but relatively blunted when positive social feedback is delivered (Jones et al., 2011; Schultz, 2006). We also found that BI versus BN adolescents used “unfriendly” and “not fun” more frequently for peers they did not select. Behavioral inhibition may reflect atypical integration via the striatum of social feedback into expectations and behavioral responses. The suggested atypical neural responsiveness in inhibited individuals is further supported by the finding that the observed BN striatal response to social acceptance closely resembles findings from typically developing adolescents (Gunther Moor et al., 2010; Guyer, Choate, Pine, et al., 2012).
At the behavioral level, early-childhood behavioral inhibition is associated with social withdrawal and difficulty engaging with peers (Fox et al., 2005; Rubin et al., 2009). Physiological studies link these early behavioral signatures to variations in cortisol, electroencephalography activity, and autonomic physiology (Fox et al., 2005). Recent work found that, among highly inhibited preschoolers who were more socially connected to peers, stress reactivity (as indexed by cortisol levels) increased across the school year, suggesting that becoming more socially integrated (e.g., having many friends, attaining popularity) may have been more challenging (Tarullo, Mliner, & Gunnar, 2011). Neuroimaging data suggest that these childhood patterns reflect associations between behavioral inhibition and functioning in two neural circuits: perturbed amygdala response to fearful faces (Perez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003) and striatal alterations and associated approach-related responses to reward anticipation or delivery (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011). Because social engagement is important for healthy adolescent development, it is not surprising that in typical adolescents the striatum responds to positive social feedback. Although more attention has focused on altered amygdala response to social threat in adolescent anxiety and behavioral inhibition (Guyer et al., 2008; McClure et al., 2007; Perez-Edgar et al., 2007; Schwartz et al., 2003), the current data suggest that altered striatal function may also play an important role in guiding appropriate affective responses in social contexts.
Socially anxious and BI youth both display striatal hyperactivity during anticipation of monetary incentives, a pattern not observed in other forms of anxiety (Guyer, Choate, Detloff, et al., 2012). Because individuals with BI and social anxiety both tend to withdraw from and avoid social interactions, striatal functional anomalies in social contexts may be seen in both BI and social anxiety. Adults with social anxiety show striatal alteration related to public speaking (Lorberbaum et al., 2004) and striatal dopamine dysfunction (Schneier et al., 2000; Tiihonen et al., 1997), although inconsistently (Schneier et al., 2009). Work in animal models also suggests that the striatum mediates altered social behavior in response to social stressors (Trainor, 2011).
In addition to the striatum, regions involved in social cognition also differed by temperament classification in response to social feedback. Beyond the striatum, multiple other brain regions have been implicated in aspects of adolescent social development. These include regions such as the fusiform and the temporal association cortex (Nelson et al., 2005). Findings in the current study did arise for some of these regions. For example, in the posterior fusiform gyrus, which is implicated in initial perceptual processing of faces (Saxe, 2006), the BI group showed heightened activation across both feedback trial types, whereas the BN group displayed minimal activation. Because the BI group’s fusiform response was invariant to trial type, it is possible that this pattern reflects a heightened sensitivity to a range of social stimuli. Given that the earliest signs of behavioral inhibition manifest as hyperresponsiveness to broad classes of sensory stimuli, this neural pattern may be a remnant of the sensory sensitivity first identified in infancy (Marshall, Reeb, & Fox, 2009). Prior work suggests social perception occurs relatively early in the visual processing stream (Adolphs, 2001; Haxby et al., 2000), before reward or goal relevance is ascertained. Alternatively, this hyperresponsiveness could arise from late-processing stream structures feeding back onto early perceptual systems (Perez-Edgar et al., 2007; Schwartz et al., 2003), although no such between-group differences in global processing were analyzed here.
BI and BN adolescents displayed similar STG activation to selected peers who reciprocated their interest. However, BI versus BN adolescents showed opposite STG response patterns to selected peers who in turn rejected them. The STG likely supports high-level integration of social information in which sensory and affective stimuli converge and social meaning is instantiated (Blakemore, 2008; Redcay, 2008). Our findings suggest that whereas BI and BN adolescents similarly integrate sensory and affective meaning for liked peers who provided positive feedback, they differ on the integration of social information gleaned from liked but rejecting peers. Such between-group differences in STG function may support underlying social cognitive biases found among BI youth (Perez-Edgar et al., 2010).
Some limitations should be considered when interpreting the results of the current study. First, only 5% of the BI group included here met clinical diagnosis criteria for SAD. To some degree, exclusion criteria limited our ability to study BI and BN adolescents with and without SAD. This is because we excluded participants taking medications or in need of acute treatment. Nevertheless, other factors also could have played a role. For example, the low rate of SAD may reflect the small sample size of this group or reveal resilience to this disorder at this particular age for youth with early-childhood behavioral inhibition.
Second, another limitation is that we only assessed striatal function at one point in time. As a result, it is impossible to disentangle striatal effects that are causal versus consequential of temperamental status. For example, early temperament’s association with later social experience and peer relationships could mediate the associations that were observed here. The lack of current or past peer functioning measures in our analyses is a limitation to be addressed in future work. Nonetheless, our findings do show that persistent patterns of inhibited social behavior in infancy and early childhood, as documented by multiple informants using multiple methods at multiple time points, predicts differentiated neural response patterns in late adolescence. Thus, it will be critical for future work to examine engagement of these brain regions in large samples of adolescents characterized by early temperament and SAD. It will also be important to consider the other possible mediators in children’s social environments that might contribute to the associations that were observed here.
Third, our work presented here focused solely on response to social rewards whereas our past work focused on response to nonsocial rewards (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011; Perez-Edgar et al., 2013). Although similar patterns across tasks suggest generalizability, in order to fully determine unique and common temperament-based striatal response patterns to these cues, neural responses will need to be directly compared to both anticipated and delivered social and nonsocial rewards in a single population and in a single task.
Fourth, although our hypotheses for this study centered specifically on striatal engagement to social reward, we also examined amygdala responsivity to social threat (e.g., bids of peer rejection).We did not find evidence of differences in amygdala response to peer rejection between BI and BN adolescents. In contrast, clinical studies have revealed different patterns of amygdala activity to anticipated and received peer feedback in adolescents with social anxiety (Guyer et al., 2008; Lau et al., 2011) using a variant of the present task. The lack of differential amygdala activation in the current study may be a consequence of an older sample or due to the fact that the current study’s sample did not meet the same clinical criteria for social impairment. Future work on behavioral inhibition should test hypotheses regarding neural response to social evaluation using other tasks that might probe amygdala engagement in a context that is highly relevant to this form of temperament.
Fifth, the current study is based in a relatively small sample. Future studies in larger samples would be able to explore factors within the BI or BN group that might predict more fine-grained differences in neural responding to social feedback. Such factors might include variations in psychopathology, such as anxiety, depression, or substance use, as well as social experiences, such as history of peer victimization.
Despite these limitations, the functional differences observed between BI and BN youth in the recruitment of the striatum in a socially evaluative context provide two key contributions to the study of developmental psychopathology. First, what we learn about atypical adolescent development helps to delineate the boundaries separating typical from atypical variations in development (Cicchetti & Rogosch, 2002), and this holds true at the interface of neurobiology, behavior, and experience (Cicchetti & Thomas, 2008; Cicchetti & Tucker, 1994). Peer relationships are a prominent context for adolescent development, with negative peer evaluation a typical concern for most adolescents, and a highly avoided situation for BI youth. Our ecologically valid, social evaluation neuroimaging paradigm allowed us to leverage the significance of peer contexts, and capture adolescents’ neural and affective responses just prior to and after receipt of peer evaluation. In both past work (Gunther Moor et al., 2010; Guyer, Choate, Pine, et al., 2012) and the present study, peer acceptance but not rejection is associated with increased activity in the brain’s social motivation and reward circuitry in typically developing adolescents (here, BN adolescents). These results provide clues for future research about how social stimuli deemed desirable by youth with extreme temperamental behavioral inhibition are perceived, interpreted, and responded to.
Second, by identifying neural correlates of responses to peer feedback in youth who have a history of extreme social reticence and behavioral inhibition, we can expand our knowledge of the potential mechanisms that may lead to psychopathology later in development, particularly for at-risk youth (Beesdo et al., 2007). This approach will improve our ability to generate more effective interventions for youth who are wary of, and hypersensitive to, social situations. Given the reduced engagement of reward circuitry in contexts that likely induce temperament-based social fears observed in the current study, such interventions for extremely shy and inhibited individuals might focus on learning and reinforcement of socially valued cues and increasing motivation toward social stimuli. Furthermore, given that the social difficulties differentiating these temperament groups were more apparent earlier in development, the present findings suggest that intervening with extremely socially hesitant and withdrawn young children could reshape the brain’s coding of motivated behavior toward peers. Work that used a variant of the present study’s Chatroom Task found that extremely shy 4- to 7-year-old boys showed particular sensitivity to both positive and negative bids of peer evaluation (Howarth, Guyer, & Perez-Edgar, 2013).
Conclusion
Among BN adolescents, recruitment of the striatum and other social information-processing network regions (Nelson et al., 2005) suggests increased salience of mutually reinforcing social events. In contrast, BI youth demonstrated a neural response pattern that emphasized salience of social stimuli that they had selected and relative nonresponse of the striatum to receipt of social feedback. These differential neural patterns in adolescence are likely associated with the social reticence of early-childhood behavioral inhibition (Helfinstein, Fox, & Pine, 2012). The present study cannot distinguish direct, causal relationships between social withdrawal and alterations in socially relevant neuronal networks, especially in adolescents who have accumulated extensive social experience in which expectations and behavioral patterns have become established. Nonetheless, the altered responses to novel sensory experiences documented in infancy, which may be linked to striatal function, and patterns of striatal responsivity that are similar within social and nonsocial contexts indicate that striatal functional differences represent a biological index related to early-appearing behavioral inhibition that varies in response to social experience.
Acknowledgments
This research was supported by the Intramural Research Program of NIMH, an NIMH Seymour S. Kety Memorial Award (to A.E.G.), and NIMH Grants MH080076 (to A.E.G.) and MH074454 (to N.A.F.). The authors thank Harvey Iwamoto for technical support and all of the families who participated in the study.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Allen JP, Porter MR, McFarland FC, Marsh P, McElhaney KB. The two faces of adolescents’ success with peers: Adolescent popularity, social adaptation, and deviant behavior. Child Development. 2005;76:747–760. doi: 10.1111/j.1467-8624.2005.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almas AN, Phillips DA, Henderson HA, Hane AA, Degnan KA, Moas OL, et al. The relations between infant negative reactivity, nonmaternal childcare, and children’s interactions with familiar and unfamiliar peers. Social Development. 2011;20:718–740. doi: 10.1111/j.1467-9507.2011.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asendorpf JB, Meier GH. Personality effects on children’s speech in everyday life: Sociability-mediated exposure and shyness mediated reactivity to social situations. Journal of Personality and Social Psychology. 1993;64:1072–1083. doi: 10.1037//0022-3514.64.6.1072. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nature Neuroscience. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Boivin M, Hymel S, Burkowski WM. The roles of social withdrawal, peer rejection, and victimization by peers in predicting loneliness and depressed mood in childhood. Development and Psychopathology. 1995;7:765–785. [Google Scholar]
- Brown BB. Adolescents’ relationships with peers. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. 2nd ed. Hoboken, NJ: Wiley; 2004. pp. 363–394. [Google Scholar]
- Burgess KB, Wojslawowicz JC, Rubin KH, Rose-Krasnor L, Booth-LaForce C. Social information processing and coping strategies of shy/withdrawn and aggressive children: Does friendship matter? Child Development. 2006;77:371–383. doi: 10.1111/j.1467-8624.2006.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, DeSouza AT, Chen H, Wang L. Reticent behavior and experiences in peer interactions in Chinese and Canadian children. Developmental Psychology. 2006;42:656–665. doi: 10.1037/0012-1649.42.4.656. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, et al. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70:6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Thomas KM. Imaging brain systems in normality and psychopathology. Development and Psychopathology. 2008;20:1023–1027. doi: 10.1017/S0954579408000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: Distinguishing among reticence and passive and active solitude among children. Child Development. 1994;65:129–137. [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Egger HL, Pine DS, Nelson EE, Leibenluft E, Ernst M, Towbin K, et al. The NIMH Child Emotional Faces Picture Set (NIMHChEFS): A new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research. 2011;20:145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience & Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanish LD, Guerra NG. Predictors of peer victimization among urban youth. Social Development. 2000;9:521–543. [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus–action–reward association learning. Journal of Neurophysiology. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O’Reilly RC. Neural mechanisms of acquired phasic dopamine responses in learning. Neuroscience & Biobehavioral Reviews. 2010;34:701–720. doi: 10.1016/j.neubiorev.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and noninhibited adolescents. Neuropsychologia. 2011;49:479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Fox NA, Pine DS. Approach–withdrawal and the role of the striatum in the temperament of behavioral inhibition. Developmental Psychology. 2012;48:815–826. doi: 10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- Howarth GZ, Guyer AE, Perez-Edgar K. Young children’s affective responses to acceptance and rejection from peers: A computer- based task sensitive to variation in temperamental shyness and gender. Social Development. 2013;22:146–162. doi: 10.1111/sode.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, et al. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology. 2012;92:306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, et al. Behavioral and neural properties of social reinforcement learning. Journal of Neuroscience. 2011;31:13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Galen’s prophecy: Temperament in human nature. Boulder, CO: Westview Press; 1997. [Google Scholar]
- Kagan J, Fox NA. Handbook of child psychology. Hoboken, NJ: Wiley; 2007. Biology, culture, and temperamental biases. [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School- Age Children—Present and LifetimeVersion (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Lopez N. Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26:83–94. doi: 10.1023/a:1022684520514. [DOI] [PubMed] [Google Scholar]
- Lau JY, Guyer AE, Tone EB, Jenness J, Parrish J, Pine DS, et al. Neural responses to peer rejection in anxious adolescents: Contributions from the amygdala–hippocampal complex. International Journal of Behavioral Development. 2011 doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. NeuroReport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science. 2009;12:568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld AT, Gluck MA, Stark CE. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learning & Memory. 2011;18:703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Muranishi M, Inokawa H, Yamada H, Ueda Y, Matsumoto N, Nakagawa M, et al. Inactivation of the putamen selectively impairs reward history-based action selection. Experimental Brain Research. 2011;209:235–246. doi: 10.1007/s00221-011-2545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meesters C, Merckelbach H, Sermon A, Zwakhalen S. Worry in normal children. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:703–710. doi: 10.1097/00004583-199807000-00009. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, et al. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Neural representations of subjective reward value. Behavioural Brain Research. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!” Neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Pathophysiology of childhood anxiety disorders. Biological Psychiatry. 1999;46:1555–1566. doi: 10.1016/s0006-3223(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neuroscience Biobehavioral Reviews. 2008;32:123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rimm-Kaufman SE, Kagan J. Infant predictors of kindergarten behavior: The contribution of inhibited and uninhibited temperament types. Behavioral Disorders. 2005;30:331–347. [Google Scholar]
- Rowe DC, Plomin R. Temperament in early childhood. Journal of Personality Assessment. 1977;41:150–156. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) University of Waterloo; 1989. [Google Scholar]
- Rubin KH, Bukowski WM, Parker JG. Peer interactions, relationships, and groups. In: Eisenberg NE, Damon WE, Lerner RME, editors. Handbook of child psychology: Vol 3. Emotional, and personality development. 6th ed. Hoboken, NJ: Wiley; 2006. pp. 571–645. [Google Scholar]
- Rubin KH, Coplan RJ, Bowker JC. Social withdrawal in childhood. Annual Review of Psychology. 2009;60:141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16:235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Abi-Dargham A, Martinez D, Slifstein M, Hwang DR, Liebowitz MR, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depression and Anxiety. 2009;26:411–418. doi: 10.1002/da.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M. Low dopamine D(2) receptor binding potential in social phobia. American Journal of Psychiatry. 2000;157:457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Silverman WK, La Greca AM, Wasserstein S. What do children worry about? Worries and their relation to anxiety. Child Development. 1995;66:671–686. doi: 10.1111/j.1467-8624.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Mliner S, Gunnar MR. Inhibition and exuberance in preschool classrooms: Associations with peer social experiences and changes in cortisol across the preschool year. Developmental Psycholology. 2011;47:1374–1388. doi: 10.1037/a0024093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Bergström K, Lepola U, Koponen H, Leinonen E. Dopamine reuptake site densities in patients with social phobia. American Journal of Psychiatry. 1997;154:239–242. doi: 10.1176/ajp.154.2.239. [DOI] [PubMed] [Google Scholar]
- Trainor BC. Stress responses and the mesolimbic dopamine system: Social contexts and sex differences. Hormones and Behavior. 2011;60:457–469. doi: 10.1016/j.yhbeh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal–ventral divide of the striatum. Trends in Neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]