Abstract
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide. Serum alpha-fetoprotein (AFP) is the conventional biomarker currently used in clinical diagnosis of this malignancy. However, AFP is not reliable for early diagnosis, and especially the sensitivity and specificity of AFP in HCC diagnosis are not optimal. Early detection of HCC is an important issue because of the very poor prognosis and usually no more than 6 months survival after diagnosis. Therefore, there is a need for the development of more sensitive and specific methods that can supplement AFP in the early detection of this cancer. In this study, autoantibody responses to 14-3-3ζ in HCC were evaluated by enzyme-linked immunosorbent assay (ELISA), western blot, and indirect immunofluorescence assay. Immunohistochemistry (IHC) with tissue array slides was also performed to analyze protein expression of 14-3-3ζ in HCC and control tissues. The prevalence of autoantibodies against 14-3-3ζ was 16.7 % (28/168) in HCC, which was significantly higher than that in liver cirrhosis (LC), chronic hepatitis (CH), and normal human sera (NHS) (P<0.01). The average titer of autoantibodies against 14-3-3ζ in HCC sera was higher compared to that in LC, CH, and NHS (P<0.01). In the further study, anti-14-3-3ζ antibodies have been detected in the sera from several HCC patients with serial bleeding samples. A stronger reactive band with 14-3-3ζ in western blot can be seen in sera at 9 months before the clinical diagnosis of HCC. Our preliminary data indicate that anti-14-3-3ζ autoantibodies may be potential biomarkers for early-stage HCC screening and diagnosis.
Keywords: Hepatocellular carcinoma (HCC), 14-3-3ζ, Tumor-associated antigens (TAAs), Immunodiagnosis
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death throughout the world. The overall survival of patients with HCC remains poor. The majority of people with HCC will die within 6 months of its detection. This high case fatality rate can in part be attributed to a lack of diagnostic methods that allow early detection. Although alpha-fetoprotein (AFP) is the most effective serological marker available to detect HCC, the sensitivity and specificity is not optimal. Hepatitis B or C viral infections are the main risk factors for HCC, but the molecular pathogenesis remains incompletely defined in HCC [1–6]. Many studies demonstrated that cancer sera contain antibodies which react with a unique group of autologous cellular antigens generally known as tumor-associated antigens (TAAs). During the progression from chronic liver disease to HCC, the novel autoantibodies appearing with malignant transformation will be more likely to be related to events associated with tumorigenesis, and therefore these autoantibodies can be used as reporters identifying aberrant cellular mechanisms in tumorigenesis and also serve as immunodiagnostic markers for cancer detection. Due to the poor prognosis and difficult early diagnosis of HCC, it is imperative to identify more sensitive and specific biomarkers in HCC detection.
The 14-3-3 protein is an adaptor protein that localizes to the leading edge of spreading cells, returning to the cytoplasm as spreading ceases. In mammals, there are seven distinct isoforms: β, γ, ε, ζ, η, σ, and τ that are encoded by seven different 14-3-3 genes [7]. The 14-3-3 isoforms had been linked to carcinogenesis because they are involved in various cellular processes such as cell cycle regulation, apoptosis, proliferation, and differentiation [8]. It is implicated in the regulation of a large spectrum of both general and specialized signaling pathway. The 14-3-3 proteins lack endogenous enzymatic activity; however, they function through binding to phosphorylatedserine/threonine motifs on their target proteins. Following binding, target proteins can be regulated by 14-3-3 through a number of mechanisms including changing the conformation of the protein, affecting protein activity or stability, facilitating protein complex formation, or altering protein subcellular localization. Binding generally results in the modulation of the activity of the binding partner [9]. Some studies revealed that 14-3-3 proteins can bind to specific pSer/pThr containing motifs in protein targets which indicates a role for Ser/Thr phosphorylation in the assembly of protein– protein complexes [10]. Other studies find that 14-3-3 proteins can also recognize unphosphorylated motifs [11–14]; 14-3-3ζ is a ubiquitously expressed 14-3-3 family member that has been implicated to have oncogenic potential through its interaction with target proteins involved in cancer initiation (Rafs, p85PI3K, Bad, FOXO transcription factors) as well as cancer progression (Snail, TRI). Elevated expression of 14-3-3ζ has been observed in a variety of tumor types [15].
To improve the effectiveness of diagnosis and reduce the mortality of HCC, it is important to search for tumor-specific biomarkers whose function may involve in HCC progression and which may be useful as potential biomarker of HCC early diagnosis, prognosis and therapeutic targets. In this study, a cancer-related protein, 14-3-3ζ was evaluated by immunoassay as a potential TAA in HCC, and autoantibody to this protein was also validated to be a biomarker in immunodiagnosis of HCC.
Materials and methods
Sera and general information
All sera used in this study, including 168 sera from patients with HCC, 30 sera from patients with liver cirrhosis (LC), 30 sera from patients with chronic hepatitis (CH), and 89 normal human sera (NHS) and 18 sera from three HCC patients with serial bleeding samples, were obtained from the serum bank of Cancer Autoimmunity and Epidemiology Research Laboratory at UTEP (University of Texas at El Paso). This study was approved by the Institutional Review Board of UTEP and collaborating institutions.
All HCC patients were diagnosed according to the criteria described in a previous study [16] and had not received treatment with any chemotherapy or radiotherapy. Patients with CH and LC were followed up at least 18 months after collecting blood to exclude individuals with primary biliary cirrhosis and asymptomatic or clinically undetectable HCC. Normal human sera were collected from individuals who had no obvious evidence of malignancy at the same locality during annual health examinations.
Recombinant proteins and antibodies used in this study
The 14-3-3ζ construct GST-14-3-3 WT (pGEX) (plasmid ID 1944) purchased from Addgene (Addgene Inc., Cambridge, MA) was subcloned into pET28a vector producing a fusion protein with NH-terminal 6× histidine and T7 epitope tags. The recombinant protein expressed in Escherichia coli BL21 (DE3) was purified using nickel column chromatography. Polyclonal anti-14-3-3ζ rabbit antibody and monoclonal anti-β-actin mouse antibody were purchased (Cell Signaling Technology, Inc., Danvers, MA). Horseradish peroxidase (HRP)-conjugated goat antihuman IgG, HRP-conjugated goat anti-rabbit IgG, HRP-conjugated goat anti-mouse IgG, and FITC-conjugated goat antihuman IgG were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Anti-rabbit IgG Fab2 (Alexa Fluor 488) was purchased from Cell Signaling Technology, Inc., Danvers, MA.
Cell lines and cell extracts
Nine different tumor cell lines including human non-small cell lung carcinoma (H1299), human ovarian carcinoma (SKOV3), human leukemia (KOPN63), human hepatocellular carcinoma (HepG2), human hepatocellular carcinoma (SNU449), human epitheloid cervical carcinoma (Hela), human urinary bladder carcinoma (T24), human epidermoid carcinoma (Hep2), and human small cell lung cancer (H146) were obtained from the Tumor Cell bank of our laboratory and cultured following the specific protocol for each cell line. Cells grown in monolayers were directly solubilized in Laemmli's sample buffer containing protease inhibitors and then briefly sonicated before electrophoresis on sodium dodecyl sulfate (SDS)–polyacrylamide gels.
Enzyme-linked immunosorbent assay (ELISA)
Standard protocol for ELISA was used as described in our previous study [17, 18]. In brief, a 96-well microtiter plate (ImmunoChemistry Technologies, LLC, Bloomington, MN) was coated overnight at 4 °C with recombinant 14-3-3ζ protein at a final concentration of 0.5 µg/ml in phosphate buffered saline (PBS). The antigen-coated wells were blocked with gelatin post-coating solution at room temperature for 2 h. Human sera diluted at 1:100 with serum diluent were incubated for 2 h at room temperature in the antigen-coated wells, followed by HRP-conjugated goat antihuman IgG (Caltag Laboratories, San Francisco, CA) at 1:4,000 dilution. The substrate 2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS, Sigma-Aldrich, St. Louis, MO) was used to detect the immune complexes. The average optical density (OD) value at a wavelength of 405 nm was used for data analysis. The cutoff value designating positive reaction was the mean OD value of 89 normal human sera plus three standard deviations (SD).
Western blot
Denatured recombinant 14-3-3ζ protein and cancer cell lysates were electrophoresed on 10 % SDS-PAGE and transferred to a nitrocellulose membrane. After blocking in PBS with 5 % nonfat milk and 0.05 % Tween-20 for 1 h at room temperature, the nitrocellulose membranes were incubated overnight at 4 °C with 1:200 dilution of human sera, 1:500 dilution of polyclonal anti-14-3-3ζ antibody, or 1:500 dilution of monoclonal anti-β-actin mouse antibody, separately. HRP-conjugated goat antihuman IgG, HRP-conjugated goat anti-rabbit IgG, and HRP-conjugated goat anti-mouse IgG were subsequently applied as secondary antibody at a 1:10,000 dilution. The ECL kit was used to detect immunoreactive bands according to the manufacturer's instructions (Thermo Scientific, Waltham, MA).
Indirect immunofluorescence assay (IIFA) and confocal microscopy
An indirect immunofluorescence assay was performed on Hep2 antinuclear antigen tissue slides (Bion Enterprises, Des Plaines, IL). The sera were diluted at 1:80 in phosphate-buffered saline (PBS), pH 7.4 and incubated with the slides for 30 min at room temperature. After extensive washing, the slides were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG secondary antibody (Santa Cruz Biotechnology, CA) or anti-rabbit IgG Fab2 (Alexa Fluor 488) as secondary antibody diluted with 1:100 in PBS for 1 h at room temperature. The slides were washed two times with PBS before adding a drop of mounting media containing 1.5 µg/mL 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc. Burlingame, CA). To prevent photo bleaching, the latest steps were performed in the dark. The slides were then examined under fluorescence microscopy, with LSM 700 Confocal Microscope (Zeiss), at ×400 magnification, and Zen 2009 software was used for images capture and analysis.
Absorption of antibodies with recombinant protein
The diluted human sera (1:80) were incubated overnight at 4 °C with recombinant 14-3-3ζ protein, at a final concentration of 0.03 µg/µl, and then centrifuged at 10,000×g for 10 min. The antigen-absorbed supernatant was used for the immunofluorescence assay.
Immunohistochemistry (IHC) with tissue array slides
Liver cancer tissue array slides with normal tissue controls (38 cases/80 cores, including pathological diagnosis and pathological grades, ten normal liver tissues as controls) were purchased (US Biomax, Inc., Rockville, MD) and used to detect the expression of the 14-3-3ζ protein. Tissue array slides were deparaffinized with xylene and dehydrated with ethanol. Antigen retrieval was performed by microwave heating methods in Trilogy™ pretreatment solution for 20 min. Avidin/biotin blocking solution was used to prevent nonspecific binding of antibodies. The tissue sections were incubated with polyclonal anti-14-3-3ζ antibody (1:100 dilution) for overnight at 4 °C. HRP Detection System (HRP streptavidin label and polyvalent biotinylated link) and diaminobenzidine (DAB) Substrate Kit were used as detecting reagents. After counterstaining with hematoxylin, the sections were dehydrated and mounted. The slides were observed by light microscopy (Leica DM1000, Germany).
Statistical analysis
The mean OD value of each group of patients' sera was compared using the Mann–Whitney U test; the frequency of autoantibody to TAAs in each group of patients' sera and the expression profile of 14-3-3ζ in liver cancer and normal tissue groups were compared using the chi-square test (χ2) test with Fisher's exact test, and two significant levels (0.05 and 0.01) were used.
Results
Frequency and titer of autoantibodies against 14-3-3ζ in HCC
The full-length recombinant 14-3-3ζ protein was used as coating antigen in ELISA to screen autoantibodies against 14-3-3ζ in sera from patients with HCC, LC and CH as well as NHS. In total, 168 sera from patients with HCC, 30 from LC, 30 from CH, and 89 sera from normal human individuals were used in this study. As shown in Table 1, the prevalence of autoantibody against 14-3-3ζ was 16.7 % (28/168) in HCC, which was significantly higher than that in LC, CH, and NHS (P<0.01). The titer of anti-14-3-3ζ antibodies in human sera is shown in Fig. 1. The titer of anti-14-3-3ζ antibodies in sera from some of the HCC patients was much higher than that in other groups. The average titer of autoantibody against 14-3-3ζ in HCC sera was higher than that in LC, CH, and NHS (P<0.01). The ELISA results were also confirmed by western blot analysis. Figure 2 shows that representative HCC sera with positive reaction to 14-3-3ζ in ELISA also have strong reactivity in western blot compared to LC, CH, and normal sera. Autoantibody to 14-3-3ζ in serial serum samples from three HCC patients (cases #1, 2, and 3) was also tested. The ELISA and western blot results are shown in Fig. 3. In HCC case 1 and HCC case 2, anti-14-3-3ζ autoantibody were stronger at 6 months before HCC was detected. In HCC case 3, anti-14-3-3ζ autoantibody became stronger at 9 months before the date of HCC diagnosed.
Table 1.
Frequency of autoantibody against 14-3-3ζ in human sera by ELISA
| Type of sera | No. tested | Autoantibody to 14-3-3ζ (%) |
|---|---|---|
| HCC | 168 | 28 (16.7)** |
| LC | 30 | 0 |
| CH | 30 | 0 |
| NHS | 89 | 1 (1.1) |
Cutoff value, mean+3 SD of NHS
HCC hepatocellular carcinoma, LC liver cirrhosis, CH chronic hepatitis, NHS normal human sera
P<0.01 (value relative to NHS, CH, and LC)
Fig. 1.
Titer of autoantibody against 14-3-3ζ in human sera by ELISA. The range of antibody titers to 14-3-3ζ was expressed as OD obtained from ELISA. The mean + 3 SD of NHS are shown in the relationship to all serum samples. Titer of anti-14-3-3ζ in HCC is much higher than that in other types of sera (P<0.01)
Fig. 2.

Western blot analysis with representative sera in ELISA. Lane 1, the polyclonal anti-14-3-3ζ antibody was used as positive control. Lanes 2, 3, and 4, three representative HCC sera which were positive in ELISA also had strong reactivity with 14-3-3ζ recombinant protein in western blot analysis. Lanes 5, 6, and 7, randomly selected LC, CH, and NHS sera, respectively, with negative reactivity with 14-3-3ζ recombinant protein
Fig. 3.
Autoantibody to 14-3-3ζ in serial serum samples from three HCC patients (cases 1, 2 and 3). a First HCC patient (case #1) was diagnosed on Feb 7, 1995 with strong expression of anti-14-3-3ζ autoantibody. Totally, we had six serum samples within more than 1 year of time span collected from this HCC patient, and the 14-3-3ζ reactive bands were present in all of them. It became stronger at month 6 before HCC was detected. b Total of six serum samples were acquired from the second HCC patient (case #2). All of them had 14-3-3ζ reactive bands. Anti-14-3-3ζ autoantibody became stronger at month 6 before the date (Jul 2, 1993) of HCC diagnosed. c The third HCC patient (case #3) was diagnosed at the date of Apr 9, 1992, with a strong 14-3-3ζ reactive band. During a 1-year period (Apr 25, 1991 to Jul 16, 1992), six serum samples collected from this patient showed a gradually increased reactive band with 14-3-3ζ. Clearly, a stronger reactive band can be observed in the serum at 9 months before the diagnosis of HCC
Detection of intense nuclear staining pattern showed in Hep2 cells by indirect immunofluorescence assay with representative positive HCC sera
To further confirm the reactivity of autoantibody in HCC sera to 14-3-3ζ and the intracellular location of 14-3-3ζ, commercially purchased Hep2 cell slides were used in indirect immunofluorescence assay to detect HCC sera with anti-14-3-3ζ positive in ELISA. As shown in Fig. 4a representative anti-14-3-3ζ positive HCC serum had an intense cytoplasmic staining pattern, which was similar in fluorescent staining pattern and cellular location to that shown by polyclonal anti-14-3-3ζ antibody. This fluorescence signal was significantly reduced when the same HCC serum was pre-absorbed with recombinant 14-3-3ζ protein.
Fig. 4.
Representative immunofluorescence staining pattern of anti-14-3-3ζ antibody-positive HCC serum. a NHS were used as a negative control. b Polyclonal anti-14-3-3ζ antibody which showed a cytoplasmic immunofluorescence staining pattern was used as a positive control. c A representative anti-14-3-3ζ antibody-positive HCC serum demonstrated an intense cytoplasmic staining pattern. d The same HCC serum used in panel c was pre-absorbed with recombinant 14-3-3ζ. The fluorescent staining of cytoplasm was significantly reduced
Expression of 14-3-3ζ in liver cancer tissues and normal hepatic tissues by immunohistochemistry
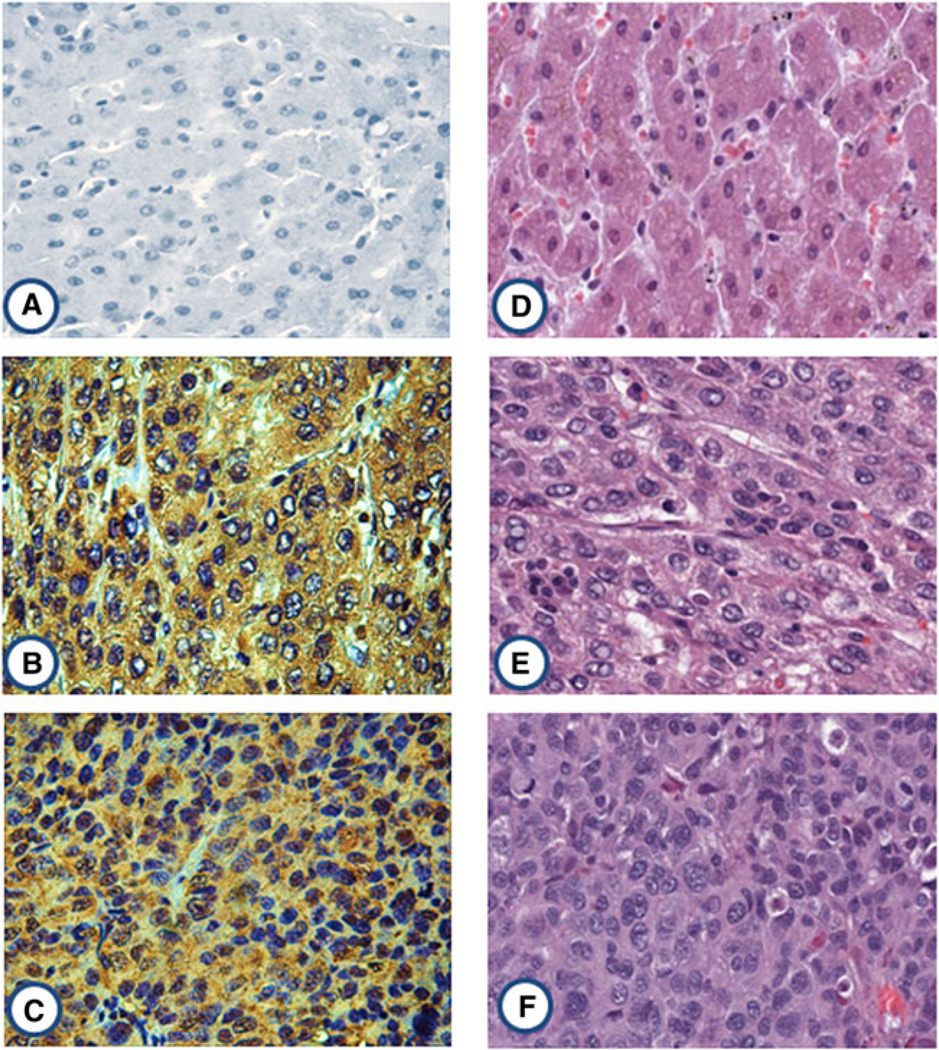
In the current study, the expression profile of 14-3-3ζ in liver cancer tissues and normal liver tissues was examined by immunohistochemistry with tissue array slides. Tissue array slides were commercially available for this study, including 30 liver tissues from HCC patients and ten from normal hepatic tissue donors. The polyclonal anti-14-3-3ζ antibody was used as primary antibody to detect the expression of 14-3-3ζ in liver cancer and normal hepatic tissues. As a result, 21 of the 30 HCC tissues were positively stained (70.0 %), and none of the ten normal hepatic tissues were positively stained (0 %). Due to the small sample size of tissues in this study, it is difficult to establish a statistical analysis. The characteristics of patients and 14-3-3ζ expression in liver cancer are shown in Table 2. The expression of 14-3-3ζ in liver cancer and normal hepatic tissues are shown in Fig. 5.
Table 2.
Characteristics of patients and 14-3-3ζ expression in liver cancer
| Variable | Frequency | Percentage | |
|---|---|---|---|
| Age | ≥60 | 7 | 23.3 |
| <60 | 23 | 76.7 | |
| Gender | Male | 20 | 66.7 |
| Female | 10 | 33.3 | |
| Grade | II | 15 | 50.0 |
| III | 15 | 50.0 | |
| Normal liver tissue | Negative | 10 | 100.0 |
| Positive | 0 | 0.0 | |
| Liver cancer | Negative | 9 | 30.0 |
| Positive | 21 | 70.0** |
P<0.01 (value of liver cancer to normal tissue)
Fig. 5.
Expression of 14-3-3ζ in liver cancer and normal hepatic tissues by immunohistochemistry. The polyclonal anti-14-3-3ζ antibody was used as primary antibody to detect the expression of 14-3-3ζ in liver cancer and normal hepatic tissues. a A normal hepatic tissue had negative staining. b HCC tissue (grade II) had positive staining. c HCC tissue (grade III) had strong positive staining. d A normal hepatic tissue with HE staining. e HCC tissue (grade II) with HE staining. f HCC tissue (grade III) with HE staining. Grade II: moderately differentiated, cells appear slightly different than normal; grade III: poorly differentiated, cells appear abnormal and tend to grow and spread more aggressively
Overexpression of 14-3-3ζ in different cancer cell lines
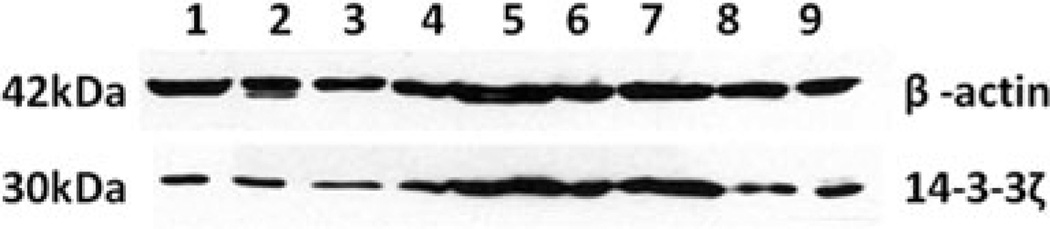
To confirm the expression of 14-3-3ζ in different tumors, nine tumor cell lines (H1299, SKOV3, KOPN63, HepG2, SNU449, Hela, T24, Hep2, H146) were analyzed by western blot. The polyclonal anti-14-3-3ζ antibody was used as probe for this study. As shown in Fig. 6, HepG2 (hepatocellular carcinoma), SNU449 (hepatocellular carcinoma), Hela (epitheloid cervix carcinoma), T24 (urinary bladder carcinoma), and H146 (small cell lung cancer) cell lines had strong reactivity with anti-14-3-3 antibody, and H1299 (non-small cell lung carcinoma), SKOV3 (ovarian carcinoma), KOPN63 (leukemia), and Hep2 (epidermoid carcinoma) cell lines had weak reactivity compared to cell lines which have clear reactive bands.
Fig. 6.
Nine types of tumor cell lines were analyzed by western blot. The polyclonal anti-14-3-3ζ antibody was used as probe. HepG2, SNU449, Hela, T24, and H146 cell lines had strong reactivity, and H1299, SKOV3, KOPN63, and Hep2 cell lines had weak reactivity compared to cell lines which have clear reactive bands. 1, H1299; 2, SKOV3; 3, KOPN63; 4, HepG2; 5, SNU449; 6, Hela; 7, T24; 8, Hep2; 9, H146
Discussion
HCC is one of the most common types of cancer worldwide. The initial hepatocellular alterations altered hepatocytes and, subsequently, dysplastic hepatocytes that form foci and nodules. These changes cause a discrepancy in the microenvironment of liver cells, which may result in changes in the protein expression profile of the cells. The changed proteins may be as a TAA which leads to humoral autoimmune response. Analysis of the autoantibodies to TAAs related to the development of HCC may help elucidate the molecular mechanisms of the disease. These anti-TAA autoantibodies may also serve as candidate biomarkers for early HCC diagnosis [19–23].
The 14-3-3 proteins are a ubiquitous class of regulatory proteins found in all eukaryotic cells and were the first class of molecules to be recognized as discrete phosphoserine/threonine binding modules. The 14-3-3 proteins function as molecular scaffolds by modulating the conformation of their binding partners. They bind a large number of different substrates to regulate a wide range of cellular signaling events including cell cycle progression and DNA damage responses, programmed cell death, cytoskeletal dynamics, and transcriptional control of gene expression, as well as processes directly related to cancer progression. The importance of 14-3-3 proteins in cancer have become apparent recently, and its exact role in cancer progression as well as the mechanisms by which 14-3-3 proteins mediate cancer cell function remains unknown [24]. A lot of research about the relationships between HCC and 14-3-3 indicated that all 14-3-3 isoforms did play an important role in HCC progression. While protein 14-3-3σ is widely accepted as a tumor suppressor, 14-3-3ζ, β, and γ isoforms have been shown to have tumor promoting effects.
Increased 14-3-3β expression is associated with subsequent extrahepatic metastasis and worse survival rates, as well as cancer progression of hepatocellular carcinoma [25]. The protein was found to be a candidate biomarker and a potential target for novel therapies against human HCC progression and metastasis [26]. The 14-3-3ε may act as an important regulator in modulating tumor metastasis by promoting epithelial–mesenchymal transition (EMT) as well as cell migration, and it may serve as a novel prognostic biomarker or therapeutic target for HCC [27]. Overexpression of 14-3-3ε in primary HCC tissues also predicts a high risk of extrahepatic metastasis and a poor prognosis, suggesting that it may be a potential therapeutic target [28]. Hypermethylation and the resulting loss of expression of the 14-3-3σ gene corresponds to one of the most common abnormalities reported to date in HCC, indicating their crucial role in the development and/or progression of HCC [29]. The 14-3-3σ silence reversed the suppressive effect of cell growth and apoptosis induced by introducing 1,3,6,7-tetrahydroxyxanthone which was identified to effectively suppress HCC cell growth [30]. The 14-3-3ζ protein was significantly overexpressed in hepatoma cell lines and human tumorous tissues of HCC patients. Knockdown with RNA interference in hepatoma cell lines with high 14-3-3ζ expression can suppress tumor cell proliferation via activation of c-Jun N-terminal kinase (JNK) and p38/MAPK. Furthermore, suppression of 14-3-3ζ can enhance the anticancer effect of cis-diammined dichloridoplatium (CDDP) in hepatoma cell lines. These results suggest that silencing of 14-3-3ζ may be an attractive target for HCC therapeutic development [31]. Despite the importance of 14-3-3 family in mediating various cell processes, the exact role and mechanism of 14-3-3ζ in HCC remains unclear [24].
Because of its possible roles in HCC formation, we try to validate whether anti-14-3-3ζ antibodies can be used as biomarkers for early HCC diagnosis in this study. In order to test the prevalence of anti-14-3-3ζ autoantibodies in HCC patients, 168 HCC cases were selected in the present study. The results demonstrated that almost 20 % of HCC sera showed immune response to 14-3-3ζ recombinant protein. The mean titer of autoantibodies against 14-3-3ζ in sera from patients with HCC was significantly higher than that in LC, CH, and normal individuals. These results were also confirmed by western blot analysis which shows that representative HCC sera with positive reaction to 14-3-3ζ in ELISA also have strong reactivity in western blot compared to normal sera. Three HCC cases with serial bleeding serum samples were available in our laboratory, and tested. The results indicated that six serum samples collected from each patient showed a gradually increased reactivity with 14-3-3ζ. Western blot analysis showed that stronger reactive bands can be observed in the serum at 6 to 9 months before the clinical diagnosis of HCC. These results indicated that autoantibodies against 14-3-3ζ in HCC sera might be potential biomarkers for early-stage HCC screening and diagnosis. Our previous studies have demonstrated that the sensitivity using a single anti-TAA antibody as biomarker in HCC diagnosis is usually not very high, and using a mini-array of multiple TAAs to enhance anti-TAA antibody detection may be an optimal approach in HCC immunodiagnosis [32–34]. In the current study, although the detection of anti-14-3-3ζ antibody itself in HCC does not have enough sensitivity for HCC diagnosis, it can still be used as an optimal TAA adding to the panel of TAAs we have selected and validated in our previous studies.
The 14-3-3ζ is upregulated in a number of cancer types. The expression of 14-3-3ζ was upregulated in stage I non-small cell lung cancer (NSCLC) and the overexpression of 14-3-3ζ correlated with histological grades, lymph node metastasis, and poor clinical outcome [35]. The 14-3-3ζ protein secreted by tumor-associated monocytes/macrophages from as cites of epithelial ovarian cancer (EOC) patients is common to the secretome of ascitic MO/MA and the ascites of advanced EOC patients [36]. Overexpression of 14-3-3ζ in esophageal hyperplasia, dysplasia, and squamous cell carcinoma, suggesting that alteration in its expression occurs in early stages and is associated with esophageal tumorigenesis [37]. One study has found that knockdown of a single zeta isoform of 14-3-3 is sufficient to restore the sensitivity of cancer cells to anoikis and impairs their anchorage-independent growth through an RNAi approach using human lung adenocarcinoma-derived A549 cells as a model system [38]. The expression of the signature genes was significantly decreased or increased upon reduction or overexpression of 14-3-3ζ in ER-positive breast cancer cells, indicating their co-regulation; 14-3-3ζ also played a critical role in the regulation of FOXM1, with 14-3-3ζ acting upstream of FOXM1 to regulate cell division-signature genes. Depletion of 14-3-3ζ markedly increased apoptosis, reduced proliferation and receptor tyrosine kinase (HER2 and EGFR) signaling, and, importantly, reversed endocrine resistance [39]; 14-3-3ζ is involved in functioning in cell signaling, cycle control, and apoptotic death [9]. The overexpression of 14-3-3ζ in HCC was also found in our present study. Seventy percent of HCC liver tissues were positively stained with anti-14-3-3ζ antibody, and HCC tissues with grade III had stronger positive staining than HCC tissues with grade II in using the immunohistochemistry approach with HCC tissue array slide; 14-3-3ζ was found in the cytoplasm of cell. IHC study with HCC tissue array has further verified that the cellular localization of 14-3-3ζ is also in the cytoplasm. The cytoplasm distribution of 14-3-3ζ is associated with its multifunction in all kinds of cell signal transduction. The 14-3-3ζ protein participates in almost all the cell functions through binding many kinds of proteins in cells. The expressions of 14-3-3ζ in different tumor cell lines were also tested in this study. The results indicated that 14-3-3ζ had strong reactivity in solid tumors and weak reactivity in hematologic malignancy. It may be due to the different mechanism of tumor genesis. Strong reactivities with anti-14-3-3ζ antibody in HepG2 cell line may indicate that 14-3-3ζ plays an important role in HCC; 14-3-3ζ is a cancer-related protein, and it can be overexpressed in several cancers. The prevalence of anti-14-3-3ζ autoantibodies in HCC patients is much higher than that in LC, CH, and NHS. If it is used as a single biomarker in HCC, it is still not high enough to be a perfect one for HCC diagnosis. Although our previous studies have identified and validated several TAAs which-may be used as potential biomarkers in HCC, the concern is that not one can be used as a single marker in HCC diagnosis. Therefore, we are still trying our best to identify and validate more TAAs and anti-TAAs autoantibodies in HCC to find a better way for early HCC diagnosis.
Overexpression of 14-3-3ζ in human cancers may contribute to transformation by inhibiting apoptosis, activating signaling pathways that promote growth, and/or sequestering tumor suppressor proteins. Many previous studies considered 14-3-3ζ as a prognostic marker or therapeutic target for cancer; 14-3-3ζ was found to be a novel androgen-responsive gene that activates proliferation, cell survival, and androgen receptor transcriptional activity and may facilitate the progression of prostate cancer [40]. Recently, the 14-3-3ζ protein was identified as a potential serum biomarker of epithelial ovarian cancer [41, 42]. However, levels of the 14-3-3ζ protein were not found to be changed significantly as a consequence of treatment [43]. In this study, we use immunologic and molecular methods to test the possibility of 14-3-3ζ as a TAA and whether anti-14-3-3ζ antibody can be used as an early diagnostic biomarker in HCC patients. All results from our present study has indicated that 14-3-3ζ can be used as a potential TAA in HCC, and the detection of anti-14-3-3ζ antibody may help in HCC early diagnosis. In a future study, 14-3-3ζ can be included into the panel of TAAs we identified previously to make a customized TAA array, and it would be helpful to increase the sensitivity for HCC diagnosis. The mechanism underlining the production of anti-14-3-3ζ antibodies in HCC still remains to be investigated.
Acknowledgments
The authors thank Dr. Eng M. Tan (The Scripps Research Institute) for his support. This work was supported by grants (SC1CA166016) from the National Institutes of Health (NIH) and by a grant of high technical personnel training item from the Beijing Health System (2011-3-083) and You'an liver disease and AIDS funding (BJYAH-2011-047, BJYAH-2011-045). We also thank the Border Biological Research Center (BBRC) Core Facilities at The University of Texas at El Paso (UTEP) for their support, which was funded by NIH grant (5G12MD007592).
Contributor Information
Mei Liu, Email: maybelake@gmail.com, Beijing You’an Hospital, Capital Medical University, Beijing 100069, China; Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Xinxin Liu, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Pengfei Ren, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Jitian Li, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Yurong Chai, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Su-Jun Zheng, Beijing You’an Hospital, Capital Medical University, Beijing 100069, China.
Yu Chen, Beijing You’an Hospital, Capital Medical University, Beijing 100069, China.
Zhong-Ping Duan, Beijing You’an Hospital, Capital Medical University, Beijing 100069, China.
Ning Li, Beijing You’an Hospital, Capital Medical University, Beijing 100069, China.
Jian-Ying Zhang, Email: jzhang@utep.edu, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
References
- 1.Simonetti RG, Camma C, Fiorello F, Politi F, D’Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–972. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg BS. Hepatitis B, virus, the vaccine, and the control of primary cancer of the liver. Proc Natl Acad Sci U S A. 1997;94:7121–7125. doi: 10.1073/pnas.94.14.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo M, Sangiovanni A. Etiology, natural history and treatment of hepatocellular carcinoma. Antivir Res. 2003;60:145–150. doi: 10.1016/j.antiviral.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Colombo M, Donato MF. Prevention of hepatocellular carcinoma. Semin Liver Dis. 2005;25:155–161. doi: 10.1055/s-2005-871195. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JY, Dai M, Wang X, Lu WQ, Li DS, Zhangt MX, et al. A case-control study of hepatitis B and C virus infection as risk factors for hepatocellular carcinoma in Henan, China. Int J Epidemiol. 1998;27:574–578. doi: 10.1093/ije/27.4.574. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JY, Wang X, Han SG, Zhuang H. A case-control study of risk factors for hepatocellular carcinoma in Henan, China. Am J Trop Med Hyg. 1998;59:947–951. doi: 10.4269/ajtmh.1998.59.947. [DOI] [PubMed] [Google Scholar]
- 7.Gardino AK, Smerdon SJ, Yaffe MB. Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: a comparison of the X-ray crystal structures of all human 14-3-3 isoforms. Semin Cancer Biol. 2006;16(3):173–182. doi: 10.1016/j.semcancer.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Lee IN, Chen CH, Sheu JC, Lee HS, Huang GT, Yu CY, et al. Identification of human hepatocellular carcinoma-related biomarkers by two-dimensional difference gel electrophoresis and mass spectrometry. J Proteome Res. 2005;4(6):2062–2069. doi: 10.1021/pr0502018. [DOI] [PubMed] [Google Scholar]
- 9.Neal CL, Yu D. 14-3-3ζ as a prognosticmarker and therapeutic target for cancer. Expert Opin Ther Targets. 2010;14(12):1343–1354. doi: 10.1517/14728222.2010.531011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 11.Fu H, Coburn J, Collier RJ. The eukaryotic host factor that activates exoenzyme S ofPseudomonas aeruginosais a member of the 14-3-3 protein family. Proc Natl Acad Sci U S A. 1993;90:2320–2324. doi: 10.1073/pnas.90.6.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell JK, Gurung R, Romero S, Speed CJ, Andrews RK, Berndt MC, et al. Activation of the 43 kDa inositol polyphosphate 5-phosphatase by 14-3-3zeta. Biochemistry. 1997;36:15363–1570. doi: 10.1021/bi9708085. [DOI] [PubMed] [Google Scholar]
- 13.Masters SC, Pederson KJ, Zhang L, Barbieri JT, Fu H. Interaction of 14-3-3 with a nonphosphorylated protein ligand, exoenzyme S of Pseudomonas aeruginosa. Biochemistry. 1999;38:5216–5221. doi: 10.1021/bi982492m. [DOI] [PubMed] [Google Scholar]
- 14.Obsil T, Obsilova V. Structural basis of 14-3-3 protein functions. Semin Cell Dev Biol. 2011;22(7):663–672. doi: 10.1016/j.semcdb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Freeman AK, Morrison DK. 14-3-3 proteins: diverse functions in cell proliferation and cancer progression. Semin Cell Dev Biol. 2011;22(7):681–687. doi: 10.1016/j.semcdb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PJ, Leung N, Cheng P, Welby C, Leung WT, Lau WY, et al. ‘Hepatoma-specific’ alphafetoprotein may permit preclinical diagnosis of malignant change in patients with chronic liver disease. Br J Cancer. 1997;75:236–240. doi: 10.1038/bjc.1997.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EKL, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomark Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 18.Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EK. Antibody detection using tumor-associated antigen mini-array in diagnosing human hepatocellular carcinoma. J Hepatol. 2007;46:107–114. doi: 10.1016/j.jhep.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JY, Chan EKL, Peng XX, Tan EM. A novel RNA-binding protein is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–1110. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himoto T, Kuriyama S, Zhang JY, Chan EK, Nishioka M, Tan EM. Significance of autoantibodies against insulin-like growth factor II mRNA-binding proteins in patients with hepatocellular carcinoma. Int J Oncol. 2005;26(2):311–317. [PubMed] [Google Scholar]
- 21.Ersvaer E, Zhang JY, McCormack E, Olsnes A, Anensen N, Tan EM, et al. Cyclin B1 is commonly expressed in the cytoplasm of primary human acute myelogenous leukemia cells and serves as a leukemia-associated antigen associated with autoantibody response in a subset of patients. Eur J Haematol. 2007;79(3):210–225. doi: 10.1111/j.1600-0609.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wang K, Zhang J, Liu SS, Dai L, Zhang JY. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10:2863–2872. doi: 10.1021/pr200141c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen XL, Zhou L, Yang J, Shen FK, Zhao SP, Wang YL. Hepatocellular carcinoma-associated protein markers investigated by MALDI-TOF MS. Mol Med Rep. 2010;3(4):589–596. doi: 10.3892/mmr_00000302. [DOI] [PubMed] [Google Scholar]
- 24.Goc A, Abdalla M, Al-Azayzih A, Somanath PR. Rac1 activation driven by 14-3-3ζ dimerization promotes prostate cancer cell-matrix interactions, motility and transendothelial migration. PLoS One. 2012;7(7):e40594. doi: 10.1371/journal.pone.0040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu TA, Jan YJ, Ko BS, Chen SC, Liang SM, Hung YL, et al. Increased expression of 14-3-3β promotes tumor progression and predicts extrahepatic metastasis and worse survival in hepatocellular carcinoma. Am J Pathol. 2011;179(6):2698–2708. doi: 10.1016/j.ajpath.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko BS, Lai IR, Chang TC, Liu TA, Chen SC, Wang J, et al. Involvement of 14-3-3γ overexpression in extrahepatic metastasis of hepatocellular carcinoma. Hum Pathol. 2011;42(1):129–135. doi: 10.1016/j.humpath.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Liu TA, Jan YJ, Ko BS, Liang SM, Chen SC, Wang J, et al. 14-3-3ε overexpression contributes to epithelial-mesenchymal transition of hepatocellular carcinoma. PLoS One. 2013;8(3):e57968. doi: 10.1371/journal.pone.0057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko BS, Chang TC, Hsu C, Chen YC, Shen TL, Chen SC, et al. Overexpression of 14-3-3ε predicts tumour metastasis and poor survival in hepatocellular carcinoma. Histopathology. 2011;58(5):705–711. doi: 10.1111/j.1365-2559.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 29.Iwata N, Yamamoto H, Sasaki S, Itoh F, Suzuki H, Kikuchi T, et al. Frequent hypermethylation of CpG islands and loss of expression of the 14-3-3 sigma gene in human hepatocellular carcinoma. Oncogene. 2000;19(46):5298–5302. doi: 10.1038/sj.onc.1203898. [DOI] [PubMed] [Google Scholar]
- 30.Fu WM, Zhang JF, Wang H, Tan HS, Wang WM, Chen SC, et al. Apoptosis induced by 1,3,6,7-tetrahydroxyxanthone in hepatocellular carcinoma and proteomic analysis. Apoptosis. 2012;17(8):842–851. doi: 10.1007/s10495-012-0729-y. [DOI] [PubMed] [Google Scholar]
- 31.Choi JE, Hur W, Jung CK, Piao LS, Lyoo K, Hong SW, et al. Silencing of 14-3-3ζ over-expression in hepatocellular carcinoma inhibits tumor growth and enhances chemosensitivity tocis-diammined dichloridoplatium. Cancer Lett. 2011;303(2):99–107. doi: 10.1016/j.canlet.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Zhou YS, Qiu SM, Wang KJ, Liu SW, Peng XX, et al. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289:32–39. doi: 10.1016/j.canlet.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao Q, Ren P, Li Y, Peng B, Dai L, Lei N, et al. Autoantibodies against glucose-regulated protein 78 as serological diagnostic biomarkers in hepatocellular carcinoma. Int J Oncol. 2012;41(3):1061–1067. doi: 10.3892/ijo.2012.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JY. Mini-array of multiple tumor-associated antigens to enhance autoantibody detection for immunodiagnosis of hepatocellular carcinoma. Autoimmun Rev. 2007;6:143–148. doi: 10.1016/j.autrev.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zang D, Li X, Zhang L. 14-3-3ζ Overexpression and abnormal β-catenin expression are associated with poor differentiation and progression in stage I non-small cell lung cancer. Clin Exp Med. 2010;10(4):221–228. doi: 10.1007/s10238-009-0089-2. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi R, Deavers M, Patenia R, Rice-Stitt T, Halbe J, Gallardo S, et al. 14-3-3 zeta protein secreted by tumor associated monocytes/macrophages from ascites of epithelial ovarian cancer patients. Cancer Immunol Immunother. 2009;58(2):247–258. doi: 10.1007/s00262-008-0549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajpai U, Sharma R, Kausar T, Dattagupta S, Chattopadhayay TK, Ralhan R. Clinical significance of 14-3-3 zeta in human esophageal cancer. Int J Biol Markers. 2008;23(4):231–237. doi: 10.1177/172460080802300406. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Zhao J, Du Y, Park HR, Sun SY, Bernal-Mizrachi L, et al. Down-regulation of 14-3-3zeta suppresses anchorage-independent growth of lung cancer cells through anoikis activation. Proc Natl Acad Sci U S A. 2008;105(1):162–167. doi: 10.1073/pnas.0710905105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergamaschi A, Christensen BL, Katzenellenbogen BS. Reversal of endocrine resistance in breast cancer: interrelationships among 14-3-3ζ, FOXM1, and a gene signature associated with mitosis. Breast Cancer Res. 2011;13(3):R70. doi: 10.1186/bcr2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murata T, Takayama K, Urano T, Fujimura T, Ashikari D, Obinata D, et al. 14-3-3ζ, a novel androgen-responsive gene, is upregulated in prostate cancer and promotes prostate cancer cell proliferation and survival. Clin Cancer Res. 2012;18(20):5617–5627. doi: 10.1158/1078-0432.CCR-12-0281. [DOI] [PubMed] [Google Scholar]
- 41.He Y, Wu X, Liu X, Yan G, Xu C. LC-MS/MS analysis of ovarian cancer metastasis-related proteins using a nude mouse model: 14-3-3 zeta as a candidate biomarker. J Proteome Res. 2010;9(12):6180–6190. doi: 10.1021/pr100822v. [DOI] [PubMed] [Google Scholar]
- 42.Waldemarson S, Krogh M, Alaiya A, Kirik U, Schedvins K, Auer G, et al. Protein expression changes in ovarian cancer during the transition from benign to malignant. J Proteome Res. 2012;11(5):2876–2889. doi: 10.1021/pr201258q. [DOI] [PubMed] [Google Scholar]
- 43.Hatzipetros I, Gocze P, Koszegi T, Jaray A, Szereday L, Polgar B, et al. Investigating the clinical potential for 14-3-3 zeta protein to serve as a biomarker for epithelial ovarian cancer. J Ovarian Res. 2013;6(1):79. doi: 10.1186/1757-2215-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]