Abstract
There is an urgent need to identify relevant tumor markers showing high sensitivity and specificity for early immunodiagnosis of breast cancer. Autoantibodies directed against tumor-associated antigens (TAAs) have been shown to be relevant tumor markers. The purpose of this study was to evaluate whether autoantibodies to a tumor-associated antigen p90/CIP2A can be used as diagnostic markers in breast cancer. In this study, autoantibody responses to p90/CIP2A were evaluated by enzyme-linked immunosorbent assay (ELISA), western blotting, and indirect immunofluorescence assay in sera from patients with breast cancer and normal human individuals. The results have demonstrated that p90/CIP2A can induce a relatively higher frequency of autoantibody response in breast cancer (19.1 %) compared to the sera of normal individuals (2.3 %). The frequency of p90/CIP2A expression in breast cancer tissues was significantly higher than that in adjacent normal tissues (P <0.01). Our preliminary results suggest that autoantibodies against p90/CIP2A may be a useful serum biomarker for early stage breast cancer screening and diagnosis.
Keywords: Breast cancer, p90/CIP2A, Immunodiagnosis, Tumor-associated antigen
Introduction
Breast cancer is the most prevalent form of cancer among women, accounting for about 28 % of all new cancer cases [1, 2], with rising incidence rates in recent years across western countries [3]. Despite improvements in breast cancer therapy, more than one fourth of patients diagnosed with breast cancer eventually die from the disease [4]. Thus, early diagnosis and effective therapies for breast cancer are necessary.
Antigenic changes in cancer cells can be recognized by the immune system of patients themselves and present as immune responses to factors involved in malignant transformation. This is manifested in several ways, one of which is the appearance of circulating autoantibodies. These autoantibodies have been called “reporters” from the immune system, which can identify the antigenic changes in cellular factors involved in the transformation process [5, 6]. In the past decade, we have used these circulating autoantibodies in cancer patients as probes in isolating the cognate tissue antigens, many of which have been shown to be cellular factors participating in known tumorigenesis pathways. These cancer-related tissue antigens are generally known as tumor-associated antigens (TAAs). There is a proposition that the cancer patient’s immune system conveying in the form of autoantibodies to TAAs should be utilized to a greater extent in identifying early signs of tumorigenesis. For example, autoantibodies to TAAs such as p53 and p16 were detected in 10–20 % of most types of cancer patients [7, 8], while autoantibodies to p62 were 21 % positive in patients with liver cancer [9]. Due to the fact that these autoantibodies do not exist or appear with a very low titer in the serum samples of healthy people, therefore, the autoantibodies may become potential biomarkers in the diagnosis of certain types of cancer.
Cancerous inhibitor of protein phosphatase 2A (CIP2A), originally named p90, has been cloned by immunoscreening a cDNA expression library with autoantibodies from a patient with hepatocellular carcinoma (HCC) [10]. p90/CIP2A is a recently characterized endogenous inhibitor of the phosphatase activity of protein phosphatase 2A (PP2A), which extends the half-life of oncogenic protein c-myc and promotes cell survival via regulating AKT dephosphorylation [11]. Furthermore, p90/CIP2A has been demonstrated as a novel oncoprotein, and a growing number of reports have shown its overexpression in many human malignancies, including breast cancer [12–18]. In the current study, we aimed to evaluate whether serum anti-p90/CIP2A autoantibody can be used as a novel biomarker in the detection of breast cancer.
Materials and methods
Patients and samples
In the current study, a total of 168 sera from patients with breast cancer and 88 sera from normal individuals were derived from the sera bank in the Cancer Autoimmunity Research Laboratory at the University of Texas, El Paso (UTEP). These sera were originally provided by our clinical collaborators. All serum samples were collected under consented individuals in the present study. Cancer sera were obtained at the time of breast cancer diagnosis by mammography screening. Pathological diagnosis of breast cancer for all patients has been confirmed with biopsy specimens. None of the breast cancer patients had received treatment with any chemotherapy or radiotherapy. This study was approved by the Institutional Review Board of UTEP and Collaborating Institutions.
Enzyme-linked immunosorbent assay (ELISA)
Serum IgG antibody against p90/CIP2A was assessed by ELISA as previously described [19]. In brief, 96-well microtiter plates were coated overnight (at least for 24 h) at 4 °C with 2 μg/ml p90/CIP2A diluted in phosphate-buffered saline (PBS). Plates were blocked with gelatin post-coating solution for 2 h at room temperature. The antigen-coated wells were incubated with human sera diluted at 1:200 with serum diluent at room temperature for 2 h. The goat antihuman IgG-HRP (Invitrogen, NY) and the substrate 2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS, Invitrogen) were used as detecting reagents. The average optical density (OD) value at a wavelength of 405 nm was applied for data analysis. The cutoff value designating positive reaction was the mean OD of 90 normal human sera (NHS) plus 3 standard deviations (SD).
Western blotting
Purified recombinant p90/CIP2A protein was electrophoresed on 10 % SDS-PAGE and transferred onto a nitrocellulose membrane paper. After blocking with PBS containing 5 % nonfat dry milk and 0.05 % Tween-20 (PBST) for 1 h at room temperature, the nitrocellulose papers were incubated for 60 min at room temperature with a 1:200 dilution of serum and 1:1,000 dilution of monoclonal anti-p90/CIP2A antibody. HRP-conjugated goat antihuman IgG and HRP-conjugated goat antimouse IgG were applied as secondary antibodies at a 1:1,000 dilution. Immunoreactive bands were detected using the ECL kit (Thermo Scientific) according to the manufacturer’s instructions.
Indirect immunofluorescence (IIF) assay
Commercially available HEp-2 cell slides (MBL International Corporation, MA) were used in IIF for identification of autoantibodies in cancer sera. Sera with 1:80 dilution and anti-p90/CIP2A antibody with 1:50 dilution were incubated for 45 min at room temperature. FITC-conjugated goat antihuman IgG and antirabbit IgG Fab2 were used as secondary antibodies at a 1:200 and 1:100 dilution, respectively. Confocal fluorescence images were acquired with a laser scanning microscope (LSM 700; Zeiss, New York, NY), using a×20 objective and processed with ZEN 2009 software (Zeiss, CA).
Absorption of antibodies with recombinant protein
The diluted human sera (1:80) were incubated with recombinant protein (final concentration of recombinant protein was 0.03 μg/μl) overnight at 4 °C and then centrifuged at 10,000×g for 10 min. The supernatant was used for immunofluorescence assay.
Immunohistochemistry (IHC) with tissue array slides
Breast cancer tissue array slides with normal tissue controls (46 cases/92 cores, including clinical stages and pathology grades) were purchased (US Biomax, Inc., Rockville, MD) and used to detect the expression of the p90/CIP2A protein. The slides were deparaffinized with xylene and dehydrated with ethanol of different strengths. Antigen retrieval was performed by microwave-heating methods in Trilogy™ pretreatment solution for 20 min and cooled down naturally for about 1 h. Three percent H2O2 and 10 % fetal bovine serum blocking solution were used to prevent nonspecific binding of antibodies for 20 min separately. The sections were incubated with monoclonal anti-p90/CIP2A antibody (1:500 dilution) overnight at 4 °C. HRP Detection System (HRP streptavidin label and polyvalent biotinylated link) and DAB Substrate Kit were used as detecting reagents. After counterstaining with hematoxylin, the sections were dehydrated and mounted. Finally, the slides were observed by a microscope (Leica, DM1000). All IHC results were read blindly by two independent researchers. A four-level scoring system (−, negative; +, low expression level; ++, moderate expression level; +++, high expression level) was used to evaluate the staining intensity.
Statistical analysis
Statistical analysis was performed using SPSS 13.0. Data were analyzed with χ2 test and represented as the mean±3 SD from ELISA. The results were considered to indicate a statistically significant difference when P values were <0.05 or <0.01.
Results
Frequency and titer of anti-p90/CIP2A autoantibody in sera from patients with breast cancer
Serum levels of anti-p90/CIP2A autoantibodies were determined by ELISA as described in the section of “Materials and methods.” In total, 168 sera from patients with breast cancer and 88 sera from normal human individuals were used in this study. As shown in Table 1, the prevalence of autoantibody against p90/CIP2A was 19.1 % (32/168) in breast cancer, which was significantly higher than that in NHS (2.3 %, 2/88) (P <0.01). Titer of anti-p90/CIP2A antibodies in human sera is shown in Fig. 1. The average titer of autoantibody against p90/CIP2A in breast cancer sera was higher than that in NHS (P <0.01). The ELISA results were also confirmed by western blot analysis. Figure 2 shows that representative breast cancer serum with positive reaction to p90/CIP2A in ELISA also has strong reactivity in western blotting compared to normal serum.
Table 1.
Frequency of autoantibody against p90/CIP2A in human sera by ELISA
| Type of sera | No. tested | Autoantibody to p90/CIP2A (%) |
|---|---|---|
| Breast cancer | 168 | 32 (19.1)** |
| Normal human serum (NHS) | 88 | 2 (2.3) |
Cut-off value: mean+3 SD of NHS; P value relative to NHS:
P <0.01
Fig. 1.
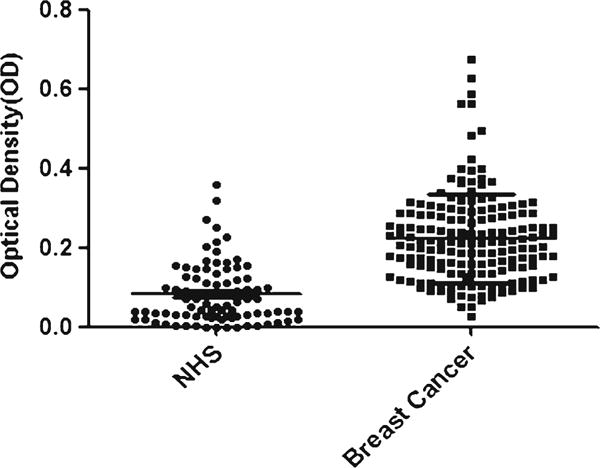
Titer of autoantibody against p90/CIP2A in human sera by ELISA. The range of antibody titers to p90/CIP2A was expressed as optical density (OD) obtained from ELISA. The mean+3 SD of NHS are shown in relationship to all serum samples. Titer of anti-p90/CIP2A in breast cancer is much higher than that in NHS (P <0.01)
Fig. 2.

Western blotting analysis showing representative breast cancer sera recognizing p90/CIP2A recombinant protein. The monoclonal anti-p90/CIP2A antibody was used as positive control; lanes 1–5, five representative breast cancer sera that were positive in ELISA test and also have strong reactivity with p90/CIP2A recombinant protein in western blotting analysis; lanes 6 and 7, normal human sera were used as negative control
Immunofluorescence staining pattern of p90/CIP2A in HEp-2 cells
To further confirm the reactivity of autoantibody against p90/CIP2A in breast cancer sera and the intracellular localization of p90/CIP2A, HEp-2 cell slides were used in indirect immunofluorescence assay to detect breast cancer sera with anti-p90/CIP2A positive in ELISA. As shown in Fig. 3, a representative anti-p90/CIP2A positive breast cancer serum had both cytoplasmic and perinuclear staining pattern, while the monoclonal anti-p90/CIP2A antibody with more intense staining in perinuclear regions. The fluorescent staining was significantly reduced when the same breast cancer serum was pre-absorbed with recombinant p90/CIP2A protein.
Fig. 3.
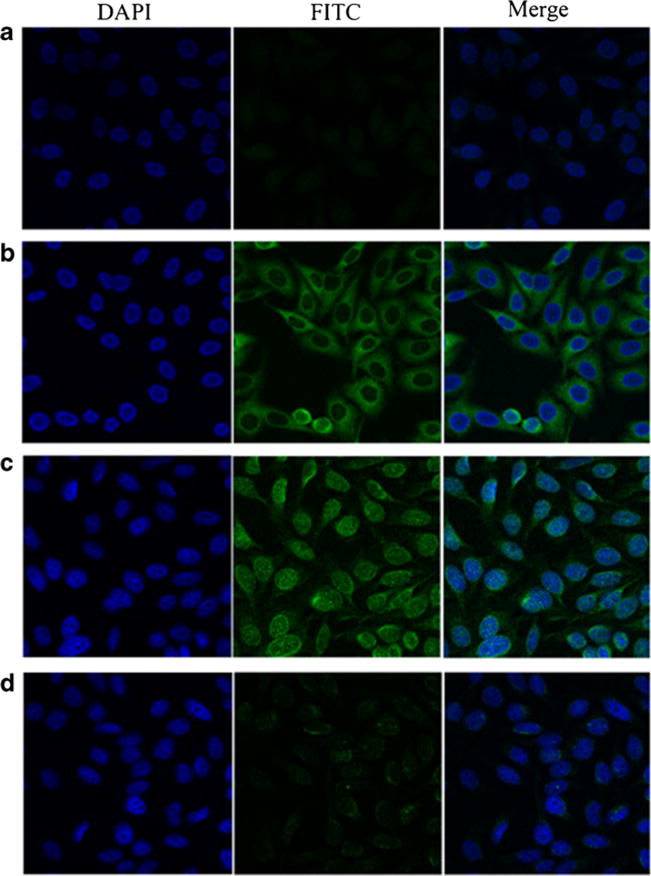
Representative immunofluorescence staining pattern of anti-p90/CIP2A antibody positive breast cancer serum. a A normal human serum (NHS) was used as negative control; b monoclonal anti-p90/CIP2A antibody that demonstrated a perinuclear immunofluorescence staining pattern was used as positive control; c a representative anti-p90/CIP2A antibody positive breast cancer serum demonstrated both cytoplasmic and perinuclear immunofluorescence staining pattern; d the same breast cancer serum that was used in c was post-absorbed with recombinant p90/CIP2A protein. The fluorescent signal was remarkably decreased
Expression of p90/CIP2A in breast cancer tissues and adjacent normal breast tissues by immunohistochemistry
In the present study, the expression of p90/CIP2A in breast cancer tissues and adjacent normal breast tissues was examined by immunohistochemistry with tissue array slides. Tissue array slides including 46 breast cancer tissue specimens and 46 adjacent normal breast tissue specimens were commercially available for this study. The monoclonal anti-p90/CIP2A antibody was used for immunostaining the tissue specimens. As shown in Table 2, all the breast cancer tissues were positively stained (100 %), and 13 of the 46 adjacent normal breast tissues were positively stained (28 %). The frequency of p90/CIP2A expression in breast cancer tissues was significantly higher than that in adjacent normal tissues (P <0.01). Figure 4 indicates positive reaction of breast cancer tissues with grades I, II, and III, respectively, while the normal breast tissue has negative staining. Analysis on the frequency of positive p90/CIP2A staining in breast cancer tissues with different clinical stages has not revealed any significant correlation between p90/CIP2A expression and cancer stages due to the limited number of tissue specimens in this study, especially the small sample size of tissues with stage I and III cancer.
Table 2.
Expression of p90/CIP2A in breast cancer tissues and cancer adjacent normal tissues
| Type of tissues | No. tested | Autoantibody to p90/CIP2A (%) |
|---|---|---|
| Breast cancer | 46 | 46 (100)** |
| Normal controls | 46 | 13 (28) |
P value of breast cancer tissues to adjacent normal tissues:
P <0.01
Fig. 4.
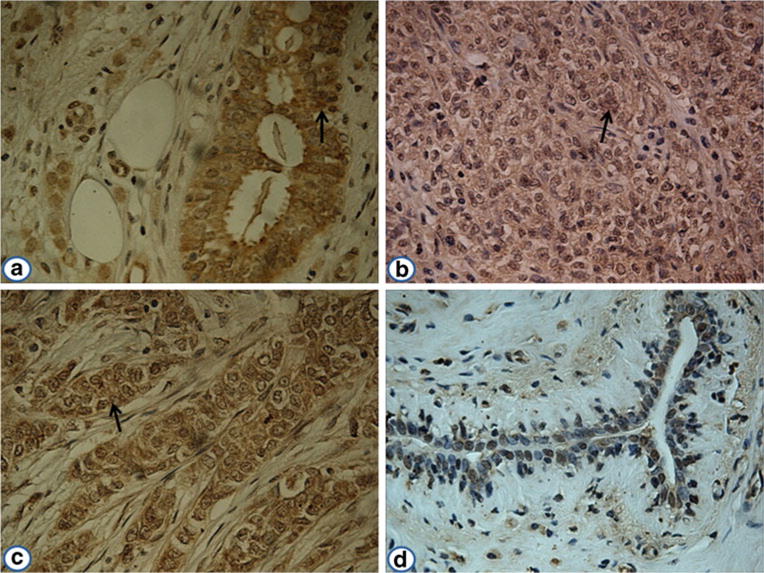
Expression of p90/CIP2A in breast cancer and adjacent normal breast tissues by immunohistochemistry. The monoclonal anti-p90/CIP2A antibody was used as primary antibody to detect the expression of p90/CIP2A in breast cancer and adjacent normal breast tissues. a Breast cancer tissue (grade I); b breast cancer tissue (grade II); c breast cancer tissue (grade III); d an adjacent normal breast tissue had negative staining (magnification, ×400). The arrows indicate p90/CIP2A positive staining
Discussion
Recent studies from several groups have highlighted that p90/CIP2A is upregulated in a wide variety of malignant tumors [20–24]. p90/CIP2A overexpression has also been reported to be associated with worse prognosis, and therefore, it can serve as a prognostic marker in numerous human cancers [11, 14]. In addition, p90/CIP2A mediates the apoptotic effect of bortezomib in breast cancer [13] and HCC [25]. p90/CIP2A promotes Ras-elicited foci formation in mouse embryo fibroblasts and supports transformation of immortalized human cells [11]. In studies with loss of function, p90/CIP2A depletion has been shown to reduce the overall tumor xenograft size in nude mice [11, 26] and to further impair clonogenicity and anchorage-independent growth of tumor cells [11, 14, 16, 20]. In breast cancer, Come et al. showed that high p90/CIP2A expression associates with high proliferation index, p53 mutation, and high Scarff–Bloom–Richardson grade [26]. In order to evaluate whether p90/CIP2A can be used as a tumor-associated antigen in breast cancer, the purified recombinant p90/CIP2A protein was used as the target antigen, and the immune response to this protein in sera with breast cancer has been investigated.
Due to the small size of the samples, whether the autoantibody response to p90/CIP2A in breast cancer sera is correlated with the clinical significance remains to be established. In the current study, the expression profiles of p90/CIP2A in breast cancer and adjacent normal breast tissues were extensively evaluated by immunohistochemistry with breast cancer tissue array slides. Our results showed that the frequency of p90/CIP2A expression in breast cancer tissues was significantly higher than that in adjacent normal tissues (P <0.01). However, 13 of the 46 adjacent normal breast tissues were also positively stained. In this study, we used the commercial breast cancer tissue array slides and detected the expression of p90/CIP2A in breast cancer tissues compared to breast cancer adjacent tissues as controls. There could be some histopathological differences between breast cancer adjacent tissues and normal breast tissues. This may be the reason why there is a relatively high expression rate of p90/CIP2A in breast cancer adjacent tissues. Analysis on the frequency of positive p90/CIP2A staining in breast cancer tissues with different clinical stages has not revealed any significant correlation between p90/CIP2A expression and cancer stages due to the limited number of tissue specimens in this study, especially the small sample size of tissues with stage I and III cancer. Moreover, most patients with breast cancer are currently still treated with some traditional regimens such as surgery and radiotherapy. The molecularly targeted drugs for breast cancer treatment such as the HER2-targeting antibody like trastuzumab, the anti-estrogen drugs like tamoxifen, and the humanized antivascular endothelial growth factor antibody like bevacizumab are currently only available for the treatment of very few patients. In this regard, there is an urgent need to identify novel potential target proteins for breast cancer therapy. The results shown in this study support the potential role of p90/CIP2A as a target protein for future cancer therapies.
Unlike traditional tumor biomarkers, anti-TAA autoantibodies are found in the sera from patients with different cancers and may represent early indicators of tumor development [27, 28]. Breast cancer is a heterogeneous disease in which tumor cells express a variety of aberrant proteins capable of eliciting an immune response. Interestingly, this immune response appears months to years before the clinical diagnosis of the tumor [29–31]. These anti-TAA antibodies may provide an in vivo amplification of an early carcinogenic signal, therefore possibly allowing earlier detection of cancer than current tumor biomarkers. Body fluids such as blood, saliva, and urine are considered as ideal sources to assess the presence of cancer biomarkers [32–35]. Many studies have demonstrated that serum samples from cancer patients contain circulating TAAs and anti-TAA antibodies related to cancer progression and development [36–41]. Although many candidate biomarkers have been identified and evaluated, current biomarker detection assays are not sufficiently sensitive or specific to provide a reliable early diagnosis. In this respect, efforts to discover novel cancer biomarkers still continue. Especially, considerable effort is being made toward the discovery of better biomarkers that may allow an early detection of breast cancer [42, 43]. In the present study, almost 20 % of breast cancer sera showed immune response to p90/CIP2A recombinant protein. The mean titer of autoantibody against p90/CIP2A in the sera from patients with breast cancer was significantly higher than that in normal individuals. Among the tremendous number of tumor markers relative to breast cancer, oncofetal protein (CEA), oncoproteins (Her2, c-myc), tumor suppressor protein (p53), carbohydrate antigens (CA15-3, CA27-29), and MUC-1 are the most commonly used markers in clinical studies [44, 45]. However, diagnosis based on single tumor marker detection of either a TAA or an anti-TAA antibody usually lacks sensitivity and specificity as noticed in most studies. Taken together, a combination of several tumor markers has been demonstrated to be a better approach than that in using any single marker for diagnosing and monitoring breast cancer. Moreover, to further evaluate that p90/CIP2A is a specific biomarker in the diagnosis of breast cancer, it is better to test more serum samples from other types of cancer to see whether the anti-p90/CIP2A antibody is also useful in the diagnosis of other cancers in a future project.
In this study, we found that there are strong cytoplasmic and perinuclear expressions of p90/CIP2A in the sera from patients with breast cancer by indirect immunofluorescence assay. When p90/CIP2Awas first identified by Soo Hoo et al., it has been demonstrated that the localization of p90/CIP2A was mainly in the perinuclear regions of the cytosol [10]. In a further study, Junttila et al. noted its overexpression with predominant cytoplasmic localization and only a weak nuclear expression in head and neck squamous cell carcinoma and colon cancer [11]. Recent studies have only addressed the cytoplasmic role of p90/CIP2A, and the biological function of nuclear p90/CIP2A is largely unknown. This raises an important question, which calls for studies about the functional significance of nuclear p90/CIP2A. Our findings suggest that nuclear p90/CIP2A protein may have an important role in breast carcinogenesis.
In conclusion, a total of 168 sera from patients with breast cancer have been tested with different immunological assays and were found that p90/CIP2A can induce autoantibody response in sera from patients with breast cancer, suggesting that the anti-p90/C1P2A autoantibody might be a potential biomarker for clinical serologic screening in the diagnosis of breast cancer. The underlying mechanism of how p90/CIP2A induces humoral immune response in breast cancer patients and how it is involved in tumorigenesis of breast cancer remain to be investigated.
Acknowledgments
The authors thank Dr. Eng M. Tan (The Scripps Research Institute) for his support. This work was supported by a grant (SC1CA166016) from the National Institutes of Health (NIH) and also by grants from the National Natural Science Foundation of China (81172086 and 81372371). They also thank the Border Biological Research Center (BBRC) Core Facilities at The University of Texas at El Paso (UTEP) for their support, which were funded by NIH grant (5G12MD007592).
Footnotes
Conflicts of interest None
Contributor Information
Xinxin Liu, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, China, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Yurong Chai, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Jitian Li, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Pengfei Ren, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, China, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Mei Liu, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Liping Dai, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, China, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Wei Qian, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
Wenjie Li, Email: lwj@zzu.edu.cn, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, China.
Jian-Ying Zhang, Email: jianyingzhang@hotmail.com, College of Public Health, Zhengzhou University, Zhengzhou, Henan 450001, China, Department of Biological Sciences, The University of Texas at El Paso, El Paso, TX 79968, USA.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–38. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 5.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–5. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–88. [PubMed] [Google Scholar]
- 8.Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006;16:1105–10. [PubMed] [Google Scholar]
- 9.Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–10. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006–15. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- 11.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Chen KF, Yeh PY, Hsu C, Hsu CH, Lu YS, Hsieh HP, et al. Bortezomib overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance in hepatocellular carcinoma cells in part through the inhibition of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2009;284:11121–33. doi: 10.1074/jbc.M806268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen KF, Liu CY, Lin YC, Yu HC, Liu TH, Hou DR, et al. CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene. 2010;29:6257–66. doi: 10.1038/onc.2010.357. [DOI] [PubMed] [Google Scholar]
- 14.Dong QZ, Wang Y, Dong XJ, Li ZX, Tang ZP, Cui QZ, et al. CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann Surg Oncol. 2011;18:857–65. doi: 10.1245/s10434-010-1313-8. [DOI] [PubMed] [Google Scholar]
- 15.Katz J, Jakymiw A, Ducksworth MK, Stewart CM, Bhattacharyya I, Cha S, et al. CIP2A expression and localization in oral carcinoma and dysplasia. Cancer Biol Ther. 2010;10:694–9. doi: 10.4161/cbt.10.7.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna A, Bockelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101:793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 17.Vaarala MH, Vaisanen MR, Ristimaki A. CIP2A expression is increased in prostate cancer. J Exp Clin Cancer Res. 2010;29:136. doi: 10.1186/1756-9966-29-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Li W, Li L, Yu X, Jia J, Chen C. CIP2A is over-expressed in acute myeloid leukaemia and associated with HL60 cells proliferation and differentiation. Int J Lab Hematol. 2011;33:290–8. doi: 10.1111/j.1751-553X.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Zhou Y, Qiu S, Wang K, Liu S, Peng XX, et al. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289:32–9. doi: 10.1016/j.canlet.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Ge Z, Liu C, Liu Z, Bjorkholm M, Jia J, et al. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722–8. doi: 10.1158/1078-0432.CCR-07-4137. [DOI] [PubMed] [Google Scholar]
- 21.Teng HW, Yang SH, Lin JK, Chen WS, Lin TC, Jiang JK, et al. CIP2A is a predictor of poor prognosis in colon cancer. J Gastrointest Surg. 2012;16:1037–47. doi: 10.1007/s11605-012-1828-3. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Li W, Yan L, Jiao W, Tian S, Li D, et al. Expression of CIP2A in renal cell carcinomas correlates with tumour invasion, metastasis and patients’ survival. Br J Cancer. 2011;105:1905–11. doi: 10.1038/bjc.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basile JR, Czerninski R. The role of CIP2A in oral squamous cell carcinoma. Cancer Biol Ther. 2010;10:700–2. doi: 10.4161/cbt.10.7.13151. [DOI] [PubMed] [Google Scholar]
- 24.Bockelman C, Koskensalo S, Hagstrom J, Lundin M, Ristimaki A, Haglund C. CIP2A overexpression is associated with c-Myc expression in colorectal cancer. Cancer Biol Ther. 2012;13:289–95. doi: 10.4161/cbt.18922. [DOI] [PubMed] [Google Scholar]
- 25.Tseng LM, Liu CY, Chang KC, Chu PY, Shiau CW, Chen KF. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res BCR. 2012;14:R68. doi: 10.1186/bcr3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15:5092–100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 27.Desmetz C, Cortijo C, Mange A, Solassol J. Humoral response to cancer as a tool for biomarker discovery. J Proteomics. 2009;72:982–8. doi: 10.1016/j.jprot.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Peng B, Lu Y, Xu W, Qian W, Zhang JY. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun Rev. 2011;10:331–5. doi: 10.1016/j.autrev.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–73. doi: 10.1093/annonc/mdm007. [DOI] [PubMed] [Google Scholar]
- 30.Desmetz C, Bibeau F, Boissiere F, Bellet V, Rouanet P, Maudelonde T, et al. Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J Proteome Res. 2008;7:3830–7. doi: 10.1021/pr800130d. [DOI] [PubMed] [Google Scholar]
- 31.Pereira-Faca SR, Kuick R, Puravs E, Zhang Q, Krasnoselsky AL, Phanstiel D, et al. Identification of 14-3-3 theta as an antigen that induces a humoral response in lung cancer. Cancer Res. 2007;67:12000–6. doi: 10.1158/0008-5472.CAN-07-2913. [DOI] [PubMed] [Google Scholar]
- 32.Park BW, Oh JW, Kim JH, Park SH, Kim KS, Lee KS. Preoperative CA 15–3 and CEA serum levels as predictor for breast cancer outcomes. Ann Oncol. 2008;19:675–81. doi: 10.1093/annonc/mdm538. [DOI] [PubMed] [Google Scholar]
- 33.Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–53. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Downes MR, Byrne JC, Dunn MJ, Fitzpatrick JM, Watson RW, Pennington SR. Application of proteomic strategies to the identification of urinary biomarkers for prostate cancer: a review. Biomark Biochem Indic Expo Response Susceptibility Chem. 2006;11:406–16. doi: 10.1080/13547500600799821. [DOI] [PubMed] [Google Scholar]
- 35.Chang JW, Kang UB, Kim DH, Yi JK, Lee JW, Noh DY, et al. Identification of circulating endorepellin LG3 fragment: potential use as a serological biomarker for breast cancer. Proteomics Clin Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- 36.Caron M, Choquet-Kastylevsky G, Joubert-Caron R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteomics MCP. 2007;6:1115–22. doi: 10.1074/mcp.R600016-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Madrid FF, Maroun MC. Serologic laboratory findings in malignancy. Rheum Dis Clin North Am. 2011;37:507–25. doi: 10.1016/j.rdc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong SH, Misek DE, Wang H, Puravs E, Giordano TJ, Greenson JK, et al. An autoantibody-mediated immune response to calreticulin isoforms in pancreatic cancer. Cancer Res. 2004;64:5504–10. doi: 10.1158/0008-5472.CAN-04-0077. [DOI] [PubMed] [Google Scholar]
- 39.Himoto T, Kuriyama S, Zhang JY, Chan EK, Nishioka M, Tan EM. Significance of autoantibodies against insulin-like growth factor II mRNA-binding proteins in patients with hepatocellular carcinoma. Int J Oncol. 2005;26:311–7. [PubMed] [Google Scholar]
- 40.Ersvaer E, Zhang JY, McCormack E, Olsnes A, Anensen N, Tan EM, et al. Cyclin B1 is commonly expressed in the cytoplasm of primary human acute myelogenous leukemia cells and serves as a leukemia-associated antigen associated with autoantibody response in a subset of patients. Eur J Haematol. 2007;79:210–25. doi: 10.1111/j.1600-0609.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Wang K, Liu SS, Dai L, Zhang JY. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10:2863–72. doi: 10.1021/pr200141c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Disis ML, Cheever MA. HER-2/neu protein: a target for antigen-specific immunotherapy of human cancer. Adv Cancer Res. 1997;71:343–71. doi: 10.1016/s0065-230x(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 43.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 44.Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–51. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 45.Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, et al. Tumor markers in breast cancer—European Group on Tumor Markers recommendations. Tumour Biol. 2005;26:281–93. doi: 10.1159/000089260. [DOI] [PubMed] [Google Scholar]


